Introduction
Oral cancer is a malignant tumor type with an
increasing prevalence worldwide, with ~350,000 new cases (2.0% of
the total cancer cases) and 170,000 mortalities (1.9% of the total
cancer mortality) reported in 2018 (1–3).
Furthermore, the 5-year overall survival rate of patients with
advanced stage oral cancer is 30–40% (1–3). Oral
squamous cell carcinoma (OSCC), which has high morbidity and
mortality rates, is the most common oral cancer type, accounting
for >90% of the histological classification in males (4,5). While
progress in OSCC diagnosis and therapies has been achieved in
recent decades, the primary treatment option for patients with
advanced stage OSCC is the combined therapeutic regimen of surgery
followed by adjuvant radiotherapy with or without chemotherapy
(6,7), and the 5-year survival rate of patients
with OSCC remains poor, despite patients responding to the
combination of chemotherapy with docetaxel, cisplatin and
5-fluorouracil (8,9).
The leading causes of OSCC treatment failure include
metastatic spread, recurrence and poor drug efficacy (10). Furthermore, previous studies have
reported that OSCC treatment failure is associated with drug
resistance, which is mainly attributed to chemotherapeutic drug
transportation, metabolic reprogramming, redox status and DNA
repair (11). The absence of
effective drugs to treat patients with OSCC and the resistance of
existing drugs indicates the urgent requirement for novel and
effective anti-OSCC drugs for clinical treatment.
It has been revealed that the proliferative ability
of OSCC cells is associated with oxidative stress (12,13).
Increments in reactive oxygen species (ROS) may induce OSCC cell
death, and a decreasing trend in ROS is observed in patients with
advanced stage OSCC, suggesting that ROS may be an important
potential antitumor target in OSCC therapeutic strategies. In
recent years, a number of medicinal herbs and their active
ingredients have been reported to induce ROS-mediated apoptosis in
OSCC cells, including curcumin (10), β-lapachone (14) and erufosine (15).
Lycorine is the major active ingredient of the
Amaryllidaceae alkaloids derived from the medicinal herb
Lycoris radiate. Lycorine and its derivatives have
previously been reported to possess various biological activities,
including antiviral, anti-inflammatory and antitumor activities.
Furthermore, lycorine may act on ovarian cancer cells (16), breast cancer cells (17), hepatoma cells (18), melanoma cells (19) and bladder cancer cells (20) to induce apoptosis and proliferation,
and inhibit tumor neovascularization. However, to the best of our
knowledge, the anti-OSCC effects of lycorine have not previously
been reported.
The present study aimed to investigate the apoptosis
of the OSCC HSC-3 cell line induced by lycorine hydrochloride (LH),
and to investigate the changes of ROS and the expression levels of
the apoptosis-related proteins, including Bax, Bim, Caspase,
poly(ADP-ribose) polymerase 1 (PARP), JNK, mitogen-activated
protein kinase kinase 4 (MKK4) and c-JUN, in order to identify the
apoptotic pathways.
Materials and methods
Materials
LH (purity ≥98%) was purchased from Man Si-Te
(http://www.cdmust.com/). Dulbecco's modified
Eagle's medium (DMEM) and fetal bovine serum (FBS) were purchased
from Gibco; Thermo Fisher Scientific, Inc. A Cell Counting kit
(CCK)-8 was purchased from Dojindo Molecular Technologies, Inc.
N-acetyl-L-cysteine (NAC; cat. no. A7250; purity ≥99%) and
propidium iodide (PI) were obtained from Sigma-Aldrich (Merck
KGaA). PhosSTOP phosphatase inhibitor cocktail, complete™ protease
inhibitor cocktail, Annexin V-FITC/PI apoptosis detection kit, JC-1
detection kit and TaqMan probe were obtained from Roche
Diagnostics, Inc. Horseradish peroxidase (HRP) chemiluminescence
kit and polyvinylidene difluoride (PVDF) membranes were obtained
from EMD Millipore. An intracellular ROS detection kit and RIPA
buffer were obtained from BI Yun-Tian (https://www.beyotime.com/index.htm).
The antibodies against GAPDH (cat. no.5174;
1:1,000), Bax (cat. no. 5023; 1:1,000), Bim (cat. no. 2933;
1:1,000), Bid (cat. no. 2002; 1:1,000), Mcl-1 (cat. no. 5453;
1:1,000), caspase9 (cat. no. 9502; 1:1,000), caspase3 (cat. no.
14220; 1:1,000), PARP (cat. no. 9542; 1:1,000), JNK (cat. no. 9252;
1:1,000), c-Jun (cat. no.9165; 1:1,000), MKK4 (cat. no. 9152;
1:2,000) and ATF2 (cat. no. 35031; 1:2,000), as well as the
phospho-JNK pathway antibody sampler kit (cat. no. 4668; 1:1,000)
and goat anti-rabbit IgG antibody (cat. no. 7074;1:2,000) were
purchased from Cell Signaling Technology, Inc.
RNAiso Plus and PrimeScript™ RT Reagent kit were
purchased from Takara Bio, Inc. The synthetic primers were
synthesized by Qingke Biological Company (http://www.tsingke.net). All other chemicals were of
analytical grade.
Cell culture
The human OSCC HSC-3, HSC-4, UM1 and UM2 cell lines
were donated by Professor Wu Mingbo's research group at the State
Key Laboratory of Biotherapy of Sichuan University (Chengdu,
Sichuan, China). The cells were cultured in DMEM supplemented with
10% FBS, and 100 U/ml penicillin and streptomycin, under humidified
conditions with 5% CO2 at 37°C, according to the culture
conditions recommended by the American Type Culture Collection.
Cells in the logarithmic growth phase, which was when the cells
reached a high density of ~80%, were used in the subsequent
experiments.
Cell proliferation assay
The cell viability was detected using a CCK-8 assay.
Viable HSC-3 cells were seeded onto a 96-well plate and incubated
with appropriate concentrations of LH, followed by the addition of
CCK-8 into each well for another 4 h at 37°C with 5%
CO2. The absorbance at 450 nm was measured using a
spectra microplate spectrophotometer (BioTek Powerwave; BioTek
Instruments, Inc.). Data acquisition and the IC50
analysis were performed using GraphPad Prism 7 software (GraphPad
Software, Inc.).
Cell cycle assay
HSC-3 cells treated with LH for 12 or 24 h were
harvested and washed briefly with ice-cold PBS. Cells were fixed in
75% ice-cold ethanol at 4°C overnight and concentrated following
the removal of ethanol. The cellular DNA was stained with PI at 4°C
for 30 min in dark. Data acquisition and analysis of the cell cycle
distribution were performed using a flow cytometer (BD FACS Accuri
C6; BD Biosciences) and CFlow Sampler v. 1.0 software (BD
Biosciences).
Cell apoptosis assay
Viable HSC-3 cells treated with different
concentrations of LH (with or without pre-treatment of 5 mM NAC for
1 h) for 24 h were harvested and washed with ice-cold PBS. Cells
were dual-stained with Annexin V-FITC and PI at 25°C for 20 min,
followed by FCM analysis according to the manufacturer's protocol
of the Annexin V-FITC/PI apoptosis detection kit. Data acquisition
and analysis of the cell apoptosis were performed using a flow
cytometer (BD FACS Accuri C6; BD Biosciences) and CFlow Sampler
v.1.0 software (BD Biosciences).
