Introduction
Colorectal cancer (CRC), which accounts for nearly a
million global deaths each year, remains a major cause of cancer
mortality due to the limited efficacy of currently available
systemic treatment for advanced disease (1). Consequently, improved understanding of
the disease is required to further optimize systemic treatment
strategies including immunotherapy. CRC is known to develop from
intestinal epithelium following progressive accumulation of genetic
alterations, which include mutations of the adenomatous polyposis
coli (APC) gene (2). In the
highly evolutionary conserved canonical Wnt signalling pathway, APC
is known to target β-catenin for cytoplasmic degradation, thus
preventing its nuclear translocation to promote tumorigenesis
(3). Most sporadic CRCs are thought
to acquire APC mutations as an early event during
tumorigenesis, prior to the development of adenomas (4). Furthermore, patients with familial
adenomatous polyposis (FAP) carry a germ-line APC mutation,
which predisposes the individual to intestinal adenomas and CRC
(5). The ApcMin/+
mouse is a model of FAP that possess a germline heterozygous Δ850
Apc mutation (6). Such mice
develop predominantly small intestinal adenomas and die at ~130
days of age from the intestinal adenoma burden (6). In addition, haematopoietic defects,
including the development of generalized atrophy of lymphoid
tissue, occur during the early stages of intestinal tumorigenesis
at ~80 days of age (7), while
myeloid defects have been reported at an advanced age in the
ApcMin/+ mouse and another mouse model that is
haplo-insufficient for Apc (8,9).
Over the past decade, immune checkpoint inhibitor
(CKI) therapy including targeting programmed cell death protein-1
(PD-1), has emerged as an effective therapeutic strategy against
several types of cancer, such as lung, melanoma and renal cell
cancer (10). More recently, CKI
therapy has been shown to be effective for the mismatch repair
(MMR)-deficient or microsatellite instability (MSI)-high subset of
various malignancies, including CRC (11,12).
However, this strategy has proven ineffective so far in the
management of the majority of MMR-proficient CRC that represent
>90% of sporadic CRCs (13). For
melanoma, higher neoantigen burden (14) and a greater extent of T lymphocyte
infiltration (15) are correlated
with enhanced responses to PD-1 inhibition. However, T lymphocyte
and MM cell infiltration have been inversely correlated with
enhanced β-catenin pathway signalling in melanoma (16). Furthermore, a study in autochthonous
mouse melanoma models with constitutive β-catenin signalling has
demonstrated the dependence of T lymphocyte infiltration on the MM
cell population (16). Therefore,
characterization of the intestinal MM cell population during
intestinal adenoma development in the ApcMin/+
model could yield insight into any early MM population changes
associated with enhanced Wnt signalling.
A review on MM cells highlighted their
heterogeneity, with no pan-MM cell marker defined, which has
compounded previous studies on MM cell populations (17). However, a mouse with EGFP knocked
into the mouse-lysozyme (M-lys) locus by homologous
recombination (M-lyslys-EGFP/lys-EGFP) was
previously generated, which utilizes EGFP expression to facilitate
studies on murine MM cells (18). In
this model, EGFP fluorescence has been observed in multiple surface
marker-defined peripheral MM sub-populations (18). The present study had two aims. First,
to determine if there is reduction of the total resident intestinal
LPMNC population during the early stage of intestinal
tumorigenesis. Furthermore, utilizing ApcMin/+
and wild-type mice bred onto the M-Lyslys-EGFP/+
background, it was investigated if there is a reduction in the
resident intestinal MM cell population during the early stage of
intestinal tumorigenesis.
Materials and methods
Mice
C57BL/6J and C57BL/6J-ApcMin/+
mice were obtained from The Jackson Laboratory.
C57BL/6J/Sv129-M-lyslys-EGFP/lys-EGFP mice
(18) were obtained from Albert
Einstein College of Medicine. All mice were bred in-house, kept
under isolator conditions at temperatures between 19 and 23°C on a
12-h light-dark cycle. They were pathogen-free by regular
bacteriological and serological testing. Relative humidity was kept
between 45 and 55%. The mice were fed on mouse complete maintenance
diet with free access to food and water. For the experiments
described here, the following mice were utilized:
Apc+/+M-Lys+/+ (n=20),
ApcMin/+M-Lys+/+ (n=23),
Apc+/+M-Lyslys-EGFP/+ (n=9) And
ApcMin/+M-Lyslys-EGFP/+ (n=12).
Mouse breeding and genotyping
Apc+/+ and
ApcMin/+ mice on the
M-Lyslys+/+ background were obtained by
mating male ApcMin/+M-Lyslys+/+
mice with female
Apc+/+M-Lyslys+/+ mice.
Furthermore, ApcMin/+ and
Apc+/+mice on the
M-Lyslys-EGFP/+ background were obtained by
mating male ApcMin/+M-Lyslys+/+
mice with female Apc+/+M-lys lys-EGFP/lys-EGFP
mice. The offspring were genotyped for Apc and M-lys
at ~30 days of age by PCR analysis of genomic DNA (18,19).
Resident peritoneal cell, splenic and
intestinal tissue collection
Each mouse was sacrificed between 30 and 138 days of
age for experiments described below by cervical dislocation,
immediately after which the peritoneal cavity was opened and
peritoneal mononuclear cells (when required) were obtained by
lavage with sterile phosphate buffer saline (PBS) at room
temperature (25°C), prior to dissection of the spleen or whole
intestine. Splenic and intestinal tissues were collected in ice
cold PBS, then stored in ice cold PBS for ≤5 min until processed as
described in subsequent sections.
