Introduction
Lung cancer is the leading cause of cancer-related
death worldwide, and the 5-year survival of patients with
advanced-stage disease is poor (1).
Currently, serological diagnosis for primary lung cancer is poor,
even when a combination of several serum tumor markers is used.
Additionally, there are few reliable tumor markers to detect
recurrence postoperatively. The lack of major improvements in the
diagnostic and survival rates for lung cancer has driven a search
for novel markers aimed at improving early detection of primary
lung cancer and recurrence after surgery using a high-specificity
antibody.
MUC1 (mucin 1, cell surface associated; CD227) is a
high molecular-weight transmembrane glycoprotein (2). The MUC1 N-terminal subunit (MUC1-N)
contains highly glycosylated tandem repeats (TRs) that are a
physical characteristic of the mucin family (3). A TR consists of a constant amino acid
sequence rich in serine (Ser) and threonine (Thr) residues
(4,5). MUC1-N forms a complex with the MUC1
C-terminal subunit at the cell membrane and is shed from the
surface of cancer cells, leading to increased plasma levels of
MUC1. Although mucins, including MUC1, are also highly expressed in
healthy epithelial cells, the sugar chain structure of MUC1 is
completely different between normal and tumor cells. Normal cells
have modifications in long-branched sugar chains, whereas cancer
cells express various simple and short sugar chain antigens called
O-glycans (e.g., Tn, sialyl-Tn and sialyl-Lewis-X). Abnormally
glycosylated mucins in malignant cells are currently being
researched due to unique post-translational modification of mucin
backbones by carbohydrates (6).
O-glycosylation is initiated by the polypeptide
N-acetylgalactosaminyltransferase, which utilizes UDP-GalNAc to add
GalNAc to Ser/Thr residues. The addition of GalNAc to Ser/Thr via
α-linkage forms the Tn antigen (GalNAcα1-O-Ser/Thr, CD175)
(7). Increased expression of Tn has
been attributed to inactive T-synthase, core 1 synthase
glycoprotein-N-acetylgalactosamine, and 3-β-galactosyltransferase
(C1GALT1) (8). Aberrant
O-glycosylation due to disruption of C1GALT1 contributes to
progression and metastasis of pancreatic cancer in mice (9). Additionally, C1GALT1 unusually requires
a private chaperone, core 1 β3-Gal-T-specific molecular chaperone
(COSMC), for folding and activity. Loss of COSMC due to somatic
mutations or hypermethylation also causes the dysregulation of
O-glycans and induces traditional oncogenic features, including
hyperproliferation, loss of tissue architecture and disruption of
basement membrane adhesion, and invasive growth of cancer (10).
It is important to understand the recognition site
of MUC1 antibody. The conventional MUC1 antibody recognizes amino
acids in the TR domain or a carbohydrate epitope of the MUC1
protein, and this lack of specificity leads to diametrically
opposite outcomes in lung cancer prognosis analysis (11–13).
Krebs von den Lungen-6 (KL-6), an important biomarker of
interstitial lung diseases (ILD), is now classified as a MUC1
protein. Regenerating type II pneumocytes are the primary cellular
source of KL-6/MUC1 in the lungs of patients with ILD. KL-6/MUC1 is
detectable in the sera of 70–100% of patients with various ILD
(14); however, it is also detected
in the sera of lung cancer patients (15), raising the false-positive rate of
lung cancer diagnosis. Further, an antibody that can selectively
recognize this cancer-specific change in sugar chain structure
would be useful for early diagnosis of lung cancer. Although
significant research on MUC1 in lung cancer has been conducted,
research evidence about the importance of MUC1-Tn as a diagnostic
and prognostic marker is lacking. Therefore, we screened a
high-specificity monoclonal antibody against MUC1 TR glycopeptides
bearing the Tn antigen (MUC1-Tn antigen epitope-defined antibody
[MUC1-Tn ED Ab]) through an innovative technique utilizing the
production of fine glycopeptides and a screening system for a
specific antibody using epitope analyses (16).
We aimed to examine the expression of the MUC1-Tn
antigen in primary lung adenocarcinoma (ADC) and assessed
relationships between its expression and clinical impact on
prognosis by an immunohistochemical (IHC) study with
paraffin-embedded tissue microarray (TMA) sections. This could lead
to the development of MUC1-Tn as a novel high-specificity
diagnostic marker and therapeutic target for lung ADC.
Materials and methods
Clinical lung cancer tissue
samples
A total of 175 lung ADC tissue samples were obtained
from patients who underwent surgery at the Hokkaido University
Hospital with the patients' informed consent. Detailed clinical and
pathological information was collected retrospectively for all
patients. The median follow-up period was 66.9 months for patients
who were alive. The median age of the patients at the time of
diagnosis was 68 years (interquartile range, 60–73 years), and
52.0% of the patients were women (Table
I). Histological diagnoses of tumors were based on the 2015
World Health Organization Classification (17). All tumors were staged according to
the pathological tumor/node/metastasis classification (8th edition)
of the Union for International Cancer Control (18). All tumors were histologically
reviewed by an experienced pathologist (K.C.H.).
 | Table I.Patient and tumor characteristics
(n=175). |
Table I.
Patient and tumor characteristics
(n=175).
| Characteristic | Value |
|---|
| Age (years), median
(IQR) | 68 (60,73) |
| Female, n (%) | 91 (52.0) |
| Tumor size (mm),
median (IQR) | 22.0 (15.0,
30.0) |
|
p-Stagea,
n |
|
| IA1-3
(p-T1a-cN0) | 99 |
| IB
(p-T2aN0) | 29 |
| IIA
(p-T2bN0) | 7 |
| IIB
(p-T3N0/p-T1a-cN1/p-T2a-bN1) | 17 |
| IIIA
(p-T1a-cN2/p-T2a-bN2) | 22 |
| IIIB
(p-T3N2/p-T4N2) | 1 |
| Preoperative serum
CEA (ng/ml), median (IQR) | 4.0 (2.7, 8.0) |
| Preoperative serum
CYFRA21-1 (ng/ml), median (IQR) | 1.3 (1.0–2.2) |
| Consolidation/tumor
ratio, median (IQR) | 1.0 (0.5, 1.0) |
|
18F-FDG-PET SUVmax,
median (IQR) | 2.4 (1.1–5.0) |
Tissue microarray construction and
immunohistochemistry
Tissue areas were selected for sampling based on
visual alignment with the corresponding hematoxylin and
eosin-stained sections on slides. TMA blocks were then constructed
using a manual tissue microarrayer (JF-4; Sakura Finetek Japan)
with a 1.5-mm diameter needle. Formalin-fixed paraffin-embedded
specimens were cut into 4-µm-thick sections, dewaxed with xylenes,
and rehydrated through a graded ethanol series. The IHC protocol
for MUC1, MUC1-Tn, Vimentin, and E-cadherin was as follows: i) for
antigen retrieval, sections were treated with Target Retrieval
Solution, Low pH (K8005; Dako) for Vimentin and High pH (EnVision
FLEX Mini Kit; Dako) for all other antibodies at 97°C for 20 min
before inhibiting endogenous peroxidase activity for 5 min at room
temperature (RT) with EnVision FLEX Peroxidase-Blocking Reagent
(Dako); ii) sections were incubated with clone SN-102 or SN-110
(1:500 and 1:100, respectively), commercially available MUC1
antibodies (Ma552, 1:100; Novocastra and Ma695, 1:1600; Biocare
Medical), anti-E-cadherin monoclonal antibody (M3612, clone NCH-38,
1:50; Dako), or anti-Vimentin monoclonal antibody (IR630, clone V9,
Ready-to-use; Dako) at the final concentration using mixed antibody
diluent (S2022; Dako) for 30 min at RT; iii) a polymer-based
detection with 3,3-Diaminobenzidine (DAB) system was used (DAB and
DAB plus Chromogen Solution, Dako); and iv) sections were
counterstained with hematoxylin. Slides were dehydrated and placed
on coverslips.