Intracellular ROS assay
To monitor the generation of intracellular ROS,
viable HSC-3 cells were pre-treated with NAC for 1 h at 37°C,
followed by 10 and 20 µM of LH for 24 h. The generation of
intracellular ROS was detected via flow cytometry with
2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) as the
peroxide-sensitive fluorescent probe, which may be converted to
DCFH and then oxidized to the fluorescent compound DCF in the
presence of ROS in cells. The cells treated with NAC and LH were
harvested, washed with PBS, mixed with 10 µM DCFH-DA and incubated
at 25°C for 30 min in the dark. The cell suspension was subjected
to the flow cytometer and the fluorescence signal was detected for
intracellular ROS measurement via flow cytometry (BD FACS Accuri
C6; BD Biosciences) and data analysis of intracellular ROS was
performed using a CFlow Sampler v.1.0 software (BD
Biosciences).
Mitochondrial membrane potential (MMP)
assay
The changes in MMP were detected via staining cells
with JC-1, a fluorescent probe for MMP detection. HSC-3 cells
treated with LH (with or without pre-treatment of 5 mM NAC for 1 h)
for 24 h were harvested, washed with ice-cold PBS and stained with
5 mg/ml JC-1 at 37°C for 30 min in the dark. Data acquisition and
analysis of MMP were performed by flow cytometry (BD FACS Accuri
C6; BD Biosciences) and CFlow Sampler v.1.0 software (BD
Biosciences).
Western blot analysis
HSC-3 cells treated with LH for 24 h were lysed in
RIPA buffer with protease inhibitor or phosphatase inhibitor, and
the lysates were centrifuged at 13,000 × g for 15 min at 4°C. The
protein concentration was determined using the BCA method. Equal
amounts (50 µg/lane) of total proteins were separated via 12%
SDS-PAGE and the proteins were transferred onto PVDF membranes via
wet electro-transfer for 150 min at 250 mA. The membranes were
blocked for 1.5 h with 5% skimmed milk at room temperature and
incubated overnight at 4°C with the aforementioned primary
antibodies, followed by incubation with the HRP-conjugated
secondary antibody for 1.5 h at room temperature. The blots were
visualized using the enhanced chemiluminescence system and
densitometry was performed using Quantity One v.4.6 analysis
software (Bio-Rad ChemiDocXRS; Bio-Rad Laboratories, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
HSC-3 cells treated with LH (with or without
pre-treatment of 5 mM NAC for 1 h) for 24 h were harvested. Total
RNA was extracted from the cultured cells using RNAiso Plus, and
cDNAs were synthesized using the PrimeScript RT reagent kit. The
conditions for RT were as follows: 42°C for 2 min, 37°C for 15 min,
85°C for 5 sec 4°C for 30 min. qPCR was performed with the Bio-Rad
CFX96 Real-Time PCR Detection system using the TaqMan probe method.
The following primers were used: c-Jun forward,
5′-CCAAAGGATAGTGCGATGTTT-3′ and reverse,
5′-CTGTCCCTCTCCACTGCAAC-3′; and GAPDH forward,
5′-AGCCACATCGCTCAGACAC-3′ and reverse,
5′-AATACGACCAAATCCGTTGACT-3′. The thermocycling conditions were as
follows: 95°C for 10 min, followed by 45 cycles at 95°C for 10 sec,
60°C for 30 sec, and at 40°C for 30 sec. The mRNA expression levels
were calculated with the 2−ΔΔCq method (21) and expressed in relative
quantification units. A control without cDNA was run in parallel
with each assay. Each reaction was performed in triplicate.
Statistical analysis
All experiments were repeated ≥3 times, unless
otherwise stated. Data are presented as the mean ± standard
deviation and were compared using a Dunnett's test for multiple
group comparisons with the control and Tukey's test for comparisons
of differences between group using GraphPad Prism 7 software
(GraphPad Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
LH inhibits the proliferation of OSCC
cells
Several OSCC cell lines, including HSC-3, HSC-4, UM1
and UM2, were originally used to investigate the suppressive effect
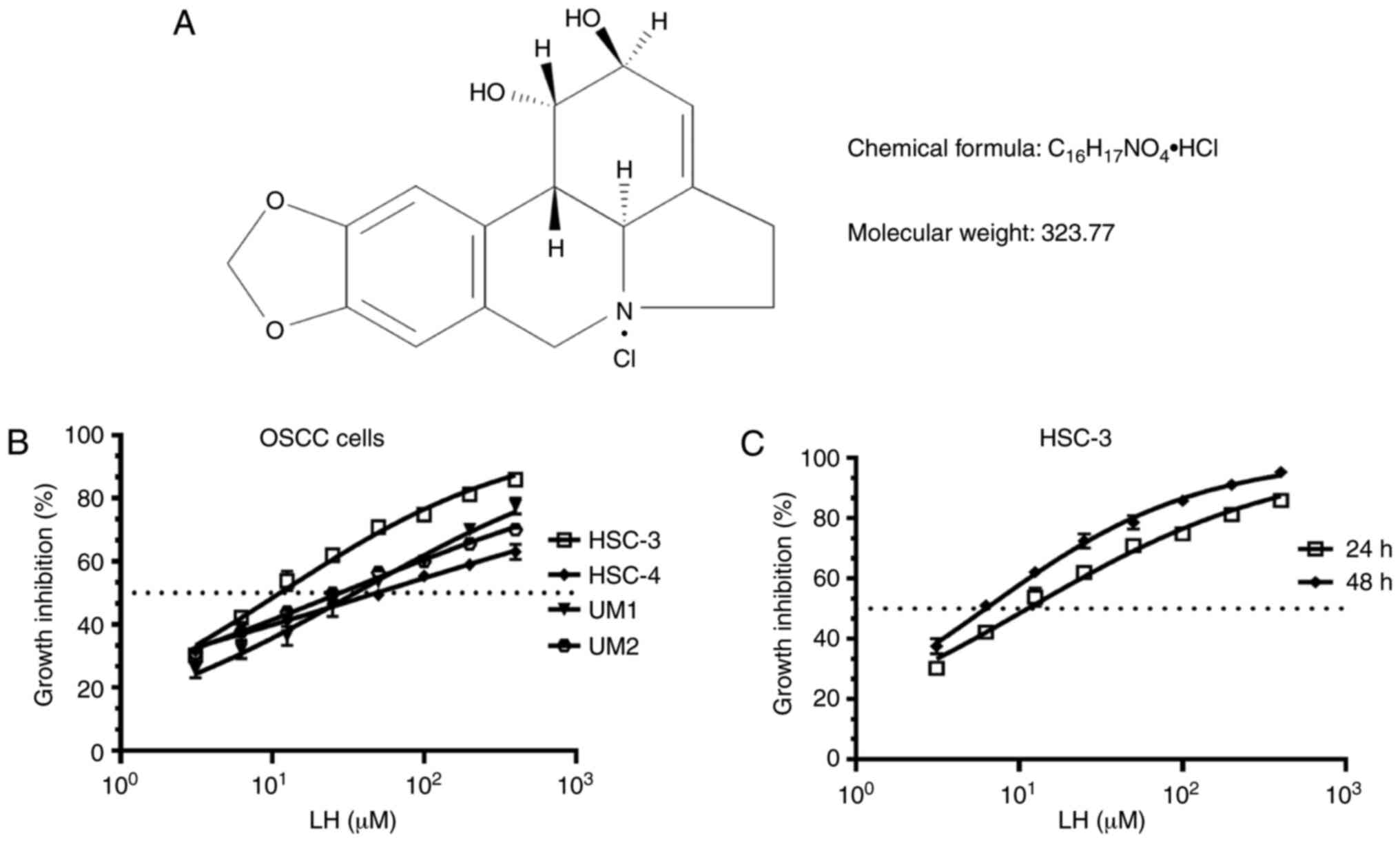
on proliferation induced by LH in vitro (Fig. 1A), based on the previously reported
antitumor effects of LH in other malignant tumors (16–20). The
results demonstrated that LH inhibited the proliferation of HSC-3,
HSC-4, UM1 and UM2 cells in vitro, with IC50
values of 15.65, 48.55, 35.32 and 27.95 µM, respectively (Fig. 1B). Comparison of the growth
inhibitory activity of LH on these four OSCC cell lines indicated
that the HSC-3 cell line had the highest sensitivity to LH. LH
inhibited HSC-3 cell proliferation in a time- and dose-dependent
manner, with a 24 h IC50 value of 15.65 µM and a 48 h
IC50 value of 6.23 µM (Fig.