Intestinal adenoma count
Following lavage for peritoneal cells and dissection
of the spleen, intestine was removed from the pylorus to the anus.
The small intestine was then separated from the caecum and colon.
Intestines were flushed out gently with PBS until no luminal
content remained, after which they were carefully cut and opened
out longitudinally to avoid adenoma disruption. For five
ApcMin/+M-Lyslys-EGFP/+ at 92±23 days
of age, adenomas were counted by naked eye examination of small
intestinal tissue.
Histology
Tissue from the small intestine and spleen of three
pairs of Apc+/+M-Lyslys-EGFP/+ and
ApcMin/+M-Lyslys-EGFP/+ mice at 93±23
days of age underwent histological evaluation and
immunohistochemistry for EGFP localisation as described below.
Tissue from an SW480 human CRC xenograft transfected with an
EGFP-expressing herpes saimiri viral vector served as positive
control due to previously demonstrated EGFP expression (20). Tissue from age matched
ApcMin/+M-Lyslys+/+ mice served
as negative control. Sections were fixed in 4% (w/v)
paraformaldehyde in PBS for 6 h at 25°C and paraffin wax embedded.
Sections were cut to 5–7-µm thickness and underwent haematoxylin
and eosin staining or immunohistochemistry (as described in the
next section). Sections were viewed using fluorescence and phase
contrast microscopy by a NIKON Eclipse E1000 fluorescence
microscope (Nikon Corporation). Images were then captured using
LUCIA GF imaging software (version 4.60) (Nikon Corporation).
Immunohistochemistry for the detection
of EGFP
Steps of the procedure were performed at room
temperature (25°C) except otherwise stated. Sections were de-waxed
progressively in xylene for 1 min each three times, then absolute
(100%) ethanol for a minute each (×3), then washed in distilled
water before endogenous tissue peroxidase activity was blocked by
immersion of slides in 2% (v/v) H2O2 in
absolute methanol for 15 min. Subsequently, slides were washed in
distilled water for 10 min. Immunohistochemical staining of
intestinal sections was carried out as previously described
(21). Serum block was with 1.5%
(v/v) goat serum (Dako; Agilent Technologies, Inc.) in PBS for 30
min. Sections were then incubated with rabbit anti-Aquorea
anti-EGFP primary antibody (1:4,000; cat. no. A-6455; Invitrogen;
Thermo Fisher Scientific, Inc.) for 20 h at 4°C, after which slides
were washed in PBS four times for 5 min each. Sections were then
incubated for 30 min with HRP/dextran polymer-conjugated goat
anti-rabbit secondary antibody (ready to use; cat. no. K4002; Dako;
Agilent Technologies, Inc.) at room temperature. Subsequently,
sections were washed in PBS four times for 5 min each. Sections
were then incubated for 10 min with 0.1% (v/v) diaminobenzidine
solution [Dako; Agilent Technologies, Inc.) in Tris-buffer (0.05 M
Tris (pH 7.6 with HCl)], containing 0.03% (v/v)
H2O2 at room temperature. Sections were
washed in tap water four times for 5 min each, counterstained with
Mayer's Haematoxylin (cat no. MHS32; Sigma-Aldrich; Merck KGaA) for
90 sec at room temperature, followed by immersion in Scott's tap
water (0.167 M and MgS04 and 0.042 M NaHCO3
in distilled water) for 1 min. Slides were then rinsed in distilled
water and the stained sections were dehydrated by immersion in
absolute ethanol for 3 min three times, then immersed in xylene for
three times 3 min each followed by mounting in DPX mountant (cat.
no. 100579; Millipore Sigma). Sections were, viewed and images were
captured as aforementioned.
Isolation and enumeration of small
intestinal lamina propria mononuclear cells
Utilizing Apc+/+
M-Lys+/+ mice as controls, intestinal LPMNCs were
isolated from ApcMin/+ M-Lys+/+ mice
at the following ages: Weaning (~30 days of age; n=4 pairs), prior
to the appearance of macroscopically visible adenomas (~70 days of
age; n=8 pairs), prior to death from macroscopic adenoma burden
(~100 days of age; n=8 pairs). LPMNCs were isolated from mouse
small intestine at room temperature (25°C) with viability and
numbers determined as previously described (22,23)
ensuring complete enzymatic digestion of the intestines of mice at
different ages.
Flow cytometric analysis of small
intestinal lamina propria mononuclear cells
Flow cytometric analysis of small intestinal LPMNCs
has been described previously for EGFP, phycoerythrin (PE) and
propidium iodide (PI) expression (23). Flow cytometry was performed at room
temperature (25°C) on intestinal LPMNCs of six
Apc+/+M-Lyslys-EGFP/+ mice and seven
ApcMin/+M-Lyslys-EGFP/+mice at 74±2
days of age. Monoclonal primary antibodies utilized were as
follows: F4/80 (1:100; cat. no. MCA497G; clone A3-1; BioRad
Laboratories, Inc.), BMDM-1, ER-HR3 (1:10), ER-MP23, ER-MP58,
ER-TR9, MOMA-1 (all 1:10) and MOMA-2. Antibodies (with the
exclusion of F4/80) were hybridoma-conditioned supernatant, a kind
gift from Professor Pieter Leenen (24). A PE-conjugated goat anti-rat antibody
was utilised (cat no. 305009; Bio-Rad Laboratories, Inc.) at 1: 100
dilution. Cells were then washed in 10% foetal calf serum (FCS)
labelled with propidium iodide (cat. no. P4864; Sigma-Aldrich;
Merck KGaA) at 1:2000 dilution. Flow cytometry was performed using
5×104 LPMNCs and a FACS Vantage cytometer
(Becton-Dickinson and Company) with Cell Quest™ software version
3.3 (Becton-Dickinson and Company). The R1 gate was set up to
exclude PI-positive cells and cells with low forward scatter
(deemed non-viable). EGFP-positive cells (fluorescence level
greater than that obtained by <0.1% of wild-type LPMNCs) and
macrophage marker positive cells ([M] with fluorescence level
greater than that obtained by <0.1% of cells with non-specific
labelling by control rat IgG) were analysed as expressing the
relevant marker. Some cells were labelled with a cocktail of
ER-HR3, F4/80 and MOMA-1 primary antibodies defined as the
‘Mac-mix’(at the same concentration as utilized for single
markers). BMDM-1, ER-MP23, ER-MP58, ER-TR9 and MOMA-2 were not
evaluated further, due to the level of expression of undiluted
hybridoma supernatant being no higher than for control rat IgG
(data not shown).
