Introduction
As the most common cause of death worldwide, breast
cancer (BC) mortality ranks first among Chinese women with
malignant tumors (1). Although
comprehensive treatments with surgery and radiotherapy are used for
BC, chemotherapy has been deemed safer and more essential for
prolonging survival time or decreasing metastases (2). Cisplatin is an effective strong-effect
and broad-spectrum chemotherapy agent for the therapy of BC
(3). Cisplatin induces apoptosis in
cancer cells by forming platinum-DNA adducts (4). However, cisplatin resistance remains a
significant factor limiting clinical efficacy (5). Therefore, sensitivity to cisplatin in
BC must be improved.
MicroRNAs (miRs/miRNAs) are a type of endogenously
expressed non-coding small RNA that can regulate the expression of
various genes (6). miRNAs have been
determined to play key roles in various biological processes,
including cell proliferation, differentiation and apoptosis
(7). In recent years, a growing
number of studies have shown that the imbalance of miRNAs may be
responsible for resistance (8–10).
miR-181a was reported to be differentially expressed in BC
(11,12). Therefore, targeted regulation of
miRNAs have become a potential method to decrease cisplatin
resistance in BC.
Autophagy plays a major homeostatic role in
controlling the quality and quantity of proteins and organelles,
which can help cells resist poor growth and promote cell survival
(13,14). Protective autophagy can also promote
chemotherapy-induced apoptosis (15). Vitamin D receptor (VDR), as a
regulator of autophagy, is a ubiquitous nuclear receptor that can
regulate the expression of numerous genes involved in cell
differentiation, proliferation and calcium/phosphate homeostasis
(16,17). Studies indicated that VDR acts as a
major transcriptional regulator and plays a crucial role in
chemotherapy sensitivity (18).
The present study investigated the function and
downstream genes of miR-181a-5p, a potential tumor suppressor, in
order to improve the efficiency of cisplatin in BC.
Materials and methods
Cell culture and transfection
Human breast cancer cell lines HS578T, HCC70,
MDA-MB-231, MDA-MB-468 and BT549 were cultured in corresponding
culture medium (HyClone; Cytiva) containing 10% fetal bovine serum
(Capricorn Scientific GmbH) and 1% penicillin-streptomycin mixture
(MDA-MB-231-L15, Base; MDA-MB-468, L15 medium; Hst578, high sugar
DMEM, HCC70, RPMI-1640; and BT549, RPMI-1640) at 37°C and 5%
CO2 and sub-cultured every 4 days.
The five cell lines were treated with different
concentrations of cisplatin (0.8, 1.6, 3.1, 6.2, 12.5, 25, 50, 100
and 200 µM) for 48 h in order to determine their IC50 of
cisplatin. Upon reaching 70% confluence, miR-181a inhibitor (100
pmol, 5′-ACUCACCGACAGCGUUGAAUGUU-3′), miR-181a mimics (100 pmol,
5′-AACAUUCAACGCUGUCGGUGAGUUCACCGACAGCGUUGAAUGUUUU-3′) or control
(100 pmol, mimics-NC:
5′-UUCUCCGAACGUGUCACGUTTACGUGACACGUUCGGAGAATT-3′; and Inhibitor NC:
5′-CAGUACUUUUGUGUAGUACAA-3′), was transfected into HS578T cells
using Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
After 8–12 h of cell culture, the medium was replaced with
serum-containing medium that was preheated at 37°C and then
cultured for another 24 h. Finally, cells were treated with
cisplatin according to different experimental groups (Table I). Cells were collected for
functional assays after 48 h.
 | Table I.Experimental grouping. |
Table I.
Experimental grouping.
| Group | a | b | c | d | e | f | g | h | i | j |
|---|
| Cisplatin-NC | + | + | + | + | + | – | – | – | – | – |
| Cisplatin | – | – | – | – | – | + | + | + | + | + |
| Mimics-NC | – | + | – | – | – | – | + | – | – | – |
| Mimics-181a | – | – | + | – | – | – | – | + | – | – |
| Inhibitor-NC | – | – | – | + | – | – | – | – | + | – |
| Inhibitor-181a | – | – | – | – | + | – | – | – | – | + |
Reverse transcription-quantitative PCR
(RT-qPCR)
TRIzol® (Roche Biotech) was used to
isolate total RNA from all cells. cDNA was synthesized using the
RevertAid First Strand cDNA Synthesis kit (Invitrogen; Thermo
Fisher Scientific, Inc.) in accordance with the manufacturer's
instructions. qPCR was performed on the ABI 7500 Real-Time system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) with the
following temperature protocol: 95°C for 10 min; 95°C for 15 sec,
60°C for 20 sec, 72°C for 25 sec for 40 cycles; and 72°C for 5 min.
