Introduction
Glioma is the most common tumour of the central
nervous system and is one of the most aggressive types of human
cancer (1). A study in Germany in
2011 showed that the median prognosis of survival for patients with
glioma is 20 to 36 months (2).
Despite advancements in glioma treatments, such as surgical
resection, radiation therapy, chemotherapy and immunotherapy, there
has been little recent improvement in survival rates (3). As such, a deeper understanding of
glioma pathogenesis and the discovery of novel molecular biomarkers
for glioma is still paramount, which will help improve the survival
rate of patients with glioma.
A large number of recent studies have shown that
interleukin 1 receptor-associated kinase 4 (IRAK4) may be an
oncogene that plays a key role in promoting proliferation and
invasion in numerous types of cancer, including colorectal cancer
and hepatocellular carcinoma (4–6). IRAK4
activates transcription factors for NF-κB, and is associated with
aggressive pancreatic ductal adenocarcinoma that has a poorer
prognosis (7). Cheng et al
(6) demonstrated that IRAK4 can
regulate the stemness and drug resistance of hepatocellular
carcinoma cells, and is associated with tumour malignancy and poor
patient survival. However, a role for IRAK4 in glioma has not been
previously reported, to the best of our knowledge. Considering the
role of IRAK4 in other cancer types, it was hypothesised that IRAK4
may also play an important role in glioma, and may be a potential
biomarker related to the diagnosis and treatment of glioma.
The present study investigated the role of IRAK4 in
glioma based on thousands of glioma samples and related clinical
information. First of all, combined with reverse
transcription-quantitative (RT-q)PCR technology and biological
information analysis, the expression levels of IRAK4 in glioma
samples and corresponding normal control samples were compared.
Subsequently, the relationship between high expression of IRAK4 and
prognosis and a series of clinical features was explored. Finally,
the Gene Set Enrichment Analysis (GSEA) method was used to explain
the regulatory mechanism of IRAK4. All in all, the current study
aimed to provide a potential biomarker for the prognostic
evaluation and treatment of glioma.
Materials and methods
Source of data and tissue samples
The Chinese Glioma Genome Atlas database (CGGA,
http://www.cgga.org.cn/) is a public database
containing high-throughput data and clinical characteristics of a
large number of glioma samples (8).
Sequencing data and corresponding clinical information of 1,018
samples from patients with glioma were obtained from the CGGA
database. After deleting samples with incomplete clinical
information, 748 samples were retained for subsequent multiple
analyses. The detailed clinical information of the sample is
presented in Table SI.
The Cancer Genome Atlas database (TCGA; http://portal.gdc.cancer.gov/) is a well-known
database containing gene sequencing data and clinical
characteristics of various human malignant tumour samples (9). The publicly available data of 666
glioma samples and five normal brain tissue samples were obtained
and used to verify the CGGA data.
The Gene Expression Profiling Interactive Analysis
(GEPIA, http://gepia.cancer-pku.cn/) database
contains tumour tissue samples from TCGA data and normal control
samples from the GTEx database (10). Data of IRAK4 expression in a variety
of tumour tissues and corresponding normal tissues was obtained
using GEPIA.
The Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) is an open
public data platform (11). Two GEO
datasets related to glioma were obtained, namely GSE15824
(tumour=12, normal=2) (12) and
GSE50161 (tumour=34, normal=13) (13). The platforms of these two GSE data
sets were GPL570.
The Human Protein Atlas (HPA, http://www.proteinatlas.org/) contains research data
related to a variety of human pathology and normal histological
sections (14). The
immunohistochemical results of glioma (sample ID: 3241) and normal
brain tissue (sample ID: 2521) were screened and obtained from this
database. The immunohistochemical antibody was CAB022077.
Tissue samples from five patients with glioblastoma
and five patients with epilepsy were collected at Henan Provincial
People's Hospital (Zhengzhou, China) from June 2019 to September
2019. Inclusion criteria included: i) Patients with glioma
diagnosed histopathologically and ii) Those who underwent surgical
treatment. Exclusion criteria included: i) Patients with glioma
patients without histopathological diagnosis, ii) patients with
spinal involvement and iii) Patients with incomplete data records.
The samples were obtained from surgical resection in the operating
room. The patient's clinical information was recorded in the
supplementary file Table SII. The
classification of clinical characteristics of glioma patients are
presented in Table SIII. The
expression levels of IRAK4 in glioma and non-tumour brain tissue
samples were further validated using by RT-qPCR. Patient tissue
samples were immediately frozen in liquid nitrogen after being
obtained, then were stored at −80°C until isolation of total RNA.
The study protocol was approved by The Ethics Committee of the
Henan Provincial People's Hospital (Zhengzhou, China) and all
experiments were performed in accordance with approved guidelines
of the Henan Provincial People's Hospital.
Cell culture
Human glioma cells (A172) and human-derived
astrocyte (A735) were purchased from The Cell Bank of Type Culture
Collection of The Chinese Academy of Sciences. All cells were grown
in incubators at 37°C and 5% carbon dioxide and cultured using DMEM
medium plus 10% FBS (both Thermo Fisher Scientific, Inc.).
Cell transfection
The small interfering RNA (siRNA) that specifically
targeted IRAK4 was purchased from Shanghai GenePharma Co., Ltd. The
RNA oligo sequence of siRNAs used in the study is presented in
Table I. In total,
0.5–2×105 cells were seeding in 500 µl of complete
medium. Cell transfection was performed when the cell confluence
was 50–60%. Complete medium was removed and replaced with
serum-free medium. Cells were transfected using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and 4 µg/ml siRNA. The same concentration of
si-negative dissolved in transfection reagent was used as
thenegative control group. The same volume of transfection reagent
was used as a blank control group. Cells were cultured at 37°C and
5% CO2 for 6 h following cell transfection. Then
serum-free medium was switched to complete medium. A series of
follow-up experiments were performed after the cells were cultured
for 48 h. The transfection efficiency was determined using RT-qPCR
at 48 h after transfection. The si-RNA with the greatest efficiency
was then selected for further experiments.
 | Table I.RNA oligo sequence of siRNA. |
Table I.
RNA oligo sequence of siRNA.
| Gene | RNA oligo sequence,
5′-3′ |
|---|
| GAPDH-F |
CAAGGTCATCCATGACAACTTTG |
| GAPDH-R |
GTCCACCACCCTGTTGCTGTAG |
| IRAK4-si-1-F |
CCUCAAUGUUGGACUAAUUTT |
| IRAK4-si-1-R |
AAUUAGUCCAACAUUGAGGTT |
| IRAK4-si-2-F |
CCAUUUCUGUUGGUGGUAATT |
| IRAK4-si-2-R |
UUACCACCAACAGAAAUGGTT |
| IRAK4-si-3-F |
CCACUUCAGUUGAAGCUAUTT |
| IRAK4-si-3-R |
AUAGCUUCAACUGAAGUGGTT |
| IRAK4-si-NC-F |
UUCUCCGAACGUGUCACGUTT |
| IRAK4-si-NC-R |
ACGUGACACGUUCGGAGAATT |
RT-qPCR
Total RNA was isolated from sample tissues and
corresponding cell lines after cell transfection using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.)
extraction. RNA quality and quantity were estimated by measuring
absorbance at 260 and 280 nm using a NanoDrop One spectrophotometer
(Thermo Fisher Scientific, Inc.). cDNA was synthesised from
isolated total RNA by using a Transcriptor First Stand cDNA
Synthesis kit (Roche Diagnostics) according to the manufacturer's
instructions. A FastStart Universal SYBR Green Master (ROX) kit
(Roche Diagnostics) was used for qPCR. The thermal cycling
conditions were as follows: Initial denaturation at 95°C for 10
min, denaturation at 95°C for 10 sec, annealing and extension at
60°C for 30 sec, for a total of 40 cycles. The RNA expression for
GAPDH was used as an internal control. The primer sequences of all
genes used in the study are recorded in Table II. Triplicates of each tissue sample
were used for RT-qPCR. Expression of IRAK4 was calculated using the
2−ΔΔCq method and GAPDH was used as the reference gene
(15). Wilcoxon rank sum test,
one-way ANOVA following by Bonferroni's correction and unpaired
t-tests were used for statistical analysis and the level of
significance was set at P<0.05.
 | Table II.Primer sequences of reverse
transcription quantitative-PCR used to verify the co-expression
analysis. |
Table II.