Evaluation of immunohistochemical
staining
Digital images of IHC-stained TMA slides were
obtained at ×4-x20 magnification using a whole slide scanner
(NanoZoomer 2.0-HT slide scanner; Hamamatsu Photonics). Annotation
of tumor regions on slides was performed by researchers (T.K and
H.U.) blinded to the clinical follow-up data using Aperio's
annotation software (ImageScope Viewing Software: Positive Pixel
Count v9.1, Aperio ImageScope®; Leica Microsystems
Inc.). The weighted intensity of staining was graded as follows:
grade 0 (negative), 1+ (WEAK positive: Intensity Threshold WEAK
[Upper Limit]=220, [lower limit]=175), 2+ (MEDIUM: [upper]=175,
[lower]=100), and 3+ (STRONG: [upper]=100, [lower]=0) by default.
The staining of SN-102 was quantified by IHC positivity, which was
calculated as the number of positive pixels stained at each
intensity level divided by the total number of pixels (the number
of positive and negative pixels). According to the IHC positivity
(Pos.), samples were finally divided into three groups based on
MUC1-Tn expression (the threshold leading to the 1st [Q1=0.04] and
3rd quartiles [Q3=0.14]): low MUC1-Tn expression was less than Q1
(MUC1-Tn-L, with a Pos. <0.04), moderate MUC1-Tn expression was
between Q1 and Q3 (MUC1-Tn-M, with 0.04≤ a Pos. <0.14), and high
MUC1-Tn expression was Q3 or greater (MUC1-Tn-H, with a Pos.
≥0.14).
The levels of E-cadherin and Vimentin staining were
independently evaluated by two investigators (T.K. and H.U.) and
supervised by an experienced pathologist (K.C.H.). Cancer cells
showing membrane and cytoplasmic staining for E-cadherin and those
showing cytoplasmic staining for Vimentin were considered positive.
The expression of E-cadherin was scored according to proportion and
intensity scores using the following criteria: i) proportion of
stained tumor cells was scored as 0 (0%), +1 (1–4%), +2 (5–49%),
and +3 (≥50%); and ii) staining intensity was scored as +1 (weak),
+2 (moderate), and +3 (strong). The scores were then multiplied to
get a final E-cadherin staining score; final scores ≥9 were
considered positive. The expression of Vimentin was evaluated
according to the proportion of Vimentin-positive cells (≥+2).
According to these results, we classified tumor samples into three
groups: epithelial (E-cadherin[+]/Vimentin[-]), intermediate
(E-cadherin[+]/Vimentin[+]) or (E-cadherin[-]/Vimentin[-]), and
mesenchymal (E-cadherin[-]/Vimentin[+]).
Statistical analysis
MUC1-Tn immunoreactivity was assessed for
association with clinicopathologic variables using the
χ2 test and Mann-Whitney U test for variables. Multiple
comparison analyses (Kruskal-Wallis test followed by Dunn's test)
were used to determine statistical significance for three groups.
Receiver operating characteristic (ROC) curves were drawn using the
data of MUC1-Tn positivity between lung cancer and normal lung, and
the area under the ROC curve (AUC) was calculated. The Kaplan-Meier
method was used to generate survival curves based on the status of
MUC1-Tn expression, and survival differences were analyzed using
the log-rank test. The primary end point was overall survival as
measured from the date of surgery to the time of death.
Disease-free survival was the period from surgery until the date of
disease relapse. Univariate and multivariate analyses were
performed using Cox proportional-hazards regression model. After
crude analysis, we adjusted for pathologic variables (pT and pN) in
the multivariate analysis. We used normal ranges for tumor markers
as cutoff values (carcinoembryonic antigen [CEA], 0–5.0 ng/ml and
cytokeratin 19 fragment [CYFRA21-1], 0–2.1 ng/ml) in hazard
analyses. The consolidation/tumor (C/T) ratio of the computed
tomography (CT) scan was dichotomized at its 1st quartile value of
0.5 because the median was 1.0 in uni- and multivariate analyses
using Cox's proportional hazards model. The maximum standardized
uptake value (SUVmax) of
18F-fluorodeoxyglucose positron emission tomography
(18F-FDG-PET) was dichotomized at its median value of
2.4. P<0.05 was considered statistically significant. All
analyses were performed using StatFlex version 6.0 for Windows
(Artech).
Results
Specificity and staining pattern of
MUC1-Tn in normal lung and lung ADC tissues
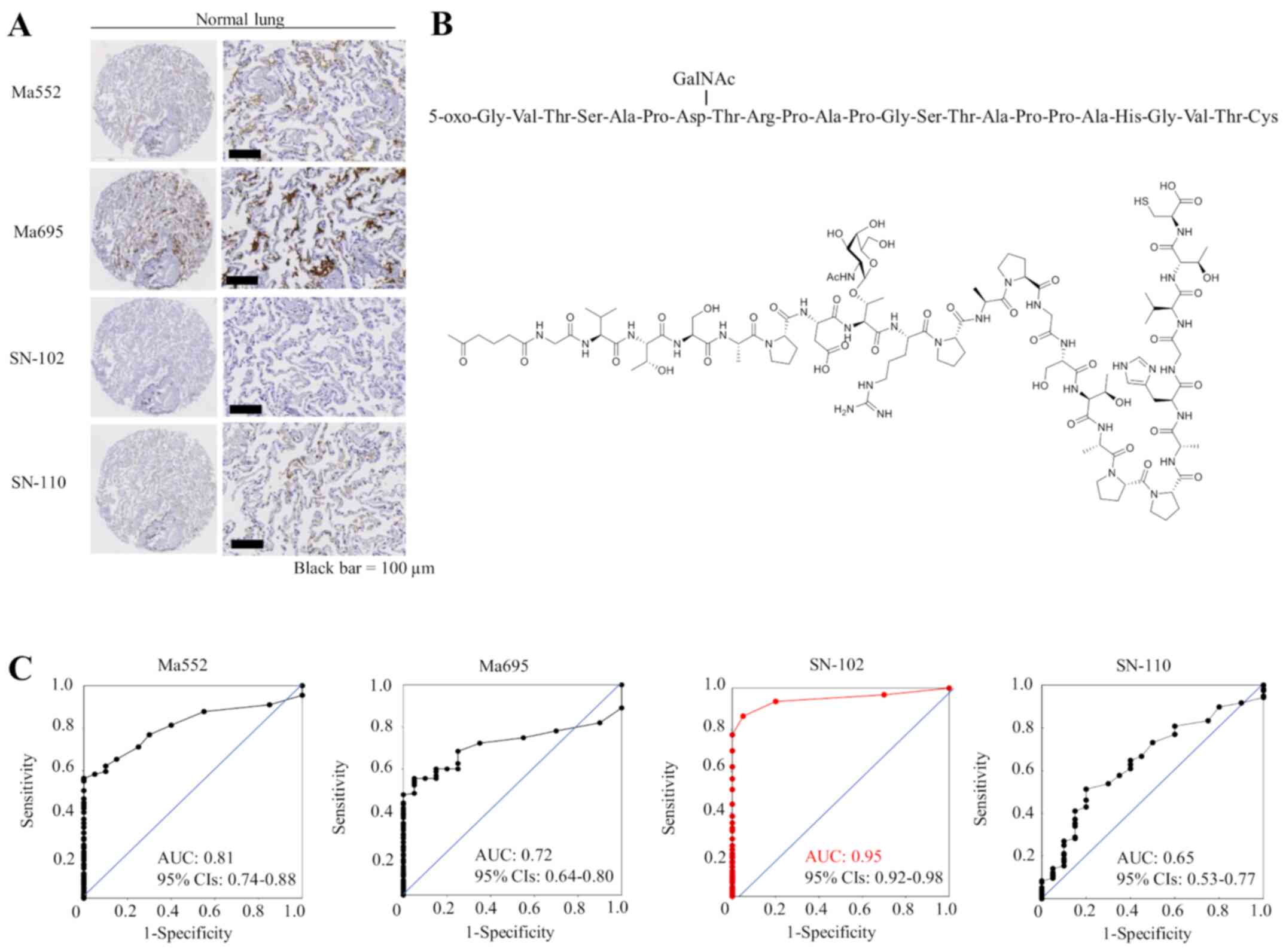
First, we examined the specificity of commercially
available MUC1 antibodies and antibodies that specifically
recognize MUC1 with cancer-associated sugar chain structures using
TMAs containing 20 normal lung tissue slides. Although commercially
available monoclonal antibodies against MUC1 (Ma552 and Ma695) and
the other MUC1-Tn ED antibody (clone SN-110) showed weak to
moderate staining in normal lung tissue, the SN-102 antibody did
not stain normal lung at all (Fig.
