Introduction
Dipyridamole, a traditional anti-platelet agent, is
an inhibitor of phosphodiesterase enzyme 3 (PDE3) and PDE5, which
results in the accumulation of cyclic adenosine monophosphate
(cAMP) and cyclic guanosine monophosphate (cGMP) (1), consequently increasing protein kinase A
(PKA) and PKG, respectively (2).
Previous studies have reported that dipyridamole enhances the
cytotoxicity of anti-tumor drugs, such as 5-flurouracil (3), cisplatin (4) and methotrexate (5,6), as well
as preventing tumor progression (7)
and decreasing the proliferative activity of cancer cells (2,8).
Although dipyridamole has been reported to inhibit the re-uptake of
adenosine and downregulate cyclin D1 and c-Myc levels (7), the precise role and the relevant
mechanism of dipyridamole in regulating the proliferation of cancer
cells is yet to be elucidated. Moreover, there is no consensus on
the anti-cancer effect of dipyridamole.
Complex signaling pathways are known to mediate the
proliferation, survival and therapeutic resistance of cancer cells
(9). Of which, cAMP-regulatory
element-binding protein (CREB) has been demonstrated to serve a
critical role in both hematologic and non-hematologic malignancies
(10,11) via the phosphorylation of various
kinases, such as Akt and PKA (12,13).
Moreover, the cAMP/PKA axis has been demonstrated to regulate
poly(ADP-ribose) polymerase-1 (PARP-1) (14), which is known to serve an important
role not only in DNA repair (15),
but also the development and progression of malignant tumors
(16,17). As dipyridamole may induce the
accumulation of cAMP and cGMP to support the survival and
proliferation of cells, the precise role of dipyridamole on the
proliferation of cancer cells requires further investigation.
Doxorubicin is a widely prescribed chemotherapeutic
drug, but its use in high doses is limited due to serious side
effects, such as myelotoxicity and cardiotoxicity (18). Considering the favorable safety
profile of dipyridamole, it will be beneficial to use dipyridamole
as adjuvant drug for enhancing the sensitivity of cancer cells to
doxorubicin. Therefore, the present study aimed to investigate the
anti-cancer effect of dipyridamole in combination with
doxorubicin.
Using human colorectal cancer cells (HCT-8),
CD133+/CD44+ stem-like subpopulation of HCT-8
cells and human monocyte histiocytic lymphoma cells (U937), it was
identified that dipyridamole increased, rather than inhibited, the
proliferation of HCT-8 and U937 cells in a dose-dependent manner.
However, the cytotoxicity of doxorubicin was enhanced by the
combined usage of dipyridamole at particular doses.
Materials and methods
Cell culture
Human colorectal cancer cells (HCT-8),
CD133+/CD44+ stem-like subpopulation of HCT-8
cells and human monocyte histiocytic lymphoma cells (U937) were
used for the experiments. Cells were maintained in RPMI-1640 medium
(Fujifilm Wako, Inc.) supplemented with 10% FBS (Corning, Inc.) and
1% penicillin/streptomycin (Fujifilm Wako, Inc.), at 37°C in a
humidified atmosphere with 5% CO2 and 95% air.
Cytotoxicity assay
Based on previous publications (2,7,8), we used 0, 10 and 20 µM dipyridamole in
this study. For doxorubicin, we used the same dose of 0, 1.0 and
3.0 µM for HCT-8 cells as our previous study (19). However, the cytotoxicity of
doxorubicin largely vary among cancer cell lines (20). As cytotoxicity of doxorubicin to U937
cells was highly indicated even at dose of 0.5 µM in our
preliminary experiment, we used 0, 0.1 and 0.2 µM doxorubicin for
U937 cells in this study. Cytotoxicity assay was performed using
the Cell Proliferation Kit I (MTT) according to the manufacturer's
protocol (Roche Diagnostics). Briefly, cells were seeded in 96-well
culture plates (5×103 cells/well) and cultured
overnight. Cells were then treated with various concentrations of
doxorubicin (Fujifilm Wako, Inc.) and dipyridamole (Sigma-Aldrich;
Merck KGaA). At 24 h after treatment, MTT was added and incubated
for another 4 h. The formation of formazan from MTT was stopped by
adding solubilization solution, and the absorbance of formazan was
measured at 570 nm using a microplate reader (iMark™ Microplate
Reader; Bio-Rad Laboratories, Inc.). The optical density (OD) value
of cells with vehicle treatment was used as a normalization control
(100%). The combination effect was analyzed with coefficient of
drug interaction [CDI = AB/(A × B)], where AB is the OD value ratio
of the combination group and vehicle groups, A is the OD value
ratio of the drug A and vehicle groups, and B is the OD value ratio
of the drug B and vehicle groups. CDI value <1 indicates a
synergistic effect, CDI value =1 indicates an additive effect and
CDI value >1 indicates an antagonistic effect. Optical density
(OD); coefficient of drug interaction (CDI).
