Introduction
Gastric cancer (GC) is one of the most common
gastrointestinal malignant tumors, with high morbidity and
mortality rates (1,2). In 2015, China reported ~498,000
mortalities from GC, accounting for 17.7% of all malignant cases
(498,000/2.814 million), and it ranked second among all types of
tumors (1). Surgery remains the most
effective treatment for GC (3,4).
However, identification of important molecular functions in the
progression of GC will contribute to the development of novel
effective treatment strategies.
Circular RNAs (circRNAs) are a type of closed
circular RNA molecule formed by the cyclization of linear RNA
sequences at the 5′ and 3′ downstream ends, which lack a 5′ cap and
3′ poly (adenylate) tail structure (5). CircRNAs are stable in nature and are
not easily degraded by nucleic acid endonuclease (6,7).
CircRNAs exist in a variety of mammalian cells and are involved in
gene transcription and post-transcriptional expression regulation
(7). Recently, several studies have
demonstrated that circRNAs play an important role in the occurrence
and development of different types of diseases (5,6,8–10),
including cancer (11,12). In addition, previous studies have
reported that the abundance of total circRNAs in cancer tissues is
associated with the degree of cancer metastasis (12,13).
CircRNAs are abundant in GC and play an important role in the
occurrence and development of GC (14–16).
Thus, the different expression levels of circRNAs and their
molecular mechanisms can provide novel insight and methods for the
diagnosis, treatment plan and prognosis of patients with GC.
In the present study, bioinformatics analysis was
performed to select an circRNA molecule in GC from the Gene
Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo). The results
demonstrated that hsa_circ_0076305 (circPGC) was downregulated in
GC, suggesting that circPGC may inhibit the proliferation, and
promote the migration and invasion of AGS cells. In addition, the
clinicopathological characteristics of patients with GC were
assessed to identify the migration-proliferation dichotomy role of
circPGC in GC development. The results of the present study
demonstrated that the novel circRNA, circPGC, affected the
progression of GC with migration-proliferation dichotomy.
Materials and methods
Patient samples and cell lines
Bioinformatics analysis was performed to select an
circRNA molecule in GC from the GEO database (GSE100170 dataset)
(13). A total of nine pairs of GC
and paracancerous tissue samples (Table
SIII) were collected from the Zhongshan Hospital of Xiamen
University between September 2012 and April 2014 and stored at
−80°C until further experimentation. In addition, 30 cDNA GC and
paracancerous tissue samples were purchased from Shanghai Outdo
Biotech Co., Ltd. (cat. no. cDNA-HStmA060CS0).
The AGS GC cell line was kindly provided by the
Shanghai Stem Cell Bank of the Chinese Academy of Sciences
(https://www.cellbank.org.cn). Cells were
maintained in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.), at 37°C with 5% CO2.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from GC and paracancerous
tissues, as well as treated AGS GC cells using TRIzol®
reagent (Takara Bio, Inc.). Total RNA was reverse transcribed into
cDNA using the PrimeScript RT reagent kit (cat. no. RR047A; Takara
Bio, Inc.). qPCR was subsequently performed on an ABI 7500 system
using ChamQ SYBR qPCR Master Mix (cat. no. Q311; Vazyme Biotech
Co., Ltd.), according to the manufacturer's instructions. The
primer sequences used for qPCR are listed in Table SII. Relative expression levels were
normalized to the internal reference gene GAPDH. Amplification and
detection were run in ABI 7500 system with an initial cycle of 95°C
for 10 sec, followed by 40 cycles of 95°C for 5 sec, 60°C for 30
sec and 72°C for 5 sec. Relative expression levels were calculated
using the threshold cycle (2−ΔΔCq) method and normalized
to GAPDH (17,18). The primer sequences used are listed
in Table SI.
Plasmid construction and
transfection
The circPGC cDNA template was extracted from AGS
cells and amplified using Prime STAR HS DNA Polymerase (cat. no.
R010A; Takara Bio, Inc.). Similarly, the no-load plasmid
pcDNA3.1(+) CircRNA Mini Vector which was purchased from the
nonprofit plasmid repository Addgene (cat. no. 60648) was
linearized via PCR. The target DNA template was extracted from AGS
cells. PrimeSTAR® HS DNA Polymerase was purchased from
Taraka (cat. no. R010A). The PCR condition was 1 cycle of 98°C for
2 min, and 40 cycles of 98°C for 10 sec, 57°C for 5 sec, 72°C for 1
min, and then 1 cycle of 72°C for 10 min followed by cooling down
to 4°C. The PCR products were visualized on SYBR green stained 1%
agarose gels. The primer sequences are listed in Table SI. The results demonstrated that
circPGC circularized with exons 3–9 of PGC mRNA (Fig. S1). The fragment of PGC exons 3–9 was
constructed into the pcDNA3.1(+) CircRNA Mini Vector via seamless
cloning, and subsequently circularized into a circular RNA
molecular structure via back splicing (19). The primer sequences used are listed
in Table SI. Agarose gel
electrophoresis was subsequently performed to separate and purify
the DNA fragment using the OMEGA Gel Extraction kit (cat. no.
D2500; Omega Bio-Tek, Inc.). The HB-infusion Master mix (Hanbio
Biotechnology Co., Ltd.) was used to construct the circPGC
expression vector, according to the manufacturer's
instructions.
AGS cells (5×106) were cultured in
RPMI-1640 medium for 24 h at 37°C until they reached 70–80%
confluence. Cells were transfected with 1 µg circPGC expression
vector (circPGC) or empty vector (pcDNA 3.1) for 48 h at 37°C using
Lipofectamine® 3000 reagent (Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. RT-qPCR
analysis was performed to identify the cyclization of circPGC and
validate the resistance of circPGC to RNase R digestion in AGS
cells 48 h post-transfection.
