Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most
common type of non-Hodgkin lymphoma worldwide representing ~40% of
all cases (1,2). DLBCL exhibits biological and clinical
heterogeneity and ~30–40% of the patients succumb to their illness
following standard first line immune-chemotherapy, including R-CHOP
(rituximab, cyclophosphamide, doxorubicin, vincristine and
prednisone) (3,4). DLBCL with rearrangement or
overexpression of MYC, BCL-2 and BCL-6 is referred to as double-hit
lymphoma (DHL) or double-expressor lymphoma (DEL). Due to high
aggressiveness and poor prognosis, the World Health Organization
recently reclassified DHL from DLBCL, not otherwise specified, or
unclassified B cells between DLBCL and Burkitt lymphoma to
high-grade B cell lymphoma (1).
DELs, including DHL, are also characterized by an aggressive
clinical course and inferior therapeutic response. In addition, DEL
is relatively more common than DHL (21–34 vs. 2–10%) in newly
diagnosed DLBCL and may be directly diagnosed by
immunohistochemistry. Therefore, it is of considerable clinical
significance to identify the underlying molecular mechanism of this
disease in order to provide individualized therapy and improve
patient survival (5–8).
Fatty acid synthase (FASN) is an enzyme crucial for
the de novo synthesis of fatty acids, which acts as a
metabolic driver in tumors (9–11). FASN
is reported to participate in aggressive cancer phenotypes, which
include the processes of uncontrolled proliferation and metastasis
(12). Previous studies have
revealed that FASN promotes cell growth and metastasis by
activating the ERK pathway (13–17).
FASN is overexpressed in DLBCL and inhibition of FASN has
demonstrated potent antiproliferative and proapoptotic effects
(18,19). Furthermore, FASN is reported to be
associated with the development of a more aggressive phenotype and
poor patient survival, including DLBCL (9,19,20).
Therefore, FASN is considered a therapeutic target in DLBCL,
implying its potential function during DEL progression as well as
its critical importance for the development of therapies in this
subtype.
In the present study, the histopathological features
of the DLBCL and DEL subtypes were investigated in association with
the expression of FASN. Subsequently, the biological function of
FASN and the inter-regulatory mechanisms were investigated in
vitro. The data demonstrated that FASN-overexpression was
associated with the DEL subtype and poor survival. Inhibition of
FASN suppressed cell growth and promoted apoptosis via the
pERK/BCL-2 signaling pathway. The present study provided evidence
that FASN may be a promising therapeutic target for DEL.
Materials and methods
Patients and tissue samples
The cohort consisted of 87 patients with primary
DLBCL and 15 patients with reactive lymphoid hyperplasia (RLH) who
were diagnosed at the Jiangxi Cancer Hospital between March 2015
and December 2017. All cases of Epstein-Barr virus positive (EBV+)
DLBCL, primary mediastinal DLBCL, primary cutaneous DLBCL,
lymphomatoid granulomatosis, T-cell/histocyte rich large B-cell
lymphoma, plasmablastic lymphoma and patients with a history of
small mature B-cell lymphoma of various types, or insufficient
follow-up period or essential clinical data were excluded. All
patients were treated with R-CHOP as first line therapy. Tumor
responses were assessed at the end of treatment and were classified
as complete response (CR), unconfirmed complete response (CRu),
partial response, stable disease or progressive disease according
to the International Workshop criteria (21). Overall response rate (ORR) was
defined as the proportion of patients achieving CR, CRu or PR.
Ethical approval for the present study was provided by the Ethics
Committee of Jiangxi Cancer Hospital. Written informed consent was
obtained from all study participants.
Immunohistochemical analysis
Immunohistochemical studies were performed using
formalin fixed with 10% formalin for 24 h at 25°C,
paraffin-embedded (FFPE) tissue sections, either at the time of
diagnosis or for the purpose of the present study. FFPE sections
were cut at a thickness of 4 µm and stained with the standard
lymphoma markers mentioned below. The following antibody working
solutions (0.2 mg/ml) were used for diagnosis: CD3 (cat. no.
ZM-0417; Origene Technologies Inc.), CD5 (cat. no. ZM-0280; Origene
Technologies Inc.), CD10 (cat. no. ZM-0283; Origene Technologies
Inc.), CD20 (cat. no. kit-0001; Maxim Biotech Inc.), BCL-6 (cat.
no. ZM-0011; Origene Technologies Inc.), MUM1 (cat. no. ZM-0401;
Origene Technologies Inc.), Ki-67 (cat. no. ZM-0166; Origene
Technologies Inc.), BCL-2 (cat. no. ZM-0010; Origene Technologies
Inc.) and MYC (cat. no. ZA-0658; Origene Technologies Inc.). A
primary rabbit anti-human polyclonal antibody against FASN was also
used (dilution, 1:500; cat. no. ab22759; Abcam). The primary
antibodies were incubated overnight at 4°C. Protein detection was
performed using a Two-step Detection kit according to the
manufacturer's protocol (cat. no. PV-8000; ZSGB-BIO).
Quantitative method
The immunostaining results were evaluated by two
independent pathologists separately with a light microscope
(Olympus BX51) (magnification, ×400). The cells of origin were
determined according to the Hansalgorithm based on CD10, BCL6 and
MUM1 immunostaining (22). Based on
previous data that examined the optimum survival cut-offs for
dichotomizing levels of expression, 40% positivity was used as a
cut-off for MYC and 50% positivity for BCL-2. In the present study,
DEL was defined as lymphomas demonstrating ≥40% MYC and ≥50% BCL-2
expression (23,24). FASN staining was determined by the
proportion and intensity of the stained cells (11–13,25,26).
FASN expression was considered positive when the stained cells were
>10%. The intensity score ranged from 0 to 3 as follows: 0, no
staining (<10%); 1, low staining (10–50%); 2, moderate staining
(51–80%); and 3, high staining (>80%). For analytical purposes,
patients with a staining score of 1–2 were categorized as low-FASN
expression subjects and patients with a staining score of 3 were
categorized as high-FASN expression subjects.
Cell culture
The diffused large cell lymphoma SU-DHL-2 and U2932
cell lines were obtained from the Key Laboratory of Carcinogenesis
and Translational Research of Peking Universtiy Cancer Hospital
& Institute. The cells were maintained in RPMI-1640 medium
(Thermo Fisher Scientific, Inc.), supplemented with 15% fetal
bovine serum (Hyclone; GE Healthcare Life Sciences), 1%
penicillin/streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.) at 37°C in 5% CO2.