Primer sequences of reverse
transcription quantitative-PCR used to verify the co-expression
analysis.
| Gene | Primer sequence,
5′-3′ |
|---|
| GAPDH-F |
CAAGGTCATCCATGACAACTTTG |
| GAPDH-R |
GTCCACCACCCTGTTGCTGTAG |
| IRAK4-F |
TCATAGGCGGCAGGAACTTA |
| IRAK4-R |
ACCCAAACACTTCCCATCAG |
| CMTM6-F |
TGGAGAACGGAGCGGTGTA |
| CMTM6-R |
AGCCGGCCCATGAAAAAGTA |
| MOB1A-F |
CAGCAGCCGCTCTTCTAAAAC |
| MOB1A-R |
CCTCAGGCAACATAACAGCTTG |
| MFSD1-F |
GTTGTCACCACTTTCCCCTCT |
| MFSD1-R |
CAGTAACAGCACCAAAAGCCG |
| CD164-F |
TCAAGTGGGGAACACGACAG |
| CD164-R |
TTCGCACAGGTTGTGAGGTT |
| CMTR2-F |
TTGCGGGAGCTTCATACAGG |
| CMTR2-R |
CAGGTCCTCAGGGGATCAGA |
| HIST3H2BB-F |
TGCCAGACCCGTCCAAAT |
| HIST3H2BB-R |
TCTTCTGTGCCTTGGTGACA |
| RPPH1-F |
TCCTGTCACTCCACTCCCAT |
| RPPH1-R |
TGGCCCTAGTCTCAGACCTT |
| TERC-F |
CGCCTTCCACCGTTCATTCTA |
| TERC-R |
TGACAGAGCCCAACTCTTCG |
| HIST1H4C-F |
GCAAAGGCGGAAAAGGCTTG |
| HIST1H4C-R |
TAGCCGGTTTTGTAATGCCCT |
| RIMS1-F |
TGGAAGTCATTAGAGCACGAAGC |
| RIMS1-R |
CCCAGACAATCACCTGAAGAACT |
GSEA
GSEA is a widely used bioinformatics analysis tool
for gene enrichment analysis of gene function annotation (16,17).
Data from high-throughput sequencing contains the expression of a
large number of genes, which can be used to investigate the
relationships between genes (18,19).
GSEA formulates a special algorithm based on the existing research
status of genes and cell signalling pathways, which can be used to
explore the cell signalling pathways that a single gene may
participate in the regulation (16,17). The
mRNA sequencing data from CGGA was used for GSEA analysis. First,
batch calibration and normalization were performed using the SVA
and limma packages of R software (version 3.6.1) (20). Then the RNA sequencing data was
divided into high expression and low expression groups according to
the expression of IRAK4. GSEA (version 4.0.3) jar software was used
for enrichment analysis. The number of permutations was set to
1,000 and Kyoto Encyclopaedia of Genes and Genomes (KEGG) was set
as the gene database. The statistical test standard was set as
P<0.05 and false discovery rate (FDR)<0.25.
Co-expression analysis and drug
correlation analysis
The co-expression analysis on IRAK4 was performed
using Pearson's correlation analysis (21). According to the P-value and the
correlation coefficient value, five genes with positive or negative
correlation with IRAK4 expression were selected. A heat map and
circle map were constructed based these analysis results.
Statistical analysis
R software (version 3.6.1) was used for all data
analysis (20). The Wilcoxon rank
sum or Kruskal-Wallis tests were used to determine if there was an
association between clinical characteristics and expression of
IRAK4 in patients with glioma. According to the World Health
Organisation (WHO) 2007 grading standard, the data from the CGGA
database were classified into ‘All grades of gliomas’, ‘WHO Grade
II’, ‘WHO Grade III’ and ‘WHO Grade IV’ (1). After that, survival analysis was
performed for each group. Kaplan-Meier and Cox regression survival
analysis were used in the three groups ‘All grades of gliomas’,
‘WHO Grade II’ and ‘WHO Grade III’. The Renyi test was used in the
‘WHO Grade IV’ group using SAS software (version 9.4; SAS
Institute, Inc.). Univariate and multivariate Cox analyses were
used to compare the effects of IRAK4 expression and other clinical
features on the survival of patients with glioma. Pearson's
correlation analysis was used to evaluate the degree of correlation
between the expression of different genes. The Wilcoxon rank sum
test was used to compare the expression of IRAK4 in the glioma and
non-cancer control groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical features and the association
between IRAK4 expression and clinical features in patients with
glioma
The glioma sample data obtained from the CGGA
database was screened and normalized. Its clinical characteristics
are presented in Table SI. The
patients were divided into different groups according to various
clinical characteristics, and the expression levels of IRAK4 in
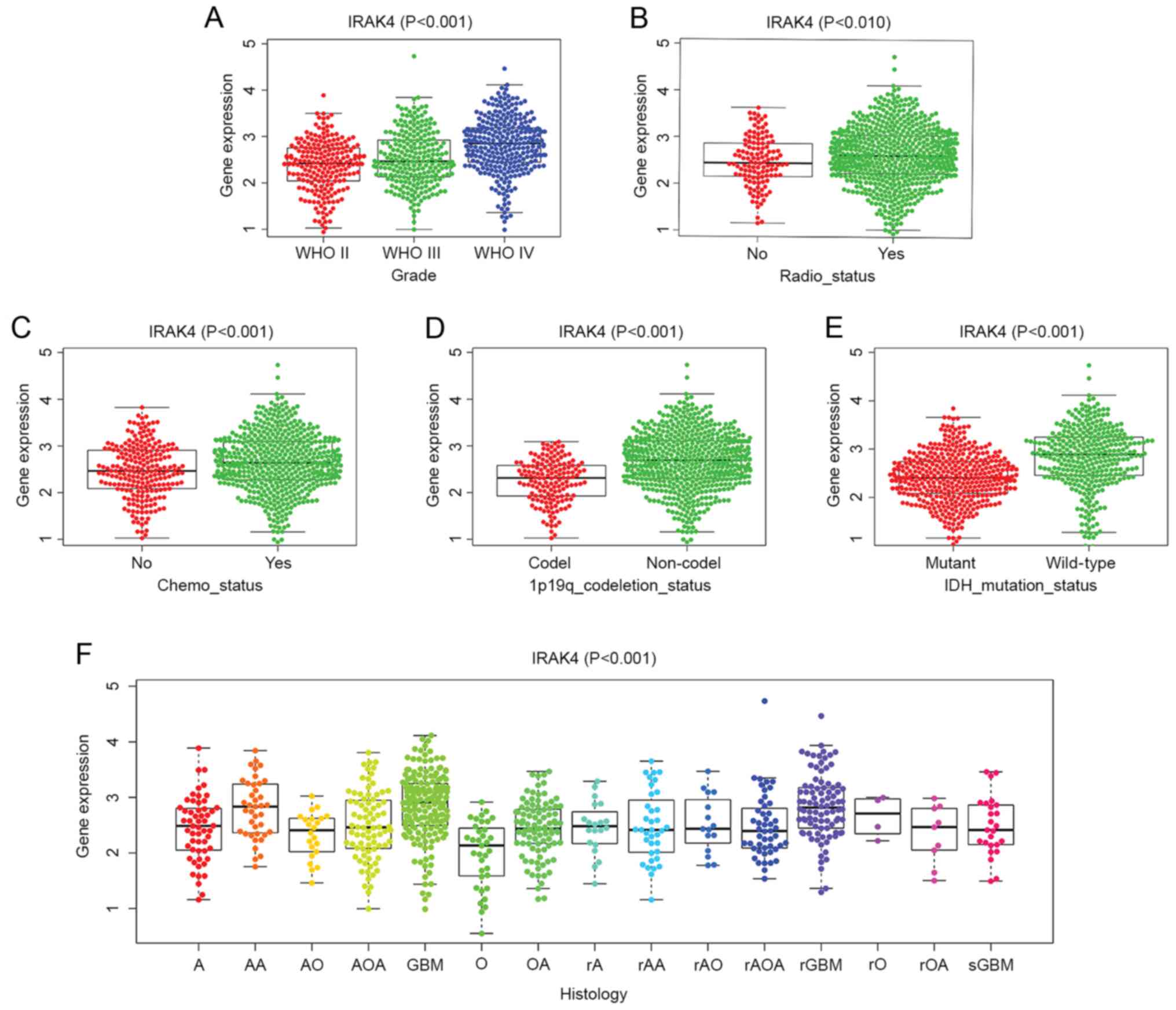
each group were compared. Fig. 1
shows that the expression level of IRAK4 in patients with glioma
from CGGA may be associated with a variety of clinical and
molecular characteristics. The expression of IRAK4 was
significantly higher in patients with higher WHO grade, isocitrate
dehydrogenase (IDH) wild-type and 1p19p non-co-deletion compared
with in patients with lower WHO Grade, IDH mutation and 1p19p
co-deletion (all P<0.001; Fig. 1A, D
and E).
