Introduction
Gastrointestinal stromal tumors (GISTs) are the most
frequently occurring mesenchymal tumors of the digestive tract and
are characterized by differentiation from the interstitial cells of
Cajal (1). GISTs predominantly
(60-70%) arise in the stomach, followed by the small bowel (20-30%)
(2). The pathological diagnosis of
GISTs is based on various morphological manifestations together
with sensitive and specific markers, including CD117, discovered on
GIST-1 (DOG1, also known as anoctamin 1) and CD34, identified by
immunostaining (3). GISTs represent
a wide spectrum of tumors, with variable disease behaviors
associated with tumor size, mitotic activity and anatomical origin
(3–5). According to these three
clinicopathological features, several recurrence risk assessment
systems have been developed and used for primary GISTs. The Chinese
Society of Clinical Oncology Expert Committee recommends the
modified US National Institutes of Health (NIH) classification,
which they consider to be particularly suitable for Asian
populations (6,7). In addition, the World Health
Organization (WHO) classification recommends the US Armed Forced
Institute of Pathology criteria, which are classified into eight
grades (grades 1, 2, 3a, 3b, 4, 5, 6a and 6b) (8,9).
It has been reported that 82–87% of GISTs harbor
gain-of-function mutations in the KIT proto-oncogene, receptor
tyrosine kinase (KIT) or platelet derived growth factor
receptor α (PDGFRA) oncogenes encoding type III receptor
tyrosine kinases (10–12). KIT mutations have been
reported to occur in 69–83% of all GISTs and PDGFRA
mutations have been detected in 12.9-14.0% of primary GISTs
(10,12,13). In
adults, ~15% of GISTs without detectable mutations in KIT or
PDGFRA are considered wild-type (WT) GISTs (14). The most frequent site of KIT
mutation is at the 5′ end of exon 11; this encodes the JM domain
which has an autoinhibitory function under ligand-free conditions.
Mutations of exon 11 disrupt this autoinhibitory function and
thereby result in ligand-independent receptor activation (15). Several studies have demonstrated that
exon 11 deletions of KIT are associated with a high risk of
relapse and metastasis. In particular, GISTs with deletions
affecting codons 557/558 exhibit a higher risk of progression
(10,11,16,17).
However, in comparison with KIT deletions, KIT exon
11 substitutions indicate an improved patient outcome (10,18).
Furthermore, GISTs with PDGFRA exon 18 mutations have a
lower risk of relapse than those with KIT deletions
(11). However, although the roles
of KIT and PDGFRA mutations in the assessment of the
response to imatinib therapy are well documented (3,7,9), the prognostic significance of these
mutations in the Chinese population has yet to be defined.
Furthermore, whether genotype analysis should be considered as an
additional prognostic approach is currently unclear.
Therefore, the aim of the present study was to
analyze the clinicopathological and mutational characteristics of
GISTs and evaluate the prognostic significance of these parameters
in a large cohort of 302 cases in North China. The findings may be
helpful for risk assessment and personalized targeted therapy.
Materials and methods
Patients and samples
A series of 302 GIST cases was retrospectively
collected from records archived in the Department of Pathology,
Peking University First Hospital (Beijing, China). The cases were
diagnosed between May 2009 and June 2019, and represent ~2.1% of
all gastrointestinal malignancies at this center. Inclusion and
exclusion criteria were as follows. Patients with primary GIST
diagnosis and primary therapy-naive tumors with curative resection
were eligible (R0 or R1). The information on clinicopathological
factors, follow-up data and the mutational status of KIT and
PDGFRA were available. Patients undergoing neoadjuvant
imatinib or chemoradiotherapy for GIST before surgery were
excluded. GIST was diagnosed based on histopathological features,
immunochemical findings and genotype according to the 5th edition
of the WHO classification (9). The
study was approved by the ethics committee of Peking University
First Hospital and was conducted in compliance with the Declaration
of Helsinki. Written patient consent for use of their tissues in
research was obtained.
For each case, the histological assessment included
location, tumor size, mitotic count per 50 high-power fields (HPF;
equivalent to 5 mm2), cell type and the presence or
absence of rupture. Risk was stratified and prognostic grades were
evaluated according to the modified NIH consensus and WHO
classification, respectively (6,9).
Immunohistochemical staining was carried out on 4-µm
thick sections form a total of 302 cases of paraffin-embedded
tissue blocks which were fixed using 10% formalin at room
temperature for 24 h. Briefly, tissue sections were incubated at
65°C for 10 min, followed by two 10-min cycles of deparaffinization
using xylene and then hydration in a graded ethanol series (100,
100, 95, 80 and 70% for 2 min, respectively). They were pretreated
to promote antigen retrieval in EDTA-Tris (pH 9.0) at 95°C for 20
min (PT Link; Dako; Agilent Technologies, Inc.) and were treated
with 3% hydrogen peroxide for 10 min to block endogenous
peroxidase. Tissues were subsequently incubated with primary
antibodies for 50 min at room temperature. The panel of protein
immunostained for GIST diagnosis was as follows: CD117 (working
solution, Maixin), DOG1 (1:100; ZSGB-BIO), CD34 (1:200; ZSGB-BIO),
Ki67 (1:100; ZSGB-BIO), S-100 (1:200; ZSGB-BIO), SDHB (1:100;
ZSGB-BIO), smooth muscle actin (1:200; ZSGB-BIO) and desmin (1:100;
ZSGB-BIO). Sections were incubated with secondary antibody using a
EnVision™, FLEX+, High pH kit (cat. no. K8002; Dako; Agilent
Technologies, Inc.) according to the manufacturer's instructions.
Strong positive expression of CD117, DOG1, CD34 or SDHB in >50%
of the tumor tissue was defined as cytoplasmic immunopositivity by
eye under a light microscope.
Follow-up were performed for 259 GIST cases and
patient information was obtained by regular outpatient visits or by
telephone. Relapse-free survival (RFS) was defined as the duration
from surgery to relapse (local recurrence or metastasis). Relapse
was identified based on biopsy and/or imaging assessment.
Gene mutation analysis
Genomic DNA was extracted from formalin-fixed,
paraffin-embedded tissue using a DNeasy Blood & Tissue Kit
(Qiagen, Inc.). Prior to extraction, histological assessment was
performed to ensure that the percentage of tumor in the specimens
by area was >80%. Mutational analysis of KIT and
PDGFRA was carried out by PCR amplification, followed by
Sanger sequencing of the amplified products. Briefly, initial
amplification was performed using Takara LA Taq polymerase (cat.
no. RR02MA; Takara Bio, Inc.). The PCR amplification program was as
follows: Denaturation at 94°C for 5 min, 45 cycles of denaturation
at 94°C for 30 sec, annealing at 56°C for 45 sec, extension at 72°C
for 20 sec, and finally, incubation at 72°C for 10 min. The
sequencing reaction products were electrophoresed on an ABI3700
genetic analyzer (Applied Biosystems; Thermo Fisher Scientific,
Inc.). KIT exons 9, 11, 13 and 17, and PDGFRA exons
12, 14 and 18 were analyzed. The primer sequences used are shown in
Table SI.
Statistical analysis
For univariate analysis, the χ2 test or
Fisher's exact test was used to compare categorical variables.
Survival analysis was carried out using the Kaplan-Meier method,
and statistical significance was determined using the log-rank
test. Univariate and multivariate Cox proportional hazard models
were used to determine the prognostic impact of variables on RFS.
The strengths of the associations are shown as hazard ratios (HRs)
and corresponding 95% confidence intervals (CIs). A 2-sided
P<0.05 was considered to indicate a statistically significant
result. Analyses were performed using IBM SPSS Statistics V23.0
software (IBM Corp.).
Results
Clinicopathological
characteristics
The detailed clinical and pathological
characteristics of the 302 patients with GISTs are provided in
Table I. There were 160 males
(53.0%) and 142 females (47.0%), who ranged in age from 13 to 84
years (median, 61.8 years), with patients >60 years old
accounting for 54.3% of cases. The majority of tumors were located
in the stomach (66.9%), and the small intestine was the second most
common location (25.5%). Only 14 cases (4.6%) had tumors in the
colon or rectum, and the remaining locations (3.0%) involved the
prostate (1 case), retroperitoneum (2 cases), abdominal cavity (5
cases) and pelvic cavity (1 case). The tumor size ranged from 0.3
to 26.0 cm (median, 4.2 cm) at initial diagnosis, and was ≤5 cm in
most cases (60.6%) and >10 cm in 41 cases (13.6%). However, the
sizes of the tumors in non-gastric sites were larger than those in
the stomach. Most GISTs (75.8%) exhibited low mitotic activity
(≤5/50 HPF), with no marked difference between gastric and
non-gastric sites. Tumor necrosis was observed in 67 cases (22.2%),
and was predominant in non-gastric sites. Only 2 cases (0.7%) had a
ruptured tumor. The predominant morphology was spindle variant
(87.4%), and only 9 cases (3.0%) presented epithelioid histology,
which was more frequently seen in tumors in the stomach. The
remaining 29 cases (9.6%) featured a combination of spindle and
epithelioid morphology (mixed variant).
 | Table I.Clinicopathological and mutational
characteristics of GISTs (n=302). |
Table I.
