Introduction
Globally, hepatocellular carcinoma (HCC) constituted
the third most common cause of cancer-related mortality in 2013
(1). Unlike the majority of other
types of cancer, liver cancer mortality rates have increased by
34.8% in the USA over the past 20 years (2), suggesting that the liver
cancer-associated health and economic burden will likely increase
substantially worldwide in the coming decades (3). HCC is characterized by a poor
prognosis, and the 5-year survival rate is ~15% (4). The main reasons contributing to the
high mortality and poor prognosis include difficulties in early
diagnosis and the complexity of tumor treatment in patients due to
the vast heterogeneity of symptoms and tumor biology (5,6).
Therefore, identifying new prognostic markers that help to improve
individual patient care is of great clinical significance.
Phosphoprotein enriched in diabetes/phosphoprotein
enriched in astrocytes-15 (PED/PEA-15) is a ubiquitously expressed
phosphoprotein in human glucose metabolism and was initially
discovered in primary cultured astrocytes (7). PED/PEA-15 serves a key role in diabetes
and glucose metabolism (8). In
addition, PED/PEA-15 regulates a range of cellular processes,
including cell proliferation, apoptosis and migration in numerous
types of cancer, such as breast and lung cancer (9,10).
Notably, PED/PEA-15 acts as a tumor-promotor or a tumor-suppressor
depending on its phosphorylation status (11). The unphosphorylated form binds
extracellular signal receptor-activated kinase 1/2 (ERK1/2),
suppressing its subsequent activation; by contrast, phosphorylation
of PED/PEA-15 at Ser116 [PED/PEA-15(S116)] releases ERK1/2,
resulting in its activation, which causes cell proliferation and
migration, and thus, tumor promotion, including lung, breast and
prostate cancer (9,10,12). In
addition, PED/PEA-15(S116) binds to the Fas-associated death domain
protein (FADD), inhibiting FADD-mediated apoptosis (13).
The p27Kip1 (p27) protein regulates cellular
functions such as differentiation, proliferation, apoptosis,
translation and adhesion (14,15).
Previous evidence has demonstrated that the absence or low levels
of p27 expression are indicators of a poor prognosis in several
types of cancer, including colon, breast, bladder and lung cancer
(16,17). Notably, the major pathway of
post-transcriptional nuclear degradation of p27 involves its
phosphorylation at Thr187 [P-p27(T187)], which results in its
suppression (18). During the
G1/S phase transition of the cell cycle, P-p27(T187)
allows the binding of S-phase kinase-associated protein 2 and the
formation of cyclin E/A-Cdk2 complexes, leading to p27
ubiquitination and degradation (19,20).
Furthermore, ERK1/2 phosphorylation upregulates p27 phosphorylation
and downregulates nuclear p27 levels (21). Despite the abundant information about
PED/PEA-15(S116) and P-p27(T187), their relationship in HCC remains
unclear. In addition, the association between PED/PEA-15(S116) and
P-p27(T187) in a clinical setting has not been studied previously.
Therefore, the present study aimed to explore the connection
between PED/PEA-15(S116) and P-p27(T187) expression and the
clinical features and prognosis in HCC.
Materials and methods
Patients and tissue specimens
A prospective single-center study was performed
between January 2011 and January 2017. Samples for laboratory
assays were obtained between January 2011 and December 2014. Tumor
and adjacent non-tumorous hepatic (2 cm from the edge of the tumor)
tissues were harvested from 60 patients with HCC who had undergone
hepatectomy by the same surgical team at Yinzhou Hospital
Affiliated to Medical School of Ningbo University (Ningbo, China).
The tissues were divided into two parts, one of which was
immediately fixed with formalin and embedded in paraffin, whereas
the other was stored in liquid nitrogen. Two independent
hepatologists confirmed the diagnosis of HCC based on postoperative
pathology. In addition, normal hepatic tissues were collected from
12 patients with benign lesions. No patient had received
preoperative administration of neoadjuvant radiotherapy,
chemotherapy or any invasive intervention, including percutaneous
ablation or chemoembolization.
In the present study, the follow-up period was up to
January 2017 or until the date of the patient's death. The data
were collected by a telephone interview or outpatient service.
Tumor-Node-Metastasis (TNM) stage and Edmondson grade were used to
analyze the clinicopathological features in patients with HCC
(22). The overall survival (OS) was
determined as the period between the surgery date and death from
any cause or the last follow-up date. The study received ethical
approval from the Research Ethics Committee of Yinzhou Hospital
(approval no. 2017006). Written informed consent was obtained from
all participants of the present study. The protocols of the present
study regarding human subjects followed the ethical standards of
the National Research Committee and the Declaration of
Helsinki.
Immunohistochemistry and evaluation of
immunostaining
HCC, adjacent non-tumorous and normal tissue samples
were fixed in a 10% neutral formalin solution immediately following
resection at room temperature for >24 h. Dehydration was
performed in an ethanol gradient series at room temperature as
follows: 70% for 1 h, 80% for 2–4 h, 95% for 2–4 h, 95% for 2–4 h,
100% for 2–4 h, and 100% for 2–4 h. Subsequently the samples were
embedded in paraffin, sectioned into 3–5-µm slices, mounted on
glass slides and dewaxed. The slides were treated for 30 min with
1% H2O2-methanol solution at room temperature
and placed in citric acid/sodium citrate buffer (0.01 M; pH 6.0) at
92–98°C, followed by microwaving for 10 min and washing three times
for 5 min with PBS. Subsequently, the samples were incubated with
1% sheep serum (cat. no. ZLI-9021; Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.) in PBS to block non-specific antigens for
20 min at room temperature, and PED/PEA-15(S116) (1:50; cat. no.
PA5-38314; Thermo Fisher Scientific, Inc.) and P-p27(T187) (1:50;
cat. no. PA5-104911; Thermo Fisher Scientific, Inc.) primary
antibodies were added in a dropwise manner and incubated at 4°C
overnight. The following day, sheep anti-rabbit IgG-HRP horseradish
peroxidase-conjugated secondary antibodies (1:1,000; cat. no.
BHR101; Beijing Bersee Science and Technology Co., Ltd.) were
added, followed by incubation at 37°C for 40 min in a humidified
chamber. Subsequently, the avidin-biotin-peroxidase complex (1:100)
(Beijing Bersee Science and Technology Co., Ltd.)) was added, and
the slides were incubated at 37°C for 40 min in a humidified
chamber. The samples were counterstained with 3,3′-diaminobenzidine
at room temperature for 60–90 sec, dehydrated, cleared and sealed.
