Introduction
Malignant pleural mesothelioma (MPM) is a highly
aggressive tumor of pleural mesothelial cell associated with
asbestos exposure (1,2) with a long latency period (~30–40
years), an incidence expected to increase worldwide prior to 2025
(3,4)
and a poor life expectancy (median survival time of ~12 months)
(2).
MPM can be divided into three major histological
subtypes: Epithelioid, accounting for ~60% of MPM and characterized
by a longer survival time (range, 12–27 months); sarcomatoid,
representing ~20% of all MPM and showing a shorter survival time
(range, 7–18 months); biphasic (a combination of epithelioid and
sarcomatoid histology), including the residual 20% with an
intermediate survival time (range, 8–21 months) (5).
Determination of prognostic markers of MPM at
diagnosis may aid in managing the patient in the therapeutic and
follow-up settings (6,7).
For determining MPM prognosis, the scoring systems
of the Cancer and Leukemia Group B (CALGB) and the European
Organization for Research and Treatment of Cancer (EORTC) are the
most useful among those currently available. These systems identify
a low performance status score, a non-epithelioid histology, weight
loss, male sex, leukocytosis and low hemoglobin as factors
associated with a poor prognosis (6–8).
However, performance status and histology are
considered, by clinicians, to have the main prognostic role
(6–8).
Despite these scoring systems, there is a
requirement for novel, highly sensitive and specific biological
markers for MPM (9).
Genetic markers with diagnostic or prognostic value
are currently under investigation in MPM tumor tissues. Chromosomal
alterations, DNA mutations, gene expression profile and the
deregulation of microRNA are very promising markers (10–12).
Moreover, interesting results derive also from studies on
circulating tumor cells, free DNA and microRNA present in body
fluids (6).
In recent years, other biomarkers have been proposed
in body fluids, including osteopontin, fibulin-3 and vascular
endothelial growth factor evaluated in plasma, serum and pleural
effusion, in addition to hyaluronic acid evaluated in serum and
pleural effusion and the high mobility group box 1 detected only in
serum (6,13,14).
They showed a low prognostic significance.
However, due to the small number of samples and the
heterogeneity of patients analyzed in the individual studies, the
clinical application of all the markers mentioned above, in MPM,
requires further confirmation (6,13,14).
The soluble mesothelin-related protein (SMRP) serves
an important role in the diagnosis of MPM as its levels in pleural
effusion (PE-SMRP) and in serum (Se-SMRP) have been found to be
significantly increased and associated with tumor size (15,16).
Therefore, the US Food and Drug Administration approved the SMRP
measurement as a routine test (Mesomark™ ELISA) to aid in
diagnosing and monitoring of patients with epithelioid or biphasic
MPM.
Although, in MPM, Se-SMRP shows a lower diagnostic
performance with respect to PE-SMRP (15), its levels have been used to
distinguish patients with MPM from individuals with benign pleural
diseases (17) and from patients
with pleural metastases of different types (18). However, the diagnostic performance of
Se-SMRP appears limited by poor sensitivity (6).
SMRP is derived from the mesothelin protein (MSLN).
MSLN is encoded by the MSLN gene (chromosome 16p13.3) as a
precursor protein of 71-kDa that is physiologically cleaved into
the 31-kDa megakaryocyte potentiating factor and the 40-kDa
mesothelin that is anchored by glycosylphosphatidylinositol to the
membrane of mesothelial cells and shed into the biological fluids
in which it is referred to as SMRP (19,20). The
production of SMRP may be due to abnormal splicing that results in
a secreted form or to cleavage from the membrane by the
TNFα-converting enzyme ADAM17 (20).
Mesothelin expression was identified in ~90% of
epithelioid MPM and in other cancer types, including lung
adenocarcinoma (60%), and breast (25%), esophageal (35%), ovarian
(60%), pancreatic, gastric (50%) and colon (40%) cancer (19,21).
Mesothelin expression is more prevalent in aggressive histological
subtypes of lung, breast (triple negative) and esophageal
(high-grade dysplasia) cancer (21).
Given that the expression of mesothelin is rather
limited in numerous normal tissues but elevated in solid tumors, it
represents a potential antigen target for therapy in various cancer
types, including mesothelioma, pancreatic, breast, colorectal and
lung cancer (5,22). In this regard, several therapeutic
approaches are under investigation which include the
anti-mesothelin immunotoxins SS1P, the chimeric monoclonal antibody
amatuximab, the antibody-drug conjugate anetumab ravtansine, the
cancer CRS-207 vaccine and chimeric antigen receptors T-cells
therapies (5,22).
The prognostic value of Se-SMRP and PE-SMRP levels
has also been investigated, but clinical results on this remain
uncertain and further studies are required to obtain definitive
conclusions (23–34).
The aim of the present study was to highlight the
prognostic significance of Se-SMRP in a cohort of Italian patients
with MPM. In addition, using a flexible, data-driven model of
survival probabilities, the functional form (linear or non-linear)
of the dose-response association between Se-SMRP levels and MPM
death rates was investigated which, to the best of our knowledge,
had not been previously reported in the literature.