Cell proliferation analysis
The effects of circPGC overexpression on cell
proliferation were assessed via the Cell Counting Kit-8 (CCK-8) and
colony formation assays. Briefly, AGS cells with stable circPGC
overexpression in the logarithmic phase were seeded into 6-well
plates at a density of 500 cells/well and incubated for 10 days at
37°C in a 5% CO2 incubator. Following incubation, cells
were fixed with 4% paraformaldehyde for 20 min and stained with
0.1% crystal violet for 2 min at room temperature. Cell colonies
were observed and counted under an inverted fluorescence microscope
(Olympus IX51; Olympus Corporation; magnification, ×200).
Similarly, the CCK-8 assay (Beyotime Institute of
Biotechnology) was performed according to the manufacturer's
instructions. AGS cells (3,000 cells/well) were incubated with
CCK-8 reagent for 3 h and cell proliferation was subsequently
analyzed at a wavelength of 450 nm.
Wound healing assay
Cells were transfected with plasmid and cultured
until they reached 100% confluence. A wound gap was carefully
scratched across the surface of the cells using a sterile 10 µl
pipette tip, and the scraped cells were removed using PBS. Cells
were re-cultured in serum-free RPMI-1640 medium and incubated at
37°C in a 5% CO2 incubator. Cells were observed under an
inverted fluorescence microscope (magnification, ×200, Olympus
IX51; Olympus Corporation).
Cell migration and invasion
assays
A 24-well chamber with an 8-µm pore size (cat. no.
3422; Corning, Inc) was used to detect the cell migratory and
invasive abilities. A total of 5×104 AGS cells were
plated in the upper chambers of Transwell plates in 200 µl
serum-free medium. For the invasion assay, Transwell membranes were
precoated with Matrigel in 4°C for 12 h (BD Biosciences). Medium
supplemented with 10% FBS was plated in the lower chambers.
Following incubation at 37°C for 12 h, cells on the upper chambers
were removed using a cotton swab, while the migratory cells were
fixed with 4% paraformaldehyde for 30 min and stained with 0.1%
crystal violet for 20 min at room temperature. Stained cells were
counted using an inverted fluorescence microscope (magnification,
×200).
Western blotting
Total cellular protein was extracted using RIPA
lysis buffer (cat. no. 20101ES60; Yeasen Biotech Shanghai) and
quantified using a BCA kit (cat. no. 23252; Thermo Fisher
Scientific, Inc.). Protein samples (50 µg) were loaded and
separated by 12% SDS-PAGE. The separated proteins were subsequently
transferred onto polyvinylidene difluoride membranes (EMD
Millipore) and blocked with 5% non-fat milk for 1 h at room
temperature. The membranes were incubated with primary antibodies
against: E-cadherin (dilution ratio 1:2,000; cat. no. SC7870; Santa
Cruz Biotechnology, Inc.), N-cadherin (dilution ratio 1:1,000; cat.
no. 22018-1; ProteinTech Group, Inc.), Snail (dilution ratio
1:2,000; cat. no. 3879; Cell Signaling Technology, Inc.), Twist
(dilution ratio 1:2,000; cat. no. ab49254; Abcam), Vimentin
(dilution ratio 1:1,000; cat. no. 10366-1; ProteinTech Group,
Inc.), β-catenin (dilution ratio 1:2,000; cat. no. 8480; Cell
Signaling Technology, Inc.), matrix metallopeptidase (MMP)14
(dilution ratio 1:2,000; cat. no. MAB3328; Merck KGaA), MMP9
(dilution ratio 1:2,000; cat. no. ab38898; Abcam), Collagen I
(dilution ratio 1:1,000; cat. no. ab138492; Abcam), proliferating
cell nuclear antigen (PCNA; dilution ratio 1:1,000; cat. no. 60097;
ProteinTech Group, Inc.), myc proto-oncogene protein (c-Myc;
dilution ratio 1:2,000; cat. no. 1472-1; Abcam), epidermal growth
factor receptor (EGFR; dilution ratio 1:2,000; cat. no. 4267; Cell
Signaling Technology, Inc.) and β-actin (dilution ratio 1:5,000;
cat. no. 60008-1; ProteinTech Group, Inc.), overnight at 4°C.
Membranes were washed twice with PBS and subsequently incubated
with secondary antibodies (dilution ratio 1:5,000; cat. nos. G21040
and G21234; Thermo Fisher Scientific, Inc.) conjugated to
horseradish peroxidase (Merck KGaA) for 1 h at room temperature.
Protein bands were visualized using enhanced chemiluminescent
reagents (Pierce; Thermo Fisher Scientific, Inc.).
Statistical analysis
GraphPad Prism 6.0 software (GraphPad Software,
Inc.) was used to perform statistical analysis. All experiments
were performed in duplicate and repeated at least three times and
data are presented as the mean ± standard error of the mean SEM.
Unpaired Student's t-test was used to compare differences between
two groups. The association between circPGC expression and the
clinicopathological characteristics of patients with GC was
assessed using Pearson's correlation and tumor-node-metastasis
(TNM) system analysis (20).
P<0.05 was considered to indicate a statistically significant
difference.
