Introduction
According to the 2018 Global Cancer Statistics, the
number of new cases of esophageal cancer (EC) in 2018 accounted for
3.2% of the total number of cancer cases, with EC-related
fatalities accounting for 5.3% of the total cancer deaths (1). There are two main histological types of
EC, namely esophageal squamous cell carcinoma (ESCC) and esophageal
adenocarcinoma (EADC). ESCC is the most common histological type of
EC worldwide, particularly in high-risk areas, such as China. China
is one of the areas exhibiting a high incidence of EC, which may be
associated with genetics and lifestyle habits. Smoking, excessive
alcohol consumption and the excessive intake of red meat and spicy
foods are considered as high-risk factors for ESCC (2–4).
Previous studies on EC cases in China, particularly in Linxian
county, which has the highest ESCC-related mortality rate
worldwide, demonstrated that a polymorphism in the TP53 gene (the
main regulator of p21) is associated with the risk of developing
ESCC (5,6). Intestinal metaplasia in Barrett's
esophagus is a major risk factor for EADC, and the inactivation of
p53 and p21 may be implicated in the progression of Barrett's
esophagus to cancer (7,8).
Radical surgery is the only curative method
currently available for the treatment of EC; however, as the early
symptoms of EC are not evident, the majority of patients are
already at an advanced stage at the time of diagnosis. Radiotherapy
and chemotherapy are the main treatment strategies for patients
with advanced EC. However, chemotherapeutic resistance and adverse
events may occur, which are the main causes of treatment failure in
EC. Immunotherapy for EC has recently emerged; PD-1/PD-L1
inhibitors have been proven to be effective in the treatment of EC
(9). Furthermore, angiogenesis
inhibitors and inhibitors of epidermal growth factor receptor, such
as bevacizumab, cetuximab, panitumumab and erlotinib, have been
reported to be useful for the treatment of EC (10). However, only limited progress has
been made in targeting drugs specific to EC. Therefore, the
identification of novel therapeutic targets for EC is the focus of
ensuing research.
It has been >20 years since p21 was discovered as
a cell cycle inhibitor regulated by p53. In 1993, el-Deiry et
al identified p21 by using subtractive hybridization
technology, which is downstream of wild-type p53, a 21-kDa protein
encoded by wild-type p53-activated fragment gene 1 (WAF1) (11). In addition, Harper et al
demonstrated that p21 is a type of cyclin-dependent kinase (CDK)
inhibitor through a two-hybrid system (12). In recent years, the therapeutic
potential of targeting the cell cycle has been widely acknowledged.
The new generation of selective CDK4/6 inhibitors, such as
palbociclib, has been approved for the treatment of breast cancer
(13). This has put forward a new
approach to the treatment of EC via targeting the cell cycle. p21,
as a cell cycle kinase inhibitor, can regulate cell cycle
progression in EC (14). Subsequent
research has identified that p21 also plays an important role in
inducing cell senescence and apoptosis, promoting DNA repair and
maintaining genome stability (15).
Therefore, it is necessary to examine the role of p21 in EC and
actively develop drugs targeting p21.
p21 regulates the cell cycle, DNA
replication and apoptosis
p21 regulates the cell cycle by
inhibiting the cyclin-CDK complex
Cyclin is a type of protein that is ubiquitous in
eukaryotic cells and can appear and disappear regularly during the
cell cycle (16). Cyclin regulates
the cell cycle by binding to and activating CDK. As shown in
Table I, the phosphorylation of
specific targets by the cyclin-CDK complex sets in motion different
processes that drive the cell cycle in a timely manner (17). Over the past two decades, numerous
studies have demonstrated that cell cycle disorders are implicated
in human cancers; the overactivation of CDK may promote tumor
development by inducing unprogrammed cell division of stem or
progenitor cells.
 | Table I.Cyclin-CDK complex can act at
different times in the cell cycle. |
Table I.
Cyclin-CDK complex can act at
different times in the cell cycle.
| Cell cycle | Cyclin-CDK
complex |
|---|
| G1 phase | Cyclin D-CDK4,
cyclin D-CDK6 |
| G1/S phase | Cyclin E-CDK2 |
| S phase | Cyclin E-CDK2,
cyclin A-CDK1, cyclin A-CDK2 |
| G2/M phase | Cyclin B-CDK1 |
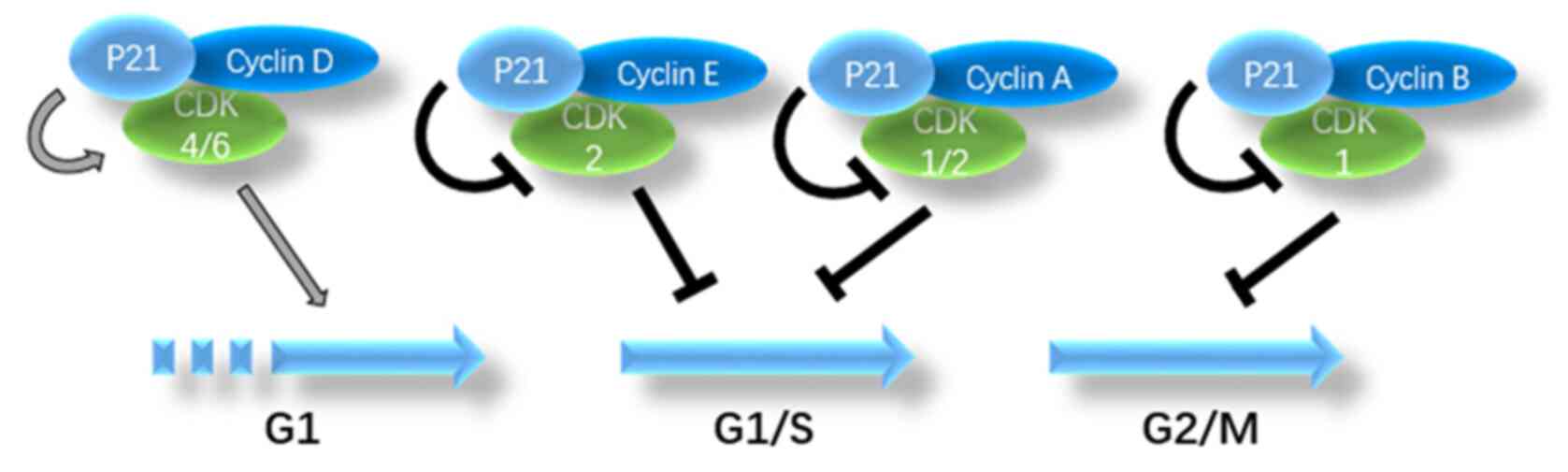
p21 is an effective CDK inhibitor that can bind to
CDK and block the cell cycle (12).
