Introduction
Breast cancer is the most common malignancy, which
accounted for ~11.6% of all neoplasms worldwide in 2018 (1), and it accounts for ~15% of new cancer
cases in women, seriously threatening their health and lives
(2). Despite great progress in
surgery, radiotherapy, chemotherapy and endocrine therapy, the
prognosis of advanced and metastatic breast cancer remains poor
(3). With the advancement of
molecular biology, cell biology and immunology research, biological
immunotherapy has become a new therapeutic method for solid tumors,
thus altering the treatment mode for a variety of cancer types,
such as lung cancer, cancer of the neck and head, renal cancer and
malignant melanoma (4). However, for
breast cancer, the clinical benefits of immunotherapy remain
unsatisfactory, except in a small number of patients with
triple-negative breast cancer (5).
Exploring the immunological mechanism of breast cancer may provide
a new theoretical basis and clinical strategy for the treatment of
breast cancer.
Interleukin-10 (IL-10) is a classic
immunosuppressive cytokine that plays an important role in
regulating the cellular immune response, inhibiting the secretion
of proinflammatory factors, and promoting the proliferation and
metastasis of tumor cells via immunosuppression (6). IL-10-mediated immunosuppression is
realized by synthesizing tumor necrosis factors, IL-1, IL-12 and
chemotactic factors, and downregulating the costimulators CD80 and
CD86 on the tumor surface (7). IL-10
can also promote the expression and synthesis of IL-6, and induce
cellular proliferation by upregulating B-cell lymphoma-2, to change
the proliferation and apoptosis of tumor cells; additionally, it
inhibits the production of IL-1b, TNF-α, IL-6 and MMP-9 in tumors
by downregulating vascular endothelial growth factors (8). IL-10 possesses both tumor-promoting and
tumor-inhibiting features, and its agonists and antagonists exert
therapeutic effects via different mechanisms (9). The immune cells in the tumor
microenvironment can secrete a large amount of IL-10, and tumor
cells can also produce IL-10. In ovarian cancer, tumor-associated
macrophages upregulate the expression of hypoxia-inducible factor
1α by releasing IL-10, which promotes the invasion and metastasis
of cancer cells (10). In papillary
thyroid carcinoma, the expression of IL-10 is upregulated, which is
associated with capsule invasion and lymph node metastasis
(11).
IL-18 is a multifunctional cytokine that was first
reported in 1995 (12). IL-18
possesses strong immunomodulatory biological activity, exerts
anti-infection, antiparasite and antitumor effects by inducing
interferon-γ (13), promotes
perforin- and FasL-mediated cytotoxicity, and directly or
indirectly inhibits and destroys malignant tumors via multiple
channels (14). Thus, IL-18 inhibits
or prevents the growth of breast cancer, bladder cancer and
neuroblastoma (15,16). According to a previous study
(17), IL-18 combined with other
cytokines, such as IL-12 and −15, can regulate the activity of
multiple types of immune cells to exert antitumor effects. However,
to the best of our knowledge, the correlation between IL-18 and −10
in breast cancer has not been reported.
Based on the aforementioned context, the present
study investigated the correlation between IL-10 and −18 in breast
cancer, and explored their association with the progression of the
disease. The results of this study might provide new insights for
the early diagnosis and treatment of breast cancer.
Materials and methods
Study subjects
The tissue samples used in the present study were
obtained from 135 patients with pathologically confirmed breast
tumors who underwent surgical resection at the Jinan Central
Hospital Affiliated to Shandong University (Jinan, China) between
November 2016 and January 2019. Among these patients, 104 had
breast cancer. The mean age of the patients with breast cancer was
53.6±5.2 years (range, 28–69 years). The inclusion criteria were as
follows (18): i) No history of
malignant tumors; ii) no breast cancer-related treatment, such as
neoadjuvant chemotherapy, endocrine therapy, targeted therapy or
radiotherapy, before surgery; and iii) complete basic information.
In addition, tumor tissues from 31 cases of breast fibroadenoma
were used as controls. The ages of these patients ranged from 28–69
years, with a mean age of 54.3±5.3 years. The disease courses
ranged from 2 months to 8 years, with a mean of 5.9±1.2 months. The
tumor diameters ranged from 6–20 mm, with 23 cases of unilateral
lesions and 8 cases of bilateral lesions. Participants with one or
more of the following criteria were excluded from the study
(19): Hematological diseases, acute
and chronic infections, thyroid diseases, and other benign or
malignant tumors. In addition, clinical pathological parameters,
such as tumor size, pathological grade, estrogen receptor (ER)
status, progesterone receptor (PR) status and HER-2 status, were
included in the medical records.
All samples were fixed with 10% formalin at 25°C for
24 h and embedded in paraffin. Next, 4-µm thick sections were cut
and conventional hematoxylin and eosin (H&E) staining and
immunohistochemistry were performed. According to the World Health
Organization (2003) classification standard (20), among the 104 cases of breast cancer,
23 cases were grade I, 47 cases were grade II and 34 cases were
grade III. According to the AJCC (2003) staging criteria (21), 23 cases were stage I, 30 cases were
stage IIa, 17 cases were stage IIb, 19 cases were stage IIIa and 15
cases were stage IIIb. Tumor metastasis was based on
Tumor-Node-Metastasis staging (22):
N0, no metastasis; N1, ispilateral single axillary lymph node
metastasis; N2, multiple axillary lymph node metastases; N3,
supraclavicular lymph node metastasis; M, distant metastasis.
This research was conducted in accordance with the
Declaration of Helsinki and approved by the Ethics Committee of
Jinan Central Hospital Affiliated to Shandong University (approval
no. 20181103). Written informed consent for research purposes was
obtained from each participant.
Main reagents
The main reagents used in the present study included
PBS solution (pH 7.4; PBS-0060 phosphate buffer powder), citrate
antigen repair buffer (pH 6.0; MVS-0066 citrate buffer), a
concentrated DAB color development kit (cat. no. DAB-0031), an
EliVision (mouse/rabbit) immunohistochemical kit (cat. no.
KIT-9922) (all from Fuzhou Maixin Biotech Co., Ltd.), and rabbit
anti-human IL-10 and −18 monoclonal antibodies (BIOSS; cat. no.
BS-20373R and BS-4988R, respectively) and poly
peroxidase-anti-rabbit/mouse IgG (Beyotime Institute of Technology;
cat. no. P0267).
H&E staining
Each paraffin block was successively sliced into 5
sections with a thickness of 4 µm and then dried at 60°C for 60
min. The sections were soaked with xylene I and II, and then
subjected to gradient washing with 100, 95, 85 and 75% alcohol.