Clinicopathological and mutational
characteristics of GISTs (n=302).
|
Characteristics | All cases
(n=302) | Gastric
(n=202) | Non-gastric
(n=100) |
|---|
| Sex |
|
Male | 160 (53.0) | 94 (46.5) | 66 (66.0) |
|
Female | 142 (47.0) | 108 (53.5) | 34 (34.0) |
| Age (years) |
| Median
(range) | 61.8 (13–84) | 62.5 (13–82) | 59.8 (31–84) |
|
≤60 | 138 (45.7) | 85 (42.1) | 53 (53.0) |
|
>60 | 164 (54.3) | 117 (57.9) | 47 (47.0) |
| Site |
|
Stomach | 202 (66.9) | 202 (100.0) | NA |
| Small
intestine | 77 (25.5) | NA | 77 (77.0) |
| Colon
or rectum | 14 (4.6) | NA | 14 (14.0) |
|
Others | 9 (3.0) | NA | 9 (9.0) |
| Tumor dimension
(cm) |
| Median
(range) | 4.2 (0.3-26.0) | 3.4 (0.3-21.0) | 5.9 (1.5-26.0) |
| ≤2 | 62 (20.5) | 56 (27.7) | 6 (6.0) |
| >2
to≤ 5 | 121 (40.1) | 85 (42.1) | 36 (36.0) |
| >5
to ≤10 | 78 (25.8) | 42 (20.8) | 36 (36.0) |
|
>10 | 41 (13.6) | 19 (9.4) | 22 (22.0) |
| Mitotic count
(/50HPF) |
| Median
(range) | 2.4 (0–80) | 2.4 (0–60) | 2.4 (0–80) |
| ≤5 | 229 (75.8) | 157 (77.7) | 72 (72.0) |
|
>5 | 73 (24.2) | 45 (22.3) | 28 (28.0) |
| Necrosis |
|
Present | 67 (22.2) | 31 (15.3) | 36 (36.0) |
|
Absent | 235 (77.8) | 171 (84.7) | 64 (64.0) |
| Morphology |
|
Spindle | 264 (87.4) | 181 (89.6) | 83 (83.0) |
|
Epithelioid | 9 (3.0) | 6 (3.0) | 3 (3.0) |
|
Mixed | 29 (9.6) | 15 (7.4) | 14 (14.0) |
| Risk
stratification |
| Very
low | 58 (19.2) | 53 (26.2) | 5 (5.0) |
|
Low | 104 (34.4) | 70 (34.7) | 34 (34.0) |
|
Intermediate | 52 (17.2) | 45 (22.3) | 7 (7.0) |
|
High | 88 (29.1) | 34 (16.8) | 54 (54.0) |
| WHO grade |
| 1 | 58 (19.2) | 53 (26.2) | 5 (5.0) |
| 2 | 108 (35.8) | 74 (36.6) | 34 (34.0) |
| 3a | 50 (16.6) | 26 (12.9) | 24 (24.0) |
| 3b | 14 (4.6) | 5 (2.5) | 9 (9.0) |
| 4 | 4 (1.3) | 3 (1.5) | 1 (1.0) |
| 5 | 14 (4.6) | 12 (5.9) | 2 (2.0) |
| 6a | 25 (8.3) | 14 (6.9) | 11 (11.0) |
| 6b | 29 (9.6) | 15 (7.4) | 14 (14.0) |
| CD117
immunostaining |
|
Positive | 287 (95.0) | 191 (94.6) | 96 (96.0) |
|
Negative | 15 (5.0) | 11 (5.4) | 4 (4.0) |
| DOG1
immunostaining |
|
Positive | 282 (93.4) | 187 (92.6) | 95 (95.0) |
|
Negative | 20 (6.6) | 15 (7.4) | 5 (5.0) |
| CD34
immunostaining |
|
Positive | 245 (81.1) | 192 (95.0) | 53 (53.0) |
|
Negative | 57 (18.9) | 10 (5.0) | 47 (47.0) |
| KIT
mutation |
| WT | 69 (22.8) | 55 (27.2) | 14 (14.0) |
| Exon
9 | 16 (5.3) | 4 (2.0) | 12 (12.0) |
| Exon
11 | 210 (69.5) | 139 (68.8) | 71 (71.0) |
|
Substitution | 79 (26.2) | 58 (28.7) | 21 (21.0) |
|
Deletiona | 110 (36.4) | 67 (33.2) | 43 (43.0) |
|
Duplication | 21 (7.0) | 14 (6.9) | 7 (7.0) |
| Exon
13 | 4 (1.3) | 2 (1.0) | 2 (2.0) |
| Exon
17 | 3 (1.0) | 2 (1.0) | 1 (1.0) |
| PDGFRA
mutation |
| WT | 272 (90.1) | 176 (87.1) | 96 (96.0) |
| Exon
12 | 1 (0.3) | 0 (0.0) | 1 (1.0) |
| Exon
14 | 2 (0.7) | 2 (1.0) | 0 (0.0) |
| Exon
18 | 27 (8.9) | 24 (11.9) | 3 (3.0) |
| WT
KIT/PDGFRA | 39 (12.9) | 29 (14.4) | 10 (10.0) |
Strong expression of CD117, DOG1 and CD34 was
detected in the majority of GIST cases (95.0, 93.4 and 81.1%,
respectively). Among these cases, triple expression was detected in
219 cases (72.5%), double expression in 74 cases (24.5%) and single
expression in 9 cases (3.0%). No triple-negative cases were
observed in the study. Notably, CD34 expression was more frequently
detected in tumors located in the stomach than in other sites (78.4
vs. 21.6%), and the loss of CD34 expression was predominant in
specimens with epithelioid and mixed variant morphology (33.3 and
34.5%, respectively).
Based on the modified NIH consensus (6), as shown in Table I, more than half of cases were
assessed as low or very low risk (162/302, 53.6%). There was very
low risk of relapse in 19.2% of cases, in which the tumors were
predominantly located in gastric sites. There was a high risk of
relapse in 88 cases (29.1%), and this assessment was more frequent
for non-gastric GISTs than for gastric GISTs. Correspondingly,
based on the prognostic assessment recommended by the WHO
classification (9), grade 6b tumors
were predominantly located outside of the stomach, while grade 1
tumors were more frequently detected in the stomach (Table I).
Genotype analysis
Analyses of KIT and PDGFRA mutations
were performed for all 302 GIST specimens in the present study. In
total, KIT and PDGFRA mutations were identified in
233 (77.2%) and 30 (9.9%) cases, respectively, and WT KIT
and PDGFRA were found in 39 specimens (12.9%). The
mutational landscape is presented in Tables I and II. The predominant genotype was KIT
exon 11 deletion (36.4%), followed by KIT exon 11
substitution (26.2%), PDGFRA exon 18 substitution (7.3%) and
KIT exon 11 duplication (7.0%).
 | Table II.Associations between KIT
mutations and clinicopathological characteristics of patients with
GISTs (n=302). |
Table II.
Associations between KIT
mutations and clinicopathological characteristics of patients with
GISTs (n=302).
|
| KIT exon11
mutation, n (%) |
| KIT mutation, n
(%) |
|
|---|
|
|
|
|
|
|
|---|
|
Characteristics | Deletion
(n=110) | Substitution
(n=79) | P-value | Del 557/558/559
(n=86) | Others (n=147) | P-value |
|---|
| Sex |
|
Male | 57 (51.8) | 37 (46.8) | 0.499 | 48 (55.8) | 74 (50.3) | 0.419 |
|
Female | 53 (48.2) | 42 (53.2) |
| 38 (44.2) | 73 (49.7) |
|
| Age (years) |
|
≤60 | 51 (46.4) | 30 (38.0) | 0.250 | 40 (46.5) | 62 (42.2) | 0.520 |
|
>60 | 59 (53.6) | 49 (62.0) |
| 46 (53.5) | 85 (57.8) |
|
| Site |
|
Gastric | 67 (60.9) | 58 (73.4) | 0.073 | 54 (62.8) | 93 (63.3) | 0.942 |
|
Non-gastric | 43 (39.1) | 21 (26.6) |
| 32 (37.2) | 54 (36.7) |
|
| Tumor dimension
(cm) |
| ≤2 | 15 (13.6) | 15 (19.0) | 0.051 | 12 (14.0) | 24 (16.3) | 0.449 |
| >2
to ≤ 5 | 44 (40.0) | 42 (53.2) |
| 34 (39.5) | 64 (43.5) |
|
| >5
to ≤10 | 30 (27.3) | 16 (20.3) |
| 22 (25.6) | 40 (27.2) |
|
|
>10 | 21 (19.1) | 6 (7.6) |
| 18 (20.9) | 19 (12.9) |
|
| Mitotic count
(/50HPFa) |
| ≤5 | 68 (61.8) | 66 (83.5) | 0.001 | 50 (58.1) | 117 (79.6) | <0.001 |
|
>5 | 42 (38.2) | 13 (16.5) |
| 36 (41.9) | 30 (20.4) |
|
| Necrosis |
|
Present | 35 (31.8) | 12 (15.2) | 0.009 | 27 (31.4) | 33 (22.4) | 0.132 |
|
Absent | 75 (68.2) | 67 (84.8) |
| 59 (68.6) | 114 (77.6) |
|
| Morphology |
|
Spindle | 97 (88.2) | 75 (94.9) | 0.246 | 74 (86.0) | 137 (93.2) | 0.072 |
|
Epithelioid | 1 (0.9) | 0 (0.0) |
| 1 (1.2) | 1 (0.7) |
|
|
Mixed | 12 (10.9) | 4 (5.1) |
| 11 (12.8) | 9 (6.1) |
|
| Risk
stratification |
| Not
highb | 66 (60.0) | 64 (81.0) | 0.002 | 50 (58.1) | 106 (72.1) | 0.029 |
|
High | 44 (40.0) | 15 (19.0) |
| 36 (41.9) | 41 (27.9) |
|
| WHO grade |
|
1/2/3a/4 | 68 (61.8) | 63 (79.7) | 0.008 | 50 (58.1) | 110 (74.8) | 0.008 |
|
3b/5/6a/6b | 42 (38.2) | 16 (20.3) |
| 36 (41.9) | 37 (25.2) |
|
As shown in Fig. 1B,
the spectrum of the exon 11 mutations involved codons ranging from
codon 548 to 589. Among these codons, the more frequent mutations
were found in codons 557–560, which harbored deletion and/or
substitution subtypes, and the less frequent mutations were
observed in codons 572–580, which exhibited duplication mutations.