PBS and normal sheep serum were used in place of the primary
antibodies as the blank and negative controls, respectively. The
staining results were observed under a light microscope with ×10,
×20 and ×40 magnification (23).
Similarly, the protein expression results were evaluated according
to the frequency of positive staining in the cytoplasm or the
nucleus of the cells (23). A total
of 10 different high magnification fields were randomly selected
for double-blind counting (100 cells/field). Protein expression was
categorized as positive when >50% of the cells were stained.
Western blotting
Protein extraction and western blotting were
performed following a standard protocol (24). Human frozen tissues (tumor tissues,
adjacent non-tumorous and normal liver tissue) were crushed into a
fine powder in a metal mortar, cooled on dry ice and lysed in RIPA
lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 1%
sodium deoxycholate, 0.1% SDS, 1 mM PMSF) with 10:1 protease
inhibitor cocktail (Sigma-Aldrich; Merck KGaA). The protein
concentration was determined by the BCA assay. The extracted
proteins were separated by SDS-PAGE (40 µg protein per lane; 12%
separation gel and 5% spacer gel) and transferred to PVDF
membranes. Following blocking with TBS-0.1% Tween-20 (TBS-T) buffer
containing 5% non-fat powder milk at room temperature for 2 h, the
membranes were immunoblotted using the following primary
antibodies: Anti-β-actin (1:200; cat. no. sc-8432; Santa Cruz
Biotechnology, Inc.), anti-PED/PEA-1 5 (1:800; cat. no. PA5-100749;
Thermo Fisher Scientific, Inc.), anti-PED/PEA-15(S116) (1:800; cat.
no. PA5-38314; Thermo Fisher Scientific, Inc.), anti-P27 (1:800;
cat. no. PA5-27188; Thermo Fisher Scientific, Inc.) and
anti-P-p27(T187) (1:800; cat. no. PA5-104911; Thermo Fisher
Scientific, Inc.), at 4°C for >12 h, and the goat anti-mouse IgG
peroxidase-conjugated secondary antibody (1:800; Pierce; Thermo
Fisher Scientific, Inc.) was added and incubated in a 37°C water
bath for 1 h. The membranes washed three times with TBS-T.
Immunoreactive bands on the blots were visualized with an ECL kit
(Beit HaEmek).
Statistical analysis
The data were analyzed using SPSS 22.0 software (IBM
Corporation). Continuous variable values were expressed as the mean
± standard error of the mean and analyzed using Student's t-test.
The analysis of multiple groups was performed by one-way ANOVA with
Tukey's post hoc test. Categorical variables were compared using
the χ2 or Fisher's exact test. In addition, Spearman's
rank correlation analysis was used to analyze the associations
between PED/PEA-15(S116) and P-p27(T187). Survival curves were
drawn using the Kaplan-Meier method and analyzed by the log-rank
test. Multivariate analysis was performed on the prognostic
variables using the Cox proportional hazard regression model with
stepwise forward selection. P<0.05 was considered to indicate a
statistically significant difference.
Results
Detection of PED/PEA-15(S116) and
P-p27(T187) protein expression
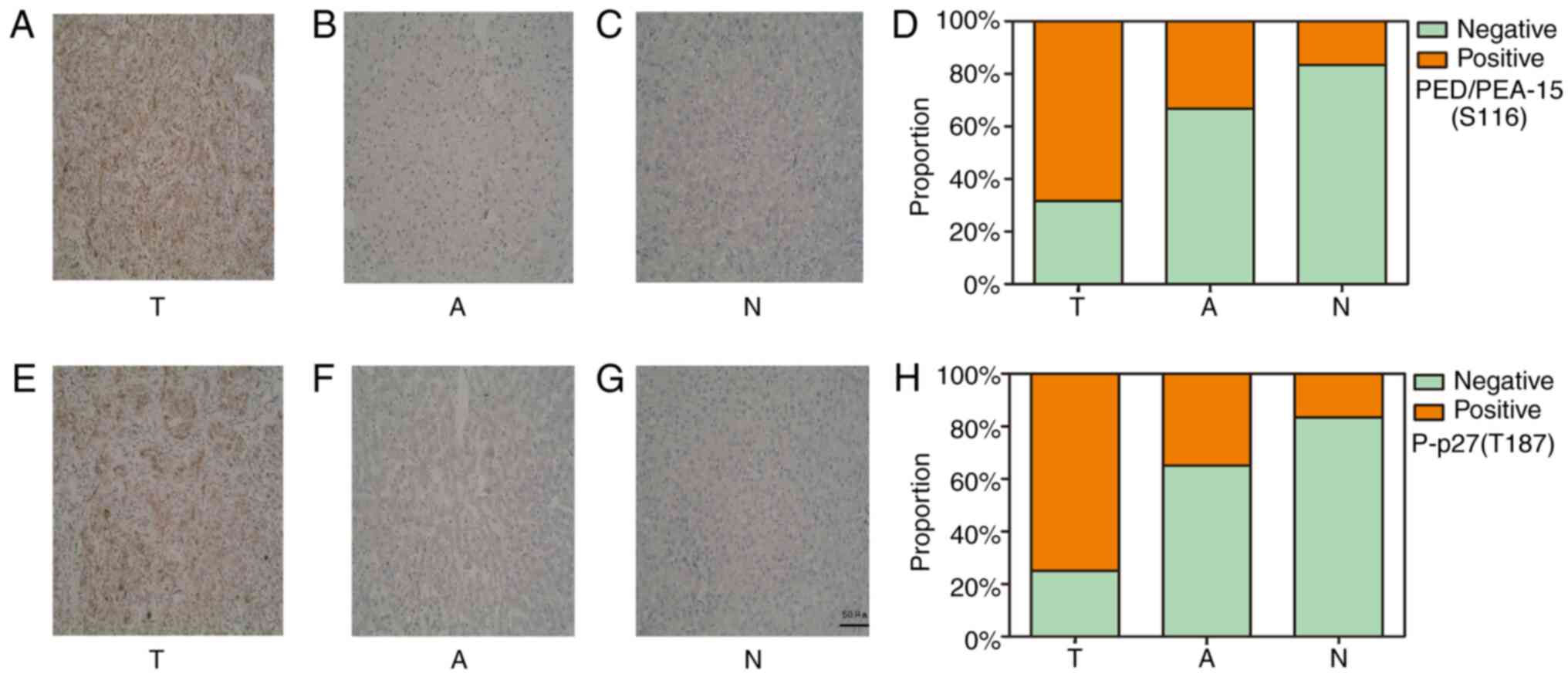
The immunohistochemical staining results revealed
positive staining for both PED/PEA-15(S116)-and P-p27(T187) in the
cytoplasm. (Fig. 1). The rates of
positive expression for PED/PEA-15(S116) and P-p27(T187) in
patients with HCC were 68.3% (41/60) and 75.0% (45/60),