Isolation of resident peritoneal
cells
Lavaged peritoneal cells from three pairs of
Apc+/+M-Lyslys-EGFP/+ and
ApcMin/+M-Lyslys-EGFP/+ mice at 93±23
days were treated with 5 mM EDTA in Hank's Balanced Salt Solution
(Invitrogen; Thermo Fisher Scientific, Inc.) for 75 min and
collagenase/dispase enzymes for 90 min at 37°C as for small
intestinal LPMNCs (23). Peritoneal
cells were then washed twice in an excess of 10% (v/v) foetal calf
serum in PBS and re-suspended at 25°C in the same culture medium as
for LPMNCs prior to been viewed by fluorescence microscopy.
Statistical analysis
The mean ± standard error of mean (SEM) was
calculated for numbers or proportions of cells belonging to
different small intestinal LPMNC sub-populations of different
groups of mice. Analysis of the difference between total LPMNCs of
the oldest and youngest mice of either genotype (parametric data)
was compared using unpaired two-tailed Student's t-tests.
Statistical comparison of the myelomonocytic sub-population and
adenoma numbers between mice was conducted using two-tailed
Mann-Whitney U tests. Statistical analysis utilized Minitab
software version 13 (Minitab, LLC). P≤0.05 was considered to
indicate a statistically significant difference.
Results
Small intestinal LPMNC numbers
decrease with age in ApcMin/+ mice
Initially, the total small intestinal LPMNC
population was characterised during intestinal tumorigenesis in the
ApcMin/+ mouse. It was determined if there were
any differences in small intestinal LPMNC numbers with age. There
were no significant differences in LPMNC viability between groups
of mice (Table I). There was no
significant difference in total intestinal LPMNC numbers with age
in Apc+/+ mice (33±1 vs. 109±2 days old; P=0.35),
though older mice had a trend to reduced total LPMNCs (Table I). By contrast, total intestinal
LPMNCs numbers significantly reduced with age in
ApcMin/+ mice (33±1 vs. 109±2 days old; P=0.05;
Table I). This suggested
significantly reduced intestinal LPMNCs with age in
ApcMin/+ mice.
 | Table I.Decreased numbers of small intestinal
LPMNCs with age in ApcMin/+ mice. |
Table I.
Decreased numbers of small intestinal
LPMNCs with age in ApcMin/+ mice.
|
| Mouse strain |
|---|
|
|
|
|---|
|
|
Apc+/+ |
ApcMin/+ |
|---|
|
|
|
|
|---|
| Age, (days) | LPMNCs,
×106 | Viability, % | LPMNCs,
×106 | Viability, % |
|---|
| 33±1 | 25.0±6.0 | 81.5±2.1 | 23.2±13.9 | 81.3±4.3 |
| 71±1 | 17.1±2.6 | 78.2±1.9 | 12.3±2.4 | 77.1±2.4 |
| 109±2 |
18.7±3.3a | 76.8±1.6 |
10.6±2.4b | 76.1±1.5 |
No significant effect of M-lys
hemizygosity on ApcMin/+ mouse small intestinal
tumorigenesis
A previous study reported no difference in the
proportion of EGFP-expressing cells in the blood and bone marrow of
Apc+/+M-Lyslys-EGFP/lys-EGFP compared
with Apc+/+M-Lyslys-EGFP/+ mice
(18). Therefore,
Apc+/+ and ApcMin/+ mice were
bred onto the M-Lyslys-EGFP/+ background to
facilitate the study of MM cell populations with intact
M-Lys function. It was determined if there was any impact of
heterozygous M-Lys deletion on intestinal tumorigenesis by
counting macroscopic adenomas from
ApcMin/+M-Lyslys-EGFP/+ intestine.
Adenoma numbers were 45.8±10 (mean ± SEM; n=5) from the small
intestine of ApcMin/+M-Lyslys-EGFP/+
mice (data not shown), similar to that of
ApcMin/+M-Lys+/+ mice previously bred
in our facility that had 53±4 tumours (mean ± SEM; n=25) (25). Consequently, hemizygous deletion of
M-Lys appeared not to have significant impact on intestinal
tumorignenesis in the ApcMin/+ mouse.
EGFP fluorescence in isolated small
intestinal LPMNCs of M-Lyslys-EGFP/+ mice
M-Lys mRNA expression has previously been
demonstrated in mouse intestine (26). The presence of EGFP protein was
tested for and fluorescence in isolated intestinal LPMNCs of
M-Lyslys-EGFP/+ mice. EGFP fluorescence in
intestinal LPMNCs of
Apc+/+M-Lyslys-EGFP/+ mice in situ as
well as in the LPMNC isolate was observed (Fig. S1).
