Introduction
Myeloid-derived suppressor cells (MDSCs) are
heterogeneous cell populations that are precursors of dendritic
cells (DCs), macrophages and/or granulocytes (1,2). Under
physiological conditions, MDSCs mature and differentiate into DCs,
macrophages and granulocytes, while their differentiation is
inhibited in the context of inflammation and tumors, leading to
their accumulation in tumors and lymphoid organs as a negative
balance mechanism to prevent excessive T cell activation (3). Tumor cells use this mechanism for
immune escape, and MDSCs are directly involved in several processes
that promote tumor development, damaging the immune response of T
cells and natural killer (NK) cells, particularly CD8+ T
cell activation and effector function (4). High levels of circulating MDSCs in
patients with tumor are associated with a worse prognosis and
disease progression (5). Thus, one
of the directions of development in cancer immunotherapy is to
target either the MDSC populations and/or the signals involved in
their recruitment and function (6–8).
MDSCs express several TLR family members, including
TLR2 (9), TLR3 (10), TLR4 (11), TLR5 (12) and TLR7/8/9 (13) in mice, and TLR2 (14) and TLR7/8 (15) in humans. MDSCs accumulate in the
tumor microenvironment (TME) and are an important target for TLR
signaling regulation (16–18). However, the effects of TLR signaling
on MDSCs and its effect on tumor growth are not yet fully
understood. Previous studies have demonstrated that TLR ligands are
inducers of MDSCs, and have emphasized that myeloid differentiation
primary response 88 (MyD88) is essential for acquiring the direct
suppressive activity of MDSCs and the ability to promote tumor
growth, whereas these abilities are inhibited by blocking
MyD88-mediated signaling (19–21). The
accumulation of MDSCs mediated by MyD88-nuclear factor kappa-B
(NF-κB) signaling increases the production of IL-10, which inhibits
the function of DCs in liver cancer (22). In addition, it has been demonstrated
that increased expression of interferon regulatory factor (IRF)4, a
negative feedback regulator of TLR signaling (23), decreases the MDSC population,
particularly the G-MDSC population (24).
Furthermore, immune-checkpoint protein V-domain
immunoglobulin suppressor of T-cell activation (VISTA) is a chief
myeloid cell-intrinsic immune-checkpoint protein that can control
antitumor immunity (25). VISTA
modulates the polyubiquitination and protein expression of TNF
receptor associated factor 6 to inhibit TLR-mediated activation of
the mitogen-activated protein kinase (MAPK)/Activator protein-1
(AP-1) and IKK/NF-κB signaling cascades, which decreases the
ability of MDSCs to produce proinflammatory mediators and enhance
their T cell-suppressive functions, thereby protumor progression
(26).
However, other groups have disputed these findings,
reporting that TLR signaling activation can decrease the
immunosuppressive activity of MDSCs (13,27,28).
Loss of MyD88 results in an increase in prostate intraepithelial
tumors and highly differentiated adenocarcinoma areas in TRAMP
transgenic mice, accompanied by an increase in the frequency of
MDSCs and the production of inducible nitric oxide synthase (iNOS),
prostate arginase 1 (Arg1), and cytokine Interleukin (IL)-10
(27). Furthermore, other studies
have reported that TLR stimulation decreases the MDSC population
and enhances their differentiation into tumoricidal macrophages
(13,28). Thus, the regulation of TLR signaling
on MDSCs is diverse. The present review summarizes the effects of
TLR signals on the number, phenotype and inhibitory activity of
MDSCs, and their role in cancer immunotherapy. It remains essential
to address this in the present and future cancer immunotherapies
for the immunosuppression of the TME.
General aspects of MDSCs and TLR
signaling
MDSCs
MDSCs can be divided into two categories, including
polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs or
G-MDSCs) that are phenotypically and morphologically similar to
neutrophils, and monocyte-myeloid-derived suppressor cells
(M-MDSCs), which are similar to monocytes. In mice, the phenotype
of G-MDSCs is CD11b+Ly6G+Ly6Clo,
and M-MDSCs is CD11b+Ly6G−Ly6Chi
(29). In humans, the phenotype of
G-MDSCs is CD11b+CD14−CD66b+ or
CD11b+CD14−CD15+, and the
phenotype of M-MDSCs is
CD11b+CD14+HLA−DR−/loCD15−
(29). Increasing evidence suggests
that MDSCs promote tumor progression and metastasis through their
immunosuppressive activity, the mechanism of which can be
summarized as i) Arg1 and iNOS produced by MDSCs consume arginine
and cysteine, which are nutrients required by lymphocytes, leading
to the downregulation of the ζ chain in the T cell receptor (TCR)
complex and inhibiting the proliferation of antigen-activated T
cells (30); ii) reactive oxygen
species (ROS) and reactive nitrogen species (RNS) generated by
MDSCs induce the formation of oxidative stress, leading to the loss
of T cell ζ chain expression and interfering with IL-2 receptor
signaling cascades (31,32); iii) MDSCs interfere with the
transportation and survival of lymphocytes, affecting the migration
of CD8+ T cells to the TME and restricting T cell
recycling to the lymph nodes (33,34) and
iv) MDSCs improve naïve CD4+ T cell differentiation into
regulatory T cells, thereby inhibiting T cell function (35). Thus, it remains essential to develop
cancer immunotherapies that aim to decrease the negative impact of
MDSCs on effector immune cells.
Previous studies have demonstrated that targeting
the MDSC population and the signals involved in their function can
delay tumor progression (36,37).
Thus, understanding the regulators and signaling pathways involved
in the survival, development, differentiation and activation of
MDSCs is essential for cancer immunotherapy targeting MDSCs.
Currently, two-signal models are used to describe
the differentiation of MDSCs. The model includes two stages: The
first stage is the expansion of immature bone marrow cells and the
inhibition of terminal differentiation; the second stage is the
activation stage, which transforms immature bone marrow cells into
MDSCs (38). Notably, the TLR
signaling pathways are involved in both stages of MDSC
differentiation, and TLR receptors are expressed positively on
MDSCs. TLR signals are considered important regulators of the
differentiation and acquisition of the immunosuppressive function
of MDSCs (39).
TLR signaling
TLRs are type I transmembrane proteins, with 10
existing in humans (TLR1-10) and 12 in mice (TLR1-9, TLR11-13)
(40). TLR1, TLR2, TLR4, TLR5 and
TLR6 are located on the cell surface and are mainly involved in the
detection of extracellular bacterial products, while TLR3, TLR7,
TLR8 and TLR9 are located in intracellular compartments that are
involved in the detection of nucleic acids from viral and bacterial
sources (40). These TLRs recognize
pathogen-associated molecular patterns to initiate the appropriate
host immune response. Binding of the TLR to the ligand results in
the activation of two major signaling pathways, the MyD88-dependent
and Toll-IL-1 receptor-domain containing adaptor-inducing
interferon-β (TRIF)-dependent signaling pathways. Excluding TLR3,
all TLRs activate MyD88-dependent signaling pathways, and both TLR3
and TLR4 activate TRIF-dependent signaling pathways. The
MyD88-dependent pathway activates nuclear factor NF-κB and the MAPK
pathway, and induces the development of inflammatory responses.
However, the TRIF-dependent pathway activates the interferon IRF
pathway and induces antiviral type 1 interferon, which is involved
in the antiviral response (40).
Currently, Bacillus Calmette-Guerin (BCG, TLR2/TLR4
agonist), Monophosphoryl lipid A (MPL, TLR4 agonist), and Imiquimod
(Imiq) (TLR7 agonist) have been approved by the Food and Drug
Administration (FDA) for clinical treatment of patients with cancer
(41). Furthermore, TLR agonists are
extensively used as adjuvants for cancer vaccines to enhance their
antitumor effects. The antitumor effect of TLR agonists is
attributable to the activation of TLR signals that enhance
antigen-specific humoral and cellular immune responses. However,
due to the existence of the immune tolerant microenvironment, TLR
agonists alone or as cancer vaccine adjuvants can only produce
moderate clinical benefits in cancer treatment (42,43).
Thus, it remains critical to identify and develop therapeutic
approaches to overcome the immunosuppressive TME and to enhance the
efficiency of current tumor immunotherapies. Understanding the
effect of TLR signaling on MDSCs will help optimize the role of TLR
agonists in antitumor therapy and provide novel insight into the
development of cancer immunotherapies.
Pro-tumorigenic effects of MDSCs induced by
TLR signaling
Increasing evidence suggests that the accumulation
and activation of MDSCs is associated with tumor progression,
recurrence and a negative clinical outcome (44). Recently, a number of studies have
demonstrated that TLR signaling induces MDSC accumulation and
enhances the ability to inhibit tumor-specific T cell responses,
resulting in tumor progression (45,46).
Related studies and potential TLR signaling pathways are summarized
in Table I and Fig. 1.