Results
circPGC expression is downregulated in
GC
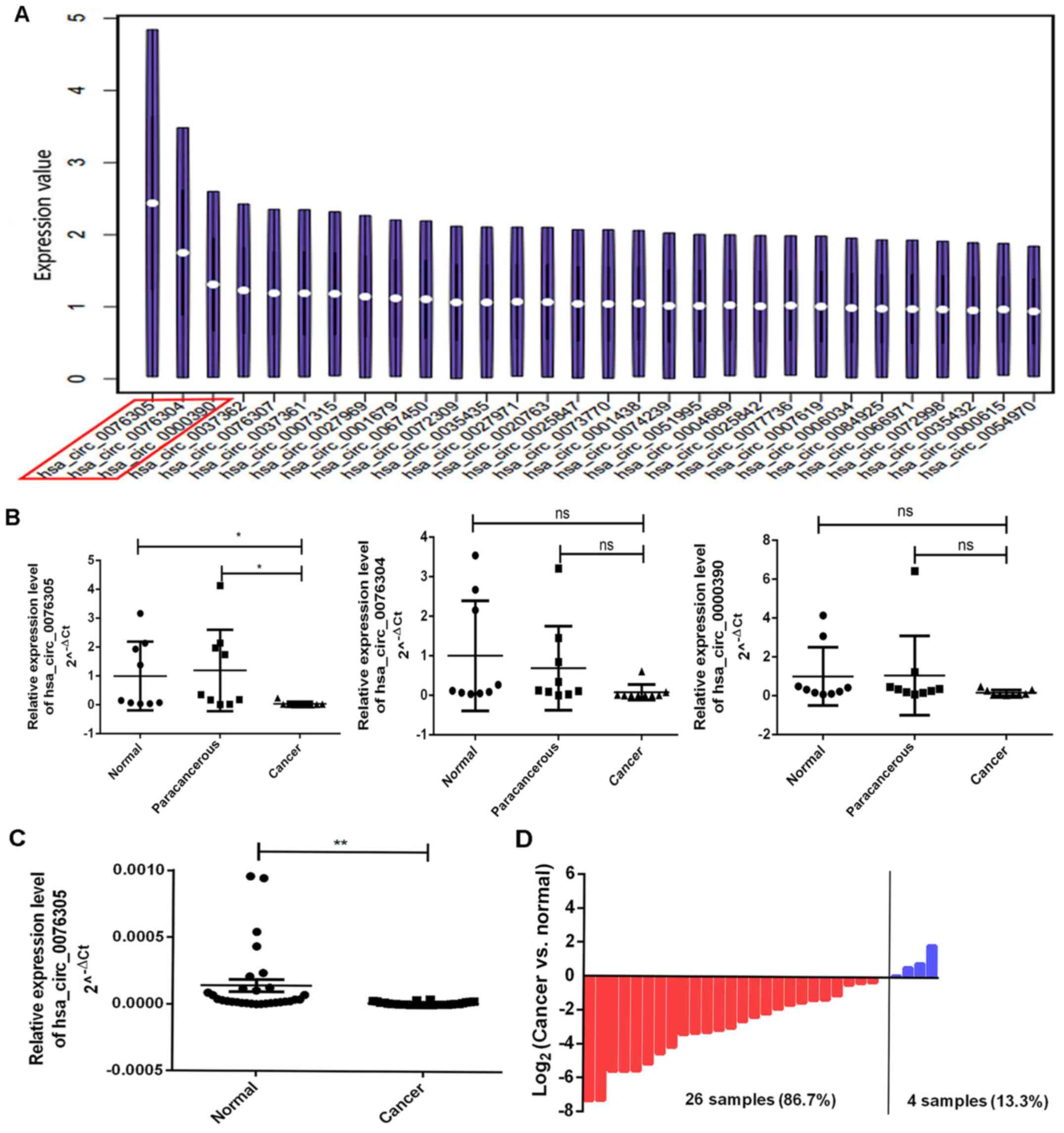
According to the GEO database, using the non-coding
RNA GSE100170 dataset of GC and paracancerous tissue samples, the
study (13) demonstrated that a
significant difference was observed in 713 circRNAs between GC and
paracancerous tissues. Among these, three circRNAs with significant
differences (circPGC, hsa_circ_0076304 and hsa_circ_0000390) were
selected for further experimentation (Fig. 1A).
To determine whether circPGC, hsa_circ_0076304 and
hsa_circ_0000390 were downregulated in GC, the expression of these
circRNAs was assessed in nine pairs of GC and paracancerous tissue
samples. The results demonstrated that only circPGC was
significantly downregulated in GC (P<0.05; Fig. 1B). Furthermore, circPGC expression
was assessed in 30 pairs of GC and paracancerous tissue samples and
the results demonstrated that circPGC expression was significantly
downregulated in GC (P<0.01; Fig.
1C). Among the 30 pairs of tissue samples, circPGC expression
was downregulated in 26 patients (86.7%) and only upregulated in 4
patients (13.3%) (Fig. 1D).
