Introduction
Glioblastoma (GBM) is the most common malignant
primary central nervous system tumor, with an average survival time
of 12–15 months (1). The failure of
conventional treatments is attributed to its highly invasive and
diffusely infiltrative nature (2).
Thus, the identification of novel therapeutic targets and
strategies to improve the efficacy of existing forms of treatment
is urgently required.
Cell polarity and intercellular adhesion play a key
role in regulating normal tissue structure and function (3). The disruption of cell polarity and cell
adhesion is usually associated with tumor formation (4). Lethal (2)-giant larvae (Lgl) is a cortical
cytoskeletal protein, which was initially identified in
Drosophila and exhibits notable effects in the establishment
and maintenance of apical-basal epithelial polarity, asymmetric
cell division, tissue integrity and cell proliferation (5). The human homologues of Lgl1 and Lgl2
are termed human giant larvae (Hugl)-1 and Hugl-2. Mutations that
cause loss of function of Lgl have been demonstrated to result in
tissue overgrowth and neoplastic tumor formation (6,7). The
Hugl-1 protein shares 62.5% similarity with Lgl (8–10). A
previous study indicated that hepatocellular carcinoma (HCC)
contains frequent mutations of Hugl-1, whereas overexpression of
HCC-derived aberrant Hugl-1 variants significantly promote HCC cell
migration and invasion (11). In
addition, Hugl-1 expression is downregulated in different types of
human cancer, including colorectal cancer, melanoma, prostate
cancer, breast cancer, endometrial cancer, lung cancer and
esophageal carcinoma (12–15). Hugl-1 expression is positively
associated with a higher survival rate in patients with pancreatic
carcinoma, suggesting its use as a reliable prognostic marker
(16). The majority of previous
studies have focused on epithelial-derived tumors (11–15),
thus the role of Hugl-1 in gliomas (glia-derived tumors) has not
yet been fully elucidated. A previous study performed by our group
has demonstrated that Hugl-1 protein levels decrease in human
glioma tissues, whereas overexpression of Hugl-1 attenuates glioma
cell proliferation in an intracranial model of nude mice; however,
it does not affect glioma cell proliferation in vitro
(17). As a regulator of cell
polarity, Hugl-1 exhibits important properties that are closely
associated with cell adhesion and cytoskeletal function and
structure (18). However, the role
of Hugl-1 in glioma migration and invasion has not yet been fully
investigated.
Cell surface adhesion molecules are the main
mediators of cell-cell interactions, which are essential for tumor
malignant biological behaviors. Reorganization of the cell
cytoskeleton and alteration of cell-cell adhesion are required
prior to cell migration (19,20).
These processes are mainly mediated by cadherin family members. It
is reported that E-cadherin is essential for the normal migration
of cranial neural crest cells in vivo, while P-cadherin,
also known as placental cadherin, is associated with malignant
invasion of esophageal squamous cells (21–24). In
most tumors, N-cadherin expression is often upregulated and can be
used as a promoter of tumor invasion (25,26).
N-cadherin expression in epithelial cells can induce morphological
changes of fibroblast phenotype and orchestrate cell-cell
communication during cell movement (27). N-cadherin is also known as an
epithelial-to-mesenchymal transition marker and exhibits several
functions according to the cell environment that can promote
adhesion or induce migration (28,29).
However, increasing evidence suggests that N-cadherin exhibits
tumor-inhibitory roles in non-epithelial derived neoplasms, such as
osteosarcoma and glioma (27,30).
Thus, the functions of N-cadherin may be tumor-type specific
(27).
The present study aimed to investigate the role and
molecular mechanism of Hugl-1 on the motility of malignant glioma
cells.
Materials and methods
Cell culture
The U251-MG glioma cell line was purchased from the
Shanghai Cell Bank, Type Culture Collection Committee, Chinese
Academy of Sciences. Cells were maintained in DMEM/F-12 media
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal
bovine serum (FBS; Biological Industries), at 37°C in 5%
CO2.
Stable transfection of Hugl-1 into
U251-MG cells
The pEGFP-C1 vector alone or the pEGFP-C1-Hugl-1
construct (provided by Professor Zhengjun Chen, Shanghai Institute
of Biochemistry and Cell Biology, Chinese Academy of Sciences) was
transfected into U251-MG cells (GFP-Vector or GFP-Hugl-1 cells,
respectively) using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
instructions. Briefly, 9 µl Lipofectamine® 2000 and 3 µg
of the Hugl-1 expression plasmid were added to 1 ml Opti-MEM
(Invitrogen; Thermo Fisher Scientific, Inc.) and incubated for 10
min at room temperature. The plasmid and Lipofectamine were mixed
together and incubated for 30 min before adding them to the U251-MG
cells. The transfectants were subsequently selected using G418
(1,200 µg/ml), and single-cell clones were obtained following 3–4
weeks of growth for expansion. The G418-resistant cells were used
for subsequent experiments. DsRed-C1 or DsRed-N-cadherin plasmids
were kindly provided by the Laboratory of Cell Biology of Northeast
Normal University (Changchun, China). DsRed-C1 or DsRed-N-cadherin
plasmids were transfected into Hugl-1 overexpressing U251-MG cells.
The specific transfection procedure was the same as that of
Hugl-1.
Digestion assay
Cultured GFP-Vector or GFP-Hugl-1 cells were
digested with trypsin simultaneously (Gibco; Thermo Fisher
Scientific, Inc.). Briefly, cells were digested with trypsin at
room temperature for 8 min and observed at designated time points
(0, 2, 4 and 8 min) under an inverted light microscope during
trypsinization at ×200 magnification (Olympus Corporation;
IX71).
Attachment assay
The attachment assay was performed using 12-well
plates. The cell suspension was added into the plates and cell
images were obtained at 3, 6, 9 and 24 h using an inverted light
microscope at ×400 magnification (Olympus Corporation; IX71).
Wound healing assay
Cell migration was assessed via the wound healing
assay, as previously described (31). Briefly, cells were seeded into 6-well
plates and cultured until they reached ~80% confluence. The cell
monolayers were scratched using a 10 µl sterile pipette tip. Cells
were subsequently washed twice with PBS to remove floating cells
and serum-free DMEM/F-12 medium (Gibco; Thermo Fisher Scientific,
Inc.) was added. Cell wound healing was observed at 0, 24 or 48 h
using an inverted light microscope at ×200 magnification (Olympus
Corporation; IX71).