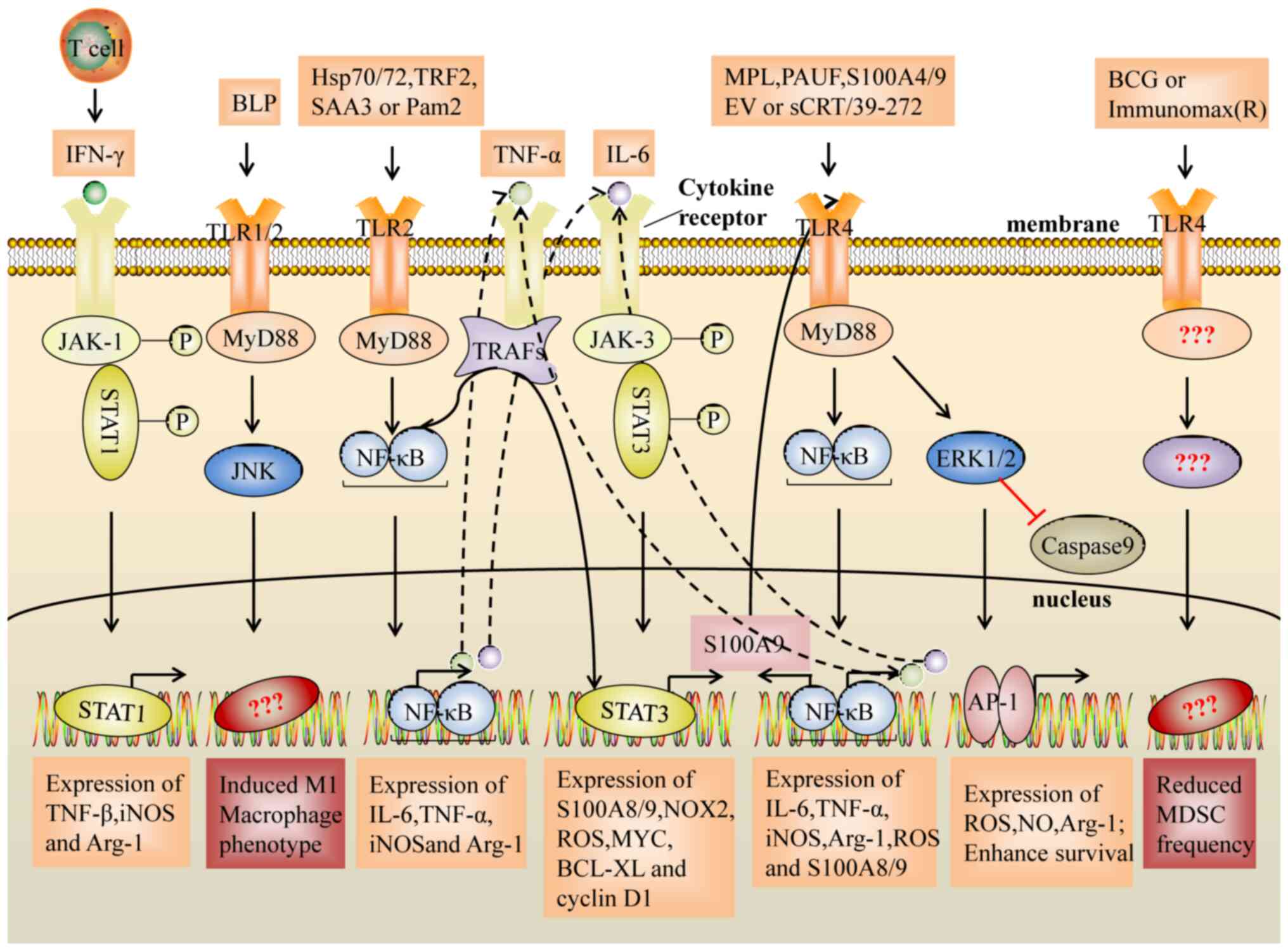 | Figure 1.Suppressive activity of MDSCs induced
by the TLR2 and TLR4 signaling pathways. The NF-κB pathway
activated by TLR2/4 induces the expression of inflammatory factors
(IL-6 and TNF-α). In turn, IL-6 and TNF-α activate the STAT3
signaling pathway and the NF-κB signaling pathway. Notably, STAT3
regulates the expression of the inflammatory factors, S100A8 and
S100A9, which act as TLR4 ligands to activate the NF-κB pathway,
resulting in upregulation of IL-6 and TNF-α expression, and they
form a loop that enhances the expansion and activation of MDSCs. In
addition, TLR2/JUK signals induce an M1-like macrophage phenotype
and decrease the immunosuppressive activity of MDSCs, whereas
transcription factors for the differentiation to M1 macrophages or
decreasing frequency on MDSC are unclear. TLR, Toll-like receptor;
BLP, bacterial lipoprotein; sCRT/39-272, Recombinant CRT fragment
39–272; PAUF, protein pancreatic adenocarcinoma upregulated factor;
SAA3, serum amyloid A3; TRF2, telomeric repeat-binding factor 2;
EV, extracellular vesicles; Hsp, heat shock protein; MPL,
monophosphoryl Lipid A; BCG, bacillus Calmette-Guerin; Pam2,
Pam2CSK4; TNFα, tumor necrosis factor-α; IL-6, interleukin-6; IFNγ,
interferon-γ; MyD88, myeloid differentiation primary response 88;
TRAF, TNF receptor associated factor; ERK, extracellular regulated
protein kinases; JNK, c-Jun kinase; JAK, Janus kinase; NF-κB,
nuclear factor kappa-B; AP-1, activator protein-1; ROS, reactive
oxygen species; iNOS, inducible nitric oxide synthase; Arg1,
arginase 1; BCl-XL, B-cell lymphoma XL. |
 | Table I.Pro-tumorigenic effects of MDSCs
induced by TLR signaling in cancer. |
Table I.
Pro-tumorigenic effects of MDSCs
induced by TLR signaling in cancer.
| TLR | Stimulus | Species | Cancer | Number and
phenotype | Function and
mediator(s) | (Refs.) |
|---|
| TLR2 | Pam2CSK | M | Lymphoma | Accumulation in
tumor sites supported survival | – | (9) |
|
|
| H | Colon, prostate,
pancreatic, liver cancer | M2-like
(25F9+/CD200R+) | Inhibited T cell
proliferation. Mediator: IL-6, IL-10 | (14) |
|
|
| M | Lung cancer and
lymphoma | Prolonged
survival | iNOS, NO | (45) |
|
| Hsp72/Hsp70 | M, H | Breast cancer
melanoma lymphoma and RCC | Expansion | Arg1, iNOS | (47–50) |
|
| TRF2 | M | OSCC | Accumulation and
activation | Triggered NK and T
cell suppression. Mediator: Arg1, IL-10, TGF-β, | (46) |
|
| SAA3 | M | Breast and CRC | Prolonged survival
inhibited MDSCs differentiation into M1 | NOS2, Arg1,
Nox2, | (51) |
| TLR4 | MPL | M | – | Accumulation | Suppressed T cell
proliferation. | (57) |
|
|
|
|
|
| Mediator: IL-10,
NO |
|
|
| S100A9 | M | CRC | – | Inhibited
CD8+T cell activity. | (53) |
|
|
|
|
|
| Mediator: Arg1 and
iNOS |
|
|
|
| M | MM | – | TNF-α, IL-6, and
IL-10 | (54) |
|
| S100A4 | M | Melanoma lung
cancer | Prolonged
survival | – | (58) |
|
| PUAF | M, H | Pancreatic
cancer |
| Arg1, NO, and
ROS | (65) |
|
| HMGB1 | M | – |
| Suppressed T cell
proliferation | (66) |
|
| sCRT/39-272 | M | Melanoma | Prolonged survival
inhibited MDSCs differentiation into DC | S100A8 and
S100A9 | (52) |
|
| EV | M, H | Melanoma |
| Upregulated PD-L1
expression | (69) |
| TLR9 | CpG | M | Pancreatic
carcinoma | Accumulation in
tumor sites |
| (59) |
| TLR7 | CL264 | M | Lung
adenocarcinoma | Accumulation in
tumor site |
| (60) |
The accumulation and survival of MDSCs
induced by TLR signaling
TLR2 and MDSCs
In EG7 tumor-bearing mice, it has been demonstrated
that Pam2CSK4, a TLR2 agonist, induces the accumulation of MDSCs
and prolongs the survival of MDSCs, leading to the suppression of
the antitumor immune response (9,45). In
addition, signal transducer and activator of transcription (STAT3)
is an important transcription factor for MDSC expansion, which is
attributed to the abnormal and continuous activation of STAT3 in
myeloid progenitor cells that prevents them from differentiating
into mature myeloid cells (4).
Recently, some studies have confirmed this effect (46–50). It
has been demonstrated that heat shock protein (Hsp72/Hsp70) in
tumor cell-derived exosomes and telomeric repeat-binding factor 2
promote the recruitment and expansion of MDSCs through the
activation of STAT3, which is induced in a
TLR2/MyD88/IL-6-dependent manner (46–50).
Furthermore, serum amyloid A3 can activate STAT3 by
TLR2/MyD88/tumor necrosis factor (TNF)α signaling, leading to the
enhanced survival of MDSCs (51).
Notably, STAT3 also regulates the expression of the inflammatory
factors S100A8 and S100A9, which act as TLR4 ligands to activate
the immunosuppressive activity of MDSCs (52–54).
TLR4 and MDSCs
The anticonvulsant drug valproic acid, which
decreases the frequency of MDSCs, is accompanied by the
downregulation of TLR4 mRNA expression (55). In addition, both tumor volume and
pulmonary recruitment of MDSCs decrease with a TLR4/MD-2 complex
antagonist (56). Previous studies
have suggested that the TLR4 signaling pathway may also be involved
in the accumulation and survival of MDSCs (55,56).