The length of the in-frame deletion ranged from 3 to 15 bp. The
most prevalent type of deletion, involving codons 557/558/559, was
identified in 86 cases (28.5%). Substitutions mostly involved
codons 559 (n=36; p.V559D, p.V559A, p.V559G), 560 (n=20; p.V560D,
p.V560E, p.V560G), 576 (n=9; p.L576P) and 557 (n=8; p.W557R,
p.W557G).
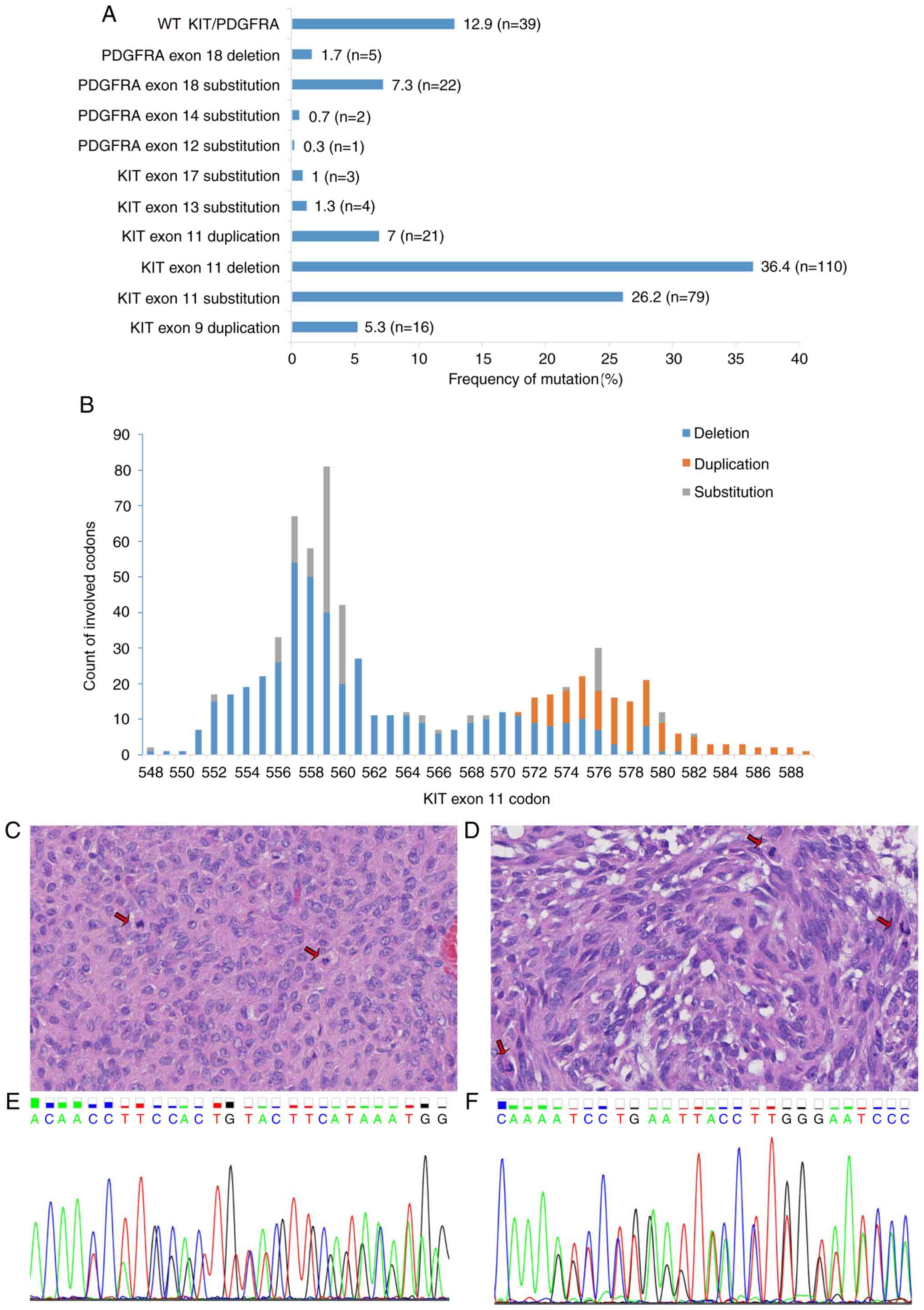 | Figure 1.Spectrum of KIT and
PDGFRA mutations in 302 cases of GISTs. (A) Distribution of
KIT and PDGFRA mutations. KIT exon 11 deletion
was the most common genotype (36.4%, n=110) among the GISTs. Less
frequent mutations included KIT exon 11 substitution (26.2%,
n=79) and PDGFRA exon 18 substitution (7.3%, n=22).
KIT exon 17 substitution, PDGFRA exon 12 substitution
and exon 14 substitution were rare genotypes. (B) Counts of
KIT exon 11 codons affected by deletion, duplication and
substitution are depicted. Deletions and substitutions frequently
involved codons 557–560, whereas duplication was observed in codons
572–580. (C-F) One patient with a GIST exhibited the coexistence of
KIT exon 11 deletion and exon 13 duplication. The specimen
of this patient presented (C) epithelioid and (D) spindle
morphology and high mitotic activity (arrows). Hematoxylin and
eosin staining (magnification, ×400). In this patient, the (E)
in-frame deletion of KIT exon 11 (codons 553–558) and (F)
in-frame insertion of exon 13 (one base pair insertion between
codons 642 and 643) were detected. KIT, KIT proto-oncogene,
receptor tyrosine kinase; PDGFRA, platelet derived growth factor
receptor α; GIST, gastrointestinal stromal tumor. |
In-frame duplication of exon 9 was detected in 16
cases (5.3%), and comprised p.A502_Y503dup (n=15) and
p.F506_F508dup (n=1). There were 4 cases with exon 13 substitution
(all p.K642E) and 3 cases with exon 17 substitutions (p.N822K,
p.N822Y). Notably, in one tumor, the coexistence of exon 11
p.Y553_K558del and exon 13 duplication (one base pair insertion
between codons 642 and 643) at a hotspot mutation site was
observed. This tumor, whose size was >10 cm and mitotic count
was >5/50 HPF, presented spindle and epithelioid morphology, and
was classified as NIH high risk and high WHO grade 6b (Fig. 1C-F). Whether this patient received
adjuvant therapy after resection is unknown.
The most frequent type of PDGFRA mutation
comprised exon 18 substitutions involving codons 842 (n=21;
p.D842V, p.D842T, p.D842Y) and 839(n=1;p.L839Q). Exon 18 deletions
were also found in 5 cases (p.D842_H845del, p.M844_S847del,
p.M844_D846del). In addition, PDGFRA exon 12 (p.Y555C) and
exon 14 (p.N659K) substitutions were detected in 1 and 2 cases,
respectively.
Genotype and clinicopathological
characteristics
The associations between genotype and
clinicopathological characteristics are presented in Tables I and II. The KIT exon 11 substitution
subtype was commonly detected in tumors at gastric sites (58/79,
73.4%), while KIT exon 9 duplication was more frequently
found in tumors at non-gastric sites (12/16, 75%). In addition,
PDGFRA exon 18 substitutions and deletions were more
frequent in tumors of the stomach (24/27, 88.9%) than in those of
other sites. Larger (>10 cm) tumors were more likely to bear
GISTs with KIT exon 11 deletions than exon 11 substitutions.
KIT exon 11 deletions were also more frequent than
KIT exon 11 substitutions in tumors with high mitotic counts
(>5/50 HPF), a high risk of relapse and high (3b/5/6a/6b) WHO
grades (Table II; all P<0.05).
Deletions involving KIT codons 557/558/559 were more
frequently identified in gastric GISTs than in non-gastric GISTS
(62.8 vs. 37.2%, respectively). Furthermore, when compared with
cases with other KIT mutations, cases with exon 11 deletions
involving codons 557/558/559 were significantly associated with
worse clinicopathological features: High mitotic activity, high
risk according to the NIH classification and high WHO grade
(Table II; all P<0.05). These
findings suggested that KIT exon 11 deletions, particularly
those involving codons 557/558/559, may contribute to poor
prognosis.
In the present study, 6 cases had two nodular masses
that were located in different sites of the abdominal cavity at the
initial diagnosis of the primary tumor. Their clinicopathological
features and genotypes are provided in Table III. Notably, in 4 cases (cases 1,
2, 5 and 6), specimens from the two different locations presented
distinct morphological appearances and genetic alterations.
