Introduction
Previously, colorectal cancer (CRC) was
characterized by an aging population in high income countries
attributed to dietary habits and lifestyle (1). However, currently CRC is the third most
commonly diagnosed malignancy and ranks fourth in terms of
mortality with ~900,000 deaths annually, including in developing
countries (2,3). While CRC rates vary widely worldwide,
three distinct temporal patterns have been identified based on
incidence and mortality rates. The pattern of increased incidence
and increased mortality is most often seen in transitioning
economies (4). One such economy that
suffers from the burden of cancer and associated mortality is
China. The incidence of CRC (12.2%) was found to be second only
after lung cancer (18.1%) (3). The
burden of the disease not only equates to the associated mortality
but also severely impacts the quality of life.
Improving the mortality rate of CRC can be
approached by improving diagnostic techniques and providing early
interventional treatment. However, CRC may in part be derived from
a variety of environmental factors, making it one of the most
heterogenous types of cancer (5).
Its complex heterogeneity is not yet completely understood and
contributes to the lack of predictive prognostic markers, except
for the RAS-mutation status (6).
Genomics and epigenomics have revolutionized cancer
diagnostics and predictive biomarker assessments (7); therefore, the present study aimed to
elucidate the influence of post-translation modifications (PTM) on
carcinogenesis in CRC. The era of -omics (genomics, transcriptomics
and epigenomics) coupled with technological advancements, such as
next generation sequencing, has greatly improved the understanding
of different cancers (8). However,
translation of these in silico findings to the field is
largely affected by the lack of available data.
One of the most common PTM is prolyl hydroxylation.
Hydroxyproline constitutes ~4% of all amino acids, with most of
them being present in collagen. In human pancreatic cancer cells
and tissues, prolyl 4-hydroxylase subunit α1 (P4HA1), is the most
predominant isoform that contributes to proline 4-hydroxylase
activity (9). Proline 4-hydroxylase
comprises tetrameric units (α2β2) and is responsible for the
4-hydroxylation of proline, ensuring folding and formation of the
collagen triple helix (10). The
role of P4HA1 has been investigated in breast cancer (11) and pancreatic cancer (9). Whilst the epigenetic modification of
P4HA1 with microRNAs (miR) have received attention in prostate
cancer (12), the contribution of
prolyl hydroxylation in CRC tumorigenesis remains to be elucidated.
It has previously been shown in both triple negative breast cancer
cells and pancreatic ductal adenocarcinoma that P4HA1 stabilizes
hypoxia inducible factor-1α (HIF1α) through modulating glycolytic
activity, such as the levels of α-ketoglutarate and succinate, thus
mediating cellular transformation (10).
It has been demonstrated that the wingless-type
mouse mammary tumor virus integration site family (Wnt) signaling
pathway is activated in CRCs (13,14). The
Wnt proteins are secreted glycoproteins that also act as ligands of
the Wnt signaling pathway. There are three distinct Wnt signaling
pathways currently known: i) β-catenin pathway; ii) the calcium
dependent pathway; and iii) planar cell polarity pathway (14). While cell growth and proliferation
are promoted through the canonical β-catenin pathway, cell motility
and polarity are governed by the latter two pathways. Surface
binding of intracytoplasmic β-catenin and Wnt ligands activates the
Wnt signaling pathway (14).
However, β-catenin can be phosphorylated in the absence of Wnt
ligands (15).
Given the aforementioned evidence, the present study
aimed to establishing the role of the most common PTM, P4HA1, in
the promotion and progression of CRC through the activation of the
β-catenin pathway. In addition, the pathway through which P4HA1
alters the cell signaling pathways leading to tumor progression was
assessed. With this objective, in silico analyses using the
Gene Expression Omnibus (GEO) database confirmed the upregulation
of P4HA1 in CRC (15). In
vitro experiments analyzed the mRNA and protein expression of
P4HA1 and its influence on cellular transformation, with respect to
cellular proliferation, stemness and chemoresistance. This is a
preliminary demonstration of the activation of the β-catenin
pathway through P4HA1.
The assessment of PTMs and their signaling
mechanisms may serve as a unique approach to expand the repertoire
of reliable diagnostic and prognostic markers in CRC. Furthermore,
these mechanisms may also serve as molecular targets for directed
intervention.
Materials and methods
GEO analysis
We analyzed gene expression of CRC patients through
two GEO databases, GEO/GDS4382 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE32323)
(16) and GEO/GDS5232 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE25071)
(17) using GEO2R method (https://www.ncbi.nlm.nih.gov/geo/info/geo2r.html).
Clinical samples
The present study was performed in accordance with
the Declaration of Helsinki and approved by the Medical Ethics
Committee of Qiqihar Medical University (no. 2015QY137). Written
informed consent was obtained from all patients. Cancer tissues and
adjacent normal tissues (≥5 cm from the edge of the tumor) were
collected by debulking surgery from 30 patients with colorectal
adenocarcinoma who were recruited into a clinical trial at the
Third Affiliated Hospital of Qiqihar Medical University between
January 2016 and January 2017. Clinicopathological information was
obtained and two pathologists independently determined diagnoses
according to World Health Organization classification of tumors of
the digestive system (18) and the
clinicopathological parameters listed in Table SI. None of the patients in the study
received chemotherapy or radiation treatment prior to surgery and
all patients were diagnosed with adenocarcinoma and other
pathological types were excluded. Of the 30 included patients, 18
were men and 12 were women. The age of patients ranged from 42 to
81 years. The cancers were classified as follows: 8
well-differentiated, 15 moderately differentiated and 7 poorly
differentiated colorectal tissues.
Human CRC cell culture
The human CRC cell lines (SW480, SW620 and HCT116
cells) were purchased from The Cell Bank of Type Culture Collection
of the Chinese Academy of Sciences and cultured in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml
streptomycin (Gibco; Thermo Fisher Scientific, Inc.). All cells
were cultured at 37°C in a 5% CO2 atmosphere. The cells
were cultured in 1% oxygen atmosphere when hypoxic conditions were
required. P4HA1 and control siRNA were purchased from Shanghai
GenePharma Co., Ltd. These siRNA duplexes (100 nmol/l) were
transfected into CRC cells using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocols. Briefly, one day before transfection, CRC
cells were incubated in growth medium without antibiotics and were
~50% confluent at the time of transfection. Lipofectamine 2000 was
mixed with Opti-MEM I Reduced Serum Medium (Invitrogen; Thermo
Fisher Scientific, Inc.) and incubated for 5 min at room
temperature, then the siRNA duplexes were mixed with the diluted
Lipofectamine 2000 and incubated for 20 min at room temperature.
Subsequent experiments were performed in CRC cells 48 h
post-transfection at 37°C in a CO2 incubator. The
following sequences of siRNA were used for transfection: P4HA1
siRNA, 5′-GAUAAAGUCUCUGUUCUAG-3′; HIF1α,
5′-AACCAAGTAGCCTGTTATCAA-3′; and control siRNA,
5′-UUCUCCGAACGUGUCACGUTT-3′.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
All commercial assays used were performed according
to the manufacturer's instructions. Total mRNA from cultured SW620
and HCT116 cells and tissue samples was extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.),. Complementary DNA was synthesized from 2 µg total RNA using
a Reverse Transcription kit (Takara Biotechnology Co., Ltd.)
according to the manufacturers' instructions. Real-time
quantitative PCR analyses were performed with SYBR-Green Real-Time
Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) on
a 7500 Fast Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) using the following thermocycling conditions:
Initial denaturation at 95°C, followed by 30 cycles at 95°C for 15
sec and 60°C for 1 min. The following primer pairs were used for
qPCR: P4HA1 forward, 5′-AGGGGTTGCTGTGGATTACC-3′ and reverse,
5′-GTCATGTACTGTAGCTCGGC-3′; and GAPDH forward,
5′-GGGCTGCTTTTAACTCTGGT-3′ and reverse, 5′-TGGCAGGTTTTTCTAGACGG-3′.