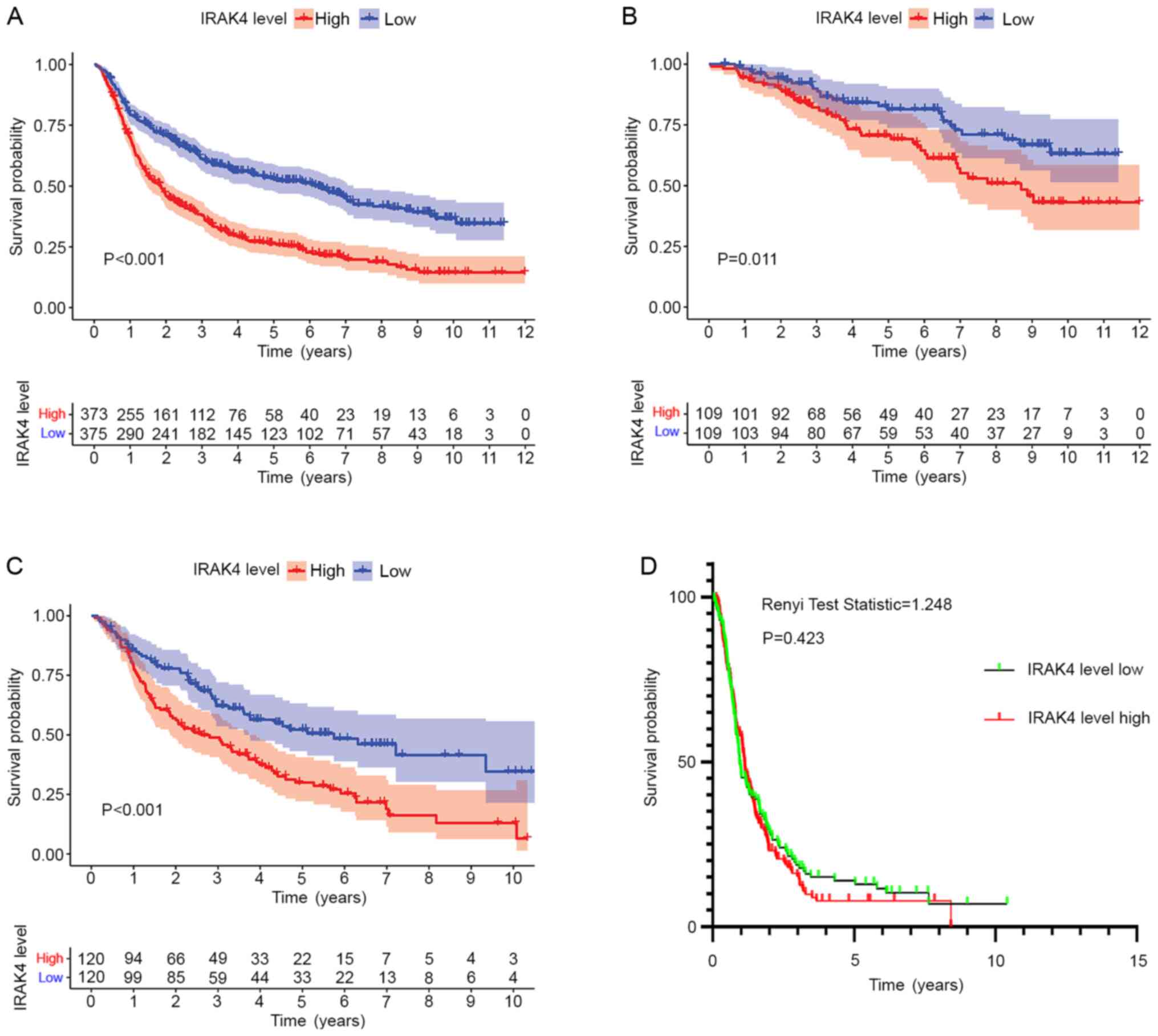
High expression of IRAK4 can lead to a
poor prognosis
According to the expression level of IRAK4, CGGA
mRNA sequencing data of glioma were divided into high and low
expression groups. After that, Kaplan Meier method was used to draw
the overall survival curve. The results showed that high IRAK4
expression group is significantly associated with a shorter
survival time of patients with glioma of all grades (P<0.001),
WHO-II grade gliomas (P=0.011) and WHO-III grade gliomas
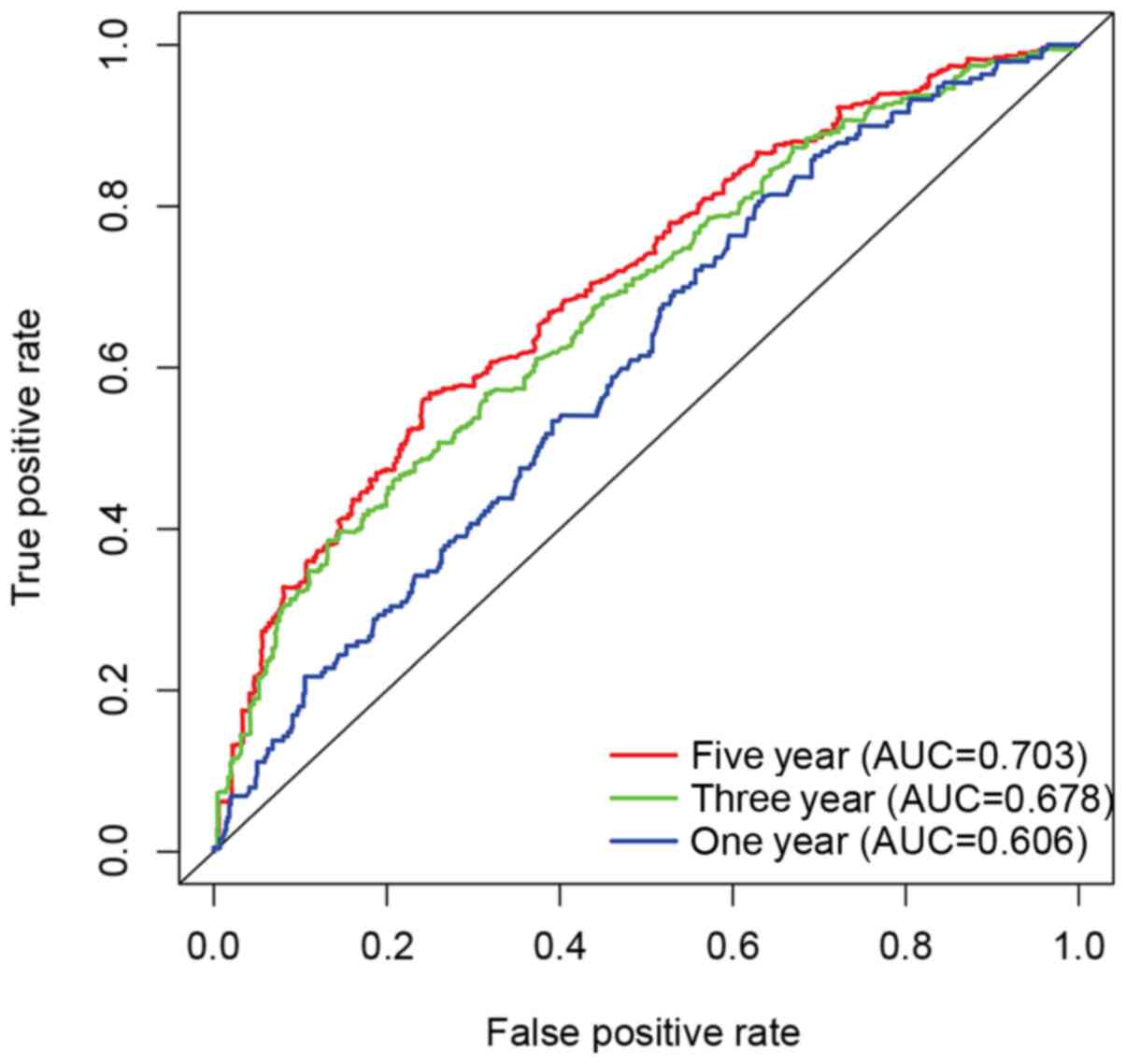
(P<0.001) (Fig. 2A-C). Receiver
operating curve (ROC) was used to explore whether the expression
level of IRAK4 had a certain prognostic value in glioma. The
results showed that the expression level of IRAK4 had a certain
predictive value for the 5-year survival rate (area under the curve
>0.7) in Fig. 3.