EGFP expression in the spleen and
small intestine of Apc+/+M-Lyslys-EGFP/+ and
ApcMin/+M-Lyslys-EGFP/+ mice
Utilizing an EGFP-expressing SW480 human CRC
xenograft as positive control, it was determined if there was any
difference in the resident intestinal MM cell localization between
Apc+/+M-Lyslys-EGFP/+ and
ApcMin/+M-Lyslys-EGFP/+ mice at ~100
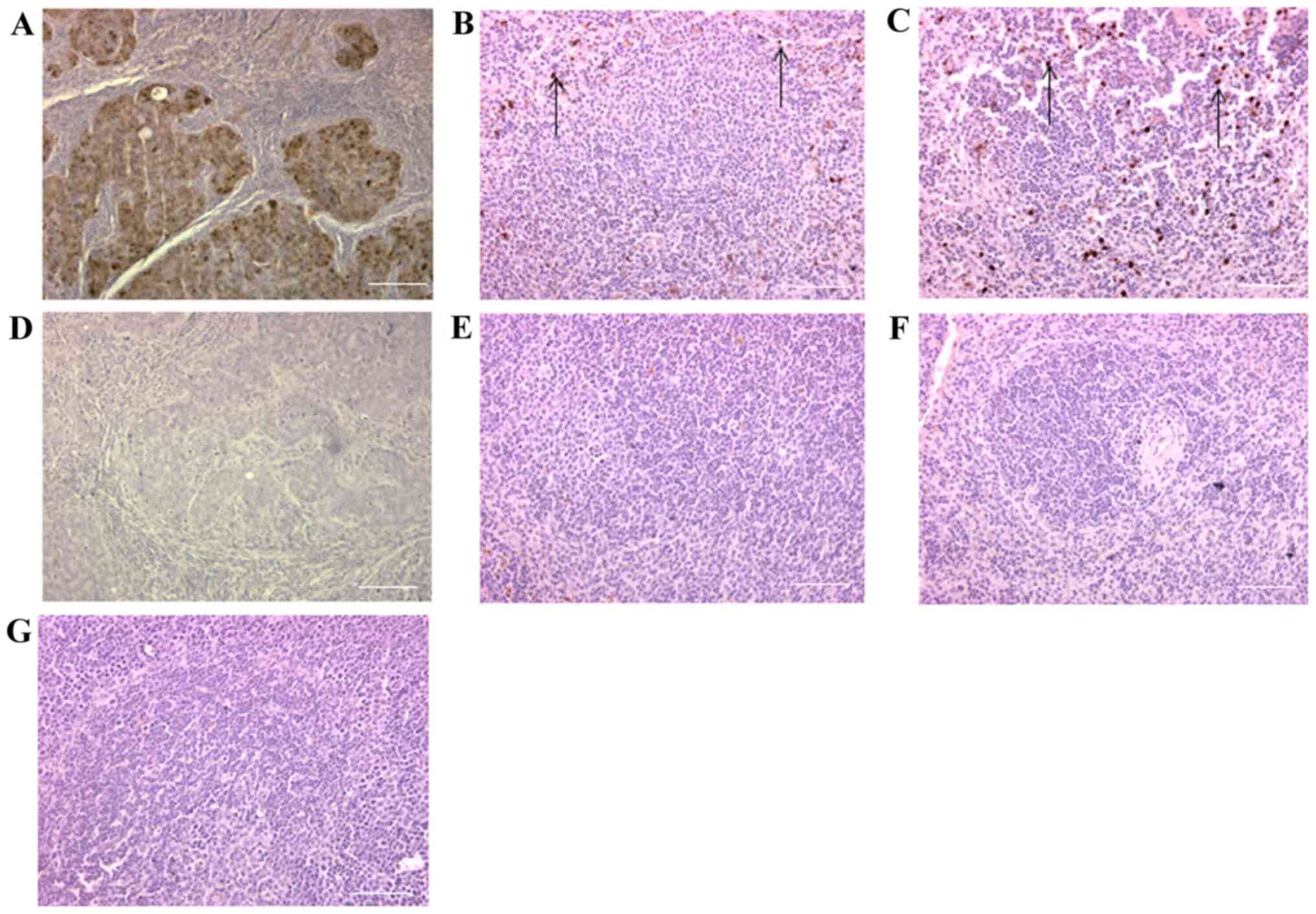
days of age (n=3 pairs) by immunohistochemistry for EGFP (Figs. 1 and 2). The spleen was studied as independent
lymphoid tissue with a sentinel MM cell population. Even though
there was some fibrotic distortion of
ApcMin/+M-Lyslys-EGFP/+ splenic
tissue, EGFP-expressing cells were localized to the marginal zone
of the spleen in both types of mice (Fig. 1B and C). In the intestine,
EGFP-expressing cells were localized to intestinal villi and in
particular lymphoid follicles of
Apc+/+M-Lyslys-EGFP/+ mice (Fig. 2Aa-c). While EGFP-expressing cells
were also localized to the intestinal villi of
ApcMin/+M-Lyslys-EGFP/+ mice and the
periphery of adenomas, lymphoid follicles were absent from the
intestine of ApcMin/+M-Lyslys-EGFP/+
mice (Fig. 2Ba-c). This suggested
loss of myelomonocytic cells and immune cell aggregates in
ApcMin/+M-Lyslys-EGFP/+ mouse
intestine
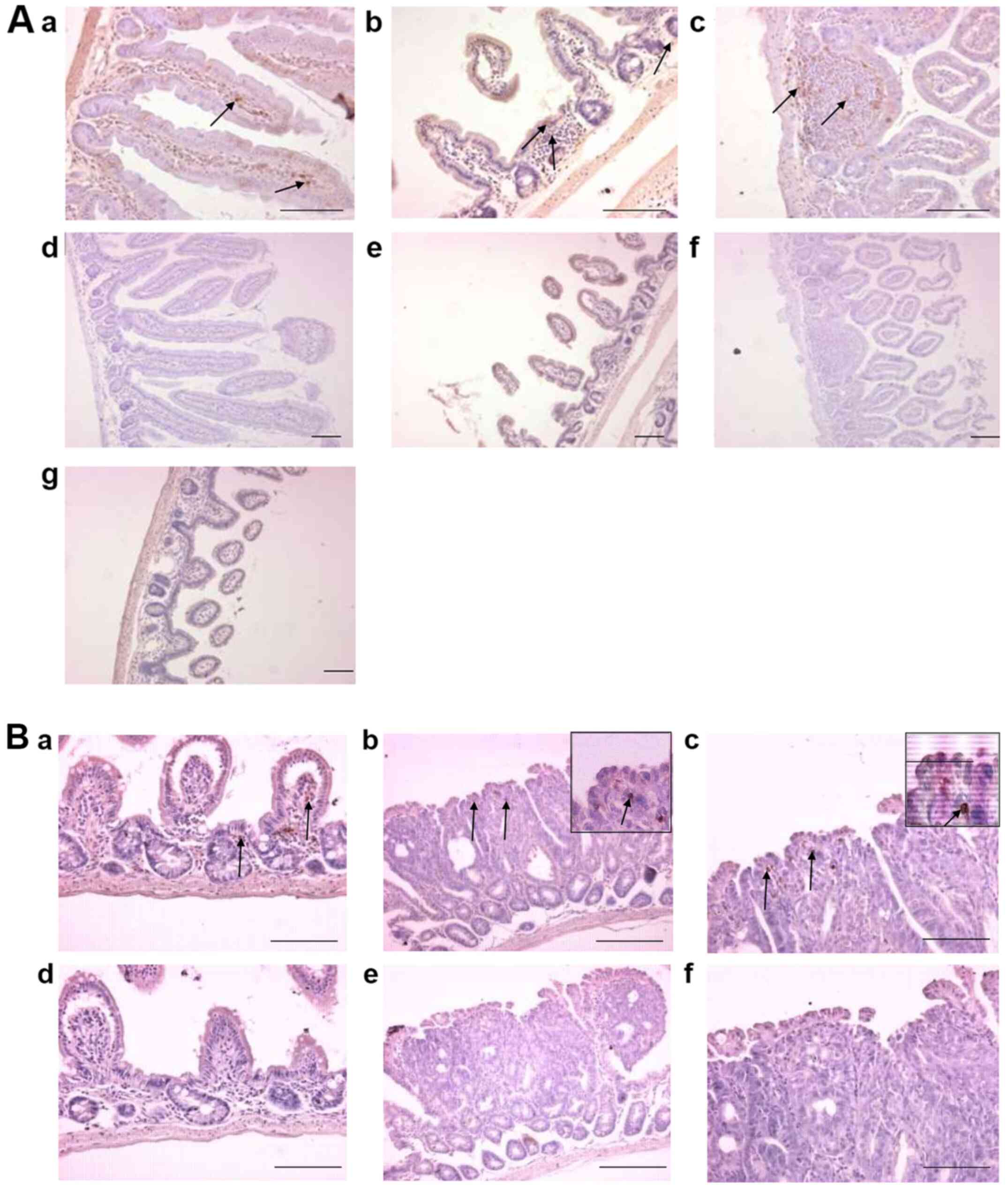 | Figure 2.Localisation of EGFP expressing cells
in Apc+/+ M-lyslys-EGFP/+ mouse small
