Introduction
Pancreatic carcinoma is a malignant tumor of the
digestive tract, which is difficult to diagnose and treat.
Approximately 90% of pancreatic cancers are ductal adenocarcinomas
derived from ductal epithelium (1).
In recent years, its morbidity and mortality have significantly
increased. To make matters worse, the surgical mortality rate is
high, and the cure rate is markedly low (2). At present, the cause of pancreatic
cancer is not clear. It has been reported that chronic pancreatitis
and diabetes may be related to the occurrence of pancreatic cancer
(3). In addition, pancreatic cancer
is a highly malignant tumor with a markedly poor prognosis. The
one-year survival rate for pancreatic cancer patients is 8%, and
the five-year survival rate is 3%. The median survival time is only
2 to 3 months (4). Early diagnosis
and early treatment is the key to improving the prognosis of
pancreatic cancer patients. Therefore, it is urgent to explore new
targets to improve the survival rate of pancreatic cancer
patients.
Long noncoding RNAs (lncRNAs) have been reported to
regulate the tumorigenesis of numerous human cancers, including
pancreatic cancer. For example, lncRNA CASC2 suppressed the
metastasis of pancreatic carcinoma cells by downregulating miR-21
(5). DUXAP8 is a pseudogene derived
lncRNA that promotes the growth of pancreatic carcinoma cells
through epigenetic silencing of CDKN1A and KLF2 (6). In the present study, the role of lncRNA
TMPO antisense RNA 1 (TMPO-AS1) in pancreatic carcinoma was
investigated, and previous studies have not been reported. The
upregulation of lncRNA TMPO-AS1 has been detected in lung
adenocarcinoma and prostate cancer (7,8).
Functionally, lncRNA TMPO-AS1 was revealed to promote the
progression of non-small cell lung cancer by regulating its natural
antisense transcript TMPO (9). In
addition, lncRNA TMPO-AS1 promoted the occurrence of osteosarcoma
by regulating the miR-199a-5p/WNT7B axis (10). However, the regulatory mechanism of
lncRNA TMPO-AS1 remains unknown in pancreatic cancer. Therefore,
the present study aimed to clarify the function of lncRNA TMPO-AS1
in pancreatic cancer.
As is well known, competitive endogenous RNA (ceRNA)
competes with miRNA target genes by binding microRNA (miRNA).
lncRNAs can affect the expression of these target genes and
ultimately regulate tumorigenesis by acting as ceRNAs (11). In the present study, it was predicted
that miR-383-5p has a binding site with lncRNA TMPO-AS1. miR-383
has been demonstrated to suppress the development of pancreatic
carcinoma by inhibiting the expression of GAB1 (12). In addition, SRY-related high-mobility
group box 11 (SOX11) was predicted to be a potential target of
miR-383-5p. The important role of SOX11 in human diseases has been
reported. It has been revealed that SOX11 is associated with poor
clinicopathological characteristics of human prostate cancer
(13). miR-145 has been reported to
suppress the growth of endometrial cancer by targeting SOX11
(14). However, the relationship
between miR-383-5p and SOX11 in pancreatic cancer has not been
investigated.
Therefore, the present study clarified the
regulatory mechanism of the lncRNA TMPO-AS1/miR-383-5p/SOX11 axis.
Concurrently, the functions of lncRNA TMPO-AS1, miR-383-5p and
SOX11 were also investigated in pancreatic cancer. This research
will help us better understand the functional mechanism of lncRNA
TMPO-AS1 in pancreatic carcinoma.
Materials and methods
Clinical tissues
Thirty-eight patients with pancreatic carcinoma
(33–81 years old; 24 males and 14 females) diagnosed at Qingdao
Hospital of Traditional Chinese Medicine (Qingdao Hiser Hospital;
Qingdao, China) were involved in the present study from January
2017 and November 2019. The inclusion criteria comprised of
patients who were diagnosed with pancreatic carcinoma and had not
received chemotherapy or other treatments. The exclusion criteria
included patients with pancreatic carcinoma who had infections,
immune system diseases, chronic diseases or other types of cancer
concurrently, such as biliary tract or pulmonary infection,
rheumatoid arthritis, uremia and leukemia. All participants only
received surgery and provided written informed consents. The study
was approved by the Institutional Ethics Committee of Qingdao
Hospital of Traditional Chinese Medicine (Qingdao Hiser Hospital).
The pancreatic carcinoma tissues and adjacent non-tumor tissues
were frozen in liquid nitrogen and stored at −80°C for further
experiments.
Cells culture and transfection
Pancreatic duct epithelial cell line HPDE6-C7 and
pancreatic cancer cells SW1990, PANC-1 (ATCC) were grown in DMEM
(Gibco; Thermo Fisher Scientific, Inc.) containing 10% fetal bovine
serum (FBS; Invitrogen Thermo Fisher Scientific, Inc.). These cells
were incubated at 37°C in an atmosphere with 5% CO2.
The miR-383-5p mimic (5′-AGAUCAGAAGGUGAUUGUGGCU-3′)
and control miRNA mimic (mimic-NC, 5′-ACUACUGAGUGACAGUAGA-3′),
miR-383-5p inhibitor (5′-AGCCACAAUCACCUUCUGAUCU-3′) and control
miRNA inhibitor (inhibitor-NC, 5′-UUCUCCGAACGUGUCACGUTT−3′) were
purchased from Shanghai GenePharma Co., Ltd. The TMPO-AS1 or SOX11
siRNA (si-TMPO-AS1, 5′-AGGTAGAAACGCAGTTTAA-3′; si-SOX11,
5′-GCTAGGTTAGAATACATTTAA−3′) and non-silencing siRNA (si-NC,
5′-CAGUACUUUUGUGUAGUACAAA-3′) oligonucleotide were also purchased
from Shanghai GenePharma Co., Ltd. The cDNA encoding TMPO-AS1 was
sub-cloned into the vector pcDNA3.1 (the pcDNA-TMPO-AS1 primers
were used as follows: Forward, 5′-GGGGTACCGGGTTGGTGCGAGCTTCC-3′ and
reverse, 5′-CCGCTCGAGCACTGTTCAAATTTAACA−3′; Invitrogen; Thermo
Fisher Scientific, Inc.). The empty pcDNA3.1 vector (pcDNA-NC) was
used as a control. Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used for the transfection into SW1990 cells
for 48 h (37°C) following to the manufacturer's protocol. Following
transfection, the cells were cultured in a humidified incubator at
37°C and 5% CO2 for 48 h before the initiation of
further experiments.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from pancreatic cancer
tissues and cells using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). The cDNA was synthesized using PrimeScript RT
reagent kit according to the manufacturer's protocol (Takara
Biotechnology Co., Ltd.; reverse transcription primer:
5′-GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACAGCCAC−3′;
protocol: 30 min at 16°C, 30 min at 42°C, and 5 min at 85°C).