Materials and methods
Patients
The present study included 60 patients with MPM
whose sera were collected at diagnosis prior to any treatment in
the Pneumology Department of Azienda Sanitaria Locale 5 (ASL 5), La
Spezia, Italy, between March 2008 and July 2011. The study period
ended in November 2016. Definitive diagnosis was made on the basis
of clinical signs, imaging data, cytological examination of PE and
tumor biopsy examination staining with hematoxylin-eosin and
immunohistochemistry. The characteristics of the patients were
collected retrospectively (Table
I).
 | Table I.Characteristics of patients with
malignant pleural mesothelioma analyzed for Se-SMRP. |
Table I.
Characteristics of patients with
malignant pleural mesothelioma analyzed for Se-SMRP.
| Patient
characteristic | n (%) |
|---|
| Age, years, at
diagnosis, median (range) | 73
(60–90) |
| Sex |
|
|
Male | 55 (91.6) |
|
Female | 5
(8.4) |
| Smoking habit |
|
|
Non-smoker | 28
(46.7) |
|
Current/former smoker | 32
(53.3) |
| Asbestos
exposure |
|
|
Unexposed | 21
(35.0) |
|
Exposed | 39
(65.0) |
| Symptoms |
|
|
Dyspnea | 35
(58.3) |
| Chest
pain | 16
(26.7) |
|
Unknown | 9
(15.0) |
| IMIG stage |
|
| I | 26
(43.3) |
| II | 18
(30.0) |
|
III/IV | 15
(25.0) |
|
Unknown | 1
(1.7) |
| ECOG-PS |
|
| 0 | 26
(43.3) |
| 1 | 22
(36.7) |
|
2/3 | 12
(20.0) |
|
Granulocyte/lymphocyte ratio, median
(range) | 3.0
(0.1–40.1) |
|
<2.70 | 23
(38.3) |
|
2.70–4.13 | 16
(26.7) |
|
>4.13 | 21
(35.0) |
| Platelet count,
×103/µl, median (range) | 285 (119–857) |
|
<245 | 18
(30.0) |
|
245–317 | 23
(38.3) |
|
>317 | 19
(31.7) |
| Histological
subtype |
|
|
Epithelioid | 43
(71.7) |
|
Sarcomatoid | 11
(18.3) |
|
Biphasic | 6
(10.0) |
| Chemotherapy |
|
|
Yes | 33
(55.0) |
| No | 13
(21.7) |
|
Unknown | 14
(23.3) |
| Se-SMRP, nM, median
(range) | 1.24
(0.05–13.5) |
|
<0.66 | 17
(28.3) |
|
0.66–1.46 | 18
(30.0) |
|
>1.46 | 25
(41.7) |
| Whole
sample | 60
(100.0) |
Patient treatment
All patients were treated in the Oncology Department
of ASL 5. Thirty-three patients (55.0%) received first-line
therapy, of which 32 (53.3%) had pemetrexed plus cisplatin or
carboplatin and 1 (1.7%) had gemcitabine plus carboplatin.
Fifteen patients (25.0%) were treated with
pemetrexed plus cisplatin in second-line therapy, while 13 (21.7%)
had only supportive care and 14 (23.3%) were treated in other
hospitals.
Soluble mesothelin-related protein
(SMRP) detection assay
The analysis of SMRP was performed in the Clinical
Pathology Department of ASL 5. Aliquots from sera were centrifuged
(1,500 × g for 10 mins at 4°C) and the supernatant was stored at
−20°C until the SMRP analysis was performed.
The determination of Se-SMRP concentrations was
performed in duplicate by an ELISA test (ELISA kit ‘Mesomark’; cat.
no. 801-900; Fujirebio Diagnostic), which has a detection limit of
0.1 ng/ml.
Statistical analyses
Overall survival (OS) time was calculated as the
interval between the date of diagnosis and the date of mortality or
the last follow-up. Kaplan-Meier analysis was applied to describe
and investigate OS probabilities according to tertile-based
categories (33rd and 66th percentile) of Se-SMRP and the log-rank
test was used to statistically assess differences among OS
probabilities. Multivariable Cox regression analysis was performed
to estimate death rate ratios (RR) and corresponding 95% confidence
limits (95% CL). Differences among death rates were assessed using
the likelihood ratio test (35). In
order to highlight a linear or non-linear dose-response
association, potentially overlooked because of the categorization
process, a Cox regression model with a restricted cubic spline
(RCS) function of Se-SMRP levels was fitted to the OS data. The use
of RCS has been widely described as a valid strategy to analyze
dose-response associations as it provides greater flexibility for
fitting data and modeling more complex associations while adjusting
for other covariates (36).
Se-SMRP levels were also log-transformed in order to
decrease the influence that outlying measurements may unduly exert
on the statistical indexes.
All tests were two tailed and a P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed using Stata (StataCorp. Stata
Statistical Software; version 14.2).