However, in the other two cases (cases 3 and 4), the same
morphological manifestations and genotypes were observed in
specimens from both locations. In case 1, the primary tumor in the
greater curvature of the stomach had spindle morphology, strong
CD117 expression and KIT p.V560D mutation, whereas the other
mass in the lesser curvature of the stomach was an epithelioid
variant with loss of CD117 expression and WT KIT (Fig. 2). In case 6, as depicted in Fig. 3, most of the specimens of the tumor
in the stomach (case 6A) exhibited anaplastic/pleomorphic
morphology with multinucleated giant cells, high mitotic activity
and necrosis, but the tumor located between the stomach and spleen
(case 6B) predominantly exhibited spindle histology. The deletion
of KIT codon 579 occurred in the former, while KIT
substitution (p.W557G) occurred in the latter. Furthermore, CD34
expression was lost in the specimen of case 6A but not in that of
case 6B. Thus, GISTs presented heterogeneous histopathological,
immunostaining and gene mutation features, suggesting the need for
comprehensive mutation detection and highlighting new challenges
for diagnosis and therapy.
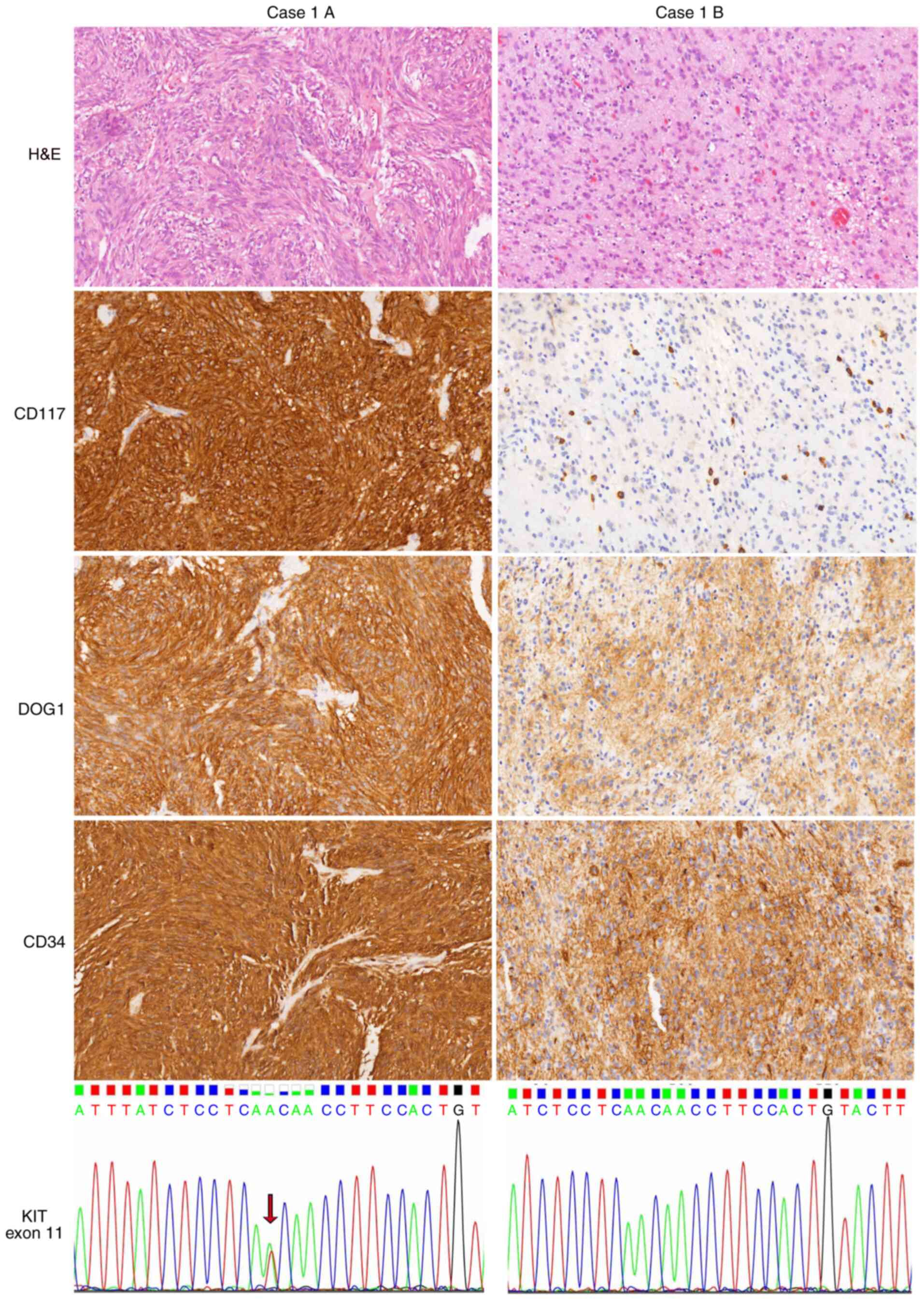 | Figure 2.Heterogeneity of GISTs in one patient
(case 1). Case 1A, a GIST at the greater curvature of the stomach,
showed spindle cell and in distinct storiform morphology, with the
strong expression of CD117, DOG1 and CD34 and the presence of
KIT exon 11 substitution (p.V560D, arrow). By contrast, case
1B, a GIST at the lesser curvature of the stomach, had epithelioid
morphology with uniform nuclei and cytoplasmic vacuoles. The tumor
was positive for DOG1 and CD34 but negative for CD117. Mast cells
served as an internal positive control. No mutation involving
KIT exon 11 was detected in case 1B. Images show H&E,
CD117, DOG1 and CD34 staining (magnification, ×200). GIST,
gastrointestinal stromal tumor; DOG1, discovered on GIST-1; KIT,
KIT proto-oncogene, receptor tyrosine kinase; H&E, hematoxylin
and eosin. |
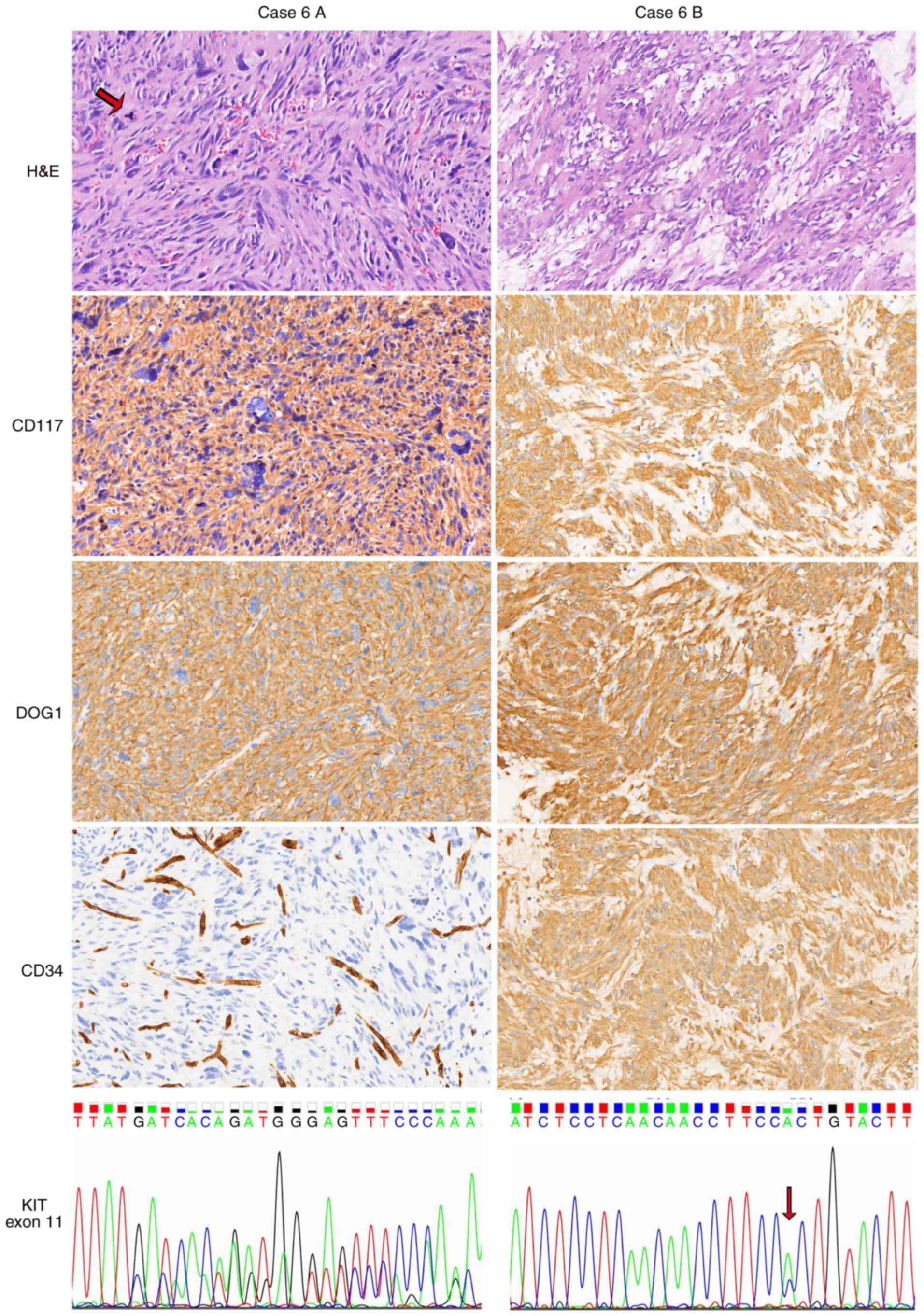 | Figure 3.Heterogeneity of GISTs in a second
patient (case 6). Hypercellular and pleomorphic histology was
visible in the specimen of case 6A, a gastric GIST. Multinucleated
giant cells and pathological mitoses (arrow) were observed. The
tumor in case 6A was positive for CD117 and DOG1 but negative for
CD34. Endothelial cells served as an internal positive control.