Recently, MPL, a TLR2 and TLR4 agonist, has been confirmed to have
this effect (57). MPL induces the
accumulation of MDSCs both in vitro and in vivo by
inhibiting DC development from myeloid cells (57). In addition, in a melanoma mouse
model, soluble calreticulin (sCRT39-272) was demonstrated to
promote the migration and survival of tumor-derived MDSCs via
interactions with TLR4 (52).
Notably, Li et al (58)
demonstrated that exogenous S100A4 upregulates TLR4 receptor
expression on MSC2 cells and protects MDSCs from apoptosis via the
TLR4/extracellular regulated protein kinases (ERK)1/2 signaling
axis, both in vitro and in vivo.
TLR7/9 and MDSCs
Notably, some intracellular TLR7/9 signaling
pathways also promote accumulation of MDSCs (59,60). CpG
ODN (CpG, TLR9 agonist) administration induces the accumulation of
tumor-infiltrating MDSCs in pancreatic ductal adenocarcinoma
(59). It has also been demonstrated
that CL264 (TLR7 agonist) directly interacts with the TLR7 receptor
on murine lung adenocarcinoma LLC-Luc cells, which promotes the
accumulation of G-MDSCs by increasing the secretion of
granulocyte/macrophage CSF and chemokine (C-C motif) ligand 2
(CCL2) in the TME, resulting in an increased number of lung
metastases and the promotion of tumor progression (60,61).
However, some studies have reported that TLR7 and TLR9 signals
weaken MDSC immune activity (16,17).
Differentiation and activation of MDSCs
induced by TLR signaling
TLR2 and MDSCs
It has been demonstrated that Pam2CSK4 inhibits
TCR-stimulated syngeneic T cell proliferation by inducing M-MDSCs
to differentiate into the M2-like
(25F9+/CD200R+) phenotype, and produce IL-6
and IL-10 (14). Another study
revealed a novel mechanism of inducing the immunosuppressive
activity of M-MDSCs: Pam2CSK4 promotes the differentiation of
M-MDSCs into CD11b+F4/80+ macrophages, which
inhibits DC-induced T cell proliferation through nitric oxide (NO)
produced by iNOS (45). In addition,
TLR2 signaling activates MDSCs by increasing the expression levels
of NOS2, Arg1, iNOS, IL-10 and transforming growth factor (TGF)-β,
thereby triggering NK and T cell suppression (46).
TLR agonists induce CD8+ T cells to
produce interferon-γ (IFN-γ), which is beneficial for killing tumor
cells (62). However, studies have
demonstrated that the IFN-γ-STAT1-IRF1 axis is essential for the
inhibitory activity obtained by M-MDSCs, and may upregulate the
expression levels of iNOS and Arg-1 (39,63).
This phenomenon has also been confirmed by Shime et al
(45), who demonstrated that the TLR
agonist, Pam2CSK4, induces IFN-γ production by CD8+ T
cells, accompanied by interferon gamma receptor 1 (IFNgR1)
expression on M-MDSCs. Thus, IFN-γ interacts with IFNgR1 on
M-MDSCs, induces iNOS expression and inhibits the proliferation of
T cells. These results suggest the rationality of targeting the
IFN-γ-STAT1-IRF1 axis in MDSCs while targeting MDSC inhibition.
TLR4 and MDSCs
SA100A8/A9 are important pro-inflammatory cytokines
that increase MSC accumulation and immunosuppressive activity in
the TME (64). De Veirman et
al (54) demonstrated that
S100A9 acts as a chemokine for multiple myeloma (MM) cells and
induces MDSCs to express and secrete inflammatory and promyeloma
cytokines, including TNF-α, IL-6 and IL-10. In addition, He et
al (52) demonstrated that TLR4
signaling inhibits MDSC differentiation into DCs and promotes their
functional maturation, and the chemotactic migration of MDSCs by
initiating the expression of S100A8 and S100A9. Recent studies on
colorectal cancer (53) and MM
(54) have demonstrated that S100A9
promotes the expression levels of Arg1, iNOS and IL-10, and ROS
production in MDSCs via TLR4-NF-κB signaling cascades, thereby
inhibiting CD8+ T cell activity and promoting tumor
progression (53). It has also been
demonstrated that the TLR4/ERK/AP-1 and TLR4-IRF axis signaling
pathways enhance the immunosuppressive function of MDSCs (65,66).
Furthermore, TLR4 signaling can increase the production of IL-10
and attenuate the production of IL-12 in MDSCs, thereby enhancing
the interaction between MDSCs and macrophages, and promoting a
shift from the tumoricidal Th1 response to the pro-tumorigenic Th2
response (67,68).
Notably, Fleming et al (69) demonstrated that ret mouse melanoma
cell-derived extracellular vesicles can induce the upregulation of
PD-L1 on bone marrow (BM)-derived murine immature myeloid cells,
the immortalized myeloid suppressor cell line, MSC-2, and normal
human CD14+ monocytes in a TLR4-MyD88/TRIF-NF-κB
signaling-dependent manner, thereby strongly suppressing
CD8+ T cell activation through PD-1/PD-L1 signaling
cascades (70,71). Similarly, Ki-67 expression in MDSCs
is upregulated by the TLR4 mAb, accompanied by increased PD-L1 and
iNOS expression on MDSCs, particularly on M-MDSCs (11). These results suggest the possibility
of MDSC inhibition and PD-1/PD-L1 signal inhibition,
synergistically breaking the immune tolerance microenvironment.
Antitumor effects of MDSCs induced by TLR
signaling
Recent studies have demonstrated that activation of
TLR signaling decreases the ability of MDSCs to inhibit T cell
proliferation, thereby inhibiting tumor growth. This effect is
mainly manifested in the decreased number of MDSCs, differentiation
of MDSCs into antigen-presenting cells and decreased production of
inhibitory mediators (72,73). Related studies and potential TLR
signaling pathways are summarized in Table II and Fig. 2.
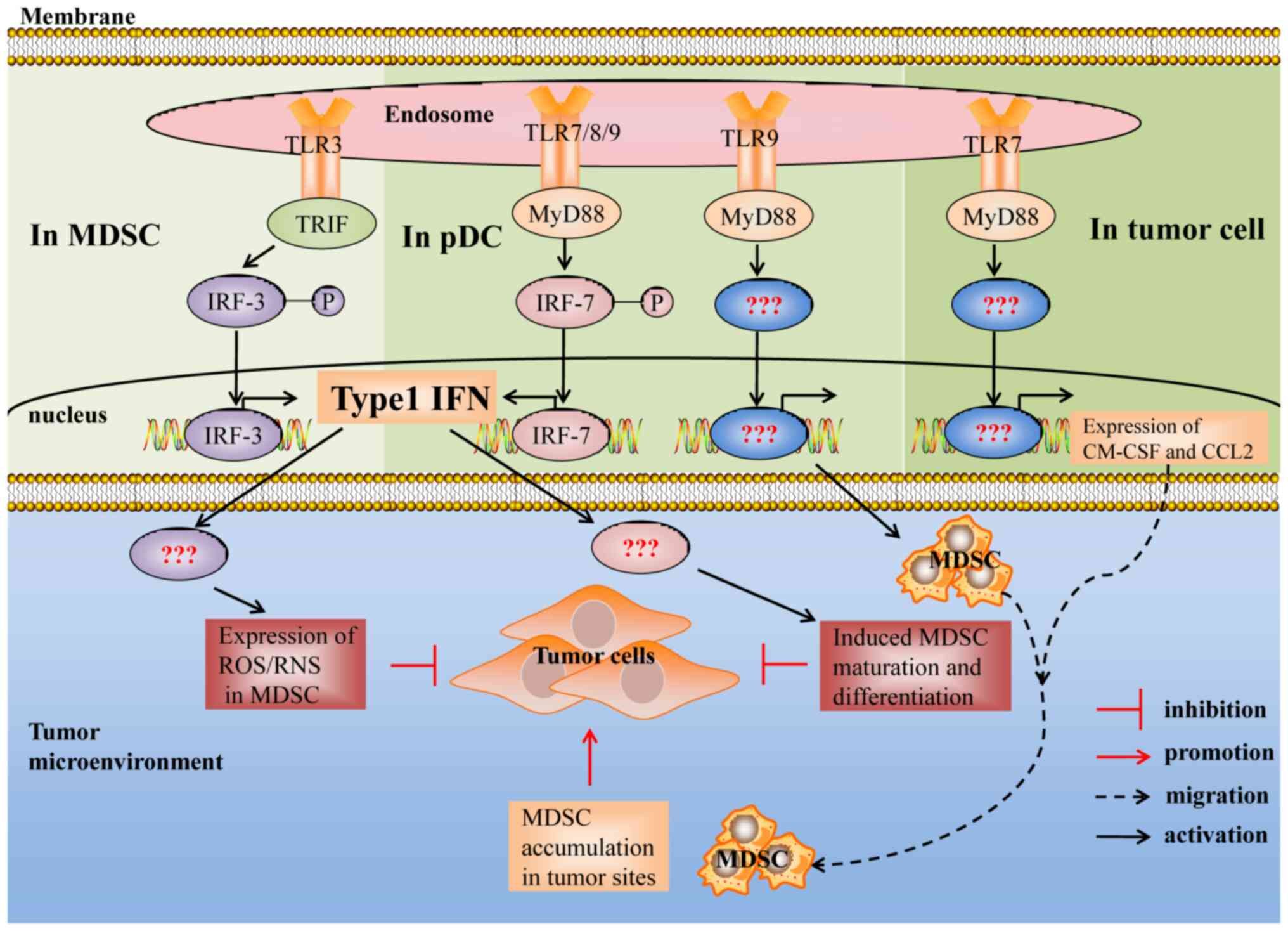 | Figure 2.Suppressive activity of MDSCs induced
by the TLR3, TLR7, TLR8 and TLR9 signaling pathways. Type I
interferon is essential for TLR3/7/8/9 signal induced MDSCs to
inhibit the growth of tumors, whereas the mechanism remains
unclear. In addition, the downstream signals and transcription
factors of TLR7/9 signal induced MDSCs that promote tumor growth
remain unclear. TLR, Toll-like receptor; MyD88, myeloid
differentiation primary response 88; TRIF, TIR adaptor-inducing
interferon-β; IRF3/7, interferon regulatory factor 3/7; ROS,
reactive oxygen species; RNS, reactive nitrogen species; GM,
granulocyte/macrophage; CCL2, chemokine (C-C motif) ligand 2. |
 | Table II.Antitumor effects of MDSCs induced by
TLR signaling in cancer. |
Table II.