Colony forming assay (CFA)
A CFA was performed to confirm the MTT assay data of
dipyridamole in regulating the proliferation of parent and
CD133+/CD44+ stem-like subpopulation of HCT-8
cells. After treatment with 20 µM dipyridamole for 24 h, the cells
were collected, re-cultured in 6-well culture plate (200
cells/well) and incubated for 10 days. Colonies were fixed with 4%
formalin (Fujifilm Wako, Inc.) for 20 min and stained with 0.5%
crystal violet solution for 1 h. Colonies were counted using ImageJ
2.1.0 software (National Institutes of Health).
Western blotting
Western blotting was performed as previously
described (21). Briefly, cells were
lysed in Laemmli's buffer. Total proteins were separated using
SDS-PAGE and were then transferred to PVDF membranes (Bio-Rad
Laboratories, Inc.). After blocking, the membranes were incubated
with primary antibodies against rabbit phosphorylated (p)CREB
(Ser133; 1:1,000, cat. no. ab32096; Abcam), rabbit PARP-1 (1:1,000
cat. no. 9542; Cell Signaling Technology, Inc.) and mouse α-tubulin
(1:1,000, cat. no. 3873; Cell Signaling Technology, Inc.) which was
followed by incubation with appropriate horseradish
peroxidase-conjugated secondary antibodies against rabbit (1:2,000,
cat. no. p0448; Dako Agilent Technologies) and mouse (1:2,000, cat.
no. p0260; Dako Agilent Technologies). The expression was
visualized using an ECL detection kit (cat. no. RPN2106; Cytiva).
Images were acquired using ImageQuant LAS 4000 Mini biomolecular