Case 6B, a GIST between the stomach and spleen, exhibited a
conventional spindle cell morphology, with the presence of myxoid
stroma and the strong expression of CD117, DOG1 and CD34.
KIT codon 579 was deleted in case 6A, whereas a KIT
exon 11 substitution (p.W557G, arrow) existed in case 6B. Images
show H&E, CD117, DOG1 and CD34 staining (magnification, ×200).
GIST, gastrointestinal stromal tumor; DOG1, discovered on GIST-1;
KIT, KIT proto-oncogene, receptor tyrosine kinase; H&E,
hematoxylin and eosin. |
 | Table III.Features of patients with GISTs of
heterogenous morphology, immunostaining and genotype. |
Table III.
Features of patients with GISTs of
heterogenous morphology, immunostaining and genotype.
|
|
|
|
|
|
|
| Expression |
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Case no. | Sex | Age (years) | Case part | Site | Morphology | Mitoses
(/50HPF)a | CD117 | DOG1 | CD34 | Ki67 (%) | KIT
mutation | PDGFRA
mutation |
|---|
| 1 | F | 66 | A | Greater curvature
of the stomach | Spindle | 4 |
(+) |
(+) |
(+) | 5 | Exon 11
p.V560D | WT |
|
|
|
| B | Lesser curvature of
the stomach | Epithelioid | 1 | (−) | (+) | (+) | 2 | WT | WT |
| 2 | M | 70 | A | Posterior of the
gastric fundus | Mixed | 2 | (+) | (−) | (+) | 4 | Exon 11
p.W557R | WT |
|
|
|
| B | Greater curvature
of the stomach | Spindle | 5 | (+) | (+) | (+) | 5 | Exon11 p.W557_K558
del | WT |
| 3 | F | 82 | A | Stomach | Spindle | 26 | (+) | (+) | (+) | 20 | Exon 11 p.P551_Q556
del | WT |
|
|
|
| B | Omentum | Spindle | 40 | (+) | (+) | (+) | 40 | Exon 11 p.P551_Q556
del | WT |
| 4 | M | 83 | A | Abdominal
cavity | Mixed | 12 | (+) | (+) | (−) | 25 | Exon 11 p.T574_Q575
insP | WT |
|
|
|
| B | Small
intestine | Mixed | 12 | (+) | (+) | (−) | 20 | Exon 11 p.T574_Q575
insP | WT |
| 5 | M | 71 | A | Stomach | Mixed | 25 | (+) | (+) | (+) | 40 | Exon 11
p.V555-V559del | WT |
|
|
|
| B | Omentum | Spindle | 20 | (+) | (+) | (+) | 30 | Exon 11
p.Q556-V560del | WT |
| 6 | F | 39 | A | Stomach | Mixed
(pleomorphic) | 6 | (+) | (+) | (−) | 5 | Exon 11
p.D579del | WT |
|
|
|
| B | Between stomach and
spleen | Spindle | 1 | (+) | (+) | (+) | 3 | Exon 11
p.W557G | WT |
To evaluate the occurrence of morphological or
genotypic heterogeneity in relapsed or metastatic GIST cases,
histological and mutational analyses were performed for 9 patients
with progression after resection (8 cases with recurrence and 1
case with hepatic metastasis). Of these cases, 3 were receiving
imatinib treatment prior to progression. Notably, the
morphological, immunohistochemical and mutational phenotypes of the
primary tumors were observed to be the same as those of the
recurrent and/or metastatic tumors (data not shown), suggesting the
existence of homogenous features in recurrent and/or metastatic
tumors, in contrast to the aforementioned primary tumors with two
masses.
Succinate dehydrogenase (SDH)-deficient GIST
accounts for approximately half of all WT GISTs. Previous studies
have demonstrated that all SDH mutations are reliably detected by
the loss of SDH subunit B (SDHB) expression using immunostaining
(19,20). Thus, SDHB-immunohistochemistry (IHC)
is an efficient method for the detection of SDH deficiency. In the
present study, the results of SDHB-IHC were obtained for 139 GIST
cases. Loss of SDHB expression was detected in 5 cases (3.6%),
suggesting the presence of SDH-deficient GIST. These SDH-deficient
GIST tumors, which were predominantly found in women (4 cases),
occurred in the stomach and in older adults (age ≥48 years). The
tumor cells of SDH-deficient GIST exhibited spindle morphology,
which is not in accordance with previous observations of the
predominance of epithelioid or mixed types.
Survival analysis
Follow-up data were available for 259 GIST cases. In
this group, the median follow-up time ranged from 12 to 129 months
(median, 45 months), and the median RFS ranged from 1 to 96 months
(median, 41 months). Of these cases, regular imatinib therapy
following surgery was received by 59 cases (22.8%), including 40
cases classified as high risk, 17 cases classified as intermediate
risk, and 2 cases classified as low risk. Disease progression was
observed in 33 cases (12.7%) and hepatic metastasis occurred in 11
cases (4.2%).
Kaplan-Meier survival analysis and log-rank tests
indicated that male sex, a larger tumor size (>5 cm), a high
mitotic index (>5/50 HPF), necrosis and epithelioid morphology
were associated with an inferior RFS (Fig. 4; P=0.017, P<0.001, P<0.001,
P<0.001, respectively). In addition, patients with non-gastric
GISTs had poorer outcomes than those with gastric tumors (Fig. 4B; P<0.001), and patients
classified as high risk and high WHO grade also exhibited
significantly lower RFS rate (Fig. 4G
and H; all P<0.001). Furthermore, KIT deletions
involving codons 557/558/559 were significantly associated with a
lower RFS rate than other genotypes (Fig. 4I; P=0.020). This adverse effect of
KIT deletions affecting codons 557/558/559 on RFS was also
observed among patients with gastric GISTs (Fig. 4J; P=0.001) but not among those with
non-gastric tumors. KIT exon 11 deletion was significantly
associated with a lower RFS rate, whereas exon 11 substitution was
associated with a favorable outcome (Fig. 4K; P=0.025). Compared with codon
557/558/559 deletion, PDGFRA exon 18 mutation was associated
with a favorable prognosis, although the association was not
statistically significant (Fig. 4L,
P=0.504).
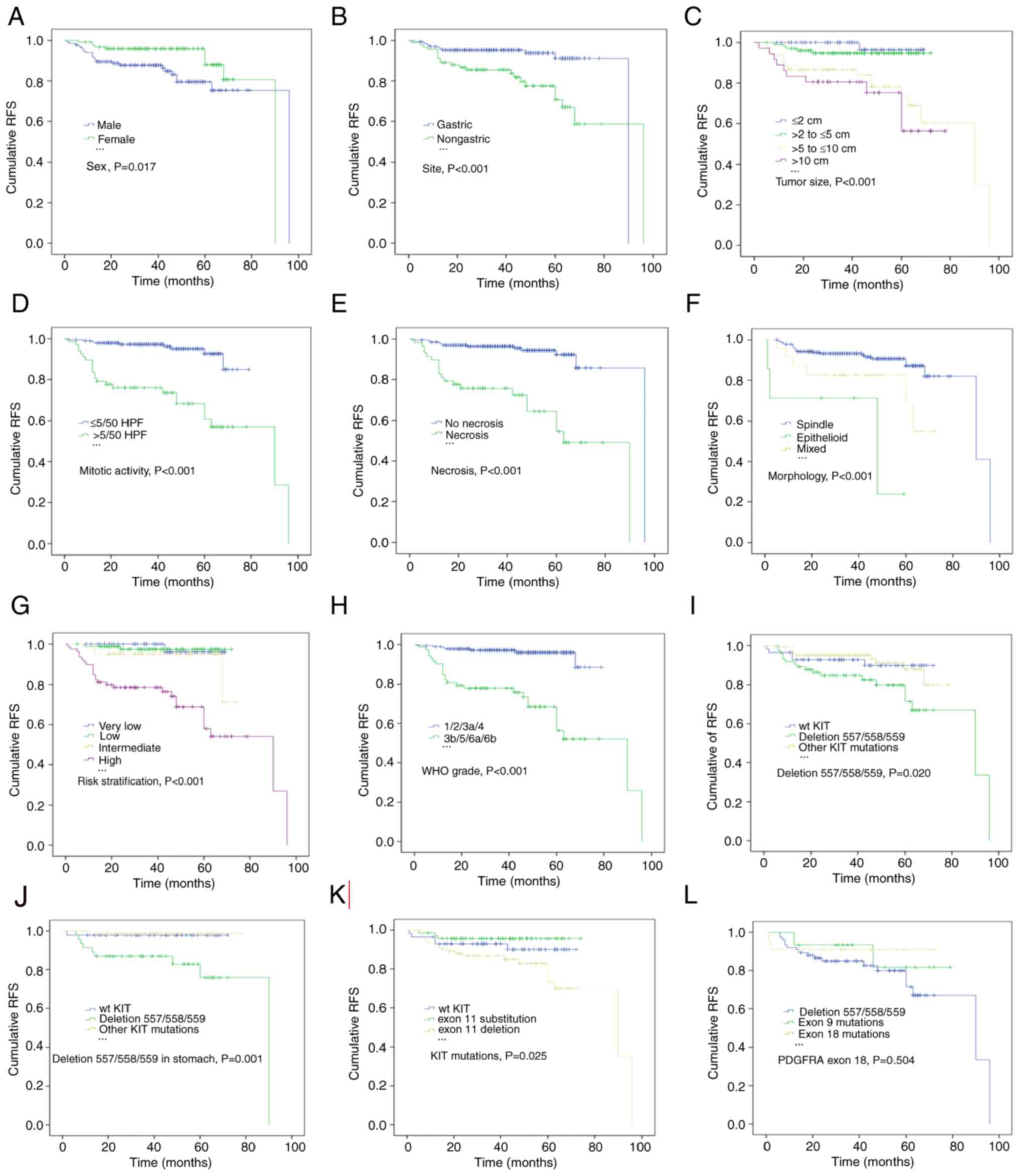 | Figure 4.Estimates of RFS in patients with
GISTs by Kaplan-Meier survival analysis with log-rank tests. (A)
Male sex, (B) non-gastric origin, (C) tumor size >5 cm, (D)
mitotic count >5/50 HPF and (E) necrosis were associated with a
lower RFS rate. (F) Compared with spindle and mixed morphology, the
epithelioid subtype was significantly associated with a shorter
RFS. According to the (G) National Institutes of Health and (H) WHO
classifications, patients classified as high risk and high grade
(3b/5/6a/6b) had significantly lower RFS rates. KIT
deletions involving codons 557/558/559 significantly reduced the
RFS rates of patients with (I) GISTs and (J) gastric GISTs compared
with those of patients with other KIT mutations or WT
KIT. (K) KIT exon 11 substitution indicated a
significantly improved RFS for patients with GISTs. (L) Patients
with PDGFRA exon 18 mutations had a higher RFS rate,
although the difference from that of patients with other
PDGFRA mutations was not statistically significant. RFS,
relapse-free survival; GIST, gastrointestinal stromal tumor; HPF,
high-power fields; WHO, World Health Organization; KIT, KIT
proto-oncogene, receptor tyrosine kinase; WT, wild-type; PDGFRA,
platelet derived growth factor receptor α. |
Univariate analysis revealed that patients who were
female or had tumors with spindle morphology had a lower risk of
relapse (Table IV; P=0.021 and
P=0.024, respectively). Conversely, non-gastric localization, high
NIH risk stratification and high WHO grade were significantly
associated with a higher risk of relapse [HR (95% CI)=3.383
(1.629-7.025), P=0.001; HR (95% CI)=10.359 (3.147-34.101),
P<0.001; HR (95% CI)=9.125 (3.930-21.187), P<0.001,
respectively; Table IV].