Transwell invasion assay
The cell invasion assay was performed as previously
described (31). Briefly, Transwell
membranes were precoated with DMEM-diluted Matrigel® (BD
Biosciences) for 3 h at 37°C. Cells (2×104) were plated
in the upper chambers of Transwell plates in 200 µl serum-free
culture DMEM/F-12 medium (Gibco; Thermo Fisher Scientific, Inc.). A
total of 500 µl DMEM/F-12 medium supplemented with 10% FBS was
plated in the lower chambers. Following incubation at room
temperature for 24 h, the invasive cells were fixed in methanol for
15 min at room temperature and subsequently stained for 15 min at
room temperature with 0.1% crystal violet. Invasive cells were
viewed and counted under an inverted light microscope at ×200
magnification (Olympus Corporation; IX71).
Western blotting
U251-MG cells were lysed with RIPA lysis buffer (50
mM Tris-HCl, 150 mM NaCl, 0.5% sodium deoxycholate and 0.1% SDS)
supplemented with protease inhibitor cocktail, and total proteins
were quantified using a BCA kit (Beyotime Institute of
Biotechnology) according to the manufacturer's protocol. Western
blotting was performed as previously described (32). Briefly, equal amounts of protein (20
µg/lane) were separated by SDS-PAGE on 8 or 10% gels, transferred
onto polyvinylidene fluoride membranes and blocked using 3% BSA
(Sangon Biotech Co., Ltd.) for 2 h at room temperature. The
membranes were then incubated with primary antibodies against
Hugl-1 (1:500) [kindly gifted by Dr ZG Luo from the Institute of
Neuroscience, Shanghai Institutes for Biological Sciences, Chinese
Academy of Sciences (33)],
N-cadherin (1:2,000; cat. no. ab76011; Abcam), β-catenin (1:5,000;
cat. no. ab32572; Abcam), integrinβ1 (1:1,000; cat. no. ab134179;
Abcam) and β-actin (1:1,000; cat. no. MABT523; EMD Millipore).
Following the primary antibody incubation at 4°C overnight,
membranes were probed with HRP-labelled goat anti-rabbit or
anti-mouse IgG secondary antibodies (1:4,000; cat. nos. sc2004 and
sc2005; Santa Cruz Biotechnology, Inc.) at room temperature for 2
h. The signal was detected using the Pierce ECL Plus Western
Blotting Substrate (Pierce; Thermo Fisher Scientific, Inc.) and
exposed to ChemiDoc Touch (Bio-Rad Laboratories, Inc.). Finally,
gray analysis was performed using ImageJ 1.48V (National Institutes
of Health) to compare the level of each protein.
Phalloidin staining
U251-MG cells were incubated for 24 h at 37°C and
cultured in DMEM/F-12 medium supplemented with 10% FBS for 30 min
at 37°C. Subsequently, cells were fixed with 4% paraformaldehyde
for 10 min at room temperature, washed twice with PBS, and 0.5%
Triton X-100 was added for 5 min at room temperature. Finally, 200
µl of the diluted phalloidin (cat. no. 94072; Sigma-Aldrich; Merck
KGaA) was added and incubated at room temperature in the dark for
30 min. Actin filaments were observed using an inverted
fluorescence microscope at ×400 magnification (Olympus Corporation;
IX71).
Statistical analysis
Statistical analysis was performed using SPSS 13.0
software (SPSS, Inc.). All experiments were performed in triplicate
and data are presented as the mean ± standard error of the mean.
Student's unpaired t-test was used to compare differences between
two groups, while one-way AVONA followed by Tukey's post hoc test
were used to compare differences between multiple groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
Effects of Hugl-1 on cell adhesive
activity
It has been demonstrated that Hugl-1 protein
expression is downregulated in human glioma tissues compared with
normal brain tissues (17). Given
that Hugl-1 acts as a tumor suppressor in several human tumors, and
its expressed at low levels in gliomas, the effect of Hugl-1
downregulation on this basis may be negligible. In addition, we
detected six different glioma cell lines in the previous study and
demonstrated that Hugl-1 protein expression was extremely low in
U251-MG cells (17). Thus, to
investigate the role of Hugl-1 in glioma, stable GFP-Hugl-1
overexpression was established in U251-MG glioma cells, and
constitutive expression was assessed via western blotting (Fig. 1A and B). Due to the high molecular
weight of Hugl-1 (115 kDa) (13),
the molecular weight of GFP-Hugl-1 fusing protein was 141 kDa (115
kDa + 26 kDa), which caused weaker GFP signaling in the GFP-Hugl-1
group compared with the GFP group. To determine the difference in
the adhesive ability between the GFP-Hugl-1 and GFP groups, cells
were trypsinized, and the results demonstrated that Hugl-1
overexpressing cells retracted more slowly compared with the GFP
control cells (Fig. 1C), suggesting
a better cell-extracellular matrix adhesive ability. Conversely,
Hugl-1 overexpressing cells extended pseudopodia faster than GFP
cells following plating (Fig. 1D).
Notably, GFP cells formed cell aggregates unlike Hugl-1
overexpressing cells (Fig. 1D, black
arrow), suggesting that upregulation of Hugl-1 decreases cell-cell
adhesive activity. The cells presented in Fig. 1D exhibited complete roundness and
good refraction, indicating that the cells were healthy. In
addition, the digested cells were placed in a culture dish and
gradually expanded.