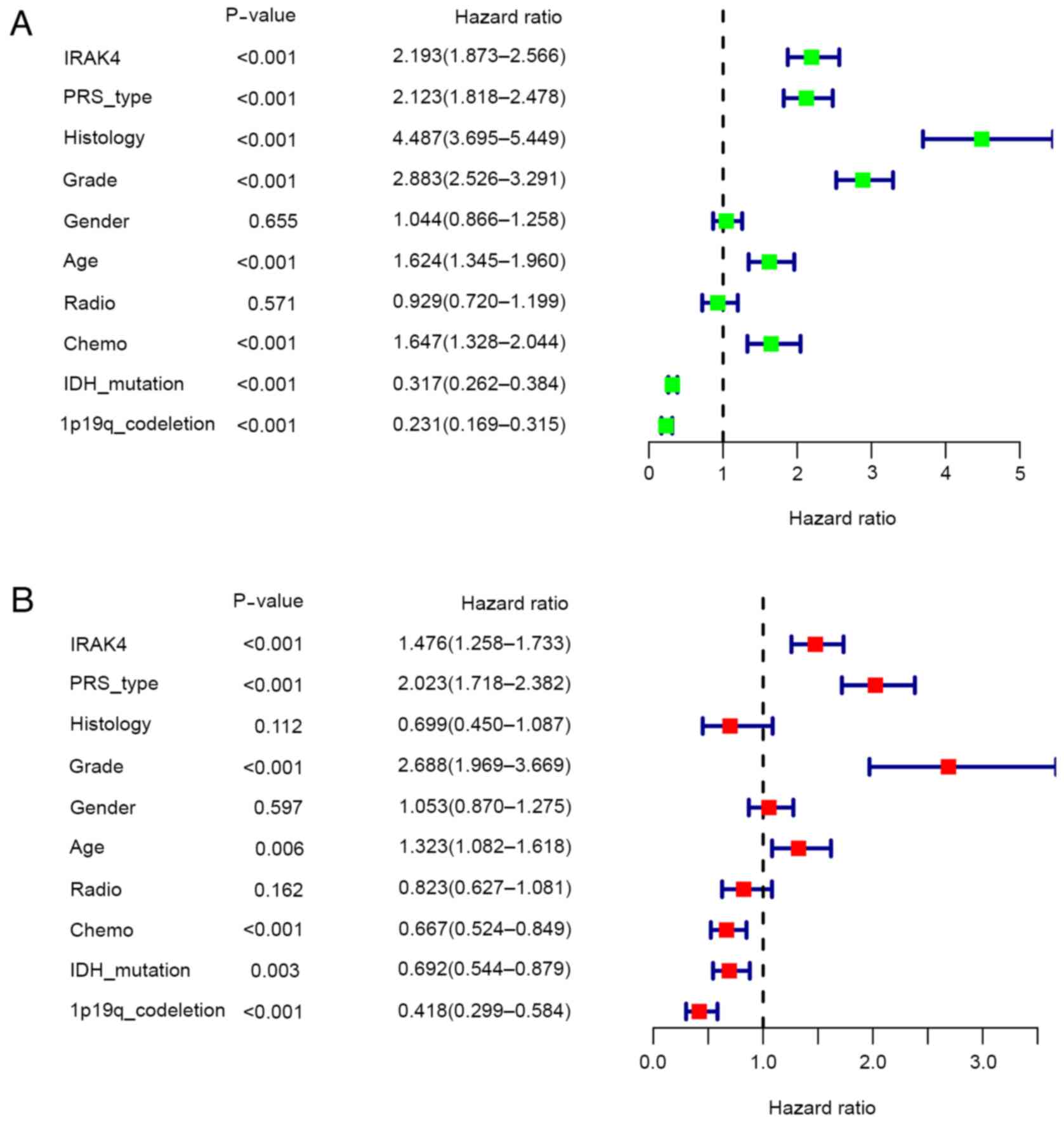
Univariate analysis and multivariate analysis were
used to determine whether the high expression of IRAK4 is an
independent risk factor leading to poor prognosis in patients with
glioma. It must be emphasized that the high expression of IRAK4,
Primary Recurrent Secondary type, higher WHO grade and age can all
be used as independent risk factors and lead to poor prognosis
[P<0.05, hazard ratio (HR)>1]. Meanwhile, postoperative
chemotherapy, IDH mutation status and 1p19q co-deletion status were
independent protective factors that lead to an improved prognosis
(P<0.05, HR<1) in Fig. 4.