Results
Patient characteristics
A total of 60 patients with MPM were studied at
diagnosis, prior to any treatment. The patients' mean age was 73
years (range, 60–90 years) and 55 (91.6%) were male. The MPM
histology was composed of 43 epithelioid, 11 sarcomatoid and 6
biphasic subtypes. According to the International Mesothelioma
Interest Group stage classification, 26 (43.3%), 18 (30.0%) and 15
(25.0%) patients had MPM at stage I, II and III/IV respectively,
while one patient (1.7%) had a missing value. Twenty-six patients
(43.3%) had an ECOG-PS score of 0, 22 patients (36.7%) had an
ECOG-PS score of 1 and 12 patients (20.0%) had an ECOG-PS score
>1. Other patient characteristics are summarized in Table I. At the end of the study period, the
median follow-up time was 13.9 months (range, 0.7–61.4 months). A
total of 54 patients (90.0%) died and the median survival time was
13.4 months (95% CL=10.1–19.4; Table
II).
 | Table II.Effect of Se-SMRP on MPM mortality
rates estimated through the Cox regression analysis. |
Table II.
Effect of Se-SMRP on MPM mortality
rates estimated through the Cox regression analysis.
|
|
|
| Follow-up time,
months | Survival time,
months |
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Se-SMRP, nM | N | Mortalities
(%) | Median | Range | Median | 95% CL | RR | 95% CL | P-value |
|---|
|
<0.66 | 17 | 16 (94.1) | 14.4 | 0.7–60.4 | 14.4 | 10.1–25.3 | 1.00 | (Ref.) | 0.199 |
|
0.66–1.46 | 18 | 15 (83.3) | 17.9 | 3.3–59.6 | 16.3 | 6.8–32.4 | 1.87 | 0.75–4.67 |
|
|
>1.46 | 25 | 23 (92.0) | 11.8 | 1.0–61.4 | 11.8 | 6.6–20.5 | 1.95 | 0.78–4.92 |