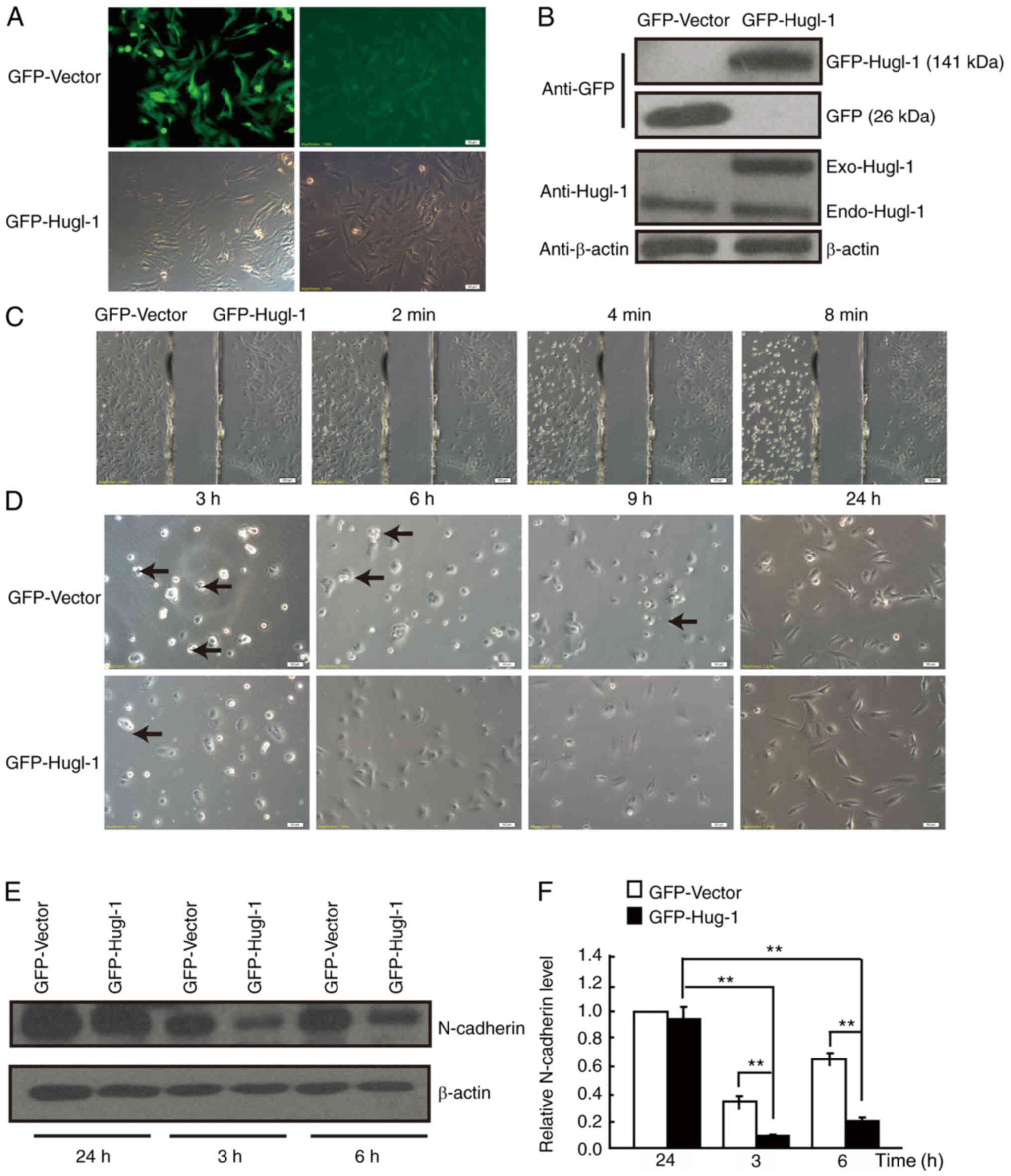 | Figure 1.Hugl-1 affects cell adhesion. (A)
GFP-Hugl-1 or GFP-Vector plasmids were transfected into U251-MG
glioma cells, followed by G418 selection. The transfection
efficiency was assessed via GFP fluorescence (scale bar, 50 µm).
(B) Western blot analysis was performed to detect Hugl-1 protein
expression. (C) Representative digital images obtained at 0, 2, 4
and 8 min during trypsinization (scale bar, 100 µm). (D)
Representative digital images obtained at 3, 6, 9 and 24 h
following plating. Black arrowheads indicate the cell aggregates
(scale bar, 50 µm). (E) N-cadherin protein levels were detected at
3, 6 and 24 h following cell attachment. (F) Quantification results
of (E). **P<0.01. Hugl-1, human giant larvae-1; GFP, green
fluorescent protein. |
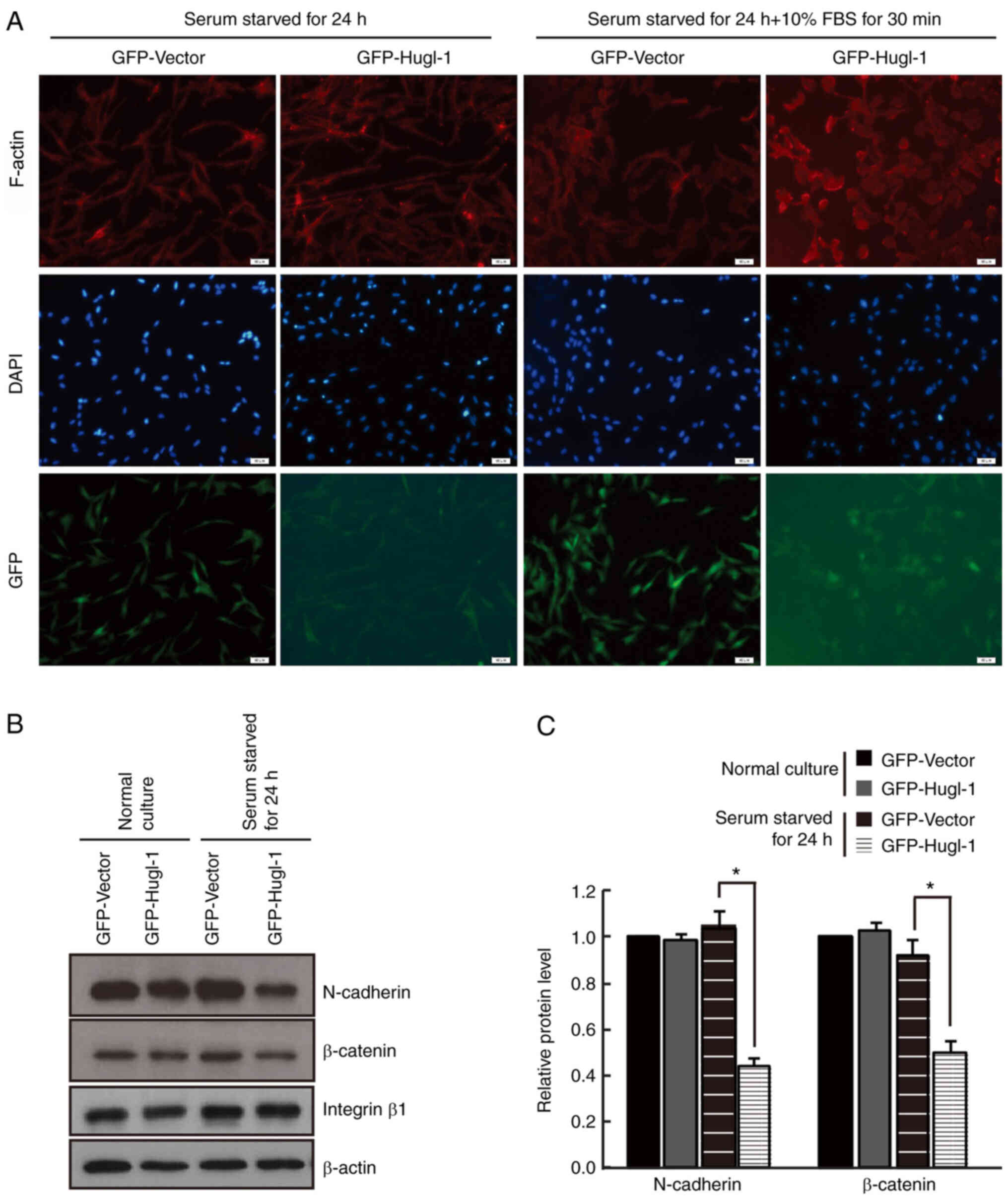
N-cadherin is a member of the calcium-dependent
adhesion molecule family, which mediates adhesion between homotypic
cells (34). Thus, N-cadherin
protein expression was detected at 3, 6 and 24 h following plating.
The results demonstrated that N-cadherin expression was lower in
Hugl-1 overexpressing cells compared with GFP cells at 3 and 6 h
following plating (Fig. 1E and
F).
Previous studies have demonstrated that adhesion
molecules play an important role in the early stage of cell
adhesion (35,36), which gradually decreases overtime
(37). In the present study, no
significant difference was observed in N-cadherin expression