1A), suggesting that antibodies (except SN-102) may be
difficult to use as therapeutic tools for lung cancer. Immunogen
for SN-102 is shown in Fig. 1B.
Although SN-102 recognizes the GalNAc region, its precise binding
site remains unknown. The ROC curves and the AUC value for each
antibody are shown in Fig. 1C,
illustrating a high AUC value (0.95) for SN-102. Therefore, we
performed subsequent analyses using SN-102. There was little
staining in lung ADC tumor tissues using Ma552 and Ma695; however,
a high level of staining was seen using SN-102, even in the same
lung cancer TMA slides (Fig. 2A).
Thus, different expression patterns were observed when MUC1-Tn and
commercially available MUC1 antibodies were used. A representative
overall view of a TMA slide is shown in Fig. 2B, illustrating high specificity of
SN-102.
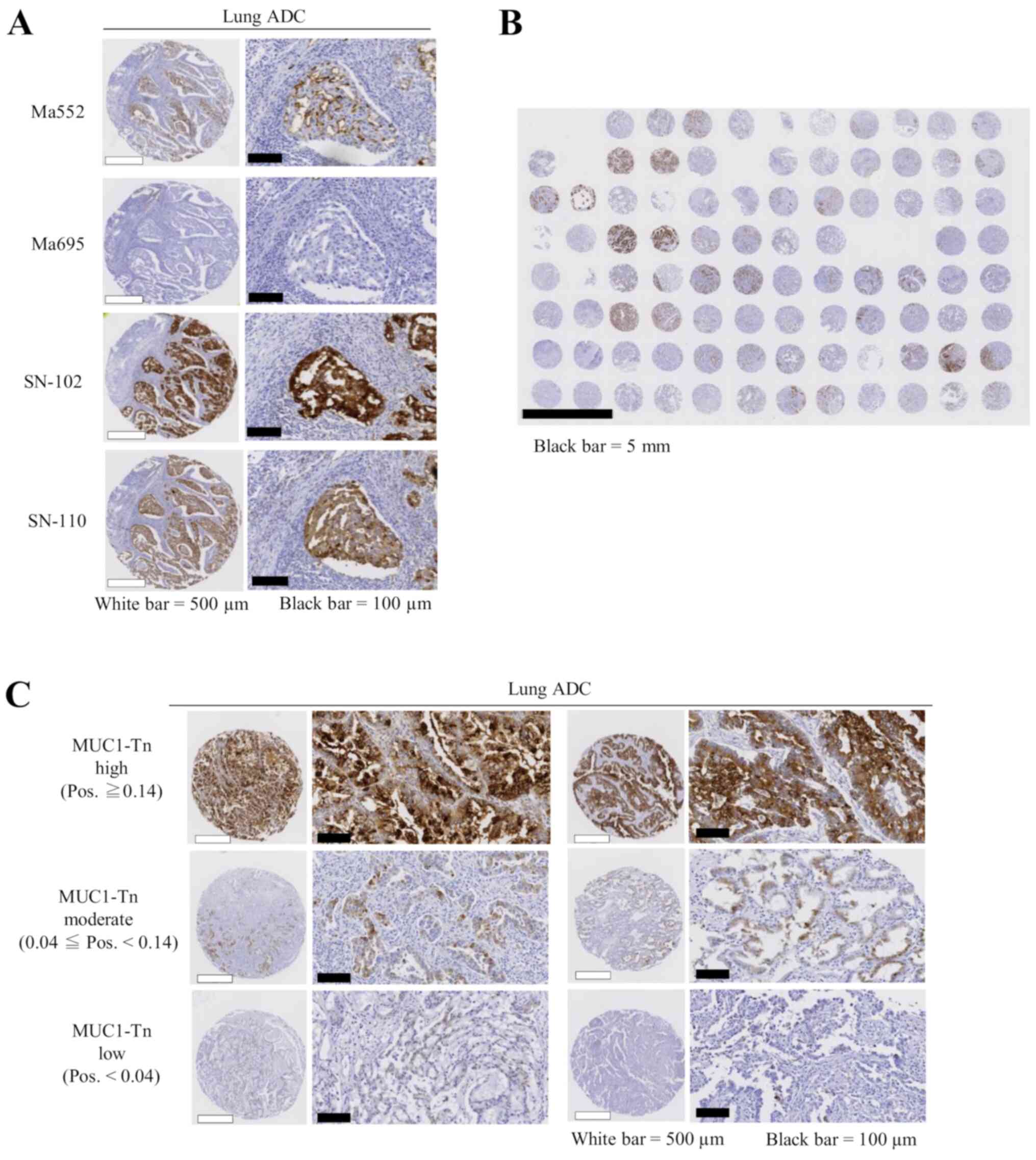 | Figure 2.Expression levels of MUC1-Tn in lung
cancer tissues. (A) MUC1-Tn exhibited stronger staining in tumor
cells than commercially available MUC1 antibodies. White scale
bars, 500 µm; black scale bars, 100 µm. (B) A representative
overall view of a tissue microarray slide for MUC1-Tn staining.