|
| Whole
sample | 60 | 54 (90.0) | 13.9 | 0.7–61.4 | 13.4 | 10.1–19.4 | 1.30a | 0.73–2.33 | 0.378 |
Association between Se-SMRP levels and
OS
The baseline median Se-SMRP concentration was 1.24
nM (range, 0.05–13.5 nM; Table I).
Kaplan-Meier OS probabilities estimated according to the
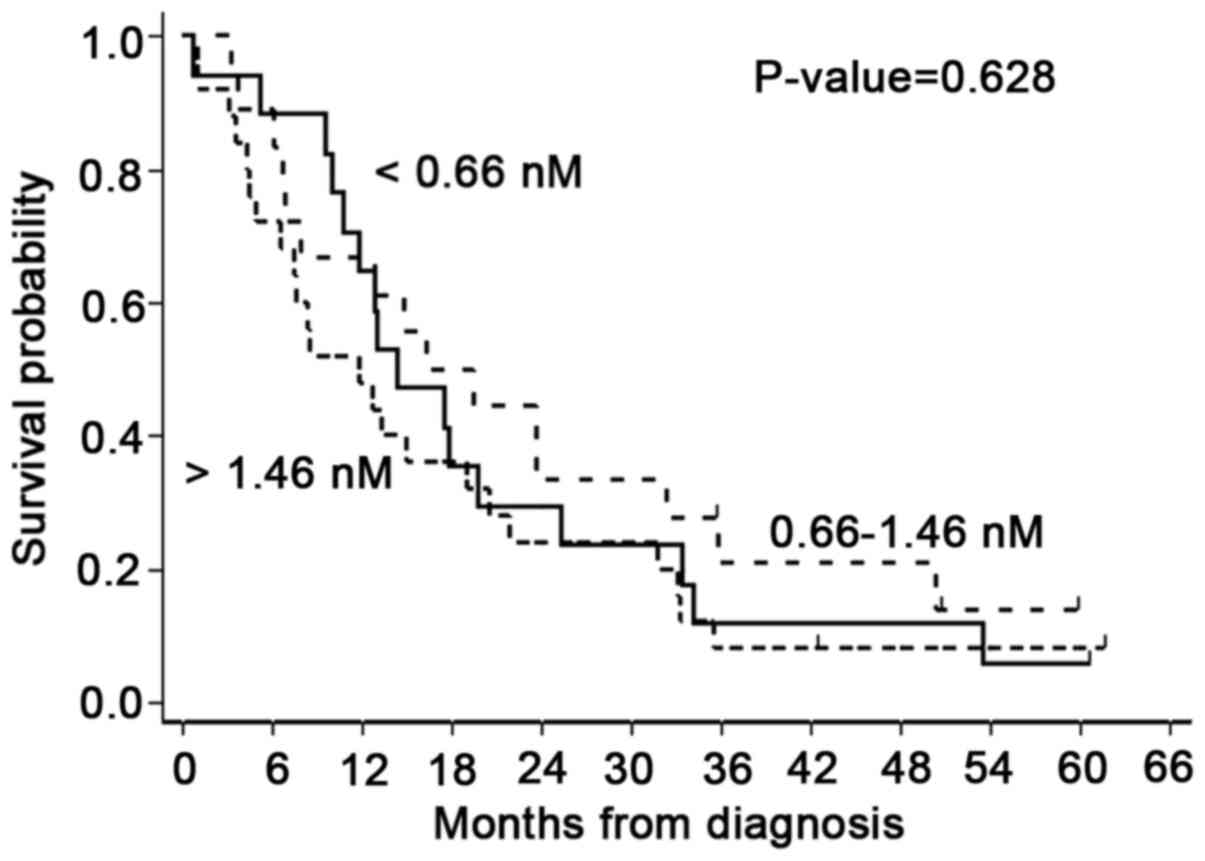
tertile-based categorization of Se-SMRP levels (<0.66 nM;
0.66–1.46 nM; >1.46 nM) did not show statistically significant
differences (P=0.628; Fig. 1). In
addition, Cox regression analysis, adjusted for sex, age at
diagnosis, disease stage, histological subtype and ECOG-PS,
highlighted that patients belonging to the intermediate and higher
categories had an RR of 1.87 (95% CL=0.75–4.67) and 1.95 (95%
CL=0.78–4.92), respectively, compared with patients in the lower
category, assumed as reference (Table
II). On average, this result corresponds to a death rate
increase of ~30% (RR=1.30; 95% CL=0.73–2.33) per 5 nM unit increase
in Se-SMPR (Table II).
Dose-response association between
Se-SMRP and mortality rates
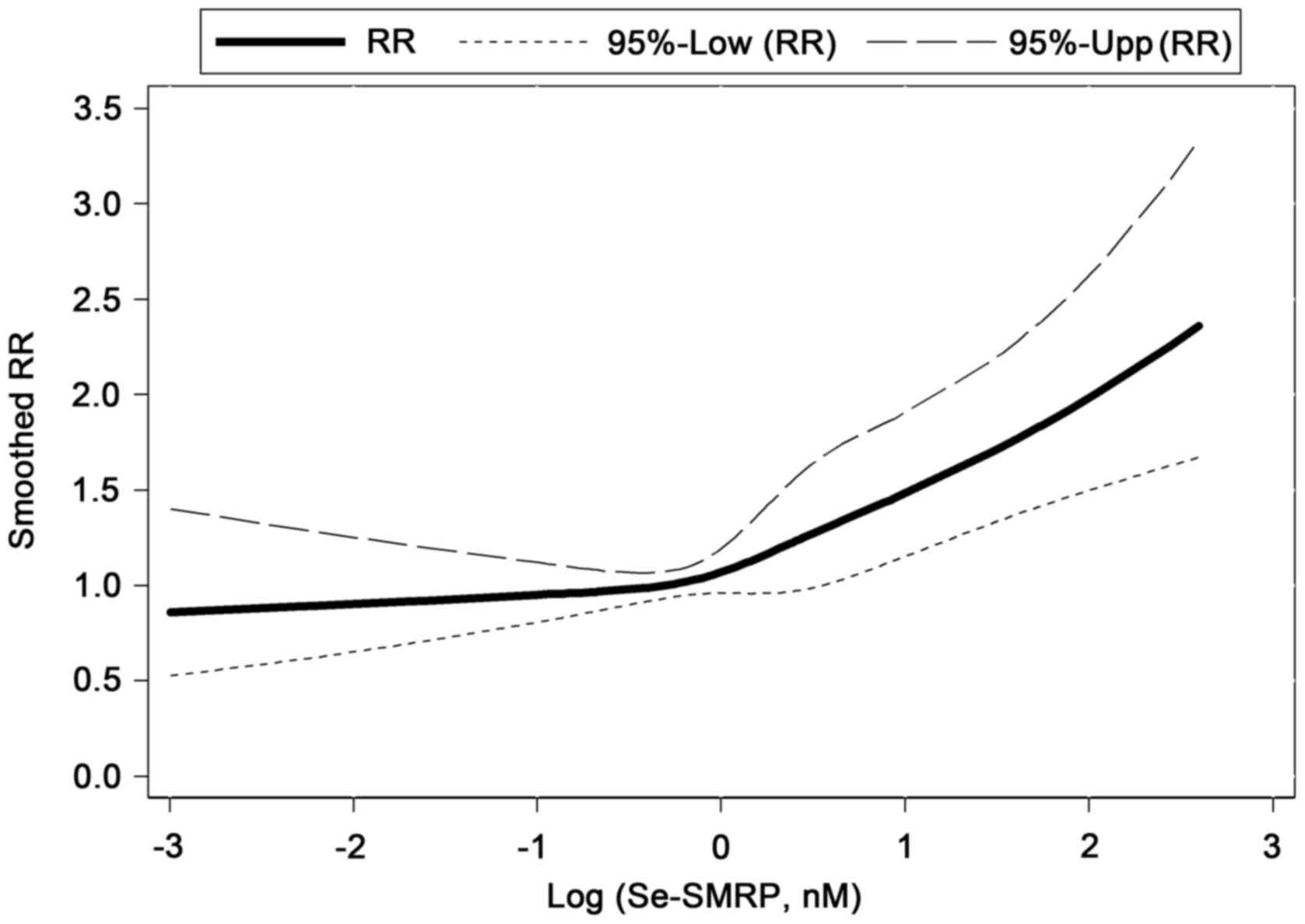
In order to identify a dose-response association
between Se-SMRP and mortality rates, a multivariable Cox regression
model with a RCS function of log-transformed Se-SMRP was used. This
method allowed further evidence of a positive association between
high Se-SMRP levels and mortality rates to be obtained. In
particular, a notably increasing non-linear tendency in mortality
rates was estimated when Se-SMRP levels exceeded 1.00 nM (Fig. 2).
