Introduction
Liver cancer ranks in the top 10 most common
malignancies for both mortality rate and incidence (1). In 2018, there were 841,080 new cases of
liver cases (4.7% of all new cancer cases) and caused 781,631
deaths (8.2% of all cancer-associated deaths) worldwide (2). The number of deaths is close to the
number of new cases. Hepatocellular carcinoma (HCC) is the most
common subtype of liver cancer (3).
Hepatitis B virus (HBV) or C virus (HCV) infections, as well as
alcohol abuse, are major risk factors for HCC (4). With the improved understanding of HBV
and HCV infections, the molecular pathogenesis of HCC has also been
extensively studied (5,6). However, the precise molecular pathways
underlying HCC development have not been fully elucidated.
The majority of studies on the molecular
pathogenesis of HCC focus on the regulation of protein-coding genes
(7–9), while the roles of most non-coding
(nc)RNAs, such as micro (mi)RNAs (miRs) and long non-coding
(lnc)RNAs, remain unclear in HCC. Although ncRNAs do not encode
proteins, they participate in the regulation of gene expression at
multiple levels to promote or suppress cancer development (10–12).
Therefore, functional characterization of critical ncRNAs in cancer
biology provides novel insights into the development of novel
targeted therapies (13). In a
recent study, lncRNA GABPB1 intronic transcript 1 (IT1) was
reported to be downregulated in non-small cell lung cancer (NSCLC)
and predicts poor survival, indicating its potential tumor
suppressive role (14). However, the
involvement of GABPB1-IT1 in other cancer types remains unknown.
miR-93, whose sequence is located in the intron of the MCM7
gene at chromosome 7q22.1, has been found to be an oncogenic micro
RNA in several cancer types, such as liver and bladder cancer
(15,16). Moreover, it has been reported that
miR-93 promotes the proliferation and invasion of bladder cancer
cells by targeting pigment epithelium-derived factor (PEDF), a
glycoprotein from the family of non-inhibitory serpins (16). Considering that the interaction
between GABPB1-IT1 and miR-93 was predicted by IntaRNA 2.0, the
present study aimed to investigate the role of GABPB1-IT1 in HCC,
and whether it functions by regulating miR-93/PEDF axis.
Materials and methods
Patients with HCC and follow-up
The present study was approved by The Ethics
Committee of 96604 Military Hospital of the Chinese People's
Liberation Army (Lanzhou, China). A total of 64 patients with HCC
(40 males and 24 females; age range, 43–67 years; mean age ±
SEM=55.1±5.1 years) were enrolled between May 2012 and May 2014.
All patients were subjected to histopathological examination to
confirm HCC. No previous history or family history of malignancies
were observed and patients with other clinical disorders were
excluded. No therapy was initiated prior to the study. All patients
provided informed written consent. Biopsies were performed on all
patients using fine needle to collect paired HCC and adjacent (3-cm
away from tumor) tissue samples from each patient. Tissue samples
were frozen in liquid nitrogen and stored at −80°C before use.
There were 12, 14, 17 and 21 cases at American Joint Committee on
Cancer stage I, II, III and IV, respectively (17). From the day of admission, all
patients were followed-up for 5 years in a monthly manner through
telephone to record their survival. The survival of patients was
recorded and all patients completed the follow-up. Overall survival
was calculated from the day of diagnosis to the day of death or
last follow-up.
HCC cells and transfection
Human HCC cell lines SNU-398 and C3A (American Type
Culture Collection) were used. SNU-398 and C3A cells were
cultivated in a cell culture medium composed of 10% FBS and 90%
RPMI-1640 (both Gibco; Thermo Fisher Scientific, Inc.). Cell
culture conditions were 5% CO2 at 37°C and 95% humidity.
Cells were collected at ~80% confluence to perform subsequent
transfections. The backbone to construct the expression vectors of
GABPB1-IT1 and pigment epithelium-derived factor (PEDF). miR-93
mimic and negative control (NC) miRNA (scrambled) were purchased
from Sigma-Aldrich; Merck KGaA. Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to transfect
miRNA (45 nM) or vector (12 nM) into SNU-398 cells with a cell
density of 106 cells/ml at 37°C for 24 h. Untransfected
cells were used as control (C) cells. NC cells were cells
transfected with empty pcDNA 3.1 vector or NC miRNA. Subsequent
experiments were carried out using cells collected 48 h after
transfection at room temperature. IntaRNA 2.0 predicted that
GABPB1-IT1 could bind to the 3′UTR region of miR-93 and then a dual
luciferase activity assay was performed to verify their
interaction. The GABPB1-IT1 luciferase reporter vector was
constructed using the pGL3 vector (Promega Corporation). SNU-398
cells were co-transfected with miR-93 mimic + GABPB1-IT1 vector
(miR-93 group) or NC miRNA + GABPB1-IT1 vector (NC miRNA group)
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.). In addition, SNU-398 cells were also co-transfected with
miRNA and GABPB1-IT1 inhibitor (Beyotime Institute of Technology)
using Lipofectamine 2000. Luciferase activity was measured 48 h
post-transfection by a Dual-Luciferase Reporter Assay kit (Promega
Corporation). The firefly luciferase activity was normalized to the
Renilla luciferase activity.