Additionally, regarding cases with KIT deletions involving
codons 557/558/559, the risk of tumor progression was almost 3-fold
higher that of cases with other KIT mutations [HR (95%
CI)=2.722 (1.235-6.003), P=0.013; Table
IV]. Multivariate Cox regression analysis also indicated that
patients bearing KIT deletions involving codons 557/558/559
had an almost 3-fold higher risk of relapse than those with other
KIT mutations [HR (95% CI)=2.794 (1.204-6.482), P=0.017;
Table IV], suggesting that this
genotype is an independent predictor of RFS. According to this
multivariate analysis, tumor location and WHO grade also markedly
influenced the risk of tumor progression [HR (95% CI)=2.420
(1.116-5.251), P=0.025; HR (95% CI)=3.166 (1.033-9.708), P=0.044;
Table IV). In addition, based on
multivariate Cox regression analysis, KIT codon 557/558/559
deletion was significantly associated with a high risk of
recurrence in the gastric location [HR (95% CI)=9.820
(1.142-84.484), P=0.037; Table V],
but not in non-gastric sites (data not shown).
 | Table IV.Univariate and multivariate analysis
of clinicopathological features and mutational status affecting the
RFS of patients with GISTs (n=259). |
Table IV.
Univariate and multivariate analysis
of clinicopathological features and mutational status affecting the
RFS of patients with GISTs (n=259).
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex |
|
Male | 1.0
(reference) |
| 1.0
(reference) |
|
|
Female | 0.412
(0.195-0.873) | 0.021 | 0.694
(0.303-1.587) | 0.387 |
| Age (years) |
|
≤60 | 1.0
(reference) |
| 1.0
(reference) |
|
|
>60 | 1.229
(0.606-2.489) | 0.568 | 1.848
(0.872-3.914) | 0.109 |
| Site |
|
Gastric | 1.0
(reference) |
| 1.0
(reference) |
|
|
Non-gastric | 3.383
(1.629-7.025) | 0.001 | 2.420
(1.116-5.251) | 0.025 |
| Morphology |
|
Mixed | 1.0
(reference) |
| 1.0
(reference) |
|
|
Spindle | 0.351
(0.142-0.870) | 0.024 | 0.911
(0.352-2.357) | 0.847 |
|
Epithelioid | 3.293
(0.914-11.865) | 0.068 | 2.605
(0.578-11.728) | 0.212 |
| Risk
stratification |
| Very
low/low | 1.0
(reference) |
| 1.0
(reference) |
|
|
Intermediate/high | 10.359
(3.147-34.101) | <0.001 | 3.528
(0.772-16.135) | 0.104 |
| WHO grade |
|
1/2/3a/4 | 1.0
(reference) |
| 1.0
(reference) |
|
|
3b/5/6a/6b | 9.125
(3.930-21.187) | <0.001 | 3.166
(1.033-9.708) | 0.044 |
| KIT
mutation |
| Other
mutations | 1.0
(reference) |
| 1.0
(reference) |
|
|
KIT del
557/558/559 | 2.722
(1.235-6.003) | 0.013 | 2.794
(1.204-6.482) | 0.017 |
| WT | 1.126
(0.385-3.298) | 0.828 | 2.087
(0.586-7.432) | 0.256 |
 | Table V.Univariate and multivariate analysis
of clinicopathological features and mutational status affecting the
RFS of patients with gastric GISTs (n=168). |
Table V.
Univariate and multivariate analysis
of clinicopathological features and mutational status affecting the
RFS of patients with gastric GISTs (n=168).
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex |
|
Male | 1.0
(reference) |
| 1.0
(reference) |
|
|
Female | 0.318
(0.082-1.232) | 0.097 | 0.407
(0.090-1.835) | 0.242 |
| Age (years) |
|
≤60 | 1.0
(reference) |
| 1.0
(reference) |
|
|
>60 | 1.757
(0.453-6.810) | 0.415 | 3.615
(0.684-19.108) | 0.130 |
| Morphology |
|
Mixed | 1.0
(reference) |
| 1.0
(reference) |
|
|
Spindle | 0.219
(0.044-1.087) | 0.063 | 0.319
(0.058-1.753) | 0.189 |
|
Epithelioid | 4.072
(0.559-29.678) | 0.166 | 6.279
(0.628-62.802) | 0.118 |
| KIT
mutation |
| Other
mutations | 1.0
(reference) |
| 1.0
(reference) |
|
|
KIT del
557/558/559 | 12.832
(1.602-102.768) | 0.016 | 9.820
(1.142-84.484) | 0.037 |
| WT | 1.612
(0.101-25.789) | 0.736 | 0.855
(0.044-16.666) | 0.918 |
Discussion
The identification of pathological and molecular
subtypes is essential in patients with GISTs for prognostic and
therapeutic purposes, particularly in the adjuvant and/or advanced
disease setting. Previous studies have revealed the prognostic
significance of KIT and PDGFRA mutational
alterations; however, studies of GISTs in the Chinese population
have only investigated small groups of patients (21,22), and
the data remain limited. In the present study, the profile and
prognostic value of pathological variables and gene mutations were
assessed in a large cohort comprising 302 GIST cases in a single
center, with a detailed description of KIT and PDGFRA
mutations and the prognostic value of specific gene
alterations.
Based on their morphological manifestations, GISTs
are classified as three variants: Spindle, epithelioid and mixed.
The present study identified spindle morphology as the predominant
variant (87.4%), which is consistent with previous reports.
However, the frequency of epithelioid morphology (3.0%), which was
mainly observed in GISTs located in the stomach, was lower than
that in previous studies (5-20%) (7,23,24).
This discrepancy may be due to selection bias. In addition, tumors
characterized by epithelioid and mixed histology showed a higher
risk of progression than those of the spindle subtype, which may be
due to the manifestation of pleomorphic/anaplastic features and
high mitotic activity observed in epithelioid and mixed-histology
tumors. In the present study, high mitotic activity and necrosis
were also associated with a lower RFS rate, which is consistent
with the study by Liu et al (25). The majority of specimens in the
present study were positive for CD117, DOG1 and CD34 (95.0, 93.4
and 81.1%, respectively). Strong triple and double expression of
these markers were detected in 72.5 and 24.5% of specimens,
respectively. CD34 negativity was more common in tumors in
non-gastric locations and with epithelioid and mixed morphology. In
a study by Hashmi et al (23), CD117 and CD34 were found to be
expressed in 46/48 (95.8%) and 34/46 (73.9%) of GISTs,
respectively. The authors also observed that CD34 negativity was
associated with epithelioid type. In a study by Liu et al
(26), the CD34 positivity rate of
primary GISTs was 92.3%. Therefore, the combined IHC detection of
CD117, DOG1 and CD34 is helpful for GIST diagnosis. In the North
American Intergroup Phase III Trial of imatinib mesylate, patients
whose tumors were CD117-negative by immunostaining were found to
have an inferior prognosis (13).