The Applied Biosystems 7500 Fast software version 1.4 (Applied
Biosystems; Thermo Fisher Scientific, Inc.) was used to analyze the
Cq values of different. Comparisons were made using the
2−ΔΔCt method (19) and
mRNAs normalized to an endogenous control GAPDH.
Immunohistochemical staining
CRC tissues were fixed in 10% formalin for one week
at room temperature and embedded in paraffin, and then cut into
5-µm-thick sections. The sections were deparaffinized in a xylene
bath, rehydrated in PBS and then subjected to heat-induced epitope
retrieval. The sections were then blocked with 3% hydrogen peroxide
for 5 min at room temperature, incubated with primary antibodies
against P4HA1 (cat. no. ab127564; 1:100; Abcam) at 4°C overnight.
The sections were then washed with PBS, followed by incubation with
HRP-conjugated secondary antibody (cat. no. sc-2357 mouse
anti-rabbit IgG-HRP; 1:1,000; Santa Cruz Biotechnology, Inc.) for 1
h at room temperature. The sections were then visualized using a
DAB (Sigma-Aldrich; Merck KGaA) according to the manufacturer's
instructions under a light microscope (Nikon Corporation;
magnification, 200×).
Western blotting
Total proteins were extracted from normal colorectal
tissues, CRC tissues and SW480, SW620 and HCT116 cell lines using
ice-cold RIPA lysis buffer [50 mM Tris (pH 7.4), 150 mM NaCl, 1%
Triton X-100, 1% sodium deoxycholate, 0.1% SDS] with
proteinase/phosphatase inhibitors (Thermo Fisher Scientific, Inc.).
The protein concentration was measured by bicinchoninic acid
method. Lysates containing 20 µg protein were separated via
SDS-PAGE (10% gel), which were then transferred to polyvinylidene
difluoride membranes (EMD Millipore). The membranes were blocked in
5% non-fat milk (Thermo Fisher Scientific, Inc.) for 1 h at room
temperature and subsequently incubated with primary antibodies
against P4HA1 (cat. no. ab127564; 1:1,000; Abcam), β-catenin (cat.
no. sc-7963; 1:1,000; Santa Cruz Biotechnology, Inc.),
phosphorylated (p)-β-catenin (cat. no. sc-101650; 1:2,000; Santa
Cruz Biotechnology, Inc.), CD133 (cat. no. sc-30220; 1:1,000; Santa
Cruz Biotechnology, Inc.), Nanog (cat. no. sc-134218; 1:1,000;
Santa Cruz Biotechnology, Inc.), axis inhibition protein 2 (Axin2;
cat. no. sc-25302; 1:1,000; Santa Cruz Biotechnology, Inc.), c-Myc
(cat. no. sc-373712; 1:1,000; Santa Cruz Biotechnology, Inc.) and
GAPDH (cat. no. sc-20357; 1:500; Santa Cruz Biotechnology, Inc.).
All antibodies were obtained from Santa Cruz Biotechnology, Inc.
The membranes were then washed 3 times in 0.1% Tween® 20
detergent and incubated with horseradish peroxidase-conjugated
mouse anti-rabbit or anti-goat secondary antibodies (cat. nos.
sc-2357 mouse anti-rabbit IgG-HRP and sc-2005 goat anti-mouse
IgG-HRP; 1:10,000; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. Protein bands were detected using an enhanced
chemiluminescence kit (Thermo Fisher Scientific, Inc.). ImageJ
software (version 1.48; National Institutes of Health, NIH) was
used to determine intensity of western blot bands normalized to
GAPDH.
MTS assay
The proliferation of SW620 and HCT116 cells was
measured using an MTS assay kit (Promega Corporation) according to
the manufacturer's instructions. Briefly, SW620 and HCT116 cells
(2,000 cells/well) were seeded into 96-well plates and, after 24 h,
the medium was replaced with fresh DMEM and the cells were further
cultured for 3 days at 37°C. MTS reagent (20 µl) was then added to
each well, and plates were incubated for 1 h at 37°C. Absorbance
was measured at 490 nm using a microplate reader (Bio-Rad
Laboratories, Inc.). Each individual experiment was performed with
six replicates three independent times.
Cell Counting Kit-8 (CCK-8) assay
CCK-8 cell proliferation kit (Beyotime Institute of
Biotechnology) was used to analyze CRC cell proliferation according
to the manufacturer's protocol. SW620 and HCT116 cells
(0.5×104/well) were seeded into 96-well plates and
cultured overnight at 37°C. The supernatant was then removed and 2
ml CCK-8 reagent, WST-8, was added into each well and incubated for
1 h to be reduced by dehydrogenase in mitochondria to orange
formazan. The absorbance was then measured at 490 nm using a
microplate reader (Bio-Rad Laboratories, Inc.).
Cell cycle
SW620 and HCT116 cells (2×106/ml) were
collected and washed twice with PBS buffer and centrifuged at 1,000
× g for 5 min at 4°C. The supernatant was discarded and cells were
fixed with ice-cold 70% ethanol (by adding dropwise to the pellet
while vortexing) for at least 30 min at 4°C. The cell solution was
centrifuged at 1,000 × g for 5 min at 4°C and cells were washed
twice with PBS and centrifuged again at 1,000 × g for 5 min at 4°C.
The supernatant was discarded and cells were incubated with 50 µl
RNase I (1 µg/ml; Sigma-Aldrich; Merck KGaA) at 37°C for 1 h in the
dark. Cells were then treated with 200 µl propidium iodide (20
µg/ml) at room temperature for 1 min according to the
manufacturer's protocol. Cell cycle analysis was performed using
the LSR II flow cytometer (BD Biosciences) and the data were
analyzed by FlowJo software (version 9; FlowJo, LLC).
Caspase-3 activity assay
Caspase-3 activity was performed using the caspase-3
Activity Assay kit (Beyotime Institute of Biotechnology) following
the manufacturer's protocol. In brief, proteins were isolated from
CRC cells and 100 µg protein were added to a reaction buffer
containing 2 mM peptide substrate acetyl-Asp-Glu-Val- Asp
p-nitroanilide (Ac-DEVD-pNA) solution from the kit, and incubated
at 37°C for 2 h. The absorbance was measured using a microplate
reader (Bio-Rad Laboratories, Inc.) at 405 nm. The caspase-3
activity was recorded as the ratio to that of the control
group.