Black scale bar, 5 mm. (C) Representative examples of MUC1-Tn
protein expression in lung adenocarcinoma. High (top), moderate
(middle) and low (bottom) expression levels of MUC1-Tn. White scale
bars, 500 µm; black scale bars, 100 µm. ADC, adenocarcinoma;
MUC1-Tn, mucin 1 Tn-antigen; Pos., positivity; MUC1, mucin 1. |
MUC1-Tn expression and correlation to
clinicopathological parameters
Positive MUC1-Tn staining of tumor cells generally
showed a membranous and cytoplasmic pattern in cancer tissue
(Fig. 2C). Of the 175 lung ADC cases
examined, MUC1-Tn-L and MUC1-Tn-M were observed in 37 (21.1%) and
94 cases (53.7%), respectively. MUC1-Tn-H was observed in 44 cases
(25.1%) and was significantly associated with male sex (P=0.016),
cigarette smoking (P=0.005), pT status (P<0.001), pleural
invasion (P=0.008), lack of lymphatic and vascular invasion
(P<0.001 and P=0.005, respectively), high preoperative serum CEA
levels (P=0.010), and high preoperative serum CYFRA21-1 levels
(P=0.014). No correlation was noted between MUC1-Tn expression and
other clinicopathological variables (Table II).
 | Table II.Association between MUC1-Tn
expression and clinicopathological features in patients with lung
ADC. |
Table II.
Association between MUC1-Tn
expression and clinicopathological features in patients with lung
ADC.
| Variables | No. of cases | No. of cases with
high MUC1-Tn expression, n (%) |
P-valueb |
|---|
| All lung ADC
cases | 175 | 44 (25.1) |
|
| Age, years |
|
≤68 | 92 | 22 (23.9) | 0.690 |
|
>68 | 83 | 22 (26.2) |
|
| Sex |
|
Male | 84 | 28 (33.3) | 0.016d |
|
Female | 91 | 16 (17.6) |
|
| Smoking
statusc |
| Current
or ex-smoker | 107 | 34 (31.8) | 0.005d |
|
Non-smoker | 64 | 8 (12.5) |
|
| NA | 4 | 2 (50.0) |
|
| pT
statusa |
|
pT1a-c | 111 | 18 (16.2) |
<0.001d |
|
pT2a-b+pT3+pT4 | 64 | 26 (40.6) |
|
| pN
statusa |
|
pN0 | 144 | 34 (23.6) | 0.314 |
|
pN1-2 | 31 | 10 (32.3) |
|
| Pleural
invasion |
|
Negative | 130 | 26 (20.0) | 0.008d |
|
Positive | 45 | 18 (40.0) |
|
| Lymphatic
invasionc |
|
Negative | 29 | 14 (48.3) |
<0.001d |
|
Positive | 64 | 9 (14.1) |
|
| NA | 82 | 21 (25.6) |
|
| Vascular
invasionc |
|
Negative | 27 | 12 (44.4) | 0.005d |
|
Positive | 66 | 11 (16.7) |
|
| NA | 82 | 21 (25.6) |
|
| Pre-op Serum CEA
level, ng/ml |
|
≤5.0 | 108 | 20 (18.5) | 0.010d |
|
>5.0 | 67 | 24 (35.8) |
|
| Pre-op Serum
CYFRA21-1 levelc,
ng/ml |
|
≤2.1 | 125 | 25 (20.0) | 0.014d |
|
>2.1 | 44 | 17 (38.6) |
|
| NA | 6 | 2 (33.3) |
|
Correlation between MUC1-Tn expression
and radiological features of tumors
Fig. 3A shows
representative CT and 18F-FDG-PET scan images of lung
ADC cases and MUC1-Tn staining of the corresponding resected
specimens. A significantly higher C/T ratio from the preoperative
CT images was observed in tumors with MUC1-Tn-H (Fig. 3B, P=0.013). The SUVmax was
also higher in tumors with MUC1-Tn-H (P<0.001; Fig. 3C).
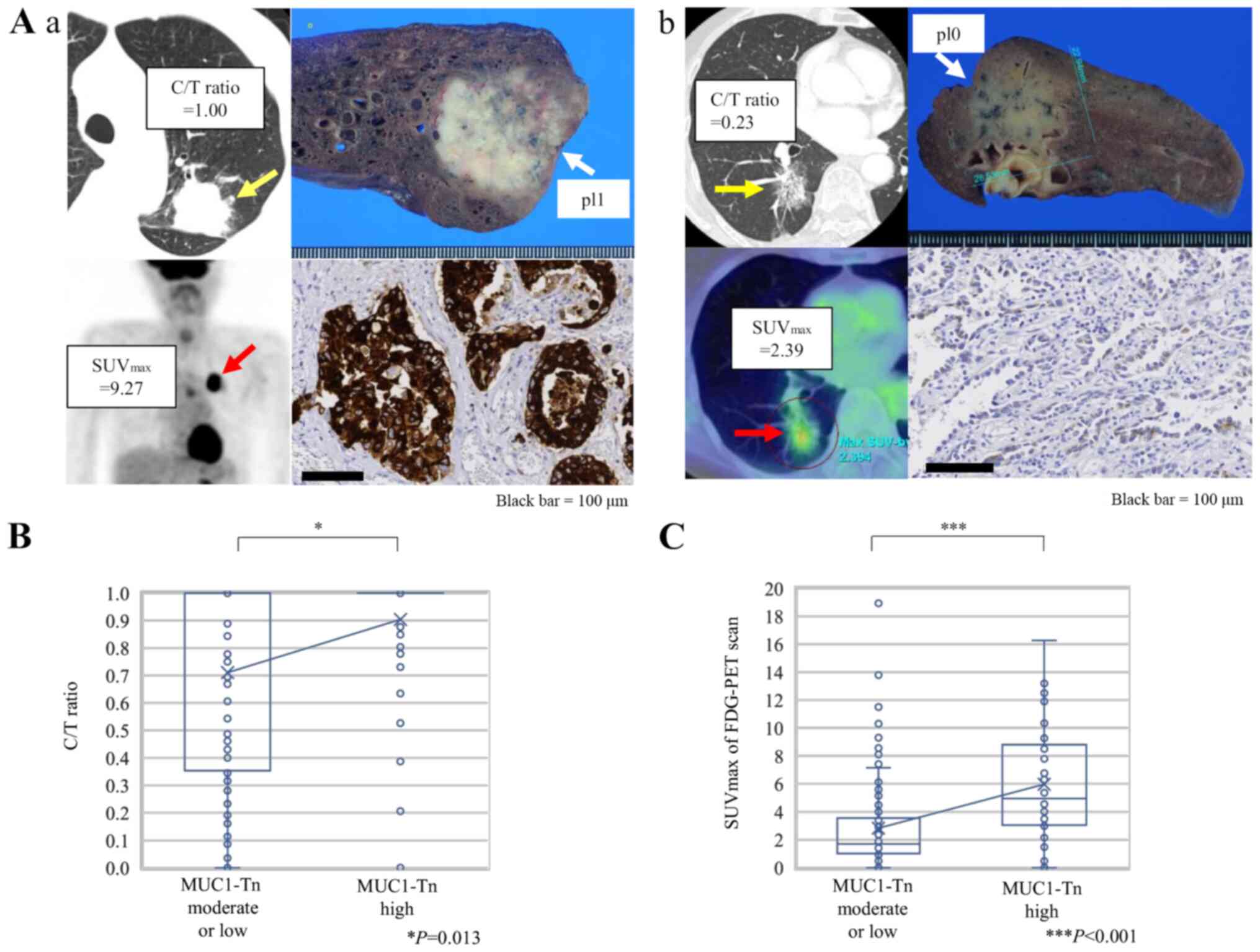 | Figure 3.Association between radiological
features and MUC1-Tn expression in lung adenocarcinoma. (A)
Representative CT and FDG-PET scan images according to MUC1-Tn
expression. (Aa) A case with high expression levels of MUC1-Tn.