respectively. These rates were higher compared with those in the
adjacent [PED/PEA-15(S116), 33.3% (20/60), P<0.05; P-p27(T187),
35% (21/60), P<0.05] and normal tissues [PED/PEA-15(S116), 16.7%
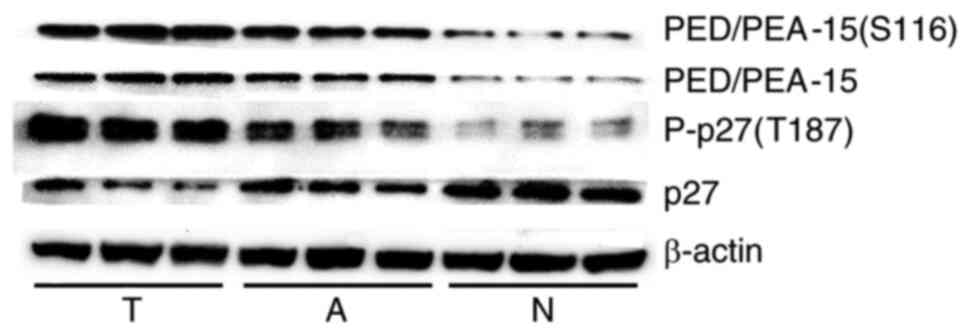
(2/12), P<0.05; P-p27(T187), 16.7% (2/12), P<0.05; Table I]. Western blot analysis revealed
higher levels of P-p27(T187) in the HCC group compared with those
in the adjacent and normal liver tissue groups. In addition, the
expression levels of PED/PEA-15 and PED/PEA-15(S116) in the HCC
group were higher compared with those in the adjacent and normal
liver tissue groups. (Fig. 2;
Table II).
 | Table I.Expression of PED/PEA-15(S116) and
P-p27(T187) by immunohistochemistry in HCC, adjacent non-cancerous
and normal tissue samples. |
Table I.
Expression of PED/PEA-15(S116) and
P-p27(T187) by immunohistochemistry in HCC, adjacent non-cancerous
and normal tissue samples.
|
|
| Positive expression
rate (%) |
χ2-value | p-value |
|---|
|
|
|
|
|
|
|---|
| Variable | N |
PED/PEA-15(S116) | P-p27(T187) | HCC vs. A | HCC vs. N | HCC vs. A | HCC vs. N |
|---|
| HCC | 60 | 41 (68.3) | 45 (75.0) |
|
|
|
|
| A | 60 | 20 (33.3) | 21 (35.0) |
|
|
|
|
| N | 12 | 2
(16.7) | 2
(16.7) |
|
|
|
|
|
PED/PEA-15(S116) |
|
|
| 14.70 | 11.09 | <0.01 | <0.01 |
| P-p27(T187) |
|
|
| 19.39 | 15.01 | <0.01 | <0.01 |
 | Table II.Expression of PED/PEA-15(S116),
P-p27(T187), PED/PEA-15, and P27 by western blotting in HCC,
adjacent non-cancerous and normal tissue samples. |
Table II.
Expression of PED/PEA-15(S116),
P-p27(T187), PED/PEA-15, and P27 by western blotting in HCC,
adjacent non-cancerous and normal tissue samples.
|
|
|
|
| t-value | P-value |
|---|
|
|
|
|
|
|
|
|---|
| Variable | HCC | A | N | HCC vs. A | HCC vs. N | HCC vs. A | HCC vs. N |
|---|
| N | 60 | 60 | 12 |
|
|
|
|
| OD ratio, mean ±
SEM |
|
|
|
|
|
|
|
|
PEA-15(S116)/actin | 0.64±0.06 | 0.35±0.07 | 0.18±0.04 | 17.80 | 22.66 | 0.01 | <0.01 |
|
P-p27(T187)/actin | 0.64±0.11 | 0.31±0.08 | 0.15±0.03 | 16.38 | 23.23 | <0.01 | <0.01 |
|
PEA-15/actin | 0.62±0.08 | 0.35±0.08 | 0.19±0.07 | 16.55 | 17.51 | <0.01 | 0.01 |
|
P27/actin | 0.21±0.06 | 0.34±0.08 | 0.66±0.14 | −10.58 | −12.54 | <0.01 | <0.01 |
|
PEA-15(S116)/PEA-15 | 1.02±0.14 | 1.00±0.21 | 0.94±0.05 |
0.84 |
0.52 | 0.04 | 0.02 |
|
P-p27(T187)/P27 | 3.03±0.31 | 0.91±0.20 | 0.23±0.05 | 42.21 | 28.48 | <0.01 | <0.01 |
Associations between PED/PEA-15(S116)
and P-p27(T187) expression and clinicopathological features in
patients with HCC
As presented in Table
III, the PED/PEA-15(S116) expression levels were associated
with the Tumor-Node-Metastasis (TNM) stage (P<0.05), Edmondson
grade (P<0.05), vascular invasion (P<0.05) and tumor
multiplicity (P<0.05). Significant associations between
P-p27(T187) expression levels and TNM stage (P<0.05), Edmondson
grade (P<0.05), vascular invasion (P<0.05) and tumor
multiplicity (P<0.05) were also identified. The expression
levels of PED/PEA-15(S116) and P-p27(T187) were not associated with
patient age, sex, tumor size, cirrhosis, α-fetoprotein level or
hepatitis B surface antigen positivity (all P>0.05).
 | Table III.Associations between PED/PEA-15(S116)
and P-p27(T187) expression levels determined by western blotting
and the clinicopathological characteristics of patients with
hepatocellular carcinoma (n=60). |
Table III.