Tumorsphere assay
SW620 and HCT116 cells were cultured in DMEM with
10% FBS in an incubator containing 5% CO2 at 37°C. When
cells reached 80% confluence, 6×105 cells were
resuspended in stem cell culture medium supplemented with 2% B27
(Gibco; Thermo Fisher Scientific, Inc.), 20 ng/ml recombinant human
epidermal growth factor (Gibco; Thermo Fisher Scientific, Inc.) and
20 ng/ml recombinant human fibroblast growth factor (Gibco; Thermo
Fisher Scientific, Inc.) in each 6-well ultralow adhesion plates
(Corning Inc.) for 6 days at 37°C in a 5% CO2
atmosphere. When the tumorspheres grew to 50 µm in diameter, the
spheres were imaged and counted under a light microscope (Nikon
Corporation). The primary spheres were dissociated into single
cells and cultured in stem cell culture medium (Gibco; Thermo
Fisher Scientific, Inc.) for 14 days at 37°C to allow each cell
forming one tumorsphere.
Chemoresistant assay
SW620 and HCT116 cells were treated with the
chemotherapeutic agent 5-fluorouracil (5-FU) at various
concentration (0, 2, 4, 8 or 16 mmol/l) for 2 h, and the cells were
then cultured for 24 h. Subsequent experiments were performed.
Statistical analysis
Each experiment was performed three times. All
results were analyzed using SPSS software, version 19.0 (IBM
Corp.). Values are expressed as the mean ± SD. Differences between
two groups were analyzed using unpaired Student's t-test or one-way
ANOVA followed by Bonferroni post hoc analysis for multiple group
comparisons, as appropriate. P<0.05 was considered to indicate a
statistically significant difference.
Results
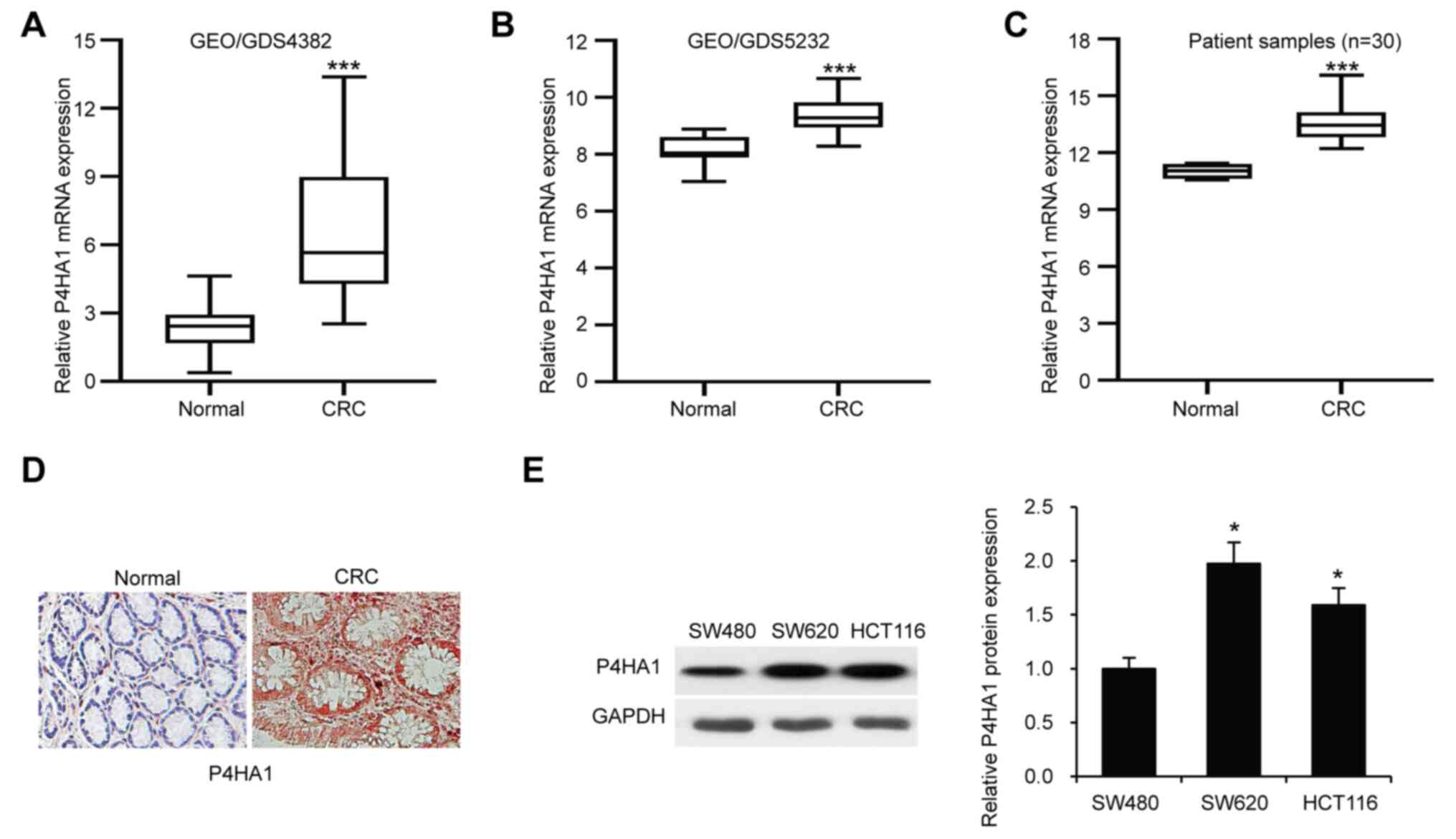
P4HA1 is upregulated in CRC tissues
and cells
The expression of P4HA1 was analyzed from the public
database GEO. P4HA1 mRNA was found to be significantly upregulated
in CRC tissues (GEO/GDS4382 and GEO/GDS5232; P<0.001) compared
with normal tissues (Fig. 1A and B).
Overexpression of P4HA1 was also confirmed in CRC clinical samples
compared with adjacent normal colorectal tissues from 30 patients
with CRC by RT-qPCR (Fig. 1C). In
addition, immunohistochemical staining analysis for P4HA1 in CRC
tissues found that P4HA1 staining was stronger in CRC tissues
compared with adjacent normal tissues (Fig. 1D). P4HA1 protein expression levels
were also analyzed in three CRC cell lines (SW480, SW620 and HCT116
cells) via western blotting (Fig.
1E). The results showed a significant difference in the
expression levels of P4HA1 in SW620 and HCT116 cells compared with
the SW480 cell line, therefore all further in vitro studies
were undertaken using these two cell lines.