RNA extraction and reverse
transcription-quantitative (RT-q)PCR assays
Ribozol (Sigma-Aldrich; Merck KGaA) was used to
isolate total RNAs from tissues and cells. miRNAs were harvested by
precipitating RNAs with 85% ethanol. RNA concentrations were
measured using NanoDrop 2000 (Thermo Fisher Scientific. Inc.) and
genomic DNAs were digested using gDNA eraser (Takara Bio, Inc.).
RNA samples with an OD 260/280 ratio ~2.0 (pure RNA) were reverse
transcribed using the PrimeScript RT Reagent kit (Takara Bio,
Inc.). The detailed workflow was as follows: 37°C For 15 min and
85°C for 5 sec then maintained at 4°C. Then the qPCR assays
performed using SYBR-Green RT-PCR kit (Vazyme Biotech Co., Ltd.) to
measure the expression levels of GABPB1-IT1 and PEDF mRNA. The
detailed workflow was as follows: 95°C For 10 min, 40 cycles of
95°C for 15 sec and 60°C for 45 sec, final annealing at 72°C for 10
min. The expression levels of mature miR-93 were measured using the
All-in-One™ miRNA RT-qPCR Detection kit (GeneCopoeia, Inc.). U6 and
GAPDH were used as the endogenous controls of miR-93 and PEDF,
respectively. PCR reactions were performed in triplicate and Cq
values were processed using the Δ-Δq (ΔΔCq) method (18).
Western-blot assay
Total proteins were isolated from SNU-398 cells
using RIPA lysis buffer (Invitrogen; Thermo Fisher Scientific,
Inc.). Protein concentrations were measured using a BCA assay
(Sigma-Aldrich; Merck KGaA). All protein samples were denatured at
95°C for 10 min, followed by loading 25 µg protein/lane onto 8%
gels and resolving using SDS-PAGE. Then these samples were
transferred to the polyvinylidene fluoride (PVDF) membranes. All
membranes were blocked with PBS containing 5% non-fat milk at room
temperature for 2 h. Primary rabbit antibody of GAPDH (1:1,000;
cat. no. ab9485) or PTEN (1:500; cat. no. ab14993) (both Abcam) was
used to incubate with the membranes at 4°C for 12 h, followed by
incubation with HRP Goat Anti-Rabbit (IgG) (1:1,000; ab97051;
Abcam) secondary antibody at room temperature for 2 h. Enhanced
chemiluminescence kit (Sigma-Aldrich; Merck KGaA) was used to
visualize the signals, which were quantified by Quantity One
software version 4.6 (Bio-Rad Laboratories, Inc.).
Cell proliferation assay
A Cell counting Kit (CCK)-8 (Dojindo Molecular
Technologies, Inc.) was used to investigate the effect of
transfection on the proliferation of SNU-398 and C3A cells. Each
well of a 96-well plate was seeded with 3,000 cells in 0.1 ml cell
culture medium, followed by cell culture under the aforementioned
conditions. Cells were collected every 24 h for 4 days. At 4 h
before cell collection, 10 µl of CCK-8 solution was added to each
well. OD values were measured at 450 nM using a microplate
reader.
In vivo animal study
In total, 20 female nude mice (4–6 weeks old, 20–22
g) were used. These mice were raised in a specific pathogen-free
room under standardized housing conditions (temperature 22–25°C,
relative humidity 40–50%, 12 h artificial light daily) with free
access to food and water. Mice were observed daily and humanely
euthanized by injection of sodium pentobarbital with humane
endpoint criteria as previously described, including severe tumor
burden (>20 mm in diameter), labored breathing, prostration,
unresponsive to external stimuli, obviously body weight loss and
body temperature drop (19,20). For investigating the role of
GABPB1-IT1, 106 C3A cells stably overexpressing
GABPB1-IT1 were suspended in mixture of PBS and Matrigel (1:1) and
subcutaneously injected into the flank of nude mice. Tumor sizes
were measured every week. The tumor size was measured using a
Vernier caliper using the formula V=1/2 (LxW2), where L
represents the length (longest dimension), and W represents the
width (shortest dimension). After 6 weeks, all mice were euthanized
by injection of sodium pentobarbital (100 mg/kg) followed by
cervical dislocation. Finally, the xenograft tissues were used for
qPCR to analyze the expression of GABPB1-IT1. The animal
experiments were approved by The Ethics Committee of 96604 Military
Hospital of the Chinese People's Liberation Army (Lanzhou, China;
approval no. 202065433).
Statistical analysis
Data from three biological replicates were expressed
as mean values ± SEM, unless otherwise shown. Unpaired t-tests were
used to compare differences between two groups. Paired t-tests were
used to compare HCC and adjacent normal tissues. ANOVA (one-way)
and Tukey's post hoc test were used to compare differences among
multiple groups. With the median expression levels of GABPB1-IT1
(1.96) in HCC tissues as cut-off value, the 64 patients were
grouped into high and low GABPB1-IT1 level groups (both n=32).