1C). Taken together, these data suggested that LH may inhibit
the proliferation of OSCC cells in vitro, particularly that
of HSC-3 cells. Therefore, HSC-3 cells were used in the subsequent
studies.
LH induces HSC-3 cell cycle arrest at
the G0/G1 phase
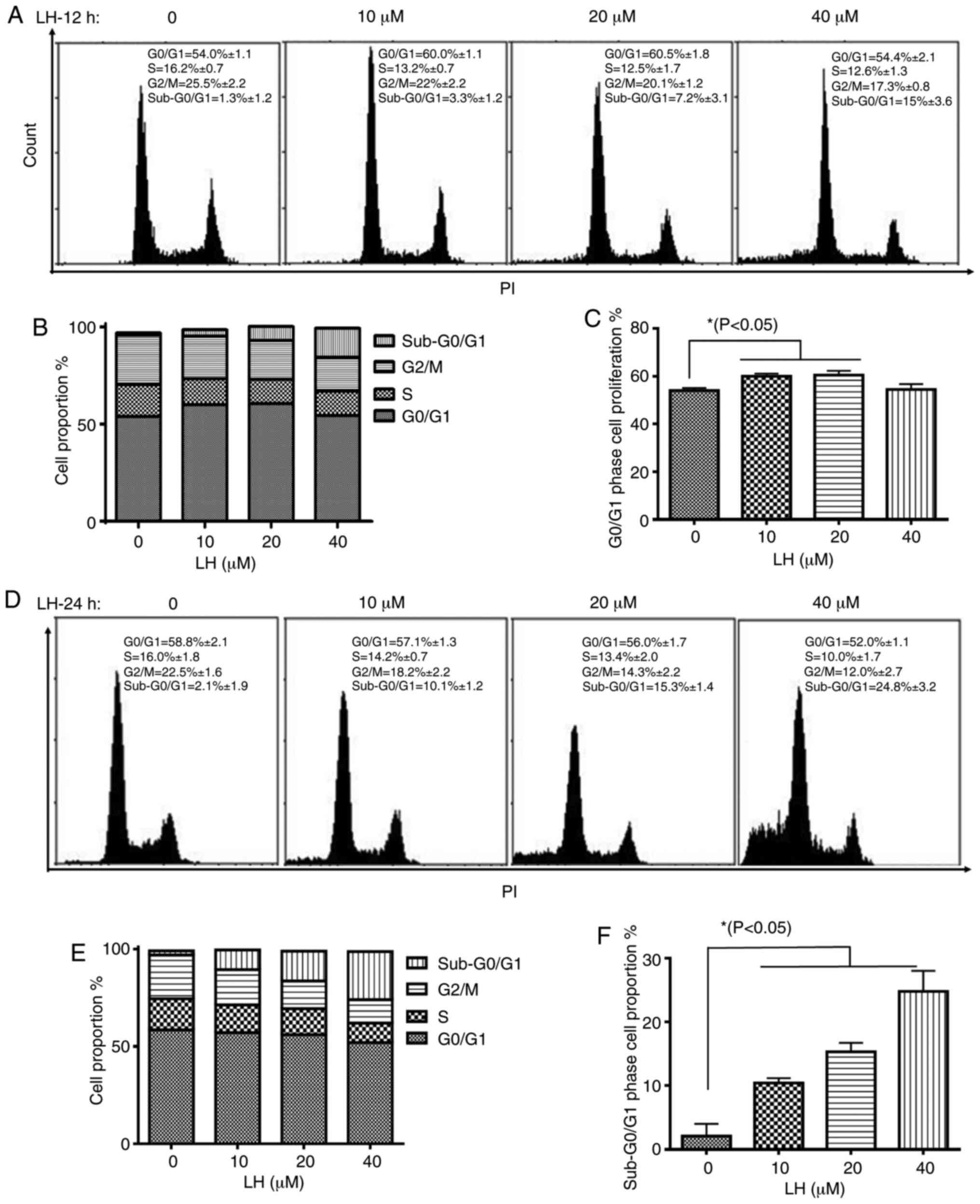
The effect of LH on cell cycle progression in HSC-3
cells was investigated to elucidate the mechanism via which LH
exerts its antiproliferative activity. HSC-3 cells were treated
with LH for 12 h, followed by the staining of the cellular DNA with
PI and flow cytometric analysis. The cell cycle assay demonstrated
that the proportion of HSC-3 cells in the S and G2/M
phases decreased from 16.2 and 25.5% (without LH) to 12.5 and 20.1%
(in the presence of 20 µM LH), respectively (Fig. 2A and B). By contrast, the percentage
of cells in the G0/G1 phase increased from
54.0% (without LH) to 60.5% (in the presence of 20 µM LH),
indicating an LH-induced cell cycle arrest at the
G0/G1 transition in HSC-3 cell cycle
progression (Fig. 2A and C).
Furthermore, the proportion of HSC-3 cells in the
sub-G0/G1 phase increased by ~12-fold in a
dose-dependent manner from 1.3% (without LH) to 15% (in the
presence of 40 µM LH) (Fig. 2A and
B). Following HSC-3 cells being treated with LH for 24 h, the
proportion of HSC-3 cells in sub-G0/G1 phases
increased significantly in a dose-dependent manner from 2.1%
(without LH) to 24.8% (in the presence of 40 µM LH; Fig. 2D-F), indicating that apoptosis may be
induced by LH in HSC-3 cells. Therefore, these data suggested that
LH induced HSC-3 cell cycle arrest at the
G0/G1 phase, and that cell apoptosis may be
the inhibitory mechanism in HSC-3 cell proliferation induced by
LH.