Validation of the association between
IRAK4 and glioma
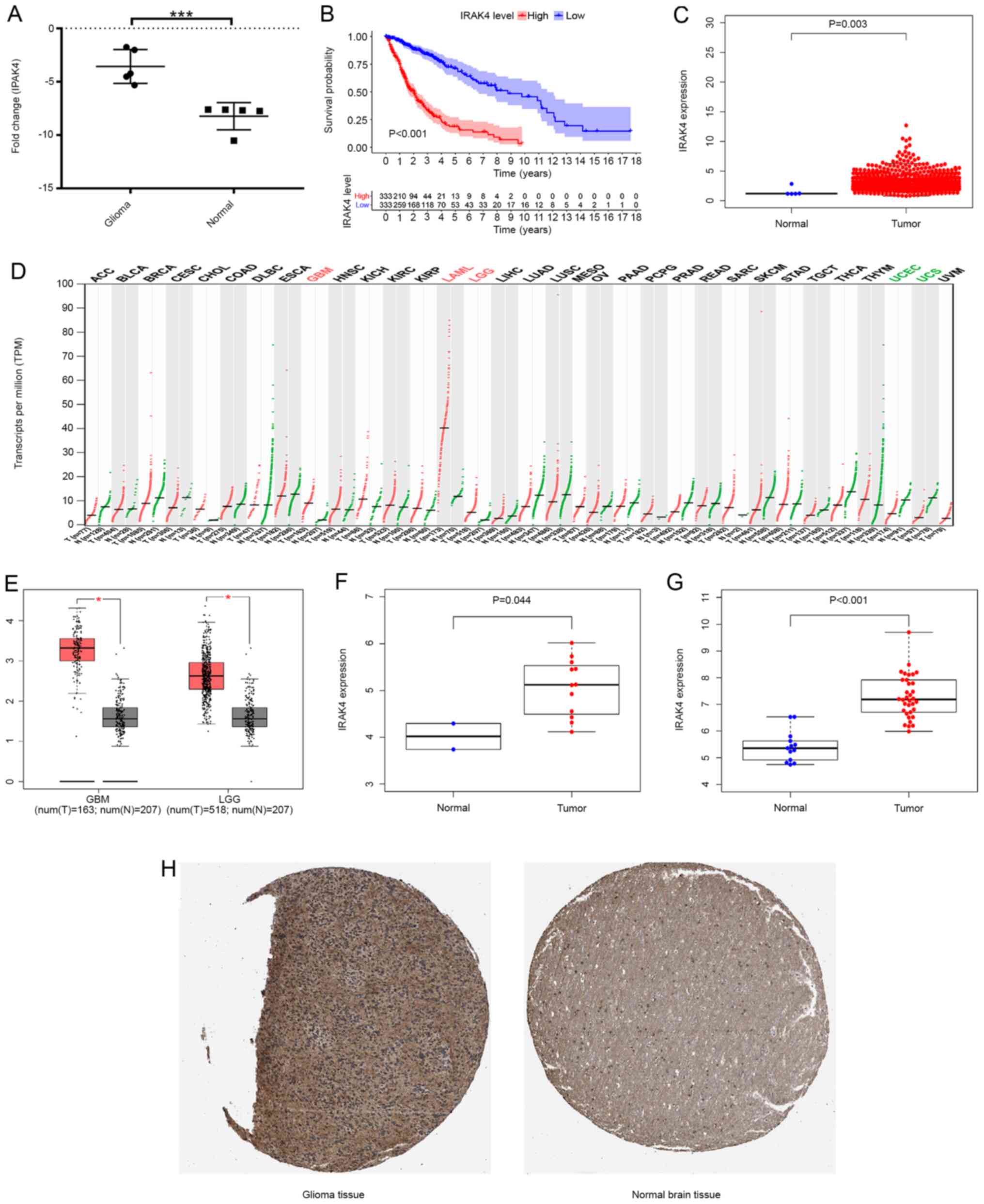
After performing RT-qPCR on clinical samples,
Fig. 5A shows that the expression
level of IRAK4 in glioma tissues (n=5) was increased (P<0.001)
compared with normal brain tissues (n=5). The results showed that
the expression level of IRAK4 was higher in glioma tissues compared
with that in normal tissues (P=0.003; Fig. 5C). The overall survival of patients
in the IRAK4 high expression group was significantly lower compared
with that of the IRAK4 high expression group, which suggested that
the abnormally high expression of IRAK4 was related to poor
prognosis (P<0.001; Fig. 5B). The
analysis of the GEPIA database showed that the expression level of
IRAK4 in GBM and LGG samples was significantly higher compared with
that in the corresponding normal control samples (P<0.05;
Fig. 5D and E). The analysis of
GSE15824 showed that the expression level of IRAK4 in glioma tissue
samples was significantly higher compared with that of normal brain
tissue (P=0.044; Fig. 5F). The
analysis of GSE50161 showed that the expression level of IRAK4 in
glioma tissue samples was significantly higher compared that of
normal brain tissue (P<0.001; Fig.
5G). In addition to detection at the mRNA level, the protein
expression level of IRAK4 was assessed using the HPA database. The
results showed that the protein expression level of IRAK4 in glioma
tissue was higher compared with that in normal brain tissue
(Fig. 5H). In summary, Fig. 5 shows that IRAK4 had abnormally high
expression relative to normal control samples at the mRNA and
protein levels from multiple data sources.
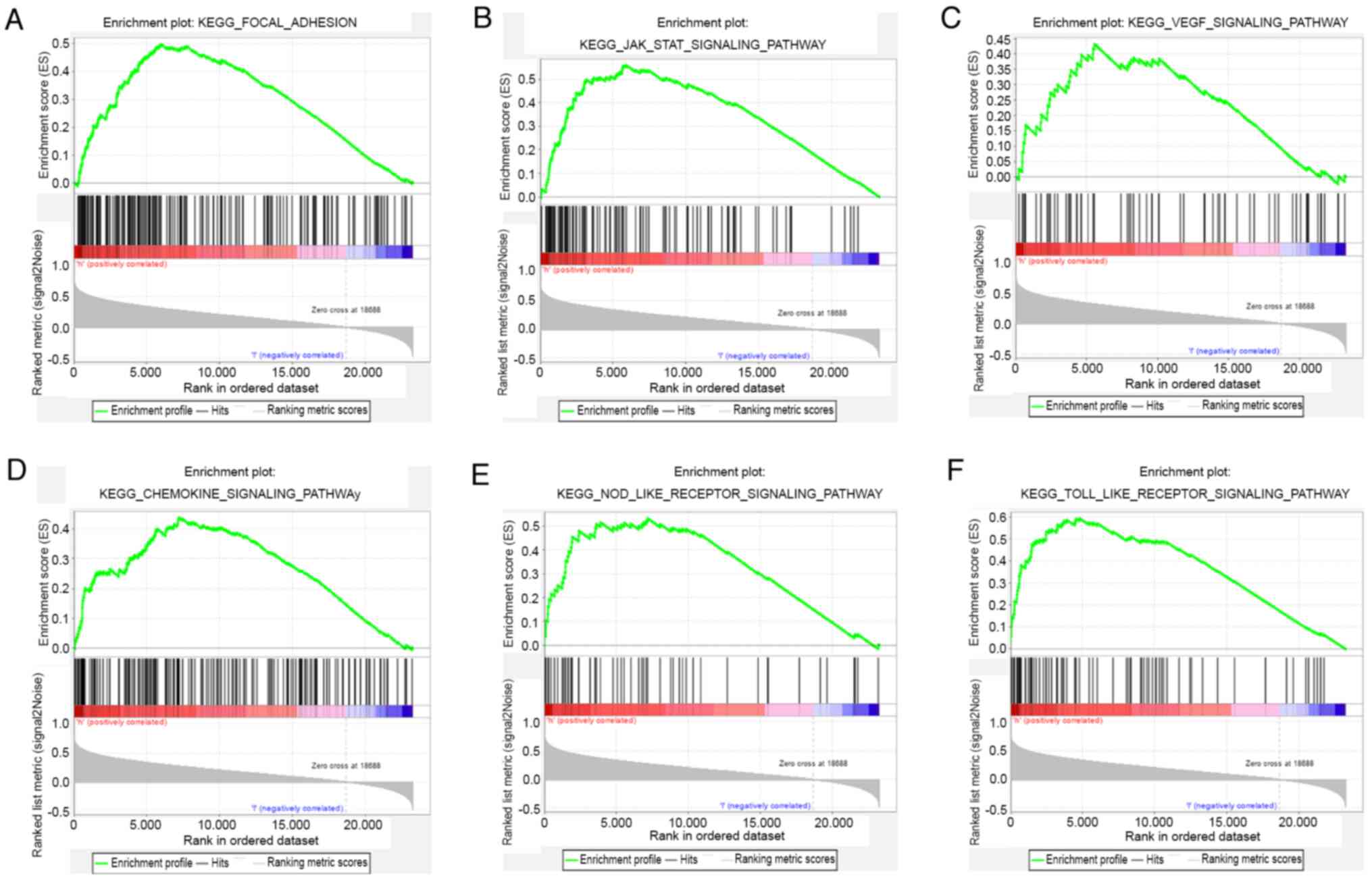
GSEA analysis
GSEA was used to form a comparison between datasets
from patients that showed either high or low expression of IRAK4
and identify signalling pathways that had different levels of
activity in glioma. There were significant differences (nominal
P<0.05 and FDR <0.25) in enrichment of pathways identified
using KEGG analysis. It was reported that focal adhesion, JAK-STAT,
VEGF, chemokine, NOD-like receptor (NLR) and Toll-like receptor
(TLR) signalling pathways were differentially enriched in the high
IRAK4 expression glioma phenotype (Fig.