p21 can be combined with and inhibit almost every cyclin-CDK
complex, such as cyclin D-CDK4, cyclin E-CDK2 and cyclin A-CDK2
(11). p21 inhibits the cyclin
E-CDK2 complex, thereby inhibiting the phosphorylation of RB and
the sequestration of E2F1 and arresting the cell cycle in the
G1 phase. p21 also inhibits the kinase activity of
cyclin A-CDK1/2, which is necessary for entering the G2
phase through the S phase. In addition, p21 inhibits the kinase
activity of cyclin B-CDK1, thereby inhibiting progression through
the G2/M phase. Under certain conditions, p21 promotes
the activity of CDK4 or CDK6, thereby promoting progression through
the G1 phase (18). The
aforementioned findings indicate that p21 plays multiple roles in
the regulation of the cell cycle. A summary of the role of p21 in
cell cycle regulation by combining with different complexes is
illustrated in Fig. 1.
p21 inhibits the binding of
proliferating cell nuclear antigen (PCNA) to polymerase δ to affect
DNA replication
The control of DNA replication is a key factor for
maintaining normal cell function, as it can affect the stability of
the genome. DNA replication that occurs in the S phase of the cell
cycle is essential for cell division (19). PCNA is an evolutionarily
well-preserved protein that exists in all eukaryotes. PCNA was
first found to be a processing factor for DNA polymerase δ, which
is required for DNA synthesis during replication (20). PCNA recognizes the primer-template
junction and conjugates with replication factor C (RFC) to assist
in the positioning of polymerase δ, and it also strengthens the
advancement of polymerase in the extension process.
Podust et al (21) found that p21 first inhibited the
RFC-catalyzed loading of PCNA onto DNA and that, second, it
prevented the binding of the DNA polymerase δ core to the PCNA
clamp assembled on the DNA; the latter was the most potent
inhibitory effect on polymerase δ enzyme activity. p21 can form a
stable complex with PCNA on DNA, preventing further interaction
with the replication proteins, RFC and DNA polymerase δ (22). In addition, Zhang et al
(23) discovered the existence of
PCNA-p21/cyclin-CDK quaternary complexes, which may be associated
with the regulation of the cell cycle.
p21 can inhibit apoptosis by
suppressing stress-activated protein kinase (SAPK) and apoptotic
signal-regulated kinase 1 (ASK1)
SAPK is a member of the subfamily of the
mitogen-activated protein (MAP) kinases, which can be activated in
response to DNA damage. During cellular stress, the N-terminus of
p21 binds to and inhibits the activity of SAPK, prevents c-Jun
phosphorylation and Ap-1 activation, thus participating in the
regulation of apoptosis. p21 located in the cytoplasm can interact
with SAPK and ASK1 to inhibit their catalytic activity, thereby
inhibiting apoptosis (24,25). ASK1, also known as MAP kinase 5
(MAP3K5), has the potential to induce cellular apoptosis under
various physiological conditions. Zhan et al (26) demonstrated that p21 interacts with
ASK1 and downregulates its kinase activity. These findings may
indicate some of the mechanisms through which p21 inhibits
apoptosis.
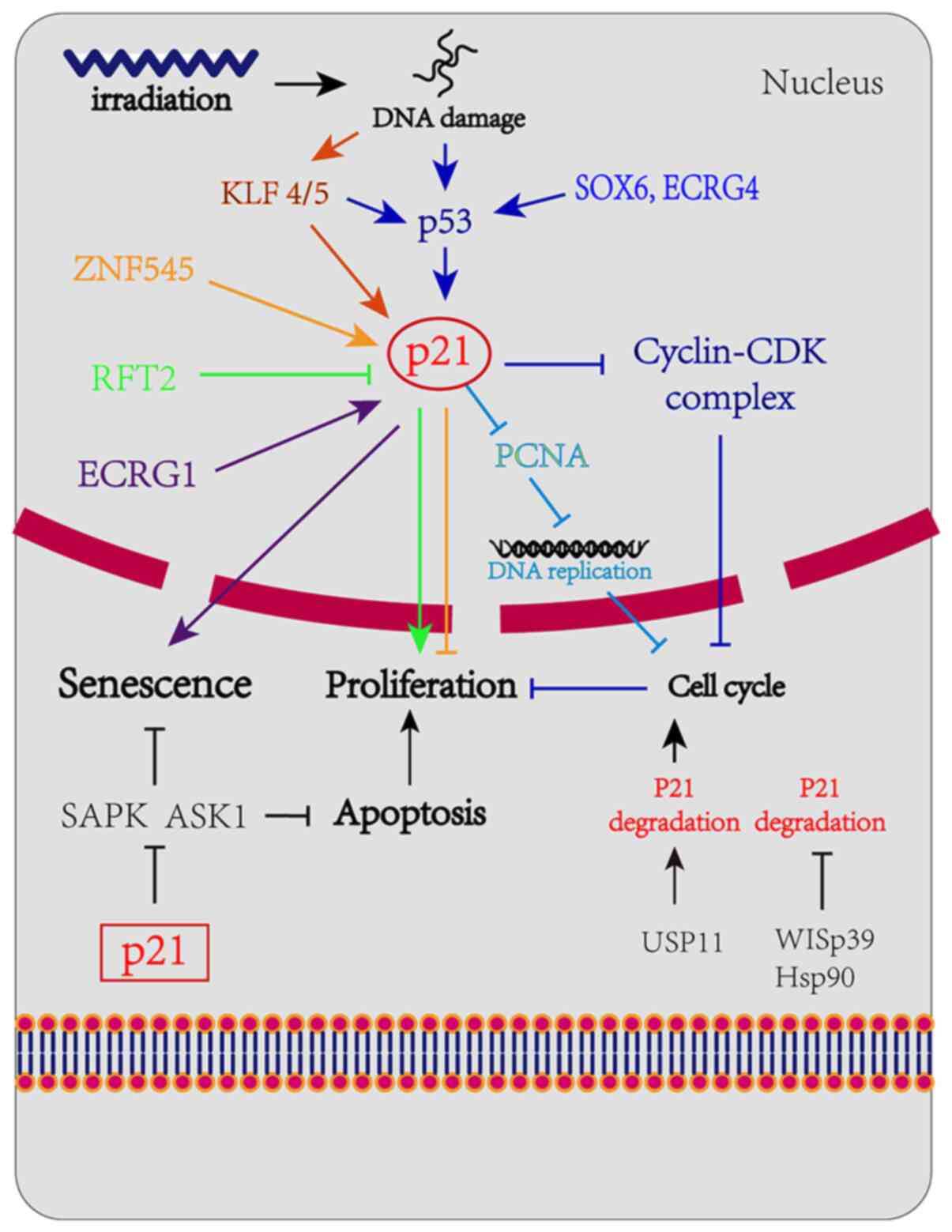
p21 may be regulated through p53-dependent
and -independent pathways
p53-dependent pathway
Numerous studies have confirmed that p21 expression
is dependent on p53 and performs part of the functions of p53 as
its downstream mediator (11,12,27,28).
When cells are exposed to radiation and DNA damage occurs, p53 and
p21 are often highly expressed in p53 wild-type cells, while
p53-deficient cells usually do not exhibit a high p21 expression,
and the cells do not undergo cell cycle arrest. At present, p21 is
known as the cell cycle inhibitory protein with the most extensive
kinase inhibitory activity. Amino acids 21–26 and 49–72 of p21 bind
to cyclin and CDK, respectively, so that the kinase activity of
cyclin-CDK complex is lost. In summary, when cells suffer DNA