U6 expression was used to normalize the relative expression of
miR-181a. The following primer pairs were used for the qPCR: U6
forward, 5′-CTCACTTCGGCAGCACATA-3′ and U6 reverse,
5′-AACTCTTCACGATTTTGTCTGTC-3′; miR-181a forward,
5′-AGCCAACATTCAACGCTGTCG-3′ and miR-181a reverse,
5′-CAGTGCAGGGTCCGAGGTATTC-3′. Data was analyzed from at least three
independent experiments and calculated using the 2−ΔΔCq
method (19).
Cell proliferation assay
Cells were seeded on a 96-well plate at a density of
5×103 cells/well and cultured overnight in 100 ml of the
corresponding medium. Following treatment with the respective
cisplatin IC50 for 48 h, Cell Counting Kit-8 (Biosharp
Life Sciences) was used to determine cell proliferation, according
to the manufacturer's protocol. The results are reported as the
ratio of cell proliferation/inhibition, and each experiment was
repeated three times. The formula used was as follows: Suppression
of proliferation rate (%)=(1-absorbance value of experimental
group/absorbance value of control group) ×100%.
Transwell assay
Following treatment with or without cisplatin for 48
h, cells were collected in each group after trypsin digestion. The
Matrigel-coated upper chamber of the Transwell insert was
inoculated with 1 ml cell suspension at a density of
5×105 cells/ml, while the lower chamber was filled with
500 µl culture medium. Cells were incubated for 24 h. After washing
with PBS, cells were fixed with polymethanol at 37°C for 30 min,
air dried and stained with 0.1% crystal violet at 37°C for 30 min.
The experiment was repeated three times. Cells were randomly
observed and counted under a light microscope (magnification, ×100;
Olympus BX53; Olympus Corporation).
Flow cytometry analysis of
apoptosis
Following treatment with or without cisplatin for 48
h, cells were collected and stained with propidium iodide and
Annexin V-FITC according to the manufacturer's protocol (Sangon
Biotech Co., Ltd.). Stained cells were analyzed by flow cytometry
(Guava EasyCyte; InCyte Software; EMD Millipore). Experiments were
repeated three times.
Western blotting
Following treatment with or without cisplatin for 48
h, cells were lysed with RIPA lysis buffer (Sangon Biotech Co.,
Ltd.). A BCA protein quantitation kit (Sangon Biotech Co., Ltd.)
was used to determine protein concentration. After 12% SDS-PAGE,
protein samples (30 µg/well) were transferred to PVDF membranes and
blocked with 5% BSA (Sigma-Aldrich; Merck KGaA) at room temperature
for 1 h. Membranes were incubated with primary antibodies against
VDR (1:100; cat. no. ab3508; Abcam) and microtubule-associated
proteins 1A/1B light chain 3B (LC3B; 1:2,000; cat. no. ab192890;
Abcam) at 37°C for 1 h and then overnight at 4°C. After washing
with PBS, membranes were incubated for 1 h at room temperature with
HRP-conjugated goat anti-rabbit secondary antibody (1:5,000; cat.
no. ab6789; Abcam). Protein signals were visualized using an ECL
system (EMD Millipore). GAPDH (1:5,000; cat. no. ab8245; Abcam) was
used as the internal control. A multimode microplate reader
(Varioskan LUX; Thermo Fisher Scientific, Inc.) was used for
densitometry.
Immunofluorescence
Following treatment with or without cisplatin for 48
h, cells were seeded onto coverslips and then fixed in 4%
paraformaldehyde at room temperature for 15 min. Cells were washed
three times with PBS, permeabilized at room temperature for 20 min
with 0.5% Triton X-100 and blocked at room temperature for 1 h with
1% BSA. Cells were incubated at 4°C overnight with rabbit
polyclonal anti-VDR antibody (1:100; cat. no. ab3508; Abcam). After
washing with PBS, cells were incubated for 2 h at room temperature
with FITC-labeled anti-rabbit secondary antibody (1:5,000; cat. no.
ab6721; Abcam). Fluorescence microscopy (Olympus Corporation)
(magnification, 100×) was used to acquire images.
Statistical analysis
All experiments were repeated at least three times.