(Ab) A case with low expression levels of MUC1-Tn. Scale bars, 100
µm. (B) Association between the C/T ratio and MUC1-Tn expression.
*P<0.05. (C) Association between SUVmax of FDG-PET
scan and MUC1-Tn expression. For the box-and-whisker plot, the ends
of the box are the upper and lower quartiles, and the median is
marked by a vertical line inside the box. The whiskers are two
lines outside the box that extend to the highest and lowest values
without outliers, which are ≥1.5 times the interquartile range. The
crosses represent the mean. ***P<0.001. MUC1-Tn, mucin 1
Tn-antigen; FDG-PET, 18F-fluorodeoxyglucose positron
emission tomography; pl0, tumor with no pleural involvement beyond
its elastic layer; pl1, tumor that has invaded beyond the elastic
layer of the visceral pleura but is not exposed on the pleural
surface; C/T ratio, consolidation/tumor ratio; SUVmax,
maximum standardized uptake value. |
Correlation between MUC1-Tn expression
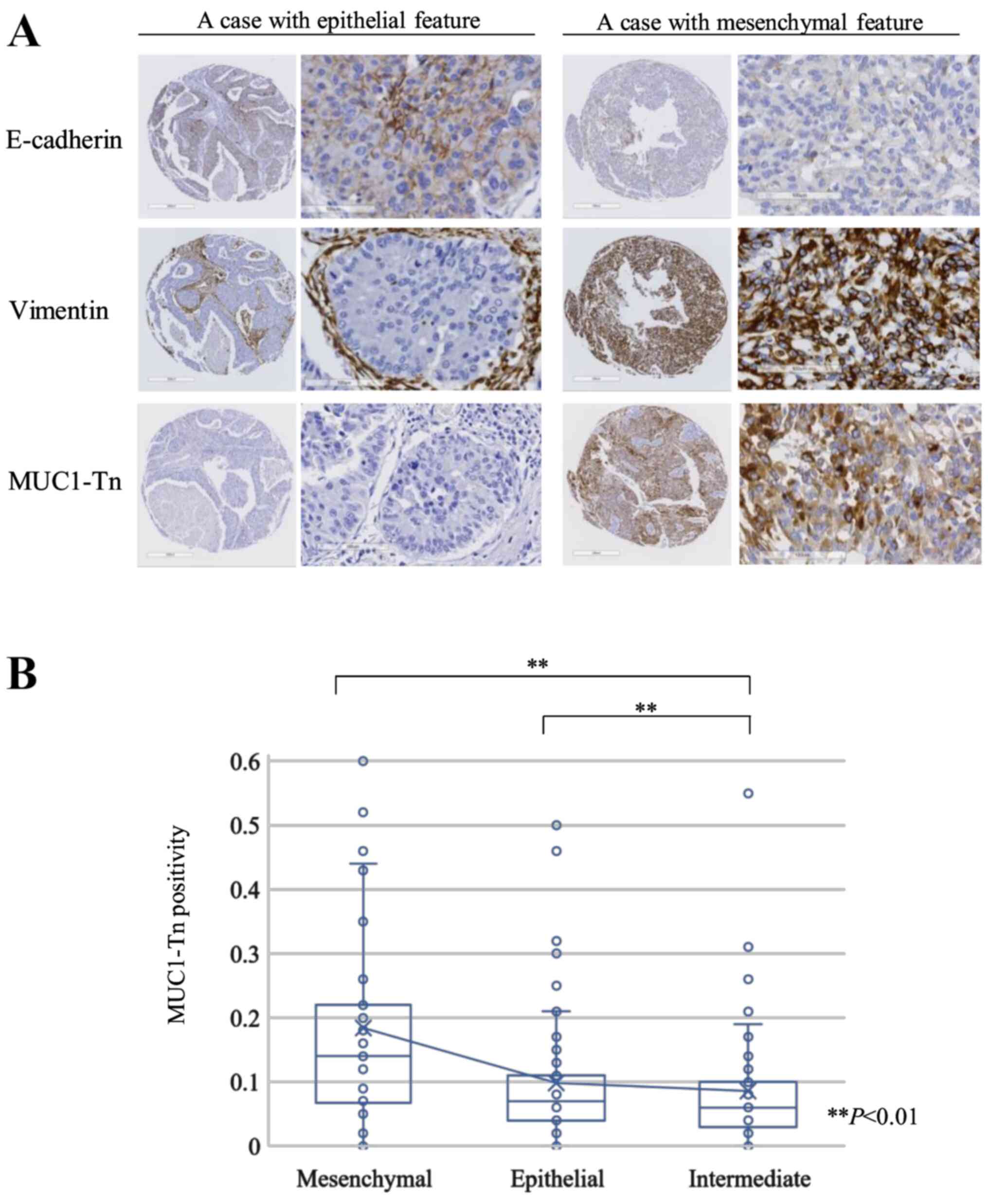
and epithelial-mesenchymal transition (EMT)
E-cadherin was detected in 97 of 171 lung ADC tumors
(56.7%), and Vimentin expression was positive in 68 tumors (39.8%).
According to the expression of E-cadherin and Vimentin, we
classified tumors into the epithelial (76 cases [44.4%]),
intermediate (49 cases [28.7%]), and mesenchymal (46 cases [26.9%])
groups. Fig. 4A shows representative
epithelial and mesenchymal tumors. The MUC1-Tn positivity of the
mesenchymal group was higher than that of the epithelial or
intermediate groups (P<0.01 and P<0.01, respectively;
Fig. 4B).