Survival curves were plotted for both groups and the log-rank test
was used to compare survival curves. Linear regression was used for
correlation analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
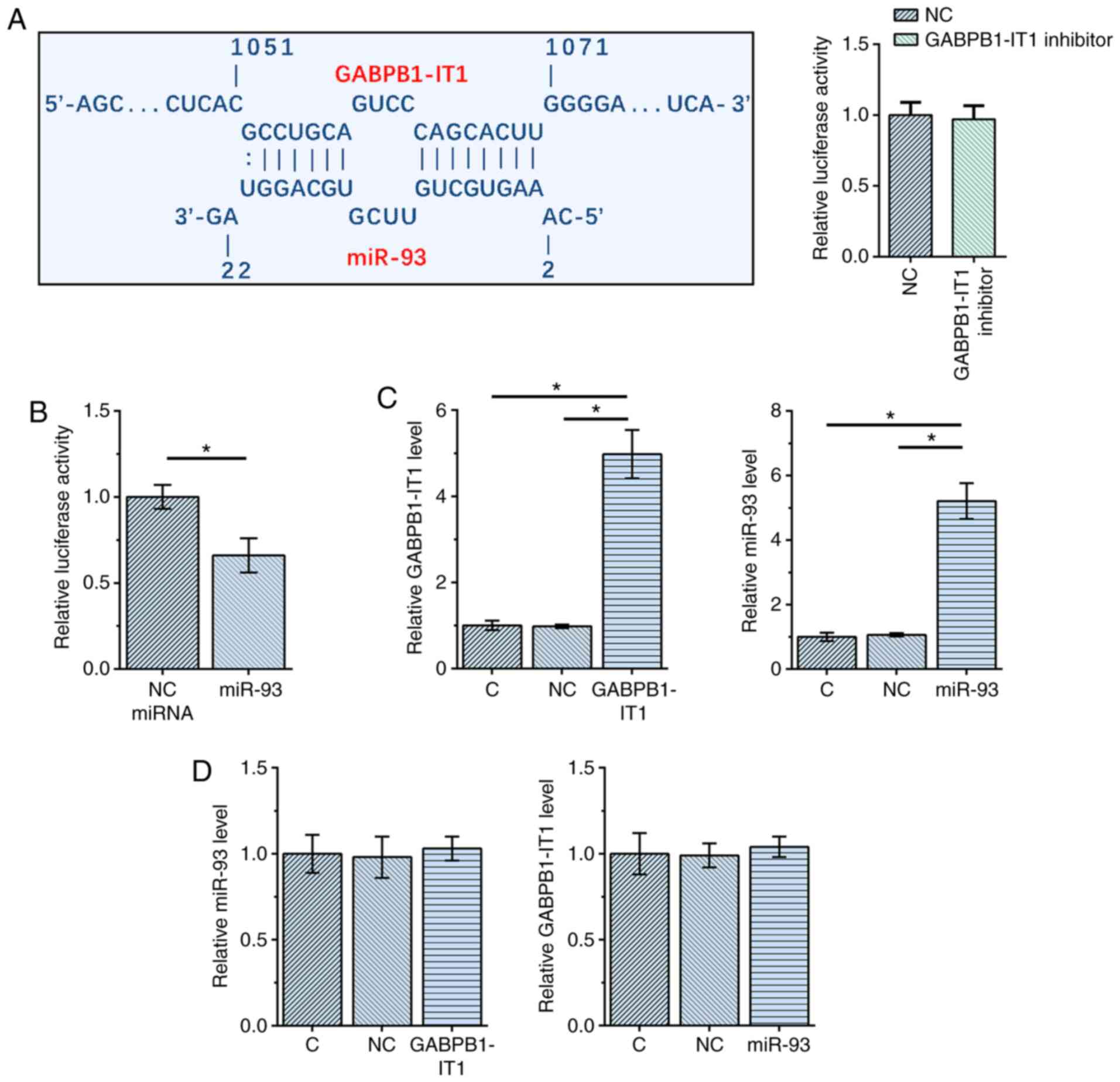
GABPB1-IT1 and miR-93 interact with
each other but do not regulate the expression of each other
The interaction between GABPB1-IT1 and miR-93 was
first predicted using IntaRNA 2.0 (21). It was observed that GABPB1-IT1 and
miR-93 could form multiple base pairs. The relative activity of
luciferase was not significantly changed by co-transfection of
miRNA and GABPB1-IT1 inhibitor (Fig.
1A). A dual luciferase activity assay was performed by
co-transfecting SNU-398 cells with miR-93 mimic + GABPB1-IT1 vector
(miR-93 group) or NC miRNA + GABPB1-IT1 vector (NC miRNA group).
Compared with NC miRNA group, the relative luciferase activity was
significantly lower in miR-93 group (P<0.05; Fig. 1B), indicating direct interaction.