The data are reported as the mean ± standard deviation. Data were
analyzed using SPSS 19.0 (IBM Corp.). One-way ANOVA and Tukey's HSD
test were used to analyze significant differences between groups or
among groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
HS578T as a cell model for
experiments
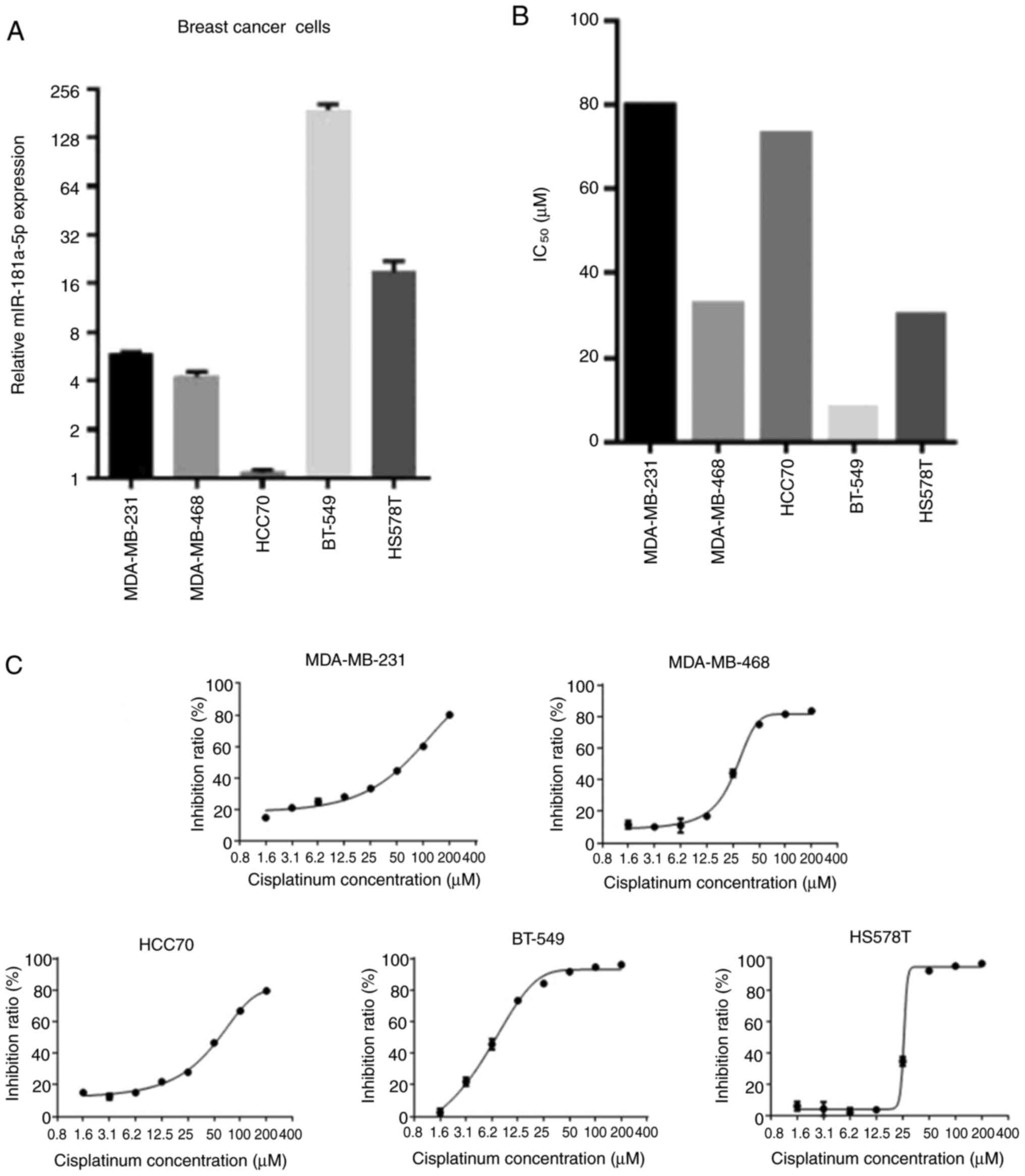
To select the most appropriate cell line suitable
for subsequent experiments, the relative expression of miR-181a-5p
and the IC50 of cisplatin was assessed in the five types
of BC cells (Fig. 1). Since HS578T
showed average miR-181a-5p expression and IC50 values
compared with other cells, this cell line was chosen for subsequent
experimentation.