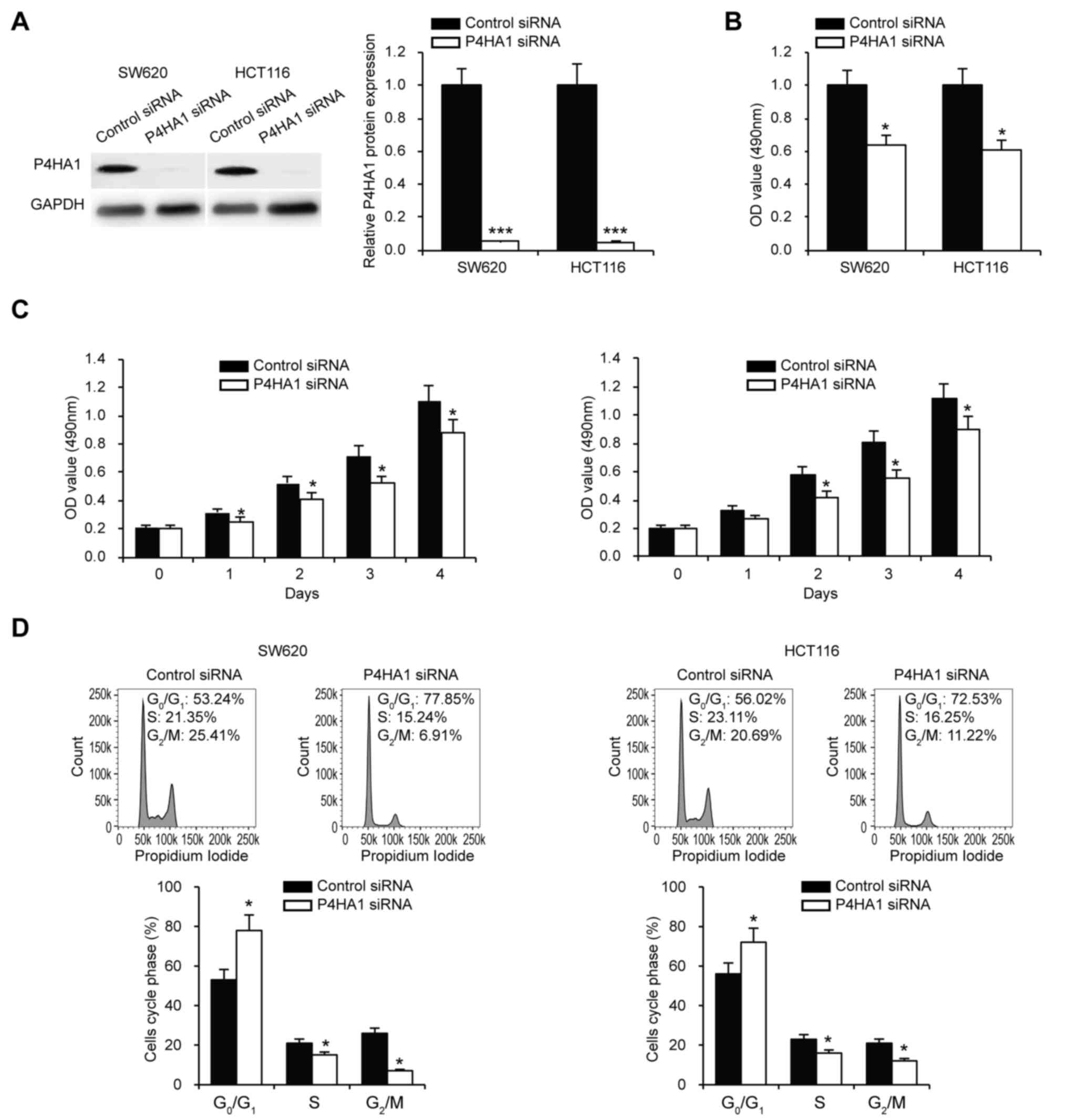
P4HA1 knockdown inhibits CRC cell
proliferation
To investigate whether P4HA1 affects the
proliferation of SW620 and HCT116 cells, P4HA1 was knocked down
using siRNA, which was confirmed via western blotting (Fig. 2A). The P4HA1-knocked down SW620 and
HCT116 cells were then subjected to CCK-8 and MTS cell
proliferation assays. The results indicated that P4HA1 knockdown
significantly decreased SW620 and HCT116 cell proliferation
(Fig. 2B and C). Cell cycle analysis
further confirmed the inhibitory effect of P4HA1 knockdown on cell
proliferation and more cells were arrested in phase
G0/G1 (Fig.
2D).
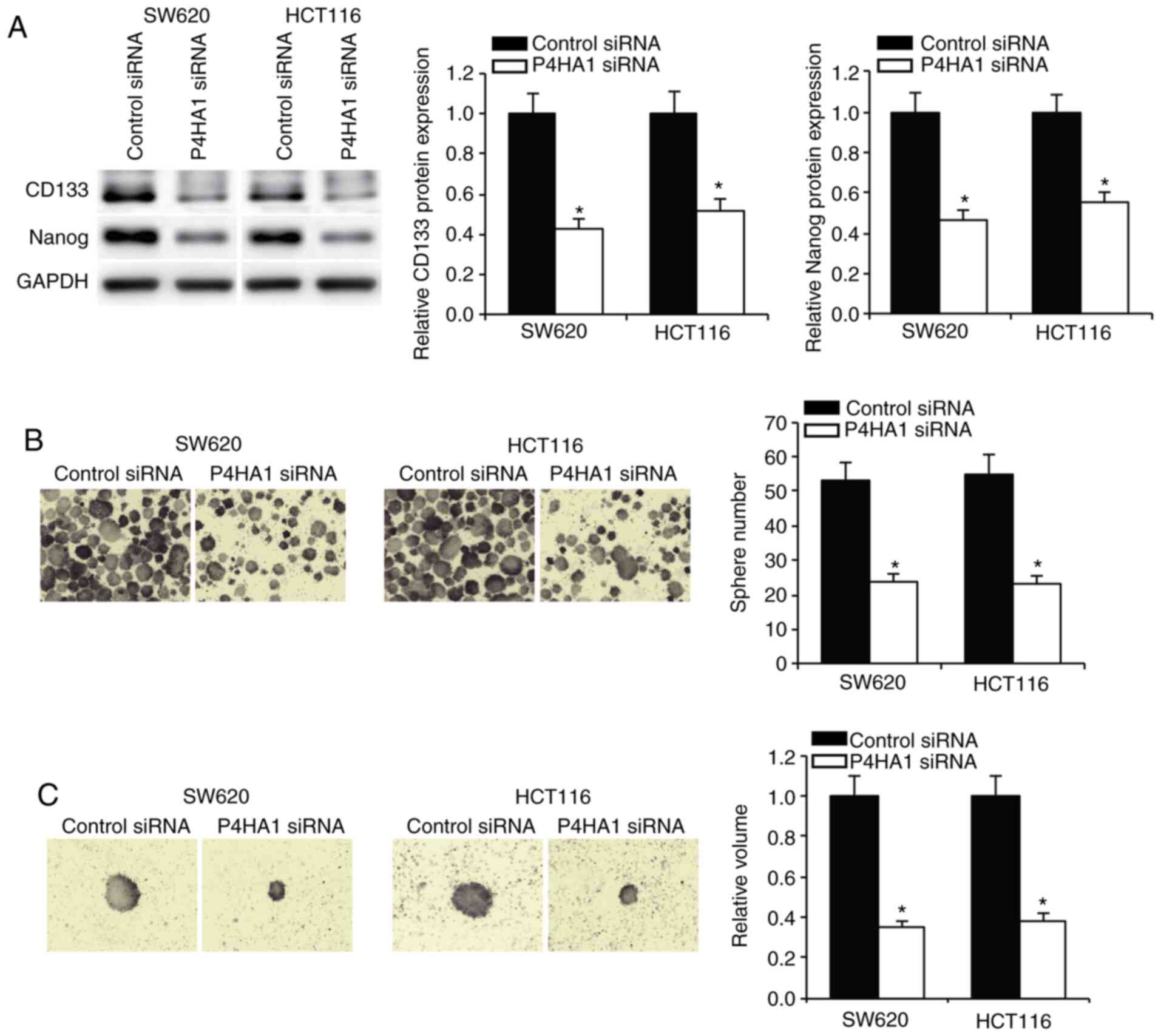
P4HA1 knockdown decreases CRC cell
stemness
In order to determine the role of P4HA1 in CRC cell
stemness, SW620 and HCT116 cells were cultured in stem cell medium,
following examination of the stem cell markers CD133 and Nanog.