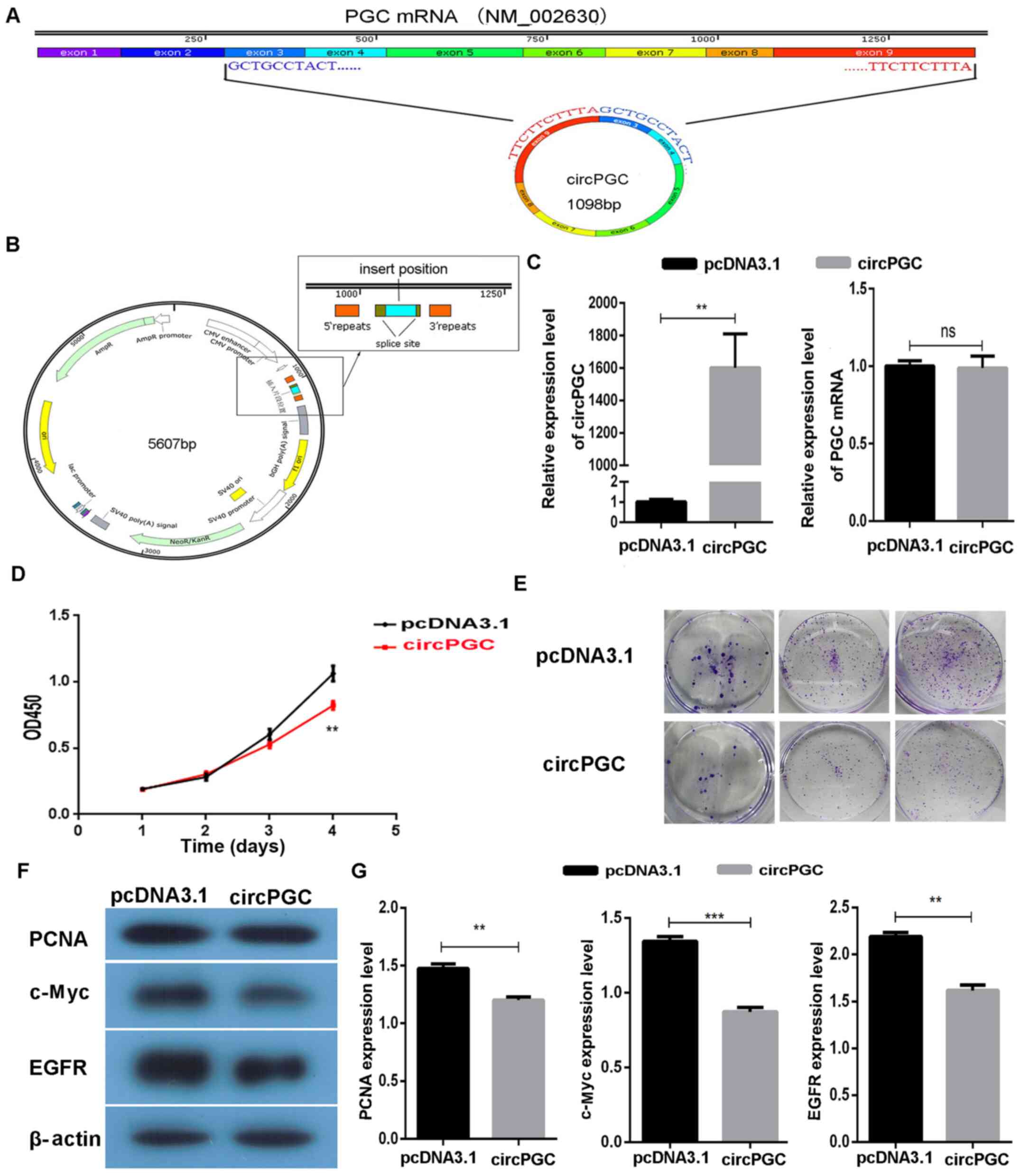
Overexpression of circPGC inhibits AGS
cell proliferation
The results demonstrated that circPGC circularized
with exons 3–9 of PGC mRNA (Fig.
2A). To study the function of circPGC in GC, the fragment of
PGC exons 3–9 was constructed into the pcDNA3.1(+) CircRNA Mini
Vector via seamless cloning (Fig.
2B). Following transient transfection of the circPGC expression
vector into AGS cells for 48 h, transfection efficiency was
detected via RT-qPCR analysis. The results demonstrated that
circPGC expression significantly increased in AGS cells following
transfection (P<0.01; Fig. 2C).
RT-qPCR analysis was also performed to detect PGC mRNA expression
following transfection with circPGC, and the results demonstrated
that PGC mRNA expression was not affected by circPGC (P>0.05;
Fig. 2C).
To assess whether overexpression of circPGC
regulated cell proliferation in GC, the CCK-8 and colony formation
assays were performed to determine the role of circPGC in AGS
cells. The results of the CCK-8 assay demonstrated that AGS cell
proliferation significantly decreased following overexpression of
circPGC (P<0.01; Fig. 2D).
Similarly, the results of the colony formation assay demonstrated
that circPGC notably inhibited the colony formation ability of AGS
cells (Fig. 2E). To determine the
molecular mechanism underlying the effect of circPGC on GC, western
blot analysis was performed to detect the expression levels of
proliferation-related proteins, including c-Myc (21), EGFR (22) and PCNA (23). The results demonstrated that
overexpression of circPGC significantly inhibited the expression
levels of c-Myc, EGFR and PCNA (P<0.05; Fig. 2F and G). Taken together, these
results suggest that circPGC inhibits AGS cell proliferation.
Overexpression of circPGC promotes
migration and invasion of AGS cells
Tumor infiltration is the process of malignant tumor
cells migrating from their original site to surrounding normal
tissues, which is closely associated with the prognosis of patients
(24). The wound healing assay was
performed to assess whether circPGC affects the migratory and
invasive abilities of GC cells. The results demonstrated that the
wound gap notably narrowed following transfection of AGS cells with
circPGC for 48 h (P<0.05; Fig.
3A). In addition, the results of the migration and invasion
assays demonstrated that overexpression of circPGC promoted cell
migration and invasion of AGS cells (P<0.05; Fig. 3B). Collectively, these results
suggest that circPGC promotes the migratory and invasive abilities
of AGS cells.