miR-181a-5p enhances the
chemosensitivity of cisplatin by regulating biological processes in
HS578T cells
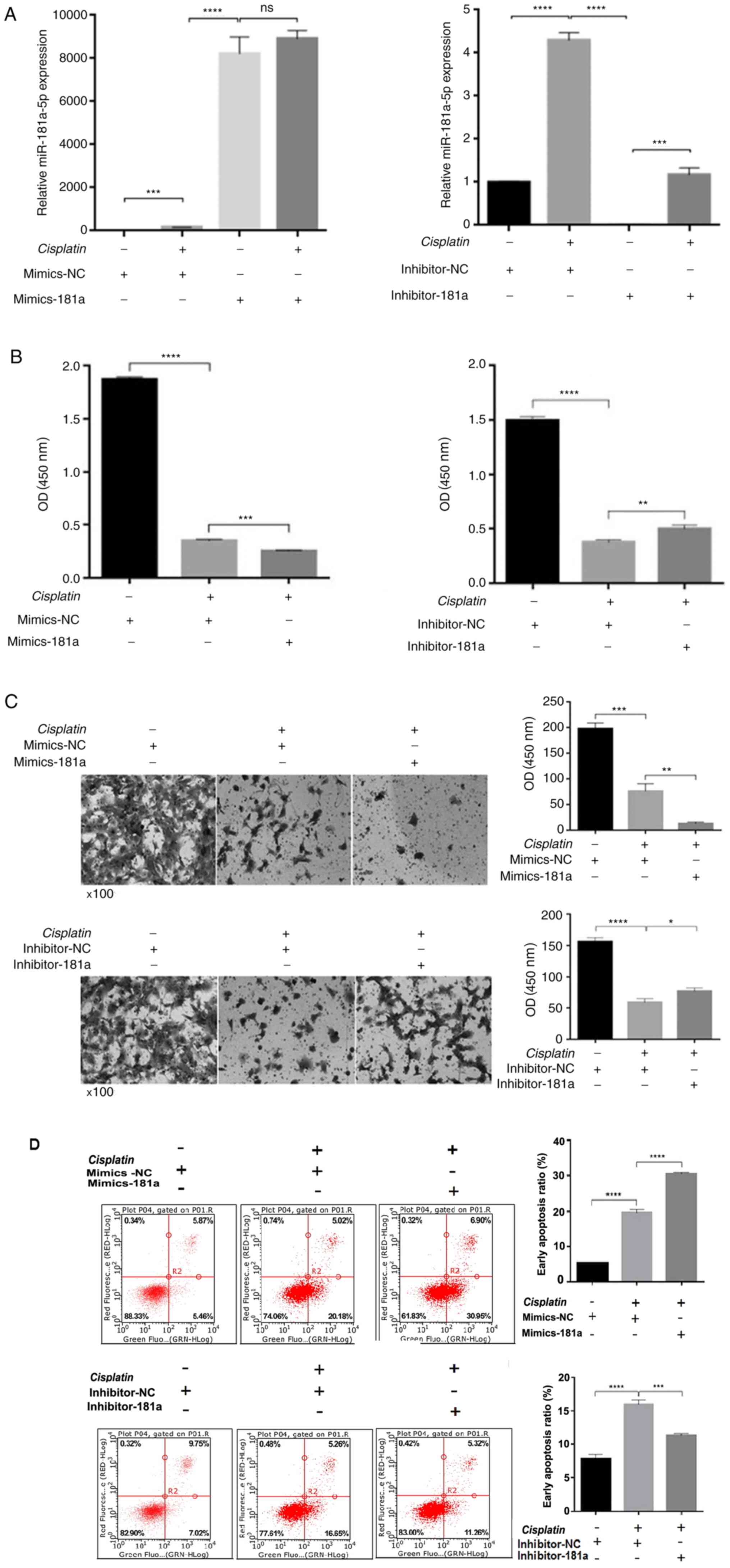
To investigate whether miR-181a-5p affected the
therapeutic effects of cisplatin by regulating proliferation,
migration and apoptosis, miR-181a-5p mimics, miR-181a-5p inhibitor
or control was transfected into HS578T cells and then treated with
or without 30.26 µM cisplatin for 48 h. As shown in Fig. 2A, miR-181a-5p expression was
significantly different among the groups, indicating successful
transfection. Cisplatin treatment increased miR-181a-5p expression,
which may be due to the synergy of the drug. Cell viability was
then detected using a cell proliferation assay. Following 48 h of
treatment with cisplatin, compared with the control group, cell
viability was significantly inhibited in the mimics-181a group,
while the cell viability recovered to some extent in the
inhibitor-181a group (Fig. 2B). When
miR-181a-5p is overexpressed, the cell invasion ability was weaker
compared with the control group. Cell invasion ability was
recovered in the inhibitor-181a group compared with the control
group (Fig. 2C). Subsequently, flow
cytometry was performed to determine whether miR-181a-5p
overexpression can enhance cisplatin-induced apoptosis. miR-181a-5p
overexpression increased cisplatin-induced apoptosis compared with
the control group. Meanwhile, in the inhibitor-181a group,
apoptosis was significantly decreased compared with the control
group (Fig. 2D).