Western blotting
The cells were washed twice with phosphate buffered
saline (PBS) and lysed in ice-cold radioimmunoprecipitation assay
buffer (Beyotime Institute of Biotechnology) with freshly added
0.01% protease inhibitor PMSF (Amresco, LLC) and incubated on ice
for 20 min. The cell lysate was centrifuged at 12,000 × g for 20
min at 4°C. The protein concentration was determined using a
Bradford assay (Bio-Rad Laboratories, Inc.). A total of 30 µg
protein from each sample were run on 10% SDS-PAGE gel (TGX™
FastCast™ Acrylamide kit; Bio-Rad Laboratories, Inc.) and
transferred onto a polyvinylidene difluoride membrane (EMD
Millipore). The membranes were blocked with 5% BSA solution for 1 h
at 25°C and subsequently incubated with the following primary
antibodies at 4°C for overnight: Rabbit anti-human FASN
(polyclonal; dilution, 1:1,000; cat. no. ab22759; Abcam), rabbit
anti-human Phospho-p44/42 MAPK (Erk1/2; Thr202/Tyr204; monoclonal;
dilution, 1:1,000; cat. no. 4370; Cell Signaling Technology, Inc.),
rabbit anti-human MYC (Y69; monoclonal; dilution, 1:1,000; cat. no.
ab32072; Abcam), rabbit anti-human BCL-2 antibody (E17; monoclonal;
dilution, 1:1,000; cat. no. ab32124; Abcam) and rabbit anti-human
β-actin (polyclonal; dilution, 1:5,000; cat. no. ab8227; Abcam). A
horseradish peroxidase-conjugated goat anti-rabbit IgG H&L
secondary antibody (1:10,000, ab205718, Abcam) was incubated at
25°C for 1 h. Protein detection was performed using an enhanced
chemiluminescence kit (ECL; Thermo Fisher Scientific, Inc.) with an
ECL Imager (Thermo Fisher Scientific, Inc.).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Reverse transcriptase
reactions were performed using cDNA and M-MLV reverse transcriptase
(Promega Corporation). The RT reaction conditions were as follows:
65°C for 2 min, 42°C for 1 h, 70°C for 10 min and 4°C for 2 min.
For mRNA detection, FASN and GAPDH mRNA expression were analyzed
using SYBR-Green qPCR reagents according to the manufacturer's
protocols (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Gene specific primers (10 µM) were used at a final concentration of
0.2 µM with cDNA (1 µl) as template. The cycling conditions were as
follows: 95°C for 2 min, followed by 39 cycles of 95°C for 15 sec
and 60°C for 60 sec. The sequences of the primers are listed in
Table I and were verified by
Primer-BLAST (NCBI). The data were analyzed using the
2−ΔΔCq relative quantification method (27).
 | Table I.Sequences of all primers. |
Table I.
Sequences of all primers.
| Primer name | Sequences
(5′-3′) |
|---|
| GAPDH (homo
sapiens) forward |
ATTGTCAGCAATGCATCCTG |
| GAPDH (homo
sapiens) reverse |
ATGGACTGTGGTCATGAGCC |
| FASN (homo sapiens)
forward |
CAACTCACGCTCCGGAAA |
| FASN (homo sapiens)
reverse |
TGTGGATGCTGTCAAGGG |
Cell Counting Kit-8 (CCK-8)
cytotoxicity assay
The proliferation of the cells was measured using
the Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies,
Inc.). SU-DHL-2 and U2932 cell suspensions were seeded onto 96-well
plates at a density of 2,000 cells/well and cultured at 37°C in 5%
CO2. A total of 20 µl CCK-8 solution was added to each
well and the plates were incubated for 4 h in a 37°C incubator at
the indicated time points (1, 2 and 3 days). The optical density
levels were measured by a microplate reader at 450 nm according to
the manufacturer's protocols.
Survival rate assay
The same number of cells (1.0×106
cells/well) was cultured in the 24-well plates for 1, 2 and 3 days.
Cell viability was quantified by adding 100 µl of 0.4% trypan blue
dye to 100 µl of cell suspension at 25°C for 5 min and counting the
numbers of stained (non-viable) and unstained (viable) cells on a
haemocytometer.
Transfection of FASN silencing
RNA
For transfection of small interfering (siRNA)
targeting FASN (FASN silencer; cat. no. s5031; Thermo Fisher
Scientific Inc.) and non-targeting control siRNA
(Silencer® Select Negative Control No. 1 siRNA; cat. no.
4390843; Thermo Fisher Scientific Inc.). The sequences of siRNA
targeting FASN were as follows: Forward,
5′-CCGUGGACCUGAUCAUCAATT-3′, and reverse,
5′-UUGAUGAUCAGGUCCACGGCG-3′. HiPerfect (Qiagen, Inc.) transfection
reagent was added to the medium without serum in order to yield a
final HiPerFect concentration of 0.5% (v/v). siFASN/negative
control (NC) was added to the aforementioned medium, mixed (final
concentration 5–10 nM), incubated for 10 min at room temperature
and finally added to the cells. Subsequently, cells were cultured
at 37°C with 5% CO2 for 48 h and collected for
subsequent experiments.
Flow cytometry
The two cell lines were transfected with silencing
RNA targeting FASN/NC (siFASN/NC) for 2 days. The FITC Annexin V
Apoptosis Detection kit with propidium iodide (cat. no. 640914;
BioLegend, Inc.) was used to detect the cell apoptotic rate. Flow
cytometry data were collected with a BD LSR Fortessa cell analyzer
and analyzed using FlowJo Software (FlowJo LLC; Version 10).
Statistical analysis
Event-free survival (EFS) was defined as the time
between diagnosis or disease progression and the time of relapse or
death from any cause or to the participant termination date (31
Dec, 2019). Patients without an event were censored at the time
point of last known follow-up. Survival curves were estimated using
the Kaplan-Meier curve and comparisons were made using the log-rank
test. Univariate analyses were made using the chi-square test and
the log-rank test. The data are presented as the mean ± standard
error of the mean. A two-tailed Student's t-test was used to
compare differences between the two groups. Statistical analysis
was performed using the SPSS version 15.0 (SPSS, Inc.) and the
graphs were generated by the GraphPad Prism version 5.0 (GraphPad
Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinicohistopathological features of
DEL and non-DEL patients
A total of 87 eligible cases of de novo DLBCL
were included in the present study. All patients were
immunocompetent. The demographic and clinicopathological data are
presented in Table II. MYC and
BCL-2 DEL was identified in 36 (41%) cases. Additionally, 44 cases
(51%) were MYC-positive and 43 cases (49%) were MYC-negative. A
total of 71 cases (82%) were BCL-2-positive and 16 cases (18%) were
BCL-2-negative. The medium age at diagnosis was 57 and 53 years in
the DEL and non-DEL groups, respectively. Age and sex distributions
were similar in the two groups. DEL was significantly associated
with late stage (Ann Arbor stage III/IV), increased lactate
dehydrogenase levels, extra nodal sites >2, PS score >1 and
IPI score ≥3. The Hans algorithm was used and the findings
indicated that the non-GCB subtype was more prevalent in the DEL
than in the non-DEL groups (89 vs. 65%; P=0.012).
 | Table II.Comparison of clinicopathological
features between non-DEL and DEL subgroup in 87 patients with
diffuse large B-cell lymphoma. |
Table II.