LH induces apoptosis mediated by ROS
in HSC-3 cells
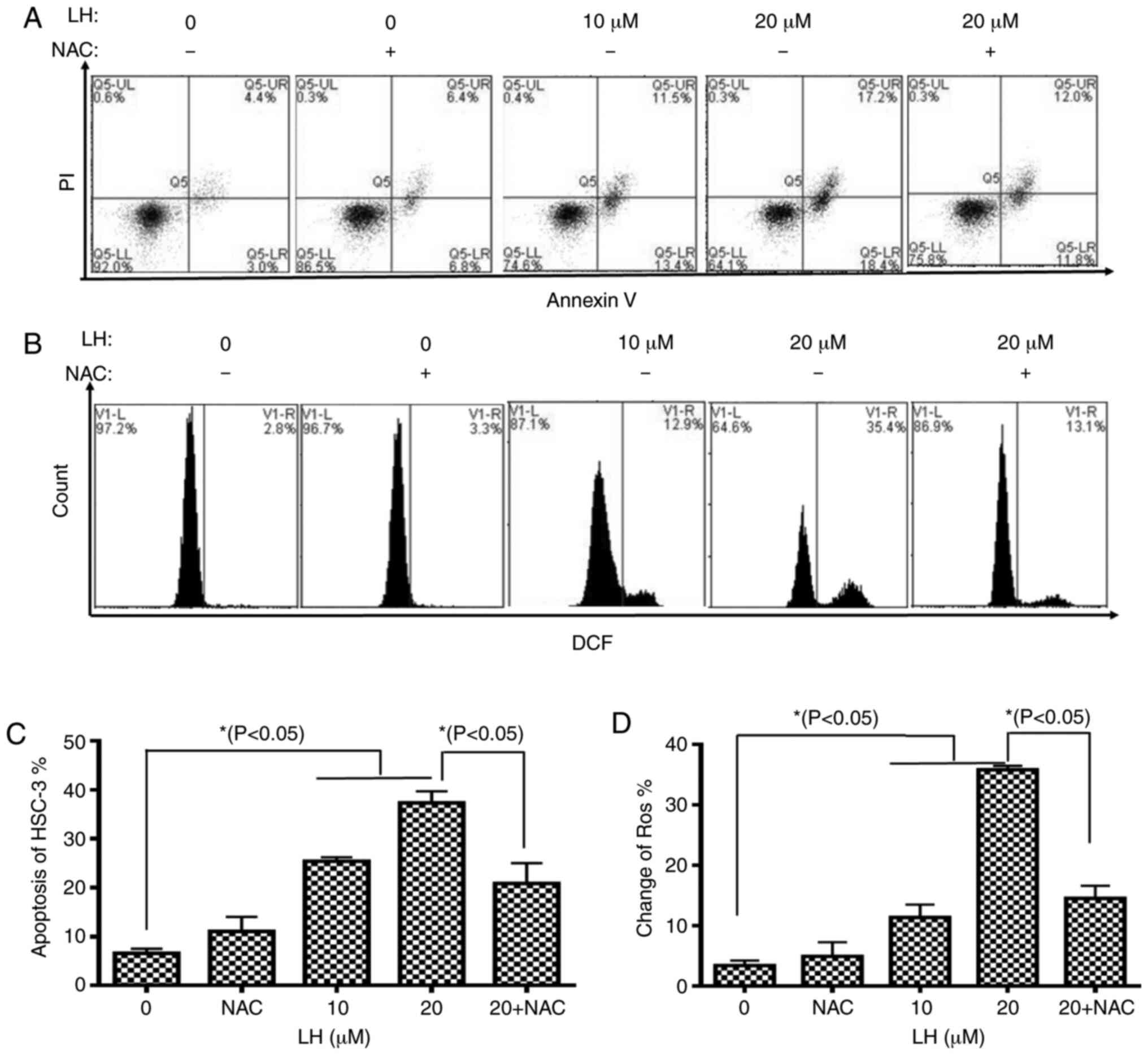
To further assess the apoptotic effect of LH, HSC-3
cells were identified via dual-staining with Annexin V-FITC and PI,
followed by flow cytometric analysis. A dose-dependent increase in
the percentage of apoptotic cells was observed in the presence of
0, 10 and 20 µM LH with apoptotic rates of 7.4, 24.9 and 35.6%,
respectively. Furthermore, the early apoptosis and late apoptosis
of HSC-3 cells increased from 3.0 and 4.4% (without LH) to 18.4 and
17.2% (in the presence of 20 µM LH), respectively. It was also
identified that NAC reversed the apoptotic rate from 35.6 to 23.8%
(Fig. 3A and C).
To investigate whether intracellular ROS, which are
well-known signaling molecules serving a pivotal role in mediating
cell apoptosis, were associated with LH-induced apoptosis in HSC-3
cells, the intracellular ROS levels were investigated using
DCFH-DA. The results indicated that LH significantly increased ROS
levels in HSC-3 cells from 2.8% (without LH) to 35.4% (in the
presence of 20 µM LH), which was a ~13-fold increase (Fig. 3B and D). By contrast, pre-treatment
with NAC, a ROS inhibitor, reversed the ROS levels from 35.4 to
13.1%, suggesting that LH had a ROS-inducing effect (Fig. 3B and D). Therefore, it was indicated
that LH may induce apoptosis mediated by ROS in HSC-3 cells.
LH induces HSC-3 cell apoptosis via a
mitochondrial pathway
The mitochondrial apoptotic pathway is a well-known,
important pathway in programmed cell death (22). To investigate whether the
mitochondrial pathway was involved in LH-induced HSC-3 cell
apoptosis, the change of MMP (ΔΨm), an important factor for
mitochondrial dysfunction, was measured with JC-1 staining via flow
cytometry. In mitochondria, the dissipated MMP may prevent the
accumulation of JC-1, leading to a shift from red (JC-1 aggregates)
to green fluorescence (JC-1 monomers). These results demonstrated
that LH depolarized the MMP in a dose-dependent manner in the
presence of 0, 10 and 20 µM LH with a ΔΨm of 5.7, 17.8 and 32.2%,
respectively. Furthermore, the depolarized MMP could be recovered
from 32.2% (in the presence of 20 µM LH) to 18.3% when the HSC-3
cells were pre-treated with NAC (Fig. 4A
and B).
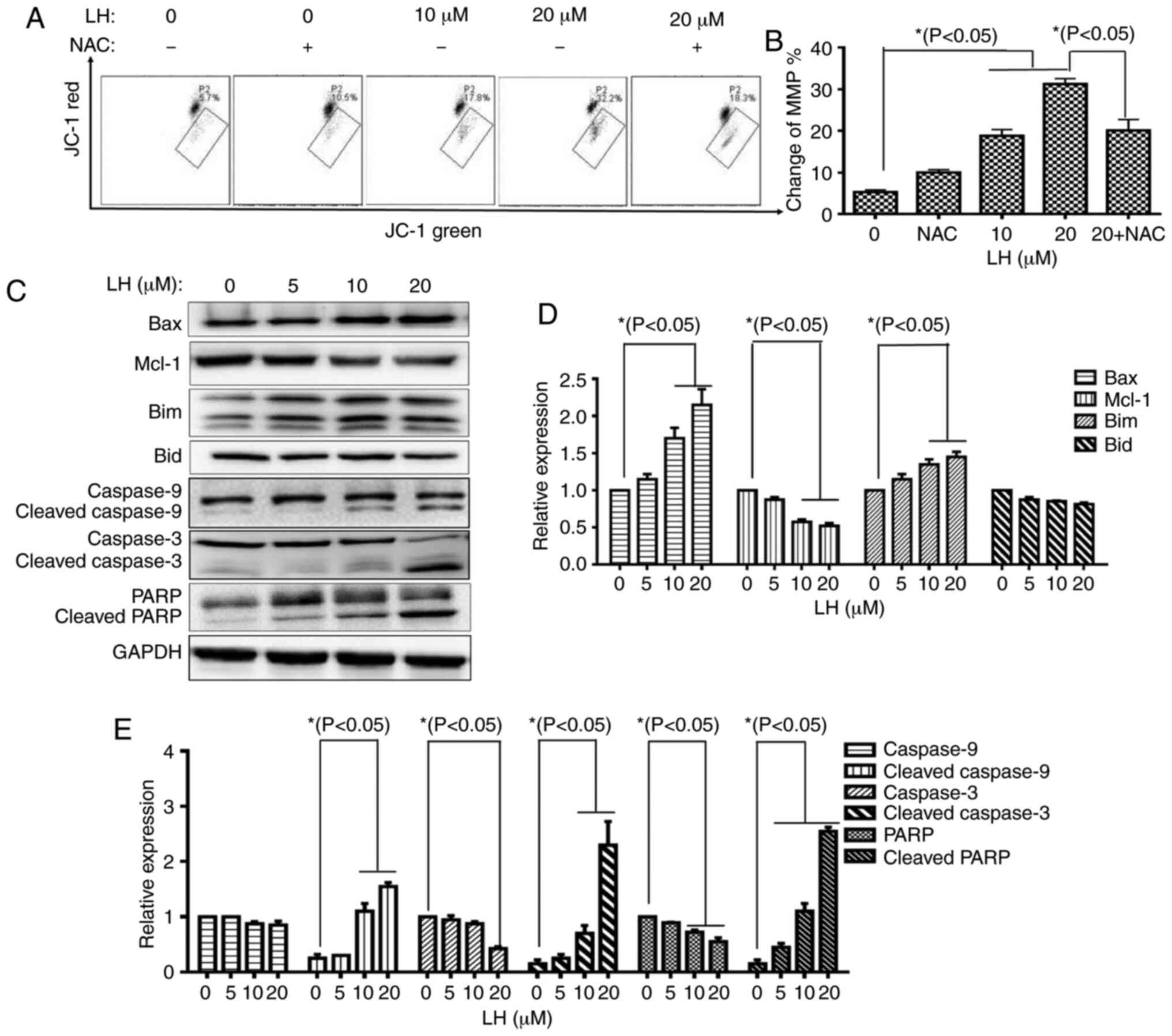 | Figure 4.LH induces HSC-3 cell apoptosis via
the mitochondrial pathway. (A) Changes in MMP in HSC-3 cells were
detected via JC-1. Data were analyzed using Accuri C6 FCM software
by measuring green (530±30 nm) and red (585±40 nm) JC-1
fluorescence. MMP loss was observed by a decrease in JC-1 red
fluorescence and an increase in JC-1 green fluorescence. In total,
≥5,000 cells were collected and counted per sample. (B) Changes in
the MMP in HSC-3 cells were investigated via histogram analyses.