Afterwards, they were stained with H&E at 25°C for 1 min,
mounted with neutral resin and then dried. The histodifferentiation
of the samples was determined based on the similarity degree
between the tumor and the normal tissue according to the
pathological section. The more similar the tumor to normal cells
indicates a higher degree of differentiation.
Immunohistochemistry
Sections from each paraffin block were dried at 60°C
for 60 min. After dewaxing with xylene, the samples were rinsed
with gradient alcohol solutions, the same procedures as
aforementioned. Afterwards, they were repaired with citrate antigen
repair buffer (pH 6.0) at 95°C for 2 min and then cooled at room
temperature. Primary antibody working solution [dilution 1:100 in
PBS-BSA (1% BSA)] at 50 µl was applied to each section for
incubation at 37°C for 90 min. After rinsing in PBS, a secondary
antibody (dilution, 1:1,000) was added and the sample was incubated
at 37°C for 20 min. Finally, the sections were subjected to
diaminobenzidine (DAB) coloration, hematoxylin counterstaining
(25°C for 2 min), dehydration and mounting.
Outcome determination
Each section was read by two pathologists using the
double-blind method. Both pathologists were not aware of the
objectives of the study. When a count difference of more than 10%
occurred, the pathologists were required to recount. The appearance
of brown-yellow coloration or brown-yellow particles in the cell
after DAB development was considered to indicate an IL-18-positive
result. The outcomes were determined using the semiquantitative
integration method (20).
Specifically, three fields under a light microscope at ×200
magnification were randomly selected, and scores were assigned
according to the proportion of positive cells and the degree of
staining. For IL-18, 1 point was assigned when <1/3 of the total
cells were stained, 2 points were assigned when 1/3 to 2/3 of the
cells were stained and 3 points were assigned when >2/3 of the
cells were stained. According to the degree of coloration, 0 points
indicated no coloration, 1 point indicated light yellow coloration,
2 points indicated a brownish yellow coloration and 3 points
indicated a brown coloration. For each section, two scores were
therefore obtained. The two scores were multiplied to obtain a
final score, based on which the positivity of IL-18 in the section
was assessed according to the following criteria: 3 points, (1+);
4–5 points, (2+); and 6–9 points, (3+). The sections with a total
IL-18 score of ≤2 points were considered to be IL-18-negative
(23,24). The expression of IL-10 was assessed
as follows: 0 points, no coloration; 1 point, light yellow
coloration; 2 points, coloration between the manifestations of 1
and 3 points; and 3 points, yellow to brownish yellow coloration.
The positive cell ratio was scored as follows: 0 points, 0%
staining; 1 point, 1–25% staining; 2 points, 26–50% staining; 3
points, 51–75% staining; and 4 points, 76–100% staining. The total
score of IL-10 was also calculated by multiplying the staining
intensity score with the positive cell ratio score, with a score of
≤2 considered to be negative and ≥3 considered to be positive. In
addition, colon fibroadenoma tissues from the Department of
Gastrointestinal Surgery of Jinan Central Hospital affiliated to
Shandong University were used as another negative control for IL-18
and IL-10.
Statistical analysis
Data are presented as percentages, and statistical
analyses were performed with SPSS 22.0 (IBM Corp.). The
χ2 test was used to compare the groups, and Spearman's
correlation analysis was performed to assess the correlation
between IL-18 and IL-10. P<0.05 was considered to indicate a
statistically significant difference.
Results
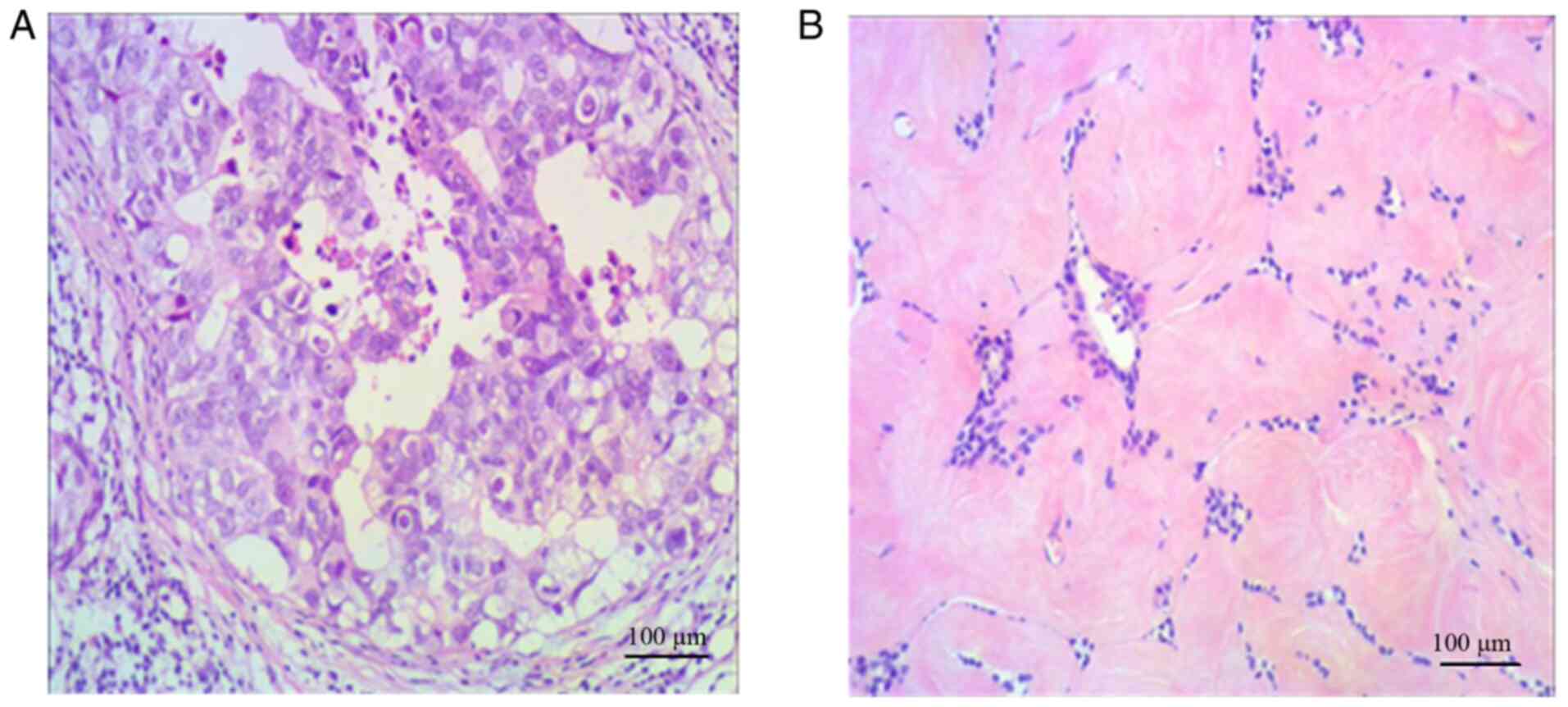
H&E staining
The H&E staining results of breast cancer and
breast fibroadenoma are shown in Fig.