Comparison of clinicopathological
features between non-DEL and DEL subgroup in 87 patients with
diffuse large B-cell lymphoma.
| Characteristic | Total n=87 (%) | Non-DEL n=51
(%) | DEL n=36 (%) | P-value |
|---|
| Median age
(range) | 55 (20–84) | 53 (24–84) | 57 (20–81) |
|
| Age >60
years | 30 (34) | 16 (31) | 14 (39) | 0.499 |
| Male:female | 46:41 | 25:26 | 21:15 | 0.531 |
| Ann Arbor stage
III/IV | 42 (42) | 18 (35) | 24 (67) | 0.004a |
| Elevated LDH | 47 (48) | 19 (37) | 28 (78) | 0.000a |
| ENS >2 | 20 (23) | 6 (12) | 14 (39) | 0.004a |
| PS score >1 | 25 (29) | 9 (18) | 17 (47) | 0.004a |
| IPI score ≥3 | 19 (22) | 6 (12) | 14 (39) | 0.004a |
| BM
involvement+ | 8 (9) | 2 (4) | 6 (17) | 0.061 |
| CNS
involvement+ | 5 (6) | 2 (4) | 3 (8) | 0.645 |
| B symptom | 9 (10) | 3 (6) | 6 (17) | 0.154 |
| GCB:non-GCB
subtype | 22:65 | 18:33 | 4:32 | 0.012a |
| Ki67 ≥80% | 27 (31) | 15 (29) | 12 (33) | 0.851 |
| ORR | 66 (76) | 46 (90) | 20 (56) | 0.000a |
FASN expression in DLBCL and DEL
patients
Immunohistochemical analyses were performed to
determine the expression levels of FASN in 87 DLBCL tissues and 15
RLH tissues. FASN expression was considered positive when the
stained cells were >10%. The tissues were divided into the three
groups according to FASN expression scores as follows: Negative,
low-FASN expression, high-FASN expression (Fig. 1A). The results revealed that FASN
protein was located in the cytoplasm and germinal center. FASN
expression was positive in all DLBCL samples and high-FASN
expression was observed in 72% of DLBCL samples. Furthermore, the
data indicated that FASN expression was significantly higher in
DLBCL compared with that in RLH tissues (2.72±0.05 vs. 1.07±0.18;
P<0.0001; Fig. 1B). In DLBCL
tissues, the expression of FASN was higher in the DEL group than in
the non-DEL group (2.86±0.06 vs. 2.57±0.08; P=0.008; Fig. 1C). The clinicohistopathological
characteristics were analyzed according to FASN expression
(Table III). High FASN expression
was significantly associated with MYC and BCL-2 combined expression
(P=0.027). In addition, high expression of FASN was associated with
MYC-positive cases (P=0.017) and those with a high Ki67 index
(P=0.043).
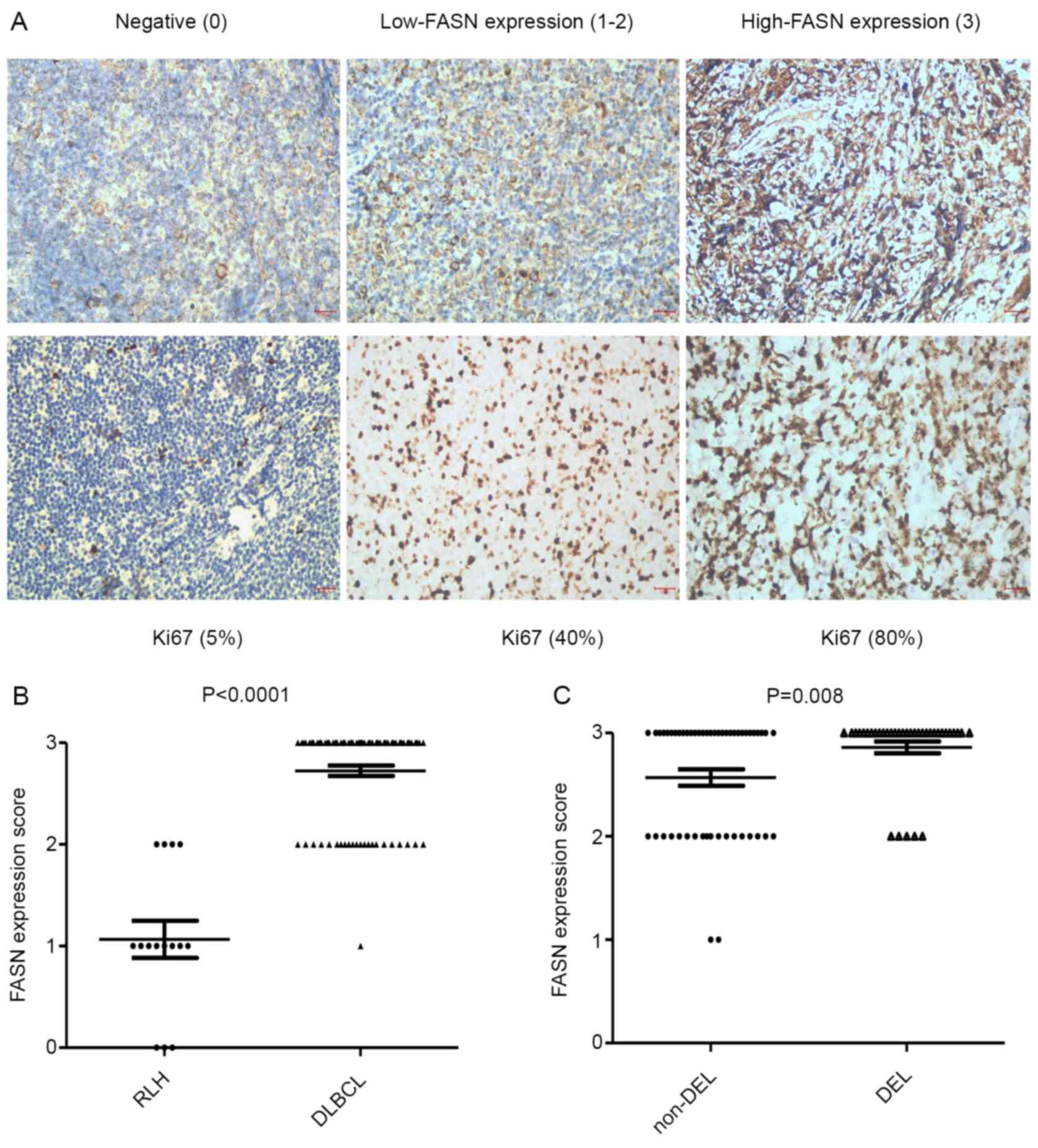 | Figure 1.Upregulation of FASN protein in DEL
patients. (A) Representative immunohistochemical staining of FASN
and Ki67 proteins in tissues (magnification, ×400). Tissues were
divided into three groups according to FASN expression intensity
scores: 0, negative (left), 1–2, low-FASN expression (middle), 3,
high-FASN expression (right). (B) The FASN expression scores in 87
patients with DLBCL, compared with 15 patients with RLH. (C) The
FASN expression score in DEL, compared with non-DEL patients.