imager (Cytiva). Semi-quantification on the relative expression of
proteins was performed using ImageJ 2.1.0 software (National
Institutes of Health).
Immunofluorescence staining
Immunofluorescence staining was performed to detect
the expression of pCREB. Briefly, cells were fixed with 4% formalin
(Fujifilm Wako, Inc.) for 10 min. After blocking, cells were
incubated with primary antibodies against pCREB (1:100) at room
temperature for 1 h, followed by incubation with an Alexa
Fluorescent 546-conjugated secondary antibody against rabbit Ig
(1:500 cat. no. A11035; Invitrogen; Thermo Fisher Scientific, Inc.)
at room temperature for 1 h in the dark. The cell nuclei were
labeled with DAPI.
Statistical analysis
Data are presented as the mean ± SEM for three
independent experiments. The data were analyzed using one-way
ANOVA, followed by Tukey's multiple comparison post-test. P<0.05
was considered to indicate a statistically significant difference.
All analyses were performed using GraphPad Prism 8.0 software or
Excel Microsoft 365.
Results
Dipyridamole increases the
proliferation of cancer cells
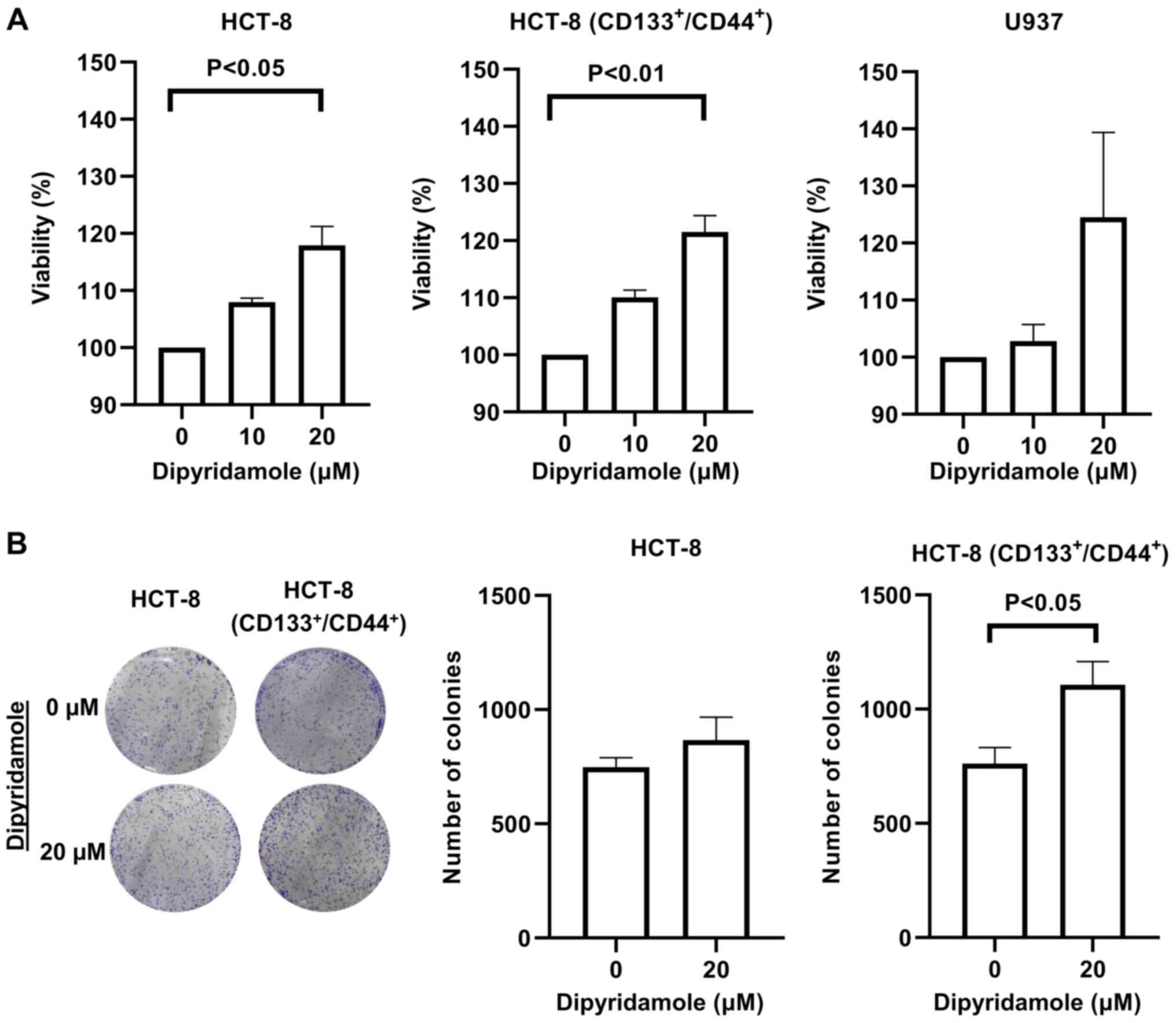
A MTT assay is used to evaluate the proliferation of
cancer cells. The addition of dipyridamole (0–20 µM) increased the
proliferation of U937 cells, parent HCT-8 cells and the
CD133+/CD44+ stem-like subpopulation of HCT-8
cells in a dose-dependent manner (Fig.
1A).
To further assess the data of the MTT assay, a