RT-qPCR was performed using SYBR-Green or TaqMan kit (TaKaRa,
Dalian, China) and primers. PCR was performed as follows: 25 cycles
of 10 min at 98°C, 10 sec at 98°C, 10 sec at 55°C and 20 sec at
72°C, with a final extension at 72°C for 5 min. The relative
expression of TMPO-AS1, miR-383-5p, SOX11 were quantified using the
2−∆∆cq method (15) and
they were normalized to GAPDH or U6. The primers used were:
TMPO-AS1 forward, 5′-AACCCAGCCCACACACTAC-3′ and reverse,
5′-GAATATGAGTGCCTGCAGAC-3′; miR-383-5p forward
5′-GGGAGATCAGAAGGTGATTGTGGCT-3′ and reverse,
5′-CAGTGCGTGTCGTGGAGT−3′; U6 forward,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′; SOX11 forward,
5′-GGTGGATAAGGATTTGGATTCG-3′ and reverse, 5′-GCTCCGGCGTGCAGTAGT−3′;
GAPDH forward, 5′-AGAAGGCTGGGGCTCATTTG-3′ and reverse,
5′-AGGGGCCATCCACAGTCTTC-3′.
Western blot analysis
Cells were lysed with RIPA cell lysis buffer
(Pierce; Thermo Fisher Scientific, Inc.). In brief, the BCA Protein
Assay Kit (Pierce; Thermo Fisher Scientific, Inc.) was used to
quantify the protein concentration. Equal amounts of protein
samples (30 µg) were loaded onto 10% SDS-PAGE gel and then
transferred onto PVDF membranes. After blocking with 5% skim milk
at room temperature for 1 h, the membranes were incubated with
SOX11 (rabbit polyclonal; dilution, 1:1,000; cat. no. ab170916) and
GAPDH (rabbit monoclonal, dilution, 1:1,000; cat. no. ab181602)
primary antibodies overnight at 4°C and then with the
HRP-conjugated secondary antibody (mouse anti-rabbit, dilution,
1:2,000; cat. no. 58802; New England BioLabs, Inc.) for 2–3 h at
room temperature. Finally, the images of the protein bands were
captured by a Tanon detection system using ECL reagent (Thermo
Fisher Scientific, Inc.).
MTT assay
Cell proliferation was measured by MTT assay.
Briefly, the transfected SW1990 cells (3×103 cells/well)
were seeded into 96-well plates. These cells were incubated at 37°C
in DMEM medium for 24, 48, 72 or 96 h, respectively. Then, the
cells were incubated at 37°C for 4 h with fresh medium containing
10% MTT. Finally, DMEM culture medium was discarded and 200 µl
dimethyl sulfoxide was then added to each well to completely
dissolve the crystals. The absorbance at 490 nm was detected by a
spectrophotometer (Bio-Rad Laboratories, Inc.).
Transwell assay
Transwell chambers (8 µm pore size; Corning, Inc.)
were used to assess migration and invasion. SW1990 transfected
cells (2×104) were seeded in the upper chamber and DMEM
medium with 10% fetal bovine serum was added in the lower chamber.
Next, these cells were incubated at 37°C with 5% CO2 for
18 h. Then the cells on the lower surface were fixed with 4%
paraformaldehyde for 20 min at room temperature and stained with
0.1% crystal violet overnight at 4°C after the cells that did not
migrate or invade were removed by a cotton swab. For the invasion
assay, the Transwell chambers were precoated with Matrigel (BD
Biosciences). Images were captured under an inverted microscope
(magnification, ×200; Olympus Corporation).
Bioinformatics analysis
To identify the putative miRNA target, we utilized
online miRNA target analysis tools TargetScan (http://www.targetscan.org/) and starBase version 2.0
(http://starbase.sysu.edu.cn/n) to
perform the prediction. The list of potential targets and binding
sites were available by searching the database.
Dual luciferase reporter assay
SW1990 cells were seeded in 24-well plates and
incubated for 24 h prior to transfection. The 3′-UTR of wild-type
or mutant TMPO-AS1 or SOX11 was inserted into the pmiR-GLO vector
(Promega Corporation). The vector was then transfected into SW1990
cells with miR-383-5p mimics or miR-NC using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). After 48 h, the
luciferase activity was measured by the dual-luciferase reporter
gene assay system (Promega Corporation). The firefly luciferase
activities were normalized to Renilla luciferase
activity.
Xenograft assays
All animal experiments were approved by the Ethics
Committee of Animal Experiments of Qingdao Hospital of Traditional
Chinese Medicine (Qingdao Hiser Hospital; Qingdao, China) (approval
no. ANI2019-56; approval date: March 6, 2019). SW1990 cells were
transfected with sh-TMPO-AS1 or empty vector (50 ng/well; Shanghai
GenePharma Co., Ltd.) using Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). Next, transfected SW1990 cells
(2×106 cells in 100 µl DMEM without FBS) were injected
subcutaneously into the upper right flank of four female nude mice
(4-week-old Balb/c/nu mice; 30 g; Changzhou Kaiwen Laboratory
Animal Co., Ltd.). The mice were maintained under specific
pathogen-free conditions and housed in ventilated cages with free
access to food and water. Tumor growth was examined every 3 days,
and the tumor volume (V) was determined by measuring the length (L)
and width (W) of the tumor with a caliper and calculated using the
formula V=(L × W2) × 0.5. Subsequently, 18 days later,
the tumors were removed and weighed. All animals were euthanized by
intraperitoneal injection of 1% sodium pentobarbital (150 mg/kg)
anesthetic. Animal death criteria included cardiac arrest,
continued absence of spontaneous breathing for 2–3 min, extreme
pupil dilation and no blink reflex in animals.