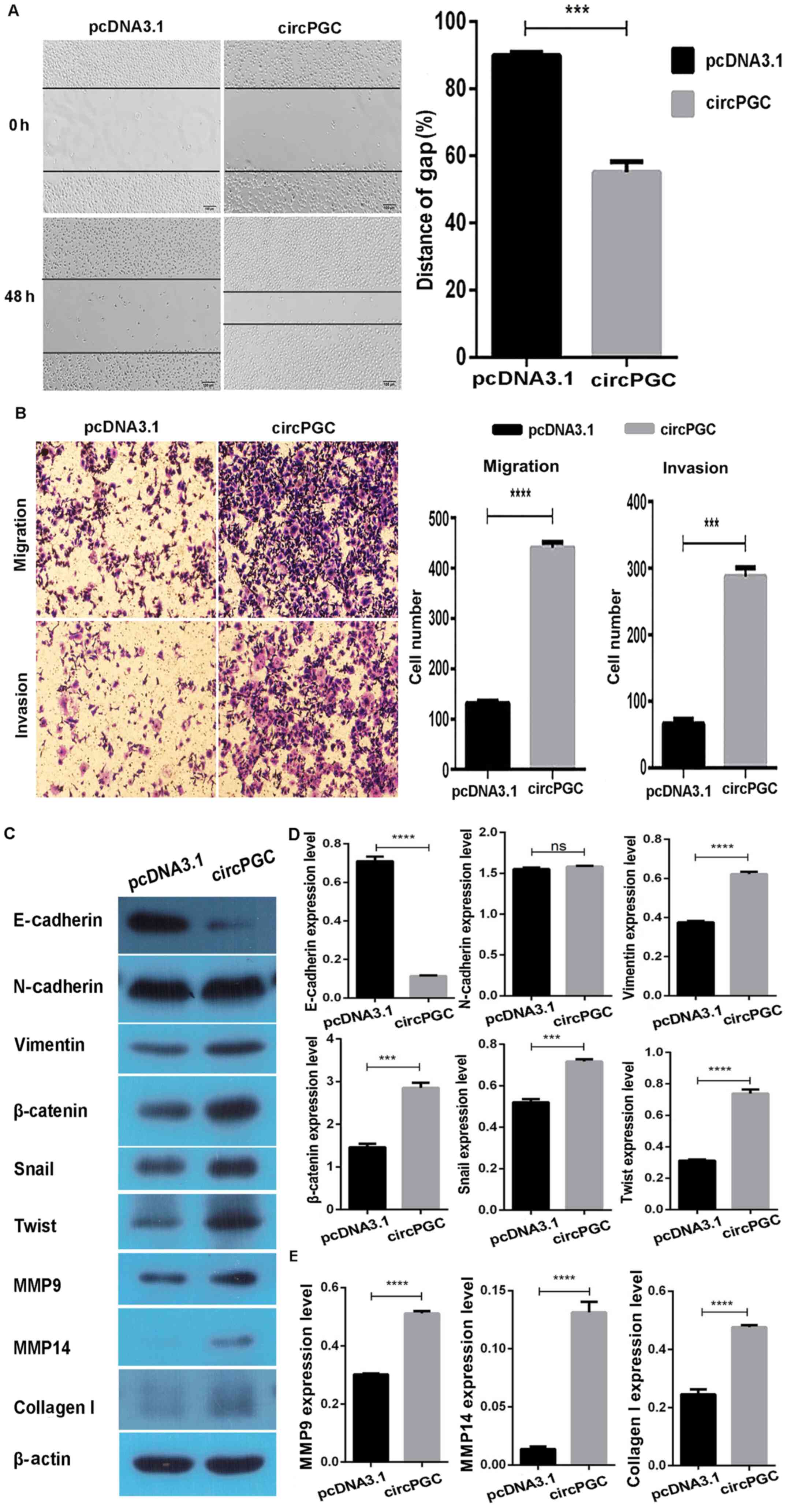 | Figure 3.Overexpression of circPGC promotes
migration and invasion of AGS cells. (A) The wound healing assay
was performed to assess the effect of circPGC on AGS cell
migration. (B) The results of the cell migration and invasion
assays demonstrated that overexpression of circPGC promoted the
cell migratory and invasive abilities of AGS cells. (C) CircPGC was
demonstrated to regulate the expression levels of E-cadherin,
Vimentin, β-catenin, Snail, Twist, MMP9, MMP14 and Collagen I. (D)
Western blot quantitative results of E-cadherin, Vimentin,
β-catenin, Snail and Twist. (E) Western blot quantitative results
of MMP9, MMP14 and Collagen I. Data are presented as the mean ±
standard error of the mean. ***P<0.001, ****P<0.0001.
CircPGC, hsa_circ_0076305; MMP, matrix metallopeptidase; ns, not
statistically significant. |
Epithelial-to-mesenchymal transition (EMT) is a
process in which epithelial cells transform into mesenchymal cells
(25), and a previous study
suggested that EMT is closely associated with tumor migration and
invasion (26). During the EMT
process, some proteins, including E-cadherin (27), β-catenin (28), Snail, Twist, Vimentin (29), and N-cadherin regulate the cell
infiltration capacity (30). In the
present study, western blot analysis demonstrated that
overexpression of circPGC significantly downregulated E-cadherin
expression and significantly upregulated the expression levels of
Vimentin, β-catenin, Snail and Twist (P<0.05), while N-cadherin
expression (P>0.05) remained unchanged (Fig. 3C and D).
Previous studies have reported that the expression
levels of MMP9, MMP14 and Collagen I also affect cell migration
(31–33). In the present study, western blot
analysis demonstrated that the expression levels of MMP9, MMP14 and
Collagen I were significantly upregulated following overexpression
of circPGC (P<0.05; Fig. 3C and
E). Taken together, these results suggest that circPGC enhances
the migratory and invasive abilities of AGS cells.