SNU-398 cells were transfected with GABPB1-IT1 expression vector or
miR-93 mimic, followed by confirmation of the overexpression of
GABPB1-IT1 and miR-93 using RT-qPCR at 48 h post-transfection (both
P<0.05 vs. NC and C; Fig. 1C).
Notably, overexpression of GABPB1-IT1 and miR-93 did not affect the
expression of each other (Fig. 1D).
Taken together, our data suggest that GABPB1-IT1 and miR-93
interact with each other but do not regulate the expression of each
other.
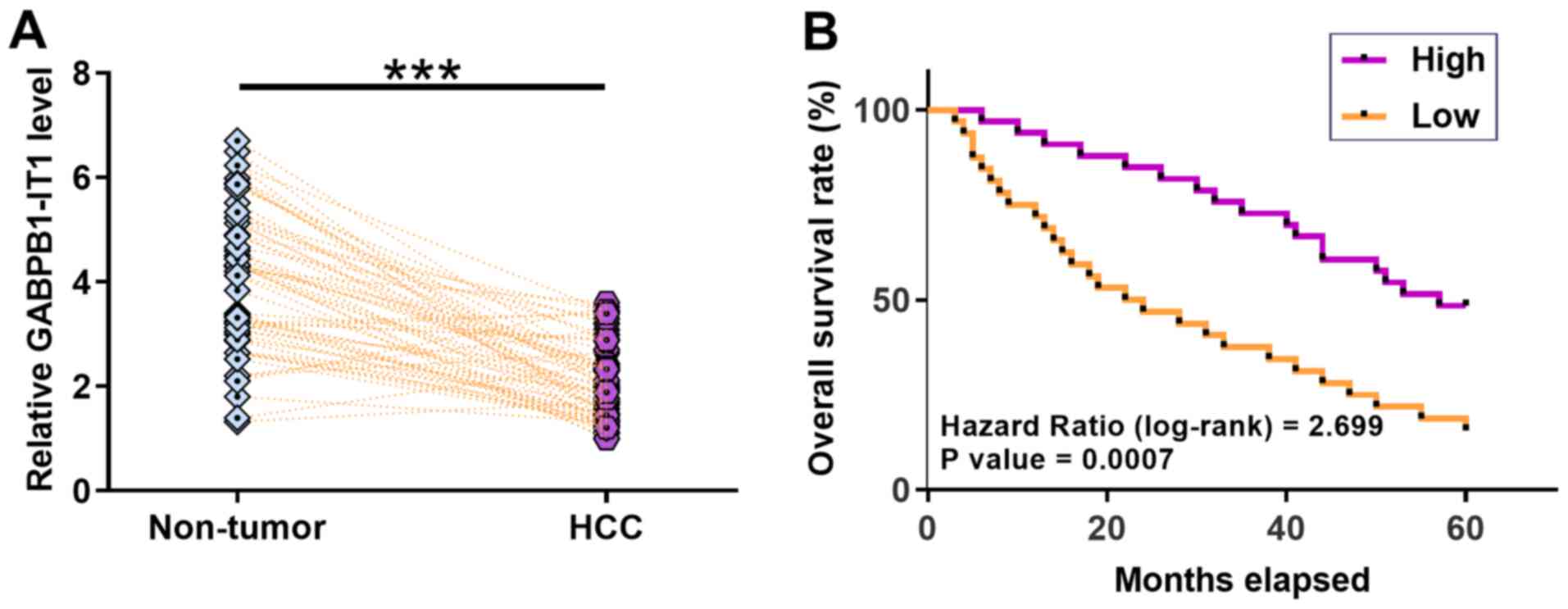
Downregulation of GABPB1-IT1 predicts
poor survival for patients with HCC
According to patients' medical records, 23 patients
were positive for HBV only, 18 were positive for HCV only, eight
were HCV and HBV positive and 15 were HCV and HBV negative. The
expression levels of GABPB1-IT1 in paired HCC and non-tumor tissues
from 64 patients with HCC were measured using RT-qPCR. It was
observed that GABPB1-IT1 was significantly downregulated in HCC
tissues compared with that in non-tumor tissues (P<0.001;
Fig. 2A). Survival curve analysis
showed that patients in the low GABPB1-IT1 level group had a lower
overall survival rate during the 5-year follow-up compared with
patients in the high GABPB1-IT1 level group (P=0.0007; Fig. 2B). miR-93 was significantly
upregulated in HCC (Fig. S1A), and
PEDF was significantly downregulated in HCC (Fig. 1SB) compared with in non-tumor tissues
(both P<0.001). No significant differences in the expression
levels of GABPB1-IT1 were observed among patients at different
clinical stages (Fig. S2). Linear
regression analysis showed that the expression of GABPB1-IT1 and
miR-93 were not significantly correlated with each other in HCC
tissues (R2=0.02 and P=0.223), while the expression of
GABPB1-I T1 and PEDF were positively correlated (R2=0.41
and P<0.001), and miR-93 and PEDF (R2=0.67 and
P<0.001) were negatively correlated in HCC tissues (Fig. S3). Taken together, our data suggest
that decreased GABPB1-IT1 predicts a poor survival for patients
with HCC.
