Introduction
Nasopharyngeal carcinoma (NPC) is a common malignant
tumor of the head and neck in southern China (1,2).
Histopathologically, unkeratinized squamous cell and
undifferentiated carcinoma are more prevalent. According to the
development of local invasion and distant metastasis, NPC can be
categorized into three distinct types: Upward (local growth and
invasion at the base of the skull), downward (distant metastasis)
and mixed progressing types (3,4). For the
upward progressing type of NPC, local invasion is dominant, and
metastasis is rare, and the prognosis is relatively good. On the
other hand, up to 90% of patients with the downward progressing
type of NPC develop cervical lymph node metastasis or distant
metastasis, even when the primary tumor was caught at an early
stage. The extent of cervical lymph node metastases is one of the
most important risk factors of distant metastasis (5) and the prognosis of downward progressing
type of NPC is significantly worse compared with that in the upward
progressing type (3,6). However, biomarkers associated with the
upward and downward progressing types of NPC have not been fully
elucidated. These two different biological behaviors of NPC have
not been significantly associated with clinical characteristics,
such as sex, age and pathological classification. Therefore, the
investigation into genetic aberration and molecular mechanisms may
lead to the identification of novel valuable biomarkers.
Type IV collagen, one of the main components of the
extracellular matrix and basement membrane, has been found to
interact with tumor cells and regulate tumor growth, proliferation,
differentiation, adhesion and metastasis (7–9), which
is important for the study of metastasis of NPC. Type IV collagen
contains three highly similar collagen precursors namely,
(α1)2α2(IV), α3α4α5(IV) and (α5)2α6(IV)
(10). The genes, COL4A1, COL4A2,
COL4A3, COL4A4, COL4A5 and COL4A6, which synthesize α1,
α2, α3, α4, α5 and α6 peptide chains, respectively, are located in
pairs on human chromosomes 13, 2 and X, respectively (11). In addition, hypermethylation of the
COL4A5/COL4A6 genes in colon cancer led to a decrease in the
expression level of the COL4A5/COL4A6 chain and the
destruction of the structural integrity of the basement membrane
(12). Similarly, it was found that
the expression level of the COL4A5/COL4A6 genes was
decreased or deleted in the invasive stage of basal cell carcinoma
and prostate cancer (13,14). However, the role of the
collagen-encoding genes in different types of NPC remains
undetermined.
In the present study, whole exon sequencing (WES)
was used to detected gene mutations associated with NPC biological
behaviors and to determine the key genes associated with the
invasion and metastasis potential of NPC. The role of COL4A3
gene expression and extracellular collagen, deposition in both the
upward and downward progressing types of NPC, were also
investigated. The inhibition of COL4A3 could promote
invasion and migration of the 5-8F NPC cell line. The present study
may provide new information in identifying novel biomarkers and
establish a molecular classification of NPC.
Materials and methods
Cell culture and transfection
The human 5-8F NPC cell line was donated by Sun
Yat-Sen University Cancer Center and was cultured in RPMI-1640
medium (supplemented with 10% fetal bovine serum, penicillin (100
U/ml) and streptomycin (100 mg-ml) (all from Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in a humidified incubator with 5%
CO2 (Sanyo Eletcric Co., Ltd). Cells in the logarithmic
growth phase were placed in a 6-well plate and incubated until
70-90% confluence.
Cells were transfected with specific short
interfering (si)RNA targeting COL4A3 (designed and
synthesized by Guangzhou Ribobio Co., Ltd.) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to manufacturers instructions. The
following siRNA was used: 5′-GGGTAATCCTGGATTTCTA-3′. A scramble
siRNA was also purchased from (Guangzhou Ribobio Co., Ltd) and used
as a control siRNA (siNC). The cells were assigned to three
experimental groups: Non-transfected group (control), siRNA
negative control group (si-NC) and COL4A3 siRNA transfected group
(si-COL4A3).
Western blot analysis
Cells were lysed in RIPA lysis buffer, containing
protease and phosphatase inhibitors (Sigma-Aldrich; Merck KGaA) on
ice for 30 min. The total protein concentration was measured using
a Bradford protein assay kit (Bio-Rad Laboratories, Inc.). Then, 60
µg per well of protein samples were separated using a 12% SDS-PAGE
(Bio-Rad Laboratories, Inc.) and transferred to a PVDF membrane
(EMD Millipore). After blocking with TBS-Tween-20 (TBST) containing
5% skimmed milk for 1 h at room temperature, the PVDF membrane was
washed with TBST for 10 min, three times. The membrane was then
incubated with primary antibodies diluted in TBST overnight at 4°C.
Antibody information: COL4A3 (cat. no. PA5-39876, 1:500 dilution)
and GAPDH (cat. no. MA5-15738-D800, 1:500 dilution) antibodies were
from Thermo Fisher Scientific, Inc. Subsequently, the membrane was
washed with TBST for 10 min thrice. Following incubation with
horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary
antibody (cat. no. A32731; 1:10,000 dilution; Thermo Fisher
Scientific, Inc.) and HRP-conjugated goat anti-mouse secondary
antibody (cat. no. 35518; 1:10,000 dilution; Thermo Fisher
Scientific, Inc.) for 2 h at room temperature, respectively. After
another three washes with TBST for 10 min, the proteins were
visualized using an ECL substrate (Pierce™ ECL Western Blotting
Substrate; Thermo Fisher Scientific, Inc.). ImageJ software version
1.48; National Institutes of Health) was used to quantify the
optical density of each protein. The relative optical density was
calculated by comparing with that in GAPDH.
Reverse transcription-quantitative
(RT-q)PCR assay
Total RNA was extracted from treated cells using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.)
Then, 2.0 µg total RNA was used for RT using a TaqMan RT kit
according to the manufacturers instructions (Applied Biosystems;
Thermo Fisher Scientific, Inc.). Subsequently, the cDNA was
amplified using qPCR, in a 20 µl total reaction volume and the Go
Tag Green Master Mix/Platinum SYBR Super mix (Invitrogen; Thermo
Fisher Scientific, Inc.). qPCR was performed using the following
conditions: Initial denaturation at 95°C for 5 min, denaturation at
95°C for 15 sec, 60°C for 30 sec, followed by 40 cycles at 72°C for
30 sec and 72°C for 10 min. The following primers were used:
COL4A3 forward, 5′-GGACTCACGGGTTCCAAAGGT-3′ and
COL4A3 reverse, 5′-CCTGCTCACCCTTAGAACCACT-3′; GAPDH
forward, 5′-CAATGACCCCTTCATTGACC-3′ and GAPDH reverse,
5′-GACAAGCTTCCCGTTCTCAG-3′. GAPDH was used as the internal
control.