damage due to environmental stimuli, p21 can inhibit the activity
of the cyclin-CDK complex as a CDK inhibitor through the
p53-dependent pathway, so that Rb protein cannot be phosphorylated,
thus arresting the cell cycle in the G1/S phase
(11). In addition, it was
previously demonstrated that p53 not only induces the expression of
p21 and exerts a tumor-suppressive effect, but also plays a role by
directly forming a complex with p21 (29,30). For
example, p53 and p21 can bind to Bcl-2 family proteins, such as
Bcl-w and Bcl-xL, and release the Bax protein through the formation
of the p53/p21/Bcl-w complex to promote tumor cell apoptosis.
In EC cells, the p53/p21 pathway is also crucial for
the regulation of cell proliferation. For example, researchers
previously used mibefradil in EC cells to inhibit the function of
calcium channels, and found that p21 increased in the p53-dependent
pathway, which ultimately inhibited the proliferation of EC cells
(31). In addition, SOX6, EC-related
gene (ECRG)4, KLF4/5 and other genes were shown to regulate the
proliferation of EC cells through the p53/p21 pathway (32–35).
p53-independent pathway
Transcriptional regulation of p21
The regulation of p21 in cells is highly complex and
diverse and p21 can also be regulated through a p53-independent
pathway. The Krüppel-associated box zinc-finger protein 545
(ZNF545) has been proven to directly regulate p21 independently of
p53 (36). It was previously
demonstrated that, in p53-mutant EC cell lines, the expression of
ZNF545 upregulated the protein expression levels of the pivotal
effectors, p21 and Bax, thereby inhibiting tumor cell proliferation
and promoting apoptosis. Human riboflavin transporter 2 and ECRG1
have also been confirmed to directly regulate the expression of
p21, thereby regulating the proliferation of EC cells (37,38).
Post-transcriptional control of
p21
p53 activates the transcription of p21 in the case
of DNA damage and creates an unstable p21, the stability of which
may improve by interacting with WISp39 and Hsp90. As previously
demonstrated, p21 cannot be upregulated in response to DNA damage
due to the lack of WISp39, suggesting that p21 transcriptional
control is insufficient to upregulate p21 protein expression
following DNA damage in the absence of p21 stability (39,40). A
2018 study demonstrated that the loss of USP11 may cause p21
instability and induce G1/S transition in cells; in
addition, the accumulation of p21 due to DNA damage was completely
eliminated in cells lacking USP11, which led to the abolition of
the G2 checkpoint and the induction of apoptosis
(41). p21 is mainly degraded
through the ubiquitination pathway (42). As an important regulator of the cell
cycle, Skp2 can specifically recognize phosphorylated substrates
and perform ubiquitin-mediated degradation. p21 is one of the
substrates of the ubiquitin proteasome pathway; it is
phosphorylated at the Ser-130 site with the participation of
CDK2-cyclin E and enters the ubiquitin degradation pathway under
the action of the accessory protein, CKS1 (43).
In addition, affecting mRNA stability and the
control of the translation of p21 mRNA are also an important part
of the p21 post-transcriptional regulatory mechanism. In 2017, Li
et al (44) silenced
methyltransferases (NSUN2, METTL3 or METTL14) and found that the
protein level of p21 decreased, although its mRNA expression was
not altered; thus, they came to the conclusion that NSUN2, METTL3
and METTL14 may affect p21 expression levels by regulating p21
translation. Another study found that HuR and AUF1 may
competitively bind with the p21 3′-untranslated region and regulate
p21 mRNA stability (45). These two
RNA-binding proteins competitively bind to p21 mRNA, but lead to
opposite results; HuR can enhance the stability of mRNA, while AUF1
accelerates p21 mRNA decay. The regulation of p21 mRNA and protein
stability has not yet been extensively investigated in EC cells.