6). These results suggested that IRAK4 may play a regulatory
role in the pathological process of glioma via the aforementioned
cellular signalling pathways.
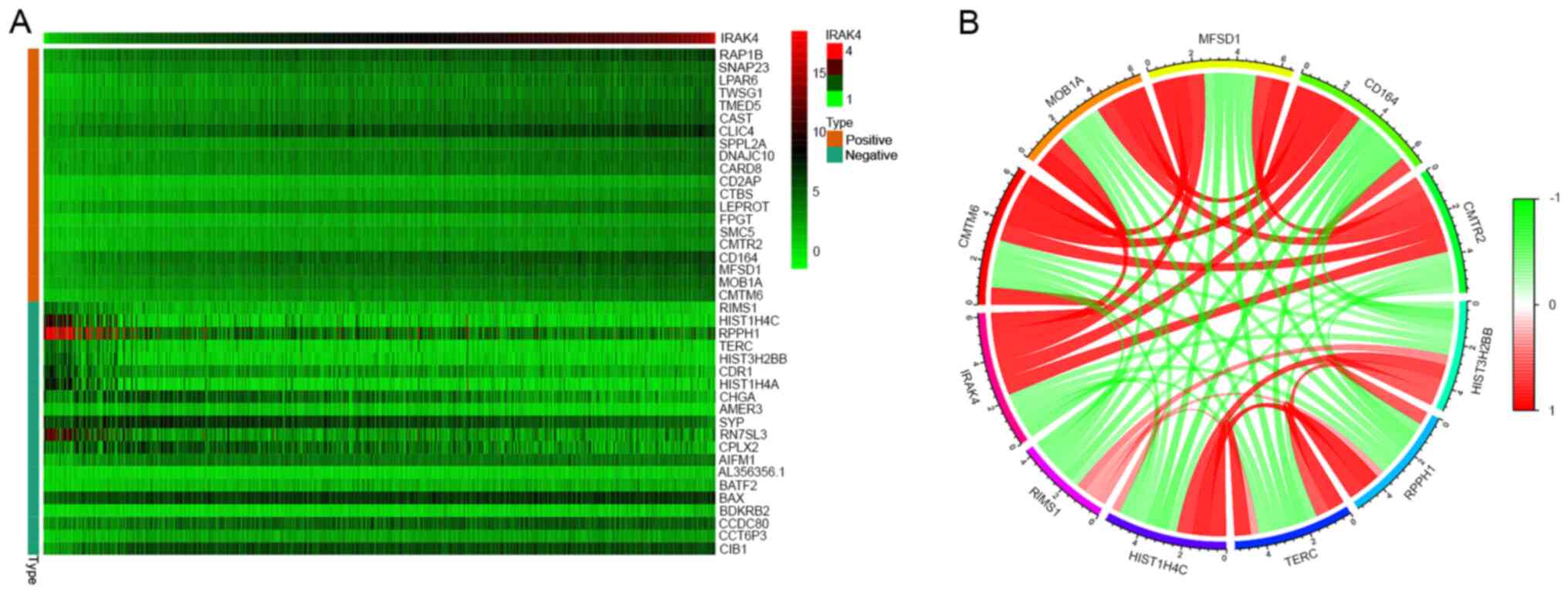
Co-expression analysis of IRAK4
A heat map of co-expression representing the top 20
genes that were most significantly negatively and positively
correlated with IRAK4 is presented in Fig. 7A. Co-expression networks were
constructed from gene expression data relative to normal levels of
expression. In the co-expression network, each node represented a
gene in the network, and gaps represented two related genes (the
shorter the distance between nodes, the stronger the correlation
between the genes). A string connection was a close correlation
between these genes and indicated that regulatory relationships may
exist. With the increasing of expression level of IRAK4, there was
a positive relationship with the expression level of CMTM6, MOB1A,
MFSD1, CD164 and CMTR2, and a negative relationship with the
expression level of HIST3H2BB, RPPH1, TERC, HIST1H4C and RIMS1
(Fig. 7B).
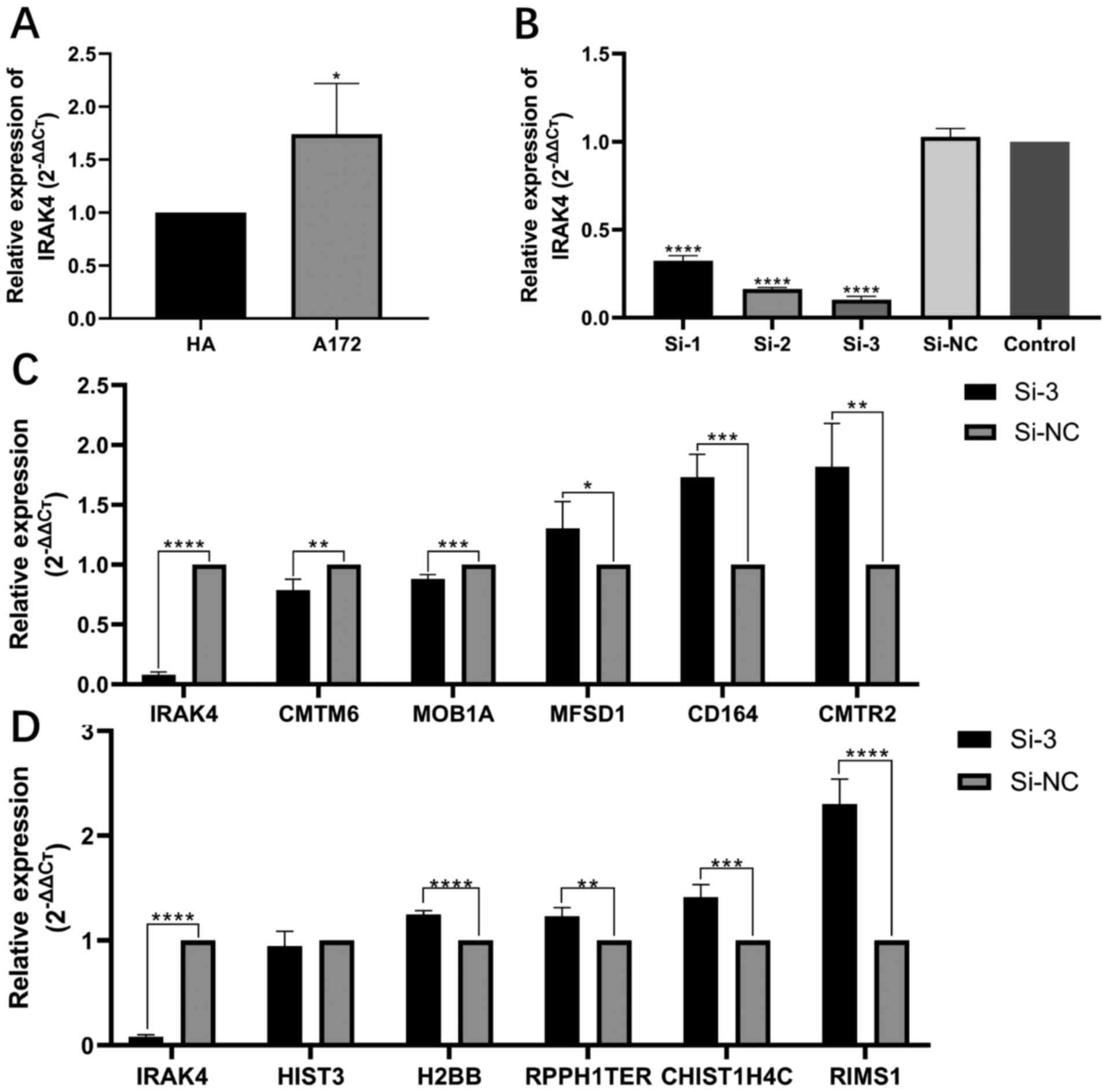
After the A172 glioma cell line was cultured and the
expression of IRAK4 was knocked down by gene interference
technology, the expression level of related genes was detected by
RT-qPCR. The expression level of IRAK4 in the A172 glioma cell line
was significantly higher compared with that in HA (P<0.05;
Fig. 8A). After gene interference,
the expression of IRAK4 was significantly decreased in A172 cells
(all P<0.0001; Fig. 8B). siRNA-3
had the highest transfection efficiency and so was selected for
further experiments. After knockdown of IRAK4 in A172 cells, the
expression levels of IRAK4 positive related genes, such as CMTM6
and MOB1A, were also decreased, which was consistent with the
results of the co-expression analysis of this study. However,
MFSD1, CD164 and CMTR2 levels were significantly increased
(P<0.05, P<0.001 and P<0.01, respectively; Fig. 8C). After knockdown of IRAK4 in A172
cells, the expression of IRAK4 negatively related genes, such as
HIST3H2BB, TERC, HIST1H4C and RIMS1, were significantly increased
(P<0.0001 or P<0.01), which was consistent with the results
of the co-expression analysis of this study., but RPPH1 did not
change significantly (Fig. 8D).