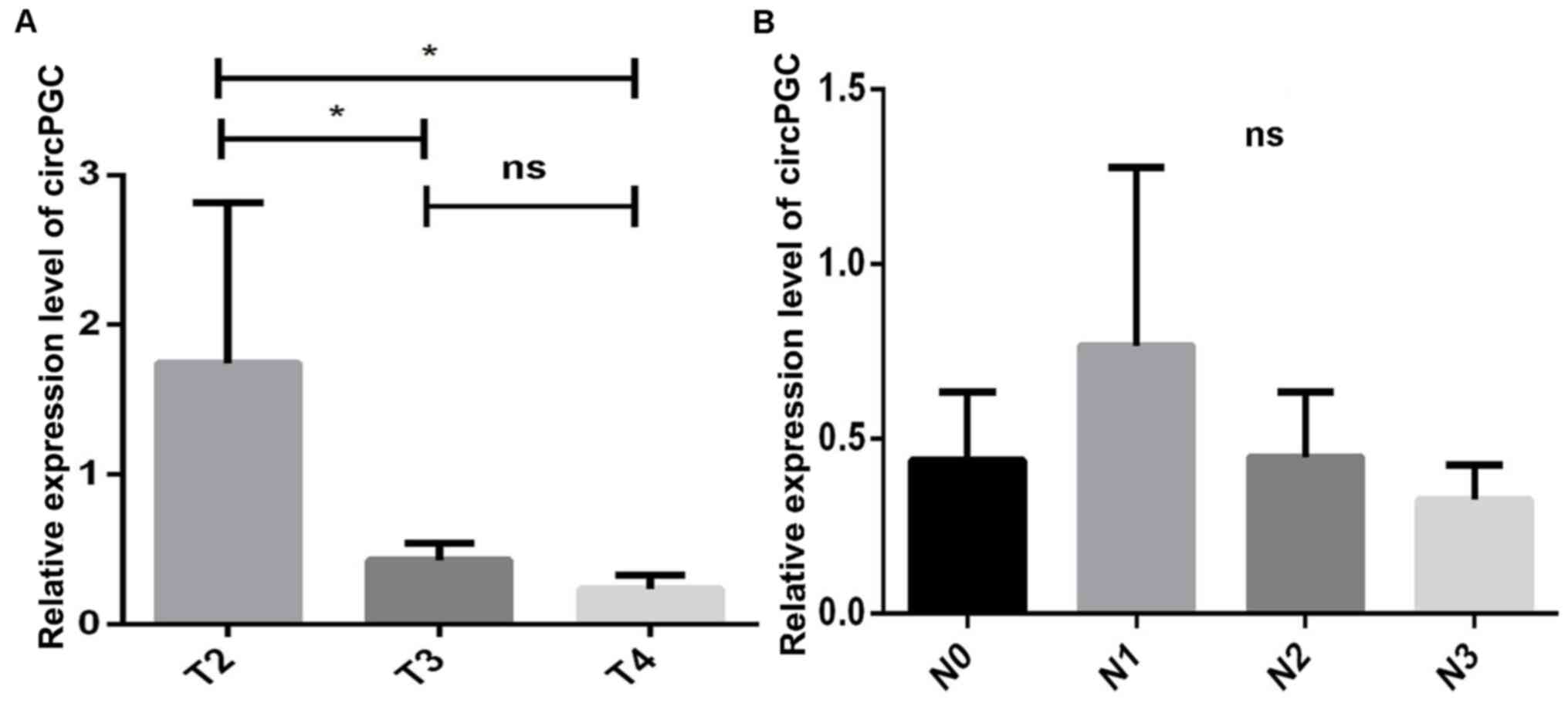
Association between circPGC expression
and clinicopathological characteristics of patients with GC
To understand the role of circPGC in GC, the
tumor-node-metastasis (TNM) system (20) was used, which is a basic criterion
for the classification of patients with cancer, their prognosis and
treatment plan (34), whereby high
TNM stages (stage III or IV) indicate a poor prognosis (20).
The association between circPGC expression and the
clinicopathological characteristics of patients with GC was
assessed using 30 cDNA GC and paracancerous tissue samples. As
presented in Table SIII, 4/13
patients (31%) with a low TNM stage (stage I or II) had high
circPGC expression, while 9/13 patients (69%) had low circPGC
expression. In addition, all patients with a high TNM stage (stage
III or IV) had low circPGC expression. The association between
circPGC expression and TNM stage suggests that circPGC is
associated with poor prognosis.
Patients in the T3 and T4 stages (advanced GC) had
low circPGC expression, while those in the T2 stage had high
circPGC expression (P<0.05; Fig.
4A). This result suggests that circPGC expression gradually
decreases with GC progressions, indicating that low circPGC
expression may promote in situ infiltration in GC cells.
Similarly, in patients with lymph node metastasis (N1-N3), circPGC
expression was demonstrated to gradually decrease; however, no
statistically significant differences were observed between the
subgroups (P>0.05; Fig. 4B).
Discussion
The results of the present study demonstrated the
dual function of circPGC in GC progression, one suppressing and the
other promoting. To the best of our knowledge, the present study
was the first to demonstrate that circPGC regulates cell
proliferation and migration dichotomy in GC cells. Overexpression
of circPGC promoted cell migration and invasion, and inhibited
proliferation of GC cells. Notably, the results of the present
study demonstrated that circPGC expression was downregulated in
advanced GC, and acted as an independent molecule for poor patient
prognosis. From the perspective of advanced GC, it was speculated
that low circPGC expression may promote the proliferation of GC
cells to affect the prognosis at this stage, instead of promoting
metastasis. Taken together, the results of the present study
suggest that low circPGC expression may be a candidate for the
prognosis of GC.
It is well-known that malignant proliferation and
metastasis are two of the dominant characteristics of cancer, which
occur simultaneously (35). However,
a phenomenon called migration-proliferation dichotomy has
demonstrated that cells do not proliferate and migrate
simultaneously (36). This dichotomy
has reported that some molecules, such as Girdin and EGFR, are key
regulators within the growth factor signaling network during cell
migration (37,38). A previous study demonstrated that
EGFR interacts with E-cadherin to promote proliferation by
activating the MAPK pathway (39).
Furthermore, patients with tumors bearing specific mutations in
EGFR or overexpressing EGFR have a good clinical response to
selective EGFR inhibitors (22,40,41).
However, in a previous study, when the tumor cells developed
resistance to the EGFR inhibitor, some cells exhibited mesenchymal
characteristics and low EGFR expression, survived and underwent EMT
(25). These findings indicate that
EGFR and E-cadherin play a dominant role in migration-proliferation
dichotomy. Consistent with previous findings (22,25,42), the
results of the present study demonstrated that both EGFR and
E-cadherin expression levels decreased following overexpression of
circPGC, demonstrating the occurrence of migration-proliferation
dichotomy.