Knockdown of P4HA1 was found to significantly decrease the
expression levels of these stem cell markers (Fig. 3A). Tumorsphere formation assay for
SW620 and HCT116 cells showed that P4HA1 knockdown resulted in a
decreased formation of spheres (Fig.
3B). Furthermore, the isolation of single CRC cells from
primary spheres and the culture of these in stem cell medium,
demonstrated that the tumorsphere volume was significantly
decreased in P4HA1 knocked down cells (Fig. 3C).
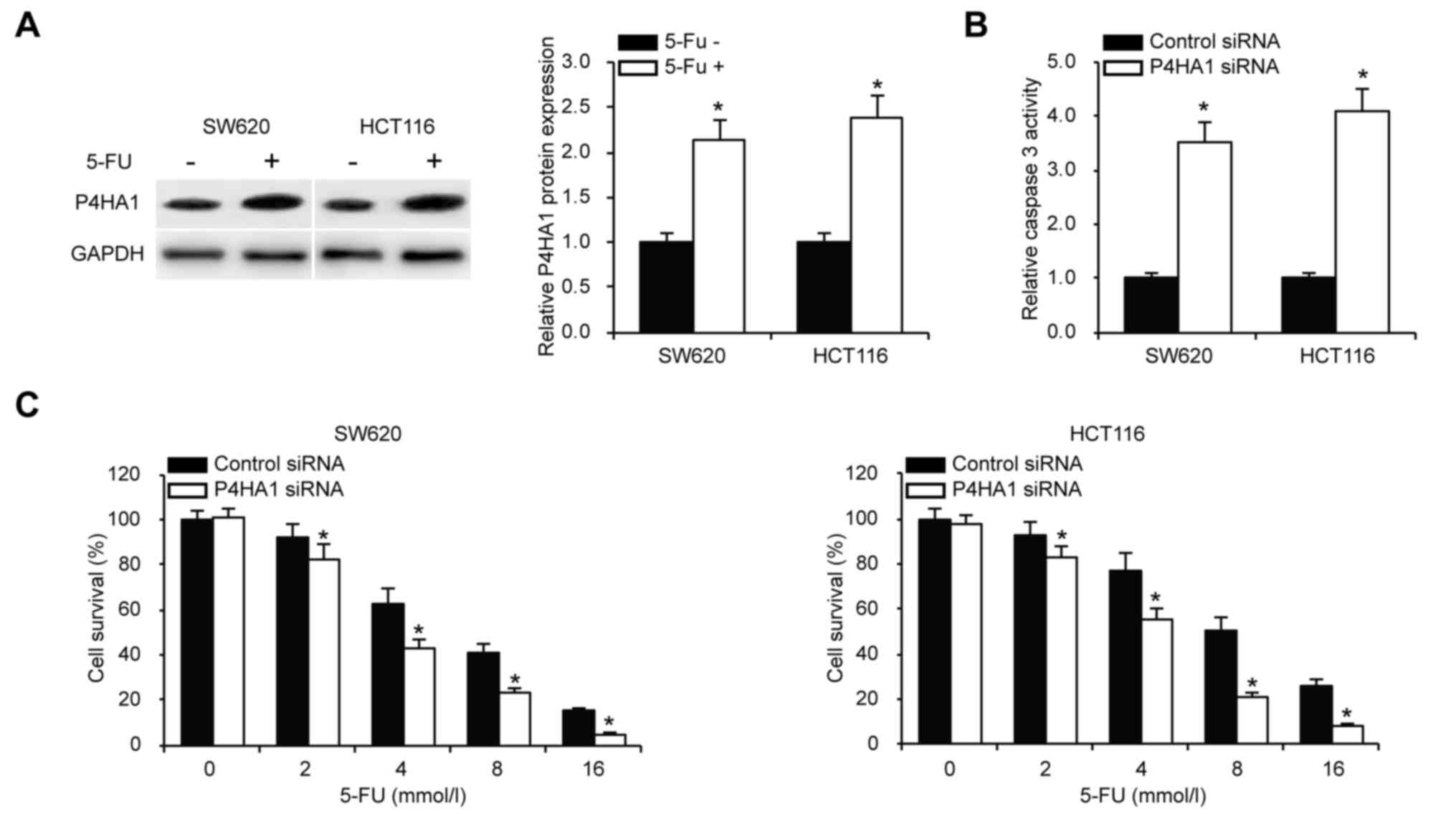
P4HA1 knockdown decreases CRC cell
chemoresistance
To examine whether P4HA1 knockdown affects SW620 and
HCT116 cells chemoresistance, cells were treated with the
chemotherapeutic agent 5-Fluorouracil (5-FU). Western blotting
analysis showed induced expression of P4HA1 after 5-FU treatment
(Fig. 4A). We tested caspase-3
activity to analyze whether P4HA1 silencing affected CRC apoptosis
following 5-FU treatment. Increased caspase-3 activity was observed
following P4HA1 knockdown (Fig. 4B).
The cell viability was analyzed by MTS assays in SW620 and HCT116
cells upon P4HA1 knockdown. The results showed that knockdown of
P4HA1 in the SW620 and HCT116 cells decreased their viability
(Fig. 4C). Notably, when comparing
the P4HA1 protein expression levels in P4HA1 knocked down cells
with or without 5-FU treatment, no obvious change in P4HA1
expression was observed (Fig. S1).
5-FU failed to affect P4HA1 expression in P4HA1 knockdown cells.
The mechanism of the effect of 5-FU treatment in CRC cells with
P4HA1 knockdown is worth investigation in the future.