Statistical analysis
Data are expressed as the mean ± standard deviation
(SD) and analyzed by SPSS 19.0 (IBM Corp.) or Graphpad Prism 6
(Graphpad Software, Inc.). Paired Student's t-test was used for
comparisons between tumor and adjacent non-tumor samples of the
same individuals, while unpaired Student's t-test was used for
other comparisons between two groups. One-way ANOVA followed by
Bonferroni's multiple comparisons post hoc test was used to
calculate the difference between multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
lncRNA TMPO-AS1 aggravates
carcinogenic behaviors of pancreatic carcinoma
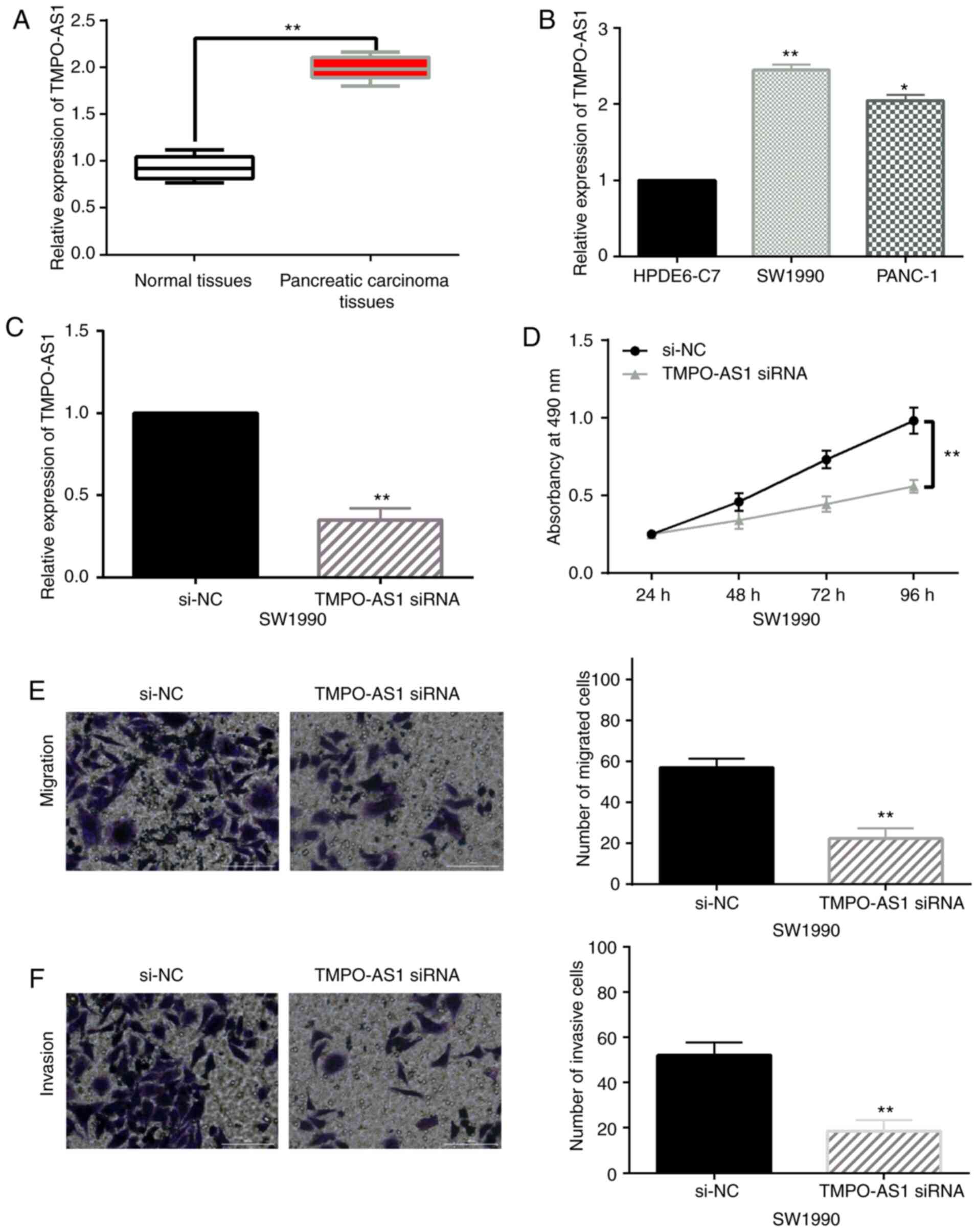
Compared with normal tissues, TMPO-AS1 expression
was upregulated in pancreatic carcinoma tissues (Fig. 1A). Similarly, the expression of
lncRNA TMPO-AS1 in SW1990 and PANC-1 pancreatic cancer cells was
higher than that in HPDE6-C7 cells (Fig.
1B). Based on the aforementioned results, SW1990 cells were
used to investigate the function of TMPO-AS1 in pancreatic
carcinoma due to the significant difference of TMPO-AS1 expression.
Next, TMPO-AS1 siRNA was transfected into SW1990 cells. The
transfection efficiency was detected by RT-qPCR (Fig. 1C). An MTT assay revealed that
downregulation of TMPO-AS1 inhibited cell proliferation in SW1990
cells (Fig. 1D). A Transwell assay
revealed that knockdown of TMPO-AS1 restrained cell migration and
invasion in SW1990 cells (Fig. 1E and
F). The aforementioned results indicated that the upregulation
of lncRNA TMPO-AS1 promoted cell proliferation and motility in
pancreatic carcinoma.
