Introduction
Sporadic colorectal cancer (CRC) is the second most
common cause of cancer death in developed countries with an
estimated 1,8 million new cases and 862,000 deaths in 2018
(1,2). Despite the overall advances in
diagnosis and therapy, survival rates for colorectal cancer remain
disappointing at approximately 65% depending on the stage. Hence,
finding molecular markers that can improve patient diagnosis and
treatment selection is necessary.
Epidermal growth factor receptor (EGFR) is a
membrane receptor of the receptor tyrosine kinase (ErbBs) family
that plays an important role in cell proliferation, survival,
differentiation and invasion in sporadic colon cancer (3,4). Several
studies have shown that EGFR is overexpressed in
approximately 50% of colon tumours (5–7) and
there is an increased level of EGFR protein at the invasive tumour
front (ITF) in comparison to the tumour centre (8,9). This
increased EGFR expression has been related to the presence of
tumour budding leading to tumour invasion and other aggressive
pathohistological features (10–13).
Overexpression of EGFR protein in CRC can be rarely attributed to
gene amplification and is more often attributed to polymorphisms in
the EGFR gene (14).
Polymorphic regions are excellent candidates as
possible prognostic biomarkers for cancer patients. Their main
advantage is that they can be easily assessed from blood and normal
and tumour tissue and determined by straightforward and
well-established methods. One of these regions is the CA
dinucleotide repeat polymorphism in intron 1 of the EGFR
gene. CA-SSR1 is important due to its close location to the second
enhancer (15) which endows it with
the ability to influence the expression of the EGFR gene
(16–21). Given that CA-SSR1 could modulate
EGFR transcription, changes in its sequence could also alter
the levels of EGFR protein. Different studies investigated the role
of CA repeats and its prognostic implication in various types of
cancer and some of them have brought into connection an increasing
number of repeats and decreasing levels of both EGFR mRNA and
protein expression (22,23). However, the results and its potential
predictive impact in CRC remain contrasting and inconclusive
(24,25).
Sporadic CRCs can be divided into
microsatellite-stable (MSS) tumours and tumours having
microsatellite instability (MSI) as a result of failure in the DNA
mismatch repair (MMR) system (26).
This failure results in changes in the length of microsatellite
sequences, potentially also affecting the EGFR CA-SSR1
polymorphism. Even though it is recognized that MSI can affect
repeat elements of the EGFR gene, and subsequent EGFR
expression (27,28), clinical and pathological significance
is still not extensively studied.
Therefore, we decided to investigate the effect of
the EGFR CA-SSR1 polymorphism on mRNA and protein expression
by considering microsatellite status in CRC tumours. Furthermore,
we wanted to examine the difference in EGFR mRNA and protein
expression between the tumour centre and invasive tumour front in
accordance to MSI status to clarify the role of EGFR in CRC tumour
progression. Additionally, we performed a correlation analysis
between the EGFR expression and CRC clinicopathological
characteristics in MSS and MSI-H tumours.
Materials and methods
Study subjects and DNA isolation
Our study included tumour samples from 160 patients
diagnosed with sporadic colon adenocarcinoma obtained from the
Croatian Tumour Bank (29). Tissue
samples were collected from 2013 to 2019 during routine surgery
performed in Merkur Clinical Hospital. Fresh tumour samples were
stored at −80°C until DNA and RNA extraction. From each patient two
samples of tumour were obtained: One corresponding to the tumour
centre (T1) and the other corresponding to the invasive tumour
front (T2) as well as adjacent normal colon tissue. If metastasis
(M) was present, samples were retrieved for the analysis. In MSS
tumours 15 and in MSI tumours 2 metastasis samples were obtained.
Before use in the study, each specimen was verified by a
pathologist (A.Š.).
DNA was extracted from the blood and tumour tissues
as well as corresponding normal tissue samples located 15 cm from
the tumour edge. DNA extraction was performed using proteinase K
digestion and phenol-chloroform extraction (30).
PCR
For MSI analysis paired normal and tumour DNA was
analysed for changes in five loci each, using previously published
Bethesda panel (26).
The primer sequences used were as follows: D2S123:
forward, 5′-AAACAGGATGCCTGCCTTTA-3′ and reverse,
5′-GGACTTTCCACCTATGGGAC-3′; D5S346: forward,
5′-ACTCACTCTAGTGATAAATCGGG-3′ and reverse,
5′-AGCAGATAAGACAGTATTACTAGTT-3′; D17S250: forward,
5′-GGAAGAATCAAATAGACAAT-3′ and reverse,
5′-GCTGGCCATATATATATTTAAACC-3′; BAT-25a: forward,
5′-TCGCCTCCAAGAATGTAAGT-3′ and reverse,
5′-TCTGCATTTTAACTATGGCTC-3′; BAT-26: forward,
5′-CTGCGGTAATCAAGTTTTTAG-3′ and reverse,
5′-AACCATTCAACATTTTTAACCC-3′. Samples were considered MSI low
(MSI-L) samples if only one marker was changed and MSI high (MSI-H)
if at least two out of five markers showed instability.
For EGFR intron 1 polymorphism genotyping,
forwar, 5′-GGGCTCACAGCAAACTTCTC-3′ and reverse,
5′-AAGCCAGACTCGCTCATGTT-3 EGFR primers were used.
Genomic DNA (100 ng) was used as a template in a
reaction volume of 25 µl containing 5 pmol of each primer, 50 µM of
each dNTP, and 1 U of Taq Gold DNA polymerase (Applied Biosystems;
Thermo Fisher Scientific, Inc.). PCR tests were carried out in an
Applied Biosystems GeneAmp PCR System 2400 for 30 cycles. Annealing
temperatures for each primer set were optimized in pilot studies
before processing experimental samples.
Short tandem repeats analysis
Polymorphic marker analysis of D2S123, D5S346,
D17S250 and EGFR intron 1 polymorphism was performed by
non-denaturing polyacrylamide electrophoresis as previously
described (31). In brief, the PCR
product was mixed with loading buffer and loaded onto a 10%
non-denaturing polyacrylamide gel. Electrophoresis was performed
for 16 h at room temperature and gels were silver stained. MSI was
defined as a visible change in the allele:allele ratio in tumours
compared with matching normal tissue.
Analysis of BAT-25 and BAT-26, as well as validation
of fragment length for the EGFR intron 1 polymorphism, was
carried out with an ABI Prism® 310 genetic analyser with
a GeneScan Analyzer (Life Technologies; Thermo Fisher Scientific,
Inc.). The primers used for the genetic analyses were labelled with
fluorescent dye. The GS500 ROX (−250 LIZ; Thermo Fisher Scientific,
Inc.) size marker was added to each sample.
RNA extraction, cDNA synthesis and
quantitative PCR (qPCR)
Total RNA was extracted from snap frozen samples of
resected colon carcinoma and corresponding normal tissues using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). To
quantify EGFR expression levels, 1 mg of RNA was used to
synthesize equal amounts of cDNA using the High-Capacity cDNA
Reverse Transcription Kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.). qPCR analysis was performed using an ABI PRISM
7300 sequence detection system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) and predeveloped TaqMan assay reagents:
Hs01076090 for the EGFR gene and Hs01060665 for the
beta-actin gene as an internal control. PCR was carried out
according to the manufacturer's protocol. The method used for
analysis was 2−ΔΔCq (32).