Associations between PED/PEA-15(S116)
and P-p27(T187) expression levels determined by western blotting
and the clinicopathological characteristics of patients with
hepatocellular carcinoma (n=60).
|
|
| OD value of protein
expression (mean ± SEM) |
|---|
|
|
|
|
|---|
| Variables | N |
PED/PEA-15(S116) | P-p27(T187) |
|---|
| Age, years |
|
| P=0.775 |
| P=0.769 |
|
≤50 | 27 | 0.598±0.017 |
| 0.625±0.022 |
|
|
>50 | 33 | 0.604±0.016 |
| 0.617±0.019 |
|
| Sex |
|
| P=0.341 |
| P=0.795 |
|
Male | 37 | 0.618±0.014 |
| 0.629±0.018 |
|
|
Female | 23 | 0.596±0.010 |
| 0.623±0.020 |
|
| HBsAg |
|
| P=0.175 |
| P=0.149 |
|
Positive | 45 | 0.602±0.018 |
| 0.624±0.011 |
|
|
Negative | 15 | 0.571±0.024 |
| 0.591±0.022 |
|
| Tumor size, cm |
|
| P=0.099 |
| P=0.145 |
| ≤5 | 38 | 0.569±0.018 |
| 0.612±0.019 |
|
|
>5 | 22 | 0.618±0.021 |
| 0.658±0.025 |
|
| Cirrhosis |
|
| P=0.083 |
| P=0.142 |
|
Yes | 42 | 0.615±0.014 |
| 0.645±0.015 |
|
| No | 18 | 0.566±0.026 |
| 0.599±0.031 |
|
| Tumor
multiplicity |
|
| P=0.004 |
| P=0.024 |
|
Single | 39 | 0.538±0.019 |
| 0.587±0.019 |
|
|
Multiple | 21 | 0.625±0.021 |
| 0.667±0.029 |
|
| AFP, µg/l |
|
| P=0.075 |
| P=0.112 |
|
≤400 | 23 | 0.594±0.019 |
| 0.626±0.028 |
|
|
>400 | 37 | 0.632±0.012 |
| 0.680±0.019 |
|
| Edmondson
grade |
|
| P=0.005 |
| P=0.005 |
|
I–II | 32 | 0.577±0.019 |
| 0.595±0.020 |
|
|
III–IV | 28 | 0.644±0.013 |
| 0.676±0.019 |
|
| TNM stage |
|
| P=0.013 |
| P=0.001 |
|
I–II | 38 | 0.578±0.017 |
| 0.597±0.017 |
|
|
III | 22 | 0.648±0.020 |
| 0.695±0.024 |
|
| Vascular
invasion |
|
| P=0.005 |
| P=0.009 |
|
Yes | 19 | 0.655±0.015 |
| 0.699±0.021 |
|
| No | 41 | 0.585±0.013 |
| 0.616±0.019 |
|
Correlation between the expression of
PED/PEA-15(S116) and P-p27(T187) in HCC
The correlation between PED/PEA-15(S116) and
P-p27(T187) expression was evaluated in HCC tissues. The Spearman