lncRNA TMPO-AS1 and miR-383-5p
expression levels are reciprocally inhibited in pancreatic
carcinoma
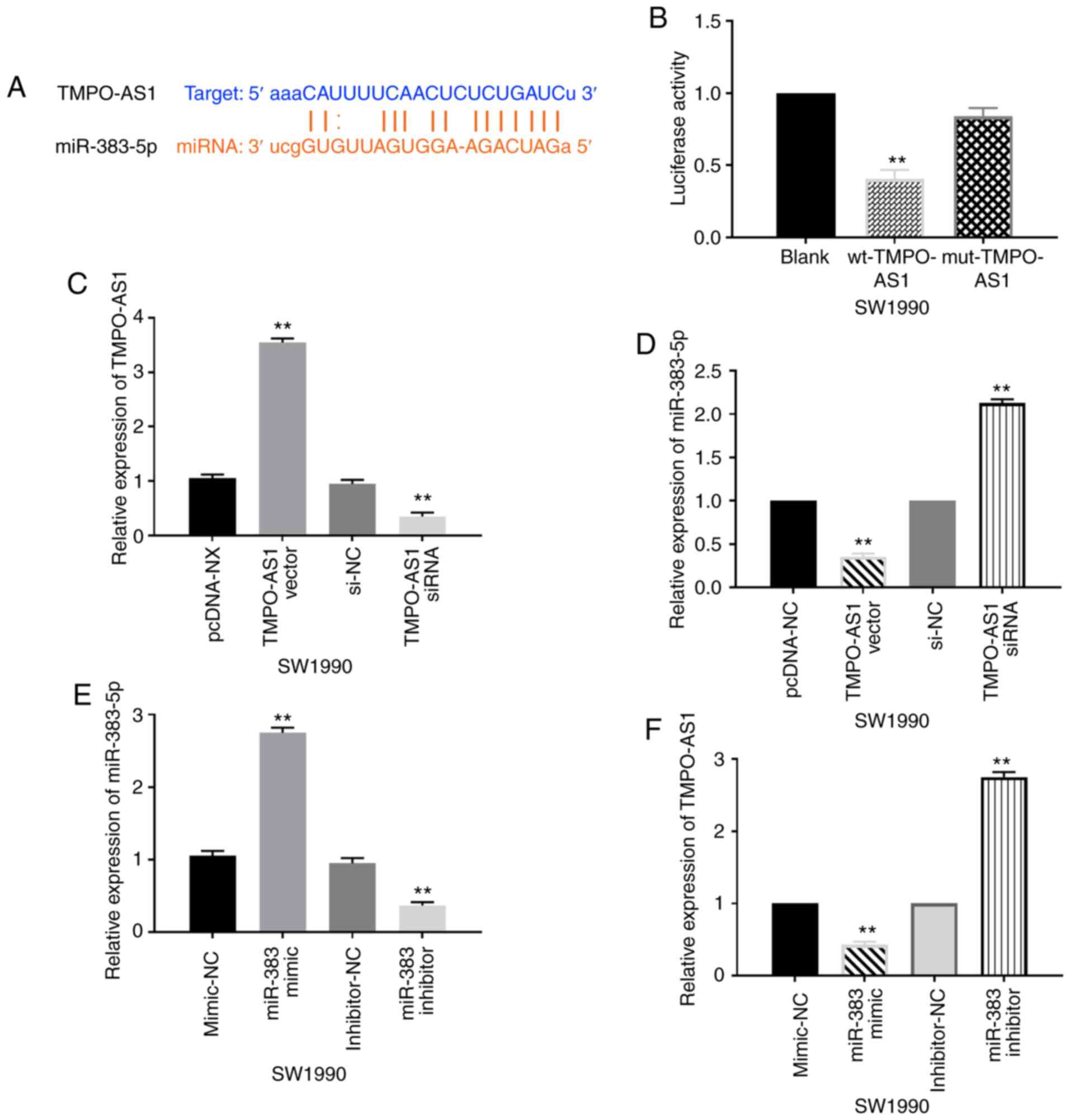
The starBase version 2.0 (http://starbase.sysu.edu.cn/n) predicted that lncRNA
TMPO-AS1 has a binding site with miR-383-5p (Fig. 2A). A dual luciferase reporter assay
revealed that miR-383-5p mimics reduced the luciferase activity of
wt-TMPO-AS1 and did not affect the luciferase activity of
mut-TMPO-AS1 in SW1990 cells (Fig.
2B). This indicated that lncRNA TMPO-AS1 directly targeted
miR-383-5p. Subsequently, the transfection efficiency of TMPO-AS1
vector and siRNA was detected in SW1990 cells by RT-qPCR (Fig. 2C). In addition, it was revealed that
the expression of miR-383-5p in SW1990 cells was downregulated by
the TMPO-AS1 vector and upregulated by TMPO-AS1 siRNA (Fig. 2D). Next, the transfection efficiency
of miR-383-5p mimics or inhibitor was detected in SW1990 cells by
RT-qPCR (Fig. 2E). Concurrently,
miR-383-5p mimics in SW1990 cells also reduced TMPO-AS1 expression,
while miR-383-5p inhibitor increased the expression of TMPO-AS1 in
SW1990 cells (Fig. 2F).
Collectively, the expression levels of lncRNA TMPO-AS1 and
miR-383-5p exhibited mutual inhibition in pancreatic carcinoma.
miR-383-5p restrains pancreatic
carcinoma cell proliferation and motility by interacting with
lncRNA TMPO-AS1
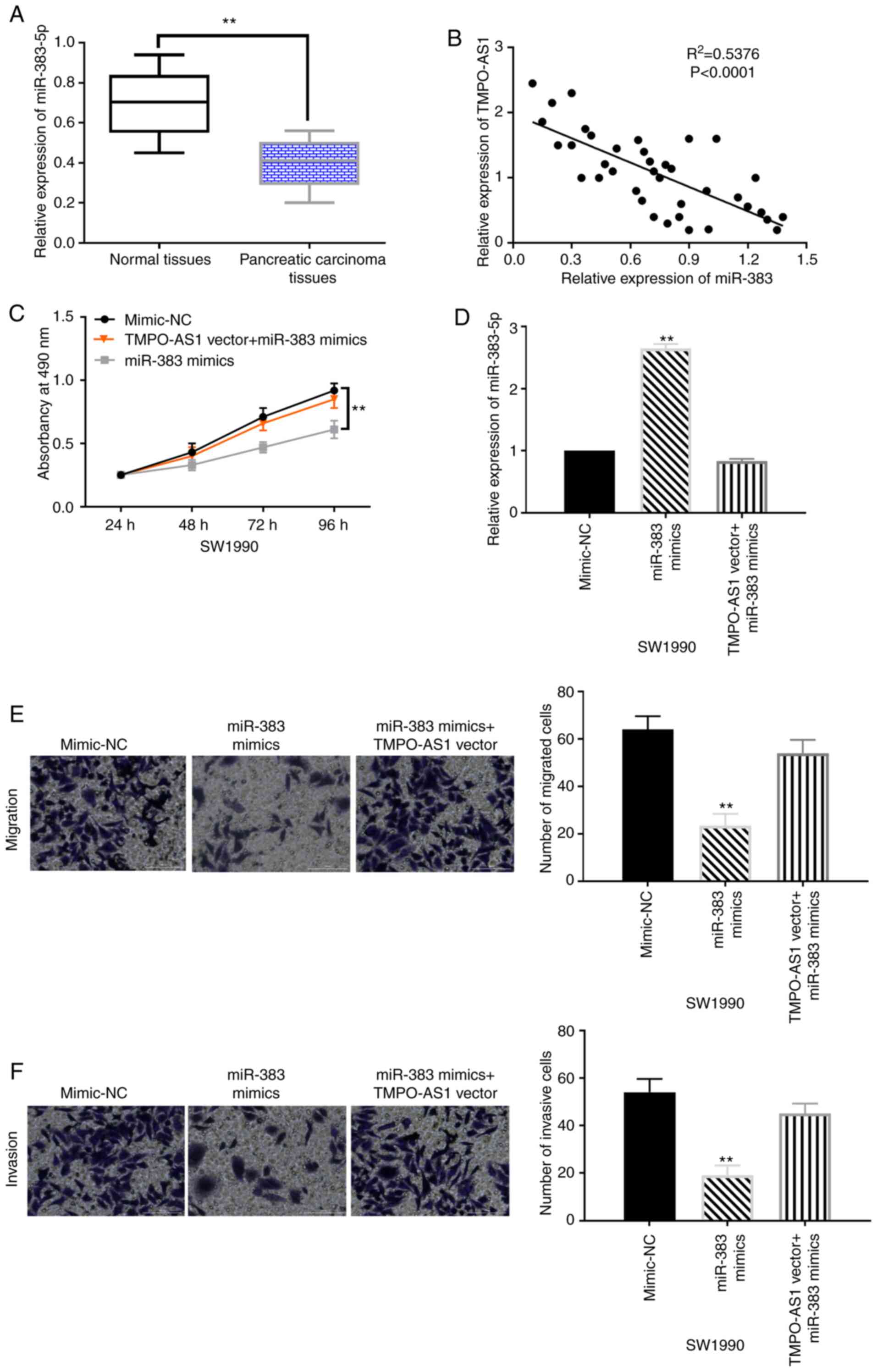
In addition, downregulation of miR-383-5p was
revealed in pancreatic carcinoma tissues compared with normal
tissues (Fig. 3A). A negative
correlation between miR-383-5p and TMPO-AS1 expression was revealed
in pancreatic carcinoma tissues (Fig.