Prognostic significance of MUC1-Tn
expression
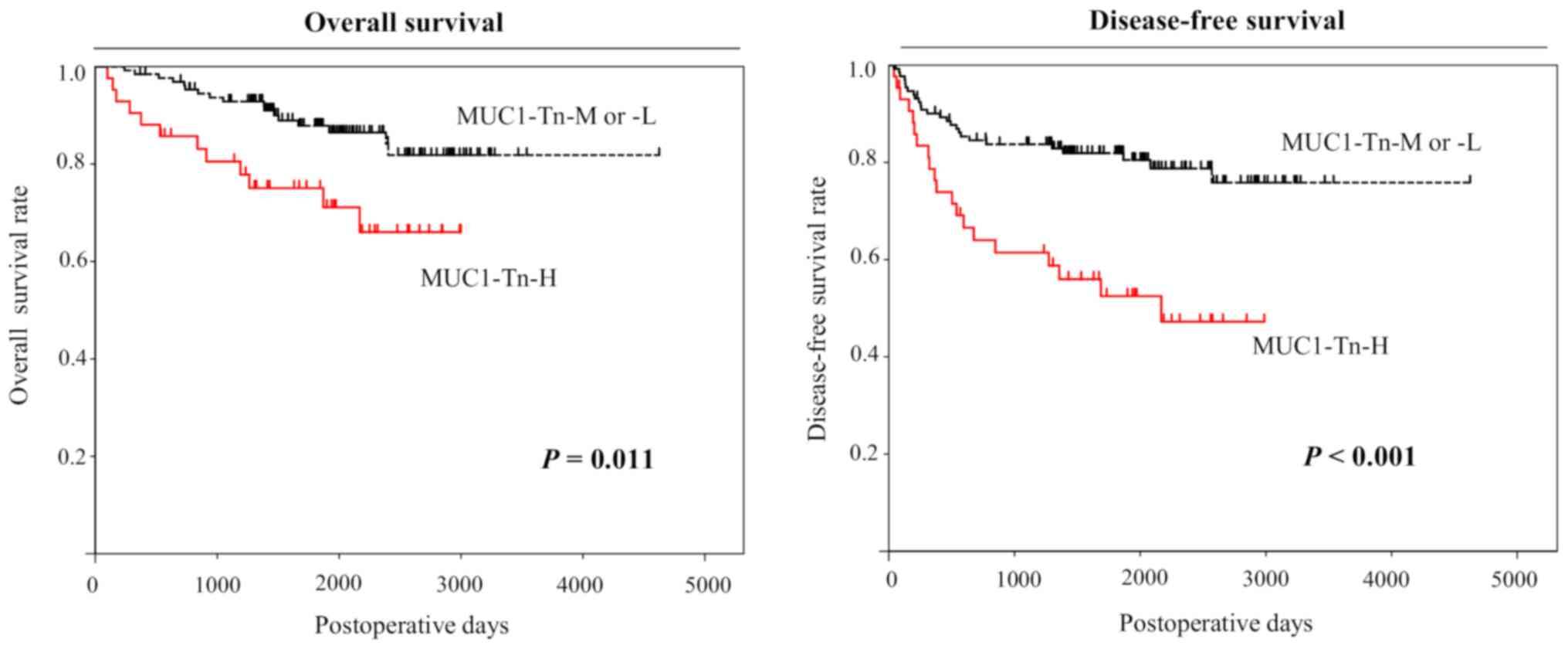
Analysis using the Kaplan-Meier method indicated
significant associations between MUC1-Tn-H in lung ADCs and 5-year
overall and disease-free survival (P=0.011 and P<0.001,
respectively, by the log-rank test; Fig.
5). We also performed univariate analysis to evaluate
associations between patient prognosis and other factors in
patients with lung ADC. Advanced pT status (P<0.001), advanced
pN status (P<0.001), advanced pleural invasion (P<0.001),
high preoperative CEA levels (P=0.002), high C/T ratio (P=0.002),
high SUVmax (P<0.001), and MUC1-Tn positivity
(P<0.001) were significantly associated with poor prognosis
(Table II). MUC1-Tn expression was
also identified as an independent prognostic factor in patients
with ADC (hazard ratio (HR) 1.965, 95% confidence intervals (CIs),
1.095–3.526, P=0.024), as were pT (HR 3.142, 95% CIs, 1.635–6.035,
P<0.001) and pN (HR 4.551, 95% CIs, 2.491–8.315, P<0.001)
status, in the pT and pN adjusted model by multivariate analysis
(Table III).
 | Table III.Cox proportional hazards model
analysis of prognostic factors in patients with adenocarcinoma
(n=175). |
Table III.
Cox proportional hazards model
analysis of prognostic factors in patients with adenocarcinoma
(n=175).
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (≤68 vs. >68
years) | 1.294 | 0.729–2.298 | 0.397 |
|
|
|
| Sex (male vs.
female) | 1.172 | 0.665–2.064 | 0.583 |
|
|
|
| Brinkman index
(≤400 vs. >400) | 1.239 | 0.699–2.196 | 0.463 |
|
|
|
| pT (pT2-4 vs.
pT1) | 5.255 | 2.843–9.715 |
<0.001a | 3.142 | 1.635–6.035 |
<0.001a |
| pN (pN1-2 vs.
pN0) | 7.013 | 3.936–12.495 |
<0.001a | 4.551 | 2.491–8.315 |
<0.001a |
| Pleural invasion
(positive vs. negative) | 3.523 | 1.933–6.226 |
<0.001a |
|
|
|
| CEA (>5.0 vs.
≤5.0 ng/ml) | 2.478 | 1.399–4.388 | 0.002a |
|
|
|
| CYRFA21-1 (>2.1
vs. ≤2.1 ng/ml) | 1.600 | 0.878–2.916 | 0.125 |
|
|
|
| C/T ratio (≤0.5 vs.
>0.5) | 9.687 | 2.348–39.955 | 0.002a |
|
|
|
| FDG-PET
SUVmax (>2.4 vs. ≤2.4) | 4.338 | 2.202–8.546 |
<0.001a |
|
|
|
| MUC1-Tn expression
(high vs. moderate/low) | 2.797 | 1.579–4.956 |
<0.001a | 1.965 | 1.095–3.526 | 0.024a |
Discussion
In the present study, we found that the MUC1-Tn
epitope-defined antibody has high specificity for lung ADC cells
and moderate or high MUC1-Tn expression was observed in
three-quarters of lung ADCs. High MUC1-Tn is strongly associated
with poor survival and the results presented herein have potential
to open up novel therapeutic target for lung ADCs.
The specificity of antibodies for cancer cells is
pivotal for antibody therapy. This is especially true in the
treatment of lung cancer, as patients with other lung disorders
such as interstitial pneumonia and emphysema sometimes have fatal
consequences once respiratory side effects develop. In this study,
a novel epitope-defined antibody that specifically recognizes MUC1
with cancer-associated carbohydrate antigens, O-glycans including
Tn and sialyl-Tn, was developed by our original epitope-mapping
analyses. Furthermore, screening normal lung and lung cancer
tissues using TMAs, it was found that SN-102, which recognizes the
Tn antigen, had high specificity for cancer tissues, and there was
no expression in normal lung tissue. Therefore, our results are
completely different compared with those reported in previous
studies regarding antibodies that simply recognize MUC1. Recently,
genetically modified T cells expressing chimeric antigen receptors
that recognized MUC1-Tn were found to have therapeutic efficacy in
xenograft models of T cell leukemia and pancreatic cancer (19). Although further investigation is
needed to validate this result, targeting cancer-associated
carbohydrate antigens can be important for the treatment of lung
cancer.
MUC1-Tn overexpression is common in multiple cancer
types, such as gastric cancer (20),
colon cancer (21,22) and breast cancer (23). Increased O-linked glycosylation of
MUC1, including linkage of the Tn antigen and sialyl-Lewis-X, has
been also reported in lung cancer (24). O-glycans (Tn, sTn and T antigen) are
synthesized in the Golgi apparatus using several
glycosyltransferases (such as T-synthase and C1GALT1). The unique
molecular chaperone of T-synthase is COSMC, which aids its folding
in the endoplasmic reticulum. Abnormal expression of C1GALT1,
somatic mutations, or hypermethylation of COSMC cause the
dysregulation of O-glycans (7,10,25). To
confirm our results using clinical specimens, we first wanted to
find MUC1-Tn-expressed cell lines to conduct an in vitro
experiment. We performed immunohistochemical analyses using a
MUC1-Tn monoclonal antibody SN-102 with cellblock materials of
several lung cancer cell lines. However, we did not find any cell
line with MUC1-Tn high expression (data not shown). To obtain a
MUC1-Tn-expressed cell line, we will need to perform cell sorting
for C1GALT1 or COSMC knockout cells (9,10).