Immunohistochemical (IHC) analysis of
EGFR expression
Formalin-fixed and paraffin-embedded blocks were cut
into 2 µm sections at least 24 h prior to immunostaining and
mounted on microscope slides. Antigen retrieval was carried out in
citrate buffer at 95°C for 5 min. Sections were incubated overnight
with anti-EGFR monoclonal antibodies (1:100, Santa Cruz
Biotechnology). Secondary detection was performed using a goat
anti-rabbit IgG antibody conjugated to horseradish peroxidase (HRP)
(ready to use; Cell Signalling Technology). Antigen-antibody
complexes were visualized by incubation in diaminobenzidine
tetrahydrochloride (Dako) for 3 min and counterstained with
haematoxylin. The EGFR stained slides were evaluated for staining
intensity which was scored from 0 (no staining) to 3 (strongest
staining) using light microscope at the magnification of ×100. All
control slides (without primary antibody staining) were negative
for staining. For further analysis, EGFR expression was divided
into a high and low category. EGFR expression was defined low when
there was either no staining (Score 0) or there was a weak positive
(light brown) staining (Score 1), and high EGFR expression was
defined when staining intensity was either intermediate (Score 2)
or strong (Score 3).
Statistical analysis
Statistical analyses were performed using the
GraphPad Prism statistical package (GraphPad Software, Inc.).
Correlations between the EGFR CA-SSR1 genotype and
EGFR mRNA expression were analysed using Spearman's
correlation coefficient and linear regression analysis. The
relationship between the sum of repeats in both alleles in the
EGFR CA-SSR1 polymorphism and EGFR protein levels was
examined by two-tailed unpaired Student's t-test and the
Mann-Whitney test. Two-way analysis of variance (ANOVA) with
Bonferroni correction was used to compare EGFR mRNA
expression between the groups. For EGFR IHC analysis and further
correlation with clinicopathological characteristics, contingency
table with Fisher's exact test was used to calculate statistical
significance. Overall survival rate was determined by Kaplan-Meier,
and statistical differences between groups were calculated with the
log-rank test. Data are presented as the mean ± SEM. Values of
*P<0.05, **P<0.01 and ***P<0.001 were considered
statistically significant.
Results
CA-SSR1 polymorphism of the EGFR gene
and microsatellite instability
The EGFR intron 1 CA repeat polymorphism
(CA-SSR1) was genotyped in 160 blood samples from patients with
sporadic colon cancer. The number of CA repeats ranged in length
from 15 to 21 and homozygous 16/16 CA repeats were the most
frequent genotype (24.5%), followed by heterozygous 16/18 (16.6%)
and 16/20 (14.4%) CA repeats. The frequencies of 10 most common
genotypes (present in 140 patients; 87%) are shown in Fig. 1A.
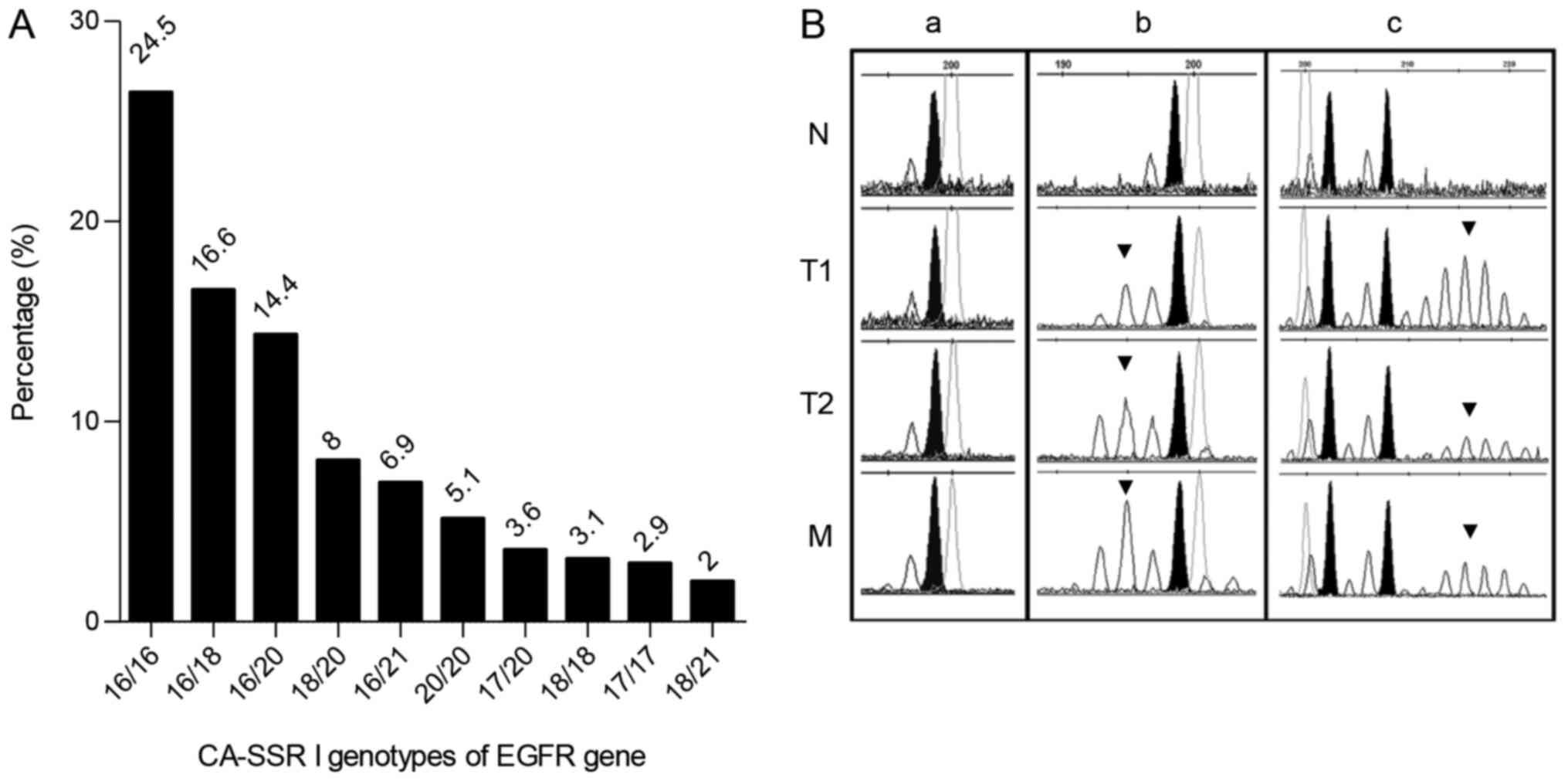 | Figure 1.CA-SSR1 polymorphism of the
EGFR gene and microsatellite instability. (A) Distribution
of the 10 most common allele frequencies of EGFR intron1
polymorphism (CA-SSR1). (B) Electropherogram of the EGFR
CA-SSR1 fragment length analysis carried out with a GeneScan
Analyzer in N, T1, T2 and M samples from (a) MSS and (b and c) MSI
high tumours. CA-SSRI analysis revealed (a and b) homozygosity and
(c) heterozygosity in the N. (a) MSS tumour exhibited no
instability in all samples of the same patient. (b and c) MSI
tumours exhibited instability with (b) additional shortened or (c)
elongated alleles (indicated by arrows) in T1, T2 and M samples.