Transwell and Matrigel assays
For the Transwell assay, 24 h following siRNA
transfection, the 5-8F cell line was harvested with trypsin and
seeded into Transwell chambers (8 µm pore size; Costar; Corning,
Inc.), at a density of 2×104 cells per well. The upper
chambers contained 200 µl serum-free RPMI-1640 medium, while the
lower chambers were filled with 600 µl RPMI-1640 medium, containing
10% FBS. For the Matrigel assay, 100 µl Matrigel (Sigma-Aldrich;
Merck KGaA) was added to the Transwell chamber, at a concentration
of 200 µg/ml. The cells on the upper surface of the membrane were
removed using a cotton swab. The invaded cells were fixed with 4%
paraformaldehyde for 30 min and stained with 1% crystal violet for
30 min at room temperature. The numbers of migrated cells were
counted in five random fields of view under a microscope at ×100
magnification using a Shunyu ICX41 inverted light microscope
(Zhejiang Kangchen Biotech Co., Ltd.) in each membrane. Finally,
cell counts were determined using the ImageJ software (National
Institutes of Health). All experiments were repeated at least three
times.
Wound-healing assay
A wound-healing assay was performed to determine the
effect of COL4A3 on cell migration. The 5-8F cell line was
seeded into 6-well plates, at a density of 2×105
cells/well, in RPMI-1640 medium, supplemented with 10% FBS, 24 h
following transfection. When the cells had reached 90–100%
confluence, a 200 µl pipette tip was used to scratch a wound on the
cells. The medium was removed, and the cells were gently washed
twice with PBS. Next, the cells were incubated with RPMI-1640
medium containing 1% FBS. Images were captured at 0 and 24 h under
a light microscope at ×40 magnification and the location and
migration of the cells was measured by determining the wound area.
The wound healing rate was quantified by using ImageJ software and
calculated with the help of following formula (Aw -
At)/Aw × 100. Where Aw is an area
of the wound at 0 h (control) and At is the area of the
wound at 24 h after scratching. All the experiments were repeated
at least three times.
Human specimens
The present study was approved by the Medical Ethics
Committee of Zhongnan Hospital of Wuhan University (approval no.
2019084). Written informed consent was obtained from each living
patient or from their relatives for deceased patients in the study.
Biopsies were collected from 20 patients with upward and downward
progressing types of NPC. In total, 17 patients were male and three
were female. The median age was 57 years (range, 42–83 years). The
following inclusion criteria were used: i) Newly diagnosed patients
with NPC from January 2003 to December 2018; ii) between 18 and 85
years of age; iii) confirmed pathological diagnosis; and iv) stages
of T3-4N0-1M0 (upward) and
Tis-1N2-3M0-1 (downward) (based on
the 8th edition of American Joint Committee on Cancer for
nasopharyngeal carcinoma staging) (15). The following exclusion criteria were
used: i) Recurrent NPC; ii) no pathological diagnosis or
pathological specimens; and iii) a history of other malignant
tumors. At the same time, the demographical and pathological
information, and imaging data were collected.
Hematoxylin and eosin (H&E)
staining
The histopathological examination was performed by a
senior pathologist at Zhongnan Hospital. The biopsies of two groups
of patients with NPC were stained with H&E, which was routinely
used in the pathology laboratory using the following procedure:
Deparaffinization with xylene, twice for 10 min each; re-hydration
with absolute ethanol, twice, 5 min each; followed by 95% ethanol
for 2 min and 70% ethanol for 2 min, followed by washing in
distilled water for 1 min; staining in H solution for 8 min at room
temperature, followed by washing in tap water for 5 min;
differentiation in acid ethanol for 30 sec, followed by washing in
tap water for 1 min; bluing in 0.2% ammonia water for 1 min,
followed by washing in tap water for 5 min. Finally, slides were
dehydrated with 85% ethanol and 95% ethanol for 5 min each and
counterstaining with E Y solution for 5 min at room temperature.
Dehydration with absolute ethanol, three times, 5 min each.
Transparency with xylene, twice, 5 min each time. The slides were
visualized under ×100 magnification using a light microscope. The
compactness of cells between upward and downward progressing types
of NPC was observed.
Masson trichromatic staining
The expression level and distribution of collagen in
the upward and downward progressing types of NPC specimens was
detected using Masson trichrome staining. The NPC specimens of the
two groups were routinely dewaxed with water by successively
putting into xylene for 20 min at room temperature, anhydrous
ethanol for 10 min, an ethanol series (95, 90, 80 and 70%), washed
with distilled water thrice, and then fixed with picric acid mixed
fixation solution for 10 min at room temperature. The nucleus was
subsequently stained with H for 5–8 min, rinsed with distilled
water thrice, and then stained with Lichun red acid Fuhong solution
for 5–7 min at room temperature. After washing with distilled
water, specimens were stained with 1% phosphomolybdic acid solution
for 5 min at room temperature and then stained directly with bright
green dye for 5 to 10 min at room temperature. Subsequently,
specimens were differentiated with 1% glacial acetic acid for 30–60
sec, dehydrated with 95% ethanol for 3 min and then dehydrated with
anhydrous ethanol for 5 min. Finally, all the specimens were made
transparent with xylene and sealed with neutral gum. The slides
were scanned using a NanoZoomer S360 Digital slide scanner
(C13220-01; Hamamatsu Co., Ltd.) and analyzed under ×10
magnification using NDP.view2 Viewing software (U12388-01;
Hamamatsu Co., Ltd.). The expression level of collagen was
quantified using Image-Pro Plus v6.0 software (Media Cybernetics,
Inc.).
Immunohistochemistry (IHC)
For IHC staining, paraffin-embedded NPC tissue
sections at 4–5 µm were deparaffinized with xylene I for 20 min,
xylene II for 20 min, and rehydrated with 100% ethanol for 10 min,
and 95, 85 and 80% ethanol for 5 min, each. Then the sections were
incubated with anti-COL4A3 antibody (cat. no. sc-52317; 1:100
dilution; Santa Cruz Biotechnology, Inc.) overnight at 4°C. The
next day, the slides were then incubated with a biotin-labeled
secondary antibody (cat. no. sc-2018; 1:100 dilutions; Santa Cruz
Biotechnology, Inc.) for 40 min at room temperature. Subsequently,
the secondary antibody was washed off with TBST (0.1% Tween-20).
The cells were then visualized using a REAL™ EnVision™Detection
System, (Peroxidase/DAB+; Rabbit/Mouse; cat. no. K5007; Dako;
Agilent Technologies, Inc.). The slides were scanned by NanoZoomer
S360 Digital slide scanner and analysed under ×300 magnification
using NDP.view2 Viewing software. The expression level of COL4A3
was quantified using Image-Pro Plus version 6.0 software (Media
Cybernetics).
WES
Sequencing and bioinformatic analysis of whole exons
from the upward and downward progressing types of NPC specimen were
completed in cooperation with Shenzhen Chengqi Biotechnology Co.,
Ltd. DNA was fragmented and hybridized using the SureSelect Human
All Exome kit V5 (Agilent Technologies, Inc.). Exome shotgun
libraries were sequenced on the SureSelect Human All Exon v.6
enrichment, Illumina NovaSeq 6000 platform (Illumina, Inc.),
generating paired end reads of 150 bp at each end. The cloud
analysis platform with FANSe algorithm (Chi-Cloud NGS Analysis
Platform) was applied for single-nucleotide variant calling
(16). Image analysis and base
calling were performed with CAVSAVR (Illumina, Inc.) using default
parameters. Adapter sequences were removed to obtain high-quality
reads. These were aligned to the NCBI human reference genome hg19
using the Burrows-Wheeler Aligner alignment algorithm. The high
frequency mutant genes associated with metastasis of NPC were
screened out. The associated amino acid replacement events and the
changes in protein coding sequence and function were predicted.