*P<0.05 was considered to indicate a statistically significant
difference using Dunnett's test for multiple group comparisons with
the control group (0 µM LH) and Tukey's test for comparisons
between group differences (groups, 20 and 20 + NAC). (C) Expression
levels of the mitochondrial pathway-related apoptotic proteins were
detected via western blot analysis. (D and E) HSC-3 cells were
treated with LH for 24 h, harvested and total protein lysate was
subjected to western blot analysis using antibodies against GAPDH,
Bax, Bim, Mcl-1, Bid, caspase-9, caspase-3 and PARP. The apoptotic
protein expression was investigated via histogram analyses.
*P<0.05 was considered to indicate a statistically significant
difference using Dunnett's test for multiple group comparisons with
the control group (0 µM LH). LH, lycorine hydrochloride; PARP,
poly(ADP-ribose) polymerase 1; Mcl-1, MCL1 apoptosis regulator,
BCL-2 family member; MMP, mitochondrial membrane potential. |
Caspase-9 is a key mediator in the intrinsic
mitochondrial-mediated apoptotic pathway, which may subsequently
activate caspase-3 and poly ADP-ribose polymerase (PARP), leading
to degradation of cellular components for apoptosis. Based on the
significant roles of these caspases involved in apoptosis, the
catalytic activities of these caspases were measured by western
blot analysis. A prominent increase in the levels of cleaved
caspases-9, caspase-3 and PARP were identified in an LH
dose-dependent manner. Furthermore, expression level changes of
several critical members of the Bcl-2 family targeting the
mitochondrial apoptotic pathway, including Bax, Bim and Mcl-1, were
analyzed. The western blot analysis results identified that LH
upregulated the expression levels of the pro-apoptotic members, Bax
and Bim, but downregulated the expression of the anti-apoptotic
protein, Mcl-1, in a dose-dependent manner, while it did not
significantly affect the expression of the pro-apoptotic member,
Bid (Fig. 4C-E). These results
indicated that the mitochondrial pathway was involved in
ROS-mediated apoptosis induced by LH.
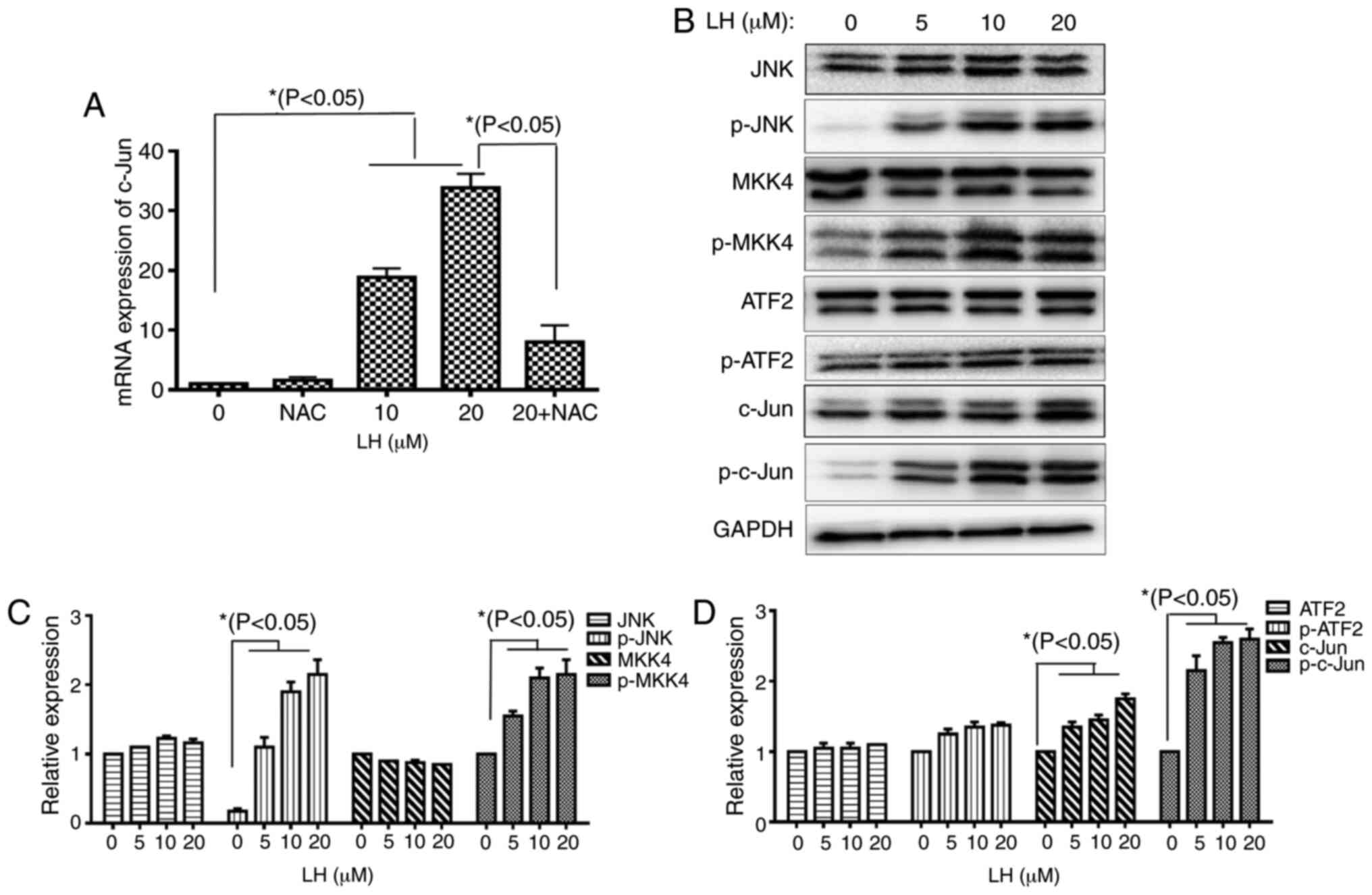
JNK signaling pathway is involved in
LH-induced apoptosis in HSC-3 cells
A previous study reported that the JNK signaling
pathway modulates mitochondrial pathway-induced cell apoptosis
triggered by oxidative stress, leading to mitochondrial dysfunction
and cell death (23). The results of
the present study demonstrated that the mRNA expression levels of
transcription factor c-Jun were increased 35-fold compared with the
control following HSC-3 cells being treated with 20 µM LH.