Black peaks indicate germline alleles; the upper axis shows
fragment sizes. N, T1, T2 and M samples were all collected from the
same individual. EGFR, epidermal growth factor receptor; N,
adjacent normal tissue; T1, tumour centre; T2, invasive tumour
front; M, metastasis; MSS, microsatellite-stable; MSI,
microsatellite instability. |
Furthermore, tumour centre (T1), invasive tumour
front (T2) and, if present, corresponding metastasis (M) samples in
comparison to corresponding normal tissues (N) were analysed for
MSI status and the EGFR CA-SSR1 polymorphism. Analysis
showed that MSI was present in 33 (20.6%) tumour samples, 13 (8.1%)
tumours were MSI-L and 20 of 160 analysed tumours (12.5%) were
MSI-H. The remaining 127 (79.4%) tumours were classified as
microsatellite stable (MSS) (Table
I). CA-SSR1 polymorphism fragment length analysis in MSS
colorectal tumours showed the same genotype in T1, T2, M and
corresponding N tissue, in all samples (Fig. 1B-a). In contrast, in all MSI-H
samples instability was detected in T1, T2 and M samples as
additional shortening or elongation of alleles of the CA-SSR1
polymorphism (Fig. 1B-b and c).
 | Table I.MSI status of tumours. |
Table I.
MSI status of tumours.
| Number of markers
exhibiting instability | Number (%) | Interpretation |
|---|
| ≥2 | 20 (12.5) | MSI-H |
| 1 | 13 (8.1) | MSI-L |
| 0 | 127 (79.4) | MSS |
Association of CA-SSR1 with EGFR mRNA
and protein expression
To determine whether the number of CA-SSR1 CA
repeats is associated with changes in EGFR transcription and
protein expression levels, 80 specimens with the most frequent
CA-SSR1 genotypes were analysed for EGFR mRNA and protein
expression with regards to MSI status.
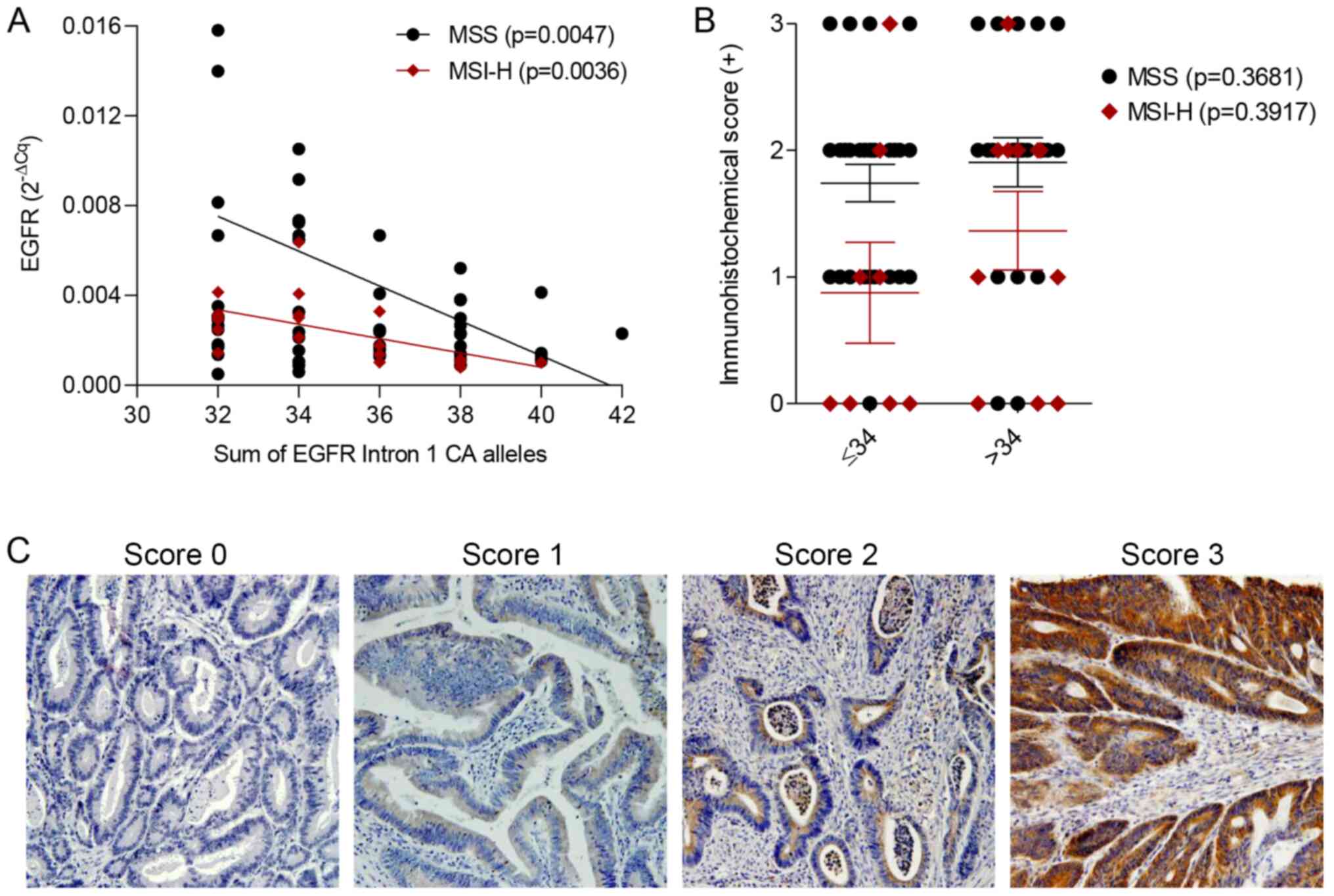
When we calculated the sum of CA repeats from both
alleles per patient, the median sum of all samples present in study
was 34 (range 31–42), with 52.2% patients having less than 34
repeats. Therefore, we classified patients as having either low
(≤34) or high (>34) numbers of CA repeats in both alleles. An
inverse correlation was found between a higher sum of CA repeats in
the EGFR CA-SSR1 polymorphism and lower EGFR mRNA
expression in both MSS (P=0.0047) and MSI-H sporadic colorectal
tumours (P=0.0036) (Fig. 2A).
However, there was no correlation between EGFR protein expression
and CA-SSR1 genotype in MSS (P=0.3681) or MSI-H (P=0.3917) tumours
(Fig. 2B). Fig. 2C shows representative
immunohistochemical staining for EGFR in the tissues histologically
classified as adenocarcinoma. Immunohistochemical staining was
scored as negative (Score 0), weak (Score 1), intermediate (Score
2) or strong (Score 3) at a magnification of ×100.