3B). Then, the miR-383-5p mimics and lncRNA TMPO-AS1 vector
were transfected into SW1990 cells to explore the role of
miR-383-5p and its interaction with lncRNA TMPO-AS1. Functionally,
upregulation of TMPO-AS1 attenuated the inhibitory effect of
miR-383-5p on cell proliferation in SW1990 cells (Fig. 3C). The results also revealed that the
increased expression of miR-383-5p induced by its mimics was
reduced by the lncRNA TMPO-AS1 vector (Fig. 3D). The TMPO-AS1 vector also reversed
the inhibition of cell migration and invasion induced by miR-383-5p
overexpression in SW1990 cells (Fig. 3E
and F). These results indicated that overexpression of
miR-383-5p inhibited cell proliferation and motility in pancreatic
carcinoma. Moreover, the upregulation of TMPO-AS1 abolished the
antitumor effects of miR-383-5p in pancreatic carcinoma.
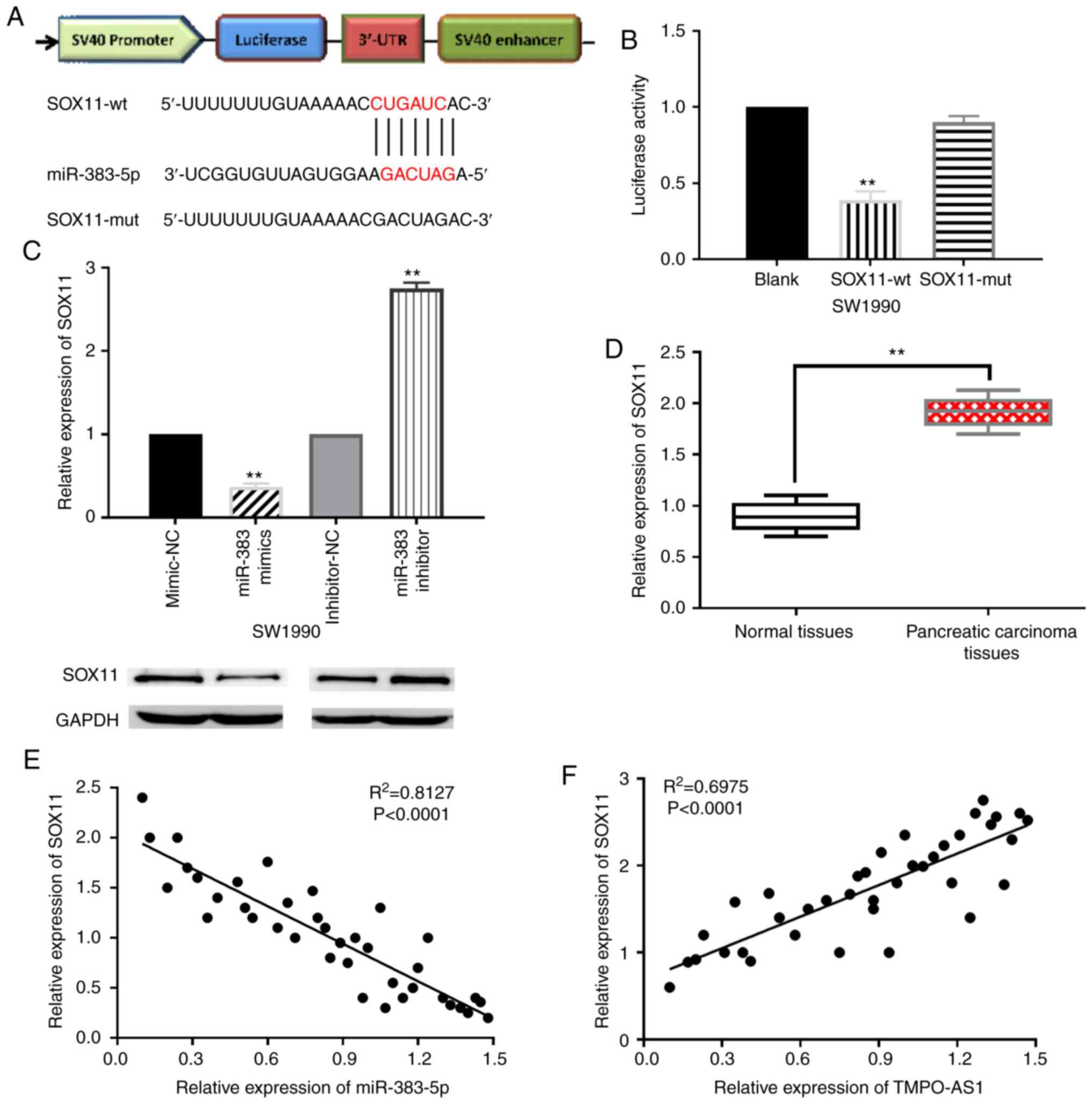
miR-383-5p directly targets SOX11
Next, potential targets of miR-383-5p were predicted
in the TargetScan database (http://www.targetscan.org). It was revealed that SOX11
has a binding site with miR-383-5p (Fig.
4A). It was also revealed that miR-383-5p mimics reduced the
luciferase activity of wt-SOX11, indicating that SOX11 is a direct
target of miR-383-5p (Fig. 4B).
RT-qPCR and western blotting revealed that miR-383-5p mimics
inhibited SOX11 expression, while miR-383-5p inhibitors promoted
SOX11 expression in SW1990 cells (Fig.
4C). In order to further confirm the relationship between SOX11
and miR-383-5p or TMPO-AS1, the expression level of SOX11 in
pancreatic carcinoma tissues was first detected. Compared with
normal tissues, SOX11 was revealed to be upregulated in pancreatic
carcinoma tissues (Fig. 4D).