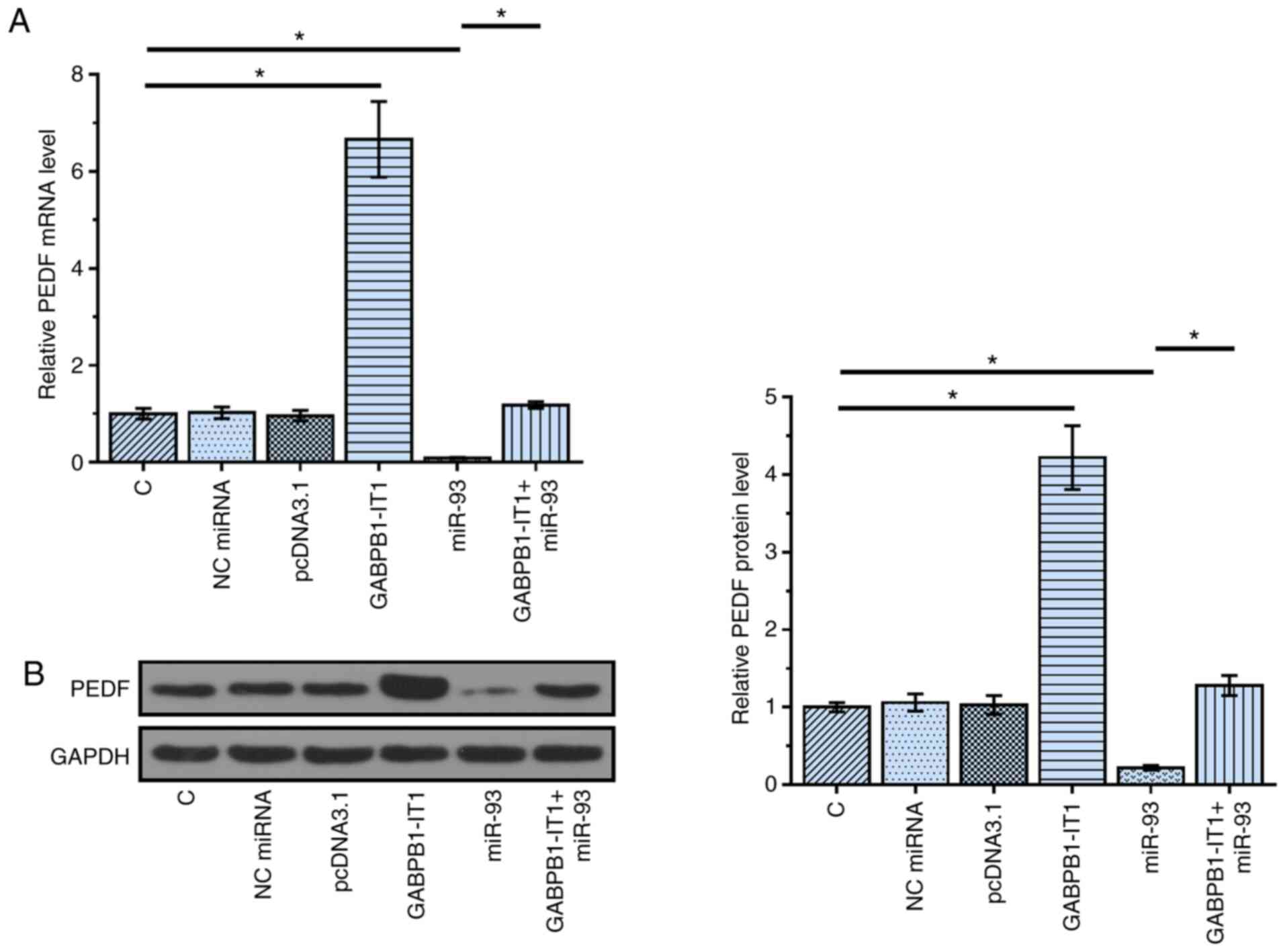
Overexpression of GABPB1-IT1
upregulates PEDF
PEDF is a direct target of miR-93 (16). The function of miRNA sponges is to
suppress the function of miRNAs, such as targeting downstream genes
(22). To test the possibility that
GABPB1-IT1 can sponge miR-93, the effects of overexpression of
GABPB1-IT1 and miR-93 on the expression of PEDF were analyzed using
RT-qPCR (Fig. 3A) and western
blotting (Fig. 3B). Compared with
the C group, overexpression of miR-93 resulted in the
downregulation of PEDF (P<0.05). In contrast, compared with the
C group, overexpression of GABPB1-IT1 in GABPB1-IT1 group led to
the upregulation of PEDF (P<0.05). Compared with the miR-93
group, overexpression of GABPB1-IT1 in GABPB1-IT1+miR-93 group
reduced the effects of miR-93 on the expression of PEDF
(P<0.05). Taken together, our data suggest that GABPB1-IT1 may
sponge miR-93 to up-regulate PEDF.