rank correlation analysis results revealed that the expression of
PED/PEA-15(S116) was positively associated with P-p27(T187)
expression (r=0.434; P<0.05) (Table
IV).
 | Table IV.Associations between PED/PEA-15(S116)
and P-p27(T187) levels in hepatocellular carcinoma determined by
immunohistochemistry. |
Table IV.
Associations between PED/PEA-15(S116)
and P-p27(T187) levels in hepatocellular carcinoma determined by
immunohistochemistry.
|
|
| P-p27(T187)
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Marker | N | Positive | Negative | r-value | P-value |
|---|
| PED/PEA-15(S116)
expression |
|
|
|
|
|
|
Positive | 41 | 36 | 5 | 0.434 | 0.001 |
|
Negative | 19 | 9 | 10 |
|
|
Association of PED/PEA-15(S116) and
P-p27(T187) expression with clinical prognosis
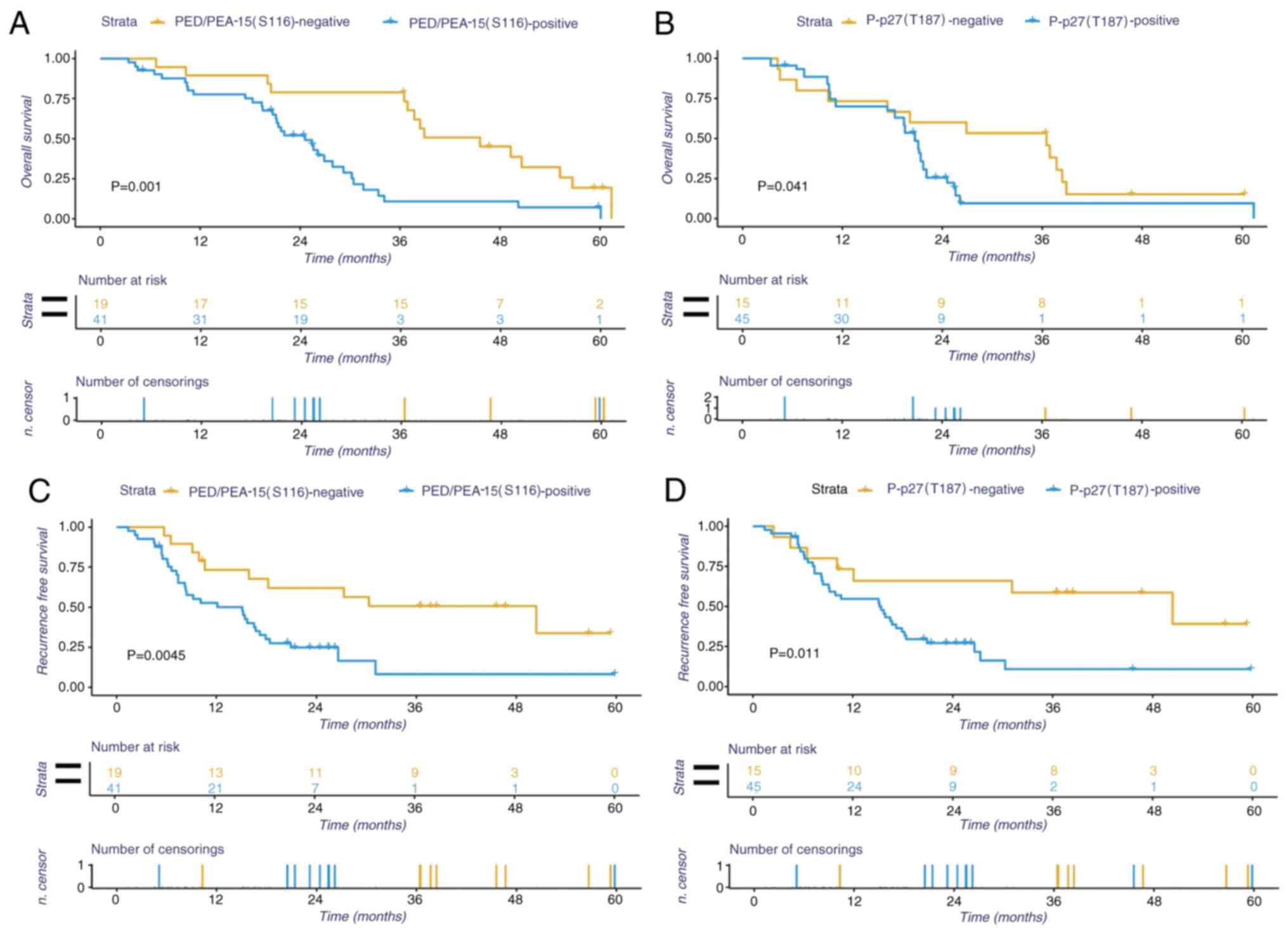
Kaplan-Meier survival analysis were performed using
data from the 60 patients with HCC to determine the effects of
PED/PEA-15(S116) and P-p27(T187) protein expression on patient
prognosis. The results revealed that the patients with HCC with
positive PED/PEA-15(S116) or P-p27(T187) expression exhibited
shorter OS times (Fig. 3C,
P<0.05). Similar trends were observed for disease-free survival,
where patients with HCC with positive PED/PEA-15(S116) or
P-p27(T187) expression exhibited shorter disease-free survival
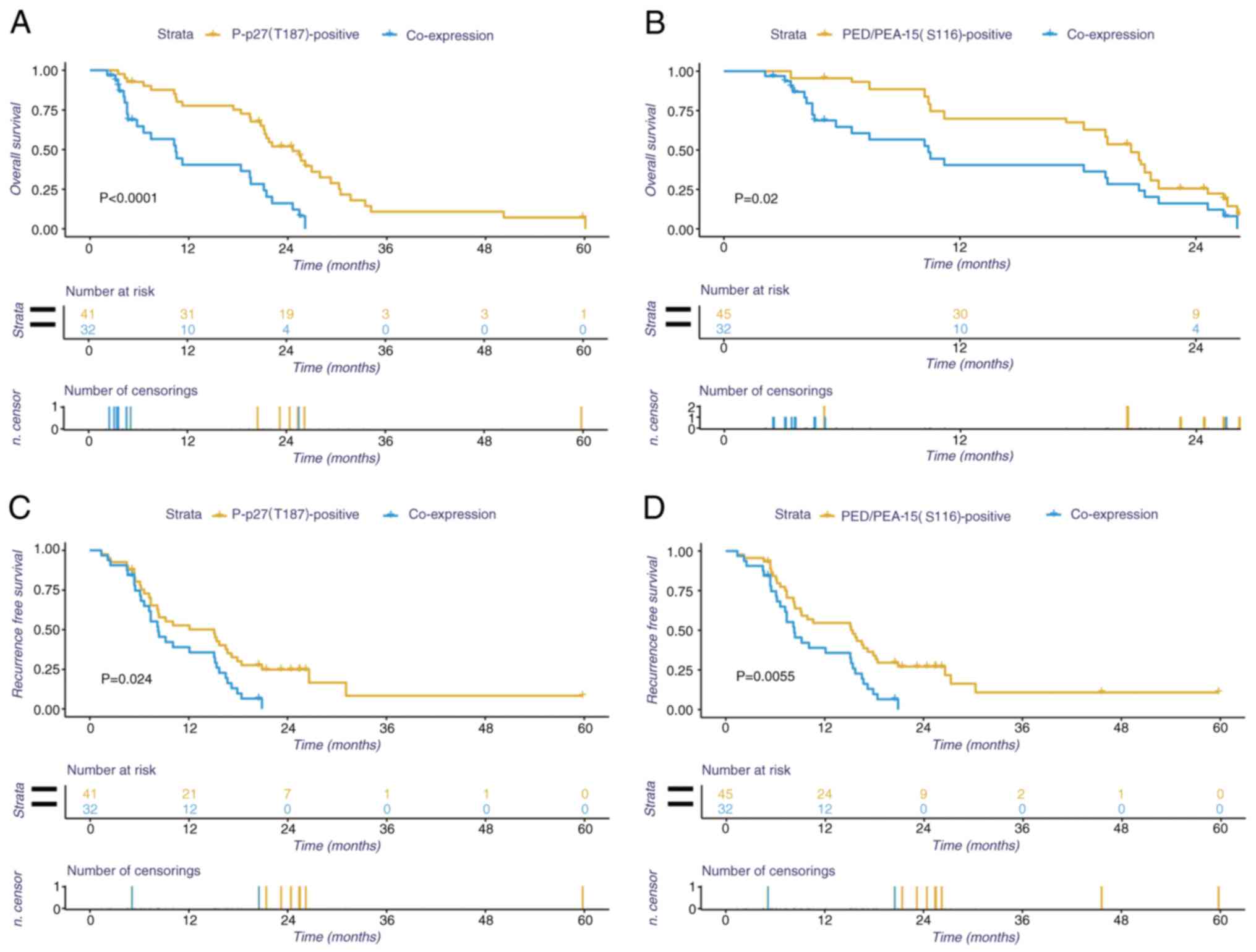
times compared with those with negative expression (Fig. 3D, P<0.05). In addition, the
effects of concomitant positive expression of P-p27(T187) and
PED/PEA-15 on OS and disease-free survival time were also
evaluated. The results revealed that patients with HCC with
positive expression of P-p27(T187) and PED/PEA-15(S116) exhibited a
poorer prognosis compared with those with positive expression of
P-p27(T187) or PED/PEA-15(S116) alone (P<0.05, Fig. 4).