colony forming assay was conducted for parent and
CD133+/CD44+ stem-like subpopulation of HCT-8
cells. Dipyridamole (20 µM) increased the number of colonies for
parent HCT-8 cells (from 748±70 to 866±172; P=0.33) and
CD133+/CD44+ stem-like subpopulation of HCT-8
cells (from 761±122 to 1106±176; P=0.04) (Fig. 1B). Based on these findings,
dipyridamole may promote, but not inhibit the proliferation of
cancer cells.
Dipyridamole enhances the expression
levels of pCREB and PARP-1
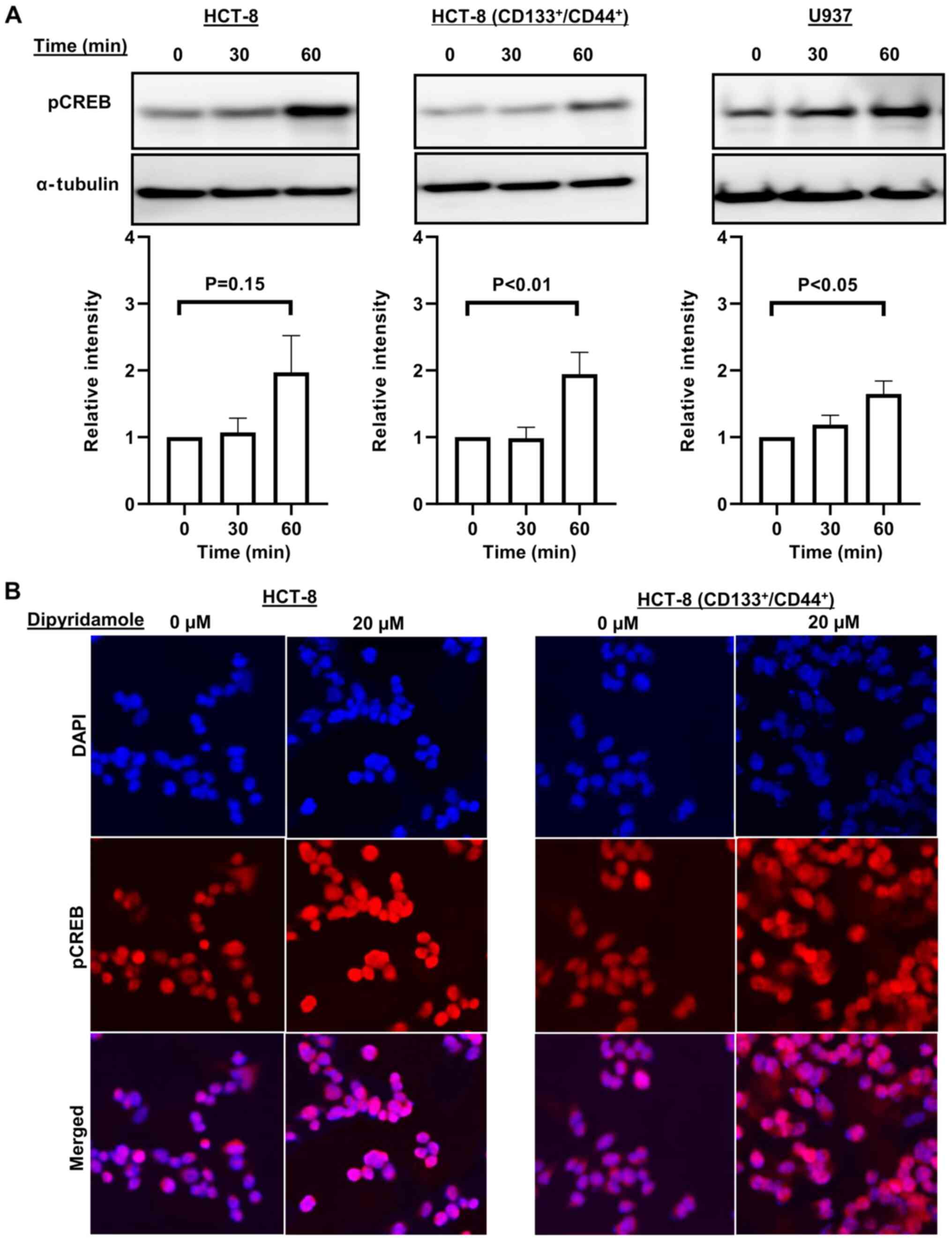
Western blotting was performed to investigate the
potential mechanism on the proliferation of cancer cells induced by
dipyridamole. It was identified that the expression of pCREB in all
cancer cells was increased at 30 or 60 min after 20 µM dipyridamole
treatment (Fig. 2A). The enhanced
expression of pCREB was also confirmed via immunofluorescence
staining (Fig. 2B). Furthermore, the
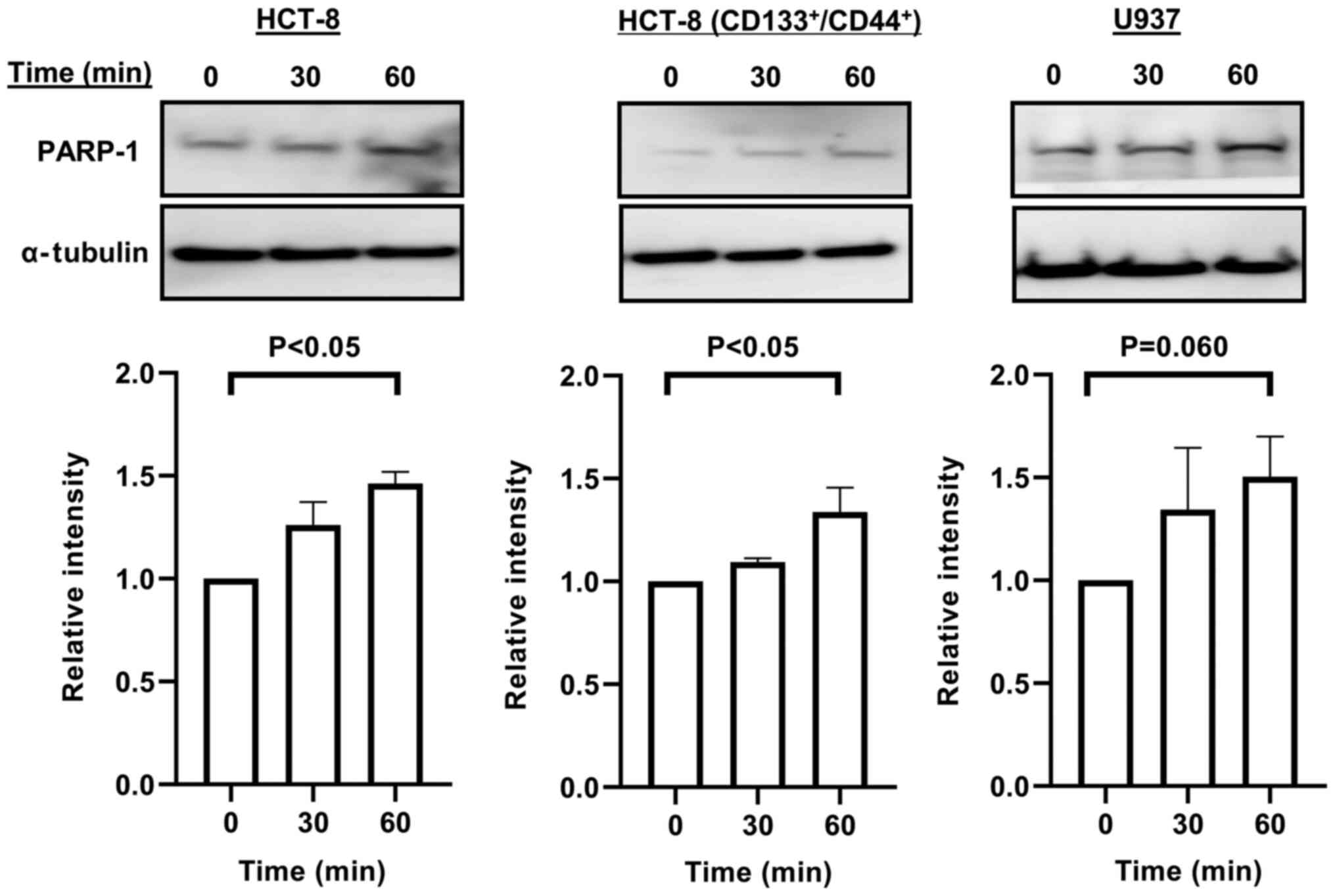
expression of PARP-1 in all cancer cells was enhanced at 30 or 60
min after 20 µM dipyridamole treatment (Fig. 3).
Cytotoxicity of doxorubicin is
enhanced by dipyridamole at particular doses
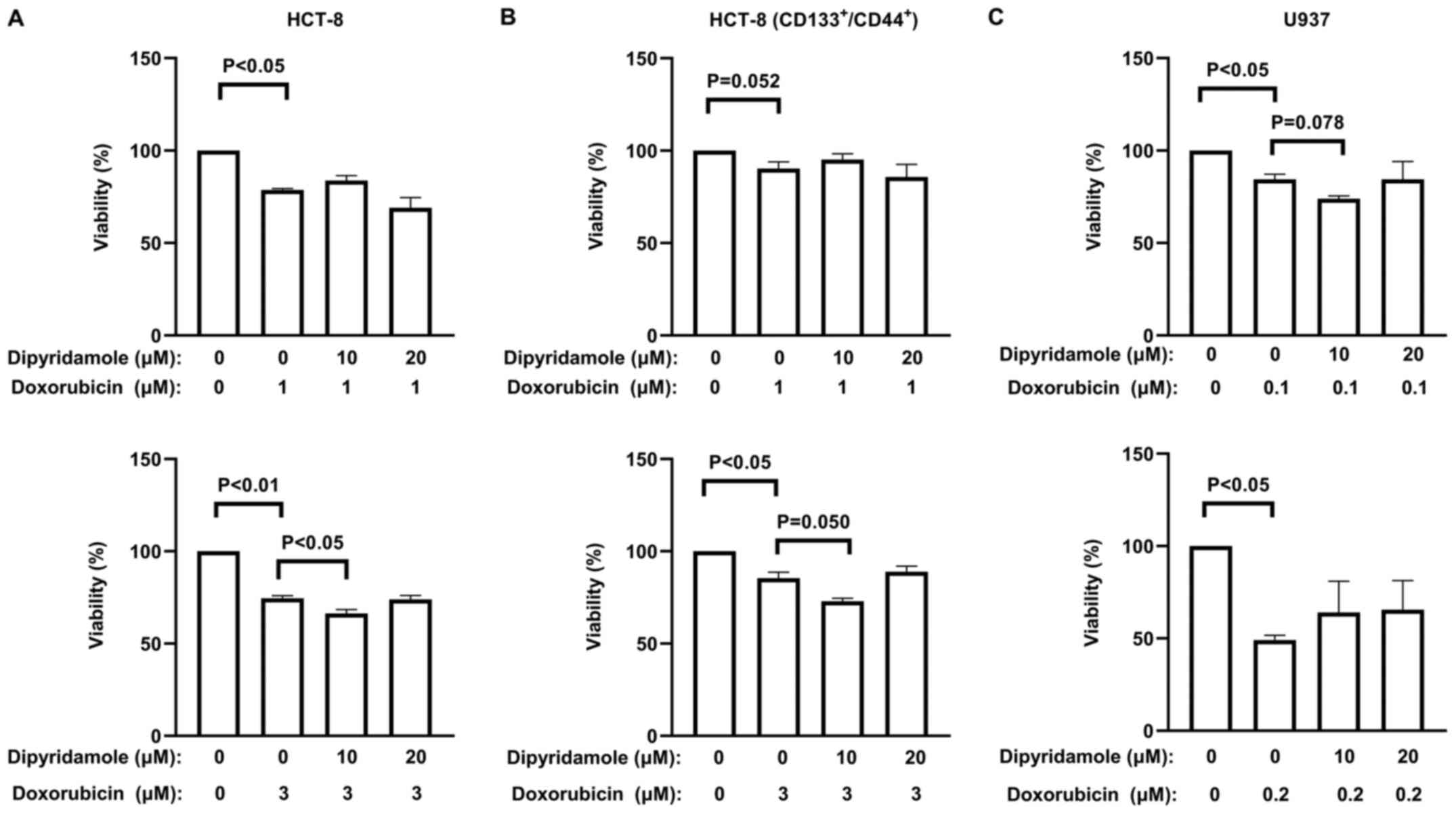
To evaluate whether dipyridamole enhances the
cytotoxicity of doxorubicin to cancer cells, various doses of
dipyridamole were added to cancer cells in combination with
doxorubicin treatment. The correct doses for both drugs were chosen
according to the reported literature and preliminary experiments
(19,20), and the IC50 values of