1. The boundaries between the cancer tissue and the surrounding
breast tissues were unclear (Fig.
1A). In the breast cancer specimens, the cells were in
nest-like or sheet-alike arrangements, with a large amount of
intercellular substances. The cells exhibited high-level atypia,
and part of the cytoplasm was transparent. A small number of tumor
thrombi were observed in the vessels. In the fibroadenoma
specimens, the boundaries between the tumor and the surrounding
tissues were clear, and the main component of the tumor was
proliferative loose fibrous stroma (Fig.
1B). The cells were round or elliptical and grew around the
vessel in the form of a vortex or cord. The nuclei did not exhibit
heterogeneity. The aggregation and sparsity zones of the cells were
alternately distributed. In the stroma, red-stained collagen fibers
with different diameters and abundant dendritic thin-walled vessels
were found.
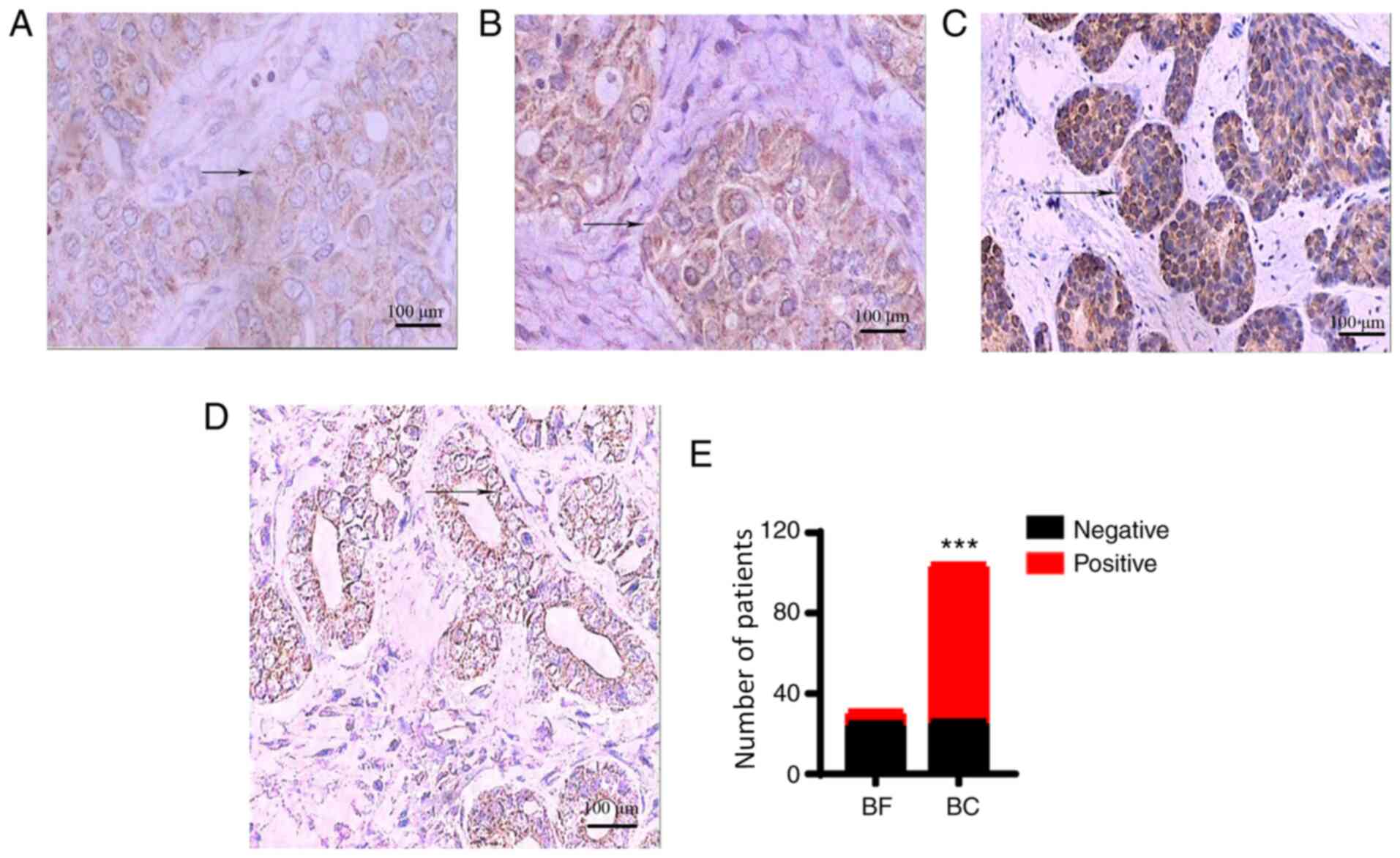
Expression of IL-18
IL-18 was positively expressed in the majority of
the breast cancer tissues (Total staining score ≥3 points), and
positive staining was mainly located in the cell membrane and
cytoplasm with a diffuse distribution (Fig. 2A). In the majority of the breast
fibroadenoma tissues, IL-18 was negatively expressed (H≤2 points;
Fig. 2B). The positive expression
rate of IL-18 in breast cancer tissues was significantly higher
than that in breast fibroadenoma tissues [75.0% (78/104) vs. 19.4%
(6/31); P<0.001; Fig. 2C, D and
E]. The negative expression of IL-18 in colon fibroadenoma
tissues is shown in Fig. S1A.