Discussion
Up to now, the etiological mechanism of glioma has
not been fully elucidated. It has been reported that gene level
changes have an important impact on the pathological process of
glioma (22). Revealing the function
of gene is helpful to clarify the pathogenesis of glioma. A large
number of studies have confirmed that IRAK4, as an oncogene, plays
an important regulatory role in tumorigenesis (4–6), but its
function in glioma has not been revealed so far (23). The present study explored the
relationship between IRAK4 and glioma. The purpose of the study was
to elucidate the effect of IRAK4 on the prognosis of patients with
glioma and to investigate the association between IRAK4 and
clinical variables.
The current study demonstrated that high expression
of IRAK4 was correlated with more advanced WHO grade, IDH wild-type
and 1p19q non-co-deletions. These three characteristics played an
important role in the 2007 and 2016 updated WHO classifications
(1,5). The previous study showed that the
higher the WHO classification, the higher the malignant degree of
glioma and the worse the prognosis (1,5). IDH
mutations and 1p19q often occur concurrently and are currently
considered to be protective factors for the prognosis of glioma
(24). This coincides with the
present univariate and multivariate Cox analysis results.
Subsequent survival analysis and ROC curves further suggested that
abnormally high expression of IRAK4 could lead to poor prognosis of
patients with glioma. Based on this, it was predicted that IRAK4
may be a potential biomarker or oncogene involved in the occurrence
and development of glioma. Therefore, to investigate this
hypothesis, the expression levels of IRAK4 mRNA and protein were
investigated in multiple cell lines, tissue samples and databases
(GEPIA, TCGA, GEO and HPA). The results demonstrated that the
expression level of IRAK4 in glioma was higher compared with that
in normal control.
The specific function of IRAK4 in glioma was still
unclear, so based on the hypothesis that IRAK4 may be an oncogene,
GSEA was used to predict the cell signalling pathways that IRAK4
may be involved in regulating. GSEA results showed that IRAK4 may
be involved in the regulation of cell signalling pathways, such as
VEGF, JAK-STAT, TLR signalling, focal adhesion, chemokine and NLR.
The important role of VEGF in tumorigenesis and development makes
it an important therapeutic target for cancer (25). Previous study has shown that
inhibition of VEGF signalling may increase overall survival time in
patients with glioma (26). Previous
studies have shown that the JAK/STAT pathway plays an important
role in the progression of glioma, and that activation of JAK-STAT
signalling is predictive of poor prognosis in patients with glioma
(27,28). A previous study also demonstrated
that IRAK4 modulates the response to temozolomide in glioma via the
TLR and NF-κB signalling pathways (29). This is consistent with results of the
present study in the correlation analysis of clinical features,
which showed that increased IRAK4 is associated with patients with
glioma receiving chemotherapy. CD155/poliovirus receptor enhances
glioma cell invasion and migration by regulating adhesion signals
and focal adhesion dynamics (30).
Numerous chemokines are potential therapeutic targets for glioma
including CCR7, which can activate matrix metalloproteinase 2/9
through NF-κB signalling to regulate invasion and migration of
TGF-β1-induced human glioma cells (31). In summary, the present GSEA predicted
cell signalling pathways that IRAK4 might be involved in
regulation.
It is well known that oncogenes can often be used as
therapeutic targets for cancer suppression therapy (32). However, changes in the expression
level of a single gene in organisms often lead to changes in the
expression levels of other genes, thus playing a synergistic role
in the regulation of disease progression (33). Therefore, to further explore the
effect of IRAK4 on other genes, the current study further performed
a co-expression analysis to identify more genes that are
potentially linked to glioma. The co-expression network suggested
that the expression levels of CMTM6, MOB1A, MFSD1, CD164 and CMTR2
were positively correlated with IRAK4, but negatively correlated
with HIST3H2BB, RPPH1, TERC, HIST1H4C and RIMS. To improve the
reliability of the prediction results, IRAK4 was knocked down in
the A172 glioma cell line, and changes in the levels of the
aforementioned co-expression genes were assessed using RT-qPCR
technology. The results showed that the expression level of MFSD1,
CD164 and CMTR2 were increased contrary to our predicted results,
while the expression level of RPPH1 did not change significantly.
Other genes co-expressed with IRAK4 had increased expression
levels, consistent with the expected outcome. These co-expression
genes may affect the pathological process of glioma through
synergistic effect with IRAK4, but the specific mechanism needs to
be further studied. This conclusion is supported by previous
report. For example, the overexpression of CMTM6 was associated
with poor prognosis in glioma and clinical features (34).
The present study used data from public databases to
fully reveal the relationship between overexpression of IRAK4 and
prognosis and clinical information of patients with glioma.
However, there were some limitations. Firstly, as the public
database aggregates information from multiple treatment centres,
there were inevitable information gaps, inconsistent data
collection and processing. In the analysis process, the impact of
the difference in the patient's detailed treatment plan, such as
the scope of surgical resection and differences in radiotherapy and
chemotherapy regimens, could not be minimised. However, because of
this, multiple databases were verified and compared, which makes
the results of the current study more objective and authentic.
Secondly, the number of healthy controls in public databases is
relatively small compared with the number of patients with glioma,
and this may introduce errors in statistical analysis. Therefore,
RT-qPCR was used to validate differences in IRAK4 expression levels
between glioma tissue and control non-cancer brain tissue. In
addition, the present research still has certain limitations. When
analysing the relationship between the expression level of IRAK4
and clinical characteristics, the two clinical characteristics of
radiotherapy and chemotherapy were introduced. However, specific
treatment modalities of radiation or chemotherapy may render
notably different results, and therefore simply categorizing by
radiotherapy or chemotherapy is of poor clinical significance.
To the best of our knowledge, the present study is
the first to link the overexpression of IRAK4 with the decreased
survival rate of patients with glioma. The current research
suggested that IRAK4 may be a potential oncogene involved in the
regulation of cell signalling pathways in glioma. IRAK4 may be a
novel biomarker for prognostic evaluation and treatment of
glioma.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgments
Not applicable.
Funding
This work was supported by The Thousand Talents Plan
of Henan province, Henan Provincial Innovation and Outstanding
Talent Program (grant no. 154200510027), The 2016 Henan Provincial
Science and Technology Research Project: Construction and Clinical
Application of Digital Precision Spine Surgery Technology System
(grant no. 162102310018) and The 2018 Henan provincial Medical
Science and Technology Tackling Program Provincial-ministerial
Co-construction Project (grant no. SBGJ2018076).
Availability of data and materials
The datasets used and/or analysed during the current
study are available in The Cancer Genome Atlas [http://www.cgga.org.cn/] and the Chinese Glioma Genome
Atlas [http://www.cgga.org.cn/] repositories.