doxorubicin were calculated as 5.95 µM for HCT-8 cells, 10.63 µM
for the subpopulation of CD133+/CD44+ HCT-8
cells and 0.29 µM for U937 cells (Fig.
S1). As indicated by the quantitative data of the MTT assay
(Fig. 4; Table SI), dipyridamole significantly
enhanced the sensitivity of cancer cells to doxorubicin, but only
at a particular dose (10 µM dipyridamole with 3 µM doxorubicin for
HCT-8 cells). However, dipyridamole at any of the other doses
failed to demonstrate a significant enhancement of doxorubicin
cytotoxicity to these cancer cells (Fig.
4; Tables SII and SIII).
Discussion
Dipyridamole, one of the most commonly used
anti-platelet agents, is also often prescribed to some patients
with cancer. Previous studies have reported that dipyridamole
sensitizes cancer cells to chemotherapeutic agents (2–7).
Although dipyridamole exerts an anti-proliferative effect on breast
and prostate cancer cells (2,7), the
anti-cancer benefit of dipyridamole to patients with cancer is yet
to be fully elucidated.
The present study aimed to investigate the precise
role on the anti-cancer benefit of dipyridamole. In contrast to
previous studies (2–7), the present results suggested that
dipyridamole increased the proliferation of parent HCT-8 cells,
CD133+/CD44+ stem-like subpopulation of HCT-8
cells and U937 cells in a dose-dependent manner. Although the
current study neither evaluated cAMP or cGMP levels, nor the
activation of cAMP/PKA or cGMP/PKG signaling pathways, the