between the two groups 24 h after plating. Taken together, these
results suggest that overexpression of Hugl-1 decreases cell-cell
adhesion, while increasing cell-extracellular matrix adhesion by
regulating N-cadherin expression.
Hugl-1 accelerates cytoskeletal
remodeling
To fully characterize the intercellular adhesion
defects observed in Hugl-1 overexpressing cells, the intracellular
organization of the cytoskeleton was assessed. Cells were incubated
for 24 h and cultured in media supplemented with 10% FBS for 30
min. Subsequently, cells were stained with phalloidin-conjugated
actin to assess actin reassembling. The results demonstrated that
Hugl-1 overexpressing cells expanded their lamellipodia earlier,
which contained concentrated F-actin, and stretched faster than GFP
cells (Fig. 2A). Previous studies
have established β-catenin as a promoter signal, which is not only
a key transcription factor in the Wnt signaling pathway but also a
structural adaptor between cadherin and actin skeleton during cell
adhesion (38,39). The present study hypothesized that
β-catenin may mediate cytoskeleton remodeling by overexpressing
Hugl-1. The results of the present study demonstrated that
β-catenin expression decreased in Hugl-1 overexpressing cells,
while the expression levels of integrin β1, another important
molecule involved in cytoskeleton remodeling and adhesion (40), remained unchanged (Fig. 2B and C). Collectively, these results
suggest that Hugl-1 may accelerate cell cytoskeleton reorganization