Antitumor effects of MDSCs induced by
TLR signaling in cancer.
| TLR | Stimulus | Species | Cancer | Number and
phenotype | Function and
mediator(s) | (Refs.) |
|---|
| TLR3 | Poly(I:C) | M | Breast cancer | Decreased MDSC
frequency, and upregulated MHC II, I-Ad, CD80 and CD86 | Decreased ROS
production | (76) |
|
|
| M | CC | Decreased the
number of MDSCs | Attenuated the
immunosuppressive activity | (28) |
|
|
| M | Lymphoma |
| Abrogated the
immunosuppressive activity | (88) |
|
| OR | M | Melanoma
lymphoma | Decreased M-MDSC
frequencies | Abrogated the
immunosuppressive activity | (10) |
| TLR7 | Imiq | M | Lung cancer | Decreased the
number of MDSCs | – | (77,78) |
|
|
| M | CC | Phenotype:
Ly6C−F4/80+ macrophage phenotype | – | (13) |
|
| SC1 | M | CC | Decreased the
number of G-MDSCs |
| (18) |
|
| s-P-sh | M | Melanoma | Decreased MDSC
proportion | – | (79) |
| TLR7/8 | R848 | M | CC | Decreased MDSC
frequency, and upregulated CD11c, F4/80, MHC-I and MHC-II | Abrogated the
immunosuppressive activity | (16) |
|
|
| M | CRC | Phenotype:
F4/80+iNOS+ M1 macrophages | TNF-α and
IL-1β | (89) |
|
|
| H | CC, prostate,
pancreatic, liver cancer | Phenotype: M1-like
(25F9+/CD200R2) | Increased the
ability to kill tumor cells and lost immunosuppressive activity.
Mediator: IL-6 and IL-12 | (14) |
| TLR8 | Resiq | M | Lymphoma | Phenotype:
F4/80+ macrophages and
CD11c+/I-Ad+ DCs | Enhanced the
proliferation of T cells | (90) |
|
| Moto | H | Melanoma, CC and
prostate | Decreased M-MDSC
frequency |
| (15) |
| TLR9 | CpG | M | CC | Decreased M-MDSC
frequency, and upregulated F4/80 and downregulated Ly6c and
Gr-1 | Abrogated
immunosuppressive activity. Mediator: IL-6, TNF-α, IL-12 | (13) |
|
|
| M | CC and
melanoma | Decreased G-MDSC
frequency, and upregulated Sca1(Ly6A/E), F4/80, MHC II and
CD11c | Abrogated
immunosuppressive activity | (17) |
|
|
| M | Hepatoma |
| Attenuated the
immunosuppressive activity. Mediator: IFN-γ | (83) |
|
| Ad-CpG/ CpG | M | Melanoma and
RCC | Decreased MDSC
frequency | – | (80,81) |
| TLR4 | BCG | M | Bladder cancer | Decreased MDSC
frequency | – | (84) |
|
| I(R) | M | Metastatic breast
cancer | Decreased MDSC
frequency | – | (85) |
| TLR1/2 | BLP | M | Lung cancer | Upregulated CD80,
CD86, MHCII, F4/80. Phenotype: M1-like macrophage | High levels of
NOS2, IL1β, IL-6 and TNF-α, and low levels of Arg1 and CD206 | (73) |
|
|
| M | Glioma | Decreased MDSC
frequency in TME | – | (87) |
| TLR2 | pAbM | M | Mammary
carcinoma | Upregulated CD86
and MHCII. Phenotype: M1 macrophage | IL-6, IL-12, TNF-α
and iNOS | (86) |
TLR signaling decreases the number of
MDSCs
Intracellular TLRs, including TLR3, TLR7, TLR8 and
TLR9, are intrinsically capable of detecting nucleic acids, in
which TLR7, TLR8 and TLR9 receptors are similar in terms of
expressed cells, recognized ligands, localization on cells and
activated pathways, and all intracellular TLRs can induce the
production of type I IFN (74,75).
Some studies have demonstrated that the TLR3/7/8/9 signaling
pathway plays a similar role in regulating MDSCs. It has been
reported that TLR3/7/8/9 agonists decrease the MDSC frequency by
activating TLR3, TLR7, TLR8 and TLR9 signaling pathways in
vivo, thereby enhancing their antitumor effects (16,17,76)
(Table II).
TLR3 and MDSCs
Poly (I:C) treatment decreased the MDSC frequency in
BM, blood, spleen and tumors (28,76). In
addition, oncolytic reovirus, which activates the TLR3 signaling
pathway, mainly decreased the M-MDSC frequency and failed to change
the G-MDSC frequency (10).
Imiquimod (77,78), SC1 (a novel synthetic agonist with
exquisite specificity for TLR7) (18) and ssRNA-Pim-3-shRNA, a synthetic
dual-function vector that triggers TLR7 receptors via ssRNA
fragments) (79) all decreased MDSCs
in the TME, thereby inhibiting the growth of tumors in mice. It has
been demonstrated that TLR7 signals decrease the number of MDSCs in
a type I IFN-dependent manner (18,79).
Similarly, TLR7/TLR9 signal-dependent type I IFN production in
plasmacytoid DCs (pDCs) is imperative for decreasing MDSC
suppressive activity, as well as promoting antitumor immunity
(17,18,79).
Thus, it was hypothesized that endosome TLR-induced MDSC inhibitory
activity is associated with TLR signaling pathway-induced type I
IFN production. However, prospective studies are required to
confirm this hypothesis and determine how type I IFN regulates MDSC
suppressive activity.
TLR7/8 and MDSCs
Systemic application of R848 (a TLR7/8 agonist)
significantly decreases the frequency of M-MDSCs in tumors, blood
and spleen instead of bone marrow, as well as the frequency of
MDSCs in a mouse subcutaneous CT26 colon cancer model and the mouse
4T1 breast cancer model; however, this decrease is not as obvious
as in the CT26 model (16). In
addition, motolimod (Moto) treatment significantly increases the
cell death of M-MDSCs in vitro and in patients with cancer
(15). Moto significantly increases
the mean fluorescence of FAS on M-MDSCs and upregulates CD69 and
FAS-L expression on the T-cell surface; therefore, Moto induces
apoptosis of M-MDSCs, in part, through the link between FAS and
FAS-L (15).
TLR9 and MDSCs
The effect of TLR9 signals on MDSCs in vivo
may be associated with the injection methods used to deliver the
TLR9 agonist; intratumoral injection of CpG decreases the
proportion of M-MDSCs in tumor-bearing mice (13), whereas subcutaneous injection of CpG
significantly decreases the amount of G-MDSCs in the spleen of mice
in vivo (17). In addition,
Ad5D24 CpG (Ad-CpG), an adenovirus targeting the TLR9 receptor,
enhanced the antitumor efficacy in a lung cancer model and
significantly decreased the total number and immunosuppressive
activation of MDSCs in tumors instead of the spleen (80). Similarly, in a mouse renal cell
carcinoma model, CpG treatment decreased the amount and frequency
of a large number of MDSCs in tumor-bearing kidney tissues instead
of renal blood vessels in vivo (81). However, CpG failed to decrease MDSCs
in patients with cancer (15,82),
which may be due to the negative expression of the TLR9 receptor on
human MDSCs (14).
Notably, Lin et al (83) demonstrated that CpG significantly
increases the M-MDSC frequency in nontumor parts of the liver and
suppresses murine hepatic tumor growth. This phenomenon was named
‘intrahepatic myeloid aggregation for T cell expansion’, which was
attributed to CpG promoting the mRNA expression of IFN-γ in both
M-MDSCs and G-MDSCs in the TME. Although these CpG-induced MDSCs
still express high levels of IL-10 and Arg-1 mRNA, presenting a
suppressive phenotype, their suppressive ability is attenuated
(83).
TLR4 and MDSCs
The TLR4 signaling pathway induces the activation
and accumulation of MDSCs; however, TLR4 signal activated by BCG
(84) and Immunomax® (IR)
(85) decreases the frequency of
MDSCs. Notably, BCG and PD-L1 blockade synergistically inhibit the
growth of bladder cancer and decrease the proportion of MDSCs
(84). BCG and PD-L1 have been
approved for individual use by the FDA for the treatment of cancer.