Based on the migration-proliferation dichotomy, it
was demonstrated that circPGC decreased the expression levels of
EGFR, PCNA and c-Myc to suppress cell proliferation.
Simultaneously, it was demonstrated that circPGC promoted cell
migration by decreasing E-cadherin expression. E-cadherin is a
well-characterized cell surface molecule expressed in epithelial
cells or certain cancer cells, which plays a major role in cell
migration and adhesion (42,43). EMT is a process by which tumor cells
lose their epithelial phenotype, tight connections and polarity,
and acquire a mesenchymal phenotype, with advanced migratory and
invasive abilities (26,44). The results of the present study
demonstrated that overexpression of circPGC increased the
expression levels of Vimentin, β-catenin, Snail and Twist, and
decreased E-cadherin expression. Notably, circPGC had no effect on
N-cadherin expression. A potential explanation for this phenomenon
is that overexpression of circPGC may only lead to partial EMT, or
there may be other potential molecular mechanisms that influence
the effect of circPGC on N-cadherin expression. ECM is an integral
part of the tumor microenvironment that provides structural support
to tumor cells (45,46). The results of the present study also
demonstrated that overexpression of circPGC degraded ECM and
basement membrane to provide a migration environment via Collagen
I, MMP9 and MMP14.
The clinicopathological characteristics of 30
patients with GC were also assessed, and the results demonstrated
that circPGC may be associated with the prognosis of patients.
Notably, patients with low circPGC expression developed advanced
GC. Thus, circPGC has the potential to be developed as a novel
target for GC prevention and therapy. Although the present study
only assessed the dual effects of circPGC on migration and
proliferation in AGS cells in vitro, the underlying
molecular mechanism of this phenomenon has not yet been determined.
In addition, the results presented here require verification
through in vivo experiments and investigations using others
GC cell lines.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from The
National Natural Science Foundation of China (grant no. 81870388)
and the Special Project for Marine Economic Development of Xiamen
City (grant no. 17GYY001NF01).
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the corresponding author upon
reasonable request.
Authors' contributions
YZ and YH designed the present study, performed the
experiments, analyzed the data and drafted the initial manuscript.
DW, LD, YL and LL interpreted and analyzed the data. DL, KZ, CW, GZ
and CH collected and assembled the clinical data and analyzed the
data. YH and GL planned the experiments, revised the manuscript for
intellectual content and submitted the manuscript for publication.
All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Clinical
Research Ethics Committee of the Zhongshan Hospital of Xiamen
University, and performed in accordance with IRB-approved
institutional protocols. Informed consent was provided by all
patients prior to the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bonelli P, Borrelli A, Tuccillo FM,
Silvestro L, Palaia R and Buonaguro FM: Precision medicine in
gastric cancer. World J Gastrointest Oncol. 11:804–829. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Symeonidis D, Diamantis A, Bompou E and
Tepetes K: Current role of lymphadenectomy in gastric cancer
surgery. J BUON. 24:1761–1767. 2019.PubMed/NCBI
|
|
4
|
Zhang F, Huang X, Song Y, Gao P, Zhou C,
Guo Z, Shi J, Wu Z and Wang Z: Conversion surgery for stage IV
gastric cancer. Front Oncol. 9:11582019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shang Q, Yang Z, Jia R and Ge S: The novel
roles of circRNAs in human cancer. Mol Cancer. 18:62019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen LL and Yang L: Regulation of circRNA
biogenesis. RNA Biol. 12:381–388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jeck WR, Sorrentino JA, Wang K, Slevin MK,
Burd CE, Liu J, Marzluff WF and Sharpless NE: Circular RNAs are
abundant, conserved, and associated with ALU repeats. RNA.
19:141–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen X, Yang T, Wang W, Xi W, Zhang T, Li
Q, Yang A and Wang T: Circular RNAs in immune responses and immune
diseases. Theranostics. 9:588–607. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rong D, Tang W, Li Z, Zhou J, Shi J, Wang
H and Cao H: Novel insights into circular RNAs in clinical
application of carcinomas. OncoTargets Ther. 10:2183–2188. 2017.
View Article : Google Scholar
|
|
10
|
Han B, Chao J and Yao H: Circular RNA and
its mechanisms in disease: From the bench to the clinic. Pharmacol
Ther. 187:31–44. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dong Y, He D, Peng Z, Peng W, Shi W, Wang
J, Li B, Zhang C and Duan C: Circular RNAs in cancer: An emerging
key player. J Hematol Oncol. 10:22017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He J, Xie Q, Xu H, Li J and Li Y: Circular
RNAs and cancer. Cancer Lett. 396:138–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dang Y, Ouyang X, Zhang F, Wang K, Lin Y,
Sun B, Wang Y, Wang L and Huang Q: Circular RNAs expression
profiles in human gastric cancer. Sci Rep. 7:90602017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sui W, Shi Z, Xue W, Ou M, Zhu Y, Chen J,
Lin H, Liu F and Dai Y: Circular RNA and gene expression profiles
in gastric cancer based on microarray chip technology. Oncol Rep.
37:1804–1814. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shen Y, Zhang J, Fu Z, Zhang B, Chen M,
Ling X and Zou X: Gene microarray analysis of the circular RNAs
expression profile in human gastric cancer. Oncol Lett.
15:9965–9972. 2018.PubMed/NCBI
|
|
16
|
Huang YS, Jie N, Zou KJ and Weng Y:
Expression profile of circular RNAs in human gastric cancer
tissues. Mol Med Rep. 16:2469–2476. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bustin SA, Benes V, Garson JA, Hellemans
J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL,
et al: The MIQE guidelines: Minimum information for publication of
quantitative real-time PCR experiments. Clin Chem. 55:611–622.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang D and Wilusz JE: Short intronic
repeat sequences facilitate circular RNA production. Genes Dev.