Univariate and multivariate analyses
of prognostic variables in HCC
Univariate and multivariate analyses were performed
to assess whether PED/PEA-15(S116) and P-p27(T187) expression
constituted an independent risk factor for HCC outcomes. The
results demonstrated that age, sex, serum α-fetoprotein level,
hepatitis B surface antigen positivity, tumor size, multiplicity,
Edmondson grade, TNM stage, vascular invasion and positive
expression of PED/PEA-15(S116) and P-p27(T187) were identified as
prognostic variables for the OS rate in patients with HCC. In the
multivariate analysis, only TNM stage (P<0.05), vascular
invasion (P<0.05), PED/PEA-15(S116) expression (P<0.05) and
P-p27(T187) expression (P<0.05) were identified as independent
prognostic variables for HCC OS (Table
V).
 | Table V.Univariate and multivariate analyses
of prognostic variables for overall survival in patients with
hepatocellular carcinoma. |
Table V.
Univariate and multivariate analyses
of prognostic variables for overall survival in patients with
hepatocellular carcinoma.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years | 0.589 | 0.324–1.069 | 0.082 |
|
|
|
| Sex | 1.193 | 0.648–2.198 | 0.571 |
|
|
|
| HBsAg | 1.737 | 0.910–3.316 | 0.094 |
|
|
|
| AFP | 0.584 | 0.319–1.069 | 0.081 |
|
|
|
| Cirrhosis | 1.759 | 0.921–3.357 | 0.087 |
|
|
|
| Tumor size, cm | 1.751 | 0.943–3.253 | 0.076 |
|
|
|
| Tumor
multiplicity | 0.269 | 0.132–0.549 | <0.001 |
|
|
|
| TNM stage | 0.237 | 0.107–0.524 | <0.001 | 2.782 | 1.136–6.802 | 0.025 |
| Edmondson
grade | 0.226 | 0.116–0.440 | <0.001 |
|
|
|
| Vascular
invasion | 2.194 | 2.136–3.154 | <0.001 | 3.255 | 0.996–10.635 | 0.017 |
|
PED/PEA-15(S116) | 2.953 | 1.517–2.749 | <0.001 | 5.801 | 2.472–13.609 | 0.001 |
| P-p27(T187) | 0.340 | 0.176–0.657 | <0.001 | 2.063 | 0.924–4.605 | 0.031 |
Discussion
In the present study, the expression levels of
PED/PEA-15(S116) and P-p27(T187) were assessed in HCC, adjacent
non-tumor and normal liver tissues. In addition, the associations
between PED/PEA-15(S116) and P-p27(T187) expression levels,
clinicopathological features and clinical outcomes of patients with
HCC were analyzed. The results demonstrated that PED/PEA-15(S116)
and P-p27(T187) expression levels were higher in HCC tissues
compared with those in adjacent and normal liver tissues. In
addition, the expression of PED/PEA-15(S116) was associated with
the expression levels of P-p27(T187) in HCC tissues, as determined
by Spearman rank correlation analysis. High expression levels of
these markers were associated with TNM stage, Edmondson grade,
vascular invasion and tumor multiplicity. Kaplan-Meier survival
analysis results demonstrated that patients with HCC with positive
expression of PED/PEA-15(S116) and P-p27(T187) exhibited poorer
clinical outcomes compared with those in patients with negative
expression. Multivariate survival analysis results demonstrated
that the expression levels of PED/PEA-15(S116) and P-p27(T187) were
independent prognostic indicators for patients with HCC.
HCC continues to be a problem in the clinic, with a
poor prognosis and limited therapeutic options (25). Thus, urgent research action is
required to aid in the identification of novel prognostic markers
for HCC surveillance and to improve individual treatment
approaches. A previous study has indicated that apoptosis-related
proteins participate in the occurrence, development and metastasis
of HCC (26). PED/PEA-15, a small
cytosolic protein, is highly conserved in mammals and is
ubiquitously expressed in human glucose metabolism. PED/PEA-15
participates in the modulation of numerous cellular processes, such
as cell proliferation, death and survival (10). This enables PED/PEA-15 to interfere
with intrinsic as well as extrinsic apoptosis pathways (27,28).
Previous studies have demonstrated that PED/PEA-15 is upregulated
in a variety of tumors, such as breast and lung cancer, in which it
facilitates tumor growth and is associated with poor survival
outcomes (9,29). By contrast, the upregulation of
PED/PEA-15 in patients with ovarian cancer is associated with a
good prognosis (30). This
difference is primarily associated with the phosphorylation status
of PED/PEA-15; in ovarian cancer, PED/PEA-15 is unphosphorylated
(31). Therefore, PED/PEA-15 acts
either as a tumor promoter or as a tumor suppressor, modulating
cell proliferation or apoptosis depending on its phosphorylation
status (11). Phosphorylated
PED/PEA-15 is associated with increased ERK1/2 activity compared
with that in non-tumorous cells, leading to increased cell growth
and migration and, consequently, tumor promotion (32). According to the results of the
present study, high expression levels of PED/PEA-15(S116) in HCC
tissues were associated with a poor prognosis and a short survival
time, which was consistent with the results of a previous study
(33).
Several studies have reported that the suppression
of p27 or its localization in the cytoplasm is associated with
increased malignancy and a poor outcome in numerous types of cancer
(16,17,31).
Uncontrolled cell proliferation is a characteristic of cancer and
arises from the loss of control over the transition from the
G1 to S phase in the cell cycle (17). P-p27(T187) triggers p27 degradation
during the G1/S phase transition through the formation
of the SCF ubiquitin ligase E3 complex, which results in the
polyubiquitination of p27 (34). The
suppression of p27 prevents efficient inhibition of cyclin-Cdk
activities, and the resulting activity of these kinases facilitates
cell proliferation (34).
Alternatively, the suppression of p27 induces the deregulation of
genes that serve a vital role in tumorigenesis and tumor metastasis
(35). By contrast, when p27 is
transported from the cell nucleus to the cytoplasm through
phosphorylation, it alters the expression of genes modulated by p27
and exerts an oncogenic function by promoting cell movement and
invasion through the modification of the cytoskeleton (36,37). In
the present study, P-p27(T187) protein was observed to be located
in the cytoplasm and upregulated in HCC tissues compared with that
in adjacent non-tumor and normal liver tissues. In addition, the
positive expression of P-p27(T187) was associated with a shorter
average survival time of patients with HCC, resulting in a poor
prognosis. Therefore, we hypothesize that increased P-p27(T187)
levels may affect HCC tumorigenesis and progression via nuclear
export and polyubiquitination-mediated degradation of p27, which is
consistent with previous findings.