Values shown are the mean ± standard error of the mean. Scale bar,
100 µm. FASN, fatty acid synthase; DLBCL, diffuse large B-cell
lymphoma; RLH, reactive lymphoid hyperplasia; DEL, double-expressor
lymphoma. |
 | Table III.Clinicohistopathological
characteristics according to FASN expression in 87 patients with
diffuse large B-cell lymphoma. |
Table III.
Clinicohistopathological
characteristics according to FASN expression in 87 patients with
diffuse large B-cell lymphoma.
|
|
| FASN |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Total n=87 (%) | Low expression n=24
(%) | High expression
n=63 (%) | P-value |
|---|
| Median (range) | 55 (20–84) | 55 (23–81) | 55 (20–84) |
|
| Age >60
years | 30 (34) | 11 (46) | 19 (30) | 0.210 |
| Male:female | 46:41 | 13:11 | 33:30 | 1.000 |
| Ann Arbor
stage | 42 (48) | 12 (50) | 30 (48) | 1.000 |
| Elevated LDH | 47 (54) | 10 (42) | 37 (59) | 0.229 |
| ENS >2 | 20 (23) | 2 (8) | 18 (29) | 0.050 |
| PS score >1 | 26 (30) | 7 (29) | 19 (30) | 1.000 |
| IPI score ≥3 | 20 (23) | 4 (17) | 16 (25) | 0.570 |
| BM
involvement+ | 8 (9) | 2 (8) | 6 (10) | 0.691 |
| CNS
involvement+ | 5 (6) | 0 (0) | 5 (8) | 0.316 |
| B symptom | 9 (10) | 2 (8) | 7 (11) | 1.000 |
| GCB:non-GCB
subtype | 22:65 | 1:2 | 2:7 | 0.287 |
| Ki67 ≥80% | 28 (32) | 4 (17) | 26 (42) | 0.043a |
| MYC + | 44 (51) | 7 (29) | 37 (59) | 0.017a |
| BCL-2 + | 71 (82) | 19 (79) | 52 (83) | 0.761 |
|
MYC+BCL-2+(DEL) | 36 (41) | 5 (21) | 31 (49) | 0.027a |
| ORR | 66 (76) | 21 (88) | 45 (71) | 0.092 |
Survival analysis
All patients in the cohort received
immunochemotherapy [rituximab plus cyclophosphamide, doxorubicin,
vincristine and prednisone (R-CHOP)] as the first line therapy. The
data showed that DEL patients exhibited an inferior ORR (56 vs.
90%) and survival (11 months vs. not reached), compared with those
noted in the non-DEL group. Subsequently, the prognostic impact of
FASN in DLBCL and DEL patients was investigated. High FASN
expression indicated poorer ORR than the low-expression group (71
vs. 88%; P=0.092; Table III). The
2-year EFS rate in the high FASN expression group was 59% (95% CI:
52–65%), while the rate in the low expression group was 83% (95%
CI: 81–91). However, the log-rank test indicated no significant
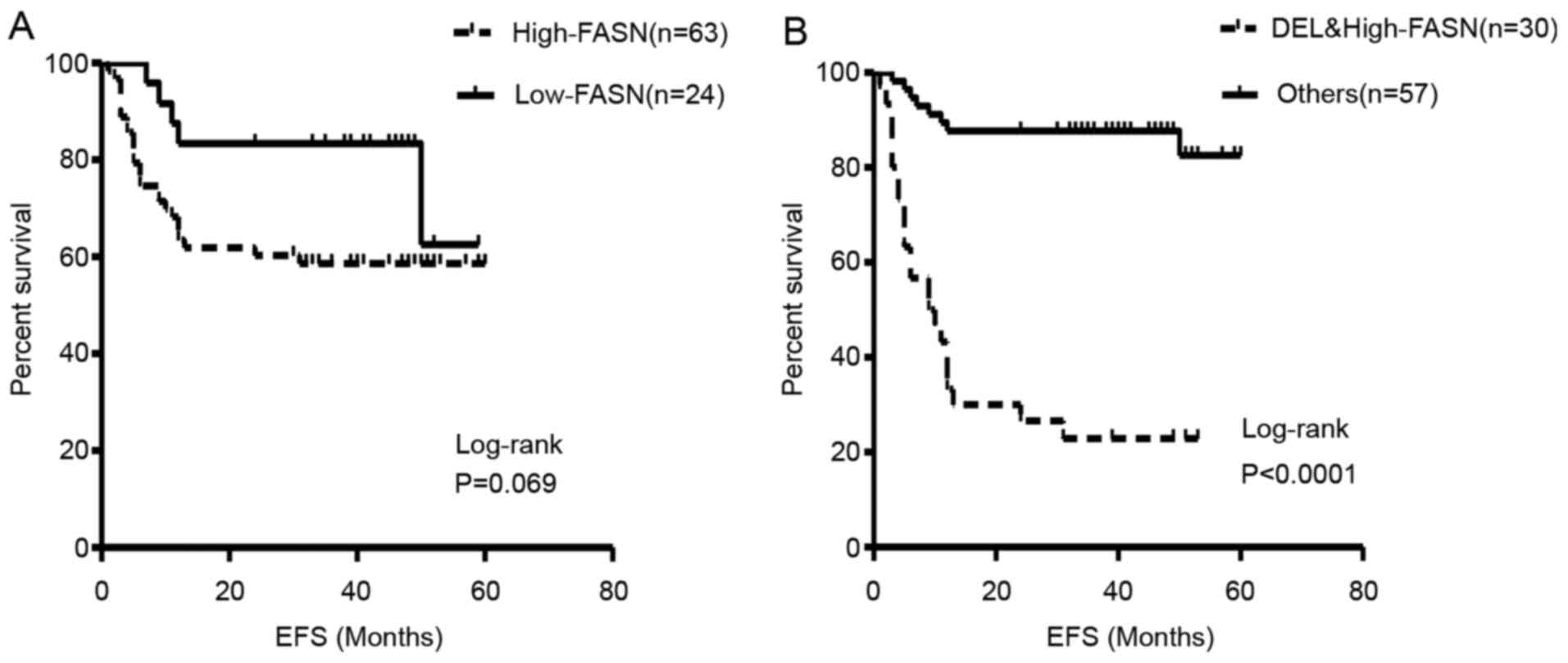
differences with regard to these parameters (P=0.069; Fig. 2A). With a median follow up of 20
months, the median EFS of the two groups had not been reached by
the termination of follow up. Subsequently, survival analysis was
performed on DEL patients with combined high FASN expression. These
patients indicated significantly poorer EFS rates and times than
the remaining patients (2-year EFS rate, 27 vs. 88%, P<0.0001;
median EFS, 9.5 months vs. not arrived; Fig. 2B). Ten factors that had significant
differences from univariate analysis were included and analyzed,
and COX regression analysis was performed. The results demonstrated
that increased LDH and double expression of MYC and BCL-2 (DEL) are
independent factors that influenced the prognosis and survival in
all the patients (Table SI).