Furthermore, this increase was significantly decreased when the
HSC-3 cells were pre-treated with NAC (Fig. 5A). This result indicated that LH
induced the mRNA expression of c-Jun via ROS in HSC-3 cells. The
protein expression levels of p-MKK4 and p-JNK, as well as the
phosphorylated transcription factors c-Jun and ATF2, were detected
to investigate whether the JNK signaling pathway serves vital roles
in apoptosis when HSC-3 cells were exposed to LH. Upregulated
expression levels of p-MKK4 and p-JNK were identified in LH-treated
HSC-3 cells. Additionally, the upregulated expression level of the
transcription factor p-c-Jun was observed. However, LH did not
notably affect p-ATF2 expression (Fig.
5B-D). Taken together, these results suggested that the JNK
signaling pathway may be another pathway involved in LH-induced
HSC-3 cell apoptosis mediated by ROS.
Discussion
OSCC is a malignant tumor type that remains a major
threat to human health and is associated with a high morbidity and
a poor 5-year survival rate. Chemotherapy is one of the methods
used to assist the multimodality treatment of advanced stage OSCC.
However, traditional chemotherapy rarely achieves a significant
response in prolonging survival. Therefore, it is important to
identify effective candidate compounds for treating OSCC. Previous
studies have focused on the active ingredients of natural products
from medicinal herbs that have significant pharmacological
activities, including potential antitumor effects, and it was
revealed that LH significantly inhibited several different tumor
cells (24–26). Therefore, the present study
investigated whether LH could effectively suppress the
proliferation of OSCC cells. In the present study, LH inhibited the
proliferation of HSC-3 cells in a time- and dose-dependent manner.
In order to identify the mechanism through which LH inhibits HSC-3
cell proliferation, cell cycle and apoptosis assays were performed
in HSC-3 cells following treatment with LH. It was identified that
the cell cycle of LH-treated cells was arrested at the
G0/G1 phase. In addition, the rate of
apoptosis of LH-treated HSC-3 cells was significantly increased,
compared with the control group. All these data indicated that LH
inhibited HSC-3 cell proliferation by inducing cellular
apoptosis.
Previous studies have reported that excessive ROS
may trigger mitochondrial dysfunction and cause cellular apoptosis
(24,27). When cells are stimulated by internal
or external stress signals, the members of the Bcl-2 superfamily
are affected, resulting in the upregulated expression of
pro-apoptotic proteins (e.g. Bax and Bim) and downregulated
expression of anti-apoptotic proteins (e.g. Mcl-1). Subsequently,
the disorder of the MMP results in the opening of mitochondrial
membrane pores, which causes the release of a large amounts of
cytochrome c from mitochondrion into the cytoplasm, forming
a complex with Apaf-1 and further activating caspase-9, which can,
in turn, activate caspase-3 and PARP, ultimately causing apoptosis
(26). The present study
demonstrated that LH caused an increase in ROS production in HSC-3
cells. LH also triggered MMP disorder, as well as an increase in
the protein expression levels of Bax, Bim, cleaved caspase-9,
cleaved caspase-3 and cleaved PARP, and a decrease in Mcl-1
expression in HSC-3 cells. Taken together, these results suggested
that LH may induce HSC-3 cell apoptosis via the ROS-mediated
mitochondrial apoptotic pathway.
The JNK signaling pathway has been demonstrated to
serve a specific role in mediating apoptosis in several types of
cancer cells (22). ROS, as an
upstream regulator of JNK, serves important roles in cell
proliferation, differentiation, necrosis and apoptosis, as well as
other stress and inflammatory responses. Furthermore, ROS
overproduction triggers mitochondrial pathway-induced cell
apoptosis by activating the JNK signaling pathway and
simultaneously activating proteins, including Bax, leading to
damaged mitochondrial dynamics, ultimately affecting mitochondrial
function and causing cell death. During this process, the
phosphorylation of JNK may lead to the activation of nuclear
transcription factors, including c-fos, c-Jun and ATF-2.
Furthermore, the activation of JNK may regulate the Bcl-2
superfamily in apoptosis, including causing the phosphorylation of
Bcl-2, resulting in Bcl-2 degradation and an inhibition of its
anti-apoptotic properties. Activated JNK may also lead to changes
in the MMP, resulting in a downstream cascade to induce apoptosis.
MKK4, JNK and c-Jun are important members of the JNK signaling
pathway. The present results suggested that the protein expression
levels of p-JNK, p-MKK4 and p-c-Jun were significantly increased in
LH-treated cells, accompanied by an increase of ROS. To investigate
the effect of ROS on the JNK pathway, NAC, a potent antioxidant,
was employed to detect the changes resulting from LH-induced
pathway activation in HSC-3 cells. The present results demonstrated
that NAC reversed the upregulation of the mRNA expression of c-Jun,
one of the most important downstream transcription factors of the
JNK signaling pathway, as well as reversing the enhanced ROS
production, the disorder of MMP and HSC-3 cell apoptosis induced by
LH. Taken together, these results indicated that LH induced HSC-3
cell apoptosis via the ROS dependent activation of the JNK
signaling pathway.
Based on the present data, a model for the mechanism
of apoptosis induced by LH was proposed and is presented in
Fig. 6. In HSC-3 cells, LH initially
triggered oxidative stress, enhanced intracellular ROS, depolarized
the MMP, increased the expression levels of the pro-apoptotic
factors Bax/Bim, inhibited the expression level of the
anti-apoptotic factor, Mcl-1, and activated the caspases cascade of
caspase-9, capsase-3 and PARP, resulting in the apoptosis of HSC-3
cells via a mitochondrial pathway. In addition, the JNK signaling
pathway was involved in the apoptosis of HSC-3 cells. The increased
intracellular ROS induced by LH successively stimulated the
phosphorylation of MKK4 and JNK in cytoplasm, followed by the
translocation of JNK into the nucleus, which further modified the
transcription factor, c-Jun, resulting in a series of
transcriptional stimulatory modifications targeting apoptosis. Of
note, previous studies have reported that activated c-Jun may
regulate Bim transcription in the nucleus, and that the activated
JNK has a distinctive feature that induces the separation of the
14-3-3 subunit from the Bax/14-3-3 protein complex in the cytoplasm
(28,29). Therefore, these results suggest the
existence of crosstalk between the mitochondrial pathway and the
JNK signaling pathway, ultimately forming an orchestrated signaling
network in HSC-3 cells.