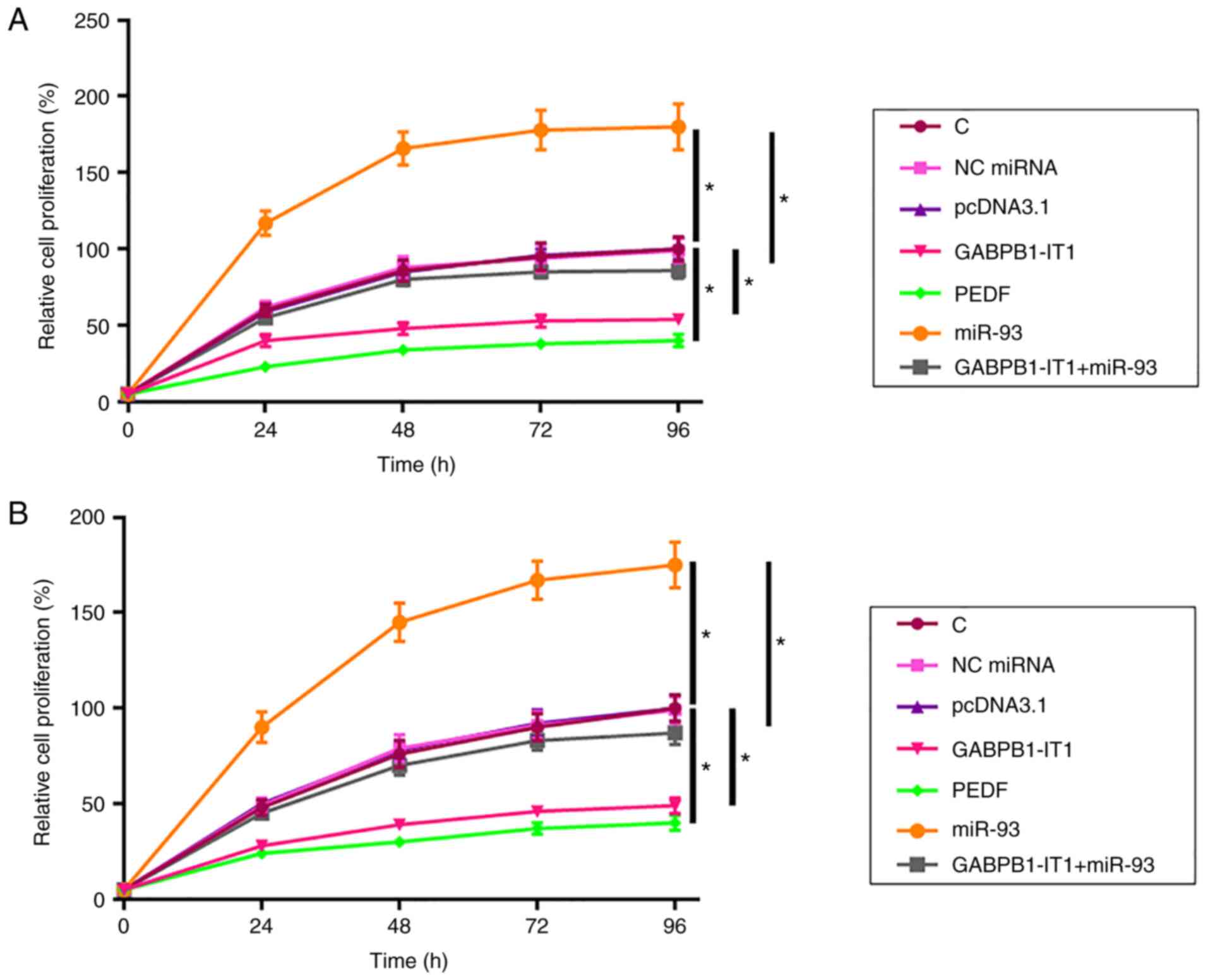
GABPB1-IT1 suppresses the
proliferation of SNU-398 and C3A cells through the miR-93/PEDF
axis
The effects of transfection on the proliferation of
SNU-398 cells were analyzed using the CCK-8 assay. Compared with
the C group, overexpression of GABPB1-IT1 and PEDF led to decreased
proliferation rate of SNU-398 cells (both P<0.05; Fig. 4A). In addition, overexpression of
GABPB1-IT1 led to decreased miR-93-mediated promotion of HCC cell
proliferation (P<0.05). C3A cells were used to repeat the cell
proliferation assay and similar results were obtained (all
P<0.05; Fig. 4B). Taken together,
our data suggest that GABPB1-IT1 inhibited HCC cell proliferation
by regulating miR-93/PEDF axis.