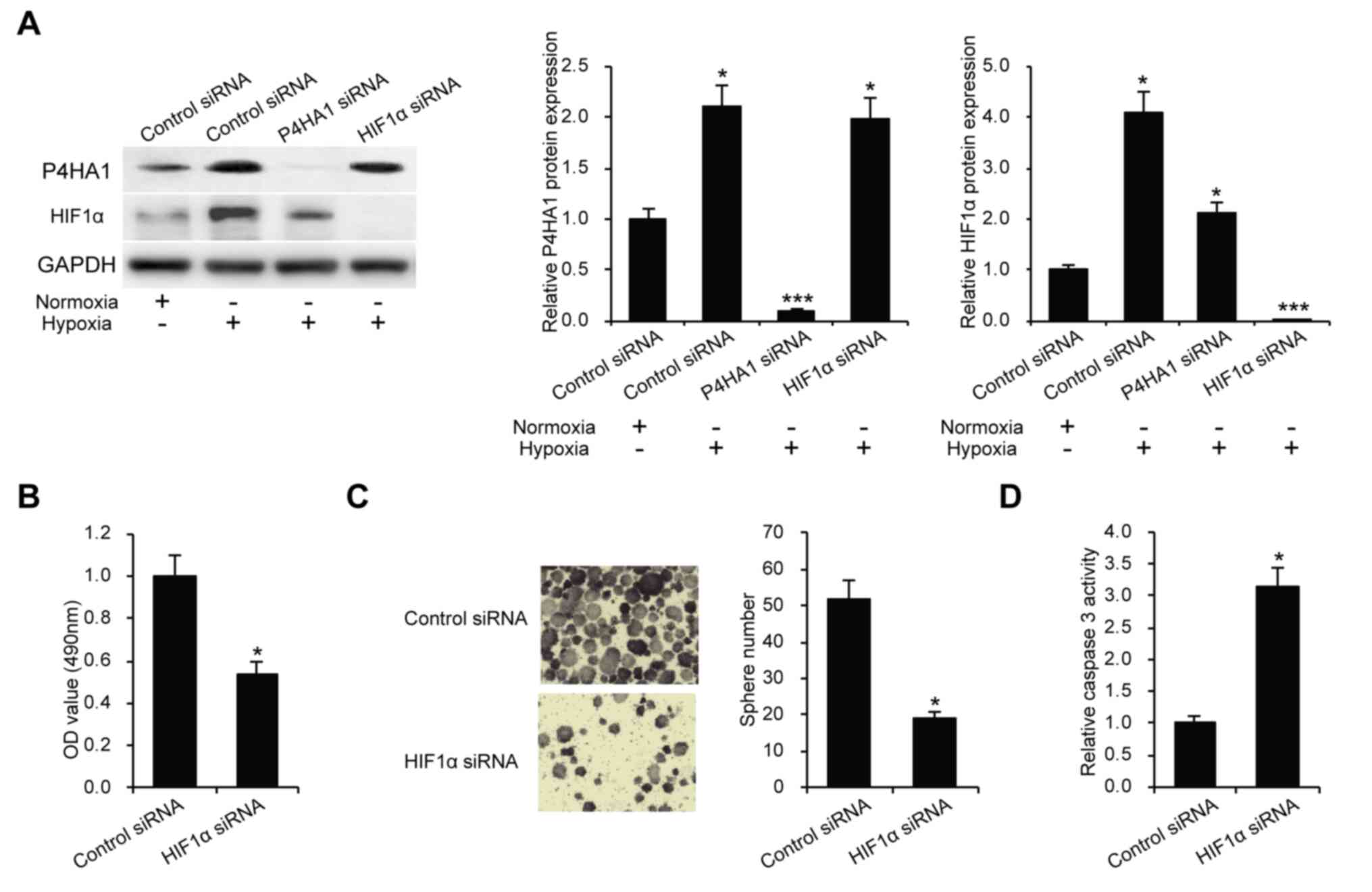
P4HA1 promotes CRC through HIF1α
To further investigate the mechanism of P4HA1 in the
regulation of CRC cell proliferation, stemness and chemoresistance,
the expression of HIF1α was analyzed in the present study. HIF1α
plays a pivotal role in CRC malignancy (20). SW620 cells were cultured under
hypoxic conditions of 1% oxygen atmosphere, and P4HA1 and HIF1α
expression levels were examined after 24 h. The results showed that
P4HA1 and HIF1α could be induced under a hypoxic environment
(Fig. 5A). Furthermore, it was
revealed that knockdown of P4HA1 resulted in a decreased level of
HIF1a, whereas the knockdown of HIF1a did not affect the expression
level of p4HA1 under hypoxic conditions (Fig. 5A). We decreased the expression of
HIF1α by siRNA method and performed MTS, tumorsphere formation and
caspase-3 activity assays. Knockdown of HIF1α significantly
inhibited CRC cell malignancy (Fig.
5B-D).
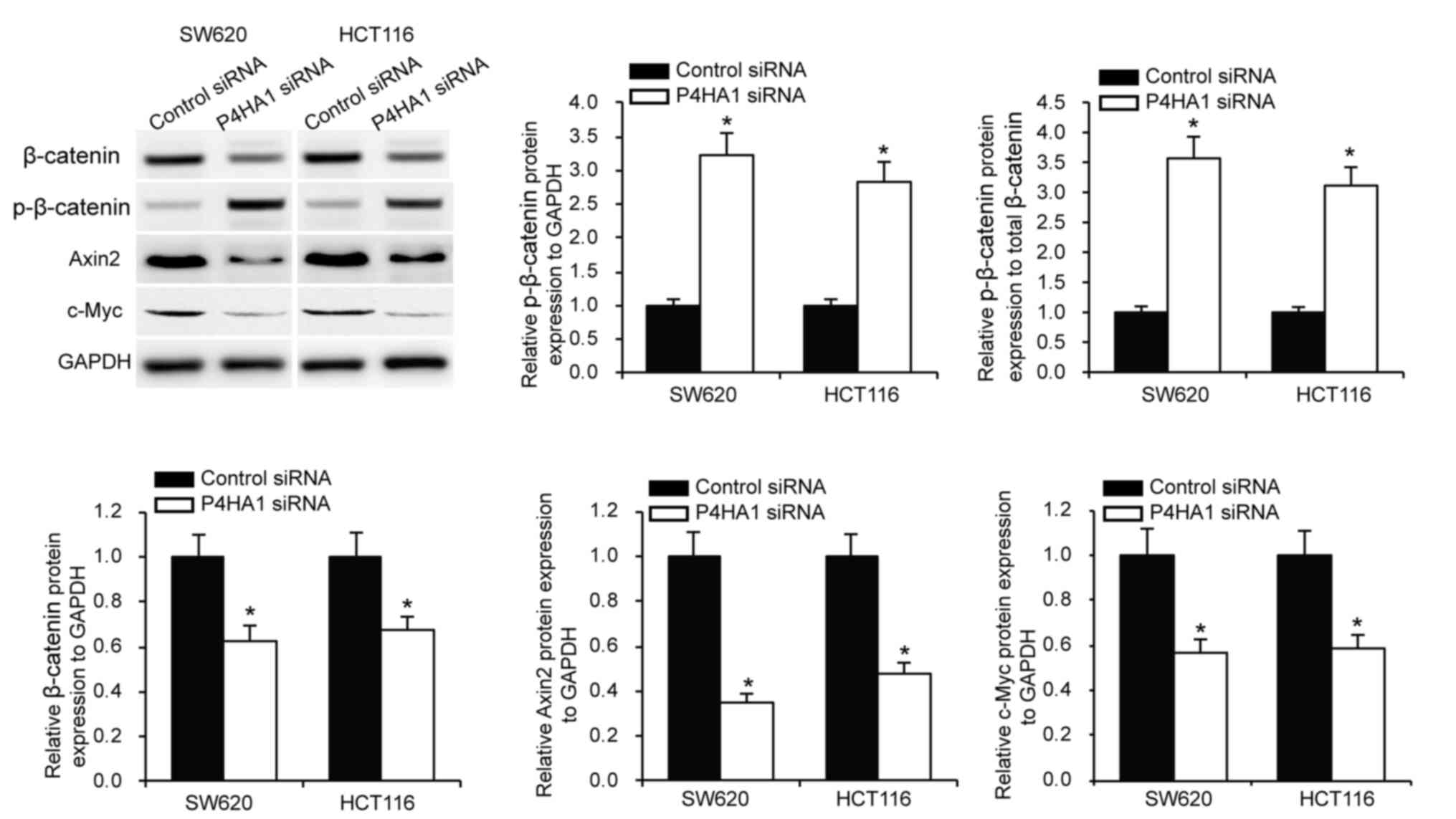
P4HA1 affects CRC cell Wnt
signaling
HIF1α is associated with Wnt signaling to regulate
cancer cell tumorigenesis (21).