The aim of the present review was mainly to explain the diversity
of the mechanisms implicated in the regulation of p21 and the key
role of p21 in complex networks. The aforementioned regulation
mechanism is shown in detail in Fig.
2.
Role of p21 in the treatment of esophageal
cancer
p21 holds promise as a prognostic
indicator and for the evaluation of treatment efficacy
From the aforementioned description of the function
of p21 and its regulatory mechanisms, it may be inferred that p21
is a key regulatory factor in EC; thus, the study of p21 is of
utmost clinical significance. In fact, some studies have confirmed
that p21 plays an important role in the prognosis of EC. A previous
meta-analysis revealed that a low p21 expression was associated
with clinicopathological characteristics such as poor
differentiation, lymph node metastasis, aggressiveness, higher
grade and clinical stage, which are associated with a poor outcome
of patients with EC (46). Apart
from this latter study, there is ample evidence indicating that
high expression of p21 is an important indicator of good prognosis
for patients with EC (47–49). These studies suggested that low p21
expression is an indicator of a highly malignant EC. The expression
of p21 is often regulated by p53; thus, comprehensive consideration
is needed when determining the prognosis of patients with EC. A
previous study demonstrated that, in p53-positive patients with EC,
the expression of p21 exerted no significant effect on the survival
rate of the patients; however, the survival rate of patients with
p21-positive tumors was significantly higher compared with that of
patients with p21-negative tumors (50).
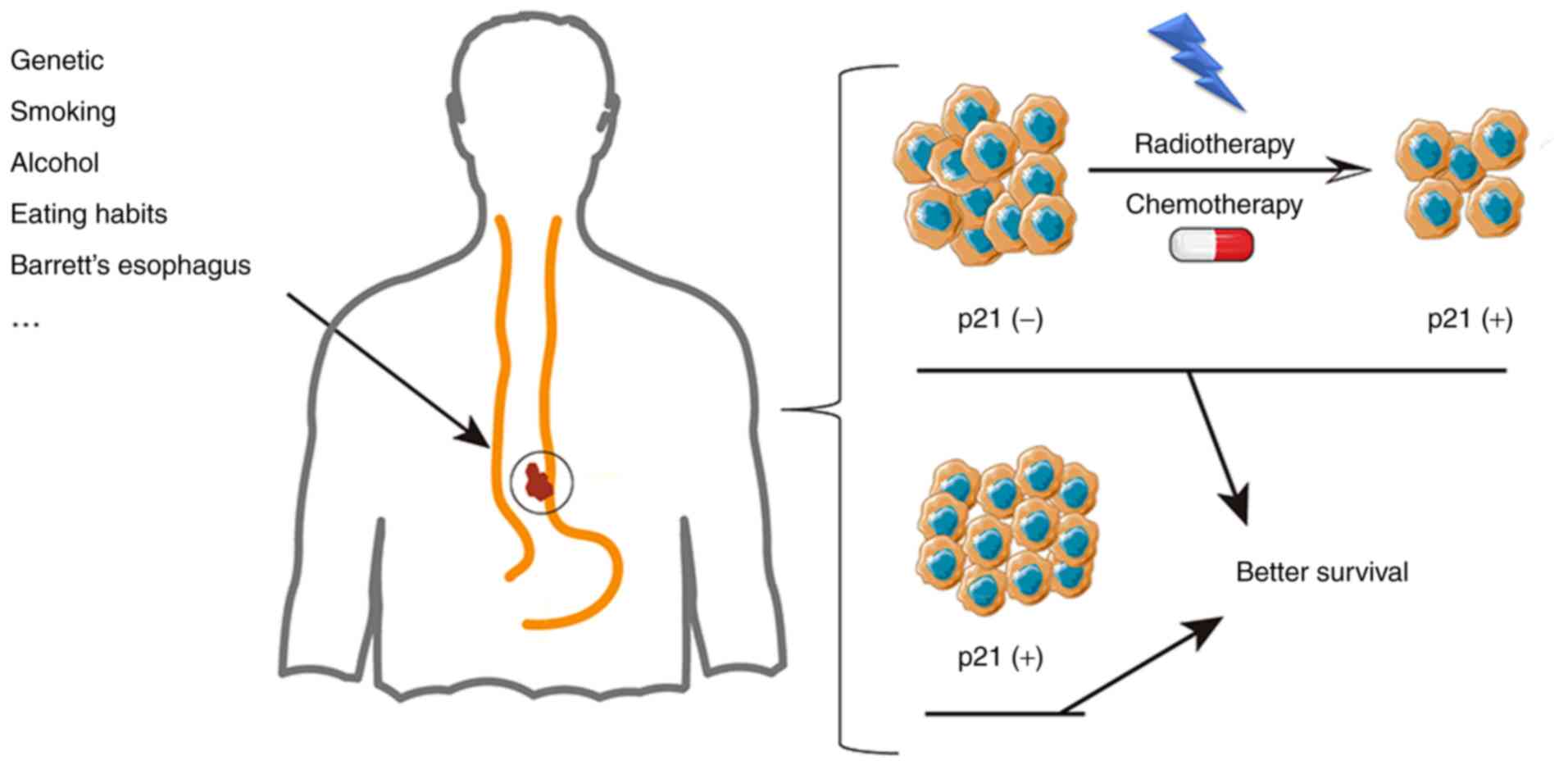
The combination of chemotherapy and radiotherapy is
the main treatment strategy for advanced EC. The expression of p53
and p21 has been proven to be an indicator of the efficacy of
radiotherapy and chemotherapy (47,50,51) for
ESCC as well as EADC. As shown in Fig.
3, a previous study indicated that the change in the p21 status
from negative to positive during treatment was accompanied by an
improvement in survival (52).