Although the role of Tn antigen in the development of cancer is
still unclear, several studies have reported the underlying
mechanisms of the relationship between MUC1-Tn and poorer
prognosis. The knockout of C1GALT1 enhanced the growth and
migration of pancreatic cancer cells (9). Additionally, COSMC knockout cells
expressing truncated O-glycans (such as Tn and/or STn) promote cell
proliferation, prevent differentiation, enhance invasive and
migratory properties, and impair cell-cell adhesion in culture
(10). Aberrant forms or amounts of
O-glycans are also thought to provide ligands that interact with
growth factors, lectins, selectins, and cell adhesion molecules in
cancer cells. The dense layers of O-glycans may also help control
the local microenvironment and protect cancer cells from adverse
growth conditions during invasion and metastasis (26). This study showed that MUC1-Tn
overexpression correlates with tumor extension and pleural
invasion. Besides, the binding between Tn antigen and macrophage
galactose-type C-type lectin (MGL) in situ, which is
expressed in dendritic cells and macrophages, may have
immunosuppressive effects and may enable the tumor escape
immunosurveillance (27–29). High expression of mucin and altered
glycosylation are related to the expression of galectin-3, which
can bind to Tn antigen, contributing to metastasis and escape from
immunosurveillance (30). This
evasion of immune surveillance may be correlated with poor
prognosis. A previous study showed that stage I lung ADCs with EMT
conversion show solid-dominant nodules on CT that are not visible
in ADCs without conversion, and the SUVmax is higher in
the mesenchymal group than in the epithelial group (31). Our study also showed that tumors with
high expression of MUC1-Tn had higher SUVmax on FDG-PET
scans, suggesting MUC1-Tn overexpression also represents a more
malignant feature of lung cancer as per radiological examinations.
Hypoxic stress causes a shift from aerobic oxidative
phosphorylation to anaerobic glycolysis, with high rates of glucose
and glutamine uptake (32). In this
context, adaptation to hypoxia and cellular energetic reprogramming
occurs mostly in a hypoxia-inducible factor-1 (HIF-1)
alpha-dependent manner and is frequently accompanied by cell
dedifferentiation and acquisition of mesenchymal features (33). Expression and/or activity levels of
glucose transporters contribute to the pattern and intensity of
18F-FDG. MUC1 is also directly regulated by HIF-1α and
affects the invasive and migratory properties of cancer cells
(34). Depolarized MUC1 expression
was significantly associated with the expression of glucose
transporters-1 (GLUT1) and poor outcomes in lung cancer (35). It is expected that the relationship
between MUC1-Tn expression and glucose transporters will be
examined in future.
Aberrant glycosylation of MUC1-N in response to
cigarette smoke initiates EMT by degrading E-cadherin and damages
cell-cell adhesions, resulting in changes in cellular polarity. The
cells become spindle-shaped and motile, an early indicator of
malignant transformation and an invasive nature (6,36).
Changes in mucin localization due to loss of epithelial cell
polarity are also associated with malignant alteration (37). In this study, we proved that high
levels of MUC1-Tn expression were correlated with smoking habits,
low E-cadherin, and high Vimentin, suggesting mesenchymal
features.
This study has some limitations. We determined the
EMT status solely via E-cadherin and Vimentin expression. This may
need further validation using a panel of EMT markers such as Snail
and Twist. Analyses of morphological characteristics or phenotypes
of EMT should be considered. Additionally, some cases had no
MUC1-Tn expression even after EMT; therefore, further
investigation, including expression of other O-glycans, may be
needed.
In conclusion, moderate to high levels of MUC1-Tn
expression were observed in three-quarters of primary lung ADC
patients, and normal lung tissue had no expression of MUC-Tn.
Overexpression of MUC1-Tn was strongly associated with EMT
potential and poor survival in patients with lung ADC. These
results strongly suggest that MUC1-Tn can be a valuable marker in
patients who are likely to show poor prognosis and can be used to
develop improved antibody immunotherapeutics for lung ADC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
TK performed the experimental design, most of the
experiments and analyses, drafted the manuscript, and was involved
in the conception and design of the study. HU contributed to the
preparation and the review of the tissue microarray. KCH
participated in the planning/design of the tissue microarray
experiments and supervised pathological review. TK, HU and KCH
assessed all raw data and confirmed the authenticity. YH was also
involved in collecting clinical tissue samples and accessing
clinical databases. AN, AO and KT conducted some supporting
immunohistochemical experiments. KN and MS were involved in the
production of MUC1-Tn ED antibody and in acquisition of data
regarding immunogen and the chemical formula for the antibody. KK,
SW, YM and YH supervised the study and were involved in the
conception and design of the study, and proofread the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The protocol was approved by the appropriate
Institutional Review Board of Hokkaido University Hospital
(approval no. #015-0367; Sapporo, Japan). Each patient provided
written informed consent at the time of surgery.
Patient consent for publication
Not applicable.
Competing interests
KN and MS are affiliated with Medicinal Chemistry
Pharmaceuticals, Co., Ltd., who made the antibody that the study is
largely based on. The other authors declare that they have no
competing interests.
References
|
1
|
National Cancer Institute: Surveillance,
Epidemiology, and End Results Program. Accessed from. https://seer.cancer.gov/statfacts/html/common.html2020
|
|
2
|
Nath S and Mukherjee P: MUC1: A
multifaceted oncoprotein with a key role in cancer progression.
Trends Mol Med. 20:332–342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parry S, Silverman HS, McDermott K, Willis
A, Hollingsworth MA and Harris A: Identification of MUC1
proteolytic cleavage sites in vivo. Biochem Biophys Res Commun.
283:715–720. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siddiqui J, Abe M, Hayes D, Shani E, Yunis
E and Kufe D: Isolation and sequencing of a cDNA coding for the
human DF3 breast carcinoma-associated antigen. Proc Natl Acad Sci
USA. 85:2320–2323. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gendler S, Taylor-Papadimitriou J, Duhig
T, Rothbard J and Burchell J: A highly immunogenic region of a
human polymorphic epithelial mucin expressed by carcinomas is made
up of tandem repeats. J Biol Chem. 263:12820–12823. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qu J, Yu H, Li F, Zhang C, Trad A, Brooks
C, Zhang B, Gong T, Guo Z, Li Y, et al: Molecular basis of antibody
binding to mucin glycopeptides in lung cancer. Int J Oncol.
48:587–594. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fu C, Zhao H, Wang Y, Cai H, Xiao Y, Zeng
Y and Chen H: Tumor-associated antigens: Tn antigen, sTn antigen,
and T antigen. HLA. 88:275–286. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ju T and Cummings RD: A unique molecular
chaperone Cosmc required for activity of the mammalian core 1 beta
3-galactosyltransferase. Proc Natl Acad Sci USA. 99:16613–16618.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chugh S, Barkeer S, Rachagani S,
Nimmakayala RK, Perumal N, Pothuraju R, Atri P, Mahapatra S, Thapa
I, Talmon GA, et al: Disruption of C1galt1 gene promotes
development and metastasis of pancreatic adenocarcinomas in mice.