intestine. (A) Sections of the small intestine of an
Apc+/+ M-lyslys-EGFP/+ mouse at 138
days of age labelled with an antibody to EGFP (brown cells). With
the primary antibody to EGFP, brown staining in (a) proximal small
intestine of the Apc+/+
M-lyslys-EGFP/+ mouse, (b) distal small intestine of
the Apc+/+ M-lyslys-EGFP/+ mouse and
(c) an intestinal lymphoid follicle of the distal small intestine
of an Apc+/+ M-lyslys-EGFP/+ mouse
(higher magnification, ×600). In sections with omission of the
primary antibody, absence of brown staining in (d) proximal small
intestine and (e and f) distal small intestine. (g) Absence of
brown staining in distal small intestine of an Apc+/+
M-lys+/+ mouse stained with the EGFP antibody. (B)
Sections of the distal small intestine of an ApcMin/+
M-lyslys-EGFP/+ mouse labelled with an antibody to
EGFP. With the primary antibody to EGFP, brown staining in (a)
LPMNCs within the villi and lamina propria of an
ApcMin/+ M-lyslys-EGFP/+ mouse, (b and
c) LPMNCs within an intestinal adenoma of an ApcMin/+
M-lyslys-EGFP/+ mouse. Inset are EGFP-expressing
cells within intestinal adenomas, shown at higher magnification
(magnification, ×600). In sections with omission of the primary
antibody, absence of brown staining in (d) distal small intestine
and (e and f) adenomas. Arrows point to EGFP-expressing cells.