HIF1α was found to be a protein that is downstream of P4HA1. In
order to investigate whether P4HA1 affects Wnt signaling, P4HA1 was
knocked down in SW620 and HCT116 cells, and β-catenin and
p-β-catenin protein expression levels were examined via western
blotting. The results revealed that knockdown of P4HA1
significantly decreased β-catenin and increased p-β-catenin
expression (Fig. 6). Subsequently,
the examination of the protein expression levels of gene targets of
the Wnt signaling pathway (Axin2 and c-Myc) showed that P4HA1
knockdown significantly decreased Axin2 and c-Myc expression in
SW620 and HCT116 cells (Fig. 6).
This finding indicated the important role of P4HA1/HIF1α/Wnt
signaling axis in CRC.
Discussion
The present study is a preliminary demonstration of
the assessment of a PTM in the carcinogenesis of CRC. The results
from the current study strengthen the genomic and epigenomic
findings in CRC biomarker discovery, suggesting that the study of
PTMs, especially P4HA1, could have an important prognostic value
and work as potential therapeutic targets.
Through a review of GEO datasets (22) the expression of P4HA1 was found to be
significantly upregulated in CRC (16,17,23).
This is also seen in in vitro assays in human CRC tissue
samples. These observations are in concordance with the findings in
triple negative human breast cancer (10,11),
adenopancreatic carcinoma (9),
gliomas (24) and oral squamous cell
carcinoma (25). P4HA1 has been
reported to be regulated by miR-124, which is a potential target in
CRC treatment (26). Further in
vitro assays using the CRC cell lines SW480, SW620 and HCT116
showed that P4HA1 is expressed in these cells. However, the
expression of P4HA1 was found to be lower in SW480 cells compared
with the other two cell lines. It has previously been shown that
the expression of proteins can significantly differ amongst primary
CRC cells (SW480) vs. metastatic colon cancer cell lines such as
(SW620 and HCT116) (27).
As aforementioned, P4HA1 is the most abundantly
found isoform of prolyl 4-hydroxylase and is responsible for
4-hydroxylation of proline residues on collagen (11). This post-translational modification
is responsible for the appropriate folding and tertiary structure
of collagen (10). Collagen is the
most abundant protein found in the extracellular matrix (ECM); the
ECM being the acellular component within tissues and organs
(28). The role of ECM extends
beyond providing merely a scaffold and/or a physical support to
cells. It also provides the appropriate milieu for biochemical cues
essential for the maintenance of tissue homeostasis (28).
Tumor-derived ECM is not only stiffer, but it is
also biochemically distinct than the normal ECM. Besides supporting
cancer cells, the role of ECM in cellular functions such as cell
proliferation, cell migration, differentiation and survival have
been well described (29,30). The role of collagen in mediating
these cellular features has been previously reported (31). It is likely that overexpression of
collagen may promote epithelial-mesenchymal transition, tumor
initiating potential and self-renewal of CSCs, thereby mediating
the stemness phenotype (29,32).
These findings may in part explain the present
results, wherein silencing of P4HA1 downregulates cellular
proliferation, reduced cancer stemness and decreased
chemoresistance plausibly through the breakdown of the ECM. As has
been demonstrated previously, it is likely that the silencing of
P4HA1 decreases the turnover of collagen in the matrix (33). The absence of collagen abrogates
activation of the focal adhesion kinase (FAK) through β1-integrin
(34). This in turn would
downregulate multiple FAK effector molecules such as the
phosphatidyl inositol 3 kinase or growth factor receptor bound
protein 7 and cell division control protein 42, thereby regulating
various cell processes, such as division and migration.
Studies in breast cancer have also found a link
between collagen hydroxylation and HIF1α activation during tumor
progression (10). Increased
expression of P4HA1 was found to enhance stability of HIF1α by
significantly reducing the levels of cellular α-ketoglutarate and
increasing the levels of succinate (11). Similar observations were also
observed in the present study, with the expression of P4HA1 being
demonstrated to be associated with increased stability of HIF1α.
Furthermore, HIF1α is associated with a poor prognosis in CRC
(35). HIF1α plays an angiogenic
role in CRC through the upregulation of angiogenic factors such as
VEGF (36).
The present findings also suggested inactivation of
the Wnt signaling pathway by silencing of P4HA1. There are two
possible explanations for the observations: i) It is likely that
P4HA1 negatively regulates the multiprotein dissociation complex of
the Wnt pathway, thereby reducing phosphorylation and consequent
proteasomal degradation of β-catenin. This in part would be
explained by the downregulation of Axin 2 upon silencing of P4HA1;
and ii) hypoxia and HIF1α regulates the expression of several
transcriptional activators, such as B cell lymphoma 9 (BCL9), which
in turn regulates a number of genes in the Wnt pathway, namely
CD44, Axin2 and survivin. It has been shown that BCL9 knockouts
have significantly decreased expression of these genes (37).
In conclusion, as the majority of CRC cases present
with an aberrated canonical Wnt signaling pathway, it would be
essential to target molecules that deregulate the Wnt pathway. The
results of the present study indicate that investigation into P4HA1
as a target biomolecule to predict prognosis of CRC could be
beneficial. Alongside this, therapeutic targets to selectively
downregulate or silence the expression of P4HA1 may be investigated
to target P4HA1-mediated CRC pathogenesis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the General program
of Qiqihar Academy of Medical Sciences (grant no.
QMSI2019M-26).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request. The data from GEO databases were cited in the article.
Authors contributions
ZY designed the study. QZ, YY, HZ, YS, WZ and ZY
performed the experiments. QZ, YY, TL and YH defined the analysis
strategy and performed the statistical analysis. QZ wrote the first
draft of the article. All authors read and approved the final
version.
Ethics approval and consent to
participate
The present study was performed in accordance with
the Declaration of Helsinki and approved by the Medical Ethics
Committee of Qiqihar Medical University (no. 2015QY137). All
patients provided written informed consent prior to the study
start.
Patient consent for publication
Not applicable.
Competing interests
The authors declared that they have no competing
interests.
References
|
1
|
Keum N and Giovannucci E: Global burden of
colorectal cancer: Emerging trends, risk factors and prevention
strategies. Nat Rev Gastroenterol Hepatol. 16:713–732. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM
and Wallace MB: Colorectal cancer. Lancet. 394:1467–1480. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuipers EJ, Grady WM, Lieberman D,
Seufferlein T, Sung JJ, Boelens PG, van de Velde CJ and Watanabe T:
Colorectal cancer. Nat Rev Dis Primers. 1:150652015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martini G, Troiani T, Cardone C, Vitiello
P, Sforza V, Ciardiello D, Napolitano S, Della Corte CM, Morgillo
F, Raucci A, et al: Present and future of metastatic colorectal
cancer treatment: A review of new candidate targets. World J
Gastroenterol. 23:4675–4688. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Locke WJ, Guanzon D, Ma C, Liew YJ,
Duesing KR, Fung KYC and Ross JP: DNA methylation cancer
biomarkers: Translation to the clinic. Front Genet. 10:11502019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chakraborty S, Hosen MI, Ahmed M and
Shekhar HU: Onco-multi-OMICS approach: A new frontier in cancer
research. Biomed Res Int. 2018:98362562018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cao XP, Cao Y, Li WJ, Zhang HH and Zhu ZM:
P4HA1/HIF1alpha feedback loop drives the glycolytic and malignant
phenotypes of pancreatic cancer. Biochem Biophys Res Commun.