miR-181a-5p potentially enhances
cisplatin chemosensitivity by negatively regulating VDR
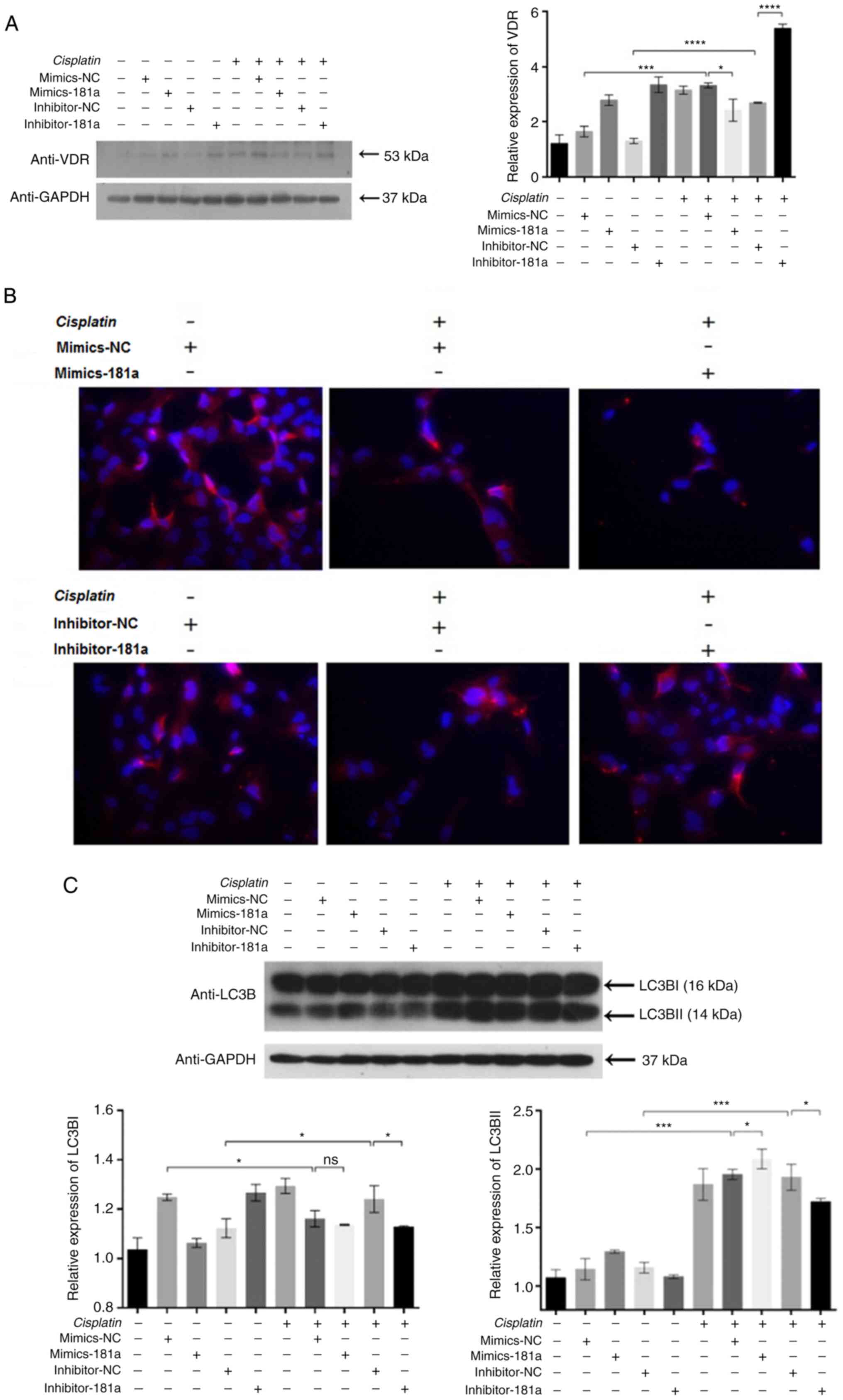
miR-181a-5p overexpression inhibited the VDR
expression in HS578T cells, as shown by western blotting (Fig. 3A). However, in the absence of
cisplatin, miR-181a-5p mimics enhanced VDR expression, which may be
due to the limited inhibitory effect of miR-181a-5p alone.