Scale bars, 100 µm. APC, adenomatous polyposis coli; EGFP, enhanced
green fluorescent protein; LPMNCs, lamina propria mononuclear
cells; M-lys, mouse lysozyme. |
Small intestinal myelomonocytic
sub-populations of Apc+/+M-Lyslys-EGFP/+ and
ApcMin/+M-Lyslys-EGFP/+ mice
To determine any differences in the intestinal MM
population during the early stages of intestinal tumorigenesis,
mice were studied at ~70 days of age, which is prior to overt
ApcMin/+ mouse lymphoid atrophy (7). This was also prior to ulceration,
bleeding and potential secondary inflammation associated with
advanced intestinal adenomas (6). As
for mice on the M-Lys+/+ background, there was a
trend towards reduced total intestinal LPMNCs in
ApcMin/+M-Lyslys-EGFP/+ mice which did
not reach statistical significance (2.65±0.23×107
Apc+/+M-Lyslys-EGFP/+ vs.
1.72±0.39×107
ApcMin/+M-Lyslys-EGFP/+ mice, P=0.12)
Typical flow cytometry plots are shown in Fig. 3. There was no significant difference
in the proportion of cells in the R1gate ([%]
Apc+/+ 35. 40±4.00 vs ApcMin/+
38.00±4.60, P=0.52). Data on the three expressed macrophage marker
antibodies, their mixture (Mac mix) and EGFP are displayed for
Apc+/+M-Lyslys-EGFP/+ and
ApcMin/+M-Lyslys-EGFP/+ mice (Table II). A higher proportion of LPMNCs
from Apc+/+ M-Lyslys-EGFP/+ mice (n=6)
expressed EGFP (P=0.11), ER-HR3 (P=0.11) or the Mac mix (P=0.18)
compared with ApcMin/+ M-Lyslys-EGFP/+
littermates (n=7) (Table II).
However, these differences were not statistically significant.
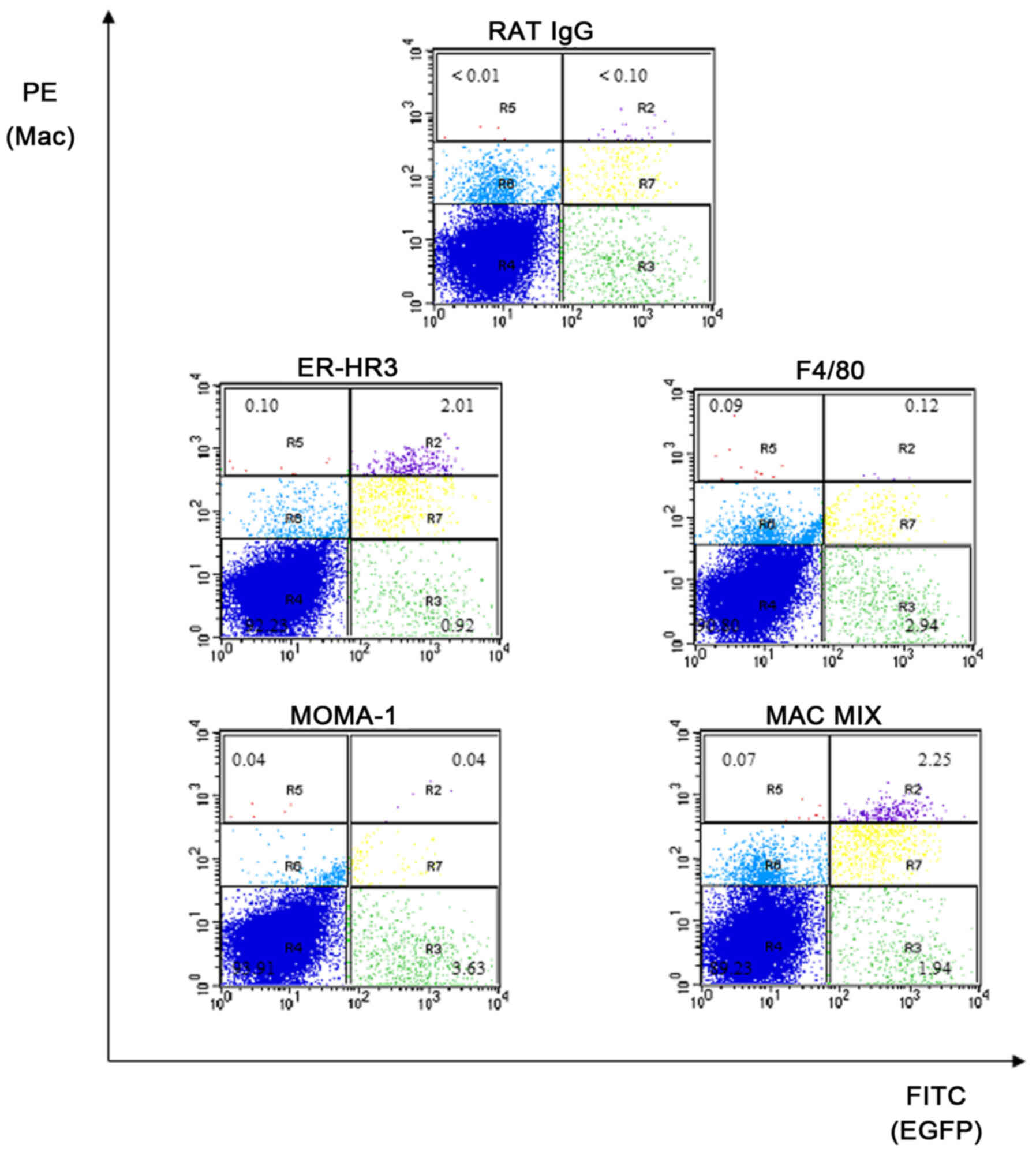 | Figure 3.Macrophage marker flow cytometry on
M-lyslys-EGFP/+ intestinal LPMNCs. Representative
flow cytometry plots from Apc+/+
M-lyslys-EGFP/+ mouse intestinal LPMNCs. Figures
show the percentage of the total population of LPMNCs in relevant
regions. EGFP fluorescence is on the abscissa, while PE
fluorescence for macrophage markers is on the ordinate. R1, viable
cell gate (not shown); R2, G+M+; R3, G+M-; R4, G-M-; R5, G-M+; R6,
LPMNCs that do not express EGFP, but were labelled by the
macrophage surface marker at a similar level to non-specific rat
IgG; R7, LPMNCs that expressed EGFP and were labelled by macrophage
surface markers at a similar level to non-specific rat IgG. LPMNCs,
lamina propria mononuclear cells; M-lys, mouse lysozyme; PE,
phycoerythrin; G+, EGFP-positive LPMNCs, G-: EGFP-negative LPMNCs.