At present, p21 is known to play an important role
in a variety of cancers and cannot be considered as a specific
marker for the diagnosis of EC. However, p21 is important for
evaluating prognosis and treatment efficacy in patients with
EC.
p21 as a novel target for EC
treatment
CDK4/6 inhibitors in clinical
treatment
p21 is a classic cell cycle regulator that acts as a
gene downstream of p53 to block the progression of the cell cycle.
This provides a basis for its use as a target for cancer treatment.
In fact, antitumor therapy targeting the cell cycle has gradually
matured. Some antitumor drugs targeting the cell cycle have entered
clinical practice, such as palbociclib, abemaciclib and ribociclib
(53). Palbociclib is an oral
targeted drug that directly acts on CDK4/6 and inhibits its
function, thereby restoring cell cycle control and inhibiting tumor
cell proliferation (54). At
present, CDK4/6 inhibitors are only approved for the treatment of
ER-positive breast cancer and attempts are being made to further
expand their use. Moreover, the identification of a novel target
for EC is required. The present review aimed to address the
possibility of using p21 as a novel therapeutic target.
Drugs targeting p21 exert therapeutic
effects on EC
Some drugs in trials have been shown to promote p21
expression, thereby inhibiting EC cell growth. A number of drugs
targeting p21 are currently under development. Diallyl disulfide
(DADS) is a lipid-soluble organic compound derived from garlic. A
previous study used various concentrations of DADS to treat cells,
and found that the proportion of EC cells in the G2/M
phase continued to increase, and the mRNA level of p21 also
increased with increasing DADS concentration. The same study
confirmed that DADS was an effective anticancer drug by suppressing
cell viability, blocking the cell cycle at the G2/M
phase and inducing the apoptosis of EC cells (55). Molecular analysis revealed that cell
cycle arrest may be due to the reduction of cyclin B1, cdc2, p-cdc2
and cdc25c, and the activation of the p53/p21 pathway. DADS was
shown to activate caspases, alter the Bax/Bcl-2 balance and inhibit
the MEK/ERK pathway to induce apoptosis (55).
Another study found that obatoclax (a type of
BH3-mimetic) induced G1/G0 arrest of EC cells
through the p38/p21 signaling pathway. Obatoclax is an indole
bipyrrole compound that can inhibit all known antiapoptotic Bcl-2
family members. Obatoclax did not alter the expression of CDKs,
including CDK2, CDK4 and CDK6, but significantly increased the
protein level of the CDK inhibitor, p21. The findings of that study
may shed new light on the anticancer activity of obatoclax and its
potential application in clinical practice (56).
Cinobufagin, isolated from traditional Chinese
herbs, exerts antitumor, anesthetic, analgesic and
anti-inflammatory effects. A previous study by Deng et al
(57) indicated that cinobufagin
induced ESCC cell cycle arrest at the G2/M phase and
promoted apoptosis by regulating the expression of p21, indicating
that traditional Chinese medicine may prove useful for the
treatment of EC.
Combined use of drugs targeting p21
can enhance the sensitivity to radiotherapy and chemotherapy
It was recently reported that crocetin combined with
cisplatin exert a synergistic anti-ESCC effect by upregulating the
p53/p21 pathway. The researchers demonstrated that the combination
of crocetin and cisplatin treatment markedly affected the
expression of p53 and p21 compared with treatment with cisplatin
alone. This type of combined treatment inhibited KYSE-150 cell
proliferation and promoted cell apoptosis in vitro (58).
Liu et al (59) demonstrated that Antrodia
cinnamomea mycelial fermentation broth blocked the cell cycle
progression of EC cells in the G2/M phase by
upregulating the expression of p21, thus rendering the cells more
sensitive to the subsequent dose of radiation. The aforementioned
simple examples demonstrate that p21-regulated drugs have exhibited
potent antitumor effects in experiments; a number of other studies
have also proven this effect (58,60–67). The
mechanisms of some of these drugs are summarized in Table II.
 | Table II.Substances used to treat esophageal
cancer by regulating p21 expression in previous experiments. |
Table II.
Substances used to treat esophageal
cancer by regulating p21 expression in previous experiments.
| Substance | Mechanism | (Refs.) |
|---|
| DADS | p53/p21
pathway | (55) |
| Obatoclax | p38/p21
pathway | (56) |
| Cinobufagin | p73/p21
pathway | (57) |
| Crocetin | p53/p21
pathway | (58) |
| AC-MFB | Upregulation of p21
expression | (59) |
| Ruthenium (II)
complex | p53/p21
pathway | (60) |
| Costunolide | p53/p21
pathway | (61) |
| Oridonin | p53/p21
pathway | (62) |
| Licochalone C | Upregulation of
p21/p27 expression | (63) |
| Suberoylanilide
hydroxamic acid | Upregulation of
p21/p27/Rb expression | (65) |
| Tanshinone IIA | p53/p21
pathway | (66) |
| Thymoquinone | p53/p21
pathway | (67) |
Conclusion
The therapeutic potential of p21 in EC warrants
further investigation. At present, the drugs targeting p21 have not
been used in clinical practice. All of the aforementioned agents
have been proven effective in the treatment of EC in vitro.
These drugs cause cell cycle arrest and induce apoptosis by
increasing the expression of p21. Furthermore, some substances
obtained from Chinese medicine have been shown to exert therapeutic
effects on EC, which also proves the value of Chinese medicine in
antitumor therapy (57).
It has been previously indicated that patients with
p21-deficient EC have a shorter survival time (46). For this group of patients, it would
be of interest to determine whether a type of p21 analog could be
produced in order to take advantage of the tumor-suppressive
effects of p21. Similar to the use of p16 as a template for the
development of palbociclib, a similar drug may be created using p21
as the template. To date, there is no study available on p21
analogues, at least to the best of our knowledge. If p21 protein
analogs can be developed to regulate the cycle of EC cells, this
may benefit a greater number of patients with EC.