by regulating the N-cadherin-β-catenin complex.
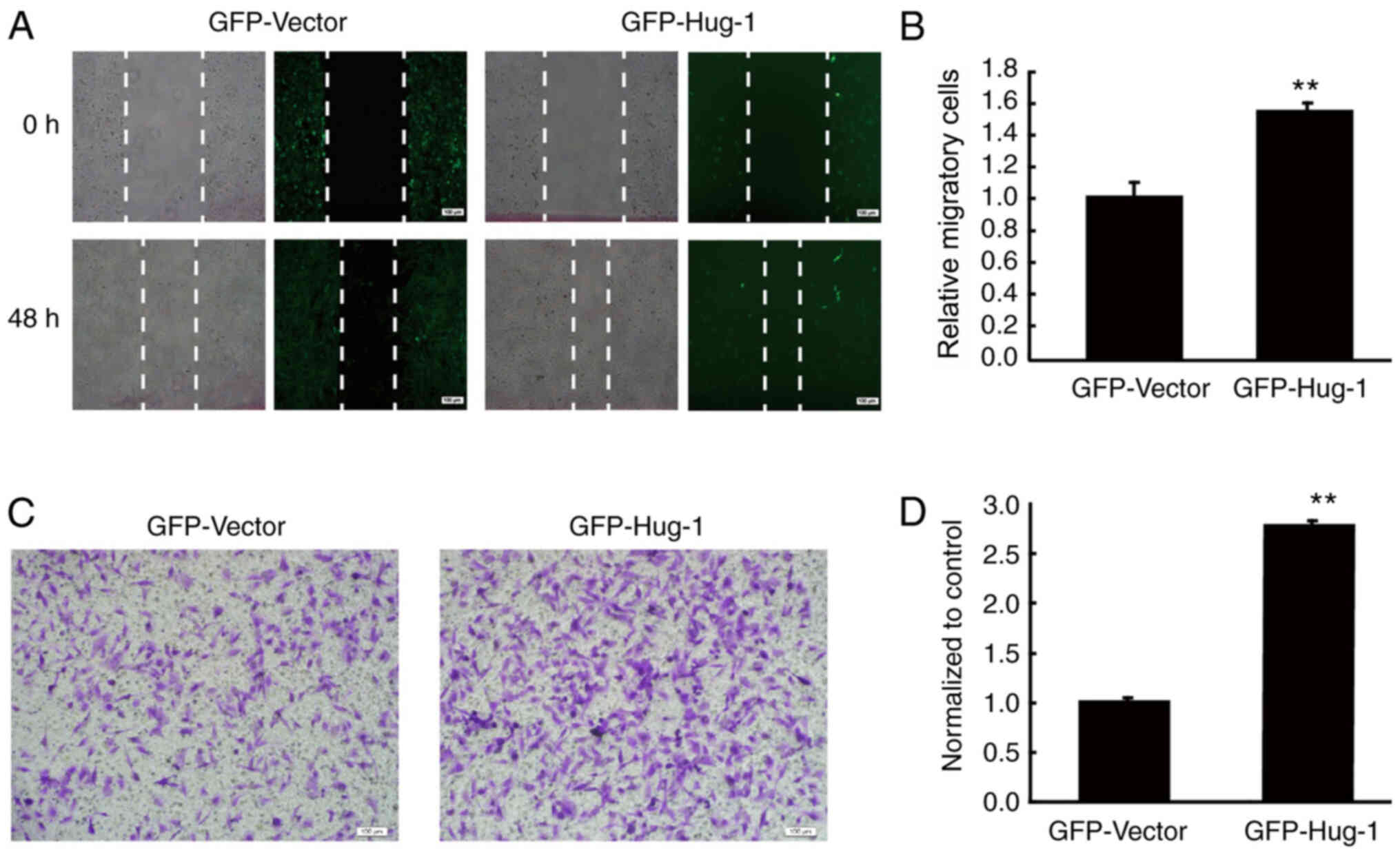
Hugl-1 promotes the migration and
invasion of glioma cells
The effect of overexpressing Hugl-1 on the migration
of glioma cells was assessed via the wound healing assay. The
results demonstrated that Hugl-1 overexpressing glioma cells
exhibited faster wound healing than GFP cells after 48 h (Fig. 3A and B), whereby the number of
migratory cells significantly increased to 30±7% (P<0.01;
Fig. 3A and B). In addition, the
effect of overexpressing Hugl-1 on the invasion of glioma cells was
assessed via the Transwell assay. The results demonstrated that the
number of invasive cells significantly increased to 139±5%
following overexpression of Hugl-1 (P<0.01; Fig. 3C and D), which confirms that Hugl-1
promotes the invasive ability of U251-MG cells.
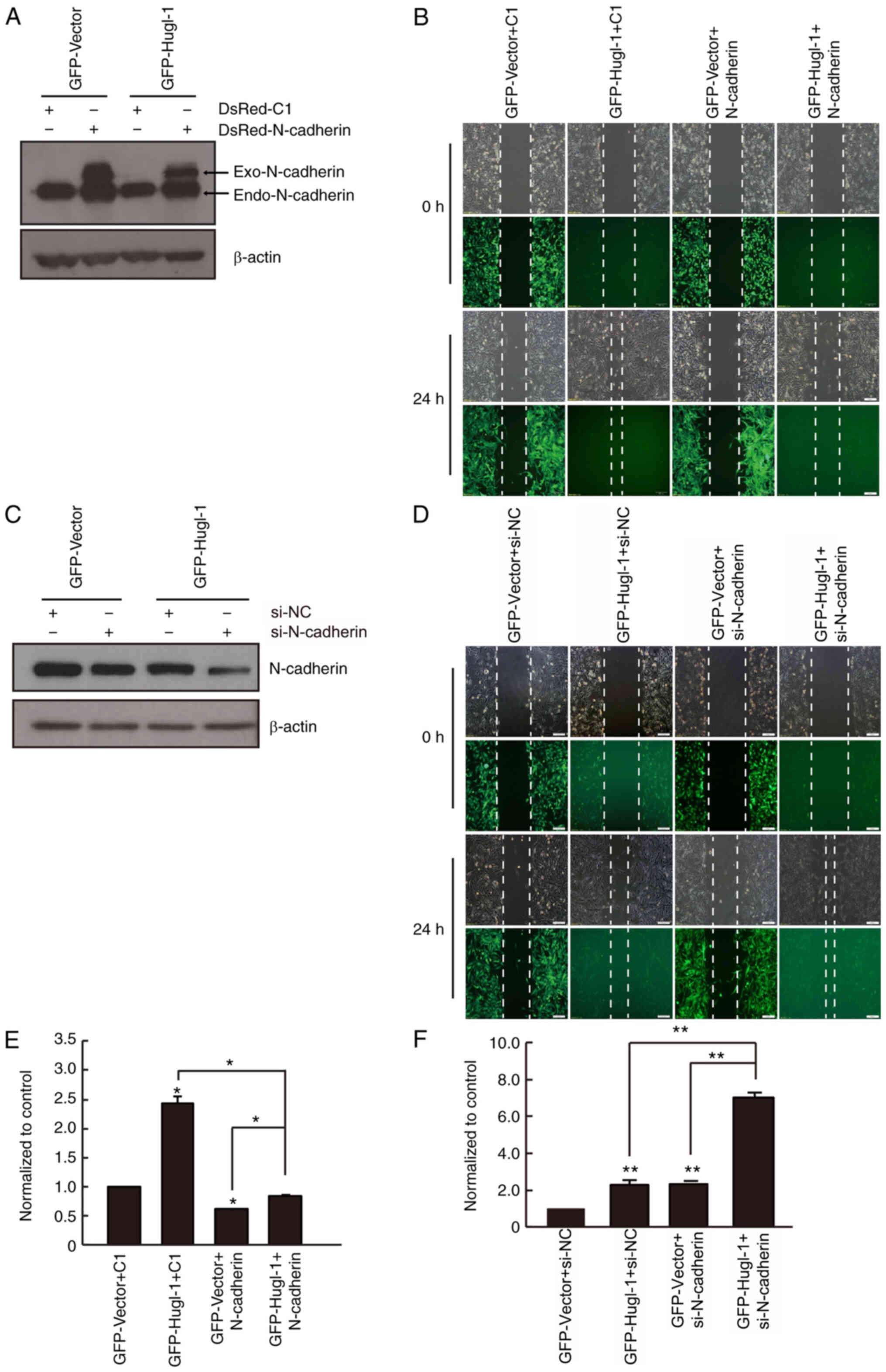
N-cadherin partially mediates the
effects of Hugl-1 expression on glioma cell migration
N-cadherin plays a key role in regulating cell
polarity and motility (27). Based
on the results presented in Fig. 1E,
whether N-cadherin mediates the effects of Hugl-1 expression on
glioma cell migration was subsequently assessed. N-cadherin was
overexpressed in GFP and Hugl-1 overexpressing cells (Fig. 4A), and the results demonstrated that
upregulation of Hugl-1 promoted cell migration, the effects of
which were reversed following overexpression of N-cadherin
(Fig. 4B and E).