M+, LPMNCs labelled by macrophage markers above levels of
non-specific binding by rat IgG. M-, LPMNCs not labelled by
macrophage markers |
 | Table II.Small intestinal myelomonocytic cell
populations of ApcMin/+
M-Lyslys-EGFP/+ and Apc+/+
M-Lyslys-EGFP/+ mice at 74±2 days of age. |
Table II.
Small intestinal myelomonocytic cell
populations of ApcMin/+
M-Lyslys-EGFP/+ and Apc+/+
M-Lyslys-EGFP/+ mice at 74±2 days of age.
| Myelomonocytic cell
marker |
Apc+/+ |
ApcMin/+ | P-value |
|---|
| EGFP (M-lys) | 4.29±0.68 | 2.90±0.47 | 0.11 |
| ERHR-3 |
|
|
|
| G- | 0.11±0.03 | 0.13±0.04 | 0.83 |
| G+ | 1.02±0.25 | 0.55±0.13 | 0.23 |
| F4/80 |
|
|
|
| G- | 0.22±0.17 | 0.31±0.18 | 0.52 |
| G+ | 0.24±0.10 | 0.14±0.07 | 0.20 |
| MOMA-1 |
|
|
|
| G- | 0.04±0.01 | 0.07±0.02 | 0.12 |
| G+ | 0.13±0.09 | 0.04±0.01 | 0.39 |
| MAC-MIX |
|
|
|
| G- | 0.16±0.10 | 0.15±0.05 | 0.48 |
| G+ | 1.20±0.34 | 0.66±0.18 | 0.28 |
Lower numbers of small intestinal
myelomonocytic cells in the
ApcMin/+M-Lyslys-EGFP/+ mice with the lowest
total small intestinal LPMNC numbers
To clarify if there was an association between the
trend to reduced ApcMin/+ total intestinal LPMNC
and MM cell numbers, the lamina propria MM cell population of mice
with the lowest total LPMNC yield were studied
(ApcMin/+M-Lyslys-EGFP/+; mice nos. 4,
5 and 7; Tables SI and SII). These three mice had an LPMNC yield
<35% compared with the average LPMNC yield of
Apc+/+ mice. For these three
ApcMin/+M-Lyslys-EGFP/+ mice, the
proportion of EGFP-expressing cells (1.78±0.26) was >2-fold
depleted compared with the mean for Apc+/+ mice
(P=0.05) while the proportion of Mac-mix expressing cells
(0.37±0.04) was ~4-fold depleted compared with the mean for
Apc+/+ mice (P=0.05). This suggested that there
was depletion of the myelomonocytic sub-population associated with
reduced intestinal LPMNCs.
Discussion
Selective EGFP fluorescence of MM cells without
significant impact on tumorigenesis in the novel
ApcMin/+M-Lyslys-EGFP/+ mouse in the
present study prompted investigation into the MM cell population.
No pan-myelomonocytic cell marker has yet been fully validated for
the murine MM cell population, which the present observation of
EGFP-negative Mac mix expressing cells corroborates. While a range
of myelomonocytic cell markers were used in the present study, rare
subsets not evaluated in the present study should not be ignored.
However, the murine M-lys-expressing MM sub-population has
been ascribed roles in phagocytosis and antigen presentation in the
intestinal micro-environment, which are crucial to the immune
response (27). A notable
observation of the current study was a reduction in the
ApcMin/+ lamina propria MM cell population early
during intestinal adenoma development, associated with loss of
intestinal lymphoid/MM cell aggregates but with retention of
splenic marginal zone MM cells. It is possible that similar to the
reduction in total intestinal LPMNC numbers observed, MM cell
depletion is progressive through adenoma development. Conventional
dendritic cells that function as antigen presenting cells in the
intestinal micro-environment are known to reside in intestinal
lymphoid follicles (28).
Consequently, the loss of lymphoid/MM cell interaction during
intestinal adenoma development could impair the development of an
immune response to tumour antigens, as previously shown in an
autochthonous melanoma model (16),
thus compromising immunosurveillance to prevent adenoma growth. In
this respect, the prognosis following resection of early CRC is
positively correlated with the extent of intra-tumoral lymphocytic
infiltration (29). Furthermore,
response to PD-1-inhibition in melanoma is associated with the
presence of a primed peri-tumoral cytotoxic lymphocyte population
(15).