Silencing of FASN inhibits cell growth
and promotes apoptosis of DLBCL cells
The two DLBCL cell lines, SU-DHL-2 and U2932, which
have been previously reported as non-GCB type and MYC/BCL-2
amplification-type, respectively, were cultured in vitro
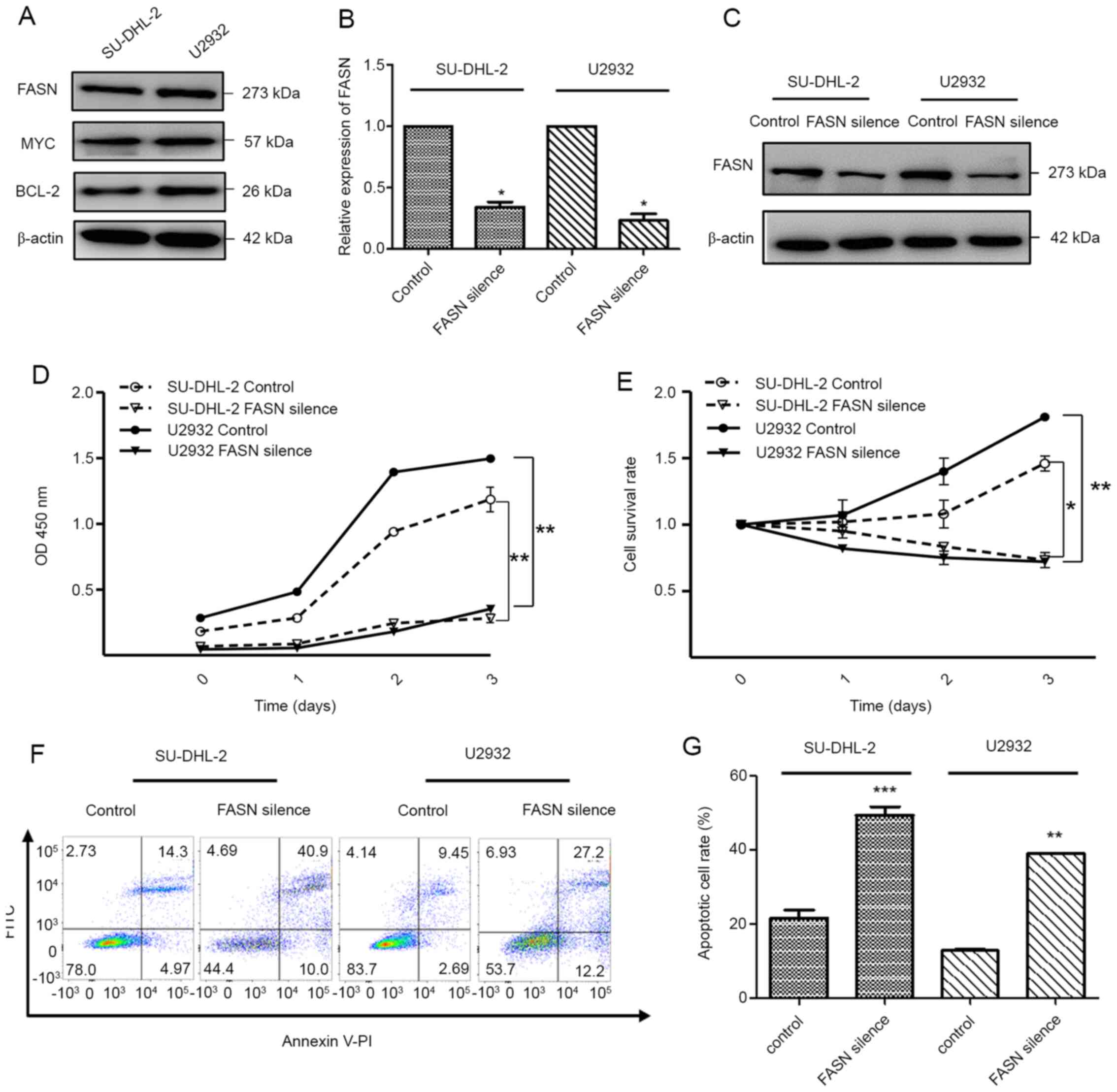
(28,29). The experimental data indicated that
SU-DHL-2 and U2932 cells exhibited high expression levels of FASN,
as well as MYC and BCL-2 protein amplification (Fig. 3A). To further investigate the
function of FASN, its silencing was achieved by siRNA targeting in
order to obtain FASN-silenced cells (Fig. 3B and C). CCK-8 assay revealed that
inhibition of FASN attenuated cell growth (Fig. 3D). The survival assay indicated that
deletion of FASN decreased the cell survival rate (Fig. 3E). The flow cytometry assay indicated
that silencing of FASN significantly accelerated the cell apoptotic
death (Fig. 3F and G), which was
accompanied by cleavage and activation of the apoptosis effector
caspase-3 (Fig. S1). These results
confirmed that FASN was required for the aggressive phenotype noted
in DLBCL.