Discussion
The CALGB and EORTC identify ECOG-PS and
histological subtypes as the two main prognostic factors of MPM.
The ECOG-PS incorporates the patient history and their physical
condition to assign a score value according to the deterioration in
their health status. However, the ECOG-PS scale is subjective,
prone to bias and affected by high inter-observer variability
(2,9). By contrast, MPM histological subtypes
are carefully defined by a team of pathologists and are considered
a more accurate and objective criterion. However, the evaluation of
the pathological assessment is performed on tumor material, usually
taken by invasive procedures at diagnosis as thoracoscopic biopsies
or computed tomography-guided biopsy. These techniques, although
tested and efficient, are associated with considerable morbidity
and cost, and cannot always be performed due to specific patient
conditions (37,38).
Furthermore, the material taken may be inadequate
because it is derived from non-tumor areas of the pleura or
quantitatively insufficient for histological analysis to be
performed.
Additionally, the biphasic subtype may not be
diagnosed because the sarcomatoid component, characterized by the
morphology of the spindle cells, can be scarce and difficult to be
detected (14,39).
Previous studies have focused on identifying novel
markers measurable in biological fluids, including serum and PE
(6,13,14).
Serum is easily obtainable with minimal stress for patients, while
PE is present in the majority of MPM patients at diagnosis and
routinely withdrawn for diagnosis and therapeutic purposes.
The ideal prognostic biomarker to be determined in
serum or PE has not yet been identified. Several biomarkers have
been proposed but, to date, none of them can be considered adequate
to evaluate the prognosis of MPM because of heterogeneous clinical
results in various published studies (6,13,14). By
contrast, SMRP may be a prognostic marker for MPM as reported for
other malignancies, including ovarian, lung, breast and esophageal
cancers (19,21).
A previous study demonstrated that PE-SMRP did not
aid in defining the prognosis of MPM. Additionally, the Cox
regression modeling, including an RCS had only showed a moderately
increasing non-linear trend in the mortality rate. Therefore, it
was concluded that PE-SMRP is not recommended for routine use in
the clinical management of patients with MPM (40).
In the present study, using the same statistical
methodology, the analysis was extended to determine whether
Se-SMRP, unlike PE-SMRP, may have a clinical application.
The prognostic value of Se-SMRP is currently highly
debated and remains unclear. In a meta-analysis based on results
from another study involving 579 patients (18,23–29),
Tian et al (30) demonstrated
that high levels of Se-SMRP represent a negative, statistically
significant prognostic factor for MPM, as assessed in univariate
analysis (pooled RR=1.96, 95% CI: 1.53–2.50, P<0.001),
Furthermore, some of these studies demonstrated that the
aforementioned association was significant in a multivariable
context (18,23–25).
Pass et al (31), using EDTA plasma, reported that
higher levels of plasma SMRP in MPM were associated with a poor
prognosis following adjusting for the ECOG-PS, histology, sex,
pre-treatment and white blood cell count and suggested SMRP as a
possible biomarker to improve the prognostic capability of the
EORTC-index (31).
Plasma-SMRP detection is to be considered in the
same way as Se-SMRP. Creaney et al (41) reported a significant correlation
between the levels of SMRP in serum and plasma (Pearson's
coefficient=0.91; P<0.001).
By contrast, other studies have supported the
hypothesis that Se-SMRP has no prognostic significance (18,32–34).
In the present study, using a Cox model including
tertile-based categorical terms for Se-SMRP (cut-off points: 0.66
and 1.46 nM), a positive association between Se-SMRP levels and
mortality rates was identified. Such an association was also
confirmed using a conservative, flexible and data-driven fitting
procedure, namely a RCS function of Se-SMRP, which highlighted a
net increase in mortality rates particularly for concentrations of
Se-SMRP higher than 1.0 nM.