The additional datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW, ZL, BL, LL and YG designed the study. JW
conducted basic experimental verification. JY, HW, LL and YG
collected the tissue samples. JW and ZL reviewed the raw data and
confirmed the authenticity of all raw data. JW and BZ performed the
analysis. XL and ZR collected data. ZL and BL drafted the
manuscript. LL and YG gave final approval of the version to be
published. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by The Ethics
Committee of the Henan Provincial People's Hospital (Zhengzhou,
China). The use of patient samples conformed to the declaration of
Helsinki. All patients provided informed written consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brandes AA, Tosoni A, Spagnolli F, Frezza
G, Leonardi M, Calbucci F and Franceschi E: Disease progression or
pseudoprogression after concomitant radiochemotherapy treatment:
Pitfalls in neurooncology. Neuro Oncol. 10:361–367. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ho VK, Reijneveld JC, Enting RH, Bienfait
HP, Robe P, Baumert BG and Visser O; Dutch Society for
Neuro-Oncology (LWNO), : Changing incidence and improved survival
of gliomas. Eur J Cancer. 50:2309–2318. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kargbo RB: PROTAC degradation of IRAK4 for
the treatment of cancer. ACS Med Chem Lett. 10:1370–1371. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Q, Chen Y, Zhang D, Grossman J, Li L,
Khurana N, Jiang H, Grierson PM, Herndon J, DeNardo DG, et al:
IRAK4 mediates colitis-induced tumorigenesis and chemoresistance in
colorectal cancer. JCI Insight. 4:e1308672019. View Article : Google Scholar
|
|
6
|
Cheng BY, Lau EY, Leung HW, Oi-Ning Leung
C, Ho NP, Gurung S, Cheng LK, Lin CH, Cheuk-Lam Lo R, Ma S, et al:
IRAK1 augments cancer stemness and drug resistance via the
AP-1/AKR1B10 signaling cascade in hepatocellular carcinoma. Cancer
Res. 78:2332–2342. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang D, Li L, Jiang H, Knolhoff BL,
Lockhart AC, Wang-Gillam A, DeNardo DG, Ruzinova MB and Lim KH:
Constitutive IRAK4 activation underlies poor prognosis and
chemoresistance in pancreatic ductal adenocarcinoma. Clin Cancer
Res. 23:1748–1759. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Z, Wang Z, Zhang C, Liu X, Li G, Liu
S, Sun L, Liang J, Hu H, Liu Y, et al: Genetic and clinical
characterization of B7-H3 (CD276) expression and epigenetic
regulation in diffuse brain glioma. Cancer Sci. 109:2697–2705.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tomczak K, Czerwińska P and Wiznerowicz M:
The cancer genome atlas (TCGA): An immeasurable source of
knowledge. Contemp Oncol (Pozn). 19:A68–77. 2015.PubMed/NCBI
|
|
10
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suzuki A, Horie T and Numabe Y:
Investigation of molecular biomarker candidates for diagnosis and
prognosis of chronic periodontitis by bioinformatics analysis of
pooled microarray gene expression datasets in gene expression
omnibus (GEO). BMC Oral Health. 19:522019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grzmil M, Morin P Jr, Lino MM, Merlo A,
Frank S, Wang Y, Moncayo G and Hemmings BA: MAP kinase-interacting
kinase 1 regulates SMAD2-dependent TGF-β signaling pathway in human
glioblastoma. Cancer Res. 71:2392–2402. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Griesinger AM, Birks DK, Donson AM, Amani
V, Hoffman LM, Waziri A, Wang M, Handler MH and Foreman NK:
Characterization of distinct immunophenotypes across pediatric
brain tumor types. J Immunol. 191:4880–4888. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Uhlen M, Zhang C, Lee S, Sjöstedt E,
Fagerberg L, Bidkhori G, Benfeitas R, Arif M, Liu Z and Edfors F: A
pathology atlas of the human cancer transcriptome. Science.
357:eaan25072017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Subramanian A, Tamayo P, Mootha VK,
Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub
TR, Lander ES and Mesirov JP: Gene set enrichment analysis: A
knowledge-based approach for interpreting genome-wide expression
profiles. Proc Natl Acad Sci USA. 102:15545–15550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mootha VK, Lindgren CM, Eriksson KF,
Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E,
Ridderstråle M, Laurila E, et al: PGC-1alpha-responsive genes
involved in oxidative phosphorylation are coordinately
downregulated in human diabetes. Nat Genet. 34:267–273. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang G, Bie F, Li G, Shi J, Zeng Y and Du
J: Study of the co-expression gene modules of non-small cell lung
cancer metastases. Cancer Biomark. Dec 3–2020.(Epub ahead of
print). doi: 10.3233/CBM-201605. View Article : Google Scholar
|
|
19
|
Li X, Liu Z, Mi M, Zhang C, Xiao Y, Liu X,
Wu G and Zhang L: Identification of hub genes and key pathways
associated with angioimmunoblastic T-cell lymphoma using weighted
gene co-expression network analysis. Cancer Manag Res.
11:5209–5220. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chan BKC: Data analysis using R
programming. Adv Exp Med Biol. 1082:47–122. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van Dam S, Vosa U, van der Graaf A, Franke
L and de Magalhães JP: Gene co-expression analysis for functional
classification and gene-disease predictions. Brief Bioinform.
19:575–592. 2018.PubMed/NCBI
|
|
22
|
Schwartzbaum JA, Fisher JL, Aldape KD and
Wrensch M: Epidemiology and molecular pathology of glioma. Nat Clin
Pract Neurol. 2:494–503. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Komori T: The 2016 WHO classification of
tumours of the central nervous system: The major points of
revision. Neurol Med Chir (Tokyo). 57:301–311. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen R, Smith-Cohn M, Cohen AL and Colman
H: Glioma subclassifications and their clinical significance.
Neurotherapeutics. 14:284–297. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Apte RS, Chen DS and Ferrara N: VEGF in
signaling and disease: Beyond discovery and development. Cell.
176:1248–1264. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yunker CK, Golembieski W, Lemke N, Schultz
CR, Cazacu S, Brodie C and Rempel SA: SPARC-Induced increase in
glioma matrix and decrease in vascularity are associated with
reduced VEGF expression and secretion. Int J Cancer. 122:2735–2743.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tu Y, Zhong Y, Fu J, Cao Y, Fu G, Tian X
and Wang B: Activation of JAK/STAT signal pathway predicts poor
prognosis of patients with gliomas. Med Oncol. 28:15–23. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang P, Chen FZ, Jia QB and Hu DF:
Upregulation of microRNA-133a and downregulation of connective
tissue growth factor suppress cell proliferation, migration, and
invasion in human glioma through the JAK/STAT signaling pathway.
IUBMB Life. 71:1857–1875. 2019. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kumar DM, Patil V, Ramachandran B, Nila
MV, Dharmalingam K and Somasundaram K: Temozolomide-Modulated
glioma proteome: Role of interleukin-1 receptor-associated kinase-4
(IRAK4) in chemosensitivity. Proteomics. 13:2113–2124. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sloan KE, Stewart JK, Treloar AF, Matthews
RT and Jay DG: CD155/PVR enhances glioma cell dispersal by
regulating adhesion signaling and focal adhesion dynamics. Cancer
Res. 65:10930–10937. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zheng Y, Miu Y, Yang X, Yang X and Zhu M:
CCR7 Mediates TGF-beta1-induced human malignant glioma invasion,
migration, and epithelial-mesenchymal transition by activating
MMP2/9 through the nuclear factor KappaB signaling pathway. DNA
Cell Biol. 36:853–861. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luo J, Solimini NL and Elledge SJ:
Principles of cancer therapy: Oncogene and non-oncogene addiction.
Cell. 136:823–837. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takada K, Kohashi K, Shimokawa M, Haro A,
Osoegawa A, Tagawa T, Seto T, Oda Y and Maehara Y: Co-Expression of
IDO1 and PD-L1 in lung squamous cell carcinoma: Potential targets
of novel combination therapy. Lung Cancer. 128:26–32. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guan X, Zhang C, Zhao J, Sun G, Song Q and
Jia W: CMTM6 overexpression is associated with molecular and
clinical characteristics of malignancy and predicts poor prognosis
in gliomas. EBioMedicine. 35:233–243. 2018. View Article : Google Scholar : PubMed/NCBI
|