Their combined application synergistically decreases the proportion
of MDSCs (84). This suggests the
possibility of a combined application of TLR agonists and
PD-1/PD-L1 inhibitors to synergistically break the immune tolerance
microenvironment.
TLR signaling decreases the
immunosuppressive activity of MDSCs
TLR1/2 and MDSCs
TLR2 signaling promotes the accumulation and
activation of MDSCs (9,48). However, some studies have
demonstrated that TLR2 signaling also weakens the inhibitory
activity of MDSCs (73,86).
It has been demonstrated that TLR1/2 agonists
decrease the immunosuppressive activity of MDSCs by inducing
M1-type macrophage characteristics in MDSCs (73,86).
Notably, Deng et al (73)
reported that TLR1/TLR2/c-Jun kinase signaling promotes M-MDSC
differentiation into M1-type macrophages, thereby preventing M-MDSC
inhibition. Furthermore, the CCL2-CCR2 signaling pathway was
implicated in the attraction of M-MDSCs to the tumor site. The
disruption of CCL2-CCR2 signaling notably decreases the monocyte
influx into the tumor, decreases the number of TAMs, and generally
delays tumor growth (72).
Similarly, Zhang et al (87)
demonstrated that the combination of adoptively transferred
antigen-specific T cells and bacterial lipoprotein decreased the
MDSC frequency in the TME, which may be associated with low CCL2
expression.
TLR5 and MDSCs
CXCL5 is the main chemokine involved in the
migration of MDSCs into tissues, including tumors. It has been
demonstrated that intratumoral injection of TLR5 ligand-secreting T
cells, engineered tumor-reactive T cells that secrete bacterial
flagellin (TLR5 ligand), resulted in a decrease in the number of
MDSCs in the spleen and tumor, particularly M-MDSCs, and
upregulated the expression levels of CD80, CD86, MHCI and MHCII on
MDSCs (12). In addition, the
decrease in MDSCs was associated with a striking reduction in CXCL5
levels (12).
However, bacterial flagellin, a TLR5 ligand, failed
to influence the ability of MDSCs to inhibit T cell proliferation
and slightly affected CD80, MHCI or MHCII expression in MDSCs. It
has been suggested that the modification of TLR agonists can change
their regulatory effect on MDSCs, which provides new ideas and a
theoretical basis for improving the antitumor effect of TLR
agonists (12).
TLR3 and MDSCs
In addition to decreasing the number of MDSCs, TLR
signals also induce MDSCs to differentiate into antigen-presenting
cells and weaken their ability to suppress T cell responses
(76). It has been reported that
TLR3 signaling activated by PolyI:C decreases the immunosuppressive
activity of MDSCs by upregulating MHC II, I-Ad, CD80 and CD86, and
decreasing the secretion of ROS in breast cancer models (76). In addition, TLR3 signaling also
abrogates the capacity of MDSCs to suppress T cell proliferation in
B16 and EL4 tumor models (10). In
addition, Shime et al (88)
demonstrated that G-MDSCs that had been activated with PolyI:C
exhibit cytotoxicity and inhibit tumor growth through the
production of ROS/RNS in a TLR3/TRIF/type I IFN-dependent
manner.
TLR7/8 and MDSCs
Increasing evidence suggests that TLR7/8 signaling
activated by Imiq (13), R848
(89) and resiquimod (16,90)
induces MDSCs to differentiate into tumoricidal M1 macrophages in
mice. Furthermore, R848 can induce M-MDSCs to differentiate into an
M1-like (25F9+/CD200R2) phenotype in patients with
cancer, induce the production of IL-6 and IL-12 in M-MDSCs,
increase their ability to kill A549 tumor cells, and lose their
ability to inhibit T cell proliferation (14). However, R848 is a topical immune
response modifier. When it was administered systemically,
undesirable side effects were observed. Thus, novel TLR7/8 (3M-055
and CL-075) agonists were designed and found to be safe when
administered to mice (91,92). Studies have demonstrated that each of
these agonists duplicates the ability of R848 to induce human
M-MDSCs to mature into M1-like macrophages, and that they are safe
when administered to mice (14,93).
These results are exciting, and they also provide new ideas for the
development of TLR agonists that decrease side effects and disrupt
the inhibition of MDSCs.
TLR9 and MDSCs
Recently, an increasing number of preclinical and
clinical trials have used CpG as a vaccine adjuvant to improve the
antitumor effect of cancer vaccines. It has been demonstrated that
CpG binding with TLR9 on MDSCs directly induces M-MDSC
differentiation into Ly6C−F4/80+ macrophages
and upregulates CD40, CD80 and CD86 expression on MDSCs in
vitro (13,81). However, another study has reported
that CpG indirectly upregulates the expression levels of CD11c,
MHCII, CD80 and F4/80 on MDSCs through type I IFN produced by pDCs
mediated by CpG (17), which may be
attributed to CpG administration. Preclinical trials in our
laboratory indicated that the recombinant mucin1-maltose-binding
protein vaccine, including recombinant mucin1-maltose-binding
protein and CpG 2006, significantly downregulated the ratio of
MDSCs in the spleen and tumor microenvironment (94). Taken together, these studies provide
a rationality for the application of CpG as a cancer vaccine
adjuvant.
TLR-TLR crosstalk and MDSCs
Previous studies have proven that a combination of
TLR agonists synergistically enhances the activity of cancer
vaccines (95,96). Thus, studying the impact of TLR-TLR
crosstalk on MDSCs is essential for understanding the synergistic
mechanism of TLR agonists and the rational combined application of
TLR agonists.
Notably, TLR7 and TLR9 signals have synergistic
effects in regulating MDSCs. 3M-052 and CpG can synergistically
decrease the frequency of tumor infiltrating M-MDSCs by nearly 90%,
and a synergistic reduction of Arg1 and Nos2 mRNA expression,
particularly Nos2 mRNA, resulting in a nearly 90% reduction
(97). Furthermore, the combination
of CpG plus 3M-052 was more successful against both CT26 colon
cancer and B16-F10 melanomas compared with CpG or 3M-052 alone, and
cure rates around 80–90% can be achieved via combination therapy
(97).
However, Triozzi et al (98) demonstrated that Imiq or CpG given
individually as an adjuvant both enhance the antitumor effect of
tumor vaccines and decrease the MDSC frequency, whereas the
combination of Imiq and CpG as adjuvants increases the frequency of
MDSCs in the spleen, and the secretion of Arg1 in MDSCs and the
production of M2-type macrophages in tumors, accompanied by a
reduction in the M1 polarized marker CXCL10, suggesting that TLR7
and TLR9 signals play an antagonistic role in the regulation of
MDSCs.
Chang et al (99) demonstrated that TLR2 and TLR9 have
synergistic effects in regulating MDSCs. It was reported that
Rlipo-E7 m, a recombinant lipoprotein that has intrinsic TLR2
agonist activity, significantly decreases MDSC frequency in the
circulation and the tumor microenvironment, and this ability to
inhibit MDSCs is enhanced when Rlipo-E7 m is combined with CpG
ODN.
Conclusions and perspectives
The regulation of MDSCs by TLR signals is a
double-edged sword in cancer. TLR signaling can activate the
immunosuppressive activity of MDSCs to promote tumor progression,
and also abrogate the immunosuppressive activity of MDSCs and
inhibit tumor growth. Although several compounds have been
investigated for the therapeutic targeting of MDSCs, finding TLR
agonists that are able to modulate the suppressive function of
tumor-expanded MDSCs could be a better choice, which represents a
desirable tipping of the balance toward an increase in
immunostimulatory activity with the concomitant loss of
immunosuppressive MDSCs. In addition, a combination of TLR agonists
and immunotherapy targeting MDSC suppression to decrease the
activation effect of MDSCs induced by TLR signaling appears to be
feasible in cancer treatment. Furthermore, targeted MDSC cancer
immunotherapy through modifying TLR ligands may be an attractive
direction, enabling enhanced immune activity, accompanying the loss
of MDSC immunosuppressive activity and reversing the
immunosuppressive microenvironment, which may be expected to cause
tumors to regress further. Studies on MDSCs and their subsets
(G-MDSC and M-MDSC) regulation by TLR is still relatively limited.