The major limitations of the present study are due
to the low statistical power and the high level of patient
heterogeneity, with particular reference to the therapeutic
treatments. Although such limitations are common to numerous other
investigations in this field, it is worth noting that they are two
important but distinct issues: The former depends mainly on the
small sample size due to the rarity of MPM; the latter on
imbalances in the individual baseline characteristics, given the
observational (non-experimental) structure of the study design. In
the first case, only further and wider investigations, supported by
meta-analyses, will be able to confirm the results of the present
study from a statistical point of view. In the second case, the use
of statistical modeling, including the multivariable Cox
regression, is able to mitigate the confounding effect that the
aforementioned imbalances can exert on the study association (life
expectancy vs. Se-SMRP levels).
In conclusion, the present study provides
interesting evidence that Se-SMRP may be considered a prognostic
biomarker in patients with MPM. Ultimately, future multicenter
studies based on larger cohorts, allowing more powerful statistical
analyses, may aid in recognizing Se-SMRP as a prognostic marker
useful in routine applications for clinical management of patients
with MPM.
Acknowledgements
The authors of the present study would like to thank
Dr E. Battolla and the staff at Laboratory of Clinical Pathology in
which the analysis was performed, the staff at the Pneumology
Department for collecting serum from patients with mesothelioma and
the staff at the Histopathology and Cytopathology Department for
performing histological diagnosis at Azienda Sanitaria Locale 5, La
Spezia, Italy.
Funding
The present study was funded by AIL La
Spezia-Sezione ‘Francesca Lanzone’, Project Code MT2019/20, to SR;
Italian Ministry of Health, 5×1000 2015, Project Code del.1410/2017
and RC-2019, Project Code M728A, IRCCS Ospedale Policlinico San
Martino, to MPP and VF.
Availability of data and materials
All datasets used and/or analyzed in the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
SR and MPP conceived and designed the study and were
responsible for supervising the study. PF, MCF and MT recruited the
patients and performed the study. AV, PAC, UG, PD and GB were
responsible for the management of the patients with mesothelioma.
SR, MPP and VF drafted the manuscript. VF and RC performed the
statistical analyses. SR, MPP, PD, RC and VF critically revised the
manuscript for important intellectual content. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Liguria Region
Ethics Committee (P.R. 207REG2014). All patients enrolled in the
study provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gibbs AR: Role of asbestos and other
fibres in the development of diffuse malignant mesothelioma.
Thorax. 45:649–654. 1990. View Article : Google Scholar
|
|
2
|
Bibby AC, Tsim S, Kanellakis N, Ball H,
Talbot DC, Blyth KG, Maskell NA and Psallidas I: Malignant pleural
mesothelioma: An update on investigation, diagnosis and treatment.
Eur Respir Rev. 25:472–486. 2016. View Article : Google Scholar
|
|
3
|
Marinaccio A, Binazzi A, Marzio DD,
Scarselli A, Verardo M, Mirabelli D, Gennaro V, Mensi C, Riboldi L,
Merler E, et al: Pleural malignant mesothelioma epidemic:
Incidence, modalities of asbestos exposure and occupations involved
from the Italian national register. Int J Cancer. 130:2146–2154.
2012. View Article : Google Scholar
|
|
4
|
Robinson BW: Malignant pleural
mesothelioma: An epidemiological perspective. Ann Cardiothorac
Surg. 1:491–496. 2012.
|
|
5
|
Yap TA, Aerts JG, Popat S and Fennell DA:
Novel insights into mesothelioma biology and implications for
therapy. Nat Rev Cancer. 17:475–488. 2017. View Article : Google Scholar
|
|
6
|
Pass HI, Alimi M, Carbone M, Yang H and
Goparaju CM: Mesothelioma biomarkers: A review highlighting
contributions from the early detection research network. Cancer
Epidemiol Biomarkers Prev. Jul 22–2020.(Epub ahead of print). doi:
10.1158/1055-9965.EPI-20-0083. View Article : Google Scholar
|
|
7
|
Billé A, Krug LM, Woo KM, Rusch VW and
Zauderer MG: Contemporary analysis of prognostic factors in
patients with unresectable malignant pleural mesothelioma. J Thorac
Oncol. 11:249–255. 2016. View Article : Google Scholar
|
|
8
|
Linton A, Pavlakis N, O'Connell R, Soeberg
M, Kao S, Clarke S, Vardy J and van Zandwijk N: Factors associated
with survival in a large series of patients with malignant pleural
mesothelioma in new south Wales. Br J Cancer. 111:1860–1869. 2014.
View Article : Google Scholar
|
|
9
|
Røe OD: Mesothelioma diagnosis and
prognosis, are we moving beyond histology and performance status
towards circulating biomarkers? J Thorac Dis. 10 (Suppl
17):S1956–S1961. 2018. View Article : Google Scholar
|
|
10
|
Jean D, Daubriac J, Le Pimpec-Barthes F,
Galateau-Salle F and Jaurand MC: Molecular changes in mesothelioma
with an impact on prognosis and treatment. Arch Pathol Lab Med.