Gastroenterology. 155:1608–1624. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Radhakrishnan P, Dabelsteen S, Madsen FB,
Francavilla C, Kopp KL, Steentoft C, Vakhrushev SY, Olsen JV,
Hansen L, Bennett EP, et al: Immature truncated O-glycophenotype of
cancer directly induces oncogenic features. Proc Natl Acad Sci USA.
111:E4066–E4075. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guddo F, Giatromanolaki A, Koukourakis MI,
Reina C, Vignola AM, Chlouverakis G, Hilkens J, Gatter KC, Harris
AL and Bonsignore G: MUC1 (episialin) expression in non-small cell
lung cancer is independent of EGFR and c-erbB-2 expression and
correlates with poor survival in node positive patients. J Clin
Pathol. 51:667–671. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nagai S, Takenaka K, Sonobe M, Ogawa E,
Wada H and Tanaka F: A novel classification of MUC1 expression is
correlated with tumor differentiation and postoperative prognosis
in non-small cell lung cancer. J Thorac Oncol. 1:46–51. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuemmel A, Single K, Bittinger F, Faldum
A, Schmidt LH, Sebastian M, Micke P, Taube C, Buhl R and Wiewrodt
R: TA-MUC1 epitope in non-small cell lung cancer. Lung Cancer.
63:98–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ishikawa N, Hattori N, Yokoyama A and
Kohno N: Utility of KL-6/MUC1 in the clinical management of
interstitial lung diseases. Respir Investig. 50:3–13. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tanaka S, Hattori N, Ishikawa N, Shoda H,
Takano A, Nishino R, Okada M, Arihiro K, Inai K, Hamada H, et al:
Krebs von den Lungen-6 (KL-6) is a prognostic biomarker in patients
with surgically resected nonsmall cell lung cancer. Int J Cancer.
130:377–387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Naito S, Takahashi T, Onoda J, Uemura S,
Ohyabu N, Takemoto H, Yamane S, Fujii I, Nishimura SI and Numata Y:
Generation of novel anti-MUC1 monoclonal antibodies with designed
carbohydrate specificities using MUC1 glycopeptide library. ACS
Omega. 2:7493–7505. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Travis W, Brambilla E, Burke AP, Marx A
and Nicholson AG: WHO Classification of Tumours of the Lung,
Pleura, Thymus and Heart. International Agency for Research on
Cancer; Lyon: 2015
|
|
18
|
Brierley JD, Gospodarowicz MK and
Wittekind C: Union for International Cancer Control (UICC): TNM
Classification of Malignant Tumours. 8th edition. Wiley-Blackwell;
Hoboken, NJ: pp. p2722017
|
|
19
|
Posey AD Jr, Schwab RD, Boesteanu AC,
Steentoft C, Mandel U, Engels B, Stone JD, Madsen TD, Schreiber K,
Haines KM, et al: Engineered CAR-T cells targeting the
cancer-associated Tn-glycoform of the membrane mucin MUC1 control
adenocarcinoma. Immunity. 44:1444–1454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li T, Mo C, Qin X, Li S, Liu Y and Liu Z:
Glycoprofiling of early gastric cancer using lectin microarray
technology. Clin Lab. 64:153–161. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Konno A, Hoshino Y, Terashima S, Motoki R
and Kawaguchi T: Carbohydrate expression profile of colorectal
cancer cells is relevant to metastatic pattern and prognosis. Clin
Exp Metastasis. 19:61–70. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Byrd JC and Bresalier RS: Mucins and mucin
binding proteins in colorectal cancer. Cancer Metastasis Rev.
23:77–99. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Welinder C, Baldetorp B, Blixt O, Grabau D
and Jansson B: Primary breast cancer tumours contain high amounts
of IgA1 immunoglobulin: An immunohistochemical analysis of a
possible carrier of the tumour-associated Tn antigen. PLoS One.
8:e617492013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pinto R, Carvalho AS, Conze T, Magalhães
A, Picco G, Burchell JM, Taylor-Papadimitriou J, Reis CA, Almeida
R, Mandel U, et al: Identification of new cancer biomarkers based
on aberrant mucin glycoforms by in situ proximity ligation. J Cell
Mol Med. 16:1474–1484. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ju T, Lanneau GS, Gautam T, Wang Y, Xia B,
Stowell SR, Willard MT, Wang W, Xia JY, Zuna RE, et al: Human tumor
antigens Tn and sialyl Tn arise from mutations in Cosmc. Cancer
Res. 68:1636–1646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Häuselmann I and Borsig L: Altered
tumor-cell glycosylation promotes metastasis. Front Oncol.
4:282014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Higashi N, Fujioka K, Denda-Nagai K,
Hashimoto S, Nagai S, Sato T, Fujita Y, Morikawa A, Tsuiji M,
Miyata-Takeuchi M, et al: The macrophage C-type lectin specific for
galactose/N-acetylgalactosamine is an endocytic receptor expressed
on monocyte-derived immature dendritic cells. J Biol Chem.
277:20686–20693. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ju T, Wang Y, Aryal RP, Lehoux SD, Ding X,
Kudelka MR, Cutler C, Zeng J, Wang J, Sun X, et al: Tn and
sialyl-Tn antigens, aberrant O-glycomics as human disease markers.
Proteomics Clin Appl. 7:618–631. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Napoletano C, Rughetti A, Agervig Tarp MP,
Coleman J, Bennett EP, Picco G, Sale P, Denda-Nagai K, Irimura T,
Mandel U, et al: Tumor-associated Tn-MUC1 glycoform is internalized
through the macrophage galactose-type C-type lectin and delivered
to the HLA class I and II compartments in dendritic cells. Cancer
Res. 67:8358–8367. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kölbl AC, Jeschke U, Friese K and
Andergassen U: The role of TF- and Tn-antigens in breast cancer
metastasis. Histol Histopathol. 31:613–621. 2016.PubMed/NCBI
|
|
31
|
Matsubara T, Tagawa T, Takada K, Toyokawa
G, Shimokawa M, Kozuma Y, Akamine T, Haro A, Osoegawa A and Mori M:
Clinical and prognostic significance of the epithelial-mesenchymal
transition in stage IA lung adenocarcinoma: A propensity
score-matched analysis. Clin Lung Cancer. 20:e504–e513. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Peixoto A, Fernandes E, Gaiteiro C, Lima
L, Azevedo R, Soares J, Cotton S, Parreira B, Neves M, Amaro T, et
al: Hypoxia enhances the malignant nature of bladder cancer cells
and concomitantly antagonizes protein O-glycosylation extension.
Oncotarget. 7:63138–63157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aubert S, Fauquette V, Hémon B, Lepoivre
R, Briez N, Bernard D, Van Seuningen I, Leroy X and Perrais M:
MUC1, a new hypoxia inducible factor target gene, is an actor in
clear renal cell carcinoma tumor progression. Cancer Res.
69:5707–5715. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kaira K, Okumura T, Nakagawa K, Ohde Y,
Takahashi T, Murakami H, Naito T, Endo M, Kondo H, Nakajima T, et
al: MUC1 expression in pulmonary metastatic tumors: A comparison of
primary lung cancer. Pathol Oncol Res. 18:439–447. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hollingsworth MA and Swanson BJ: Mucins in
cancer: Protection and control of the cell surface. Nat Rev Cancer.
4:45–60. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kufe DW: Mucins in cancer: Function,
prognosis and therapy. Nat Rev Cancer. 9:874–885. 2009. View Article : Google Scholar : PubMed/NCBI
|