Meanwhile, miR-181a-5p and cisplatin exerts synergistic effects to
effectively inhibit VDR expression. Inhibition of miR-181a-5p
expression resulted in an increase in VDR expression (column 5 vs.
column 10). TargetScan prediction revealed VDR as a potential
target gene of miR-181a-5p. Since vitamin D signals via VDR and is
involved with preventing cancer (20), the present study focused on
investigating VDR. To verify the effects of miR-181a-5p on VDR
expression, western blotting and immunofluorescence was performed.
Western blotting results showed that miR-181a-5p overexpression
inhibited the expression of VDR levels in HS578T cells (Fig. 3A). Immunofluorescence assay showed
that VDR is located in the cytoplasm, and the expression levels
were consistent with those observed in western blot assays
(Fig. 3B). These results indicated
that following the administration of cisplatin, miR-181a-5p
directly suppressed the endogenous expression of VDR in HS578T
cells. Based on the association between miR-181a-5p with VDR, and
since VDR signaling is an essential mediator of autophagy, the
present study hypothesized that miR-181a-5p might inhibit or induce
autophagy through regulating VDR. Subsequently, western blotting
was used for detecting the levels of autophagy. Following cisplatin
treatment, autophagy levels were lower in the inhibitor-181a group
compared with the control group (Fig.
3C). These data showed that VDR downregulation enhanced the
level of autophagy. In conclusion, the results support that
miR-181a-5p overexpression could increase the chemosensitivity of
HS578T cells to cisplatin by inhibiting VDR-mediated cell
autophagy.
Discussion
There are 280,000 new cases of BC and 66,000 deaths
each year in China, accounting for 12.2% of all newly diagnosed
cases and 9.6% of all deaths from BC worldwide (1). With the advancement in medical
technology, more treatment options are available for improving the
quality of life of patients with BC. As an essential
chemotherapeutic agent, cisplatin has successfully enhanced the
survival rate of patients with cancer, such as gastric (21), testicular cancer (22) and BC (23). However, lower cisplatin sensitivity
still exists due to chemoresistance and leads to poor prognosis
(24). Recent studies have indicated
that altered of miRNAs act as a regulator of chemosensitivity in
BC, which has become a research focus (25,26).
The present study investigated the potential role of
miR-181a-5p in altering cisplatin resistance in BC (11,12).
Five types of BC cell lines were chosen to identify a suitable cell
line for subsequent experiments, which included HS578T, HCC70,
MDA-MB-231, MDA-MB-468 and BT549 cells. miR-181a-5p expression and
cisplatin IC50 in these cell lines were determined.
Finally, HS578T chosen for subsequent experiments, due to the
average levels of miR-181a-5p and cisplatin IC50
measured compared with other cells. HS578T cells were transfected
of miR-181a-5p mimic, miR-181a-5p inhibitor or control and then
treated with or without cisplatin. Compared with the control group,
the cell viability and invasion abilities were significantly
inhibited in the mimics-181a group, while viability and invasion
was recovered to a certain extent in the inhibitor-181a group. It
was found that cisplatin treatment in the mimics-181a group
resulted in high levels of HS578T cell death. Compared with the
control group, miR-181a-5p overexpression increased
cisplatin-induced apoptosis, while in the inhibitor-181a group,
apoptosis was significantly decreased. In conclusion, upregulation
of miR-181a-5p resulted in an increased apoptosis ratio in HS578T
cells, while proliferation and migration abilities were inhibited.
Meanwhile, the sensitivity of HS578T cells to cisplatin
increased.
TargetScan prediction revealed VDR as a potential
target gene of miR-181a-5p.VDR is expressed in a wide variety of
tissues. Vitamin D signals through VDR, where 1,25-dihydroxy
vitamin D3 binds with VDR to modulate target gene transcription. In
cancer cells, the modulation includes preventing cell
differentiation and proliferation and the regulation of programmed
cell death, such as apoptosis and autophagy (27,28).