Notably, miR-383-5p was revealed to negatively regulate SOX11
expression in pancreatic carcinoma tissues (Fig. 4E). Conversely, SOX11 expression was
positively correlated with TMPO-AS1 expression in pancreatic
carcinoma tissues (Fig. 4F).
Therefore, SOX11 was revealed to be a direct target of miR-383-5p
and TMPO-AS1 could positively regulate the expression of SOX11 in
pancreatic carcinoma.
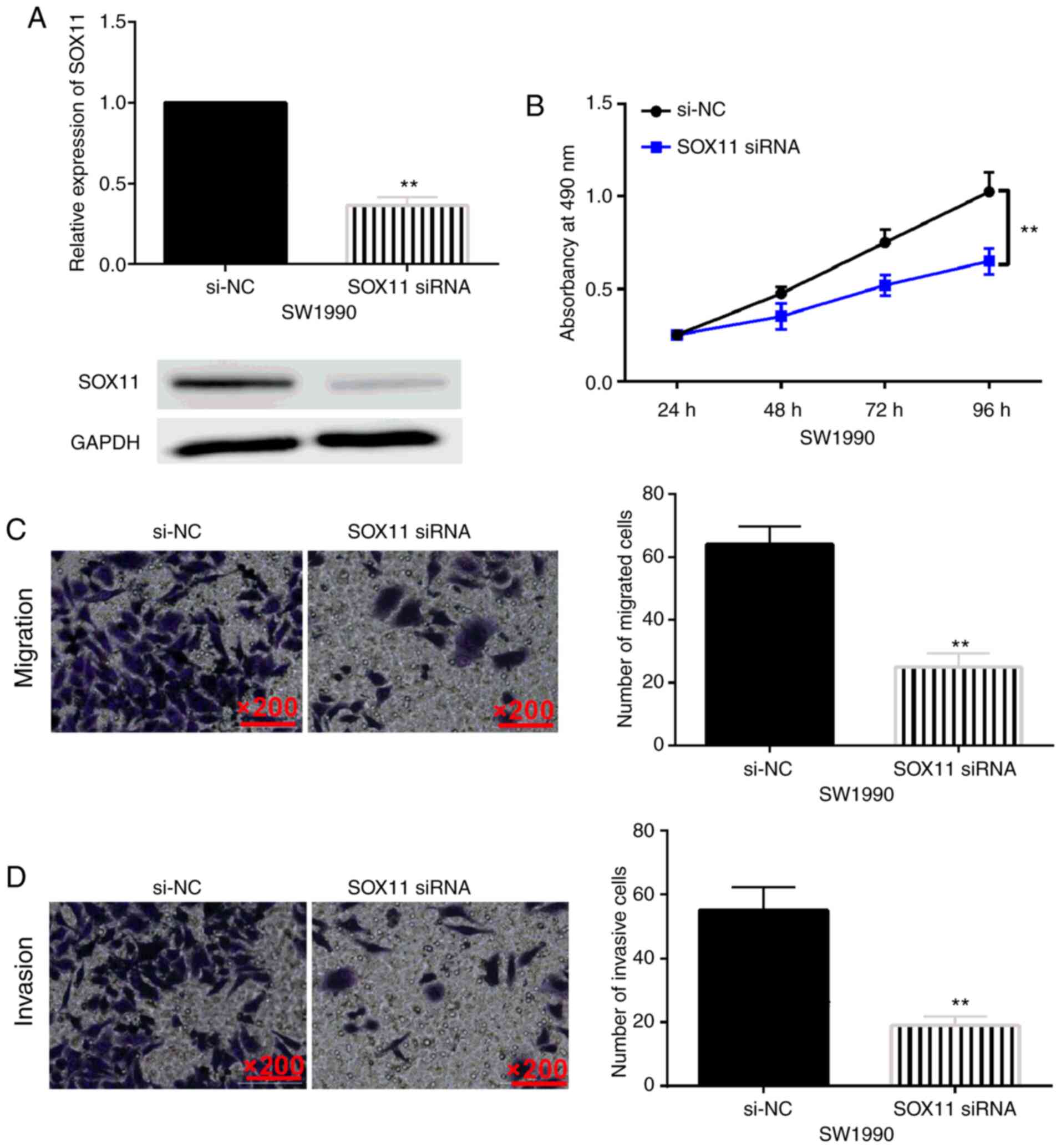
SOX11 is involved in the progression
of pancreatic carcinoma
To explore the role of SOX11 in pancreatic
carcinoma, SOX11 siRNA was transfected into SW1990 cells. RT-qPCR
and western blotting revealed that SOX11 siRNA reduced its
expression in SW1990 cells (Fig.
5A). In particular, it was determined that SOX11 downregulation
inhibited cell proliferation (Fig.
5B). In addition, knockdown of SOX11 also suppressed cell
migration and invasion in SW1990 cells (Fig. 5C and D). Collectively, SOX11 was
revealed to play a carcinogenic role in pancreatic carcinoma by
promoting cell proliferation, migration and invasion.