However, no prognostic significance of CD117 expression was
observed in the present study.
The frequency of gene mutations in the present study
cohort was 87.1%, including 77.2% KIT mutations and 9.9%
PDGFRA mutations. This frequency is in accordance with those
detected in two phase III clinical trials of imatinib, conducted by
the American College of Surgeons Oncology Group (ACOSOG) (87.4%)
(12) and the European Organization
for Research and Treatment of Cancer (86.2%) (27). The mutation rates in these trials are
higher than those in a study based on the Polish Clinical GIST
Registry (82.2%) (10) and a
European multicenter analysis (85.1%) (11), but lower than that in a study of
Chinese cases (93.8% overall, 89.1% for KIT and 4.7% for
PDGFRA) conducted by Wang et al (28). KIT mutations have been
reported to be associated with a high risk of progression (18,24,27). The
proportion of high-risk subtypes in the single-center study
conducted by Wang et al (28)
was higher than that in the present series (42.5 vs. 29.1%,
respectively), which explains the relatively lower frequency of
KIT mutations in the present data. In one study of patients
with GISTs in China, KIT mutations were identified in 76.1%
of CD117-positive GIST cases (21).
In another Chinese study, Du et al (22) found the frequency of KIT and
PDGFRA mutations in GISTs was 76.6 and 2.8%, respectively.
Thus, the frequency of PDGFRA mutations in the present study
was higher than that in previous studies. In addition, the
percentages of patients with tumors >10 cm in diameter and with
a mitotic index >5/50 HPF were lower in the present study than
in previous studies (10,12), further suggesting that relatively few
cases were in a high-risk prognostic group, in contrast with the
observations in the aforementioned phase III clinical trials. The
spectrum of KIT and PDGFRA mutations was similar to
that described in the literature (10,28). For
example, the predominant mutated area of KIT exon 11
involved codons 557–560 with deletions and/or substitutions,
followed by codons 572–580 with duplication. In addition to the
common duplication of codons 502 and 503 of KIT exon 9, a
rare duplication of codons 506–508 was detected in one case. A less
common genotype with PDGFRA exon 18 deletion was observed in
5 cases (1.7%), but its significance remains unclear.
Importantly, the findings of the present study
demonstrated that KIT deletions affecting codons 557/558/559
are independent adverse predictors of RFS in patients with GISTs.
Patients with deletions involving codons 557/558/559 had a
significantly shorter RFS, higher mitotic activity, high risk of
relapse (according to the NIH classification) and high WHO grade
compared with those of patients with other KIT mutations.
Previous studies have reported that deletions affecting codons
557/558 are associated with metastasis and poor patient outcomes
(17,29). In the Polish Clinical GIST Registry
study, patients whose tumors had KIT deletions encompassing
codons 557/558 had a lower 5-year RFS rate than those with other
exon 11 mutations or exon 11 deletions not involving codons 557/558
(10). The study also found that
deletions involving codons 557/558 were more frequently present in
tumors of larger size, with a higher mitotic count and high risk of
relapse. Regarding gastric GISTs, the patients with KIT
deletions in codons 557/558/559 had an almost 6-fold higher risk of
relapse than patients with WT KIT (30). In a European multicenter study,
KIT del-inc557/558 was a predictor of inferior outcomes
inpatients with gastric GISTs but not those with non-gastric GISTs
(11). However, in the ACOSOG Z9001
trial, deletion of codons 557 and/or 558 did not independently
affect RFS in either the placebo or imatinib arm (12). In addition, Wang et al
(28) demonstrated that codon
557/558 deletion was associated with a high mitotic rate but not
with 5-year RFS. In the present study, KIT deletions
involving codons 557/558/559 were significantly associated with a
high risk of recurrence in the stomach but not in non-gastric
locations. Furthermore, this type of deletion was more frequently
identified in gastric GISTs than in non-gastric cases and had an
adverse effect on RFS in patients with gastric GISTs. Both the
present study and previous studies identified non-gastric origin as
an adverse indicator of GIST progression. Thus, we hypothesize that
the adverse effect of deletions involving codons 557/558/559 may be
associated with malignant features of the tumors, such as a higher
mitotic count, which may result from the robust activation of
KIT signaling. The loss of the side chains of amino acids
encoded by codons 553, 557, 559 or 560 may be associated with
increased phosphorylation. Deletions of these codons, which encode
a juxta-membrane residue, may disrupt the conformation of the KIT
protein and induce a loss of inhibitory control of the kinase
activity in the KIT receptor. However, the detailed molecular
mechanism requires further study.
The results of the present study suggest that
another common molecular alteration, KIT exon 11
substitution, was a clinical predictor of an indolent tumor,
namely, a tumor of small size, with a low mitotic count, low risk
of relapse and low WHO grade. In the survival analysis, patients
with exon 11 substitution had a significantly longer RFS than those
with exon 11 deletions or WTKIT. In a Norwegian
population-based study, GISTs with KIT substitutions
exhibited low mitotic activity (18). In the Polish Clinical GIST Registry
study, the 5-year RFS rate of patients with KIT exon 11
point mutations was improved compared with that of patients with
other KIT exon 11 mutations; the study also documented that
tumors with PDGFRA mutations had a lower risk of recurrence
(10). In the present study, the
presence of a PDGFRA exon 18 mutation appeared to have a
favorable influence on GIST relapse, although the effect was not
found to be statistically significant. Thus, KIT exon 11
substitution and PDGFRA exon 18 mutation may be positive
prognostic indicators for disease progression.
Notably, the coexistence of exon 11 deletion and
exon 13 duplication was observed in the GIST of one female patient
and was characterized by adverse prognostic indicators: larger
tumor size, high mitotic activity and high risk of
recurrence/relapse. The treatment and prognosis of this patient
after resection is unknown as she was lost to follow-up. This
molecular alteration is rarely seen, and has been reported in one
imatinib-resistant tumor also featuring factors indicative of an
inferior prognosis (31). Moreover,
the cases in the present study and previous report exhibited a
similar morphology (spindle and epithelioid histology). Activating
mutations of KIT exon 11, particularly deletions, result in
ligand-independent activation, ultimately increasing cell
proliferation and inhibiting apoptosis, and mutations in KIT
exon 13, which encodes the ATP-binding region of the protein, are
considered to be associated with the autoinhibitory function of the
JM domain (15). Thus, we
hypothesize that the combination of exon 11 deletion and exon 13
duplication may increase the malignant behavior of GIST and
adversely influence patient prognosis.
The existence of tumor heterogeneity with regard to
morphology, immunostaining and genotype was observed in the present
study. Four cases had tumors with distinct gene mutations and
histology at two different sites, and three of these cases also had
distinct immunostaining phenotypes. All these distinct gene
mutations occurred in KIT exon 11, including KIT exon
11 p.V560D vs. WT, p.W557R vs. p.W557_K558 del and p.D579 del vs.
p.W557G. A previous report emphasized the heterogeneity of clinical
resistance to tyrosine kinase inhibitors in GIST (32). Recently, Serrano et al
(33) detected the presence of
secondary KIT mutations in patients whose GISTs were
resistant to imatinib, and suggested that these distinct mutations
may have resulted from tumor subclones that emerged after imatinib
therapy. In the present study, the four cases with tumor
heterogeneity did not receive any therapy prior to surgery.
Furthermore, no heterogeneity was observed between the primary and
recurrent and/or metastatic tumors of 9 patients, 3 of whom
received imatinib therapy before progression. The observation of
heterogeneous morphology, immunostaining and genotype in GISTs may
be due to the presence of different tumor subclones, providing
further challenges for diagnosis and therapy.
In summary, the present study found that KIT
and PDGFRA mutations were frequent in GISTs. KIT exon
11 deletions, particularly deletions affecting codons 557/558/559,
were genotypes indicative of more aggressive tumors and were
associated with a higher risk of relapse. Furthermore, the
emergence of specific genotypes, such as multiple coexisting
mutations and heterogeneous genetic alterations, is challenging for
individual targeted therapy. Based on different pathological
features and KIT/PDGFRA mutations, the results contribute to
the identification of patients with different subtypes of GIST for
tailored adjuvant treatments, and support the notion that specific
molecular phenotypes should be included in the present risk
classification system.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by a research grant
(grant no. 2018SF089) from the Scientific Research Seed Fund of
Peking University First Hospital.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TL designed the study. LL, LN and YD collected and
analyzed the patient data. LL, PL and TL evaluated and interpreted
the clinicopathological data and mutation phenotypes. XL was
responsible for the technical operation of PCR and Sanger
sequencing. DL performed the immunohistochemical staining. JL and
SH performed the statistical analysis and interpreted the results.
LL and TL wrote the manuscript. TL revised the manuscript. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of Peking University First Hospital and in compliance
with the Declaration of Helsinki. Written patient consent for use
of their tissues in research was obtained.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Huizinga JD, Thuneberg L, Klüppel M,
Malysz J, Mikkelsen HB and Bernstein A: W/kit gene required for
interstitial cells of Cajal and for intestinal pacemaker activity.
Nature. 373:347–349. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Joensuu H, Hohenberger P and Corless CL:
Gastrointestinal stromal tumour. Lancet. 382:973–983. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamamoto H and Oda Y: Gastrointestinal
stromal tumor: Recent advances in pathology and genetics. Pathol
Int. 65:9–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
DeMatteo RP, Lewis JJ, Leung D, Mudan SS,
Woodruff JM and Brennan MF: Two hundred gastrointestinal stromal
tumors: Recurrence patterns and prognostic factors for survival.