Generally, the exogenous protein level is higher
than that of the endogenous level. However, considering the high
molecular weight of GFP-Hugl-1 (141 kDa) and N-cadherin (127 kDa),
and the relatively low transit transfection efficiency (12,13), the
exogenous protein level was lower than the endogenous level in the
present study (Fig. 4A). Notably,
the induction effect of Hugl-1 on glioma cell migration was
partially abolished following overexpression of N-cadherin,
compared with that of the Hugl-1 overexpressing cells (Fig. 4B and E). Conversely, N-cadherin
knockdown promoted cell migration, and the effects were similar to
those noted in the Hugl-1 overexpressing cells (Fig. 4C, D and F). Furthermore, the
increased migratory ability induced by Hugl-1 upregulation was
significantly enhanced following downregulation of N-cadherin in
glioma cells (Fig. 4D and F). Taken
together, these results suggest that N-cadherin partially mediates
the effects of Hugl-1 expression on glioma cell migration.
Discussion
Intercellular adhesion plays a crucial role in the
maintenance of cell polarity to regulate normal tissue architecture
and function (41,42). This process is often disrupted in
neoplastic tumors (43,44). Loss of polarity is considered one of
the trigger signals for tumorigenesis and invasion of surrounding
tissues (45,46). As a cell polarity regulator, Hugl-1
expression is downregulated in several types of human cancer, such
as squamous cell lung carcinoma (15), esophageal carcinoma (47), pancreatic carcinoma (16), endometrial cancer (14) and malignant melanoma (12), and is inversely associated with
patient prognosis (11–15). We previously demonstrated that Hugl-1
can inhibit tumor progression in vivo, while no significant
effects on cell proliferation were observed in vitro
(17).
The results of the present study demonstrated that
overexpression of Hugl-1 decreased cell-cell adhesion, probably by
regulating N-cadherin protein expression. In addition,
overexpression of Hugl-1 promoted glioma cell migration and
invasion. Notably, overexpression or knockdown of N-cadherin
partially suppressed or enhanced the induction effect of Hugl-1
expression on glioma cell migration and invasion, respectively.
Taken together, these results suggest that Hugl-1 promotes
migration and invasion of malignant glioma cells by decreasing
N-cadherin expression, thus Hugl-1 may act as a novel therapeutic
target in patients with GBM, and function as a marker of GBM
prognosis.
Schimanski et al (13) demonstrated that Hugl-1 expression is
lost in 75% of tumor samples and that these deletions are
associated with advanced disease stage, particularly with lymph
node metastasis. Similarly, loss of Hugl-1 expression in
endometrial cancer may contribute to lymph node metastasis
(14). Notably, overexpression of
wild-type Hugl-1 inhibits HCC migration and invasion (11). Kuphal et al (12) reported that upregulation of Hugl-1
increases cell adhesion and decreases cell migration in malignant
melanoma. However, the results of the present study demonstrated
that overexpression of Hugl-1 promoted glioma cell migration and
invasion. Although Hugl-1 expression decreases in malignant
melanoma, HCC and gliomas, it exhibits opposite effects on cell
migration and invasion (promotion versus inhibition) in different
types of tumors (11,12,17).
These differences may be due to the different cell types used in
each experiment under specific conditions. Kuphal et al
(12) and Lu et al (11) used Mel Im or SK-HEP-1 cells, which
are epithelial cells, while the present study used U251-MG glioma
cells, a cell type that belongs to glia-derived cells (48). However, whether the functions of
Hugl-1 are cell-type specific remains unknown and should be
investigated in prospective studies.
Cell migration and invasion include multiple
processes, such as extracellular matrix degradation, cytoskeletal
reorganization, de-adhesion and adhesion (49,50).
Cytoskeletal reorganization is an important process that affects
assembly and disassembly of cell-cell adhesions and leads to
morphological and motility changes of tumor cells (50). It is well-known that low expression
levels of Hugl-1 in gliomas decrease cell-cell adhesion, promote
cell migration and ultimately contribute to cancer cell
dissemination and tumor progression (51). However, the results of the present
study are not consistent with these conclusions, suggesting that
the balance of polar proteins may be the optimum condition for
maintaining cell homeostasis (52).
In addition, the results of the present study demonstrated that
overexpression of Hugl-1 significantly promoted pseudopodia
formation and supported the enhanced cell-extracellular matrix
adhesion. Asano et al (53)
reported that N-cadherin expression is negatively associated with
tumor invasion. The results of the present study demonstrated that
overexpression of Hugl-1 decreased cell-cell adhesion and increased
cell migration, which was consistent with the decreased protein
levels of N-cadherin. Recently, Jossin et al (54) reported that LLGL1 directly binds to
N-cadherin and is able to promote its internalization, while
disrupting the N-cadherin-LLGL1 interaction, which results in
cortical heterotopias. The results of the present study are
consistent with these findings.
In conclusion, the results of the present study
demonstrated that Hugl-1 promoted glioma cell migration and
invasion by decreasing N-cadherin expression. Combined with our
previous studies, the results presented here provide a novel role
for Hugl-1, which includes inhibition of cell proliferation, while
promoting cell migration in glioma, suggesting that Hugl-1 may play
two-sided roles in malignant biological processes. In addition, the
results presented here provide useful information for the clinical
diagnosis of malignant GBM and the prognosis of patients with GBM.