EGFR expression in the tumour centre
and at the invasive margin
Since it is known that EGFR can promote cell
migration, invasion, and metastatic dissemination we also evaluated
EGFR mRNA and IHC protein levels in the tumour centre (T1)
and invasive tumour front (T2) samples.
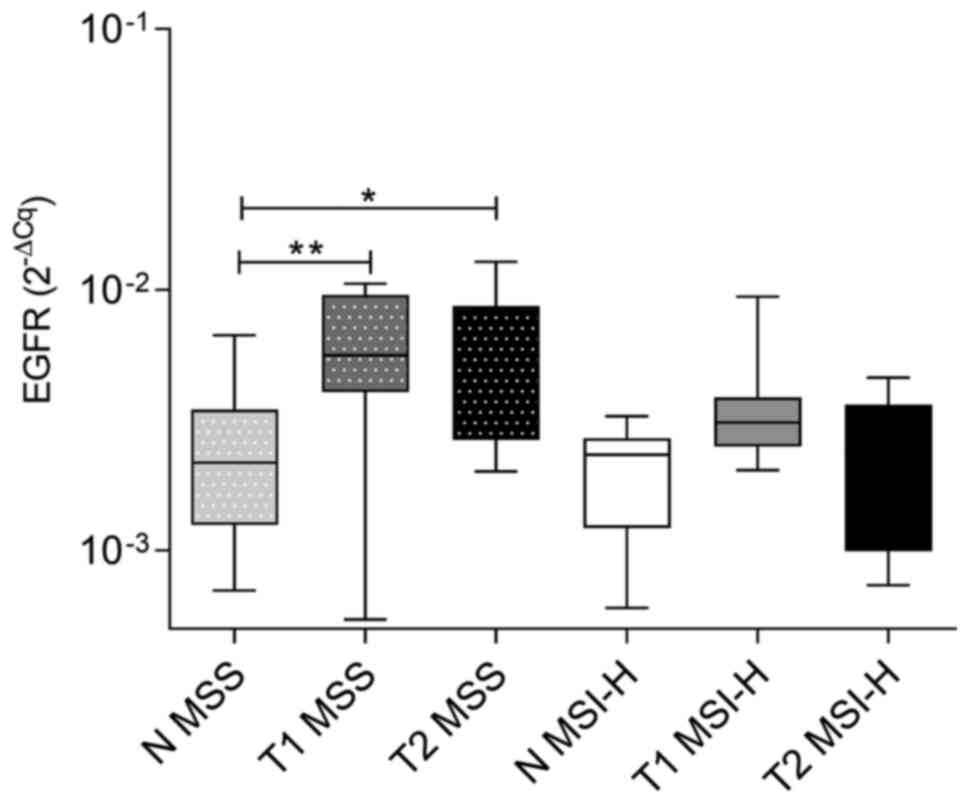
The analysis showed that EGFR mRNA levels
were significantly higher in both T1 (P=0.009) and T2 (P=0.024)
samples of MSS tumours than in normal tissues. However, in MSI-H
tumours, EGFR mRNA levels were not significantly increased
in either T1 or T2 samples in comparison to adjacent normal tissue
samples (P>0.999). Both T1 and T2 of MSI-H tumours showed
decreased EGFR mRNA levels in comparison to those in MSS
tumour, nevertheless, this was not statistically significant. There
was no difference in the expression of EGFR mRNA between
normal tissues adjacent to MSS and normal tissues adjacent to MSI-H
tumours (Fig. 3).
Immunohistochemical analysis further confirmed these
results. EGFR protein expression was detected in 6 (35.5%) adjacent
normal tissues, 50 (92.6%) T1 tissues and in 39 (97.5%) T2 tissues
of MSS tumours as well as in 2 (28.5%) adjacent normal tissues, 12
(66.7%) T1 tissues and 6 (54.5%) T2 tissues of MSI-H tumours.
EGFR protein expression was significantly increased
in tumour tissues in comparison to adjacent normal tissues in MSS
tumours (P<0.0001). Moreover, the analysis showed a difference
in EGFR protein expression between T1 and T2 samples of MSS tumours
(P=0.001) (Table II and Fig. 4). There was also significantly higher
EGFR expression in MSS tumours than in MSI-H tumours in both T1 and
T2 samples (P=0.040, P=0.001, respectively) (Table II and Fig. 4). However, there was no difference in
the expression of EGFR protein in tumour tissues in comparison to
adjacent normal tissues in MSI-H tumours or between normal tissues
adjacent to MSS and normal tissues adjacent to MSI-H tumours
(Table II and Figs. 4, S1
and S2). Immunohistochemical
staining showed similar EGFR protein expression in T2 and liver or
lymph node metastasis samples (Figs.
4, S1 and S2).
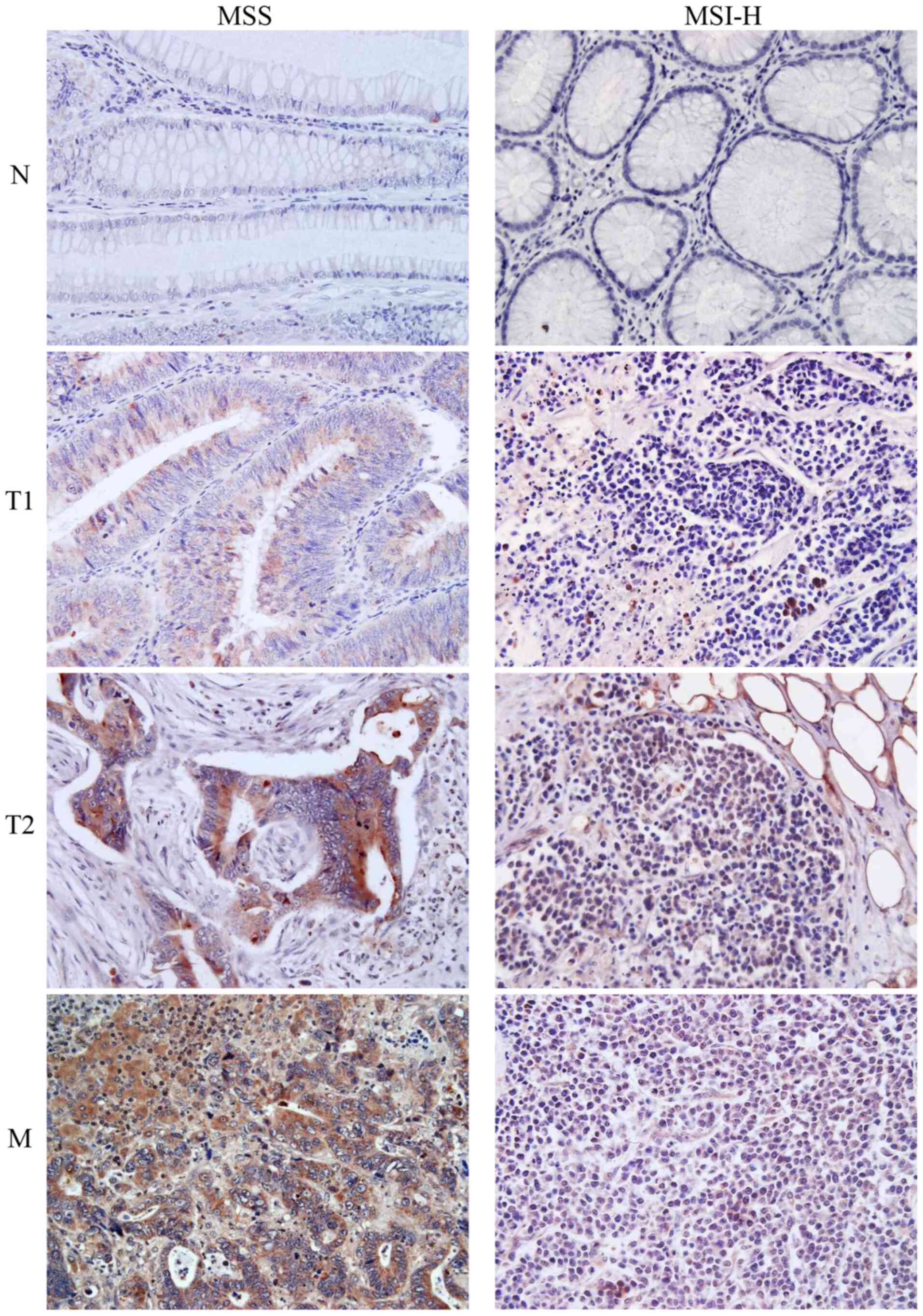 | Figure 4.Immunohistochemical staining of
epidermal growth factor receptor protein expression. Images show N,
T1 and T2 samples from MSS and MSI-H tumours, as well as a liver M
sample from an MSS sample and lymph node M sample from an MSI-H
tumour sample. N, T1, T2 and M samples were all collected from the
same individual (magnification, ×400). N, adjacent normal tissue;
T1, tumour centre; T2, invasive tumour front; M, metastasis; MSS,
microsatellite-stable; MSI-H, microsatellite instability high. |
 | Table II.EGFR protein immunohistochemical
expression in N, T1 and T2 samples from MSS and MSI-H tumours. |
Table II.