G-MDSC and M-MDSC utilize different molecular mechanisms to
suppress the immune response in the TME. Thus, understanding the
effect of TLR signaling on MDSCs subsets is beneficial to provide
new ideas for the development of cancer immunotherapies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
HZ drafted the initial manuscript, edited and
critically revised the manuscript. MJ, HY and WN contributed
substantially in drafting the manuscript, editing and critically
revising the manuscript for intellectual content. GT put forward
the concept, critically revised the article for intellectual
content, and was responsible for the organization, revision and
submission of the manuscript. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AP-1
|
activator protein 1
|
|
Arg1
|
arginase 1
|
|
BCG
|
Bacillus Calmette-Guerin
|
|
BLP
|
bacterial lipoprotein
|
|
BM
|
bone marrow
|
|
CCL2
|
chemokine (C-C motif) ligand 2
|
|
DCs
|
dendritic cells
|
|
ERK
|
extracellular regulated protein
kinases
|
|
Hsp
|
heat shock protein
|
|
IFNgR1
|
interferon gamma receptor 1
|
|
IFN-γ
|
interferon-γ
|
|
Imiq
|
imiquimod
|
|
iNOS
|
inducible nitric oxide synthase
|
|
IRF
|
interferon regulatory factor
|
|
MAPK
|
mitogen-activated protein kinase
|
|
M-MDSCs
|
monocyte-myeloid-derived suppressor
cells
|
|
MM
|
multiple myeloma
|
|
Moto
|
motolimod
|
|
MPL
|
monophosphoryl lipid A
|
|
MyD88
|
myeloid differentiation primary
response 88
|
|
NF-κB
|
nuclear factor kappa-B
|
|
NK
|
natural killer
|
|
pDCs
|
plasmacytoid DCs
|
|
RNS
|
reactive nitrogen species
|
|
ROS
|
reactive oxygen species
|
|
TCR
|
T cell receptor
|
|
TLR
|
Toll-like receptor
|
|
TME
|
tumor microenvironment
|
|
TRIF
|
Toll-IL-1 receptor-domain containing
adaptor-inducing interferon-β
|
|
VISTA
|
V-domain immunoglobulin suppressor of
T-cell activation
|
References
|
1
|
Gabrilovich DI: Myeloid-derived suppressor
cells. Cancer Immunol Res. 5:3–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kumar V, Patel S, Tcyganov E and
Gabrilovich DI: The nature of myeloid-derived suppressor cells in
the tumor microenvironment. Trends Immunol. 37:208–220. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sica A and Bronte V: Altered macrophage
differentiation and immune dysfunction in tumor development. J Clin
Invest. 117:1155–1166. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gabrilovich DI and Nagaraj S:
Myeloid-derived suppressor cells as regulators of the immune
system. Nat Rev Immunol. 9:162–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Diaz-Montero CM, Salem ML, Nishimura MI,
Garrett-Mayer E, Cole DJ and Montero AJ: Increased circulating
myeloid-derived suppressor cells correlate with clinical cancer
stage, metastatic tumor burden, and doxorubicin-cyclophosphamide
chemotherapy. Cancer Immunol Immunother. 58:49–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Orillion A, Hashimoto A, Damayanti N, Shen
L, Adelaiye-Ogala R, Arisa S, Chintala S, Ordentlich P, Kao C,
Elzey B, et al: Entinostat neutralizes myeloid-derived suppressor
cells and enhances the antitumor effect of PD-1 inhibition in
murine models of lung and renal cell carcinoma. Clin Cancer Res.
23:5187–5201. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim K, Skora AD, Li Z, Liu Q, Tam AJ,
Blosser RL, Diaz LA Jr, Papadopoulos N, Kinzler KW, Vogelstein B
and Zhou S: Eradication of metastatic mouse cancers resistant to
immune checkpoint blockade by suppression of myeloid-derived cells.
Proc Natl Acad Sci USA. 111:11774–11779. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Q, Hossain DM, Duttagupta P, Moreira
D, Zhao X, Won H, Buettner R, Nechaev S, Majka M, Zhang B, et al:
Serum-resistant CpG-STAT3 decoy for targeting survival and immune
checkpoint signaling in acute myeloid leukemia. Blood.
127:1687–1700. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maruyama A, Shime H, Takeda Y, Azuma M,
Matsumoto M and Seya T: Pam2 lipopeptides systemically increase
myeloid-derived suppressor cells through TLR2 signaling. Biochem
Biophys Res Commun. 457:445–450. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Katayama Y, Tachibana M, Kurisu N, Oya Y,
Terasawa Y, Goda H, Kobiyama K, Ishii KJ, Akira S, Mizuguchi H and
Sakurai F: Oncolytic reovirus inhibits immunosuppressive activity
of myeloid-derived suppressor cells in a TLR3-dependent manner. J
Immunol. 200:2987–2999. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsukamoto H, Kozakai S, Kobayashi Y,
Takanashi R, Aoyagi T, Numasaki M, Ohta S and Tomioka Y: Impaired
antigen-specific lymphocyte priming in mice after Toll-like
receptor 4 activation via induction of monocytic myeloid-derived
suppressor cells. Eur J Immunol. 49:546–563. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Geng D, Kaczanowska S, Tsai A, Younger K,
Ochoa A, Rapoport AP, Ostrand-Rosenberg S and Davila E: TLR5
ligand-secreting T cells reshape the tumor microenvironment and
enhance antitumor activity. Cancer Res. 75:1959–1971. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shirota Y, Shirota H and Klinman DM:
Intratumoral injection of CpG oligonucleotides induces the
differentiation and reduces the immunosuppressive activity of
myeloid-derived suppressor cells. J Immunol. 188:1592–1599. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang J, Shirota Y, Bayik D, Shirota H,
Tross D, Gulley JL, Wood LV, Berzofsky JA and Klinman DM: Effect of
TLR agonists on the differentiation and function of human monocytic
myeloid-derived suppressor cells. J Immunol. 194:4215–4221. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dang Y, Rutnam ZJ, Dietsch G, Lu H, Yang
Y, Hershberg R and Disis ML: TLR8 ligation induces apoptosis of
monocytic myeloid-derived suppressor cells. J Leukoc Biol.
103:157–164. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Spinetti T, Spagnuolo L, Mottas I,
Secondini C, Treinies M, Rüegg C, Hotz C and Bourquin C: TLR7-based
cancer immunotherapy decreases intratumoral myeloid-derived
suppressor cells and blocks their immunosuppressive function.
Oncoimmunology. 5:e12305782016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zoglmeier C, Bauer H, Noerenberg D,
Wedekind G, Bittner P, Sandholzer N, Rapp M, Anz D, Endres S and
Bourquin C: CpG blocks immunosuppression by myeloid-derived
suppressor cells in tumor-bearing mice. Clin Cancer Res.
17:1765–1775. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vascotto F, Petschenka J, Walzer KC,
Vormehr M, Brkic M, Strobl S, Rösemann R, Diken M, Kreiter S,
Türeci Ö and Sahin U: Intravenous delivery of the toll-like
receptor 7 agonist SC1 confers tumor control by inducing a CD8+ T
cell response. Oncoimmunology. 8:16014802019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hong EH, Chang SY, Lee BR, Kim YS, Lee JM,
Kang CY, Kweon MN and Ko HJ: Blockade of Myd88 signaling induces
antitumor effects by skewing the immunosuppressive function of
myeloid-derived suppressor cells. Int J Cancer. 132:2839–2848.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Delano MJ, Scumpia PO, Weinstein JS, Coco
D, Nagaraj S, Kelly-Scumpia KM, O'Malley KA, Wynn JL, Antonenko S,
Al-Quran SZ, et al: MyD88-dependent expansion of an immature
GR-1(+)CD11b(+) population induces T cell suppression and Th2
polarization in sepsis. J Exp Med. 204:1463–1474. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Llitjos JF, Auffray C, Alby-Laurent F,
Rousseau C, Merdji H, Bonilla N, Toubiana J, Belaïdouni N, Mira JP,
Lucas B, et al: Sepsis-induced expansion of granulocytic
myeloid-derived suppressor cells promotes tumour growth through
Toll-like receptor 4. J Pathol. 239:473–483. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu CE, Gan J, Zhang RD, Cheng YR and Huang
GJ: Up-regulated myeloid-derived suppressor cell contributes to
hepatocellular carcinoma development by impairing dendritic cell
function. Scand J Gastroenterol. 46:156–164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Savitsky D, Tamura T, Yanai H and
Taniguchi T: Regulation of immunity and oncogenesis by the IRF
transcription factor family. Cancer Immunol Immunother. 59:489–510.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nam S, Kang K, Cha JS, Kim JW, Lee HG, Kim
Y, Yang Y, Lee MS and Lim JS: Interferon regulatory factor 4 (IRF4)
controls myeloid-derived suppressor cell (MDSC) differentiation and
function. J Leukoc Biol. 100:1273–1284. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu W, Hiếu T, Malarkannan S and Wang L:
The structure, expression, and multifaceted role of
immune-checkpoint protein VISTA as a critical regulator of
anti-tumor immunity, autoimmunity, and inflammation. Cell Mol
Immunol. 15:438–446. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu W, Dong J, Zheng Y, Zhou J, Yuan Y, Ta
HM, Miller HE, Olson M, Rajasekaran K, Ernstoff MS, et al:
Immune-checkpoint protein VISTA regulates antitumor immunity by
controlling myeloid cell-mediated inflammation and
immunosuppression. Cancer Immunol Res. 7:1497–1510. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peek EM, Song W, Zhang H, Huang J and Chin
AI: Loss of MyD88 leads to more aggressive TRAMP prostate cancer
and influences tumor infiltrating lymphocytes. Prostate.
75:463–473. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Di S, Zhou M, Pan Z, Sun R, Chen M, Jiang
H, Shi B, Luo H and Li Z: Combined adjuvant of poly I:C improves
antitumor effects of CAR-T cells. Front Oncol. 9:2412019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bronte V, Brandau S, Chen SH, Colombo MP,
Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A,
Ostrand-Rosenberg S, et al: Recommendations for myeloid-derived
suppressor cell nomenclature and characterization standards. Nat
Commun. 7:121502016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rodriguez PC, Quiceno DG, Zabaleta J,
Ortiz B, Zea AH, Piazuelo MB, Delgado A, Correa P, Brayer J,
Sotomayor EM, et al: Arginase I production in the tumor
microenvironment by mature myeloid cells inhibits T-cell receptor
expression and antigen-specific T-cell responses. Cancer Res.