516:606–612. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu R: P4HA1 is a new regulator of the
HIF-1 pathway in breast cancer. Cell Stress. 3:27–28. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiong G, Stewart RL, Chen J, Gao T, Scott
TL, Samayoa LM, O'Connor K, Lane AN and Xu R: Collagen prolyl
4-hydroxylase 1 is essential for HIF-1alpha stabilization and TNBC
chemoresistance. Nat Commun. 9:44562018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chakravarthi BV, Pathi SS, Goswami MT,
Cieślik M, Zheng H, Nallasivam S, Arekapudi SR, Jing X, Siddiqui J,
Athanikar J, et al: The miR-124-prolyl hydroxylase P4HA1-MMP1 axis
plays a critical role in prostate cancer progression. Oncotarget.
5:6654–6669. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schatoff EM, Leach BI and Dow LE: Wnt
signaling and colorectal cancer. Curr Colorectal Cancer Rep.
13:101–110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhan T, Rindtorff N and Boutros M: Wnt
signaling in cancer. Oncogene. 36:1461–1473. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoshida N, Kinugasa T, Ohshima K, Yuge K,
Ohchi T, Fujino S, Shiraiwa S, Katagiri M and Akagi Y: Analysis of
Wnt and beta-catenin expression in advanced colorectal cancer.
Anticancer Res. 35:4403–4410. 2015.PubMed/NCBI
|
|
16
|
Khamas A, Ishikawa T, Shimokawa K, Mogushi
K, Iida S, Ishiguro M, Mizushima H, Tanaka H, Uetake H and Sugihara
K: Screening for epigenetically masked genes in colorectal cancer
Using 5-Aza-2′-deoxycytidine, microarray and gene expression
profile. Cancer Genomics Proteomics. 9:67–75. 2012.PubMed/NCBI
|
|
17
|
Danielsen SA, Cekaite L, Ågesen TH, Sveen
A, Nesbakken A, Thiis-Evensen E, Skotheim RI, Lind GE and Lothe RA:
Phospholipase C isozymes are deregulated in colorectal
cancer-insights gained from gene set enrichment analysis of the
transcriptome. PLoS One. 6:e244192011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Flejou JF: WHO Classification of digestive
tumors: The fourth edition. Ann Pathol. 31 (Suppl 5):S27–S31.
2011.(In French). PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nagaraju GP, Bramhachari PV, Raghu G and
El-Rayes BF: Hypoxia inducible factor-1alpha: Its role in
colorectal carcinogenesis and metastasis. Cancer Lett. 366:11–18.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Giles RH, Lolkema MP, Snijckers CM,
Belderbos M, van der Groep P, Mans DA, van Beest M, van Noort M,
Goldschmeding R, van Diest PJ, et al: Interplay between
VHL/HIF1alpha and Wnt/beta-catenin pathways during colorectal
tumorigenesis. Oncogene. 25:3065–3070. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barrett T, Wilhite SE, Ledoux P,
Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH,
Sherman PM, Holko M, et al: NCBI GEO: Archive for functional
genomics data sets-update. Nucleic Acids Res. 41((Database issue)):
D991–D995. 2013.PubMed/NCBI
|
|
23
|
Ågesen TH, Berg M, Clancy T, Thiis-Evensen
E, Cekaite L, Lind GE, Nesland JM, Bakka A, Mala T, Hauss HJ, et
al: CLC and IFNAR1 are differentially expressed and a global
immunity score is distinct between early- and late-onset colorectal
cancer. Genes Immun. 12:653–662. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu WM, Zhang J, Sun SX, Xi SY, Chen ZJ,
Jiang XB, Lin FH, Chen ZH, Chen YS, Wang J, et al: Identification
of P4HA1 as a prognostic biomarker for high-grade gliomas. Pathol
Res Pract. 213:1365–1369. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kappler M, Kotrba J, Kaune T, Bache M, Rot
S, Bethmann D, Wichmann H, Güttler A, Bilkenroth U, Horter S, et
al: P4HA1: A single-gene surrogate of hypoxia signatures in oral
squamous cell carcinoma patients. Clin Transl Radiat Oncol. 5:6–11.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Agarwal S, Behring M, Kim HG, Bajpai P,
Chakravarthi BVSK, Gupta N, Elkholy A, Al Diffalha S, Varambally S
and Manne U: Targeting P4HA1 with a small molecule inhibitor in a
colorectal cancer PDX model. Transl Oncol. 13:1007542020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiang L, Mou J, Shao B, Wei Y, Liang H,
Takano N, Semenza GL and Xie G: Glutaminase 1 expression in
colorectal cancer cells is induced by hypoxia and required for
tumor growth, invasion, and metastatic colonization. Cell Death
Dis. 10:402019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Frantz C, Stewart KM and Weaver VM: The
extracellular matrix at a glance. J Cell Sci. 123:4195–4200. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Begum A, Ewachiw T, Jung C, Huang A,
Norberg KJ, Marchionni L, McMillan R, Penchev V, Rajeshkumar NV,
Maitra A, et al: The extracellular matrix and focal adhesion kinase
signaling regulate cancer stem cell function in pancreatic ductal
adenocarcinoma. PLoS One. 12:e01801812017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Poltavets V, Kochetkova M, Pitson SM and
Samuel MS: The role of the extracellular matrix and its molecular
and cellular regulators in cancer cell plasticity. Front Oncol.
8:4312018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Somaiah C, Kumar A, Mawrie D, Sharma A,
Patil SD, Bhattacharyya J, Swaminathan R and Jaganathan BG:
Collagen promotes higher adhesion, survival and proliferation of
mesenchymal stem cells. PLoS One. 10:e01450682015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nallanthighal S, Heiserman JP and Cheon
DJ: The role of the extracellular matrix in cancer stemness. Front
Cell Dev Biol. 7:862019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zou Y, Donkervoort S, Salo AM, Foley AR,
Barnes AM, Hu Y, Makareeva E, Leach ME, Mohassel P, Dastgir J, et
al: P4HA1 mutations cause a unique congenital disorder of
connective tissue involving tendon, bone, muscle and the eye. Hum
Mol Genet. 26:2207–2217. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao X and Guan JL: Focal adhesion kinase
and its signaling pathways in cell migration and angiogenesis. Adv
Drug Deliv Rev. 63:610–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Baba Y, Nosho K, Shima K, Irahara N, Chan
AT, Meyerhardt JA, Chung DC, Giovannucci EL, Fuchs CS and Ogino S:
HIF1A overexpression is associated with poor prognosis in a cohort
of 731 colorectal cancers. Am J Pathol. 176:2292–2301. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang ZY, Zhang LH, Zhao C, Liu R, Tong H,
Gan C, Lan T, Tang CW and Gao JH: High HIF-1α expression predicts
poor prognosis of patients with colon adenocarcinoma. Int J Clin
Exp Pathol. 11:5635–5646. 2018.PubMed/NCBI
|
|
37
|
Xu W, Zhou W, Cheng M, Wang J, Liu Z, He
S, Luo X, Huang W, Chen T, Yan W and Xiao J: Hypoxia activates
Wnt/β-catenin signaling by regulating the expression of BCL9 in
human hepatocellular carcinoma. Sci Rep. 7:404462017. View Article : Google Scholar : PubMed/NCBI
|