EGFR protein immunohistochemical
expression in N, T1 and T2 samples from MSS and MSI-H tumours.
|
| EGFR
immunohistochemical score (+) | P-values |
|---|
|
|
|
|
|---|
| MSI status | 0, n (%) | 1, n (%) | 2, n (%) | 3, n (%) | N/T1 or T2 | T1/T2 | MSS/MSI-H |
|---|
| MSS |
| N | 11 (64.7) | 5 (29.4) | 1 (5.9) | 0 (0) |
|
|
|
| T1 | 4 (7.4) | 12 (22.2) | 24 (44.5) | 14 (25.9) |
<0.0001a | 0.001e |
|
| T2 | 1 (2.5) | 2 (5.0) | 15 (37.5) | 22 (55.0) |
<0.0001b |
| 0.799g |
| MSI-H |
|
|
|
|
|
| 0.040h |
| N | 5 (71.4) | 2 (28.6) | 0 (0) | 0 (0) |
|
| 0.001i |
| T1 | 6 (33.3) | 4 (22.3) | 6 (33.3) | 2 (11.1) | 0.182c | 0.466f |
|
| T2 | 5 (45.5) | 1 (9.1) | 2 (18.2) | 3 (27.2) | 0.197d |
|
|
There was no correlation between EGFR protein
expression and tumour size, histological grade, Dukes' stage of
tumours (P>0.05) (Table III) in
either MSS or MSI-H tumours. The survival of the two MSS tumour
subgroups, based on EGFR immunohistochemical score, had no
statistically significant difference (P=0.717) (Table IV).
 | Table III.Clinicopathological characteristics
of 147 patients with sporadic adenocarcinoma stratified by MSI
status. |
Table III.
Clinicopathological characteristics
of 147 patients with sporadic adenocarcinoma stratified by MSI
status.
|
| MSI status |
|
|---|
|
|
|
|
|---|
|
| MSS, n=127
(86.4%) | MSI-H, n=20
(13.6%) |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | Low EGFR
expression, n (%) | High EGFR
expression, n (%) | Low EGFR
expression, n (%) | High EGFR
expression, n (%) | P-value |
|---|
| Tumor size, cm |
|
|
|
| 0.3239 |
|
<5 | 11 (42.3) | 15 (57.7) | 3 (50.0) | 3 (50.0) |
|
| ≥5 | 7 (26.9) | 19 (73.1) | 8 (61.5) | 5 (38.5) |
|
| Histological
grade |
|
|
|
| 0.4226 |
| Well
(1) | 8 (40.0) | 12 (60.0) | 2 (50.0) | 2 (50.0) |
|
|
Moderate and poor (2 and
3) | 9 (74.3) | 26 (25.7) | 8 (53.3) | 7 (46.7) |
|
| Dukes' stage |
|
|
|
| 0.5305 |
|
A+B | 5 (21.7) | 18 (78.3) | 7 (70.0) | 3 (30.0) |
|
|
C+D | 9 (30.0) | 21 (70.0) | 4 (44.4) | 5 (55.6) |
|
 | Table IV.Survival of patients with
microsatellite stable sporadic adenocarcinoma stratified by EGFR
expression. |
Table IV.
Survival of patients with
microsatellite stable sporadic adenocarcinoma stratified by EGFR
expression.
| EGFR
expression | Cases, n | Deaths, n | Mean survival,
months | P-value |
|---|
| Low | 5 | 3 | 21.6 | 0.7171 |
| High | 11 | 5 | 39.4 |
|
Discussion
EGFR receptor overexpression is found in a wide
range of cancers, including CRC, and it is associated with an
aggressive tumour phenotype and poor prognosis (33). Nonetheless, the mechanisms regulating
the levels of EGFR expression in cancer have not been fully
characterized. The results of our study show that the CA-SSR1 of
the EGFR gene is altered in MSI-H sporadic colorectal
tumours and that it has an effect on EGFR expression at the mRNA
level but not at the protein level in both MSS and MSI-H tumours.
Moreover, we demonstrated that MSI-H tumours have lower EGFR
mRNA and protein levels in the tumour centre and invasive tumour
front than MSS tumours. Additionally, we confirmed that in MSS
tumours EGFR expression is higher in the invasive tumour front than
in the tumour centre.
In recent years, EGFR gene intron 1 length
has been considered a factor affecting expression through
modification of EGFR transcription. In this regard, it has been
suggested that CA-SSR1 has an effect on gene transcription. This
hypothesis was tested in several cell lines (20,22,23) as
well as in head and neck, lung, pancreas, colon and mammary tumours
(17–20,34,35), but
the results were inconsistent and somewhat contradictory, most
likely due to the limited number of analysed samples. We found the
same distribution of CA-SSR1 alleles in normal tissues as
previously reported (18,28,36), and
the most common genotype was 16/16.
The distribution of CA-SSR1 alleles and MSI status,
were analysed in normal and tumour tissues (tumour centre and
invasive tumour front) as well as in corresponding metastasis
samples when available. Our results showed for the first time that
all MSI-H tumours showed instability in the CA-SSR 1 polymorphism.