64:5839–5849. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schmielau J and Finn OJ: Activated
granulocytes and granulocyte-derived hydrogen peroxide are the
underlying mechanism of suppression of t-cell function in advanced
cancer patients. Cancer Res. 61:4756–4760. 2001.PubMed/NCBI
|
|
32
|
Mazzoni A, Bronte V, Visintin A, Spitzer
JH, Apolloni E, Serafini P, Zanovello P and Segal DM: Myeloid
suppressor lines inhibit T cell responses by an NO-dependent
mechanism. J Immunol. 168:689–695. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hanson EM, Clements VK, Sinha P, Ilkovitch
D and Ostrand-Rosenberg S: Myeloid-derived suppressor cells
down-regulate L-selectin expression on CD4+ and CD8+ T cells. J
Immunol. 183:937–944. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Molon B, Ugel S, Del Pozzo F, Soldani C,
Zilio S, Avella D, De Palma A, Mauri P, Monegal A, Rescigno M, et
al: Chemokine nitration prevents intratumoral infiltration of
antigen-specific T cells. J Exp Med. 208:1949–1962. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang B, Pan PY, Li Q, Sato AI, Levy DE,
Bromberg J, Divino CM and Chen SH: Gr-1+CD115+ immature myeloid
suppressor cells mediate the development of tumor-induced T
regulatory cells and T-cell anergy in tumor-bearing host. Cancer
Res. 66:1123–1131. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Serafini P, Meckel K, Kelso M, Noonan K,
Califano J, Koch W, Dolcetti L, Bronte V and Borrello I:
Phosphodiesterase-5 inhibition augments endogenous antitumor
immunity by reducing myeloid-derived suppressor cell function. J
Exp Med. 203:2691–2702. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Montero AJ, Diaz-Montero CM, Kyriakopoulos
CE, Bronte V and Mandruzzato S: Myeloid-derived suppressor cells in
cancer patients: A clinical perspective. J Immunother. 35:107–115.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Condamine T and Gabrilovich DI: Molecular
mechanisms regulating myeloid-derived suppressor cell
differentiation and function. Trends Immunol. 32:19–25. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Condamine T, Mastio J and Gabrilovich DI:
Transcriptional regulation of myeloid-derived suppressor cells. J
Leukoc Biol. 98:913–922. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dowling JK and Mansell A: Toll-like
receptors: The swiss army knife of immunity and vaccine
development. Clin Transl Immunology. 5:e852016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Urban-Wojciuk Z, Khan MM, Oyler BL,
Fåhraeus R, Marek-Trzonkowska N, Nita-Lazar A, Hupp TR and Goodlett
DR: The role of TLRs in anti-cancer immunity and tumor rejection.
Front Immunol. 10:23882019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Carpentier A, Metellus P, Ursu R, Zohar S,
Lafitte F, Barrié M, Meng Y, Richard M, Parizot C, Laigle-Donadey
F, et al: Intracerebral administration of CpG oligonucleotide for
patients with recurrent glioblastoma: A phase II study. Neuro
Oncol. 12:401–408. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Carpentier A, Laigle-Donadey F, Zohar S,
Capelle L, Behin A, Tibi A, Martin-Duverneuil N, Sanson M,
Lacomblez L, Taillibert S, et al: Phase 1 trial of a CpG
oligodeoxynucleotide for patients with recurrent glioblastoma.
Neuro Oncol. 8:60–66. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fleming V, Hu X, Weber R, Nagibin V, Groth
C, Altevogt P, Utikal J and Umansky V: Targeting myeloid-derived
suppressor cells to bypass tumor-induced immunosuppression. Front
Immunol. 9:3982018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shime H, Maruyama A, Yoshida S, Takeda Y,
Matsumoto M and Seya T: Toll-like receptor 2 ligand and
interferon-γ suppress anti-tumor T cell responses by enhancing the
immunosuppressive activity of monocytic myeloid-derived suppressor
cells. Oncoimmunology. 7:e13732312017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cherfils-Vicini J, Iltis C, Cervera L,
Pisano S, Croce O, Sadouni N, Győrffy B, Collet R, Renault VM,
Rey-Millet M, et al: Cancer cells induce immune escape via
glycocalyx changes controlled by the telomeric protein TRF2. EMBO
J. 38:e1000122019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gobbo J, Marcion G, Cordonnier M, Dias
AMM, Pernet N, Hammann A, Richaud S, Mjahed H, Isambert N, Clausse
V, et al: Restoring anticancer immune response by targeting
tumor-derived exosomes with a HSP70 peptide aptamer. J Natl Cancer
Inst. 108:2015.PubMed/NCBI
|
|
48
|
Xiang X, Liu Y, Zhuang X, Zhang S,
Michalek S, Taylor DD, Grizzle W and Zhang HG: TLR2-mediated
expansion of MDSCs is dependent on the source of tumor exosomes. Am
J Pathol. 177:1606–1610. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chalmin F, Ladoire S, Mignot G, Vincent J,
Bruchard M, Remy-Martin JP, Boireau W, Rouleau A, Simon B, Lanneau
D, et al: Membrane-associated Hsp72 from tumor-derived exosomes
mediates STAT3-dependent immunosuppressive function of mouse and
human myeloid-derived suppressor cells. J Clin Invest. 120:457–471.
2010.PubMed/NCBI
|
|
50
|
Diao J, Yang X, Song X, Chen S, He Y, Wang
Q, Chen G, Luo C, Wu X and Zhang Y: Exosomal Hsp70 mediates
immunosuppressive activity of the myeloid-derived suppressor cells
via phosphorylation of Stat3. Med Oncol. 32:4532015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lee JM, Kim EK, Seo H, Jeon I, Chae MJ,
Park YJ, Song B, Kim YS, Kim YJ, Ko HJ and Kang CY: Serum amyloid
A3 exacerbates cancer by enhancing the suppressive capacity of
myeloid-derived suppressor cells via TLR2-dependent STAT3
activation. Eur J Immunol. 44:1672–1684. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
He XY, Gong FY, Chen Y, Zhou Z, Gong Z and
Gao XM: Calreticulin fragment 39–272 promotes B16 melanoma
malignancy through myeloid-derived suppressor cells in vivo. Front
Immunol. 8:13062017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Huang M, Wu R, Chen L, Peng Q, Li S, Zhang
Y, Zhou L and Duan L: S100A9 regulates MDSCs-mediated immune
suppression via the RAGE and TLR4 signaling pathways in colorectal
carcinoma. Front Immunol. 10:22432019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
De Veirman K, De Beule N, Maes K, Menu E,
De Bruyne E, De Raeve H, Fostier K, Moreaux J, Kassambara A, Hose
D, et al: Extracellular S100A9 protein in bone marrow supports
multiple myeloma survival by stimulating angiogenesis and cytokine
secretion. Cancer Immunol Res. 5:839–846. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xie Z, Ago Y, Okada N and Tachibana M:
Valproic acid attenuates immunosuppressive function of
myeloid-derived suppressor cells. J Pharmacol Sci. 137:359–365.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Deguchi A, Tomita T, Ohto U, Takemura K,
Kitao A, Akashi-Takamura S, Miyake K and Maru Y: Eritoran inhibits
S100A8-mediated TLR4/MD-2 activation and tumor growth by changing
the immune microenvironment. Oncogene. 35:1445–1456. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen J, Sun B, Zhao X, Liang D, Liu J,
Huang Y, Lei W, Chen M and Sun W: Monophosphoryl lipid A induces
bone marrow precursor cells to differentiate into myeloid-derived
suppressor cells. Mol Med Rep. 8:1074–1078. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li Q, Dai C, Xue R, Wang P, Chen L, Han Y,
Erben U and Qin Z: S100A4 protects myeloid-derived suppressor cells
from intrinsic apoptosis via TLR4-ERK1/2 signaling. Front Immunol.
9:3882018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zambirinis CP, Levie E, Nguy S, Avanzi A,
Barilla R, Xu Y, Seifert L, Daley D, Greco SH, Deutsch M, et al:
TLR9 ligation in pancreatic stellate cells promotes tumorigenesis.
J Exp Med. 212:2077–2094. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Dajon M, Iribarren K, Petitprez F, Marmier
S, Lupo A, Gillard M, Ouakrim H, Victor N, Vincenzo DB, Joubert PE,
et al: Toll like receptor 7 expressed by malignant cells promotes
tumor progression and metastasis through the recruitment of myeloid
derived suppressor cells. Oncoimmunology. 8:e15051742018.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Dajon M, Iribarren K and Cremer I: Dual
roles of TLR7 in the lung cancer microenvironment. Oncoimmunology.
4:e9916152015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Jie J, Zhang Y, Zhou H, Zhai X, Zhang N,
Yuan H, Ni W and Tai G: CpG ODN1826 as a promising
mucin1-maltose-binding protein vaccine adjuvant induced DC
maturation and enhanced antitumor immunity. Int J Mol Sci.
19:9202018. View Article : Google Scholar
|
|
63
|
Schouppe E, Mommer C, Movahedi K, Laoui D,
Morias Y, Gysemans C, Luyckx A, De Baetselier P and Van
Ginderachter JA: Tumor-induced myeloid-derived suppressor cell
subsets exert either inhibitory or stimulatory effects on distinct
CD8+ T-cell activation events. Eur J Immunol. 43:2930–2942. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sinha P, Okoro C, Foell D, Freeze HH,
Ostrand-Rosenberg S and Srikrishna G: Proinflammatory S100 proteins
regulate the accumulation of myeloid-derived suppressor cells. J
Immunol. 181:4666–4675. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Song J, Lee J, Kim J, Jo S, Kim YJ, Baek
JE, Kwon ES, Lee KP, Yang S, Kwon KS, et al: Pancreatic
adenocarcinoma up-regulated factor (PAUF) enhances the accumulation
and functional activity of myeloid-derived suppressor cells (MDSCs)
in pancreatic cancer. Oncotarget. 7:51840–51853. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Tachibana M: The immunosuppressive
function of myeloid-derived suppressor cells is regulated by the
HMGB1-TLR4 axis. Yakugaku Zasshi. 138:143–148. 2018.(In Japanese).