Georgakilas et al (15) described p21 as a ‘two-faced genome
guardian’; this indicates that it has different functions in
different conditions in tumors. In the absence of p53, the
long-term overexpression of p21 enables some cells to escape
senescence (68). There are a number
of difficulties encountered in the identification of the mechanisms
of p21 due to its multiple roles. However, current research
indicates that p21 mainly plays a role in inhibiting cell
proliferation in EC. In addition, the prognosis of patients with EC
can be evaluated and the efficacy of chemotherapy and radiotherapy
can be determined by detecting the expression of p21 (69). Thus, investigating the mechanism of
p21 in EC should be the focus of future research. If p21 can be
used as a prognostic indicator for EC, the treatment efficacy of EC
may improve, and more patients will reap the benefits.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81872264).
Availability of data and materials
Not applicable.
Authors' contributions
LW and HH were involved in designing the study, in
the literature review and in the drafting of the manuscript. LW was
also responsible for designing the figures. LD and ZW participated
in the acquisition and analysis of data, and in the discussion of
the manuscript. YQ designed the study and critically revised the
manuscript. All the authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qu X, Ben Q and Jiang Y: Consumption of
red and processed meat and risk for esophageal squamous cell
carcinoma based on a meta-analysis. Ann Epidemiol. 23:762–770.e1.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Prabhu A, Obi KO and Rubenstein JH: The
synergistic effects of alcohol and tobacco consumption on the risk
of esophageal squamous cell carcinoma: A meta-analysis. Am J
Gastroenterol. 109:822–827. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Andrici J and Eslick GD: Hot food and
beverage consumption and the risk of esophageal cancer: A
meta-analysis. Am J Prev Med. 49:952–960. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu C, Wang Z, Song X, Feng XS, Abnet CC,
He J, Hu N, Zuo XB, Tan W, Zhan Q, et al: Joint analysis of three
genome-wide association studies of esophageal squamous cell
carcinoma in Chinese populations. Nat Genet. 46:1001–1006. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang SM, Abnet CC and Qiao YL: What have
we learned from Linxian esophageal cancer etiological studies?
Thorac Cancer. 10:1036–1042. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Spechler SJ: Barrett's esophagus. Curr
Opin Gastroenterol. 15:352–358. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Woodward TA, Klingler PD, Genko PV and
Wolfe JT: Barrett's esophagus, apoptosis and cell cycle regulation:
Correlation of p53 with Bax, Bcl-2 and p21 protein expression.
Anticancer Res. 20:2427–2432. 2000.PubMed/NCBI
|
|
9
|
Hong Y and Ding ZY: PD-1 inhibitors in the
advanced esophageal cancer. Front Pharmacol. 10:14182019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
el-Deiry WS, Tokino T, Velculescu VE, Levy
DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW and
Vogelstein B: WAF1, a potential mediator of p53 tumor suppression.
Cell. 75:817–825. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Harper JW, Adami GR, Wei N, Keyomarsi K
and Elledge SJ: The p21 Cdk-interacting protein Cip1 is a potent
inhibitor of G1 cyclin-dependent kinases. Cell. 75:805–816. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Petroni G, Formenti SC, Chen-Kiang S and
Galluzzi L: Immunomodulation by anticancer cell cycle inhibitors.
Nat Rev Immunol. 20:669–679. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Miao Y, Shang M, Liu M, Liu R,
Pan E, Pu Y and Yin L: LincRNA-p21 leads to G1 arrest by p53
pathway in esophageal squamous cell carcinoma. Cancer Manag Res.
11:6201–6214. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Georgakilas AG, Martin OA and Bonner WM:
p21: A two-faced genome guardian. Trends Mol Med. 23:310–319. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Evans T, Rosenthal ET, Youngblom J, Distel
D and Hunt T: Cyclin: A protein specified by maternal mRNA in sea
urchin eggs that is destroyed at each cleavage division. Cell.
33:389–396. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bloom J and Cross FR: Multiple levels of
cyclin specificity in cell-cycle control. Nat Rev Mol Cell Biol.
8:149–160. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
LaBaer J, Garrett MD, Stevenson LF,
Slingerland JM, Sandhu C, Chou HS, Fattaey A and Harlow E: New
functional activities for the p21 family of CDK inhibitors. Genes
Dev. 11:847–862. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Waga S and Stillman B: The DNA replication
fork in eukaryotic cells. Annu Rev Biochem. 67:721–751. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Celis JE, Madsen P, Celis A, Nielsen HV
and Gesser B: Cyclin (PCNA, auxiliary protein of DNA polymerase
delta) is a central component of the pathway(s) leading to DNA
replication and cell division. FEBS Lett. 220:1–7. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Podust VN, Podust LM, Goubin F, Ducommun B
and Hübscher U: Mechanism of inhibition of proliferating cell
nuclear antigen-dependent DNA synthesis by the cyclin-dependent
kinase inhibitor p21. Biochemistry. 34:8869–8875. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Waga S, Hannon GJ, Beach D and Stillman B:
The p21 inhibitor of cyclin-dependent kinases controls DNA
replication by interaction with PCNA. Nature. 369:574–578. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang H, Xiong Y and Beach D:
Proliferating cell nuclear antigen and p21 are components of
multiple cell cycle kinase complexes. Mol Biol Cell. 4:897–906.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Asada M, Yamada T, Ichijo H, Delia D,
Miyazono K, Fukumuro K and Mizutani S: Apoptosis inhibitory
activity of cytoplasmic p21(Cip1/WAF1) in monocytic
differentiation. EMBO J. 18:1223–1234. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tanaka H, Yamashita T, Asada M, Mizutani
S, Yoshikawa H and Tohyama M: Cytoplasmic p21(Cip1/WAF1) regulates
neurite remodeling by inhibiting Rho-kinase activity. J Cell Biol.
158:321–329. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhan J, Easton JB, Huang S, Mishra A, Xiao
L, Lacy ER, Kriwacki RW and Houghton PJ: Negative regulation of
ASK1 by p21Cip1 involves a small domain that includes Serine 98
that is phosphorylated by ASK1 in vivo. Mol Cell Biol.