136:277–293. 2012. View Article : Google Scholar
|
|
11
|
Bueno R, Stawiski EW, Goldstein LD,
Durinck S, De Rienzo A, Modrusan Z, Gnad F, Nguyen TT, Jaiswal BS,
Chirieac LR, et al: Comprehensive genomic analysis of malignant
pleural mesothelioma identifies recurrent mutations, gene fusions
and splicing alterations. Nat Genet. 48:407–416. 2016. View Article : Google Scholar
|
|
12
|
Lo Russo G, Tessari A, Capece M, Galli G,
de Braud F, Garassino MC and Palmieri D: MicroRNAs for the
diagnosis and management of malignant pleural mesothelioma: A
literature review. Front Oncol. 8:6502018. View Article : Google Scholar
|
|
13
|
Cavallari I, Urso L, Sharova E, Pasello G
and Ciminale V: Liquid biopsy in malignant pleural mesothelioma:
State of the art, pitfalls, and perspectives. Front Oncol.
9:7402019. View Article : Google Scholar
|
|
14
|
Arnold DT, De Fonseka D, Hamilton FW,
Rahman NM and Maskell NA: Prognostication and monitoring of
mesothelioma using biomarkers: A systematic review. Br J Cancer.
116:731–741. 2017. View Article : Google Scholar
|
|
15
|
Ferro P, Canessa PA, Battolla E, Dessanti
P, Franceschini MC, Chiaffi L, Morabito A, Fontana V, Pezzi R,
Fedeli F, et al: Mesothelin is more useful in pleural effusion than
in serum in the diagnosis of pleural mesothelioma. Anticancer Res.
33:2707–2713. 2013.
|
|
16
|
Fontana V, Vigani A, Pistillo MP, Giannoni
U, Rosemberg I, Canessa PA, Berisso G, Ferro P, Franceschini M,
Tonarelli M and Roncella S: The correlation of serum mesothelin
level with pleural thickness in malignant pleural mesothelioma
makes it a valuable tool for monitoring tumor progression. J Thorac
Oncol. 14:e92–e94. 2019. View Article : Google Scholar
|
|
17
|
Luo L, Shi HZ, Liang QL, Jiang J, Qin SM
and Deng JM: Diagnostic value of soluble mesothelin-related
peptides for malignant mesothelioma: A meta-analysis. Respir Med.
104:149–156. 2010. View Article : Google Scholar
|
|
18
|
Schneider J, Hoffmann H and Dienemann H,
Herth FJ, Meister M, Muley T and Dienemann H: Diagnostic and
prognostic value of soluble mesothelin-related proteins in patients
with malignant pleural mesothelioma in comparison with benign
asbestosis and lung cancer. J Thorac Oncol. 3:1317–1324. 2008.
View Article : Google Scholar
|
|
19
|
Chang K and Pastan I: Molecular cloning of
mesothelin, a differentiation antigen present on mesothelium,
mesotheliomas, and ovarian cancers. Proc Natl Acad Sci USA.
93:136–140. 1996. View Article : Google Scholar
|
|
20
|
Lv J and Li P: Mesothelin as a biomarker
for targeted therapy. Biomark Res. 7:182019. View Article : Google Scholar
|
|
21
|
Morello A, Sadelain M and Adusumilli PS:
Mesothelin-targeted CARs: Driving T cells to solid tumors. Cancer
Discov. 6:133–146. 2016. View Article : Google Scholar
|
|
22
|
Hassan R, Thomas A, Alewine C, Le DT,
Jaffee EM and Pastan I: Mesothelin immunotherapy for cancer: Ready
for prime time? J Clin Oncol. 34:4171–4179. 2016. View Article : Google Scholar
|
|
23
|
Creaney J, Francis RJ, Dick IM, Musk AW,
Robinson BW, Byrne MJ and Nowak AK: Serum soluble mesothelin
concentrations in malignant pleural mesothelioma: Relationship to
tumor volume, clinical stage and changes in tumor burden. Clin
Cancer Res. 17:1181–1189. 2011. View Article : Google Scholar
|
|
24
|
Grigoriu BD, Scherpereel A, Devos P,
Chahine B, Letourneux M, Lebailly P, Grégoire M, Porte H, Copin MC
and Lassalle P: Utility of osteopontin and serum mesothelin in
malignant pleural mesothelioma diagnosis and prognosis assessment.
Clin Cancer Res. 13:2928–2935. 2007. View Article : Google Scholar
|
|
25
|
Cristaudo A, Foddis R, Vivaldi A,
Guglielmi G, Dipalma N, Filiberti R, Neri M, Ceppi M, Paganuzzi M,
Ivaldi GP, et al: Clinical significance of serum mesothelin in
patients with mesothelioma and lung cancer. Clin Cancer Res.