Ann Surg. 231:51–58. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rossi S, Miceli R, Messerini L, Bearzi I,
Mazzoleni G, Capella C, Arrigoni G, Sonzogni A, Sidoni A,
Toffolatti L, et al: Natural history of imatinib-naive GISTs: A
retrospective analysis of 929 cases with long-term follow-up and
development of a survival nomogram based on mitotic index and size
as continuous variables. Am J Surg Pathol. 35:1646–1456. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Joensuu H: Risk stratification of patients
diagnosed with gastrointestinal stromal tumor. Hum Pathol.
39:1411–1419. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li J, Ye Y, Wang J, Zhang B, Qin S, Shi Y,
He Y, Liang X, Liu X, Zhou Y, et al: Chinese consensus guidelines
for diagnosis and management of gastrointestinal stromal tumor.
Chin J Cancer Res. 29:281–293. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goh BK, Chow PK, Yap WM, Kesavan SM, Song
IC, Paul PG, Ooi BS, Chung YF and Wong WK: Which is the optimal
risk stratification system for surgically treated localized primary
GIST? Comparison of three contemporary prognostic criteria in 171
tumors and a proposal for a modified Armed Forces Institute of
Pathology risk criteria. Ann Surg Oncol. 15:2153–2163. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lokuhetty D, White VA and Watanabe R: WHO
classfication of digestive system tumours. 5th edition. Lyon: IARC
Press; pp. 439–443. 2019
|
|
10
|
Wozniak A, Rutkowski P, Piskorz A,
Ciwoniuk M, Osuch C, Bylina E, Sygut J, Chosia M, Rys J, Urbanczyk
K, et al: Prognostic value of KIT/PDGFRA mutations in
gastrointestinal stromal tumours (GIST): Polish Clinical GIST
Registry experience. Ann Oncol. 23:353–360. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wozniak A, Rutkowski P, Schöffski P,
Ray-Coquard I, Hostein I, Schildhaus HU, Le Cesne A, Bylina E,
Limon J, Blay JY, et al: Tumor genotype is an independent
prognostic factor in primary gastrointestinal stromal tumors of
gastric origin: A european multicenter analysis based on
ConticaGIST. Clin Cancer Res. 20:6105–6116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Corless CL, Ballman KV, Antonescu CR,
Kolesnikova V, Maki RG, Pisters PW, Blackstein ME, Blanke CD,
Demetri GD, Heinrich MC, et al: Pathologic and molecular features
correlate with long-term outcome after adjuvant therapy of resected
primary GI stromal tumor: The ACOSOG Z9001 trial. J Clin Oncol.
32:1563–1570. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heinrich MC, Owzar K, Corless CL, Hollis
D, Borden EC, Fletcher CD, Ryan CW, von Mehren M, Blanke CD, Rankin
C, et al: Correlation of kinase genotype and clinical outcome in
the North American intergroup phase III Trial of imatinib mesylate
for treatment of advanced gastrointestinal stromal tumor: CALGB
150105 study by cancer and leukemia group B and southwest oncology
group. J Clin Oncol. 26:5360–5367. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Szucs Z, Thway K, Fisher C, Bulusu R,
Constantinidou A, Benson C, van der Graaf WT and Jones RL:
Molecular subtypes of gastrointestinal stromal tumors and their
prognostic and therapeutic implications. Future Oncol. 13:93–107.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lennartsson J, Jelacic T, Linnekin D and
Shivakrupa R: Normal and oncogenic forms of the receptor tyrosine
kinase kit. Stem Cells. 23:16–43. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martin-Broto J, Gutierrez A,
Garcia-Del-Muro X, Lopez-Guerrero JA, Martinez-Trufero J, de Sande
LM, Lainez N, Maurel J, De Juan A, Losa F, et al: Prognostic time
dependence of deletions affecting codons 557 and/or 558 of KIT gene
for relapse-free survival (RFS) in localized GIST: A Spanish group
for sarcoma research (GEIS) study. Ann Oncol. 21:1552–1557. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martín J, Poveda A, Llombart-Bosch A,
Ramos R, López-Guerrero JA, García del Muro J, Maurel J, Calabuig
S, Gutierrez A, González de Sande JL, et al: Deletions affecting
codons 557–558 of the c-KIT gene indicate a poor prognosis in
patients with completely resected gastrointestinal stromal tumors:
A study by the Spanish group for sarcoma research (GEIS). J Clin
Oncol. 23:6190–6198. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Steigen SE, Eide TJ, Wasag B, Lasota J,
Lasota J and Miettinen M: Mutations in gastrointestinal stromal
tumors-a population-based study from Northern Norway. APMIS.
115:289–298. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gill AJ: Succinate dehydrogenase (SDH) and
mitochondrial driven neoplasia. Pathology. 44:285–292. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miettinen M, Wang ZF, Sarlomo-Rikala M,
Osuch C, Rutkowski P and Lasota J: Succinate
dehydrogenase-deficient GISTs: A clinicopathologic,
immunohistochemical, and molecular genetic study of 66 gastric
GISTs with predilection to young age. Am J Surg Pathol.
35:1712–1721. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He HY, Fang WG, Zhong HH, Li Y, Zheng J,
Du J, Heng WJ and Wu BQ: Status and clinical implication of c-kit
and PDGFRA mutations in 165 cases of gastrointestinal stromal tumor
(GIST). Zhonghua Bing Li Xue Za Zhi. 35:262–266. 2006.(In Chinese).
PubMed/NCBI
|
|
22
|
Du CY, Shi YQ, Zhou Y, Fu H and Zhao G:
The analysis of status and clinical implication of KIT and PDGFRA
mutations in gastrointestinal stromal tumor (GIST). J Surg Oncol.
98:175–178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hashmi AA, Faraz M, Nauman Z, Qureshi MU,
Hashmi SK, Waseem HF, Edhi MM, Faridi N and Khan A:
Clinicopathologic features and prognostic grouping of
gastrointestinal stromal tumors (GISTs) in Pakistani patients: An
institutional perspective. BMC Res Notes. 11:4572018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mayr P, Märkl B, Agaimy A, Kriening B,
Dintner S, Schenkirsch G and Schneider-Stock R: Malignancies
associated with GIST: A retrospective study with molecular analysis
of KIT and PDGFRA. Langenbecks Arch Surg. 404:605–613. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu X, Qiu H, Zhang P, Feng X, Chen T, Li
Y, Tao K, Li G, Sun X and Zhou Z; China Gastrointestinal Stromal
Tumor Study Group (CN-GIST), : Prognostic role of tumor necrosis in
patients undergoing curative resection for gastric gastrointestinal
stromal tumor: A multicenter analysis of 740 cases in China. Cancer
Med. 6:2796–2803. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu FY, Qi JP, Xu FL and Wu AP:
Clinicopathological and immunohistochemical analysis of
gastrointestinal stromal tumor. World J Gastroenterol.
12:4161–4165. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Debiec-Rychter M, Sciot R, Le Cesne A,
Schlemmer M, Hohenberger P, van Oosterom AT, Blay JY, Leyvraz S,
Stul M, Casali PG, et al: KIT mutations and dose selection for
imatinib in patients with advanced gastrointestinal stromal
tumours. Eur J Cancer. 42:1093–1103. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang M, Xu J, Zhao W, Tu L, Qiu W, Wang C,
Shen Y, Liu Q and Cao H: Prognostic value of mutational
characteristics in gastrointestinal stromal tumors: A single-center
experience in 275 cases. Med Oncol. 31:8192014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wardelmann E, Losen I, Hans V, Neidt I,
Speidel N, Bierhoff E, Heinicke T, Pietsch T, Büttner R and
Merkelbach-Bruse S: Deletion of Trp-557 and Lys-558 in the
juxtamembrane domain of the c-kit protooncogene is associated with
metastatic behavior of gastrointestinal stromal tumors. Int J
Cancer. 106:887–895. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Capelli L, Petracci E, Quagliuolo V,
Saragoni L, Colombo P, Morgagni P, Calistri D, Tomezzoli A, Di
Cosmo M, Roviello F, et al: Gastric GISTs: Analysis of c-Kit,
PDGFRA and BRAF mutations in relation to prognosis and clinical
pathological characteristics of patients-A GIRCG study. Eur J Surg
Oncol. 42:1206–1214. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Antonescu CR, Romeo S, Zhang L, Nafa K,
Hornick JL, Nielsen GP, Mino-Kenudson M, Huang HY, Mosquera JM, Dei
Tos PA and Fletcher CD: Dedifferentiation in gastrointestinal
stromal tumor to an anaplastic KIT-negative phenotype: A diagnostic
pitfall: Morphologic and molecular characterization of 8 cases
occurring either de novo or after imatinib therapy. Am J Surg
Pathol. 37:385–392. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liegl B, Kepten I, Le C, Zhu M, Demetri
GD, Heinrich MC, Fletcher CD, Corless CL and Fletcher JA:
Heterogeneity of kinase inhibitor resistance mechanisms in GIST. J
Pathol. 216:64–74. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Serrano C, Mariño-Enríquez A, Tao DL,
Ketzer J, Eilers G, Zhu M, Yu C, Mannan AM, Rubin BP, Demetri GD,
et al: Complementary activity of tyrosine kinase inhibitors against
secondary kit mutations in imatinib-resistant gastrointestinal
stromal tumours. Br J Cancer. 120:612–620. 2019. View Article : Google Scholar : PubMed/NCBI
|


