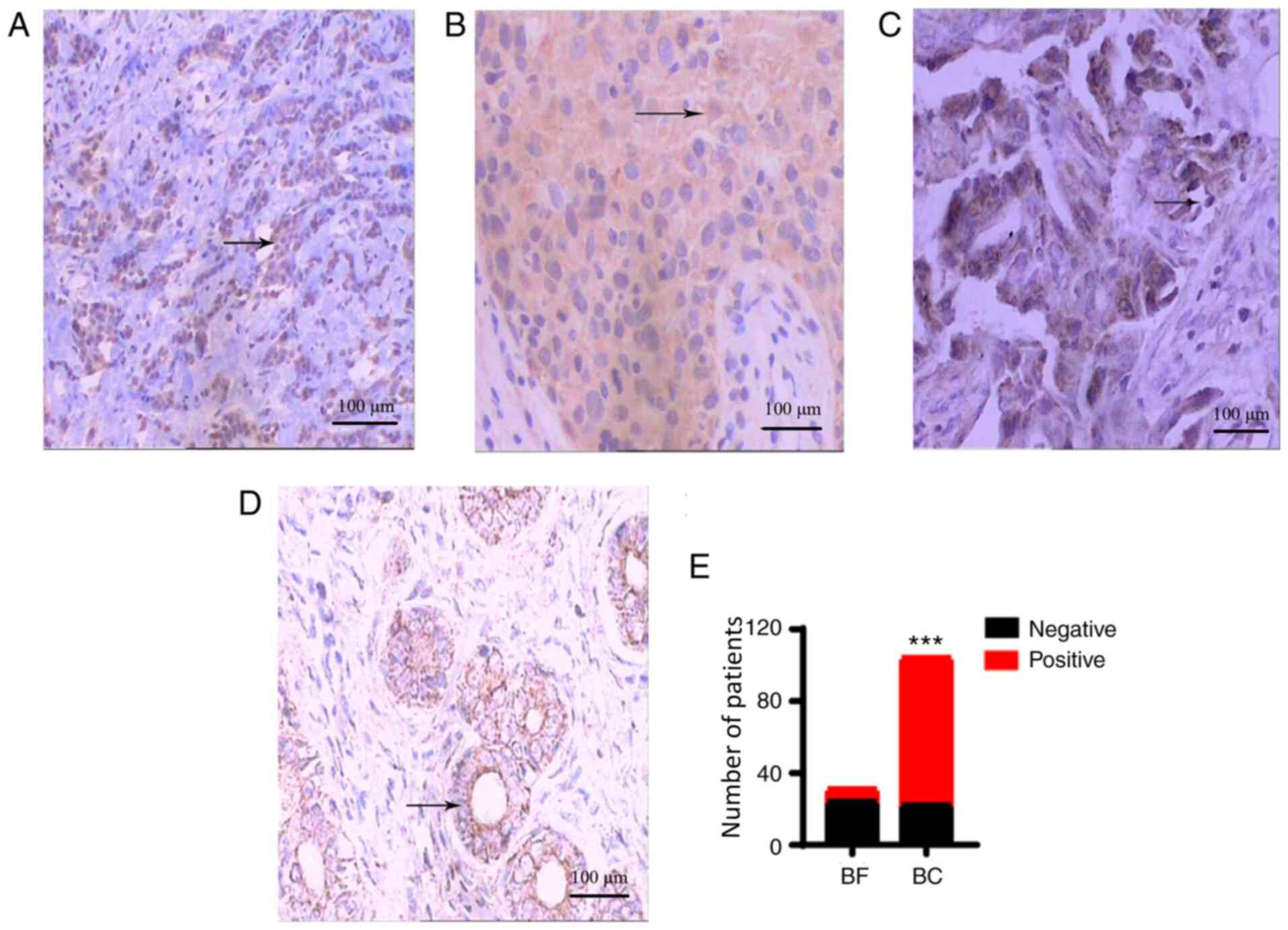
Expression of IL-10
IL-10 was positively expressed in the majority of
the breast cancer tissues (H≥3 points), and positive staining was
mainly located in the cytoplasm (Fig.
3A). In the majority of the breast fibroadenoma tissues, the
expression of IL-10 was relatively low (H≤2 points; Fig. 3B). The positive expression rate of
IL-10 in breast cancer tissues was significantly higher than that
in breast fibroadenoma tissues [78.8% (82/104) vs. 22.6% (7/31);
P<0.001; Fig. 3C, D and E]. The
negative expression of IL-10 in colon fibroadenoma tissues is shown
in Fig. S1B.
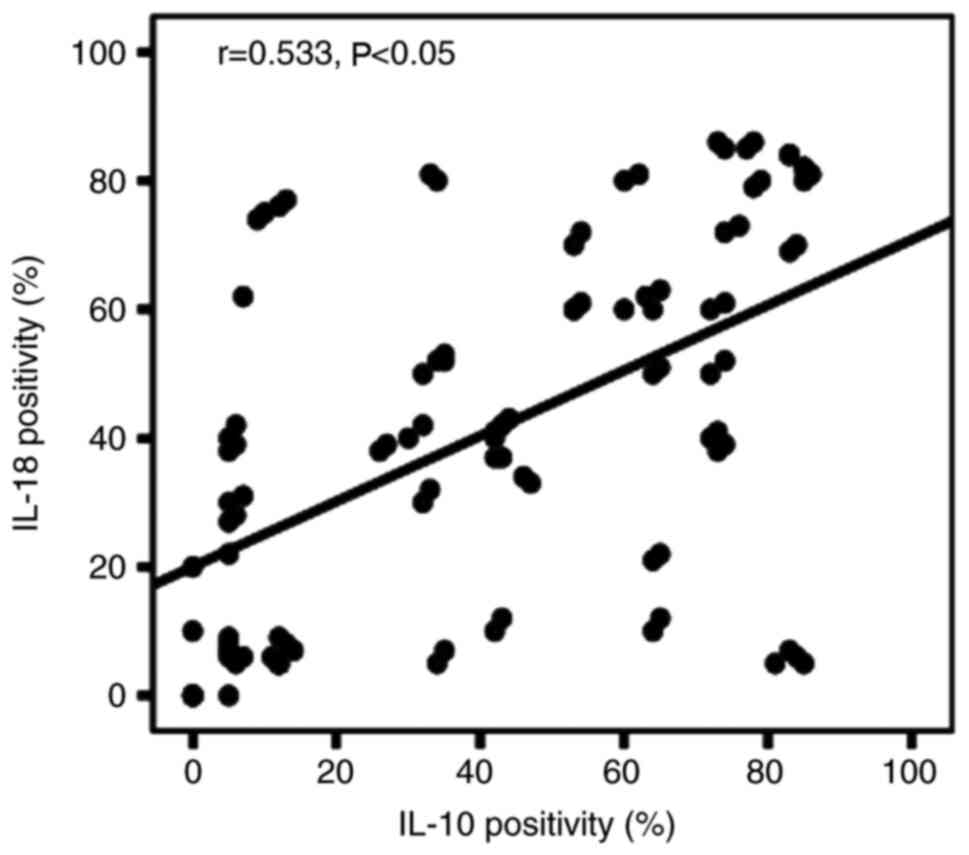
Correlation between IL-18 and
IL-10
The samples were divided into the following four
categories according to IL-18 and IL-10 expression in the same
sample: i) Both IL-18 and IL-10 were expressed positively (n=70);
ii) IL-18 expression was negative, while IL-10 was expressed
positively (n=12); iii) IL-18 was expressed positively, while IL-10
expression was negative (n=8); and iv) IL-18 and −10 expression was
negative (n=14) (Table I).