Silencing of FASN suppresses the
pERK1/2/Bcl-2 signaling pathway in the U2932 cell line
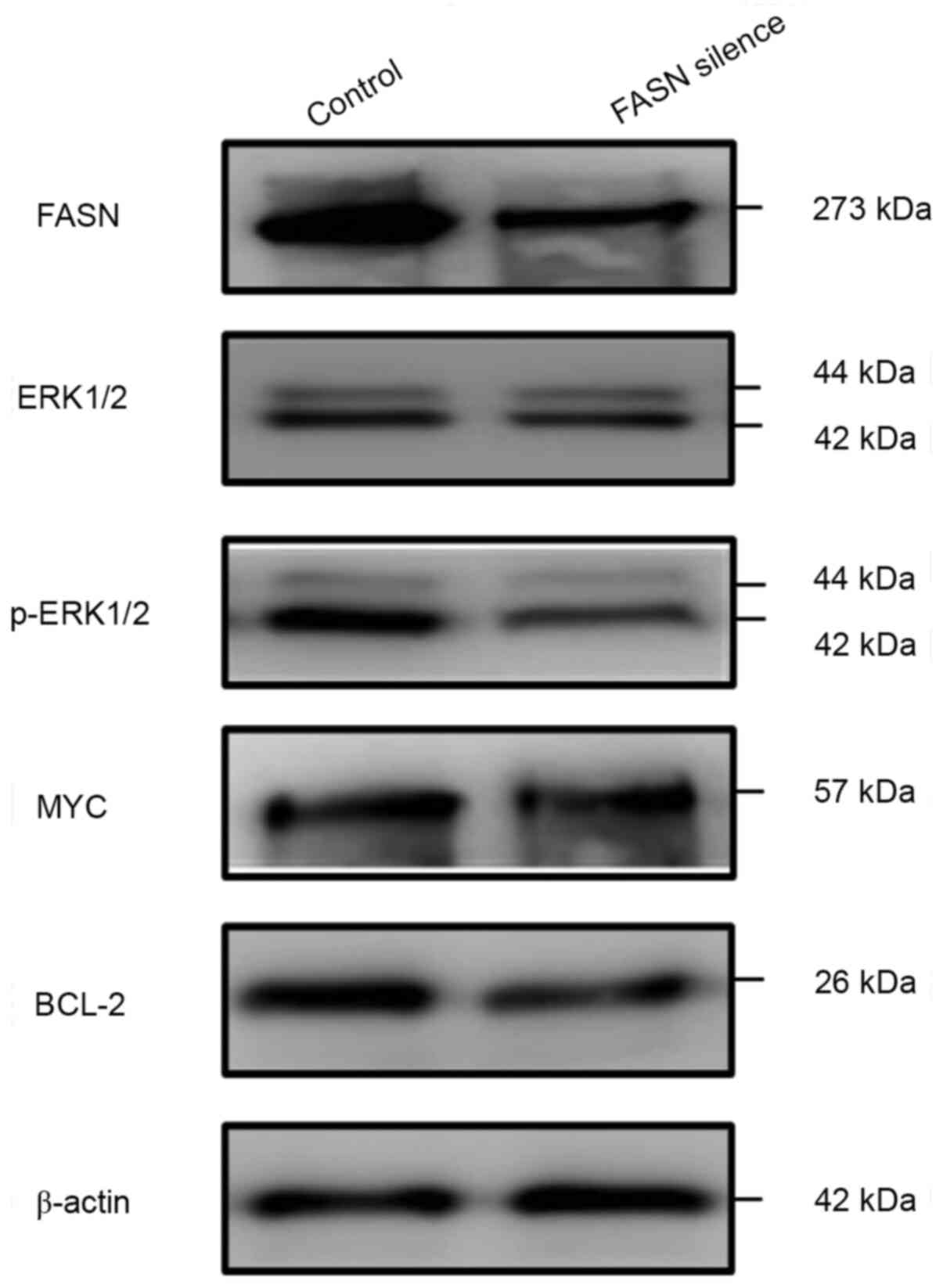
The molecular pathway of FASN was investigated in
combination with the induction of apoptosis in U2932 cells.
Previous studies have demonstrated that FASN may regulate ERK1/2
phosphorylation and the expression of the anti-apoptotic proteins,
including BCL-xl (14,15). Therefore, additional experiments were
performed to determine whether FASN exerts it functions by
regulating the pERK1/2-associated signaling pathway in DLBCL.
Silencing of FASN decreased the levels of p-ERK1/2 and BCL-2, while
total ERK1/2 levels remained unchanged, indicating that it may
suppress ERK1/2 phosphorylation and further decrease BCL-2
expression. Nevertheless, the expression of MYC was not
significantly altered (Fig. 4).
Discussion
DEL associated with co-expression of MYC and BCL-2
is recognized as a poor prognostic subgroup of DLBCL. The present
study assessed the clinicopathological features of 87 patients with
DLBCL who were recruited between March 2015 and December 2017 in
Jiangxi Cancer Hospital. MYC and BCL-2 DEL cases were identified in
41% of all cases. DEL patients exhibited a decreased ORR (56 vs.
90%) and survival time (11 months vs. not arrived), compared with
that of non-DEL patients. These findings were mostly consistent
with the results of previous studies (23,24,30–32).
Treatment of DEL is mainly restricted to R-CHOP treatment. Despite
several efforts, the therapeutic efficacy of current targeted
agents with the exception of rituximab remains controversial
(33–36). Therefore, particular focus has been
given to the identification of novel molecular targets for this
specific subtype.
FASN is considered an oncogene in carcinogenesis and
has emerged as a potential therapeutic target for lymphoma
(37,38). In the present study, substantial
evidence is presented regarding the association of FASN expression
with DLBCL. To begin with, the data indicated that FASN was
overexpressed in DLBCL, compared with RLH samples, while it was
present in highly proliferative tissues characterized by high Ki-67
index (P=0.043). Furthermore, FASN protein distribution was
different between the DLBCL and RLH tissues. In RLH tissues, FASN
expression was restricted in the germinal center B-cells,
confirming the association between FASN and BCL-2. However, FASN
expression was not restricted in the germinal center and was widely
distributed in the DLBCL tissues. In addition, FASN expression was
significantly higher in DEL than non-DEL tissues. FASN was reported
to be associated with cancer progression (14–16). The
present findings provided evidence of an active role of FASN in
DLBCL progression to a more aggressive phenotype (DEL).
Survival analysis demonstrated that DEL patients
with high-FASN expression exhibited poorer EFS, compared with that
noted in other patients (2-year EFS rate, 27 vs. 88%, P<0.0001;
median EFS, 9.5 months vs. not reached). However, following the
analysis it was not possible to demonstrate a significant and
direct association between FASN and EFS. This finding may be
associated with the retrospective nature of the present study and
the relatively small number of the patients involved. The findings
suggested that high-FASN expression patients revealed lower ORR (71
vs. 88%, P=0.092) and 2-year EFS (59 vs. 83%, P=0.065), compared
with that of low-FASN expression patients. Future studies should
focus on a larger number of uniformly-treated patients that may aid
the clarification of the prognostic and predictive value of FASN in
DEL.