However, the genotype varied from shortening to elongation, with
presence of one additional allele to several of them, regardless of
the length of the alleles present in normal tissues. CA-SSR1
instability was present in the tumour centre as well as in invasive
tumour front and metastasis samples. To further characterize the
possible effect of the CA-SSR1 polymorphism of the EGFR
gene, and its changes in MSI-H tumours, we measured EGFR
mRNA and protein levels. Since there is a lack of consensus
regarding cut off values defining shorter versus longer CA repeats
(37–40), we decided to take the median sum of
the CA repeats from both alleles per patient from all samples
present in our study. The results showed that EGFR mRNA
expression levels declined with an increasing sum of CA-SSR1
alleles which is in line with other papers (22,23),
possibly due to changes in DNA secondary structure. However, this
effect was absent at the protein level, an effect seen also by
McKay et al (41) and Buisine
et al (28). This could be
explained by posttranscriptional regulation via miRNAs (42,43) and
possibly other regulatory mechanisms on protein levels like EGFR
dimerization, internalization, degradation or recycling (44–46).
Interestingly, MSI tumours had lower levels of EGFR on both mRNA
and protein levels in comparison to MSS tumours, however, our
results show that this is not mediated by EGFR CA-SSR1
polymorphism but via some other mechanism that should be further
investigated.
To highlight the potential relationship between EGFR
overexpression and tumour invasion, we analysed tumour centre and
invasive tumour front samples from each patient. Our results showed
for the first time that EGFR mRNA and protein levels were
lower in both the tumour centre and invasive tumour front of MSI-H
tumours than in such samples from MSS tumours. This is in
accordance with several studies that showed a smaller number of
metastatic lymph nodes in MSI-H patients than MSS patients
(9,47), and it could also clarify why MSI-H
metastatic CRC is rare (48). In
addition to increased host immunity (49,50), a
decrease in EGFR expression in MSI-H CRC could partially explain
why these cancers are less aggressive and have a more promising
prognosis. Additionally, in MSS tumours, there was a higher
expression of EGFR at the invasive tumour front in comparison to
the tumour centre which confirms the putative role of EGFR in
tumour invasiveness and the development of metastasis in CRC. This
indicates that even though changes in non-coding regions are
usually background effects, intron 1 polymorphism could play a role
since it is located near the region that has a regulatory function
in the EGFR gene (51).
Correlation between EGFR expression and colon cancer
staging along with histological grade and tumour size is still at
issue (8). In our study, we found no
association between tumour size, histological grade, or Dukes stage
with a level of EGFR expression either in MSS or in MSI-H tumours.
Several studies addressed the possible relationship between EGFR
overexpression and tumour stage and/or histological grade. McKay
et al (41) reported a
significant association between the histological grade and EGFR,
however, they showed no correlation between EGFR expression and the
Dukes' stage. On the other hand, Theodoropoulos et al
(52) reported an association
between advanced tumour stage and high EGFR expression, excluding
correlation with tumour grade. Whereas, Del Carmen et al
(42) showed no correlation with
either tumour size, TNM stage or tumour differentiation altogether
leading to the conclusion that EGFR remains a controversial
prognostic factor. Even though survival analysis for MSS tumours
showed no statistical significance, it should be taken in
consideration that we had a very small dataset for MSS tumours and
a complete lack of survival data for MSI-H tumours. Therefore, this
aspect of our research should be expanded in the future.
In conclusion, the present study identified the
CA-SSR polymorphism in intron 1 of the EGFR gene as a
potential new CRC marker that is exclusively altered in MSI-H
colorectal tumours. Additionally, we showed higher EGFR expression
in MSS than in MSI-H tumours. The highest EGFR expression found in
invasive tumour front of MSS tumours suggests more important role
of EGFR in MSS tumours progression than in MSI-H tumours where MSI
is a dominant molecular genetic change. Furthermore, instability in
EGFR CA-SSR1 polymorphism that correlates with decreased
EGFR expression suggests that it could serve as an indicator of
sporadic MSI-H CRC progression.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Croatian
Science Foundation (grant no. HRZZ-IP-2016-06-1430).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SK is the PI on the project HRZZ-IP-2016-06-1430 and
designed the study. SM, KV, SP and LP performed the experiments and
the statistical analyses. AŠ and MP collected the samples and
analysed the immunohistological data. SK and SM wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients included in the present study. The present study was
approved by the Ethics Committee of Merkur Clinical Hospital,
Zagreb (Zagreb, Croatia) and Medical School, University of Zagreb
(Zagreb, Croatia) and was performed in accordance with the ethical
standards of the Helsinki Declaration.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM
and Wallace MB: Colorectal cancer. Lancet. 394:1467–1480. 2019.
View Article : Google Scholar
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar
|
|
3
|
Cohen S: Isolation and biological effects
of an epidermal growth-stimulating protein. Natl Cancer Inst
Monogr. 13:13–37. 1964.
|
|
4
|
Park JH, Han SW, Oh DY, Im SA, Jeong SY,
Park KJ, Kim TY, Bang YJ and Park JG: Analysis of KRAS, BRAF, PTEN,
IGF1R, EGFR intron 1 CA status in both primary tumors and paired
metastases in determining benefit from cetuximab therapy in colon
cancer. Cancer Chemother Pharmacol. 68:1045–1055. 2011. View Article : Google Scholar
|
|
5
|
Spano JP, Lagorce C, Atlan D, Milano G,
Domont J, Benamouzig R, Attar A, Benichou J, Martin A, Morere JF,
et al: Impact of EGFR expression on colorectal cancer patient
prognosis and survival. Ann Oncol. 16:102–108. 2005. View Article : Google Scholar
|
|
6
|
Rego RL, Foster NR, Smyrk TC, Le M,
O'Connell MJ, Sargent DJ, Windschitl H and Sinicrope FA: Prognostic
effect of activated EGFR expression in human colon carcinomas:
Comparison with EGFR status. Br J Cancer. 102:165–172. 2010.
View Article : Google Scholar
|
|
7
|
Lee WS, Baek JH, Lee JN and Lee WK:
Mutations in K-ras and epidermal growth factor receptor expression
in Korean patients with stages III and IV colorectal cancer. Int J
Surg Pathol. 19:145–151. 2011. View Article : Google Scholar
|
|
8
|
Ljuslinder I, Melin B, Henriksson ML,
Oberg A and Palmqvist R: Increased epidermal growth factor receptor
expression at the invasive margin is a negative prognostic factor
in colorectal cancer. Int J Cancer. 128:2031–2037. 2011. View Article : Google Scholar
|
|
9
|
Kang YJ, Jung CK, Choi Y, Lee KY, Kim HJ
and Kang WK: Clinicopathologic significances of EGFR expression at
invasive front of colorectal cancer. Korean J Pathol. 44:16–21.
2010. View Article : Google Scholar
|
|
10
|
Ueno H, Mochizuki H, Hashiguchi Y,
Shimazaki H, Aida S, Hase K, Matsukuma S, Kanai T, Kurihara H,
Ozawa K, et al: Risk factors for an adverse outcome in early
invasive colorectal carcinoma. Gastroenterology. 127:385–394. 2004.