Spearman's correlation analysis was performed according to a
previously described method (25).
The results showed that the positive expression rate of IL-18 was
correlated with that of IL-10 (r=0.533; P<0.05; Fig. 4).
 | Table I.Expression of IL-18 and IL-10 in 104
patients with breast cancer. |
Table I.
Expression of IL-18 and IL-10 in 104
patients with breast cancer.
| Expression | Patients, n |
|---|
|
IL-18+/IL-10+ | 70 |
|
IL-18−/IL-10+ | 12 |
|
IL-18+/IL-10− | 8 |
|
IL-18−/IL-10− | 14 |
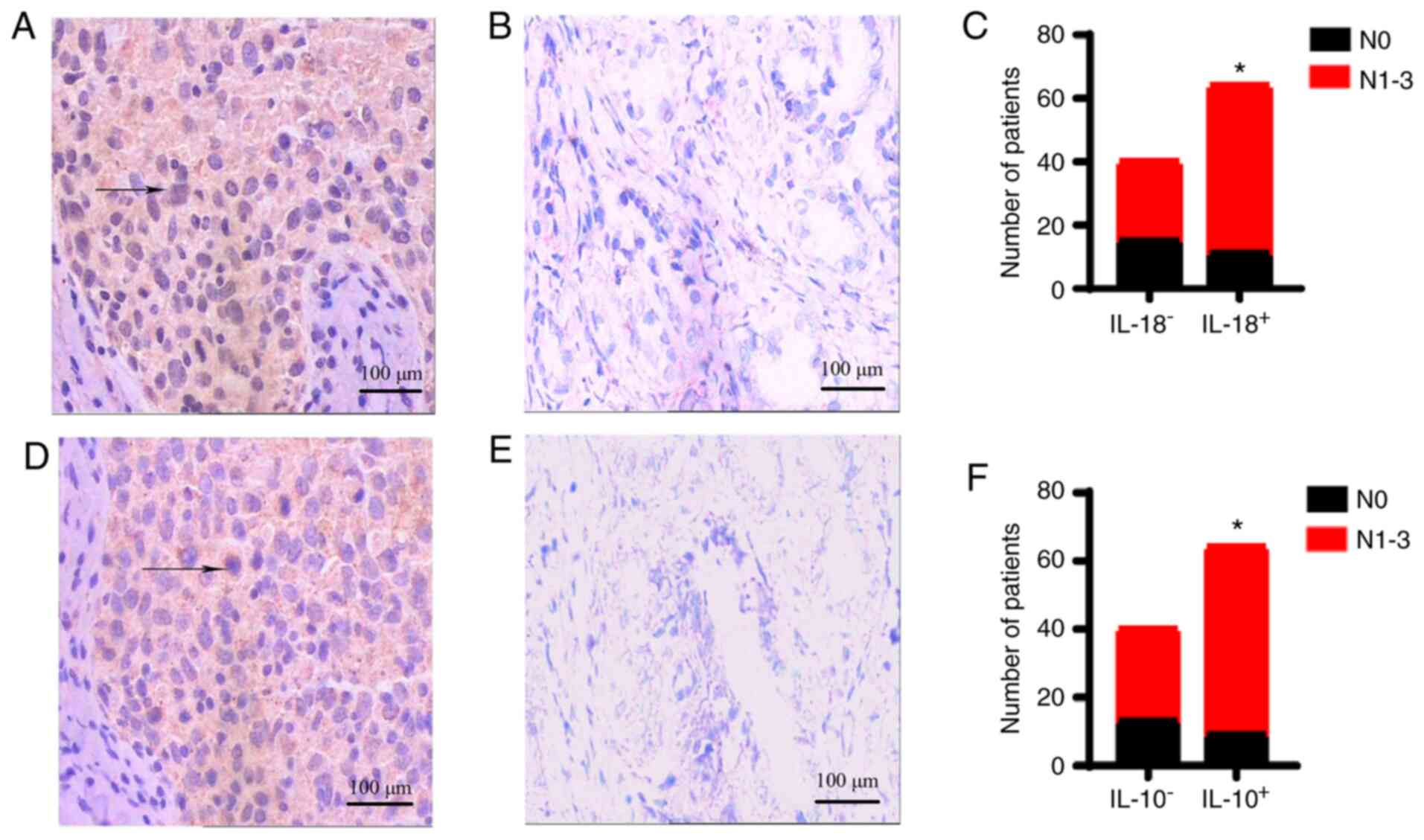
Association of the expression of IL-18
and −10 with lymph node metastasis
The expression of IL-18 and −10 in the lymph node
metastasis group was significantly higher than that in the
non-lymph node metastasis group (both P<0.05; Fig. 5). However, significant differences
were not observed in the age, tumor size, histological type,
differentiation degree, clinical grade, ER status, PR status or
HER-2 status between these two groups (Table II).
 | Table II.Associations of the expression of
IL-18 and −10 with clinical pathological indices. |
Table II.
Associations of the expression of
IL-18 and −10 with clinical pathological indices.