A previous study suggested that FASN inhibitors may
trigger apoptosis and suppress the expression of the c-Met kinase
in DLBCL (19). The present study
aimed to elucidate the biological function of FASN and the
associated molecular mechanisms in vitro. MYC/BCL-2
amplification has been reported in SU-DHL-2 and U2932 cell lines
and as a result these two cell lines were used as DEL cells. The
results indicated that FASN was highly expressed in SU-DHL-2 and
U2932 cells and that its knockdown decreased tumor growth and
promoted apoptosis. These results confirmed that FASN promoted cell
growth and apoptosis resistance in DEL, as well as in DLBCL
(19,37). The FASN/ERK pathway is a vital
signaling pathway in cancer (14–15). The
present study indicated that silencing FASN decreased p-ERK and
BCL-2 expression, suggesting that FASN may regulate cell growth and
apoptosis via the p-ERK/BCL-2 signaling pathway in DEL. However,
MYC expression levels remained unaltered. This finding may be
attributed to the fact that MYC is regulated by several genes,
whereas FASN is not its direct upstream target.
In addition, there are certain limitations to the
present study. The biological function of FASN and the exact
mechanism linking p-ERK/BCL-2 signaling to FASN expression requires
further confirmation by ectopic expression of FASN in the negative
control cell lines. In the following gain-functional assay, two
cell lines with low expression of FASN should be added to confirm
the function with ectopic expression of FASN. The length of FASN
mRNA is ~8.4 kb and its full-length cloning is difficult. The
establishment of FASN overexpression models is a difficult task and
our previous studies used FASN cDNA ORF (NCBI Ref Seq BC007909) to
upregulate FASN, suggesting that this method is possible.
Therefore, the gain/loss of function experiments on FASN are
designed to be implemented in future studies. It has been
highlighted that FASN-overexpression in tumor tissues is associated
with the serum levels of FASN (26,39,40). In
the present study, the association between the clinical prognostic
factors of DEL and FASN expression levels (tumor and serum) was
investigated.
The present study highlighted that deregulation of
FASN may be associated with the pathogenesis of DEL and provided a
rational preclinical setting for the therapeutic applications of
FASN inhibitors in DEL. Future clinical studies should focus on the
assessment of the effectiveness of FASN inhibitors in combination
with R-CHOP as therapeutic agents for the treatment of DEL.
Therefore, FASN may serve as a potential therapeutic target in DEL
patients.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Project of
Health Commission of Jiangxi Province (grant no. 20203535).
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ, YS and JGL designed the study. XZ obtained
funding, purchased the reagents and materials, and provided other
support. XZ, ZL and QL acquired the data. XZ and WZ analyzed and
interpreted the data, XZ drafted the manuscript. XZ, WZ, JGL and YS
critically revised the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Jiangxi Cancer Hospital and was performed in
accordance with the Declaration of Helsinki and the guidelines of
the Ethics Committee of Jiangxi Cancer Hospital. Written informed
consent was obtained from all patients for the use of their
clinical tissues.
Patient consent for publication
Written informed consent was obtained from all
patients for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cazzola M: Introduction to a review
series: The 2016 revision of the WHO classification of tumors of
hematopoietic and lymphoid tissues. Blood. 127:2361–2364. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lagunas-Rangel FA: A new history: The 2016
revision of the WHO classification of tumors of hematopoietic and
lymphoid tissues. Austin Leuk. 1:10012016.
|
|
3
|
Lukenbill J and Hill B:
Relapsed/Refractory diffuse large B-cell lymphoma: Review of the
management of transplant-eligible patients. Leuk Lymphoma.
56:293–300. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jain P, Fayad LE, Rosenwald A, Young KH
and O'Brien S: Recent advances in de novo CD5+ diffuse
large B cell lymphoma. Am J Hematol. 88:798–802. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reagan PM and Davies A: Current treatment
of double hit and double expressor lymphoma. Hematology Am Soc
Hematol Educ Program. 2017:295–297. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saumyaranjan M, Arun P, Moien L, Ansh G,
Parashant R, Minakshi S, Ajay G, Ranjit S and Atul S:
Clinicopathological profile of primary double expressor lymphoma.
Clin Lymphoma Myeloma Leuk. 19 (Suppl 1):S2572019. View Article : Google Scholar
|
|
7
|
Choe JY, Park M, Yun JY, Na HY, Go H, Kim
HJ, Oh S and Kim JE: PELI1 expression is correlated with MYC and
BCL6 expression and associated with poor prognosis in diffuse large
B-cell lymphoma. Mod Pathol. 29:1313–1323. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smith SM: Aggressive B-cell lymphoma: The
double-hit and double-expressor phenotypes. Clin Adv Hematol Oncol.
15:40–42. 2017.PubMed/NCBI
|
|
9
|
Danilova OV, Dumont LJ, Levy NB, Lansigan
F, Kinlaw WB, Danilov AV and Kaur P: FASN and CD36 predict survival
in rituximab-treated diffuse large B-cell lymphoma. J Hematop.
6:11–18. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Menendez JA and Lupu R: Fatty acid
synthase (FASN) as a therapeutic target in breast cancer. Expert
OpinTher Targets. 21:1001–1016. 2017. View Article : Google Scholar
|
|
11
|
Wang H, Xi Q and Wu G: Fatty acid synthase
regulates invasion and metastasis of colorectal cancer via wnt
signaling pathway. Cancer Med. 5:1599–1606. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Menendez JA and Lupu R: Fatty acid
synthase and the lipogenic phenotype in cancer pathogenesis. Nat
Rev Cancer. 7:763–777. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu ZL, Wang G, Peng AF, Luo QF, Zhou Y
and Huang SH: Fatty acid synthase expression in osteosarcoma and
its correlation with pulmonary metastasis. Oncol Lett. 4:878–882.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun TH, Zhong X, Song HH, Liu JM, Li JG,
Leung F, Lu WW and Liu ZL: Anoikis resistant mediated by FASN
promoted growth and metastasis of osteosarcoma. Cell Death Dis.
10:2982019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu N, Li Y, Zhao Y, Wang Q, You Jc, Zhang
Xd and Ye Lh: A novel positive feedback loop involving
FASN/p-ERK1/2/5-LOX/LTB4/FASN sustains high growth of breast cancer
cells. Acta Pharmacol Sin. 32:921–929. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bian Y, Yu Y, Wang S and Li L:
Up-regulation of fatty acid synthase induced by EGFR/ERK activation
promotes tumor growth in pancreatic cancer. Biochem Biophys Res
Commun. 463:612–617. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Akiyama T, Choong PF and Dass CR: RANK-Fc
inhibits malignancy via inhibiting ERK activation and evoking
caspase-3-mediated anoikis in human osteosarcoma cells. Clin Exp
Metastasis. 27:207–215. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kapadia B, Nanaji NM, Bhalla K, Bhandary
B, Lapidus R, Beheshti A, Evens AM and Gartenhaus RB: Fatty acid
synthase induced S6Kinase facilitates USP11-eIF4B complex formation
for sustained oncogenic translation in DLBCL. Nat Commun.