13:5076–5081. 2007. View Article : Google Scholar
|
|
26
|
Dipalma N, Luisi V, Di Serio F, Fontana A,
Maggiolini P, Licchelli B, Mera E, Bisceglia L, Galise I, Loizzi M,
et al: Biomarkers in malignant mesothelioma: Diagnostic and
prognostic role of soluble mesothelin-related peptide. Int J Biol
Markers. 26:160–165. 2011. View Article : Google Scholar
|
|
27
|
Grigoriu BD, Chahine B, Vachani A, Gey T,
Conti M, Sterman DH, Marchandise G, Porte H, Albelda SM and
Scherpereel A: Kinetics of soluble mesothelin in patients with
malignant pleural mesothelioma during treatment. Am J Respir Crit
Care Med. 179:950–954. 2009. View Article : Google Scholar
|
|
28
|
Creaney J, Yeoman D, Naumoff LK, Hof M,
Segal A, Musk AW, De Klerk N, Horick N, Skates SJ and Robinson BW:
Soluble mesothelin in effusions: A useful tool for the diagnosis of
malignant mesothelioma. Thorax. 62:569–576. 2007. View Article : Google Scholar
|
|
29
|
Linch M, Gennatas S, Kazikin S, Iqbal J,
Gunapala R, Priest K, Severn J, Norton A, Ayite B, Bhosle J, et al:
A serum mesothelin level is a prognostic indicator for patients
with malignant mesothelioma in routine clinical practice. BMC
Cancer. 14:6742014. View Article : Google Scholar
|
|
30
|
Tian L, Zeng R, Wang X, Shen C, Lai Y,
Wang M and Che G: Prognostic significance of soluble mesothelin in
malignant pleural mesothelioma: A meta-analysis. Oncotarget.
8:46425–46435. 2017. View Article : Google Scholar
|
|
31
|
Pass HI, Goparaju C, Espin-Garcia O,
Donington J, Carbone M, Patel D, Chen Z, Feld R, Cho J, Gadgeel S,
et al: Plasma biomarker enrichment of clinical prognostic indices
in malignant pleural mesothelioma. J Thorac Oncol. 11:900–909.
2016. View Article : Google Scholar
|
|
32
|
Hollevoet K, Nackaerts K, Thas O, Thimpont
J, Germonpré P, De Vuyst P, Bosquée L, Legrand C, Kellen E, Kishi
Y, et al: The effect of clinical covariates on the diagnostic and
prognostic value of soluble mesothelin and megakaryocyte
potentiating factor. Chest. 141:477–484. 2012. View Article : Google Scholar
|
|
33
|
Ak G, Tada Y, Shimada H, Metintas S, Ito
M, Hiroshima K, Tagawa M and Metintas M: Midkine is a potential
novel marker for malignant mesothelioma with different prognostic
and diagnostic values from mesothelin. BMC Cancer. 17:2122017.
View Article : Google Scholar
|
|
34
|
Creaney J, Dick IM, Meniawy TM, Leong SL,
Leon JS, Demelker Y, Segal A, Musk AW, Lee YC, Skates SJ, et al:
Comparison of fibulin-3 and mesothelin as markers in malignant
mesothelioma. Thorax. 69:895–902. 2014. View Article : Google Scholar
|
|
35
|
Marubini E and Valsecchi MG: Analyzing
Survival Data from Clinical Trials and Observational Studies. John
Wiley and Sons; New York, NY: 1995
|
|
36
|
Harrell FE Jr: Regression modelling
strategies. 2nd edition. Springer Verlag; New York, NY: pp. 22–28.
2015
|
|
37
|
Walters J and Maskell NA: Biopsy
techniques for the diagnosis of mesothelioma. Recent Results Cancer
Res. 189:45–55. 2011. View Article : Google Scholar
|
|
38
|
Tsao MS, Carbone M, Galateau-Salle F,
Moreira AL, Nicholson AG, Roden AC, Adjei AA, Aubry MC, Fennell DA,
Gomez D, et al: Pathologic considerations and standardization in
mesothelioma clinical trials. J Thorac Oncol. 14:1704–1717. 2019.
View Article : Google Scholar
|
|
39
|
Galateau Salle F, Le Stang N, Nicholson
AG, Pissaloux D, Churg A, Klebe S, Roggli VL, Tazelaar HD, Vignaud
JM, Attanoos R, et al: New insights on diagnostic reproducibility
of biphasic mesotheliomas: A multi-institutional evaluation by the
international mesothelioma panel from the MESOPATH reference
center. J Thorac Oncol. 13:1189–1203. 2018. View Article : Google Scholar
|
|
40
|
Fontana V, Pistillo MP, Vigani A, Canessa
PA, Berisso G, Giannoni U, Ferro P, Franceschini MC, Carosio R,
Tonarelli M, et al: Determination of mesothelin levels in pleural
effusion does not help predict survival of patients with malignant
pleural mesothelioma. Anticancer Res. 39:5219–5223. 2019.
View Article : Google Scholar
|
|
41
|
Creaney J, Yeoman D, Musk AW, de Klerk N,
Skates SJ and Robinson BW: Plasma versus serum levels of
osteopontin and mesothelin in patients with malignant
mesothelioma-which is best? Lung Cancer. 74:55–60. 2011. View Article : Google Scholar
|