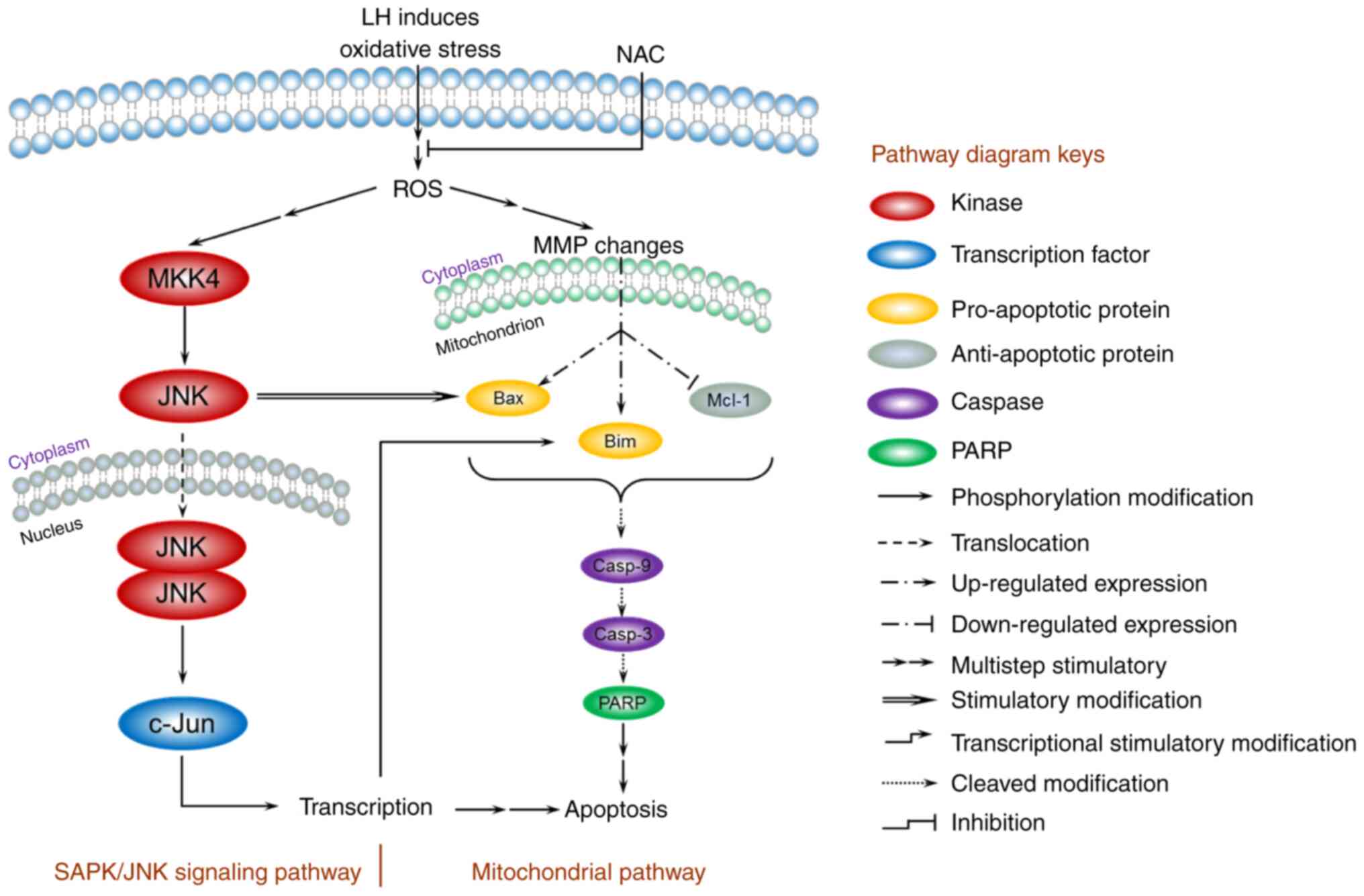 | Figure 6.A model for the ROS-mediated
apoptosis of LH in HSC-3 cells. The mitochondrial pathway and JNK
signaling pathway are involved in the regulation of apoptosis
induced by LH. LH induces oxidative stress, enhances intracellular
ROS, depolarizes the MMP, promotes expression of Bax/Bim, inhibits
expression of Mcl-1 and activates the caspases cascade of
caspase-9, caspase-3 and PARP, resulting in apoptosis of HSC-3
cells via the mitochondrial pathway. Simultaneously, LH
successively promotes MKK4, JNK and c-Jun phosphorylation,
resulting in apoptosis via the JNK signaling pathway. Furthermore,
these two pathways may be connected via the interactions indicated
in this model between JNK and Bax, c-Jun and Bim. ROS, reactive
oxygen species; LH, lycorine hydrochloride; MMP, MMP, mitochondrial
membrane potential; PARP, poly(ADP-ribose) polymerase 1. |
However, there are several limitations to the
present study that should be noted. To begin with, the potential
therapeutic target of LH remains unknown. Additionally, the
anti-OSCC effects of LH were investigated only in the OSCC HSC-3
cell line, and the antitumor effects of LH in other OSCC cell lines
and primary OSCC cells were not determined. Finally, the effect of
LH in an OSCC xenograft nude mouse model has not been investigated.
Further studies are currently being conducted using additional OSCC
cell lines and primary cells, as well as performing experiments in
a nude mouse model, to validate the effects of LH.
In conclusion, the present study demonstrated the
inhibitory effect of LH on the proliferation of OSCC HSC-3 cells.
Furthermore, the apoptosis-induced effect of LH mediated by ROS via
the mitochondrial apoptotic pathway and the JNK signaling pathway
could be rescued by NAC pretreatment. Therefore, these results
suggested that LH has the potential as an anticancer agent for
oxidative stress-mediated OSCC therapy, based on the results of the
present cell line study.
Acknowledgements
The authors would like to thank Professor Mingbo Wu
(State Key Laboratory of Biotherapy, Sichuan University) for
providing human OSCC HSC-3, HSC-4, UM1 and UM2 cell lines.
Funding
The present study was supported by grants from the
Sichuan Science and Technology Program (grant no. 20ZDYFS0321) and
the Applied Basic Research Program (grant no. 2017JY0173) of
Science and Technology Department of Sichuan Province, the Research
and Innovation Fund for Postgraduates of Chengdu Medical College
(grant no. YCX2020-16), the National Undergraduates Innovating
Experimentation Project of China (grant nos. 201713705007,
201713705009, 201813705002 and 201913705003), the National Natural
Science Foundation of China (grant no. 81872451).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MHL, PY and CL conceived and designed the
experiments; XL, CL, TTW, YSS KY, PWJ, STS, WXZ performed the
experiments; PY, MHL and KZ analyzed the data and wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for participation
Not applicable.
Competing interests
The authors declare that that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Colombet M, Soerjomataram I,
Mathers C, Parkin DM, Piñeros M, Znaor A and Bray F: Estimating the
global cancer incidence and mortality in 2018: GLOBOCAN sources and
methods. Int J Cancer. 144:1941–1953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wei M, Wu Y, Liu H and Xie C: Genipin
induces autophagy and suppresses cell growth of oral squamous cell
carcinoma via PI3K/AKT/MTOR pathway. Drug Des Devel Ther.
14:395–405. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Su L, Wang S, Yuan T, Xie X, Fu X, Ji P,
Zhong L and Liu W: Anti-oral squamous cell carcinoma effects of a
potent TAZ inhibitor AR-42. J Cancer. 11:364–373. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu Z, Jiang P and He S: Identification for
exploring underlying pathogenesis and therapy strategy of oral
squamous cell carcinoma by bioinformatics analysis. Med Sci Monit.