increased cell proliferation induced by dipyridamole could be
explained as follows: PDE3 and PDE5 are extensively expressed in
healthy tissue cells (22), and
upregulated in multiple cancer cells, including HCT-8 and U937
(13,23). As an inhibitor of PDE3 and PDE5,
dipyridamole usually induces the accumulation of cAMP and cGMP in
cells (1). cAMP and cGMP are
generally known to activate PKA and PKG, respectively, which
induces the phosphorylation of CREB (13,24) and
pCREB regulates the expression of several genes involved in the
metabolism, proliferation, differentiation and survival of cells
(12). Thus, we speculate that the
increased cellular levels of cAMP and cGMP contribute to the small
beneficial effect of dipyridamole on the cell
survival/proliferation (up to around 20% by MTT assay). Moreover,
the present results indicated that dipyridamole increased the
expression of pCREB in U937 lymphoma cells, parent HCT-8 colorectal
cancer cells and the CD133+/CD44+ stem-like
subpopulation from HCT-8 cells. Although we have not yet
investigated, it is possible that other PDE3 and PDE5 inhibitors
may also beneficial of cell proliferation. The current findings
suggested that dipyridamole enhanced the expression of PARP-1,
which known to support the survival and proliferation of cancer
cells. Thus, dipyridamole may promote, rather than inhibit, the
survival and proliferation of cells, but further details of the
relevant mechanisms require additional investigations.