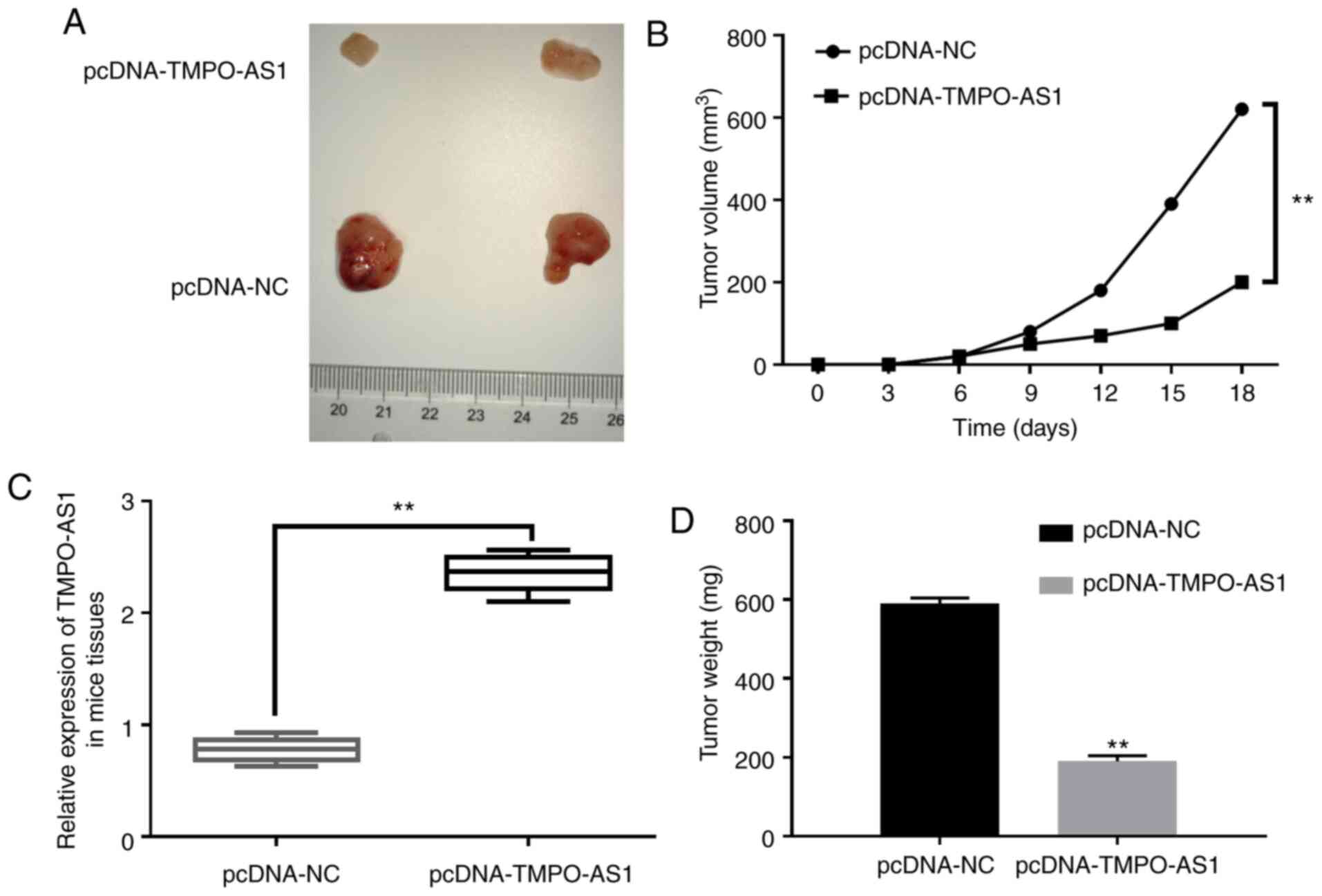
lncRNA TMPO-AS1 regulates pancreatic
carcinoma tumor growth in vivo
In order to verify whether TMPO-AS1 expression
regulates pancreatic carcinoma tumorigenesis in vivo,
xenograft analysis was performed. Consistent with previous in
vitro experiments, tumor growth in the pcDNA-TMPO-AS1 group was
significantly slower than the pcDNA-NC group (Fig. 6A and B). In addition, it was also
revealed that lncRNA TMPO-AS1 expression in the mice tissues of the
pcDNA-TMPO-AS1 group was higher than that in the pcDNA-NC group
(Fig. 6C). After nude mice were
sacrificed and tumors were removed, and the tumor weight in the
pcDNA-TMPO-AS1 group was revealed to be lower than that in the
pcDNA-NC group (Fig. 6D). These data
demonstrated that lncRNA TMPO-AS1 downregulation significantly
inhibited the growth of pancreatic carcinoma tumor xenograft.
Discussion
Recently, it has been discovered that an increasing
number of lncRNAs play important roles in pancreatic carcinoma,
including MACC1-AS1 and PVT1 (16,17). In
particular, the upregulation of lncRNA PANDAR has been identified
in pancreatic carcinoma and increased expression of lncRNA PANDAR
promoted cell proliferation and inhibited cell apoptosis in
pancreatic carcinoma (18). In the
present study, lncRNA TMPO-AS1 was also upregulated in pancreatic
cancer tissues and cells. The upregulation of TMPO-AS1 promoted
cell viability, migration and invasion in pancreatic carcinoma.
Notably, lncRNA TMPO-AS1 promoted the development of pancreatic
carcinoma by regulating the miR-383-5p/SOX11 axis. These results
indicated that lncRNA TMPO-AS1 acts as a tumor promoter in
pancreatic carcinoma.
Consistent with our results, high expression of
TMPO-AS1 was also detected in colorectal cancer and lung
adenocarcinoma (19,20). In addition, TMPO-AS1 was revealed to
promote the progression of cervical cancer by sponging miR-577 and
upregulating RAB14 (21). In the
present study, miR-383-5p was confirmed to act as a sponge for
lncRNA TMPO-AS1. The overexpression of miR-383-5p inhibited the
progression of pancreatic carcinoma. It has been reported that
miR-383 suppressed the development of pancreatic carcinoma
(12), similar to our results. In
addition, it was revealed that lncRNA TMPO-AS1 functioned as an
oncogene in pancreatic carcinoma by sponging miR-383-5p. Similarly,
LINC01128 accelerated the progression of cervical cancer by
regulating the miR-383-5p/SFN axis (22). In the present study, lncRNA TMPO-AS1
was revealed to promote the progression of pancreatic carcinoma by
mediating the miR-383-5p/SOX11 axis. Furthermore, SOX11 was
confirmed to be a direct target of miR-383-5p.
It has been demonstrated that SOX11 is overexpressed
in endometrial cancer and mantle cell lymphoma (23,24). In
the present study, SOX11 was also upregulated in pancreatic
carcinoma. In addition, knockdown of SOX11 inhibited cell
proliferation, migration and invasion in pancreatic carcinoma.
Similar to our study, it has been reported that the downregulation
of SOX11 hindered the proliferation, migration, and invasion of
thyroid tumor cells (25). It was
revealed that miR-383-5p restrained the progression of pancreatic
carcinoma by targeting SOX11. miR-145 also targeted SOX11 to
suppress the growth of endometrial cancer (14). In addition, circular RNA CEP128 was
proposed as a sponge of miR-145-5p, which promoted the progression
of bladder cancer by regulating SOX11 (26). In the present research, lncRNA
TMPO-AS1 was revealed to promote the development of pancreatic
carcinoma by sponging miR-383-5p and upregulating SOX11. However,
there is a limitation in the present study. Our results were only
investigated in SW1990 cells, and the results should be verified in
an additional cell line in the future.
In conclusion, the present study demonstrated that
lncRNA TMPO-AS1 has carcinogenic effects in pancreatic carcinoma.