25:9216–9226. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bernier J, Domenge C, Ozsahin M,
Matuszewska K, Lefèbvre JL, Greiner RH, Giralt J, Maingon P,
Rolland F, Bolla M, et al: Postoperative irradiation with or
without concomitant chemotherapy for locally advanced head and neck
cancer. N Engl J Med. 350:1945–1952. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bernier J, Cooper JS, Pajak TF, van
Glabbeke M, Bourhis J, Forastiere A, Ozsahin EM, Jacobs JR, Jassem
J, Ang KK and Lefèbvre JL: Defining risk levels in locally advanced
head and neck cancers: A comparative analysis of concurrent
postoperative radiation plus chemotherapy trials of the EORTC
(#22931) and RTOG (# 9501). Head Neck. 27:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ju WT, Ma HL, Zhao TC, Liang SY, Zhu DW,
Wang LZ, Li J, Zhang ZY, Zhou G and Zhong LP: Stathmin guides
personalized therapy in oral squamous cell carcinoma. Cancer Sci.
111:1303–1313. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thiagarajan S, Dhar H, Bhattacharjee A,
Fatehi KS, Shah SB, Chaukar D, Nair D, Deshmukh A, Prabhash K,
Joshi A, et al: Patterns of failure and outcomes in cT4 Oral
squamous cell carcinoma (OSCC) undergoing upfront surgery in
comparison to neo-adjuvant chemotherapy (NACT) followed by surgery:
A matched pair analysis. Oral Oncol. 100:1044552020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Utaipan T, Boonyanuphong P, Chuprajob T,
Suksamrarn A and Chunglok W: A trienone analog of curcumin,
1,7-bis(3-hydroxyphenyl)-1,4,6-heptatrien-3-one, possesses ROS- and
caspase-mediated apoptosis in human oral squamous cell carcinoma
cells in vitro. Appl Biol Chem. 63:72020. View Article : Google Scholar
|
|
11
|
Wang C, Liu XQ, Hou JS, Wang JN and Huang
HZ: Molecular mechanisms of chemoresistance in oral cancer. Chin J
Dent Res. 19:25–33. 2016.PubMed/NCBI
|
|
12
|
Shah O'Brien P, Xi Y, Miller JR, Brownell
AL, Zeng Q, Yoo GH, Garshott DM, O'Brien MB, Galinato AE, Cai P, et
al: Disulfiram (Antabuse) activates ROS-dependent ER stress and
apoptosis in oral cavity squamous cell carcinoma. J Clin Med.
8:6112019. View Article : Google Scholar
|
|
13
|
Yu CI, Chen CY, Liu W, Chang PC, Huang CW,
Han KF, Lin IP, Lin MY and Lee CH: Sandensolide induces oxidative
stress-mediated apoptosis in oral cancer cells and in zebrafish
xenograft model. Mar Drugs. 16:3872018. View Article : Google Scholar
|
|
14
|
Dias RB, de Araújo TBS, de Freitas RD,
Rodrigues ACBDC, Sousa LP, Sales CBS, Valverde LF, Soares MBP, Dos
Reis MG, Coletta RD, et al: β-Lapachone and its iodine derivatives
cause cell cycle arrest at G 2/M phase and reactive oxygen
species-mediated apoptosis in human oral squamous cell carcinoma
cells. Free Radic Biol Med. 126:87–100. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ansari SS, Sharma AK, Soni H, Ali DM, Tews
B, König R, Eibl H and Berger MR: Induction of ER and mitochondrial
stress by the alkylphosphocholine erufosine in oral squamous cell
carcinoma cells. Cell Death Dis. 9:2962018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cao Z, Yu D, Fu S, Zhang G, Pan Y, Bao M,
Tu J, Shang B, Guo P, Yang P and Zhou Q: Lycorine hydrochloride
selectively inhibits human ovarian cancer cell proliferation and
tumor neovascularization with very low toxicity. Toxicol Lett.
218:174–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ji Y, Yu M, Qi Z, Cui D, Xin G, Wang B,
Jia W and Chang L: Study on apoptosis effect of human breast cancer
cell MCF-7 induced by lycorine hydrochloride via death receptor
pathway. Saudi Pharm J. 25:633–637. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xin G, Yu M, Hu Y, Gao S, Sun Y, Yu W, He
J and Ji Y: Effect of lycorine on the structure and function of
hepatoma cell membrane in vitro and in vivo. Biotech Biotechnol
Equip. 34:104–114. 2020. View Article : Google Scholar
|
|
19
|
Lamoral-Theys D, Andolfi A, Van
Goietsenoven G, Cimmino A, Le Calvé B, Wauthoz N, Mégalizzi V, Gras
T, Bruyère C, Dubois J, et al: Lycorine, the main phenanthridine
Amaryllidaceae alkaloid, exhibits significant antitumor activity in
cancer cells that display resistance to proapoptotic stimuli: An
investigation of structure-activity relationship and mechanistic
insight. J Med Chem. 52:6244–6256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang C, Wang Q, Li X, Jin Z, Xu P, Xu N,
Xu A, Xu Y, Zheng S, Zheng J, et al: Lycorine induces apoptosis of
bladder cancer T24 cells by inhibiting phospho-Akt and activating
the intrinsic apoptotic cascade. Biochem Biophys Res Commun.
483:197–202. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sharma A, Boise LH and Shanmugam M: Cancer
metabolism and the evasion of apoptotic cell death. Cancers
(Basel). 11:11442019. View Article : Google Scholar
|
|
23
|
L Z: Progress on comprehensive treatment
of nasopharyngeal cancer. Cancer Res Prev Treat. 46:667–671.
2019.
|
|
24
|
An W, Lai H, Zhang Y, Liu M, Lin X and Cao
S: Apoptotic pathway as the therapeutic target for anticancer
traditional Chinese medicines. Front Pharmacol. 10:7582019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roy M, Liang L, Xiao X, Feng P, Ye M and
Liu J: Lycorine: A prospective natural lead for anticancer drug
discovery. Biomed Pharmacother. 107:615–624. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao G, Wang Y, Yang C, Zhao L, Guo L, Li
L and Wei Z: Interplay between autophagy and apoptosis in lycorine
hydrochloride-induced cytotoxicity of HCT116 cells. Nat Prod
Commun. 14:1934578X19862102019. View Article : Google Scholar
|
|
27
|
Li MH, Yang P, Yang T, Zhang K, Liu Y, Liu
J, Li LM, Luo XY, Yang SX, Zou Q and Zhang CJ: A novel
water-soluble benzothiazole derivative BD926 triggers ROS-mediated
B lymphoma cell apoptosis via mitochondrial and endoplasmic
reticulum signaling pathways. Int J Oncol. 49:2127–2134. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tomicic MT, Meise R, Aasland D, Berte N,
Kitzinger R, Krämer OH, Kaina B and Christmann M: Apoptosis induced
by temozolomide and nimustine in glioblastoma cells is supported by
JNK/c-Jun-mediated induction of the BH3-only protein BIM.
Oncotarget. 6:33755–33768. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Uzu M, Sato H, Shimizu A, Shibata Y, Ueno
K and Hisaka A: Connexin 43 enhances Bax activation via JNK
activation in sunitinib-induced apoptosis in mesothelioma cells. J
Pharmacol Sci. 134:101–107. 2017. View Article : Google Scholar : PubMed/NCBI
|