9:8292018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Uddin S, Hussain AR, Ahmed M, Bu R, Ahmed
SO, Ajarim D, Al-Dayel F, Bavi P and Al-Kuraya KS: Inhibition of
fatty acid synthase suppresses c-met receptor kinase and induces
apoptosis in diffuse large B-cell lymphoma. Mol Cancer Ther.
9:1244–1255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gonzalez-Guerrico A and Lupu R: Fatty acid
synthase (FASN) as a therapeutic target for breast cancer
metastasis. Cancer Res. 69 (Suppl 24):61602009.
|
|
21
|
Cheson BD, Fisher RI, Barrington SF,
Cavalli F, Schwartz LH, Zucca E and Lister TA: Recommendations for
initial evaluation, staging, and response assessment of hodgkin and
non-hodgkin lymphoma: The lugano classification. J Clin Oncol.
32:3059–3068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hans CP, Weisenburger DD, Greiner TC,
Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E,
Braziel RM, Jaffe ES, et al: Conformation of molecular
classification of diffuse large B-cell lymphoma by
immunohistochemistry using a tissue microarray. Blood. 103:275–282.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Johnson NA, Slack GW, Savage KJ, Connors
JM, Ben-Neriah S, Rogic S, Scott DW, Tan KL, Steidl C, Sehn LH, et
al: Concurrent expression of MYC and BCL2 in diffuse large B-cell
lymphoma treated with rituximab plus cyclophosphamide, doxorubicin,
vincristine, and prednisone. J Clin Oncol. 30:3452–3459. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bogusz AM, Kovach AE, Le LP, Feng D,
Baxter RH and Sohani AR: Diffuse large B-cell lymphoma with
concurrent high MYC and BCL2 expression shows evidence of active
B-cell receptor signaling by quantitative immunofluorescence. PLoS
One. 12:e01723642017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Giró-Perafita A, Sarrats A, Pérez-Bueno F,
Oliveras G, Buxó M, Brunet J, Viñas G and Miquel TP: Fatty acid
synthase expression and its association with
clinico-histopathological features in triple-negative breast
cancer. Oncotarget. 8:74391–74405. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Puig T, Blancafort A, Casoliva G, Oliveras
G, Casas M, Buxo M, Saiz E, Viñas G, Dorca J and Porta R:
Prospective analysis of fatty acid synthase (FASN) in breast cancer
tissue of early-stage breast cancer patients. Cancer Res. 72 (24
Suppl):P4-09-10. 2012.PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta DeltaC(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yao S, Xu F, Chen Y, Ge Y, Zhang F, Huang
H, Li L, Lin D, Luo X, Xu J, et al: Fbw7 regulates apoptosis in
activated B-cell like diffuse large B-cell lymphoma by targeting
Stat3 for ubiquitylation and degradation. J Exp Clin Cancer Res.
36:102017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tarantelli C, Gaudio E, Kwee I, Cascione
L, Bernasconi E, Hillmann P, Stathis A, Stussi G, Fabbro D, Wicki
A, et al: The dual PI3K/mTOR inhibitor PQR309 has synergistic
activity with other targeted agents in diffuse large B cell
lymphomas. Blood. 126:40052015. View Article : Google Scholar
|
|
30
|
Takahashi H, Miura K, Nakagawa M, Sugitani
M, Amano Y, Kurita D, Sakagami M, Ohtake S, Uchino Y, Kodaira H, et
al: Negative impact of concurrent overexpression of MYC and BCL2 in
patients with advanced diffuse large B-cell lymphoma treated with
dose-intensified immunochemotherapy. Leuk Lymphoma. 57:2784–2790.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li L, Zhang X, Zhang T, Song Z, Hu G, Li
W, Li L, Qiu L, Qian Z, Zhou S, et al: Prognostic significance of
BCL-2 and BCL-6 expression in MYC-positive DLBCL. Clin Lymphoma
Myeloma Leuk. 18:e381–e389. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ma Z, Niu J, Cao Y, Pang X, Cui W, Zhang W
and Li X: Clinical significance of ‘double-hit’ and
‘double-expression’ lymphomas. J Clin Pathol. 73:126–138. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Johnston PB, LaPlant B, McPhail E,
Habermann TM, Inwards DJ, Micallef IN, Colgan JP, Nowakowski GS,
Ansell SM and Witzig TE: Everolimus combined with R-CHOP-21 for
new, untreated, diffuse large B-cell lymphoma (NCCTG 1085
[Alliance]): Safety and efficacy results of a phase 1 and
feasibility trial. Lancet Haematol. 3:e309–e316. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chiappella A, Witzig TE, Vitolo U,
Gascoyne R, Russo J, Amoroso B, Hudak K, Ogunkanmi A, Xu Y, Ruiz W,
et al: ROBUST: Phase III randomized study of LENALIDOMIDE/R-CHOP VS
PLACEBO/R-chop in untreated ABC-type diffuse large B-cell lymphoma
and feasibility of cell of origin subtyping. Hematol Oncol. 35:419.
2017. View
Article : Google Scholar
|
|
35
|
Brower V: Ibrutinib promising in subtype
of DLBCL. Lancet Oncol. 16:e4282015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Westin JR, Nastoupil L, Hagemeister F,
Fayad L, Young K, McDonnell T, Chuang H, Ahmed S, Nair R, Steiner
R, et al: SMART start: Rituximab, lenalidomide, and ibrutinib alone
prior to combination with chemotherapy for patients with newly
diagnosed diffuse large B-cell lymphoma. Hematol Oncol. 37:78–79.
2019. View Article : Google Scholar
|
|
37
|
Gelebart P, Zak Z, Anand M, Belch A and
Lai R: Blockade of fatty acid synthase triggers significant
apoptosis in mantle cell lymphoma. PLoS One. 7:e337382012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kant S, Kumar A and Singh SM: Myelopoietic
efficacy of orlistat in murine hosts bearing T cell lymphoma:
Implication in macrophage differentiation and activation. PLoS One.
8:e823962013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Long QQ, Yi Yx, Qiu J, Xu CJ and Huang PL:
Fatty acid synthase (FASN) levels in serum of colorectal cancer
patients: Correlation with clinical outcomes. Tumour Biol.
35:3855–3859. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ezzeddini R, Taghikhani M, Somi MH, Samadi
N and Rasaee MJ: Clinical importance of FASN in relation to HIF-1α
and SREBP-1c in gastric adenocarcinoma. Life Sci. 224:169–176.
2019. View Article : Google Scholar : PubMed/NCBI
|