View Article : Google Scholar
|
|
11
|
Morodomi T, Isomoto H, Shirouzu K,
Kakegawa K, Irie K and Morimatsu M: An index for estimating the
probability of lymph node metastasis in rectal cancers. Lymph node
metastasis and the histopathology of actively invasive regions of
cancer. Cancer. 63:539–543. 1989. View Article : Google Scholar
|
|
12
|
Sohn DK, Chang HJ, Park JW, Choi DH, Han
KS, Hong CW, Jung KH, Kim DY, Lim SB, Choi HS and Jeong SY:
Histopathological risk factors for lymph node metastasis in
submucosal invasive colorectal carcinoma of pedunculated or
semipedunculated type. J Clin Pathol. 60:912–915. 2007. View Article : Google Scholar
|
|
13
|
Kawachi H, Eishi Y, Ueno H, Nemoto T,
Fujimori T, Iwashita A, Ajioka Y, Ochiai A, Ishiguro S, Shimoda T,
et al: A three-tier classification system based on the depth of
submucosal invasion and budding/sprouting can improve the treatment
strategy for T1 colorectal cancer: A retrospective multicenter
study. Mod Pathol. 28:872–879. 2015. View Article : Google Scholar
|
|
14
|
Shia J, Klimstra DS, Li AR, Qin J, Saltz
L, Teruya-Feldstein J, Akram M, Chung KY, Yao D, Paty PB, et al:
Epidermal growth factor receptor expression and gene amplification
in colorectal carcinoma: An immunohistochemical and chromogenic in
situ hybridization study. Mod Pathol. 18:1350–1356. 2005.
View Article : Google Scholar
|
|
15
|
Brandt B, Meyer-Staeckling S, Schmidt H,
Agelopoulos K and Buerger H: Mechanisms of egfr gene transcription
modulation: Relationship to cancer risk and therapy response. Clin
Cancer Res. 12:7252–7260. 2006. View Article : Google Scholar
|
|
16
|
Choi JE, Park SH, Kim KM, Lee WK, Kam S,
Cha SI, Kim CH, Kang YM, Kim YC, Han SB, et al: Polymorphisms in
the epidermal growth factor receptor gene and the risk of primary
lung cancer: Acase-control study. BMC Cancer. 7:1992007. View Article : Google Scholar
|
|
17
|
Loupakis F, Cremolini C, Fontanini G,
Stasi I, Salvatore L and Falcone A: Beyond KRAS: Perspectives on
new potential markers of intrinsic and acquired resistance to
epidermal growth factor receptor inhibitors in metastatic
colorectal cancer. Ther Adv Med Oncol. 1:167–181. 2009. View Article : Google Scholar
|
|
18
|
Etienne-Grimaldi MC, Pereira S, Magné N,
Formento JL, Francoual M, Fontana X, Demard F, Dassonville O,
Poissonnet G, Santini J, et al: Analysis of the dinucleotide repeat
polymorphism in the epidermal growth factor receptor (EGFR) gene in
head and neck cancer patients. Ann Oncol. 16:934–941. 2005.
View Article : Google Scholar
|
|
19
|
Frolov A, Liles JS, Kossenkov AV, Tzeng
CW, Jhala N, Kulesza P, Varadarajulu S, Eloubeidi M, Heslin MJ and
Arnoletti JP: Epidermal growth factor receptor (EGFR) intron 1
polymorphism and clinical outcome in pancreatic adenocarcinoma. Am
J Surg. 200:398–405. 2010. View Article : Google Scholar
|
|
20
|
Kersting C, Agelopoulos K, Schmidt H,
Korsching E, August C, Gosheger G, Dirksen U, Juergens H,
Winkelmann W, Brandt B, et al: Biological importance of a
polymorphic CA sequence within intron 1 of the epidermal growth
factor receptor gene (EGFR) in high grade central osteosarcomas.
Genes Chromosomes Cancer. 47:657–664. 2008. View Article : Google Scholar
|
|
21
|
Buerger H, Gebhardt F, Schmidt H, Beckmann
A, Hutmacher K, Simon R, Lelle R, Boecker W and Brandt B: Length
and loss of heterozygosity of an intron 1 polymorphic sequence of
egfr is related to cytogenetic alterations and epithelial growth
factor receptor expression. Cancer Res. 60:854–857. 2000.
|
|
22
|
Amador ML, Oppenheimer D, Perea S, Maitra
A, Cusatis G, Iacobuzio-Donahue C, Baker SD, Ashfaq R, Takimoto C,
Forastiere A and Hidalgo M: An epidermal growth factor receptor
intron 1 polymorphism mediates response to epidermal growth factor
receptor inhibitors. Cancer Res. 64:9139–9143. 2004. View Article : Google Scholar
|
|
23
|
Gebhardt F, Zanker KS and Brandt B:
Modulation of epidermal growth factor receptor gene transcription
by a polymorphic dinucleotide repeat in intron 1. J Biol Chem.
274:13176–13180. 1999. View Article : Google Scholar
|
|
24
|
Lurje G, Nagashima F, Zhang W, Yang D,
Chang HM, Gordon MA, El-Khoueiry A, Husain H, Wilson PM, Ladner RD,
et al: Polymorphisms in cyclooxygenase-2 and epidermal growth
factor receptor are associated with progression-free survival
independent of K-ras in metastatic colorectal cancer patients
treated with single-agent cetuximab. Clin Cancer Res. 14:7884–7895.
2008. View Article : Google Scholar
|
|
25
|
Garm Spindler KL, Pallisgaard N, Rasmussen
AA, Lindebjerg J, Andersen RF, Crüger D and Jakobsen A: The
importance of KRAS mutations and EGF61A>G polymorphism to the
effect of cetuximab and irinotecan in metastatic colorectal cancer.
Ann Oncol. 20:879–884. 2009. View Article : Google Scholar
|
|
26
|
Boland CR and Goel A: Microsatellite
instability in colorectal cancer. Gastroenterology.
138:2073–2087.e3. 2010. View Article : Google Scholar
|
|
27
|
Yuan Z, Shin J, Wilson A, Goel S, Ling YH,
Ahmed N, Dopeso H, Jhawer M, Nasser S, Montagna C, et al: An A13
repeat within the 3′-untranslated region of epidermal growth factor
receptor (EGFR) is frequently mutated in microsatellite instability
colon cancers and is associated with increased EGFR expression.
Cancer Res. 69:7811–7818. 2009. View Article : Google Scholar
|
|
28
|
Buisine MP, Wacrenier A, Mariette C,
Leteurtre E, Escande F, Aissi S, Ketele A, Leclercq A, Porchet N
and Lesuffleur T: Frequent mutations of the CA simple sequence
repeat in intron 1 of EGFR in mismatch repair-deficient colorectal
cancers. World J Gastroenterol. 14:1053–1059. 2008. View Article : Google Scholar
|
|
29
|
Spaventi R, Pecur L, Pavelic K, Pavelic
ZP, Spaventi S and Stambrook PJ: Human tumour bank in Croatia: A
possible model for a small bank as part of the future European
tumour bank network. Eur J Cancer. (30A): 4191994. View Article : Google Scholar
|
|
30
|
Green MR and Sambrook J: Isolation of
high-molecular-weight dna from mammalian blood using proteinase k
and phenol. Cold Spring Harb Protoc. 2017:pdb prot093492. 2017.