28:2233–2247. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sellers AH: The clinical classification of
malignant tumours: The TNM system. Can Med Assoc J. 105:836passim.
1971.PubMed/NCBI
|
|
21
|
Dang CV: MYC on the path to cancer. Cell.
149:22–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mitchell RA, Luwor RB and Burgess AW:
Epidermal growth factor receptor: Structure-function informing the
design of anticancer therapeutics. Exp Cell Res. 371:1–19. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Juríková M, Danihel Ľ, Polák Š and Varga
I: Ki67, PCNA, and MCM proteins: Markers of proliferation in the
diagnosis of breast cancer. Acta Histochem. 118:544–552. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ahn HS, Lee HJ, Hahn S, Kim WH, Lee KU,
Sano T, Edge SB and Yang HK: Evaluation of the seventh American
Joint Committee on Cancer/International Union Against Cancer
Classification of gastric adenocarcinoma in comparison with the
sixth classification. Cancer. 116:5592–5598. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tulchinsky E, Demidov O, Kriajevska M,
Barlev NA and Imyanitov E: EMT: A mechanism for escape from
EGFR-targeted therapy in lung cancer. Biochim Biophys Acta Rev
Cancer. 1871:29–39. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: Emt: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Christofori G and Semb H: The role of the
cell-adhesion molecule E-cadherin as a tumour-suppressor gene.
Trends Biochem Sci. 24:73–76. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim K, Lu Z and Hay ED: Direct evidence
for a role of beta-catenin/LEF-1 signaling pathway in induction of
EMT. Cell Biol Int. 26:463–476. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mendez MG, Kojima S and Goldman RD:
Vimentin induces changes in cell shape, motility, and adhesion
during the epithelial to mesenchymal transition. FASEB J.
24:1838–1851. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hazan RB, Phillips GR, Qiao RF, Norton L
and Aaronson SA: Exogenous expression of N-cadherin in breast
cancer cells induces cell migration, invasion, and metastasis. J
Cell Biol. 148:779–790. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang K, Corsa CA, Ponik SM, Prior JL,
Piwnica-Worms D, Eliceiri KW, Keely PJ and Longmore GD: The
collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to
facilitate breast cancer metastasis. Nat Cell Biol. 15:677–687.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin CY, Tsai PH, Kandaswami CC, Lee PP,
Huang CJ, Hwang JJ and Lee MT: Matrix metalloproteinase-9
cooperates with transcription factor Snail to induce
epithelial-mesenchymal transition. Cancer Sci. 102:815–827. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yan T, Lin Z, Jiang J, Lu S, Chen M, Que
H, He X, Que G, Mao J, Xiao J, et al: MMP14 regulates cell
migration and invasion through epithelial-mesenchymal transition in
nasopharyngeal carcinoma. Am J Transl Res. 7:950–958.
2015.PubMed/NCBI
|
|
34
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The Eighth Edition AJCC Cancer Staging Manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van Denderen BJ and Thompson EW: Cancer:
The to and fro of tumour spread. Nature. 493:487–488. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ghosh P, Beas AO, Bornheimer SJ,
Garcia-Marcos M, Forry EP, Johannson C, Ear J, Jung BH, Cabrera B,
Carethers JM, et al: A G{alpha}i-GIV molecular complex binds
epidermal growth factor receptor and determines whether cells
migrate or proliferate. Mol Biol Cell. 21:2338–2354. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ghosh P, Garcia-Marcos M, Bornheimer SJ
and Farquhar MG: Activation of Galphai3 triggers cell migration via
regulation of GIV. J Cell Biol. 182:381–393. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Beas AO, Taupin V, Teodorof C, Nguyen LT,
Garcia-Marcos M and Farquhar MG: Gαs promotes EEA1 endosome
maturation and shuts down proliferative signaling through
interaction with GIV (Girdin). Mol Biol Cell. 23:4623–4634. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pece S and Gutkind JS: Signaling from
E-cadherins to the MAPK pathway by the recruitment and activation
of epidermal growth factor receptors upon cell-cell contact
formation. J Biol Chem. 275:41227–41233. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Moiseyenko VM, Procenko SA, Levchenko EV,
Barchuk AS, Moiseyenko FV, Iyevleva AG, Mitiushkina NV, Togo AV,
Semionov II, Ivantsov AO, et al: High efficacy of first-line
gefitinib in non-Asian patients with EGFR-mutated lung
adenocarcinoma. Onkologie. 33:231–238. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Edinger N, Lebendiker M, Klein S, Zigler
M, Langut Y and Levitzki A: Targeting polyIC to EGFR
over-expressing cells using a dsRNA binding protein domain tethered
to EGF. PLoS One. 11:e01623212016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Qian X, Karpova T, Sheppard AM, McNally J
and Lowy DR: E-cadherin-mediated adhesion inhibits ligand-dependent
activation of diverse receptor tyrosine kinases. EMBO J.
23:1739–1748. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen D, Wu Z, Luo LJ, Huang X, Qian WQ,
Wang H, Li SH and Liu J: E-cadherin maintains the activity of
neural stem cells and inhibits the migration. Int J Clin Exp
Pathol. 8:14247–14251. 2015.PubMed/NCBI
|
|
44
|
Boyer B, Vallés AM and Edme N: Induction
and regulation of epithelial-mesenchymal transitions. Biochem
Pharmacol. 60:1091–1099. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hynes RO: The extracellular matrix: Not
just pretty fibrils. Science. 326:1216–1219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Venning FA, Wullkopf L and Erler JT:
Targeting ECM Disrupts Cancer Progression. Front Oncol. 5:2242015.
View Article : Google Scholar : PubMed/NCBI
|