Studies have shown that VDR regulates autophagy in luminal BC cells
(29) and other cells (16,30). To
verify whether VDR can regulate autophagy as the target gene of
miR-181a-5p, immunofluorescence and western blot assays were
performed. The western blotting results showed that miR-181a-5p
overexpression inhibited the expression of VDR levels in HS578T
cells, and VDR expression significantly increased in the
inhibitor-181a group compared with the control group.
Immunofluorescence assay results were consistent with the western
blotting. Due to time constraints, luciferase reporter assay was
not performed to directly prove that miR-181a-5p directly
suppresses VDR expression. However, both western blotting and
immunofluorescence assays results showed that miR-181a-5p could
negatively regulate VDR. A luciferase reporter assay will be
performed in future studies. LC3 immunoblotting was performed to
determine cell autophagy. It was shown that decreased expression of
VDR resulted in the increased autophagy of HS578T cells.
Additionally, the administration of cisplatin increased autophagy
in cells. This indicated that VDR inhibits the occurrence of
autophagy, and the administration of cisplatin can increase the
levels of miR-181a-5p. Increasing miR-181a-5p expression can
increase autophagy. As aforementioned, the synergy of miR-181a-5p
and cisplatin can promote HS578T cell apoptosis and inhibit
migration. Since all tests were performed under the same
conditions, it can be concluded that the increase in autophagy
could enhance cisplatin chemosensitivity in HS578T cells.
To conclude, miR-181a-5p upregulation could increase
chemosensitivity to cisplatin, decrease VDR expression and result
in increased autophagy in HS578T cells. These data indicated that
there was an inverse association between VDR and autophagy. This
was consistent with a previously published study showing that VDR
can directly regulate autophagy in BC cells. miR-181a-5p increased
the chemical sensitivity of HS578T cells to cisplatin by inhibiting
VDR to promote autophagy. However, further research must be
performed to determine the underlying mechanism. Furthermore,
miR-181a-5p and VDR might be attractive candidates for a novel
method for overcoming cisplatin resistance in BC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Youth Project
of Fujian Health Commission (grant no. 2018-2-71) and the Xiamen
Youth Innovation Talents Project (grant no. 2015-A-03).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HY, JL and ZS provided the study concept and design.
JL, XC and MS wrote and revised the manuscript. JL, XC, MS, XQ and
YW participated in discussion and made a significant contribution
to the interpretation of the results. JL, CL, XL and LZ collected
the data, and performed the experiments. XC and MS performed the
statistical analyses. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fan L, Strasser-Weippl K, Li JJ, St Louis
J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM and Goss PE: Breast
cancer in China. Lancet Oncol. 15:e279–e289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Macpherson IR, Spiliopoulou P, Rafii S,
Saggese M, Baird RD, Garcia-Corbacho J, Italiano A, Bonneterre J,
Campone M, Cresti N, et al: A phase I/II study of epertinib plus
trastuzumab with or without chemotherapy in patients with
HER2-positive metastatic breast cancer. Breast Cancer Res.
22:12019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qin T, Li B, Feng X, Fan S, Liu L, Liu D,
Mao J, Lu Y, Yang J, Yu X, et al: Abnormally elevated USP37
expression in breast cancer stem cells regulates stemness,
epithelial-mesenchymal transition and cisplatin sensitivity. J Exp
Clin Cancer Res. 37:2872018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
O'Grady S, Finn SP, Cuffe S, Richard DJ,
O'Byrne KJ and Barr MP: The role of DNA repair pathways in
cisplatin resistant lung cancer. Cancer Treat Rev. 40:1161–1170.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu G, Zhou W, Pan X, Sun Y, Xu H, Shi P,
Li J, Gao L and Tian X: miR-100 reverses cisplatin resistance in
breast cancer by suppressing HAX-1. Cell Physiol Biochem.
47:2077–2087. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kwon Y, Kim Y, Jung HS and Jeoung D: Role
of HDAC3-miRNA-CAGE network in anti-cancer drug-resistance. Int J
Mol Sci. 20:512018. View Article : Google Scholar
|
|
9
|
Yang F, Ning Z, Ma L, Liu W, Shao C, Shu Y
and Shen H: Exosomal miRNAs and miRNA dysregulation in
cancer-associated fibroblasts. Mol Cancer. 16:1482017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun Z, Shi K, Yang S, Liu J, Zhou Q, Wang
G, Song J, Li Z, Zhang Z and Yuan W: Effect of exosomal miRNA on
cancer biology and clinical applications. Mol Cancer. 17:1472018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tian Y, Fu X, Li Q, Wang Y, Fan D, Zhou Q,
Kuang W and Shen L: MicroRNA-181 serves an oncogenic role in breast
cancer via the inhibition of SPRY4. Mol Med Rep. 18:5603–5613.