Doxorubicin is commonly used for in vitro
experiments and clinics (21,25–30).
However, doxorubicin is rarely prescribed to patients with
colorectal cancer as the expression of P-glycoprotein in colorectal
cancer contributes to doxorubicin resistance (23,26). As
dipyridamole has been reported to inhibit P-glycoprotein (28), synergistic effects of dipyridamole
and doxorubicin are expected for patients with colon cancer. The
present study investigated whether dipyridamole could enhance the
cytotoxicity of doxorubicin to these cancer cells. Interestingly,
the cytotoxicity of doxorubicin was significantly enhanced by
dipyridamole, only to HCT-8 cells in particular dose. A previous
study also reported that dipyridamole alone or in combination with
methotrexate failed to increase the cytotoxicity in leukemia cells
(29). In fact, the small compound
of dipyridamole not only inhibits PDE3 and PDE5, but also regulates
multiple cell signaling pathways (2,7).
Therefore, the anti-cancer effect of dipyridamole used alone or
with doxorubicin may largely depend on the cell types and other
conditions (30). Further basic
experiments using additional cancer cell lines, as well as clinical
data, are required to confirm the potential anti-cancer effect of
dipyridamole.
In conclusion, to the best of our knowledge, the
present preliminary data from in vitro experiments indicated
for the first time that dipyridamole enhanced doxorubicin
sensitivity at particular doses. As dipyridamole was also found to
improve the survival and proliferation of cancer cells, it may be
prescribed cautiously for patients with cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Egyptian
ministry of Higher Education (grant no. CAM-751-FM-06-01).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LA performed the experiments, acquired, analyzed and
interpreted the data, and drafted the manuscript. NEM designed the
current study and gave final approval for the manuscript to be
published. TK and SG performed the experiments. TSL conceived the
current study, performed the experiments and wrote and reviewed the
final manuscript. TSL also gave final approval for the manuscript
to be published and supervised the study. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gresele P, Momi S and Falcinelli E:
Anti-platelet therapy: Phosphodiesterase inhibitors. Br J Clin
Pharmacol. 72:634–646. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thomé MP, Pereira LC, Onzi GR, Rohden F,
Ilha M, Guma FT, Wink MR and Lenz G: Dipyridamole impairs
autophagic flux and exerts antiproliferative activity on prostate
cancer cells. Exp Cell Res. 382:1114562019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kohnoe S, Maehara Y, Takahashi I, Emi Y,
Baba H and Sugimachi K: Treatment of advanced gastric cancer with
5-fluorouracil and cisplatin in combination with dipyridamole. Int
J Oncol. 13:1203–1206. 1998.PubMed/NCBI
|
|
4
|
Rodrigues M, Barbosa F Jr and Perussi JR:
Dipyridamole increases the cytotoxicity of cisplatin in human
larynx cancer cells in vitro. Braz J Med Biol Res. 37:591–599.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kennedy DG, Van den Berg HW, Clarke R and
Murphy RF: Enhancement of methotrexate cytotoxicity towards the
MDA.MB.436 human breast cancer cell line by dipyridamole. The role
of methotrexate polyglutamates. Biochem Pharmacol. 35:3053–3056.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Mouwerik TJ, Pangallo CA, Willson JK
and Fischer PH: Augmentation of methotrexate cytotoxicity in human
colon cancer cells achieved through inhibition of thymidine salvage
by dipyridamole. Biochem Pharmacol. 36:809–814. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Spano D, Marshall JC, Marino N, De Martino
D, Romano A, Scoppettuolo MN, Bello AM, Di Dato V, Navas L, De Vita
G, et al: Dipyridamole prevents triple-negative breast-cancer
progression. Clin Exp Metastasis. 30:47–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ge SM, Zhan DL, Zhang SH, Song LQ and Han
WW: Reverse screening approach to identify potential anti-cancer
targets of dipyridamole. Am J Transl Res. 8:5187–5198.
2016.PubMed/NCBI
|
|
9
|
Sakamoto KM and Frank DA: CREB in the
pathophysiology of cancer: Implications for targeting transcription
factors for cancer therapy. Clin Cancer Res. 15:2583–2587. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Crans HN and Sakamoto KM: Transcription
factors and translocations in lymphoid and myeloid leukemia.