|
|
| IL-18 | IL-10 |
|---|
|
|
|
|
|
|---|
| Index | Patients, n | +, n | -, n | P-value | +, n | -, n | P-value |
|---|
| Age, years |
|
|
| 0.141 |
|
| 0.093 |
|
≥60 | 32 | 21 | 11 |
| 22 | 10 |
|
|
<60 | 72 | 57 | 15 |
| 60 | 12 |
|
| Max diameter of
tumor, cm |
|
|
| 0.063 |
|
| 0.820 |
|
>3 | 40 | 34 | 6 |
| 32 | 8 |
|
| ≤3 | 64 | 44 | 20 |
| 50 | 14 |
|
| Histological
type |
|
|
| 0.549 |
|
| 0.283 |
|
Infiltrating duct | 86 | 66 | 20 |
| 70 | 16 |
|
|
Lobular | 18 | 12 | 6 |
| 12 | 6 |
|
| Differentiation
degree |
|
|
| 0.579 |
|
| 0.429 |
|
Moderate/high | 82 | 60 | 22 |
| 66 | 16 |
|
|
Low | 22 | 18 | 4 |
| 16 | 6 |
|
| Staging |
|
|
| 0.809 |
|
| 0.168 |
|
I–II | 70 | 52 | 18 |
| 52 | 18 |
|
|
III–IV | 34 | 26 | 8 |
| 30 | 4 |
|
| ER |
|
|
| 0.240 |
|
| 0.328 |
|
Positive | 66 | 52 | 14 |
| 54 | 12 |
|
|
Negative | 38 | 26 | 12 |
| 28 | 10 |
|
| PR |
|
|
| 0.504 |
|
| 0.148 |
|
Positive | 58 | 45 | 13 |
| 49 | 9 |
|
|
Negative | 46 | 33 | 13 |
| 33 | 13 |
|
| HER-2/neu |
|
|
| 0.282 |
|
| 0.599 |
|
Positive | 80 | 62 | 18 |
| 64 | 16 |
|
|
Negative | 24 | 16 | 8 |
| 18 | 6 |
|
| Lymph node
metastasis |
|
|
| 0.035a |
|
| 0.047a |
| N0 | 40 | 25 | 15 |
| 27 | 13 |
|
|
N1-3 | 64 | 53 | 11 |
| 55 | 9 |
|
Discussion
The present study investigated the correlation
between IL-18 and −10 in breast cancer, as well as the association
of their expression with the clinical pathological indices of
breast cancer.
IL-18 is a multifunctional immunomodulatory cytokine
that exists in the form of an inactive precursor; it exerts
biological activities only after being sheared by IL-1β invertase
(26). In the present study, IL-18
was primarily expressed in breast cancer cells, and it was highly
expressed in tumor cells in the majority of the patients with
breast cancer. Moreover, its positive expression was associated
with lymph node metastasis. By contrast, in benign breast
fibroadenoma, IL-18 expression was at low levels or was negative.
The level of serum IL-18 in patients with lung cancer, gastric
cancer and hepatocarcinoma is significantly higher than that in the
healthy population (27). The level
of serum IL-18 in patients with breast cancer is also significantly
higher than that in the healthy population (28). The present results were consistent
with those reported in the literature (27,28). The
number of regulatory T (Treg) cells significantly increases in
breast cancer patients with lymph node metastasis, and the
accumulation of Treg cells is associated with a short overall
survival time in these patients (29). Therefore, the frequency of Treg cells
is considered an independent predictor for a high risk of
recurrence after breast cancer treatment. IL-18 has been
hypothesized to promote the cytotoxicity of Th1 cells, which
aggravates the apoptosis of cytotoxic T-lymphocytes (CTLs) and
weakens their response mechanisms, thereby promoting tumor growth
(17). The present results suggest
that high expression of IL-18 in breast cancer cells may serve as a
driving factor for the progression of breast cancer and represent
an indicator of poor prognosis in patients with this condition.
IL-10 is a protein with a molecular weight of 35–40
kDa that was discovered by Fiorentino in 1989 (30). IL-10 is a multifunctional negative
regulator that is primarily secreted by monocytes, helper T
lymphocytes (Th2), macrophages and activated B cells; it exerts an
immunosuppressive function during malignancy development, which
effectively reduces the participation of tumor-immune cytokines,
thereby inducing immune escape and promoting tumor growth (31). In the present study, IL-10 was
positively expressed in the majority of the breast cancer tissues,
but expressed at low levels in the breast fibroadenoma tissues. The
positive expression rate in breast cancer tissues was significantly
higher than that in breast fibroadenoma tissues. In addition, the
study also showed that the expression of IL-10 and −18 in patients
with metastatic breast cancer was significantly higher than that in
patients without metastasis. A meta-analysis based on 11,170
patients showed that IL-10 was associated with human
papillomavirus-related tumors in Asia (32). The level of serum IL-10 in patients
with gynecological neoplasms was significantly higher than that in
healthy individuals (33). The
mechanism underlying this finding may be as follows: Tumor cells,
tumor-associated macrophages and regulatory T cells in the tumor
microenvironment are all able to release IL-10, which inhibits the
production of IL-2, TNF-α and INF-γ, thereby suppressing the
proliferation and killing ability of T cells (34,35).
IL-10 is an immunosuppressive factor that suppresses antitumor
immune response by acting upon immunocytes to promote tumor growth
and metastasis. IL-10 also upregulates the expression of PDL1 of
myeloid cells, which binds with PD1 (an inhibitory receptor of T
cells) to deactivate T cells, thereby inhibiting the antitumor
function of T cells (36,37). The present results also suggested
that IL-10 may have a tumor-promoting effect in breast cancer
tissue. Therefore, it can be used as a diagnostic index of breast
cancer; it may also function as a potential target of breast cancer
treatment. However, considering that monocytes, Th2 cells,
macrophages and activated B cells are all able to secrete IL-10,
further studies are needed to determine the source of IL-10 using
the Th2 trace-labeling method (38)
to further validate the findings of this study.
According to a previous study (39), IL-18 can function with other
cytokines, such as IL-12, to exert tumor-promoting or
tumor-suppressing effects. IL-18 may upregulate the expression and
activity of NF-κB to inhibit IL-10, thereby exerting a
tumor-suppressing effect (40).
Notably, IL-10 can activate the expression of IL-18 through NF-κB
transcription (41). According to Li
et al (42), higher
expression of IL-18 and −10 in colorectal cancer indicates a higher
cancer reoccurrence rate, a poorer prognosis and a shorter survival
time, thus indicating the potential as a prognostic indicator for
colorectal cancer. Nevertheless, to the best of our knowledge, the
regulatory association between IL-18 and −10 has not been reported.
The present study found that the expression of IL-10 was positively
correlated with that of IL-18 in breast cancer. Although ER, PR and
HER-2 are all important clinical parameters associated with breast
cancer, the results did not show a noticeable correlation between
these parameters and the expression of IL-18 and −10 (P>0.05).
In addition, the expression of IL-18 and −10 did not show a
noticeable correlation with patient age, tumor size or pathological
grade (P>0.05). By contrast, IL-18 and −10 expression was
associated with lymph node metastasis in breast cancer. The
expression of IL-18 and −10 in the patients with lymph node
metastasis (N1-3) was significantly higher than that in the
patients without metastasis (N0) (P<0.05). In addition, the
positive expression rates of ER, PR and HER-2 in the metastatic
group were noticeably higher than those in the non-metastatic
group, which suggested that the positive expression levels of ER,
PR and HER-2 may be associated with lymph node metastasis. Thus,
the underlying mechanisms may be based on the ability of IL-18 and
−10 to regulate each other and their joint participation in the
development of breast cancer.