Gene Ontology (GO) and Kyoto
Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of
putative signaling pathways
The designated genes were uploaded into the function
annotation portal of The Database for Annotation, Visualization,
and Integrated Discovery (DAVID), online bioinformatics resources
for investigating the biological signaling pathways enriched by
input genes (17). GO (http://geneontology.org/) and KEGG (https://www.kegg.jp/) corpus were adopted to identify
putative signaling pathways which were visualized via R
clusterProfiler package version 3.18.0 (18).
Survival analysis
COL4A3 gene expression, as determined using
RNA sequencing and survival data from patients with head and neck
squamous cell carcinoma were obtained and analyzed using the
Kaplan-Meier Plotter tool (http://kmplot.com/analysis) (19). Due to a lack of NPC data, the data of
head and neck squamous cell carcinoma (N=499) were used instead.
Patients were divided by selecting the auto select best cut-off.
When the auto select best cut-off is selected, all possible cut-off
values between the lower and upper quartiles are computed, and the
best performing threshold is used as a cut-off. The auto select
best cut-off value was 35. Patients of both sexes, different
ethnicities, disease stages and grades were included. Hazard ratio
(HR) with 95% CI were analyzed and the median survivals times were
compared between the high and low expression level groups.
Statistical analysis
Quantitative results are expressed as the mean ± SD.
A Wilcoxon rank sum test was performed to analyze data obtained
from experiments on biopsy samples (IHC and Masson trichromatic
staining data). One-way ANOVA test was used to compare quantitative
data from in vitro experiments containing multiple groups.
Post-hoc test between si-NC and si-COL4A3 groups were adjusted with
Bonferroni correction. Kaplan-Meier survival plots were generated,
and a log-rank test was used for comparisons between survival
curves. GraphPad Prism version 6.0 (GraphPad Software, Inc.) was
used to analyze all the data. P<0.05 was considered to indicate
a statistically significant difference.
Results
Clinical and pathological
characteristics of patients with the upward and downward
progressing types of NPC
Between January 2003 and December 2018, 20 patients
with NPC were eligible for analysis and were included in the
present study. A total of 17 patients were male. The median age was
57 years (range, 42–83 years) (Table
I). A total of 8 patients with the upward progressing type of
NPC and 4 patients with the downward progressing type of NPC were
included in the WES analysis. These patients and another eight
patients were also included to investigate COL4A3 expression level
and collagen deposition.
 | Table I.Clinical characteristic of patients
with nasopharyngeal carcinoma. |
Table I.
Clinical characteristic of patients
with nasopharyngeal carcinoma.
| A, Upward |
|---|
|
|---|
| ID | WES | Sex | Age, years | T
stagea | N
stagea | M
stagea | WHO
typeb |
|---|
| Patient 1 | Yes | Male | 66 | 4 | 0 | 0 | II |
| Patient 2 | Yes | Male | 42 | 3 | 1 | 0 | II |
| Patient 3 | Yes | Male | 59 | 3 | 1 | 0 | II |
| Patient 4 | Yes | Male | 55 | 3 | 1 | 0 | II |
| Patient 5 | Yes | Male | 68 | 4 | 1 | 0 | II |
| Patient 6 | Yes | Male | 53 | 4 | 1 | 0 | II |
| Patient 7 | Yes | Male | 54 | 4 | 0 | 0 | II |
| Patient 8 | Yes | Male | 67 | 4 | 1 | 0 | II |
| Patient 9 | No | Male | 49 | 4 | 1 | 0 | II |
| Patient 10 | No | Male | 78 | 4 | 1 | 0 | II |
| Patient 11 | No | Male | 62 | 4 | 1 | 0 | II |
| Patient 12 | No | Female | 54 | 3 | 1 | 0 | II |
| Patient 13 | No | Male | 62 | 3 | 1 | 0 | II |
| Patient 14 | No | Female | 44 | 4 | 1 | 0 | I |
| Patient 15 | No | Male | 73 | 4 | 0 | 0 | II |
|
| B,
Downward |
|
| ID | WES | Sex | Age,
years | T
stagea | N
stagea | M
stagea | WHO
typeb |
|
| Patient 16 | Yes | Male | 63 | 1 | 2 | 0 | II |
| Patient 17 | Yes | Male | 53 | 1 | 3 | 0 | II |
| Patient 18 | Yes | Male | 54 | 1 | 3 | 1 | II |
| Patient 19 | Yes | Male | 83 | 1 | 3 | 0 | II |
| Patient 20 | No | Female | 44 | 1 | 3 | 0 | I |
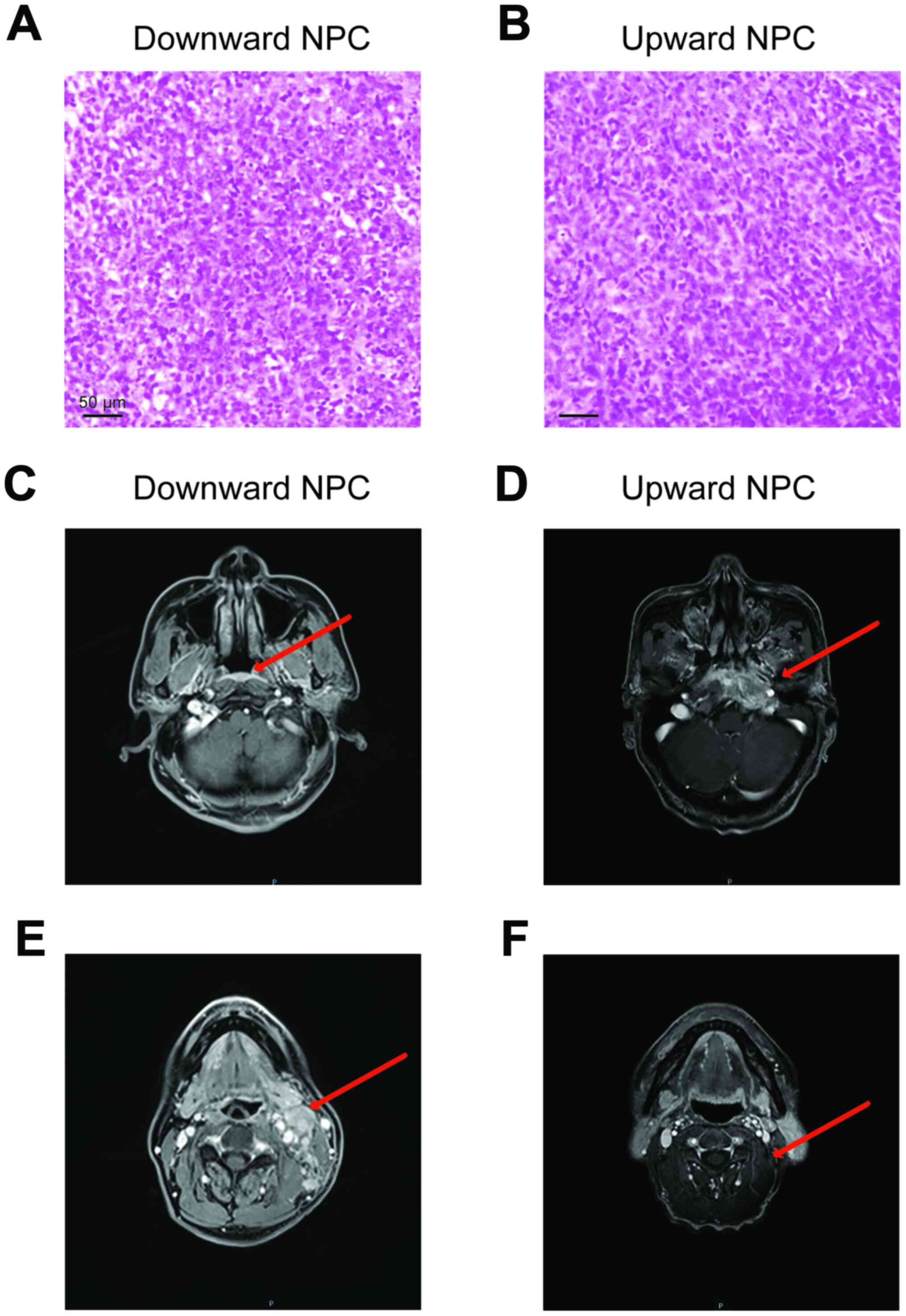
With respect the pathological features of the
patients, the arrangement of the tumor cells in the downward
progressing type of NPC was loose, as opposed to that in the upward
progressing type of the NPC cells (Fig.