27:3530–3541. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiong Y, Hannon GJ, Zhang H, Casso D,
Kobayashi R and Beach D: p21 is a universal inhibitor of cyclin
kinases. Nature. 366:701–704. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu S, Bishop WR and Liu M: Differential
effects of cell cycle regulatory protein p21(WAF1/Cip1) on
apoptosis and sensitivity to cancer chemotherapy. Drug Resist
Updat. 6:183–195. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim J, Bae S, An S, Park JK, Kim EM, Hwang
SG, Kim WJ and Um HD: Cooperative actions of p21WAF1 and p53 induce
Slug protein degradation and suppress cell invasion. EMBO Rep.
15:1062–1068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim EM, Jung CH, Kim J, Hwang SG, Park JK
and Um HD: The p53/p21 complex regulates cancer cell invasion and
apoptosis by targeting Bcl-2 family proteins. Cancer Res.
77:3092–3100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu F, Chen H, Zhou C, Liu S, Guo M, Chen
P, Zhuang H, Xie D and Wu S: T-type Ca2+ channel expression in
human esophageal carcinomas: A functional role in proliferation.
Cell Calcium. 43:49–58. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li H, Zheng D, Zhang B, Liu L, Ou J, Chen
W, Xiong S, Gu Y and Yang J: Mir-208 promotes cell proliferation by
repressing SOX6 expression in human esophageal squamous cell
carcinoma. J Transl Med. 12:1962014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li L, Zhang C, Li X, Lu S and Zhou Y: The
candidate tumor suppressor gene ECRG4 inhibits cancer cells
migration and invasion in esophageal carcinoma. J Exp Clin Cancer
Res. 29:1332010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qin YR, Tang H, Xie F, Liu H, Zhu Y, Ai J,
Chen L, Li Y, Kwong DL, Fu L and Guan XY: Characterization of
tumor-suppressive function of SOX6 in human esophageal squamous
cell carcinoma. Clin Cancer Res. 17:46–55. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang Y, Goldstein BG, Chao HH and Katz JP:
KLF4 and KLF5 regulate proliferation, apoptosis and invasion in
esophageal cancer cells. Cancer Biol Ther. 4:1216–1221. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fan Y, Wang Y, Fu S, Liu D and Lin S:
Methylation-regulated ZNF545 inhibits growth of the p53-mutant
KYSE150 cell line by inducing p21 and Bax. Exp Ther Med.
18:1563–1570. 2019.PubMed/NCBI
|
|
37
|
Jiang XR, Yu XY, Fan JH, Guo L, Zhu C,
Jiang W and Lu SH: RFT2 is overexpressed in esophageal squamous
cell carcinoma and promotes tumorigenesis by sustaining cell
proliferation and protecting against cell death. Cancer Lett.
353:78–86. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao N, Huang G, Guo L and Lu SH: ECRG1, a
novel candidate of tumor suppressor gene in the esophageal
carcinoma, triggers a senescent program in NIH3T3 cells. Exp Biol
Med (Maywood). 231:84–90. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jascur T, Brickner H, Salles-Passador I,
Barbier V, El Khissiin A, Smith B, Fotedar R and Fotedar A:
Regulation of p21(WAF1/CIP1) stability by WISp39, a Hsp90 binding
TPR protein. Mol Cell. 17:237–249. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu G and Lozano G: p21 stability: Linking
chaperones to a cell cycle checkpoint. Cancer Cell. 7:113–114.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Deng T, Yan G, Song X, Xie L, Zhou Y, Li
J, Hu X, Li Z, Hu J, Zhang Y, et al: Deubiquitylation and
stabilization of p21 by USP11 is critical for cell-cycle
progression and DNA damage responses. Proc Natl Acad Sci USA.
115:4678–4683. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang W, Nacusi L, Sheaff RJ and Liu X:
Ubiquitination of p21Cip1/WAF1 by SCFSkp2: Substrate requirement
and ubiquitination site selection. Biochemistry. 44:14553–14564.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bornstein G, Bloom J, Sitry-Shevah D,
Nakayama K, Pagano M and Hershko A: Role of the SCFSkp2 ubiquitin
ligase in the degradation of p21Cip1 in S phase. J Biol Chem.
278:25752–25757. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li Q, Li X, Tang H, Jiang B, Dou Y,
Gorospe M and Wang W: NSUN2-mediated m5C methylation and
METTL3/METTL14-mediated m6A methylation cooperatively enhance p21
translation. J Cell Biochem. 118:2587–2598. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lal A, Mazan-Mamczarz K, Kawai T, Yang X,
Martindale JL and Gorospe M: Concurrent versus individual binding
of HuR and AUF1 to common labile target mRNAs. EMBO J.
23:3092–3102. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu J, Liu L, Wu F, Qiu L, Luo M, Ke Q,
Deng X and Luo Z: Clinical and prognostic implications of P21
(WAF1/CIP1) expression in patients with esophageal cancer: A
systematic review and meta-analysis. Dis Markers. 2020:65202592020.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ishida M, Morita M, Saeki H, Ohga T,
Sadanaga N, Watanabe M, Kakeji Y and Maehara Y: Expression of p53
and p21 and the clinical response for hyperthermochemoradiotherapy
in patients with squamous cell carcinoma of the esophagus.
Anticancer Res. 27:3501–3506. 2007.PubMed/NCBI
|
|
48
|
Kuwahara M, Hirai T, Yoshida K, Yamashita
Y, Hihara J, Inoue H and Toge T: p53, p21(Waf1/Cip1) and cyclin D1
protein expression and prognosis in esophageal cancer. Dis
Esophagus. 12:116–119. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lin Y, Shen LY, Fu H, Dong B, Yang HL, Yan
WP, Kang XZ, Dai L, Zhou HT, Yang YB, et al: P21, COX-2, and
E-cadherin are potential prognostic factors for esophageal squamous
cell carcinoma. Dis Esophagus. 30:1–10. 2017.
|
|
50
|
Nakamura T, Hayashi K, Ota M, Ide H,
Takasaki K and Mitsuhashi M: Expression of p21(Waf1/Cip1) predicts
response and survival of esophageal cancer patients treated by
chemoradiotherapy. Dis Esophagus. 17:315–321. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sohda M, Ishikawa H, Masuda N, Kato H,
Miyazaki T, Nakajima M, Fukuchi M, Manda R, Fukai Y, Sakurai H and
Kuwano H: Pretreatment evaluation of combined HIF-1alpha, p53 and
p21 expression is a useful and sensitive indicator of response to
radiation and chemotherapy in esophageal cancer. Int J Cancer.