The present study has some limitations. First, all
patients included in the study were from the same center and the
sample size was small. Therefore, multicenter studies with a larger
sample size need to be performed. Second, the patients included in
the study received treatment between 2016 and 2019; therefore, the
postoperative recurrence and survival of these patients could not
be analyzed due to a short follow-up time. In the future, long-term
follow-ups for these patients will be conducted to further explore
the correlation of IL-18 and −10 with the prognosis of patients
with breast cancer. Third, although immunohistochemistry showed
that IL-18 and −10 were highly expressed in human breast cancer
tissues and lymph node tissues at the protein level and that they
were positively correlated, the mechanisms underlying their joint
actions were not clear. Last, the present study was preliminary and
did not detect the expression of IL-18 and −10 in peritumor tissues
or determine the correlations between their expression and clinical
outcomes. These issues warrant further research in the future.
In conclusion, IL-10 is highly expressed in breast
cancer tissues, and its expression correlates positively with that
of IL-18. Both IL-18 and −10 correlate positively with lymph node
metastasis in breast cancer, suggesting a possible synergistic
effect between them that promotes the development, infiltration and
migration of breast cancer. Combined detection of the expression of
IL-18 and −10 may provide new indicators for the early diagnosis
and prognosis of breast cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Health and
Family Planning Commission of Jinan (grant no. 2017-2-21).
Availability of data and materials
The data used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TM designed the study and led the writing of the
article. TM and MK conducted the experiments and collected the
data. MK conducted the data analysis. TM and MK confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
This research was conducted in accordance with the
Declaration of Helsinki and approved by the Ethics Committee of
Jinan Central Hospital Affiliated to Shandong University, Jinan,
China (approval no. 20181103). TM previously worked at Jinan
Central Hospital Affiliated to Shandong University and transferred
to the Fifth People's Hospital of Jinan (Jinan, China) in July
2020. Written informed consent was obtained from each
participant.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
IIyas AB, Bahaj RK, Shaikh AA, Khawandanah
BS, AI-Foheidi M and Omer TY: Breast cancer patients' perceptions
of their experience with chemotherapy-induced nausea and vomiting
and its impact on quality of life in Jeddah Saudi Arabia. Cureus.
12:e120382020.PubMed/NCBI
|
|
2
|
Bary F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nome ME, Euceda LR, Jabeen S, Debik J,
Bathen TF, Giskeødegård GF, Taskén KA, Maelandsmo GM, Halvorsen B,
Yndestad A, et al: Serum levels of inflammation-related markers and
metabolites predict response to neoadjuvant chemotherapy with and
without bevacizumab in breast cancers. Int J Cancer. 146:223–235.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang B, Zhu S and Song Q: New progress in
immunotherapy for breast cancer. Herald Med. 38:1013–1016.
2019.
|
|
5
|
Thakur V and Kutty RV: Recent advances in
nanotheranostics for triple negative breast cancer treatment. J Exp
Clin Cancer Res. 38:4302019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yuan XH, Li YM, Shen YY, Yang J and Jin Y:
Clinical and Th1/Th2 immune response features of hospitalized
children with human rhinovirus infection. J Med Virol. 92:26–33.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ortiz Wilczyñski JM, Olexen CM, Errasti
AE, Schattner M, Rothlin CV, Correale J and Silva EA: GAS6
signaling tempers Th17 development in patients with multiple
sclerosis and helminth infection. PLoS Pathog. 16:e10091762020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fröschen FS, Schell S, Schildberg FA,
Klausing A, Kohlhof H, Gravius S and Randau TM: Analysis of
synovial biomarkers with a multiplex protein microarray in patients
with PJI undergoing revision arthroplasty of the hip or knee joint.
Arch Orthop Trauma Surg. 140:1883–1890. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sheikhpour E, Noorbakhsh P, Foroughi E,
Farahnak S, Nasiri R and Neamatzadeh H: A survey on the role of
interleukin-10 in breast cancer: A narrative. Reports Biochem Mol
Biol. 7:30–37. 2018.
|
|
10
|
Li Q, Yin RT, Zhou L, Gao Q and Niu YZ:
Study on immune adhesins for IL-10 and tumor associated macrophages
on the invasion of ovarian cancer. West China Journal of
Pharmaceutical Sciences. 32:257–259. 2017.
|
|
11
|
Wang XF, Li J, Lu CH, Wang GQ, Wang ZH,
Liu XF, Liu B, Wang G, Zhang Q and Yang Q: IL-10-producing B cells
in differentiated thyroid cancer suppress the effector function of
T cells but improve their survival upon activation. Exp Cell Res.
376:192–197. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shirato K, Imaizumi K, Sakurai T,
Ogasawara J, Ohno H and Kizaki T: Regular voluntary exercise
potentiates Interleukin-1β and Interleukin-18 secretion by
increasing caspase-1 expression in murine macrophages. Mediators
Inflamm. 2017:92904162017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Niu XL, Huang Y, Gao YL, Sun YZ, Han Y,
Chen HD, Gao XH and Qi RQ: Interleukin-18 exacerbates skin
inflammation and affects micro abscesses and scale formation in a
mouse model of imiquimod-induced psoriasis. Chin Med J (Engl).
132:690–698. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhong DD, Ma HH and Liu JS: Study on
correlation between IL-18 level expression and IL-18 gene
polymorphism and asthma. Cell Mol Immunol. 35:480–484. 2019.
|
|
15
|
Leifsdottir K, Mehmet H, Eksborg S and
Herlenius E: Fas-ligand and interleukin-6 in the cerebrospinal
fluid are early predictors of hypoxic-ischemic encephalopathy and
long-term outcomes after birth asphyxia in term infants. J
Neuroinflammation. 15:2232018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang W, Dang SQ, Zhang GJ, He HQ and We
XP: Genetic polymorphisms of IL-10, IL-18 and IL12B are associated
with risk of non-small cell lung cancer in a Chinese han
population. Int Immunopharmacol. 77:1059382019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mostafavi E, Esmaeil B and Foroushani SM:
Evaluation of cytokines and sialic acids contents in horses
naturally infected with theileria equi. Comp Immunol Microb Infect
Dis. 70:1014532020. View Article : Google Scholar
|
|
18
|
Strube F, Infanger M, Wehland M,
Delvinioti X, Romswinkel A, Dietz C and Kraus A: Alteration of
cytoskeleton morphology and gene expression in human breast cancer
cells under simulated microgravity. Cell J. 22:106–114.