View Article : Google Scholar
|
|
31
|
Cacev T, Radosevic S, Spaventi R, Pavelic
K and Kapitanovic S: NF1 gene loss of heterozygosity and expression
analysis in sporadic colon cancer. Gut. 54:1129–1135. 2005.
View Article : Google Scholar
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
33
|
Mauri G, Pizzutilo EG, Amatu A, Bencardino
K, Palmeri L, Bonazzina EF, Tosi F, Carlo Stella G, Burrafato G,
Scaglione F, et al: Retreatment with anti-EGFR monoclonal
antibodies in metastatic colorectal cancer: Systematic review of
different strategies. Cancer Treat Rev. 73:41–53. 2019. View Article : Google Scholar
|
|
34
|
Tzeng CW, Frolov A, Frolova N, Jhala NC,
Howard JH, Vickers SM, Buchsbaum DJ, Heslin MJ and Arnoletti JP:
Pancreatic cancer epidermal growth factor receptor (EGFR) intron 1
polymorphism influences postoperative patient survival and in vitro
erlotinib response. Ann Surg Oncol. 14:2150–2158. 2007. View Article : Google Scholar
|
|
35
|
Shitara M, Sasaki H, Yokota K, Okuda K,
Hikosaka Y, Moriyama S, Yano M, Kawaguchi T, Kubo A, Takada M, et
al: Polymorphisms in intron 1 of the EGFR gene in non-small cell
lung cancer patients. Exp Ther Med. 4:785–789. 2012. View Article : Google Scholar
|
|
36
|
Arifin M, Hiyama K, Tanimoto K, Wiyono WH,
Hiyama E and Nishiyama M: EGFR activating aberration occurs
independently of other genetic aberrations or telomerase activation
in adenocarcinoma of the lung. Oncol Rep. 17:1405–1411. 2007.
|
|
37
|
Vashist YK, Trump F, Gebauer F, Kutup A,
Güngör C, Kalinin V, Muddasar R, Vettorazzi E, Yekebas EF, Brandt
B, et al: EGFR intron-1 CA repeat polymorphism is a predictor of
relapse and survival in complete resected only surgically treated
esophageal cancer. Target Oncol. 9:43–52. 2014. View Article : Google Scholar
|
|
38
|
Huang SF, Chien HT, Chuang WY, Lai CH,
Cheng SD, Liao CT and Wang HM: Epidermal growth factor receptor
intron-1 CA repeat polymorphism on protein expression and clinical
outcome in Taiwanese oral squamous cell carcinoma. Sci Rep.
7:49632017. View Article : Google Scholar
|
|
39
|
Han SW, Oh DY, Im SA, Park SR, Lee KW,
Song HS, Lee NS, Lee KH, Choi IS, Lee MH, et al: Epidermal growth
factor receptor intron 1 CA dinucleotide repeat polymorphism and
survival of advanced gastric cancer patients treated with cetuximab
plus modified FOLFOX6. Cancer Sci. 101:793–799. 2010. View Article : Google Scholar
|
|
40
|
Zhang W, Weissfeld JL, Romkes M, Land SR,
Grandis JR and Siegfried JM: Association of the EGFR intron 1 CA
repeat length with lung cancer risk. Mol Carcinog. 46:372–380.
2007. View Article : Google Scholar
|
|
41
|
McKay JA, Murray LJ, Curran S, Ross VG,
Clark C, Murray GI, Cassidy J and McLeod HL: Evaluation of the
epidermal growth factor receptor (EGFR) in colorectal tumours and
lymph node metastases. Eur J Cancer. 38:2258–2264. 2002. View Article : Google Scholar
|
|
42
|
Del Carmen S, Corchete LA, Gervas R,
Rodriguez A, Garcia M, Álcazar JA, García J, Bengoechea O,
Muñoz-Bellvis L, Sayagués JM and Abad M: Prognostic implications of
EGFR protein expression in sporadic colorectal tumors: Correlation
with copy number status, mRNA levels and miRNA regulation. Sci Rep.
10:46622020. View Article : Google Scholar
|
|
43
|
Teixeira AL, Gomes M and Medeiros R: EGFR
signaling pathway and related-miRNAs in age-related diseases: The
example of miR-221 and miR-222. Front Genet. 3:2862012. View Article : Google Scholar
|
|
44
|
Jurisic V, Vukovic V, Obradovic J,
Gulyaeva LF, Kushlinskii NE and Djordjevic N: EGFR Polymorphism and
survival of NSCLC patients treated with TKIs: A systematic review
and meta-analysis. J Oncol. 2020:19732412020. View Article : Google Scholar
|
|
45
|
Tomas A, Futter CE and Eden ER: EGF
receptor trafficking: Consequences for signaling and cancer. Trends
Cell Biol. 24:26–34. 2014. View Article : Google Scholar
|
|
46
|
Seth D, Shaw K, Jazayeri J and Leedman PJ:
Complex post-transcriptional regulation of EGF-receptor expression
by EGF and TGF-alpha in human prostate cancer cells. Br J Cancer.
80:657–669. 1999. View Article : Google Scholar
|
|
47
|
Buckowitz A, Knaebel HP, Benner A, Bläker
H, Gebert J, Kienle P, von Knebel Doeberitz M and Kloor M:
Microsatellite instability in colorectal cancer is associated with
local lymphocyte infiltration and low frequency of distant
metastases. Br J Cancer. 92:1746–1753. 2005. View Article : Google Scholar
|
|
48
|
Goldstein J, Tran B, Ensor J, Gibbs P,
Wong HL, Wong SF, Vilar E, Tie J, Broaddus R, Kopetz S, et al:
Multicenter retrospective analysis of metastatic colorectal cancer
(CRC) with high-level microsatellite instability (MSI-H). Ann
Oncol. 25:1032–1038. 2014. View Article : Google Scholar
|
|
49
|
Xiao Y and Freeman GJ: The microsatellite
instable subset of colorectal cancer is a particularly good
candidate for checkpoint blockade immunotherapy. Cancer Discov.
5:16–18. 2015. View Article : Google Scholar
|
|
50
|
Kloor M and von Knebel Doeberitz M: The
immune biology of microsatellite-unstable cancer. Trends Cancer.
2:121–133. 2016. View Article : Google Scholar
|
|
51
|
Perucho M: Tumors with microsatellite
instability: Many mutations, targets and paradoxes. Oncogene.
22:2223–2225. 2003. View Article : Google Scholar
|
|
52
|
Theodoropoulos GE, Karafoka E, Papailiou
JG, Stamopoulos P, Zambirinis CP, Bramis K, Panoussopoulos SG,
Leandros E and Bramis J: P53 and EGFR expression in colorectal
cancer: A reappraisal of ‘old’ tissue markers in patients with long
follow-up. Anticancer Res. 29:785–791. 2009.
|