110:838–844. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Heeren PA, Kloppenberg FW, Hollema H,
Mulder NH, Nap RE and Plukker JT: Predictive effect of p53 and p21
alteration on chemotherapy response and survival in locally
advanced adenocarcinoma of the esophagus. Anticancer Res.
24:2579–2883. 2004.PubMed/NCBI
|
|
53
|
Ingham M and Schwartz GK: Cell-cycle
therapeutics come of age. J Clin Oncol. 35:2949–2959. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Clark AS, Karasic TB, DeMichele A, Vaughn
DJ, O'Hara M, Perini R, Zhang P, Lal P, Feldman M, Gallagher M and
O'Dwyer PJ: Palbociclib (PD0332991)-a selective and potent
cyclin-dependent kinase inhibitor: A review of pharmacodynamics and
clinical development. JAMA Oncol. 2:253–260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yin X, Zhang R, Feng C, Zhang J, Liu D, Xu
K, Wang X, Zhang S, Li Z, Liu X and Ma H: Diallyl disulfide induces
G2/M arrest and promotes apoptosis through the p53/p21 and MEK-ERK
pathways in human esophageal squamous cell carcinoma. Oncol Rep.
32:1748–1756. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhong D, Gu C, Shi L, Xun T, Li X, Liu S
and Yu L: Obatoclax induces G1/G0-phase arrest via
p38/p21(waf1/Cip1) signaling pathway in human esophageal cancer
cells. J Cell Biochem. 115:1624–1635. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Deng X, Sheng J, Liu H, Wang N, Dai C,
Wang Z, Zhang J, Zhao J and Dai E: Cinobufagin promotes cell cycle
arrest and apoptosis to block human esophageal squamous cell
carcinoma cells growth via the p73 signalling pathway. Biol Pharm
Bull. 42:1500–1509. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li S, Shen XY, Ouyang T, Qu Y, Luo T and
Wang HQ: Synergistic anticancer effect of combined crocetin and
cisplatin on KYSE-150 cells via p53/p21 pathway. Cancer Cell Int.
17:982017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liu YM, Liu YK, Huang PI, Tsai TH and Chen
YJ: Antrodia cinnamomea mycelial fermentation broth inhibits
the epithelial-mesenchymal transition of human esophageal
adenocarcinoma cancer cells. Food Chem Toxicol. 119:380–386. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Guo L, Lv G, Qiu L, Yang H, Zhang L, Yu H,
Zou M and Lin J: Insights into anticancer activity and mechanism of
action of a ruthenium(II) complex in human esophageal squamous
carcinoma EC109 cells. Eur J Pharmacol. 786:60–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hua P, Sun M, Zhang G, Zhang Y, Song G,
Liu Z, Li X, Zhang X and Li B: Costunolide induces apoptosis
through generation of ROS and activation of P53 in human esophageal
cancer Eca-109 cells. J Biochem Mol Toxicol. 30:462–469. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Jiang JH, Pi J, Jin H and Cai JY:
Oridonin-induced mitochondria-dependent apoptosis in esophageal
cancer cells by inhibiting PI3K/AKT/mTOR and Ras/Raf pathways. J
Cell Biochem. 120:3736–3746. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kwak AW, Choi JS, Liu K, Lee MH, Jeon YJ,
Cho SS, Yoon G, Oh HN, Chae JI and Shim JH: Licochalcone C induces
cell cycle G1 arrest and apoptosis in human esophageal squamous
carcinoma cells by activation of the ROS/MAPK signaling pathway. J
Chemother. 32:132–143. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Liu YM, Liu YK, Wang LW, Huang YC, Huang
PI, Tsai TH and Chen YJ: The medicinal fungus Antrodia
cinnamomea regulates DNA repair and enhances the
radiosensitivity of human esophageal cancer cells. Onco Targets
Ther. 9:6651–6661. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tzao C, Jin JS, Chen BH, Chung HY, Chang
CC, Hsu TY and Sun GH: Anticancer effects of suberoylanilide
hydroxamic acid in esophageal squamous cancer cells in vitro and in
vivo. Dis Esophagus. 27:693–702. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang JF, Feng JG, Han J, Zhang BB and Mao
WM: The molecular mechanisms of Tanshinone IIA on the apoptosis and
arrest of human esophageal carcinoma cells. Biomed Res Int.
2014:5827302014.PubMed/NCBI
|
|
67
|
Ma J, Zhang Y, Deng H, Liu Y, Lei X, He P
and Dong W: Thymoquinone inhibits the proliferation and invasion of
esophageal cancer cells by disrupting the AKT/GSK-3β/Wnt signaling
pathway via PTEN upregulation. Phytother Res. Sep 9–2020.(Epub
ahead of print). doi: 10.1002/ptr.6795. View Article : Google Scholar
|
|
68
|
Galanos P, Vougas K, Walter D, Polyzos A,
Maya-Mendoza A, Haagensen EJ, Kokkalis A, Roumelioti FM, Gagos S,
Tzetis M, et al: Chronic p53-independent p21 expression causes
genomic instability by deregulating replication licensing. Nat Cell
Biol. 18:777–789. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
El-Deiry WS: p21(WAF1) mediates cell-cycle
inhibition, relevant to cancer suppression and therapy. Cancer Res.
76:5189–5191. 2016. View Article : Google Scholar : PubMed/NCBI
|