1A and B). In the magnetic resonance imaging (MRI) analysis,
the downward progressing type of NPC showed small primary tumors
(Tis-T1), confined in the nasopharyngeal cavity, and did not invade
into the surrounding tissue (Fig. 1C and
E). However, notable cervical lymph node metastasis or distant
metastasis occurred even when the primary tumor was in early
stages. On the other hand, the upward progressing type of NPC
(T3-T4) showed significant infiltration and destruction of the
surrounding tissue by the primary tumor, with documented clinical
manifestations of space and nerve involvement associated with tumor
invasions (Fig. 1D and F).
Nonetheless, cervical lymph node metastasis of the upward
progressing type was not significant (N0-N1) and no evidence of
distant metastasis was detected.
COL4A3 was identified as the target
gene
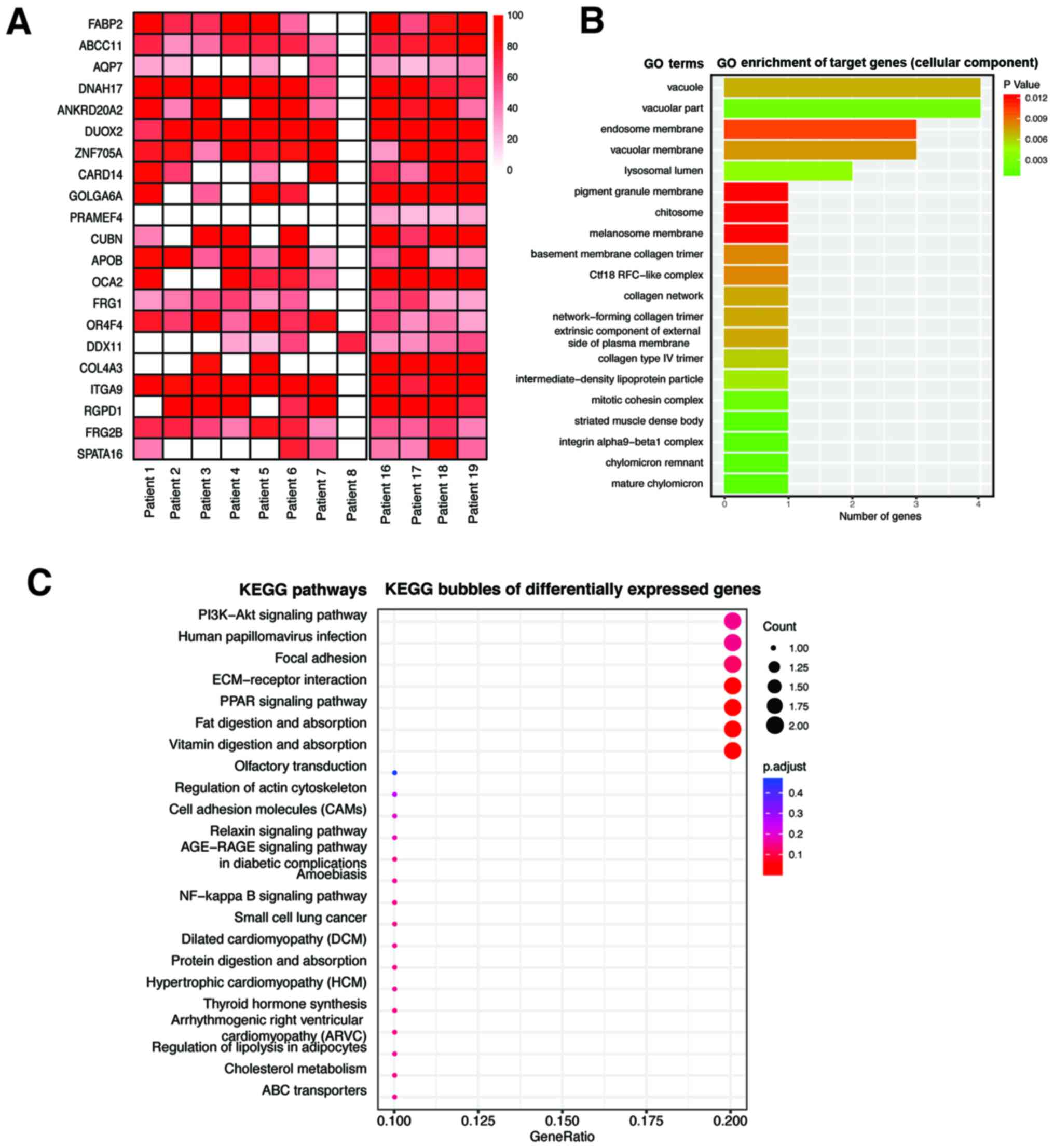
A total of 8 upward NPC specimens and 4 downward NPC
specimens were sequenced, from which 4,125 differential mutation
sites were found, involving 2,511 genes. The molecular events such
as single nucleotide variations, insertions/deletions, copy number
variations and gene fusion, with significant differences in
mutation frequency between the upward and downward progressing
types of NPC were detected. After harmful predictive filtration,
there were 21 residual differential mutation sites, involving 21
genes (Table II; Fig. 2A). Single nucleotide variations
between upward and downward progressing types of NPC, detected by
WES and Genome-Wide Association Studies, were used to analyze
differential gene expression. P<0.05 was considered
statistically significant and eight single nucleotide variations
showed significant difference between the two types of NPC
(Table II). The genes associated
with tumor metastasis, in the mutant genes were further
investigated by GO analysis and it was found that the
differentially mutated genes between the upward and downward
progressing types of NPC were enriched in the genes associated with
collagen expression (Fig. 2B). KEGG
signaling pathway analysis found that the differentially mutated
genes were significantly enriched in extracellular
matrix-associated signaling pathways (Fig. 2C). Within these identified genes, the
target gene COL4A3, which was found to be associated with
tumor metastasis, was investigated further with respect to its role
in the metastasis of NPC.
 | Table II.Whole exon sequencing results of
patients with nasopharyngeal carcinoma. |
Table II.