2020.PubMed/NCBI
|
|
19
|
Brownstone ND, Celie KB, Spigland NA and
Otterburn DM: Pediatric breast fibroadenomas: A systematic review
and algorithm for treatment. Ann Plas Surg. 83:601–605. 2019.
View Article : Google Scholar
|
|
20
|
Tavassoli FA and Devilee P: Pathology and
Genetics of Tumours of the Breast and Female Genital Organs. World
Health Organization Classification of Tumours. Volume IV. IARC
Press; Lyon, France: pp. 116–119. 2003
|
|
21
|
Kwan ML, Haque R, Lee VS, Chung WL, Avila
CC, Clancy HA, Quinn VP and Kushi LH: Validation of AJCC TNM
staging for breast tumors diagnosed before 2004 in cancer
registries. Cancer Causes Control. 23:1587–1591. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jayaprakasam VS, Yeh R, Ku GY, Petkovska
I, Fuqua JL III, Gollub M and Paroder V: Role of imaging in
esophageal cancer management in 2020: Update for radiologists. AJR
Am J Roentgenol. 215:1072–1084. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu J, Sun YP, Wang YS, Liu HP and Zhang
XL: The significance of expression of B7-H4 in breast cancer. Chin
Clin Oncol. 12:830–832, 835. 2017.
|
|
24
|
Dinarvand N, Khanahmad H, Hakimian S,
Sheikhi A, Rashidi B, Bakhtiari H and Pourfarzam M: Expression and
clinicopathological significance of lipin-1 in human breast cancer
and its association with p53 tumor suppressor gene. J Cell Physiol.
235:5835–5846. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suzuki S, Dobashi Y, Hatakeyama Y, Tajiri
R, Fujimura T, Heldin CH and Ooi A: Clinicopathological
significance of platelet-derived growth factor(PDGF)-B and vascular
endothelial growth factor-A expression, PDGF receptor-beta
phosphorylation, and microvessel density in gastric cancer. BMC
Cancer. 10:6592010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shiels RG, Hewage W, Pennell EN, Vidimce
J, Grant G, Pearson AG, Wagner KH, Morgan M and Bulmer AC:
Biliverdin and bilirubin sulfonate inhibit monosodium urate induced
sterile inflammation in the rat. Eur J Pharm Sci. 155:1055462020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao M, Zhang P and Huang L: Is NLRP3 or
NLRP6 inflammasome activation associated with inflammation-related
lung tumorigenesis induced by benzo(a)pyrene and
lipopolysaccharide? Ecotoxicol Environ Safety. 185:1096872019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang Y, Cheon S, Jung MK, Song SB, Kim D,
Kim HJ, Park H, Bang SI and Cho D: Interleukin-18 enhances breast
cancer cell migration via down-regulation of claudin-12 and
induction of the p38 MAPK pathway. Biochem Biophys Res Commun.
459:379–386. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hashemi V, Aghebati L, Esmaily M, Masjedi
A, Ghalamfarsa G, Namdar A, Yousefi M, Yousefi B and Jadidi-Niaragh
F: Regulatory T cells in breast cancer as a potent anti-cancer
therapeutic target. Int Immunopharmacol. 78:1060872020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fiorentino DF, Zlotnik A, Mosmann TR,
Howard M and O'Garra A: IL-10 inhibits cytokine production by
activated macrophages. J Immunol. 147:3815–3822. 1991.PubMed/NCBI
|
|
31
|
Del Giúdice A, Pagura L, Capitani MC,
Mainetti LE, Scharovsky OG, Di Masso RJ, Rico MJ and Rozados VR:
Nonclassical roles for IFN-γ and IL-10 in a murine model of
immunoedition. Future Sci OA. 6:FSO5892020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Qu K, Pang Q, Lin T, Zhang L, Gu ML, Niu
WQ, Liu C and Zhang M: Circulating interleukin-10 levels and human
papilloma virus and epstein-barr virus-associated cancers: Evidence
from a Mendelian randomization meta-analysis based on 11,170
subjects. Onco Targets Ther. 7:1251–1267. 2016. View Article : Google Scholar
|
|
33
|
Coosemans AN, Baert T, D'Heygere V,
Wouters R, DE Laet L, VAN Hoylandt A, Thirion G, Ceusters J, Laenen
A, Vandecaveye V and Vergote I: Increased immunosuppression is
related to increased amounts of ascites and inferior prognosis in
ovarian cancer. Anticancer Res. 39:5953–5962. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mohammad GRKS, Ghahremanloo A, Soltani A,
Fathi E and Hashemy SI: Cytokines as potential combination agents
with PD-1/PD-L1 blockade for cancer treatment. J Cell Physiol.
235:5449–5460. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song HS, Liu AZ, Liu GX, Wu F and Li ZT: T
follicular regulatory cells suppress Tfh-mediated B cell help and
synergistically increase IL-10-producing B cells in breast
carcinoma. Immunol Res. 67:416–423. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dennis KL, Blatner NR, Gounari F and
Khazaie K: Current status of interleukin-10 and regulatory T-cells
in cancer. Curr Opin Oncol. 25:637–645. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Butte MJ, Keir ME, Phamduy TB, Sharpe AH
and Freeman GJ: Programmed death-1 ligand 1 interacts specifically
with the B7-1 costimulatory molecule to inhibit T cell responses.
Immunity. 27:111–122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee M, Lee YH, Song J, Kim G, Jo Y, Min H,
Kim CH and Park Y: Deep-learning based three-dimensional label-free
tracking and analysis of immunological synapses of CAR-T cells.
Elife. 9:e490232020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hoshino T, Wiltrout RH and Young HA: IL-18
is a potent coinducer of IL-13 in NK and T cells: A new potential
role for IL-18 in modulating the immune response. J Immunol.
162:5070–5077. 1999.PubMed/NCBI
|
|
40
|
Al-Tamimi YZ, Bhargava D, Orsi NM, Teraifi
A, Cummings M, Ekbote UV, Quinn AC, Homer-Vanniasinkam S and Ross
S: Compartmentalisation of the inflammatory response following
aneurysmal subarachnoid haemorrhage. Cytokine. 123:1547782019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
May MJ and Ghhosh S: Signal transduction
through NF-kappa B. Immunol Today. 19:80–88. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li B, Wang F, Ma C, Hao T, Geng L and
Jiang H: Predictive value of IL-18 and IL-10 in the prognosis of
patients with colorectal cancer. Oncol Lett. 18:713–719.
2019.PubMed/NCBI
|