Overexpression of GABPB1-IT1 inhibits
HCC tumor growth in vivo
The effect of GABPB1-IT1 on tumorigenesis in a
xenograft model was examined. C3A cells stably overexpressing
GABPB1-IT1 were subcutaneously injected into the flank of the nude
mice. Tumor sizes were measured every week. As shown Fig. 5A, GABPB1-IT1 was significantly
downregulated in the xenografts formed by the overexpression of
GABPB1-IT1 transfected cells (P<0.01). As shown in Fig. 5B and C, the xenograft formed by
overexpression of GABPB1-IT1 had a significantly smaller tumor
volume compared with that in the control group (P<0.05). As
shown in Fig. 5D, compared with
control group, no significant change in bodyweight was observed in
mice injected with C3A cells stably overexpressing GABPB1-IT1
compared with the C group. Taken together, our data suggest that
GABPB1-IT1 overexpression represses HCC tumor growth in
vivo.
Discussion
The present study investigated the role of
GABPB1-IT1 in HCC. It was demonstrated that downregulation of
GABPB1-IT1 in HCC was associated with poor survival. In addition,
GABPB1-IT1 may upregulate PEDF by sponging miR-93 to suppress
cancer cell proliferation.
A recent study reported that GABPB1-IT1 was
downregulated in NSCLC and predicted poor survival (14). However, the function of GABPB1-IT1 in
cancer biology, such as the regulation of cancer cell behaviors,
remains unknown. The current study demonstrated the downregulation
of GABPB1-IT1 in HCC. In addition, it was reported that GABPB1-IT1
inhibited the proliferation of HCC cells. Therefore, GABPB1-IT1 is
likely a tumor suppressor in HCC.
Due to the lack of early diagnostic markers, most
patients with HCC are diagnosed at advanced stages, and the overall
survival rate is generally poor (23,24). For
example, the 5-year overall survival rate of HCC is only 30–40%
(25). The present study showed that
the low expression levels of GABPB1-IT1 in HCC tissues before
therapy could be used to predict the survival of patients with HCC
patients. Therefore, GABPB1-IT1 may serve as a prognostic marker
for HCC to improve survival by guiding treatment decisions and the
development of personalized care programs. However, the accuracy
GABPB1-IT1 as a prognostic remains to be further confirmed.
It has been reported that miR-93 can target PEDF to
promote the proliferation of bladder cancer cells (16). Moreover, miR-93-3p is a likely clear
cell renal cell carcinoma oncogene that acts by regulating PEDF
(26). The present study showed that
miR-93 could also target PEDF and increased the proliferation rate
of HCC cells. Therefore, different cancer types may share similar
molecular pathogenesis and miR-93 may serve as a target for the
treatment of multiple types. The present study demonstrated that
miR-93 and GABPB1-IT1 could directly interact with each other;
however, the expression levels of each were not affected. Instead,
overexpression of GABPB1-IT1 upregulated the expression of PEDF, a
characterized target of miR-93 (16). It has been well established that
miRNA sponging suppresses the function of miRNAs but may not
regulate the expression of the target miRNA (22). Therefore, the current data suggested
that GABPB1-IT1 could serve as an endogenous sponge of miR-93 to
suppress its function. However, other mechanisms may also exist.
For instance, GABPB1-IT1 may form certain tertiary structure to
participate in the migration and invasion of cancer cells (27).
There were some limitations to the present study.
Firstly, the sample size of patients with HCC was small and more
samples are needed to confirm the current findings. In addition,
the study only investigated the effect of GABPB1-IT1 on HCC cell
proliferation. Future studies should investigate the involvement of
GABPB1-IT1 in other cellular functions, such as cell migration and
invasion.
In conclusion, GABPB1-IT1 is downregulated in HCC
and may regulate the miR-93/PEDF axis to suppress cancer cell
proliferation.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BL and YW performed the experiments, analyzed the
data, interpreted the results and wrote the manuscript. QG and YD
performed the experiments. LG designed the study and revise the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Ethical approval was obtained from The 96604
Military Hospital of The Chinese People's Liberation Army Medical
Research Ethics Committee (approval no. 2012-06-027), and informed
written consent was obtained from all patients. The animal
experiments were approved by The Ethics Committee of 96604 Military
Hospital of the Chinese People's Liberation Army (approval no.
202065433).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ryerson AB, Eheman CR, Altekruse SF, Ward
JW, Jemal A, Sherman RL, Henley SJ, Holtzman D, Lake A, Noone AM,
et al: Annual report to the nation on the status of cancer,
1975–2012, featuring the increasing incidence of liver cancer.
Cancer. 122:1312–1337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Llovet JM, Zucman-Rossi J, Pikarsky E,
Sangro B, Schwartz M, Sherman M and Gores G: Hepatocellular
carcinoma. Nat Rev Dis Primers. 2:160182016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Janevska D, Chaloska-Ivanova V and
Janevski V: Hepatocellular carcinoma: Risk factors, diagnosis and
treatment. Open Access Maced J Med Sci. 3:732–736. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Negash AA, Olson RM, Griffin S and Gale M
Jr: Modulation of calcium signaling pathway by hepatitis C virus
core protein stimulates NLRP3 inflammasome activation. PLoS Pathog.