Whole exon sequencing results of
patients with nasopharyngeal carcinoma.
| Chromosome | Position
(1-based) | Gene name | Original | Mutation | Original
(peptide) | Mutation
(peptide) | Annotation |
P-valuea |
|---|
| chr10 | 135440216 | FRG2B | G | A | H | Y | FSHD region gene 2
family member B | 0.87870 |
| chr10 | 17147521 | CUBN | G | T | P | T | Cubilin | 0.01335 |
| chr1 | 12939765 | PRAMEF4 | A | G | L | P | PRAME family member
4 | 0.00000 |
| chr12 | 31242081 | DDX11 | G | A | R | Q | DEAD/H-box helicase
11 | 0.27880 |
| chr12 | 8327016 | ZNF705A | T | C | A | T | zinc finger protein
705A | 0.01544 |
| chr15 | 102463117 | OR4F4 | T | G | H | P | Olfactory receptor
family 4 subfamily F member 4 | 0.49690 |
| chr15 | 28197037 | OCA2 | T | C | H | R | OCA2
melanosomaltrans-membrane protein | 0.01397 |
| chr15 | 45392075 | DUOX2 | G | A | S | L | Dual oxidase 2 | 0.21210 |
| chr15 | 74374822 | GOLGA6A | T | G | H | P | golgin A6 family
member A | 0.00665 |
| chr16 | 48258198 | ABCC11 | C | T | G | R | ATP binding
cassette subfamily C member 11 | 0.07139 |
| chr17 | 76433898 | DNAH17 | T | C | H | R | Dyneinaxonemal
heavy chain 17 | 0.20260 |
| chr17 | 78178893 | CARD14 | C | T | R | W | Caspase recruitment
domain family member 14 | 0.07064 |
| chr2 | 21231524 | APOB | G | A | P | L | Apolipoprotein
B | 0.30960 |
| chr2 | 228135631 | COL4A3 | C | T | P | L | Collagen type IV
alpha 3 chain | 0.00149 |
| chr2 | 87214281 | RGPD1 | T | G | V | G | RANBP2-like and
GRIP domain containing 1 | 0.05126 |
| chr3 | 172835082 | SPATA16 | C | T | G | E | Spermatogenesis
associated 16 | 0.08735 |
| chr3 | 37574951 | ITGA9 | G | A | G | E | Integrin subunit
alpha 9 | 0.28760 |
| chr4 | 120241902 | FABP2 | T | C | T | A | Fatty acid binding
protein 2 | 0.03159 |
| chr4 | 190881957 | FRG1 | G | T | D | Y | FSHD region gene
1 | 0.05745 |
| chr9 | 33386465 | AQP7 | A | G | Y | H | Aquaporin 7 | 0.04698 |
| chr9 | 42376286 | ANKRD20A2 | C | T | A | V | Ankyrin repeat
domain 20 family member A2 | 0.08193 |
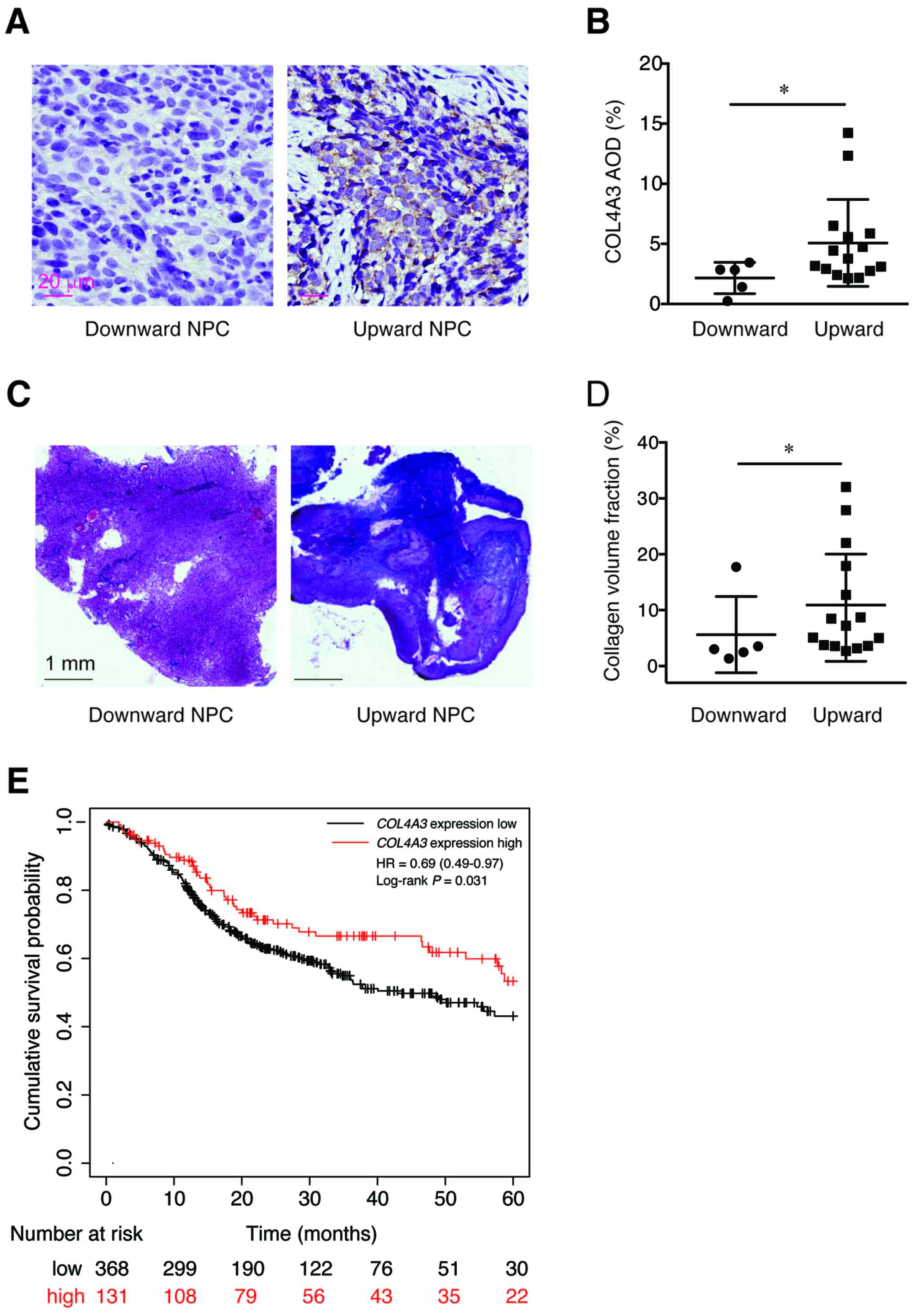
Expression of COL4A3 and deposition of
collagen in NPC
The protein expression level of COL4A3, in
patients with the two types of NPC, was detected using IHC methods.
It was found that the expression level of COL4A3 in the
downward progressing type of NPC was significantly lower compared
with that in the upward progressing type of NPC (2.161±1.306 vs.