Further studies are required to determine the exact role and
precise molecular mechanism of the cell polarity molecule, Hugl-1,
for the effective treatment of glioma. Although the present study
investigated the role of Hugl-1 in glioma cells, it still presented
some limitations. The current experiments were all completed at the
cellular level; therefore, there is a lack of detection in animal
experiments, which should be further explored in future studies to
confirm the present findings.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81672489, 81872053
and 81902526) and the Postgraduate Research & Practice
Innovation Program of Jiangsu Province (grant no. KYCX20_2460).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW wrote the manuscript and contributed to data
analysis. YZ, BS and XZ performed the experiments. RY contributed
to the study design. XZ contributed to the study design, reviewed
and edited the manuscript. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gould J: Breaking down the epidemiology of
brain cancer. Nature. 561:S40–S41. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meyer MA: Malignant gliomas in adults. N
Engl J Med. 359:18502008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Osswald M and Morais-de-Sá E: Dealing with
apical-basal polarity and intercellular junctions: A
multidimensional challenge for epithelial cell division. Curr Opin
Cell Biol. 60:75–83. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martin-Belmonte F and Perez-Moreno M:
Epithelial cell polarity, stem cells and cancer. Nat Rev Cancer.
12:23–38. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hariharan IK and Bilder D: Regulation of
imaginal disc growth by tumor-suppressor genes in
Drosophila. Annu Rev Genet. 40:335–361. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kashyap A, Zimmerman T, Ergül N,
Bosserhoff A, Hartman U, Alla V, Bataille F, Galle PR, Strand S and
Strand D: The human Lgl polarity gene, Hugl-2, induces MET and
suppresses Snail tumorigenesis. Oncogene. 32:1396–1407. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zimmermann T, Kashyap A, Hartmann U, Otto
G, Galle PR, Strand S and Strand D: Cloning and characterization of
the promoter of Hugl-2, the human homologue of Drosophila
lethal giant larvae (lgl) polarity gene. Biochem Biophys Res
Commun. 366:1067–1073. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grifoni D, Garoia F, Bellosta P, Parisi F,
De Biase D, Collina G, Strand D, Cavicchi S and Pession A: aPKCzeta
cortical loading is associated with Lgl cytoplasmic release and
tumor growth in Drosophila and human epithelia. Oncogene.
26:5960–5965. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ohshiro T, Yagami T, Zhang C and Matsuzaki
F: Role of cortical tumour-suppressor proteins in asymmetric
division of Drosophila neuroblast. Nature. 408:593–596.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vasioukhin V: Lethal giant puzzle of Lgl.
Dev Neurosci. 28:13–24. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu X, Feng X, Man X, Yang G, Tang L, Du D,
Zhang F, Yuan H, Huang Q, Zhang Z, et al: Aberrant splicing of
Hugl-1 is associated with hepatocellular carcinoma progression.
Clin Cancer Res. 15:3287–3296. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuphal S, Wallner S, Schimanski CC,
Bataille F, Hofer P, Strand S, Strand D and Bosserhoff AK:
Expression of Hugl-1 is strongly reduced in malignant melanoma.
Oncogene. 25:103–110. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schimanski CC, Schmitz G, Kashyap A,
Bosserhoff AK, Bataille F, Schäfer SC, Lehr HA, Berger MR, Galle
PR, Strand S and Strand D: Reduced expression of Hugl-1, the human
homologue of Drosophila tumour suppressor gene lgl,
contributes to progression of colorectal cancer. Oncogene.
24:3100–3109. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsuruga T, Nakagawa S, Watanabe M,
Takizawa S, Matsumoto Y, Nagasaka K, Sone K, Hiraike H, Miyamoto Y,
Hiraike O, et al: Loss of Hugl-1 expression associates with lymph
node metastasis in endometrial cancer. Oncol Res. 16:431–435. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsuzaki T, Takekoshi S, Toriumi K,
Kitatani K, Nitou M, Imamura N, Ogura G, Masuda R, Nakamura N and
Iwazaki M: Reduced expression of Hugl 1 contributes to the
progression of lung squamous cell carcinoma. Tokai J Exp Clin Med.
40:169–177. 2015.PubMed/NCBI
|
|
16
|
Biesterfeld S, Kauhausen A, Kost C, Gockel
I, Schimanski CC and Galle PR: Preservation of HUGL-1 expression as
a favourable prognostic factor in pancreatic carcinoma. Anticancer
Res. 32:3153–3159. 2012.PubMed/NCBI
|
|
17
|
Liu X, Lu D, Ma P, Liu H, Cao Y, Sang B,
Zhu X, Shi Q, Hu J, Yu R and Zhou X: Hugl-1 inhibits glioma cell
growth in intracranial model. J Neurooncol. 125:113–121. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Massimi P, Narayan N, Thomas M, Gammoh N,
Strand S, Strand D and Banks L: Regulation of the
hDlg/hScrib/Hugl-1 tumour suppressor complex. Exp Cell Res.
314:3306–3317. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhong XL and Rescorla FJ: Cell surface
adhesion molecules and adhesion-initiated signaling: Understanding
of anoikis resistance mechanisms and therapeutic opportunities.
Cell Signal. 24:393–401. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fife CM, McCarroll JA and Kavallaris M:
Movers and shakers: Cell cytoskeleton in cancer metastasis. Brit J
Pharmacol. 171:5507–5523. 2014. View Article : Google Scholar
|
|
21
|
Gloushankova NA, Rubtsova SN and Zhitnyak
IY: Cadherin-mediated cell-cell interactions in normal and cancer
cells. Tissue Barriers. 5:e13569002017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pal M, Bhattacharya S, Kalyan G and Hazra
S: Cadherin profiling for therapeutic interventions in Epithelial
Mesenchymal Transition (EMT) and tumorigenesis. Exp Cell Res.
368:137–146. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nair KS, Naidoo R and Chetty R: Expression
of cell adhesion molecules in oesophageal carcinoma and its
prognostic value. J Clin Pathol. 58:343–351. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang C, Kratzer MC, Wedlich D and Kashef
J: E-cadherin is required for cranial neural crest migration in
Xenopus laevis. Dev Biol. 411:159–171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bremmer F, Schallenberg S, Jarry H, Küffer
S, Kaulfuss S, Burfeind P, Strauß A, Thelen P, Radzun HJ, Ströbel
P, et al: Role of N-cadherin in proliferation, migration, and
invasion of germ cell tumours. Oncotarget. 6:33426–33437. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mrozik KM, Blaschuk OW, Cheong CM,
Zannettino ACW and Vandyke K: N-cadherin in cancer metastasis, its
emerging role in haematological malignancies and potential as a
therapeutic target in cancer. BMC Cancer. 18:9392018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Camand E, Peglion F, Osmani N, Sanson M
and Etienne-Manneville S: N-cadherin expression level modulates
integrin-mediated polarity and strongly impacts on the speed and
directionality of glial cell migration. J Cell Sci. 125:844–857.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gheldof A and Berx G: Cadherins and
epithelial-to-mesenchymal transition. Prog Mol Biol Transl Sci.