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Li J, Yang F, Wei F and Ren X: The role of
toll-like receptor 4 in tumor microenvironment. Oncotarget.
8:66656–66667. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Bunt SK, Clements VK, Hanson EM, Sinha P
and Ostrand-Rosenberg S: Inflammation enhances myeloid-derived
suppressor cell cross-talk by signaling through Toll-like receptor
4. J Leukoc Biol. 85:996–1004. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Fleming V, Hu X, Weller C, Weber R, Groth
C, Riester Z, Hüser L, Sun Q, Nagibin V, Kirschning C, et al:
Melanoma extracellular vesicles generate immunosuppressive myeloid
cells by upregulating PD-L1 via TLR4 signaling. Cancer Res.
79:4715–4728. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Karwacz K, Bricogne C, MacDonald D, Arce
F, Bennett CL, Collins M and Escors D: PD-L1 co-stimulation
contributes to ligand-induced T cell receptor down-modulation on
CD8+ T cells. EMBO Mol Med. 3:581–592. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Xu-Monette ZY, Zhang M, Li J and Young KH:
PD-1/PD-L1 blockade: Have we found the key to unleash the antitumor
immune response? Front Immunol. 8:15972017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Tcyganov E, Mastio J, Chen E and
Gabrilovich DI: Plasticity of myeloid-derived suppressor cells in
cancer. Curr Opin Immunol. 51:76–82. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Deng Y, Yang J, Qian J, Liu R, Huang E,
Wang Y, Luo F and Chu Y: TLR1/TLR2 signaling blocks the suppression
of monocytic myeloid-derived suppressor cell by promoting its
differentiation into M1-type macrophage. Mol Immunol. 112:266–273.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Blasius AL and Beutler B: Intracellular
toll-like receptors. Immunity. 32:305–315. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Boozari M, Butler AE and Sahebkar A:
Impact of curcumin on toll-like receptors. J Cell Physiol.
234:12471–12482. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Forghani P and Waller EK: Poly (I: C)
modulates the immunosuppressive activity of myeloid-derived
suppressor cells in a murine model of breast cancer. Breast Cancer
Res Treat. 153:21–30. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Chuang CM, Monie A, Hung CF and Wu TC:
Treatment with imiquimod enhances antitumor immunity induced by
therapeutic HPV DNA vaccination. J Biomed Sci. 17:322010.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Cho JH, Lee HJ, Ko HJ, Yoon BI, Choe J,
Kim KC, Hahn TW, Han JA, Choi SS, Jung YM, et al: The TLR7 agonist
imiquimod induces anti-cancer effects via autophagic cell death and
enhances anti-tumoral and systemic immunity during radiotherapy for
melanoma. Oncotarget. 8:24932–24948. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Liu J, Hu Y, Guo Q, Yu X, Shao L and Zhang
C: Enhanced anti-melanoma efficacy of a Pim-3-targeting
bifunctional small hairpin RNA via single-stranded RNA-mediated
activation of plasmacytoid dendritic cells. Front Immunol.
10:27212019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Cerullo V, Diaconu I, Romano V, Hirvinen
M, Ugolini M, Escutenaire S, Holm SL, Kipar A, Kanerva A and
Hemminki A: An oncolytic adenovirus enhanced for toll-like receptor
9 stimulation increases antitumor immune responses and tumor
clearance. Mol Ther. 20:2076–2086. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
James BR, Anderson KG, Brincks EL, Kucaba
TA, Norian LA, Masopust D and Griffith TS: CpG-mediated modulation
of MDSC contributes to the efficacy of Ad5-TRAIL therapy against
renal cell carcinoma. Cancer Immunol Immunother. 63:1213–1227.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Tarhini AA, Butterfield LH, Shuai Y,
Gooding WE, Kalinski P and Kirkwood JM: Differing patterns of
circulating regulatory T cells and myeloid-derived suppressor cells
in metastatic melanoma patients receiving anti-CTLA4 antibody and
interferon-alpha or TLR-9 agonist and GM-CSF with peptide
vaccination. J Immunother. 35:702–710. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Lin YC, Hsu CY, Huang SK, Fan YH, Huang
CH, Yang CK, Su WT, Chang PC, Dutta A, Liu YJ, et al: Induction of
liver-specific intrahepatic myeloid cells aggregation expands CD8 T
cell and inhibits growth of murine hepatoma. Oncoimmunology.
7:e15021292018.PubMed/NCBI
|
|
84
|
Wang Y, Liu J, Yang X, Liu Y, Liu Y, Li Y,
Sun L, Yang X and Niu H: Bacillus Calmette-Guérin and anti-PD-L1
combination therapy boosts immune response against bladder cancer.
Onco Targets Ther. 11:2891–2899. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Ghochikyan A, Pichugin A, Bagaev A,
Davtyan A, Hovakimyan A, Tukhvatulin A, Davtyan H, Shcheblyakov D,
Logunov D, Chulkina M, et al: Targeting TLR-4 with a novel
pharmaceutical grade plant derived agonist, Immunomax®,
as a therapeutic strategy for metastatic breast cancer. J Transl
Med. 12:3222014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Liu Y, Zhang L, Zhu X, Wang Y, Liu W and
Gong W: Polysaccharide Agaricus blazei Murill stimulates myeloid
derived suppressor cell differentiation from M2 to M1 type, which
mediates inhibition of tumour immune-evasion via the Toll-like
receptor 2 pathway. Immunology. 146:379–391. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhang Y, Luo F, Li A, Qian J, Yao Z, Feng
X and Chu Y: Systemic injection of TLR1/2 agonist improves adoptive
antigen-specific T cell therapy in glioma-bearing mice. Clin
Immunol. 154:26–36. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Shime H, Matsumoto M and Seya T:
Double-stranded RNA promotes CTL-independent tumor cytolysis
mediated by CD11b+Ly6G+ intratumor myeloid
cells through the TICAM-1 signaling pathway. Cell Death Differ.
24:385–396. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Liu Z, Xie Y, Xiong Y, Liu S, Qiu C, Zhu
Z, Mao H, Yu M and Wang X: TLR 7/8 agonist reverses oxaliplatin
resistance in colorectal cancer via directing the myeloid-derived
suppressor cells to tumoricidal M1-macrophages. Cancer Lett.
469:173–185. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lee M, Park CS, Lee YR, Im SA, Song S and
Lee CK: Resiquimod, a TLR7/8 agonist, promotes differentiation of
myeloid-derived suppressor cells into macrophages and dendritic
cells. Arch Pharm Res. 37:1234–1240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Butchi NB, Pourciau S, Du M, Morgan TW and
Peterson KE: Analysis of the neuroinflammatory response to TLR7
stimulation in the brain: Comparison of multiple TLR7 and/or TLR8
agonists. J Immunol. 180:7604–7612. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Gorden KB, Gorski KS, Gibson SJ, Kedl RM,
Kieper WC, Qiu X, Tomai MA, Alkan SS and Vasilakos JP: Synthetic
TLR agonists reveal functional differences between human TLR7 and
TLR8. J Immunol. 174:1259–1268. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Le Mercier I, Poujol D, Sanlaville A,
Sisirak V, Gobert M, Durand I, Dubois B, Treilleux I, Marvel J,
Vlach J, et al: Tumor promotion by intratumoral plasmacytoid
dendritic cells is reversed by TLR7 ligand treatment. Cancer Res.
73:4629–4640. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Zhou H, Zhang Z, Liu G, Jiang M, Wang J,
Liu Y and Tai G: The effect of different immunization cycles of a
recombinant mucin1-maltose-binding protein vaccine on T cell
responses to B16-MUC1 melanoma in mice. Int J Mol Sci. 21:58102020.
View Article : Google Scholar
|
|
95
|
Kawai T and Akira S: Toll-like receptors
and their crosstalk with other innate receptors in infection and
immunity. Immunity. 34:637–650. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Tan RS, Ho B, Leung BP and Ding JL: TLR
cross-talk confers specificity to innate immunity. Int Rev Immunol.
33:443–453. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zhao BG, Vasilakos JP, Tross D, Smirnov D
and Klinman DM: Combination therapy targeting toll like receptors
7, 8 and 9 eliminates large established tumors. J Immunother
Cancer. 2:122014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Triozzi PL, Aldrich W and Ponnazhagan S:
Regulation of the activity of an adeno-associated virus vector
cancer vaccine administered with synthetic Toll-like receptor
agonists. Vaccine. 28:7837–7843. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Chang LS, Leng CH, Yeh YC, Wu CC, Chen HW,
Huang HM and Liu SJ: Toll-like receptor 9 agonist enhances
anti-tumor immunity and inhibits tumor-associated immunosuppressive
cells numbers in a mouse cervical cancer model following
recombinant lipoprotein therapy. Mol Cancer. 13:602014. View Article : Google Scholar : PubMed/NCBI
|
