5.077±3.619; P<0.05; Fig. 3A and
B). Next, the extracellular collagen deposition was determined
using Masson trichromatic staining. Notably, the deposition of blue
precipitate (collagen) in the downward progressing type of NPC was
significantly lower compared with that in the upward progressing
type of NPC (5.63±6.83 vs. 10.94±9.60; P<0.05; Fig. 3C and D). In addition, the high
expression level of COL4A3 was associated with favorable
prognosis of head and neck squamous cell carcinoma (HR, 0.69; 95%
CI, 0.49–0.97; P=0.031; Fig. 3E) by
analyzing the gene expression level and patient survival data using
the Kaplan-Meier plotter database.
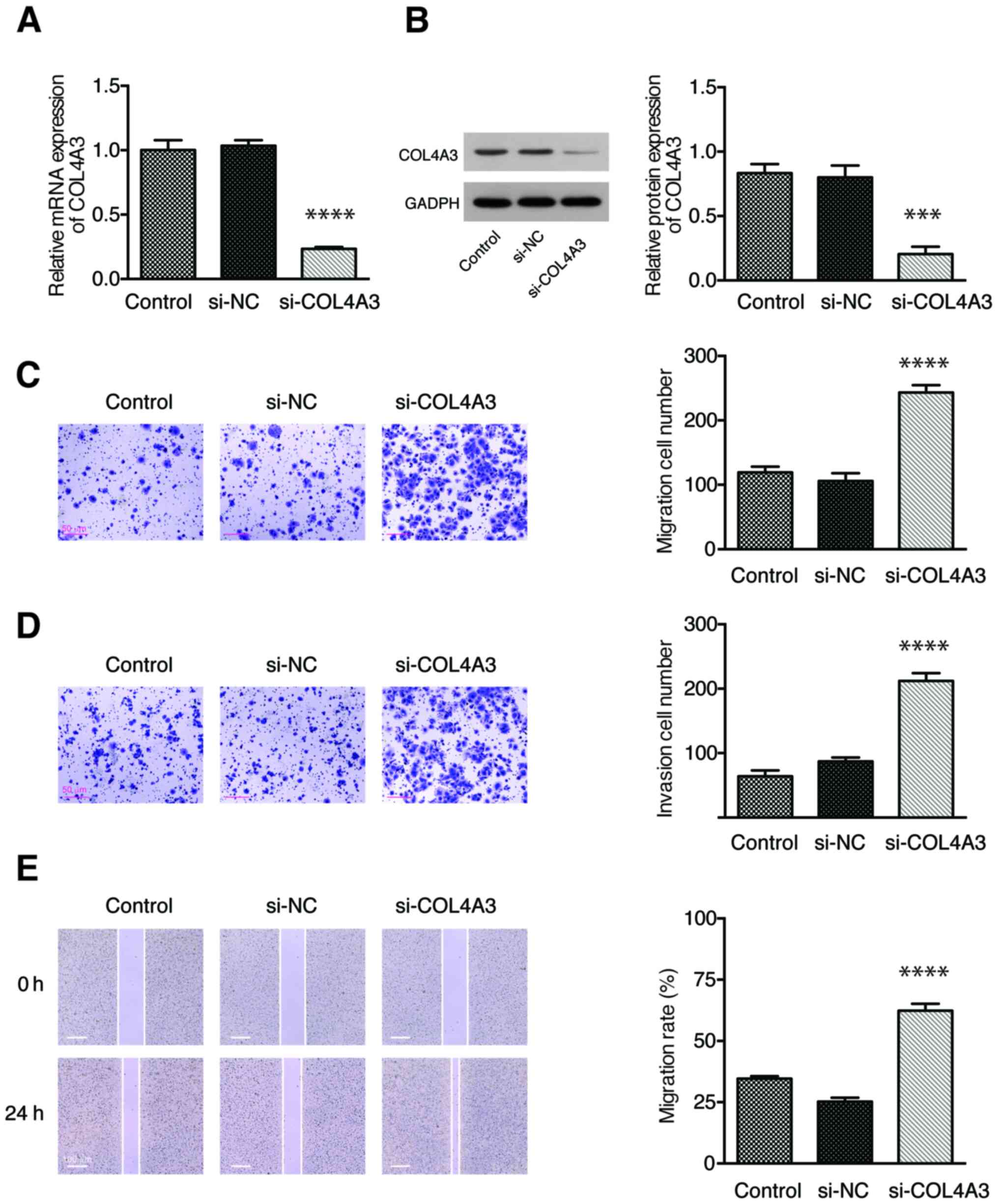
Knockdown of COL4A3 promotes the
migration and invasion of NPC cells
To further confirm the role of COL4A3 in cell
migration and invasion, the expression of COL4A3 was
inhibited in 5-8F cells by siRNA. The expression of COL4A3
was significantly downregulated in the si-COL4A3 group compared
with the si-NC group, both at mRNA (Fig.
4A) and protein (Fig. 4B)
levels. The Transwell assay showed that the number of cell
migration (Fig. 4C) and invasion
(Fig. 4D) in the si-COL4A3 group was
increased significantly compared with that in the si-NC group. A
wound-healing assay was used to detect the migration ability of
each group of 5-8F cell line. The migration rate of the cells in
the si-COL4A3 group was significantly higher than that in the si-NC
group (Fig. 4E). These results
demonstrated that the inhibition of COL4A3 could promote the
migration and invasion abilities of the 5-8F cell line.
Discussion
Metastasis is the major cause of death in patients
with cancer. Cancer metastasis is considered to be primarily
associated with changes in the tumor microenvironment, particularly
in the remodeling of extracellular matrix characterized by the
degradation, deposition and cross-linking of type IV collagen
(20–22). The extracellular matrix is a barrier
for tumor cell metastasis. Type IV collagen, from the extracellular
matrix, is the primary component of the basement membrane. The
expression of type IV collagen affects the deposition of the tumor
extracellular matrix; thus, affecting the potential of tumor
metastasis. The investigation into the effect of type IV collagen
on extracellular matrix deposition and its function is beneficial
for understanding the molecular mechanism of tumor metastasis and
provide novel ideas for identifying molecular markers and effective
treatment for tumor metastasis.
The COL4A3 domain binds and inhibits the
proliferation of melanoma and other epithelial tumor cell lines
in vitro (23). It is well
known that the α3 (IV) chain encoded by COL4A3, is lysed by matrix
metalloproteinase-9 (MMP9) to produce the primary bioactive
fragment, tumstatin, which can inhibit the formation of blood
vessels in vivo and inhibit the proliferation and metastasis
of tumors (24). In the early stage
of tumorigenesis, the MMP-mediated mechanism promotes the release
of tumstatin (also an endogenous angiogenic inhibitor), by
separating it from the basement membrane, to permit its
anti-angiogenesis and antitumor activities (25). These two antitumor properties have
been found to be regulated by an ITGB3-mediated RGD-independent
mechanism. Once tumstatin has been dissociated from the basement
membrane, it interacts with integrin αVβ3 in endothelial cells,
which leads to the arrest of the cell cycle or apoptosis (26), and has been found to cause apoptosis
(27,28) and block the proliferation of
endothelial cells (29).