In particular, upregulation of lncRNA TMPO-AS1 promoted cell
proliferation, migration and invasion in pancreatic carcinoma by
downregulating miR-383-5p and upregulating SOX11. This research may
provide a new direction for the treatment of pancreatic
carcinoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
FX and TZ designed the experiments. XS and SZ
conducted the experiments. MN, and YC performed the animal
experiments and prepared the figures. YW analyzed the data. FX and
TZ wrote and edited the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Institutional Ethics
Committee of Qingdao Hospital of Traditional Chinese Medicine
(Qingdao Hiser Hospital; Qingdao, China) (approval no. HUN2017-12;
approval date: January 5, 2017). All participants provided written
informed consents. All animal experiments were approved by the
Ethics Committee of Animal Experiments of Qingdao Hospital of
Traditional Chinese Medicine (Qingdao Hiser Hospital; Qingdao,
China) (approval no. ANI2019-56; approval date: March 6, 2019).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lin QJ, Yang F, Jin C and Fu DL: Current
status and progress of pancreatic cancer in China. World J
Gastroenterol. 21:7988–8003. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zeng Y: Advances in mechanism and
treatment strategy of cancer. Cell Mol Biol (Noisy-le-grand).
64:1–3. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
King J, Bouvet M, Singh G and Williams J:
Improving theranostics in pancreatic cancer. J Surg Oncol.
116:104–113. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wolfgang CL, Herman JM, Laheru DA, Klein
AP, Erdek MA, Fishman EK and Hruban RH: Recent progress in
pancreatic cancer. CA Cancer J Clin. 63:318–348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang H, Feng X, Zhang M, Liu A, Tian L,
Bo W, Wang H and Hu Y: Long non-coding RNA CASC2 upregulates PTEN
to suppress pancreatic carcinoma cell metastasis by downregulating
miR-21. Cancer Cell Int. 19:182019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lian Y, Yang J, Lian Y, Xiao C, Hu X and
Xu H: DUXAP8, a pseudogene derived lncRNA, promotes growth of
pancreatic carcinoma cells by epigenetically silencing CDKN1A and
KLF2. Cancer Commun (Lond). 38:642018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li DS, Ainiwaer JL, Sheyhiding I, Zhang Z
and Zhang LW: Identification of key long non-coding RNAs as
competing endogenous RNAs for miRNA-mRNA in lung adenocarcinoma.
Eur Rev Med Pharmacol Sci. 20:2285–2295. 2016.PubMed/NCBI
|
|
8
|
Huang W, Su X, Yan W, Kong Z, Wang D,
Huang Y, Zhai Q, Zhang X, Wu H, Li Y, et al: Overexpression of
AR-regulated lncRNA TMPO-AS1 correlates with tumor progression and
poor prognosis in prostate cancer. Prostate. 78:1248–1261. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qin Z, Zheng X and Fang Y: Long noncoding
RNA TMPO-AS1 promotes progression of non-small cell lung cancer
through regulating its natural antisense transcript TMPO. Biochem
Biophys Res Commun. 516:486–493. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cui H and Zhao J: lncRNA TMPO-AS1 serves
as a ceRNA to promote osteosarcoma tumorigenesis by regulating
miR-199a-5p/WNT7B axis. J Cell Biochem. 121:2284–2293. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kartha RV and Subramanian S: Competing
endogenous RNAs (ceRNAs): New entrants to the intricacies of gene
regulation. Front Genet. 5:82014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Su QL, Zhao HJ, Song CF, Zhao S, Tian ZS
and Zhou JJ: MicroRNA-383 suppresses pancreatic carcinoma
development via inhibition of GAB1 expression. Eur Rev Med
Pharmacol Sci. 23:10729–10739. 2019.PubMed/NCBI
|
|
13
|
Pugongchai A, Bychkov A and Sampatanukul
P: Promoter hypermethylation of SOX11 correlates with adverse
clinicopathological features of human prostate cancer. Int J Exp
Pathol. 98:341–346. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang L, Yuan Z, Shi H, Bian Y and Guo R:
miR-145 targets the SOX11 3′UTR to suppress endometrial cancer
growth. Am J Cancer Res. 7:2305–2317. 2017.PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qi C, Xiaofeng C, Dongen L, Liang Y,
Liping X, Yue H and Jianshuai J: Long non-coding RNA MACC1-AS1
promoted pancreatic carcinoma progression through activation of
PAX8/NOTCH1 signaling pathway. J Exp Clin Cancer Res. 38:3442019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao L, Kong H, Sun H, Chen Z, Chen B and
Zhou M: lncRNA-PVT1 promotes pancreatic cancer cells proliferation
and migration through acting as a molecular sponge to regulate
miR-448. J Cell Physiol. 233:4044–4055. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang Y, Feng E, Sun L, Jin W, You Y, Yao
Y and Xu Y: An increased expression of long non-coding RNA PANDAR
promotes cell proliferation and inhibits cell apoptosis in
pancreatic ductal adenocarcinoma. Biomed Pharmacother. 95:685–691.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mohammadrezakhani H, Baradaran B,
Shanehbandi D, Asadi M, Hashemzadeh S, Hajiasgharzadeh K and
Safaralizadeh R: Overexpression and clinicopathological correlation
of long noncoding RNA TMPO-AS1 in colorectal cancer patients. J
Gastrointest Cancer. 51:952–956. 2019. View Article : Google Scholar
|
|
20
|
Wang Y, Fu J, Wang Z, Lv Z, Fan Z and Lei
T: Screening key lncRNAs for human lung adenocarcinoma based on
machine learning and weighted gene co-expression network analysis.
Cancer Biomark. 25:313–324. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang J, Liang B and Hou S: TMPO-AS1
promotes cervical cancer progression by upregulating RAB14 via
sponging miR-577. J Gene Med. 21:e31252019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu Y, Ma Y, Liu J, Cai Y, Zhang M and Fang
X: LINC01128 expedites cervical cancer progression by regulating
miR-383-5p/SFN axis. BMC Cancer. 19:11572019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shan T, Uyar DS, Wang LS, Mutch DG, Huang
TH, Rader JS, Sheng X and Huang YW: SOX11 hypermethylation as a
tumor biomarker in endometrial cancer. Biochimie. 162:8–14. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu B, Li J, Wang H, Wu Q and Liu H:
MiR-132-3p serves as a tumor suppressor in mantle cell lymphoma via
directly targeting SOX11. Naunyn Schmiedebergs Arch Pharmacol.
393:2197–2208. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang L, Shen YF, Shi ZM, Shang XJ, Jin DL
and Xi F: Overexpression miR-211-5p hinders the proliferation,
migration, and invasion of thyroid tumor cells by downregulating
SOX11. J Clin Lab Anal. 32:e222932018. View Article : Google Scholar
|
|
26
|
Wu Z, Huang W, Wang X, Wang T, Chen Y,
Chen B, Liu R, Bai P and Xing J: Circular RNA CEP128 acts as a
sponge of miR-145-5p in promoting the bladder cancer progression
via regulating SOX11. Mol Med. 24:402018. View Article : Google Scholar : PubMed/NCBI
|