15:e10075932019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang F, Yu X, Zhou C, Mao R, Zhu M, Zhu H,
Ma Z, Mitra B, Zhao G, Huang Y, et al: Hepatitis B e antigen
induces the expansion of monocytic myeloid-derived suppressor cells
to dampen T-cell function in chronic hepatitis B virus infection.
PLoS Pathog. 15:e10076902019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi L, Zhang W, Zou F, Mei L, Wu G and
Teng Y: KLHL21, a novel gene that contributes to the progression of
hepatocellular carcinoma. BMC Cancer. 16:8152016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen HC, Jeng YM, Yuan RH, Hsu HC and Chen
YL: SIRT1 promotes tumorigenesis and resistance to chemotherapy in
hepatocellular carcinoma and its expression predicts poor
prognosis. Ann Surg Oncol. 19:2011–2019. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee JW, Soung YH, Kim SY, Lee HW, Park WS,
Nam SW, Kim SH, Lee JY, Yoo NJ and Lee SH: PIK3CA gene is
frequently mutated in breast carcinomas and hepatocellular
carcinomas. Oncogene. 24:1477–1480. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mattick JS and Makunin IV: Non-coding RNA.
Hum Mol Genet. April 15;200 (Epub ahead of print). doi:
10.1093/hmg/ddl046.
|
|
11
|
Hauptman N and Glavac D: Long non-coding
RNA in cancer. Int J Mol Sci. 14:4655–4669. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reddy KB: MicroRNA (miRNA) in cancer.
Cancer Cell Int. 15:382015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qi P and Du X: The long non-coding RNAs, a
new cancer diagnostic and therapeutic gold mine. Mod Pathol.
26:155–165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xie J, Xie G, Chen Q, Xu Z, Bai W and Chen
M: Identification of a novel lncRNA GABPB1-IT1 that is
downregulated and predicts a poor prognosis in non-small cell lung
cancer. Oncol Lett. 18:838–845. 2019.PubMed/NCBI
|
|
15
|
Ohta K, Hoshino H, Wang J, Ono S, Iida Y,
Hata K, Huang SK, Colquhoun S and Hoon DS: MicroRNA-93 activates
c-Met/PI3K/Akt pathway activity in hepatocellular carcinoma by
directly inhibiting PTEN and CDKN1A. Oncotarget. 6:3211–3224. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang H, Bu Q, Zeng M, Xia D and Wu A:
MicroRNA-93 promotes bladder cancer proliferation and invasion by
targeting PEDF. Urol Oncol. 37:150–157. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lei HJ, Chau GY, Lui WY, Tsay SH, King KL,
Loong CC and Wu CW: Prognostic value and clinical relevance of the
6th Edition 2002 American Joint Committee on Cancer staging system
in patients with resectable hepatocellular carcinoma. J Am Coll
Surg. 203:426–435. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Higuchi T, Oshiro H, Zhang Z, Miyake K,
Sugisawa N, Katsuya Y, Yamamoto N, Hayashi K, Kimura H, Miwa S, et
al: Osimertinib regresses an EGFR-mutant cisplatinum-resistant lung
adenocarcinoma growing in the brain in nude mice. Transl Oncol.
12:640–645. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun X, Zheng G, Li C and Liu C: Long
non-coding RNA Fer-1-like family member 4 suppresses hepatocellular
carcinoma cell proliferation by regulating PTEN in vitro and
in vivo. Mol Med Rep. 19:685–692. 2019.PubMed/NCBI
|
|
21
|
Mann M, Wright PR and Backofen R: IntaRNA
2.0: Enhanced and customizable prediction of RNA-RNA interactions.
Nucleic Acids Res. 45:W435–W439. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hausser J and Zavolan M: Identification
and consequences of miRNA-target interactions-beyond repression of
gene expression. Nat Rev Genet. 15:599–612. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Forner A and Bruix J: Biomarkers for early
diagnosis of hepatocellular carcinoma. Lancet Oncol. 13:750–751.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Altekruse SF, Henley SJ, Cucinelli JE and
McGlynn KA: Changing hepatocellular carcinoma incidence and liver
cancer mortality rates in the United States. Am J Gastroenterol.
109:542–553. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li X, Yao W, Yuan Y, Chen P, Li B, Li J,
Chu R, Song H, Xie D, Jiang X and Wang H: Targeting of
tumour-infiltrating macrophages via CCL2/CCR2 signalling as a
therapeutic strategy against hepatocellular carcinoma. Gut.
66:157–167. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang L, Yang G, Zhu X, Wang Z, Wang H, Bai
Y, Sun P, Peng L, Wei W, Chen G, et al: miR-93-3p inhibition
suppresses clear cell renal cell carcinoma proliferation,
metastasis and invasion. Oncotarget. 8:82824–82834. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu J, Leontis NB, Zirbel CL, Bisaro DM and
Ding B: A three-dimensional RNA motif mediates directional
trafficking of Potato spindle tuber viroid from epidermal to
palisade mesophyll cells in Nicotiana benthamiana. PLoS Pathog.
15:e10081472019. View Article : Google Scholar : PubMed/NCBI
|