Overexpression of the tumstatin domain, in a mouse melanoma model,
inhibited tumor cell invasive properties (30). Tumstatin is also the most effective
type IV collagen family and is expected to become a potentially
beneficial therapeutic molecule to inhibit tumor growth. Therefore,
the expression level and the association of COL4A3 and typeIV
collagen play an important role in tumor metastasis; however, the
role of COL4A3 in the metastasis of NPC is not clear, and
associated studies have not been reported.
Previous studies have demonstrated that specific
COL4A3 mutations could lead to loss of function in several
diseases. For example, a novel missense mutation (3725G>A,
G1242D) in exon 42 of COL4A3 played a causative role in
Alport syndrome and thin basement membrane nephropathy (31). Other mutations were also recently
identified in a consanguineous family with autosomal recessive
Alport syndrome, including IVS 22-5 T>A in the COL4A3
gene, and R1677C and R1682Q in the COL4A4 gene (32), confirming that COL4A3 and
COL4A4 mutations might cause different forms of nephropathy.
However, the role of COL4A3 mutations in NPC pathogenesis
remains undetermined. In the present study, whole exons were
sequenced and analyzed in the upward and downward progressing types
of NPC. It was found that the mutation rate of the COL4A3
gene was significantly increased and the expression level of the
COL4A3 protein was significantly decreased in the downward
progressing type of NPC. In the KEGG signaling pathway analysis,
six associated signaling pathways were identified: PI3K-Akt
signaling pathway, focal adhesion, ECM-receptor interaction, PPAR
signaling pathway, cell adhesion molecules and NF-κB signaling
pathway. Among these pathways, ECM-receptor interaction was
considered to be the most effective signaling pathway for the
regulation of expression of COL4A3. It has been demonstrated
that MMP-9 can degrade COL4A3 in the basement membrane and
extracellular matrix, resulting in decreased collagen in the
cornea, thinning the central cornea and forming keratoconus
(33). Indeed, there are significant
association between genotypic and allelic distributions of
COL4A3 (G/T) and MMP-9 (A/G) polymorphisms (34). Reciprocally, the major gene variant
COL4A3 may also affect the expression of associated
collagens or ECM proteins, thus decreasing the amount of ECM in
corneas and resulting in keratoconus (35). Therefore, ECM-receptor interaction
pathway is potentially involved in the regulation of COLA43.
Nonetheless, it requires more mechanistic studies to confirm the
hypothesis of the present study. Currently, there is still no data
on how gene mutations in COL4A3 would impact on the protein
expression level. It is hypothesized that the mutation in the
COL4A3 gene, in metastatic NPC might lead to the decrease in
COL4A3 protein expression level and the deposition of type IV
collagen. Therefore, the biopsies of the upward and downward
progressing types of NPC were analyzed using IHC methods. COL4A3
protein expression was significantly lower in the downward
progressing type of NPC, suggesting that abnormal COL4A3 expression
could be associated with the metastasis of NPC. The results from
the present study were in accordance with previous studies showing
that abnormal expression of COL4A3 was found in different types of
tumor (36,37). At the same time, the deposition of
the extracellular matrix in downward progressing type of NPC was
significantly decreased. The results indicated that the knockdown
of COL4A3 might promote the invasion and migration of NPC
cells. The low expression level of COL4A3 in the downward
progressing type of NPC was associated with increased risk of
distant metastasis, suggesting that COL4A3 expression might
be a good biomarker for NPC metastasis.
However, there are still some limitations in the
present study. Firstly, a small number of matched samples were
collected in this study. This was partly due to availability of
eligible samples, since samples that were suitable for WES should
be relatively recent to ensure DNA integrity. Therefore, the number
of samples was relatively small in the current study. A larger
number of samples should be performed to verify the results of the
present study and further clarify the gene mutation spectrum
affecting the metastatic risk of NPC by WES. Next, the function of
certain genetic mutation should be examined in detail. The mutation
status of the aforementioned genes should be verified by PCR and
Sanger sequencing. Differentially expressed genes between upward
and downward progressing types of NPC could potentially be detected
by performing transcriptomic sequencing. Thirdly, in vivo
experiments were lacking in the present study, which help validate
the hypothesis, prove the essential role of COL4A3 in NPC
metastasis and uncover potential molecular mechanisms. A plasmid
harboring targeted gene mutation will be constructed in the future
for the detection of protein expression. The in vivo effect
of COL4A3 should also be studied by manipulating its expression and
functions.
In conclusion, although several limitations exist,
the present study showed that the COL4A3 gene was highly expressed
in the upward progressing type of NPC, but was low in the downward
progressing type. This was associated with the degree of collagen
fibrosis, thus affecting the migration and invasion of NPC. COL4A3
may be a novel biomarker in the diagnosis and treatment of
metastatic NPC, which could be beneficial to establish a potential
molecular typing method and identify the target for individualized
therapy for metastatic NPC.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
Scientific Research Project of Hubei Provincial Health and Family
Planning Commission (grant no. WJ2019H064) and from the National
Natural Science Foundation of China (grant no. 81803061).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors contributions
XY, QW and YZ conceived the study and drafted the
manuscript. XY collected the clinical tissues, was responsible for
the study design and provided the data. QW performed the
statistical analysis. FW performed the bioinformatic data
collection, integration analysis and figure processing. All authors
have made substantial contributions to this manuscript. All authors
confirmed and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Zhongnan Hospital of Wuhan University (Ethical number:
2019084). Written informed consent was obtained from each living
patient or from their relatives for deceased patients in the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carioli G, Negri E, Kawakita D, Garavello
W, La Vecchia C and Malvezzi M: Global trends in nasopharyngeal
cancer mortality since 1970 and predictions for 2020: Focus on
low-risk areas. Int J Cancer. 140:2256–2264. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun P, Chen C, Chen XL, Cheng YK, Zeng L,
Zeng ZJ, Liu LZ, Su Y and Gu MF: Proposal of a clinical typing
system and generation of a prognostic model in patients with
nasopharyngeal carcinoma from Southern China. J BUON. 19:474–483.
2014.PubMed/NCBI
|
|
4
|
Liang WJ, Qiu F, Hong MH, Guo L, Qin HD,
Liu QC, Zhang XS, Mai HQ, Xiang YQ, Min HQ, et al: Differentially
expressed genes between upward and downward progressing types of
nasopharyngeal carcinoma. Chin J Cancer. 27:460–465. 2008.(In
Chinese).
|
|
5
|
Mo L, Weng J, Zeng F, Li X, Liu B, Li Z
and Kuang G: The relationship between extend types and distant
metastasis of nasopharyngeal carcinoma. Lin Chung Er Bi Yan Hou Tou
Jing Wai Ke Za Zhi. 24:554–555, 558. 2010.(In Chinese). PubMed/NCBI
|
|
6
|
Chew MM, Gan SY, Khoo AS and Tan EL:
Interleukins, laminin and Epstein - Barr virus latent membrane
protein 1 (EBV LMP1) promote metastatic phenotype in nasopharyngeal
carcinoma. BMC Cancer. 10:5742010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morrissey MA, Jayadev R, Miley GR, Blebea
CA, Chi Q, Ihara S and Sherwood DR: SPARC promotes cell invasion in
vivo by decreasing type IV collagen levels in the basement
membrane. PLoS Genet. 12:e10059052016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanjore H and Kalluri R: The role of type
IV collagen and basement membranes in cancer progression and
metastasis. Am J Pathol. 168:715–717. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Timpl R, Wiedemann H, van Delden V,
Furthmayr H and Kühn K: A network model for the organization of
type IV collagen molecules in basement membranes. Eur J Biochem.