The results of the present study also identified a
positive association between PED/PEA-15(S116) and P-p27(T187) in
HCC. Therefore, we hypothesized that PED/PEA-15(S116) may increase
ERK phosphorylation, which regulates nucleocytoplasmic p27 export
and polyubiquitination degradation, thus reducing the p27 protein
level via P-p27(T187) (21,38), and this phosphorylation cascade may
serve an essential role in the MAPK/ERK pathway and contribute to
the occurrence, development and prognosis of HCC.
The current study has several potential limitations.
First, the number of samples was limited, which affected the power
of the statistical analyses. Second, this was a retrospective study
using medical data from a single institution; thus, certain
inherent bias may exist. It is important that future prospective
studies using larger sample sizes are conducted to validate the
current findings. Finally, the present findings solely relied on
histological examination and no further studies were performed for
the two proteins; therefore, the phosphorylation cascade
interactions in the MAPK/ERK pathway between PED/PEA-15(S116) and
P-p27(T187) in in vivo and in vitro HCC models need
to be further studied and validated.
In summary, the results of the present study
suggested that PED/PEA-15(S116) and P-p27(T187) may be prospective
prognostic biomarkers for HCC. However, further evidence is needed
to validate their application. Based on the results of the current
study, there was a significant association between the expression
levels of PED/PEA-15(S116) and P-p27(T187) proteins in HCC;
however, their complicated interactions require additional research
to determine their molecular mechanisms via the MAPK/ERK signaling
pathway, which may unveil novel potential targets for HCC
therapy.
Acknowledgements
Not applicable.
Funding
This work was funded by The Provincial Natural
Science Foundation of Zhejiang (grant no. LY16H160004) and The
Science Foundation of Zhejiang province (grant no.
LQ18H160006).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW, SZ, XL and XX conceived the present study. YZ,
XL, SZ and ZL drafted the manuscript. YW, MC, ZL, XZ, XL, SZ and XX
made substantial contributions to the interpretation and analysis
of the data, drafted the study and revised it critically for
important intellectual content. YW and XX were major contributors
in the revision of the manuscript. YW and XX confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study received ethical approval from the
Research Ethics Committee of Yinzhou Hospital (Ningbo, China;
approval no. 2017006). Written informed consent was obtained from
all participants of the present study. The protocols of the present
study regarding human subjects followed the ethical standards of
the National Research Committee and the Declaration of
Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mittal S and El-Serag HB: Epidemiology of
hepatocellular carcinoma: Consider the population. J Clin
Gastroenterol. 47 (Suppl):S2–S6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bertuccio P, Turati F, Carioli G,
Rodriguez T, La Vecchia C, Malvezzi M and Negri E: Global trends
and predictions in hepatocellular carcinoma mortality. J Hepatol.
67:302–309. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singal AG and El-Serag HB: Hepatocellular
carcinoma from epidemiology to prevention: Translating knowledge
into practice. Clin Gastroenterol Hepatol. 13:2140–2151. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Llovet JM, Zucman-Rossi J, Pikarsky E,
Sangro B, Schwartz M and Sherman M: Gores: Hepatocellular
carcinoma. Nat Rev Dis Primers. 2:160182016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cai SH, Lu SX, Liu LL, Zhang CZ and Yun
JP: Increased expression of hepatocyte nuclear factor 4 alpha
transcribed by promoter 2 indicates a poor prognosis in
hepatocellular carcinoma. Therap Adv Gastroenterol. 10:761–771.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wong SS, Kim KM, Ting JC, Yu K, Fu J, Liu
S, Cristescu R, Nebozhyn M, Gong L, Yue YG, et al: Genomic
landscape and genetic heterogeneity in gastric adenocarcinoma
revealed by whole-genome sequencing. Nat Commun. 5:54772014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Danziger N, Yokoyama M, Jay T, Cordier J,
Glowinski J and Chneiweiss H: Cellular expression, developmental
regulation, and phylogenic conservation of PEA-15, the astrocytic
major phosphoprotein and protein kinase C substrate. J Neurochem.
64:1016–1025. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Greig FH and Nixon GF: Phosphoprotein
enriched in astrocytes (PEA)-15: A potential therapeutic target in
multiple disease states. Pharmacol Ther. 143:265–274. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Quintavalle C, Di Costanzo S, Zanca C,
Tasset I, Fraldi A, Incoronato M, Mirabelli P, Monti M, Ballabio A,
Pucci P, et al: Phosphorylation-regulated degradation of the
tumor-suppressor form of PED by chaperone-mediated autophagy in
lung cancer cells. J Cell Physiol. 229:1359–1368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie X, Tang H, Pengliu, Kong Y, Wu M, Xiao
X, Yang L, Gao J, Wei W, Lee J, et al: Development of PEA-15 using
a potent non-viral vector for therapeutic application in breast
cancer. Cancer Lett. 356:374–381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Crespo-Flores SL, Cabezas A, Hassan S and
Wei Y: PEA-15 C-terminal tail allosterically modulates
death-effector domain conformation and facilitates protein-protein
interactions. Int J Mol Sci. 20:33352019. View Article : Google Scholar
|
|
12
|
Mace PD, Wallez Y, Egger MF, Dobaczewska
MK, Robinson H, Pasquale EB and Riedl SJ: Structure of ERK2 bound
to PEA-15 reveals a mechanism for rapid release of activated MAPK.