116:317–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kourtidis A, Lu R, Pence LJ and
Anastasiadis PZ: A central role for cadherin signaling in cancer.
Exp Cell Res. 358:78–85. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kashima T, Kawaguchi J, Takeshita S,
Kuroda M, Takanashi M, Horiuchi H, Imamura T, Ishikawa Y, Ishida T,
Mori S, et al: Anomalous cadherin expression in osteosarcoma.
Possible relationships to metastasis and morphogenesis. Am J
Pathol. 155:1549–1555. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Han ZX, Wang XX, Zhang SN, Wu JX, Qian HY,
Wen YY, Tian H, Pei DS and Zheng JN: Downregulation of PAK5
inhibits glioma cell migration and invasion potentially through the
PAK5-Egr1-MMP2 signaling pathway. Brain Tumor Pathol. 31:234–241.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li F, Jin D, Guan L, Zhang CC, Wu T, Wang
YJ and Gao DS: CEP55 promoted the migration, invasion and
neuroshpere formation of the glioma cell line U251. Neurosci Lett.
705:80–86. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang T, Liu Y, Xu XH, Deng CY, Wu KY, Zhu
J, Fu XQ, He M and Luo ZG: Lgl1 activation of rab10 promotes axonal
membrane trafficking underlying neuronal polarization. Dev Cell.
21:431–444. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wicki A, Lehembre F, Wick N, Hantusch B,
Kerjaschki D and Christofori G: Tumor invasion in the absence of
epithelial-mesenchymal transition: Podoplanin-mediated remodeling
of the actin cytoskeleton. Cancer Cell. 9:261–272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li N, Chen G, Liu J, Xia Y, Chen H, Tang
H, Zhang F and Gu N: Effect of surface topography and bioactive
properties on early adhesion and growth behavior of mouse
preosteoblast MC3T3-E1 cells. ACS Appl Mater Interfaces.
6:17134–17143. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lewczuk Ł, Pryczynicz A and
Guzińska-Ustymowicz K: Cell adhesion molecules in endometrial
cancer-A systematic review. Adv Med Sci. 64:423–429. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
McKeown SJ, Wallace AS and Anderson RB:
Expression and function of cell adhesion molecules during neural
crest migration. Dev Biol. 373:244–257. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhan T, Rindtorff N and Boutros M: Wnt
signaling in cancer. Oncogene. 36:1461–1473. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Taciak B, Pruszynska I, Kiraga L, Bialasek
M and Krol M: Wnt signaling pathway in development and cancer. J
Physiol Pharmacol. 69:2018.PubMed/NCBI
|
|
40
|
Li ZH, Zhou Y, Ding YX, Guo QL and Zhao L:
Roles of integrin in tumor development and the target inhibitors.
Chin J Nat Med. 17:241–251. 2019.PubMed/NCBI
|
|
41
|
Li JC, Cheng LC and Jiang HY: Cell shape
and intercellular adhesion regulate mitotic spindle orientation.
Mol Biol Cell. 30:2458–2468. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mack NA and Georgiou M: The
interdependence of the Rho GTPases and apicobasal cell polarity.
Small GTPases. 5:102014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bonastre E, Brambilla E and
Sanchez-Cespedes M: Cell adhesion and polarity in squamous cell
carcinoma of the lung. J Pathol. 238:606–616. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Waghmare I and Kango-Singh M: Loss of cell
adhesion increases tumorigenic potential of polarity deficient
scribble mutant cells. PLoS One. 11:e01580812016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wan S, Meyer AS, Weiler SME, Rupp C, Tóth
M, Sticht C, Singer S, Thomann S, Roessler S, Schorpp-Kistner M, et
al: Cytoplasmic localization of the cell polarity factor scribble
supports liver tumor formation and tumor cell invasiveness.
Hepatology. 67:1842–1856. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bhattacharya S: Cell polarity: A link to
epithelial-mesenchymal transition and vascular mimicry. Crit Rev
Eukar Gene. 28:101–105. 2018. View Article : Google Scholar
|
|
47
|
Song J, Peng XL, Ji MY, Ai MH, Zhang JX
and Dong WG: Hugl-1 induces apoptosis in esophageal carcinoma cells
both in vitro and in vivo. World J Gastroenterol. 19:4127–4136.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ke LD, Shi YX and Yung WK: VEGF(121),
VEGF(165) overexpression enhances tumorigenicity in U251 MG but not
in NG-1 glioma cells. Cancer Res. 62:1854–1861. 2002.PubMed/NCBI
|
|
49
|
Painter KJ, Armstrong NJ and Sherratt JA:
The impact of adhesion on cellular invasion processes in cancer and
development. J Theor Biol. 264:1057–1067. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cavallaro U and Christofori G: Cell
adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev
Cancer. 4:118–132. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Grifoni D, Garoia F, Schimanski CC,
Schmitz G, Laurenti E, Galle PR, Pession A, Cavicchi S and Strand
D: The human protein Hugl-1 substitutes for Drosophila
lethal giant larvae tumour suppressor function in vivo. Oncogene.
23:8688–8694. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yu FX, Zhao B and Guan KL: Hippo pathway
in organ size control, tissue homeostasis, and cancer. Cell.
163:811–828. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Asano K, Duntsch CD, Zhou Q, Weimar JD,
Bordelon D, Robertson JH and Pourmotabbed T: Correlation of
N-cadherin expression in high grade gliomas with tissue invasion. J
Neurooncol. 70:3–15. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jossin Y, Lee M, Klezovitch O, Kon E,
Cossard A, Lien WH, Fernandez TE, Cooper JA and Vasioukhin V: Llgl1
connects cell polarity with cell-cell adhesion in embryonic neural
stem cells. Dev Cell. 41:481–495.e5. 2017. View Article : Google Scholar : PubMed/NCBI
|