120:203–211. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mundel TM and Kalluri R: Type IV
collagen-derived angiogenesis inhibitors. Microvasc Res. 74:85–89.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khoshnoodi J, Pedchenko V and Hudson BG:
Mammalian collagen IV. Microsc Res Tech. 71:357–370. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ikeda K, Iyama K, Ishikawa N, Egami H,
Nakao M, Sado Y, Ninomiya Y and Baba H: Loss of expression of type
IV collagen alpha5 and alpha6 chains in colorectal cancer
associated with the hypermethylation of their promoter region. Am J
Pathol. 168:856–865. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tanaka K, Iyama K, Kitaoka M, Ninomiya Y,
Oohashi T, Sado Y and Ono T: Differential expression of alpha
1(IV), alpha 2(IV), alpha 5(IV) and alpha 6(IV) collagen chains in
the basement membrane of basal cell carcinoma. Histochem J.
29:563–570. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dehan P, Waltregny D, Beschin A, Noel A,
Castronovo V, Tryggvason K, De Leval J and Foidart JM: Loss of type
IV collagen alpha 5 and alpha 6 chains in human invasive prostate
carcinomas. Am J Pathol. 151:1097–1104. 1997.PubMed/NCBI
|
|
15
|
Pan XX, Tong LH, Chen YF, Li FL, Tang WB,
Liu YJ and Yang WA: simplified T classification based on the 8th
edition of the UICC/AJCC staging system for nasopharyngeal
carcinoma. Cancer Manag Res. 11:3163–3169. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang G, Fedyunin I, Kirchner S, Xiao C,
Valleriani A and Ignatova Z: FANSe: An accurate algorithm for
quantitative mapping of large scale sequencing reads. Nucleic Acids
Res. 40:e832012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu G, Wang LG, Han Y and He QY:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nagy Á, Lánczky A, Menyhárt O and Győrffy
B: Validation of miRNA prognostic power in hepatocellular carcinoma
using expression data of independent datasets. Sci Rep. 8:92272018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wei X, Li S, He J, Du H, Liu Y, Yu W, Hu
H, Han L, Wang C, Li H, et al: Tumor-secreted PAI-1 promotes breast
cancer metastasis via the induction of adipocyte-derived collagen
remodeling. Cell Commun Signal. 17:582019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo S and Deng CX: Effect ofstromal cells
in tumor microenvironment on metastasis initiation. Int J Biol Sci.
14:2083–2093. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kaur A, Ecker BL, Douglass SM, Kugel CH
III, Webster MR, Almeida FV, Somasundaram R, Hayden J, Ban E,
Ahmadzadeh H, et al: Remodeling of the collagen matrix in aging
skin promotes melanoma metastasis and affects immune cell motility.
Cancer Discov. 9:64–81. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chelberg MK, Tsilibary EC, Hauser AR and
McCarthy JB: Type IV collagen-mediated melanoma cell adhesion and
migration: Involvement of multiple, distinct domains of the
collagen molecule. Cancer Res. 49:4796–4802. 1989.PubMed/NCBI
|
|
24
|
Hamano Y, Zeisberg M, Sugimoto H, Lively
JC, Maeshima Y, Yang C, Hynes RO, Werb Z, Sudhakar A and Kalluri R:
Physiological levels of tumstatin, a fragment of collagen IV alpha3
chain, are generated by MMP-9 proteolysis and suppress angiogenesis
via alphaV beta3 integrin. Cancer Cell. 3:589–601. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hamano Y and Kalluri R: Tumstatin, the NC1
domain of alpha3 chain of type IV collagen, is an endogenous
inhibitor of pathological angiogenesis and suppresses tumor growth.
Biochem Biophys Res Commun. 333:292–298. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maeshima Y, Colorado PC and Kalluri R: Two
RGD-independent alpha vbeta 3 integrin binding sites on tumstatin
regulate distinct anti-tumor properties. J Biol Chem.
275:23745–23750. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang GM, Zhang YM, Fu SB, Liu XH, Fu X,
Yu Y and Zhang ZY: Effects of cloned tumstatin-related and
angiogenesis-inhibitory peptides on proliferation and apoptosis of
endothelial cells. Chin Med J (Engl). 121:2324–2330. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Maeshima Y, Yerramalla UL, Dhanabal M,
Holthaus KA, Barbashov S, Kharbanda S, Reimer C, Manfredi M,
Dickerson WM and Kalluri R: Extracellular matrix-derived peptide
binds to alphavbeta3 integrin and inhibits
angiogenesis. J Biol Chem. 276:31959–31968. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maeshima Y, Sudhakar A, Lively JC, Ueki K,
Kharbanda S, Kahn CR, Sonenberg N, Hynes RO and Kalluri R:
Tumstatin, an endothelial cell-specific inhibitor of protein
synthesis. Science. 295:140–143. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pasco S, Ramont L, Venteo L, Pluot M,
Maquart FX and Monboisse JC: In vivo overexpression of tumstatin
domains by tumor cells inhibits their invasive properties in a
mouse melanoma model. Exp Cell Res. 301:251–265. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hou P, Chen Y, Ding J, Li G and Zhang H: A
novel mutation of COL4A3 presents a different contribution to
Alport syndrome and thin basement membrane nephropathy. Am J
Nephrol. 27:538–544. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rana K, Tonna S, Wang YY, Sin L, Lin T,
Shaw E, Mookerjee I and Savige J: Nine novel COL4A3 and COL4A4
mutations and polymorphisms identified in inherited membrane
diseases. Pediatr Nephrol. 22:652–657. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Saravani R, Yari D, Saravani S and
Hasanian-Langroudi F: Correlation between the COL4A3, MMP-9, and
TIMP-1 polymorphisms and risk of keratoconus. Jpn J Ophthalmol.
61:218–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Saravani S, Yari D, Saravani R and Azadi
Ahmadabadi C: Association of COL4A3 (rs55703767), MMP-9
(rs17576)and TIMP-1 (rs6609533) gene polymorphisms with
susceptibility to type 2 diabetes. Biomed Rep. 6:329–334. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hao XD, Chen XN, Zhang YY, Chen P, Wei C,
Shi WY and Gao H: Multi-level consistent changes of the ECM pathway
identified in a typical keratoconus twins family by multi-omics
analysis. Orphanet J Rare Dis. 15:2272020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang CP, Wu BH, Chen SP, Fu MY, Yang M,
Liu F and Wang BQ: High COL4A3 expression correlates with poor
prognosis after cisplatin plus gemcitabine chemotherapy in
non-small cell lung cancer. Tumour Biol. 34:415–420. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nie XC, Wang JP, Zhu W, Xu XY, Xing YN, Yu
M, Liu YP, Takano Y and Zheng HC: COL4A3 expression correlates with
pathogenesis, pathologic behaviors, and prognosis of gastric
carcinomas. Hum Pathol. 44:77–86. 2013. View Article : Google Scholar : PubMed/NCBI
|