Leukemia. 15:313–331. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seo H, Liu DD, Bekele BN, Kim MK, Pisters
K, Lippman SM, Wistuba II and Koo JS: CREB Overexpression: A
Feature Associated with Negative Prognosis in Never-Smokers with
NSCLC. Cancer Res. 68:6065–6073. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shaywitz AJ and Greenberg ME: CREB: A
stimulus-induced transcription factor activated by a diverse array
of extracellular signals. Annu Rev Biochem. 68:821–861. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fajardo AM, Piazza GA and Tinsley HN: The
role of cyclic nucleotide signaling pathways in cancer: Targets for
prevention and treatment. Cancers (Basel). 6:436–458. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brunyanszki A, Olah G, Coletta C, Szczesny
B and Szabo C: Regulation of mitochondrial poly(ADP-Ribose)
polymerase activation by the β-adrenoceptor/cAMP/protein kinase A
axis during oxidative stress. Mol Pharmacol. 86:450–462. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De Vos M, Schreiber V and Dantzer F: The
diverse roles and clinical relevance of PARPs in DNA damage repair:
Current state of the art. Biochem Pharmacol. 84:137–146. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Addioui A, Belounis A, Cournoyer S,
Nyalendo C, Brito RM, Beaunoyer M, Teira P and Sartelet H:
Preclinical study of a PARP inhibitor in neuroblastoma. J Clin
Oncol. 30 (Suppl 15):95702012. View Article : Google Scholar
|
|
17
|
Brenner JC, Feng FY, Han S, Patel S, Goyal
SV, Bou-Maroun LM, Liu M, Lonigro R, Prensner JR, Tomlins SA, et
al: PARP-1 inhibition as a targeted strategy to treat Ewing's
sarcoma. Cancer Res. 72:1608–1613. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zaremba T, Thomas H, Cole M, Plummer ER
and Curtin NJ: Doxorubicin-induced suppression of poly(ADP-ribose)
polymerase-1 (PARP-1) activity and expression and its implication
for PARP inhibitors in clinical trials. Cancer Chemother Pharmacol.
66:807–812. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yan C, Luo L, Guo CY, Goto S, Urata Y,
Shao JH and Li TS: Doxorubicin-induced mitophagy contributes to
drug resistance in cancer stem cells from HCT8 human colorectal
cancer cells. Cancer Lett. 388:34–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kibria G, Hatakeyama H, Akiyama K, Hida K
and Harashima H: Comparative study of the sensitivities of cancer
cells to doxorubicin, and relationships between the effect of the
drug-efflux pump P-gp. Biol Pharm Bull. 37:1926–1935. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan C, Luo L, Goto S, Urata Y, Guo CY, Doi
H, Kitazato K and Li TS: Enhanced autophagy in colorectal cancer
stem cells does not contribute to radio-resistance. Oncotarget.
7:45112–45121. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Omori K and Kotera J: Overview of PDEs and
their regulation. Circ Res. 100:309–327. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Klijn C, Durinck S, Stawiski EW, Haverty
PM, Jiang Z, Liu H, Degenhardt J, Mayba O, Gnad F, Liu J, et al: A
comprehensive transcriptional portrait of human cancer cell lines.
Nat Biotechnol. 33:306–312. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wong JC, Bathina M and Fiscus RR: Cyclic
GMP/protein kinase G type-Iα (PKG-Iα) signaling pathway promotes
CREB phosphorylation and maintains higher c-IAP1, livin, survivin,
and Mcl-1 expression and the inhibition of PKG-Iα kinase activity
synergizes with cisplatin in non-small cell lung cancer cells. J
Cell Biochem. 113:3587–3598. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ragazzi E, Berti E, Chiodo R, Dancona S
and Berti T: Dipyridamole as a modulator of multidrug-resistance in
tumor-cells in-vitro. Int J Oncol. 6:659–662. 1995.PubMed/NCBI
|
|
26
|
Weinländer G, Kornek G, Raderer M, Hejna
M, Tetzner C and Scheithauer W: Treatment of advanced colorectal
cancer with doxorubicin combined with two potential
multidrug-resistance-reversing agents: High-dose oral tamoxifen and
dexverapamil. J Cancer Res Clin Oncol. 123:452–455. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Krishan A, Sridhar KS, Mou C, Stein WD,
Lyubimov E, Hu YP and Fernandez H: Synergistic effect of
prochlorperazine and dipyridamole on the cellular retention and
cytotoxicity of doxorubicin. Clin Cancer Res. 6:1508–1517, 2,000.
PubMed/NCBI
|
|
28
|
Wessler JD, Grip LT, Mendell J and
Giugliano RP: The P-glycoprotein transport system and
cardiovascular drugs. J Am Coll Cardiol. 61:2495–2502. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nelson JA and Drake S: Potentiation of
methotrexate toxicity by dipyridamole. Cancer Res. 44:2493–2496.
1984.PubMed/NCBI
|
|
30
|
Solomon EI, Augustine AJ and Yoon J:
O2 reduction to H2O by the multicopper
oxidases. Dalton Trans. 30:3921–3932. 2008. View Article : Google Scholar
|