Nat Commun. 4:16812013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fiory F, Formisano P, Perruolo G and
Beguinot F: Frontiers: PED/PEA-15, a multifunctional protein
controlling cell survival and glucose metabolism. Am J Physiol
Endocrinol Metab. 297:E592–E601. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Besson A, Dowdy SF and Roberts JM: CDK
inhibitors: Cell cycle regulators and beyond. Dev Cell. 14:159–169.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bencivenga D, Caldarelli I, Stampone E,
Mancini FP, Balestrieri ML, Della Ragione F and Borriello A:
p27(Kip1) and human cancers: A reappraisal of a still enigmatic
protein. Cancer Lett. 403:354–365. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gupta A, Saltarski JM, White MA, Scaglioni
PP and Gerber DE: Therapeutic targeting of nuclear export
inhibition in lung cancer. J Thorac Oncol. 12:1446–1450. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peng M, Wang J, Zhang D, Jin H, Li J, Wu
XR and Huang C: PHLPP2 stabilization by p27 mediates its inhibition
of bladder cancer invasion by promoting autophagic degradation of
MMP2 protein. Oncogene. 37:5735–5748. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He W, Wang X, Chen L and Guan X: A
crosstalk imbalance between p27(Kip1) and its interacting molecules
enhances breast carcinogenesis. Cancer Biother Radiopharm.
27:399–402. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grimmler M, Wang Y, Mund T, Cilensek Z,
Keidel EM, Waddell MB, Jäkel H, Kullmann M, Kriwacki RW and Hengst
L: Cdk-inhibitory activity and stability of p27Kip1 are directly
regulated by oncogenic tyrosine kinases. Cell. 128:269–280. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gallastegui E, Bicer A, Orlando S, Besson
A, Pujol MJ and Bachs O: p27(Kip1) represses the Pitx2-mediated
expression of p21(Cip1) and regulates DNA replication during cell
cycle progression. Oncogene. 36:350–361. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim JE and Kang TC: Nucleocytoplasmic
p27(Kip1) export is required for ERK1/2-mediated reactive
astroglial proliferation following status epilepticus. Front Cell
Neurosci. 12:1522018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou L, Rui JA, Ye DX, Wang SB, Chen SG
and Qu Q: Edmondson-Steiner grading increases the predictive
efficiency of TNM staging for long-term survival of patients with
hepatocellular carcinoma after curative resection. World J Surg.
32:1748–1756. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang GK, Li SH, Zhao ZM, Liu SX, Zhang GX,
Yang F, Wang Y, Wu F, Zhao XX and Xu ZY: Inhibition of heat shock
protein 90 improves pulmonary arteriole remodeling in pulmonary
arterial hypertension. Oncotarget. 7:54263–54273. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Andreozzi M, Quintavalle C, Benz D,
Quagliata L, Matter M, Calabrese D, Tosti N, Ruiz C, Trapani F,
Tornillo L, et al: HMGA1 expression in human hepatocellular
carcinoma correlates with poor prognosis and promotes tumor growth
and migration in in vitro models. Neoplasia. 18:724–731. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen SL, Liu LL, Lu SX, Luo RZ, Wang CH,
Wang H, Cai SH, Yang X, Xie D, Zhang CZ and Yun JP: HBx-mediated
decrease of AIM2 contributes to hepatocellular carcinoma
metastasis. Mol Oncol. 11:1225–1240. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morofuji N, Ojima H, Hiraoka N, Okusaka T,
Esaki M, Nara S, Shimada K, Kishi Y and Kondo T: Antibody-based
proteomics to identify an apoptosis signature for early recurrence
of hepatocellular carcinoma. Clin Proteomics. 13:282016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang X, Zhang C, Li W, Jiang D, Wei Z, Lv
M, Xie X and Sun X: PEA-15 contributes to the clinicopathology and
AKT-regulated cisplatin resistance in gastric cancer. Oncol Rep.
41:1949–1959. 2019.PubMed/NCBI
|
|
28
|
Fiory F, Parrillo L, Raciti GA, Zatterale
F, Nigro C, Mirra P, Falco R, Ulianich L, Di Jeso B, Formisano P,
et al: PED/PEA-15 inhibits hydrogen peroxide-induced apoptosis in
Ins-1E pancreatic beta-cells via PLD-1. PLoS One. 9:e1136552014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mohammed HN, Pickard MR and
Mourtada-Maarabouni M: The protein phosphatase 4 - PEA15 axis
regulates the survival of breast cancer cells. Cell Signal.
28:1389–1400. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bartholomeusz C, Rosen D, Wei C, Kazansky
A, Yamasaki F, Takahashi T, Itamochi H, Kondo S, Liu J and Ueno NT:
PEA-15 induces autophagy in human ovarian cancer cells and is
associated with prolonged overall survival. Cancer Res.
68:9302–9310. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee J, Bartholomeusz C, Krishnamurthy S,
Liu P, Saso H, Lafortune TA, Hortobagyi GN and Ueno NT: PEA-15
unphosphorylated at both serine 104 and serine 116 inhibits ovarian
cancer cell tumorigenicity and progression through blocking
β-catenin. Oncogenesis. 1:e222012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Weijman JF, Riedl SJ and Mace PD:
Structural studies of ERK2 protein complexes. Methods Mol Biol.
1487:53–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Quintavalle C, Hindupur SK, Quagliata L,
Pallante P, Nigro C, Condorelli G, Andersen JB, Tagscherer KE, Roth
W, Beguinot F, et al: Phosphoprotein enriched in diabetes
(PED/PEA15) promotes migration in hepatocellular carcinoma and
confers resistance to sorafenib. Cell Death Dis. 8:e31382017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hoellein A, Graf S, Bassermann F,
Schoeffmann S, Platz U, Holzlwimmer G, Kröger M, Peschel C,
Oostendorp R, Quintanilla-Fend L and Keller U: Cks1 promotion of S
phase entry and proliferation is independent of p27Kip1
suppression. Mol Cell Biol. 32:2416–2427. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pippa R, Espinosa L, Gundem G,
Garcia-Escudero R, Dominguez A, Orlando S, Gallastegui E, Saiz C,
Besson A, Pujol MJ, et al: p27Kip1 represses transcription by
direct interaction with p130/E2F4 at the promoters of target genes.
Oncogene. 31:4207–4220. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Besson A, Assoian RK and Roberts JM:
Regulation of the cytoskeleton: An oncogenic function for CDK
inhibitors? Nat Rev Cancer. 4:948–955. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Besson A, Gurian-West M, Schmidt A, Hall A
and Roberts JM: p27Kip1 modulates cell migration through the
regulation of RhoA activation. Genes Dev. 18:862–876. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shin M, Lee KE, Yang EG, Jeon H and Song
HK: PEA-15 facilitates EGFR dephosphorylation via ERK sequestration
at increased ER-PM contacts in TNBC cells. FEBS Lett.
589:1033–1039. 2015. View Article : Google Scholar : PubMed/NCBI
|