2018.PubMed/NCBI
|
|
12
|
Strotbek M, Schmid S, Sánchez-González I,
Boerries M, Busch H and Olayioye MA: miR-181 elevates Akt signaling
by co-targeting PHLPP2 and INPP4B phosphatases in luminal breast
cancer. Int J Cancer. 140:2310–2320. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim KH and Lee MS: Autophagy-a key player
in cellular and body metabolism. Nat Rev Endocrinol. 10:322–337.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Amaravadi R, Kimmelman AC and White E:
Recent insights into the function of autophagy in cancer. Genes
Dev. 30:1913–1930. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo XL, Hu F, Wang H, Fang JM, Zhu ZΖ, Wei
LΧ and Xu Q: Inhibition of autophagy in hepatocarcinoma cells
promotes chemotherapeutic agent-induced apoptosis during nutrient
deprivation. Oncol Rep. 39:773–783. 2018.PubMed/NCBI
|
|
16
|
Sun J: VDR/vitamin D receptor regulates
autophagic activity through ATG16L1. Autophagy. 12:1057–1058. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Zhu J and DeLuca HF: Where is the
vitamin D receptor? Arch Biochem Biophys. 523:123–133. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sherman MH, Yu RT, Engle DD, Ding N,
Atkins AR, Tiriac H, Collisson EA, Connor F, Van Dyke T, Kozlov S,
et al: Vitamin D receptor-mediated stromal reprogramming suppresses
pancreatitis and enhances pancreatic cancer therapy. Cell.
159:80–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bulgakova O, Zhabayeva D, Kussainova A,
Pulliero A, Izzotti A and Bersimbaev R: miR-19 in blood plasma
reflects lung cancer occurrence but is not specifically associated
with radon exposure. Oncol Lett. 15:8816–8824. 2018.PubMed/NCBI
|
|
20
|
Feldman D, Krishnan AV, Swami S,
Giovannucci E and Feldman BJ: The role of vitamin D in reducing
cancer risk and progression. Nat Rev Cancer. 14:342–357. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mahlberg R, Lorenzen S, Thuss-Patience P,
Heinemann V, Pfeiffer P and Möhler M: New perspectives in the
treatment of advanced gastric cancer: S-1 as a novel oral 5-FU
therapy in combination with cisplatin. Chemotherapy. 62:62–70.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Albrecht W: Long-term effects of
cisplatin-based chemotherapy in testicular cancer patients-what is
important? Urologe A. 58:1212–1216. 2019.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Al-Bahlani S, Al-Lawati H, Al-Adawi M,
Al-Abri N, Al-Dhahli B and Al-Adawi K: Fatty acid synthase
regulates the chemosensitivity of breast cancer cells to
cisplatin-induced apoptosis. Apoptosis. 22:865–876. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Amable L: Cisplatin resistance and
opportunities for precision medicine. Pharmacol Res. 106:27–36.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Adhami M, Haghdoost AA, Sadeghi B and
Malekpour Afshar R: Candidate miRNAs in human breast cancer
biomarkers: A systematic review. Breast Cancer. 25:198–205. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang YW, Zhang W and Ma R: Bioinformatic
identification of chemoresistance-associated microRNAs in breast
cancer based on microarray data. Oncol Rep. 39:1003–1010.
2018.PubMed/NCBI
|
|
27
|
Høyer-Hansen M, Nordbrandt SP and Jäättelä
M: Autophagy as a basis for the health-promoting effects of vitamin
D. Trends Mol Med. 16:295–302. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Welsh J: Vitamin D and cancer: Integration
of cellular biology, molecular mechanisms and animal models. Scand
J Clin Lab Invest Suppl. 243:103–111. 2012.PubMed/NCBI
|
|
29
|
Tavera-Mendoza LE, Westerling T, Libby E,
Marusyk A, Cato L, Cassani R, Cameron LA, Ficarro SB, Marto JA,
Klawitter J and Brown M: Vitamin D receptor regulates autophagy in
the normal mammary gland and in luminal breast cancer cells. Proc
Natl Acad Sci USA. 114:E2186–E2194. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Paik S, Kim JK, Chung C and Jo EK:
Autophagy: A new strategy for host-directed therapy of
tuberculosis. Virulence. 10:448–459. 2019. View Article : Google Scholar : PubMed/NCBI
|