ApcMin/+ mice ≤84 days of age did
not have evidence of reduced peripheral MM cells or lymphocytes
(8). Consequently, total intestinal
lamina propria and intestinal MM cell depletion in 70-day old
ApcMin/+ mice appeared to be due to factors in
the intestinal micro-environment. After weaning in mice,
F4/80-positive resident intestinal macrophages of embryonic origin
are now known to be replaced by F4/80-negative macrophages, which
are constantly re-populated from the peripheral circulation and
adopt an anti-inflammatory phenotype in the intestinal
microenvironment (30,31). We previously demonstrated that the
resident intestinal MM population is the highest PGE2-secreting
intestinal LPMNC sub-population in mice. Furthermore, there is a
trend for higher PGE2 production by the ApcMin/+
mouse resident intestinal MM population compared with the
Apc+/+ mouse (23). PGE2 has previously been ascribed an
immunosuppressive role (32,33), which would be consistent with an
anti-inflammatory MM cell phenotype. It is noteworthy that MM cell
conversion to an immunosuppressive phenotype has been shown to take
up to 96 h after MM cell migration into the intestinal
micro-environment; with dendritic cells typically surviving for
several days while macrophages typically survive for several weeks
(28,34). Consequently, in this respect,
enhanced Wnt signalling may be physiologically relevant to signal
exclusion of MM cells prior to their conversion to an
immunosuppressive phenotype in the ApcMin/+
intestinal milieu. However, other factors, such as cyclooxygenase-2
(35), TGF-β and T regulatory cells
(36) may play a role in MM cell
depletion from the intestinal micro-environment.
Out of the murine macrophage surface markers
evaluated in the present study, ER-HR3 was the most widely
expressed by intestinal lamina propria macrophages, irrespective of
Apc genotype. ER-HR3 is known to be expressed by a sub-set
of peripheral and resident tissue macrophages including those in
sentinels, such as the skin, spleen and lymph nodes (37). Functionally, ER-HR3-expressing
macrophages have been shown to be phagocytic and associated with
immune latent milieu, such as the granulomata of BCG infected mice
and with the relative exclusion of F4/80-expressing macrophages,
which are more common in other murine tissue (38).
In conclusion, the present study demonstrated that
the loss of MM/lymphoid interaction occurs during the early stages
of ApcMin/+ intestinal tumorigenesis, which may
be due to enhanced micro-environmental Wnt signalling. Subsequent
anergy to emerging tumour neoantigens could compromise tumour
immunosurveillance in the ApcMin/+ model. The
relevance of this to colorectal adenomas and cancer requires
evaluation, as well as the possibility that, similar to advanced
melanoma, immune changes secondary to enhanced Wnt signalling may
persist in MMR-proficient CRC. Furthermore, the mechanism to MM
cell depletion requires further evaluation. Such studies may
contribute to understanding the resistance to CKI therapy of
MMR-proficient CRC despite a tendency for higher neoantigen burden
compared with other malignancies, such as renal or bladder cancer
(14).
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Sarah Holwell
and Mr David Brooke (University of Leeds) who assisted with mouse
genotyping, Dr Peter Smith, Ms Deborah Clarke and Ms Liz
Straszynski (University of Leeds) for help with EGFP-expressing
SW480 colorectal cell line tissue, L-cell conditioned medium and
flow cytometric analysis, respectively, Professor Thomas Graf
(Albert Einstein College of Medicine) for supplying
C57BL/6J/Sv129-M-lyslys-EGFP/lys-EGFP mice and
Professor Pieter Leenen (Erasmus University) for providing
hybridoma supernatant antibodies to murine macrophage surface
markers. The abstract was presented 4th-6th November 2018 at the UK
Annual National Cancer Research Institute conference in Glasgow,
UK.
Funding
OOF was funded by an Overseas Research Student
Scholarship. Work on intestinal tumorigenesis at St. James's
University Hospital was funded by Yorkshire Cancer Research (grant
no. L283).
Availability of data and materials
The datasets generated and/or analysed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
OOF performed experiments and wrote the initial
version of the manuscript. OOF, MAH, AFM, CB and PLC were involved
in study design, interpretation of the data and statistical
analysis. All authors confirm authenticity of the data and agreed
to the final version of the manuscript.
Ethics approval and consent to
participate
All animal work was carried out under the Animals
(Scientific Procedures) Act 1986 (PPL 40/3291 UK Home Office).
Ethical approval to conduct the study was obtained from The
University of Leeds Animal Welfare and Ethical Review
Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Apc
|
adenomatous polyposis coli gene
|
|
CKI
|
checkpoint inhibitor
|
|
CRC
|
colorectal cancer
|
|
EGFP
|
enhanced green fluorescent
protein
|
|
FAP
|
familial adenomatous polyposis
|
|
LPMNC
|
lamina propria mononuclear cell
|
|
Mac-mix
|
mixture of antibodies to macrophage
markers (ER-HR3, F4/80 and MOMA-1)
|
|
MSI
|
microsatellite instability
|
|
MMR
|
mismatch repair
|
|
MM
|
myelomonocytic
|
|
PD-1
|
programmed cell death protein-1
|
|
PGE2
|
prostaglandin E2
|
|
SEM
|
standard error of the mean
|
|
Treg
|
regulatory T lymphocyte
|
|
v/v
|
volume in total volume of
solution
|
|
w/v
|
weight in total volume of
solution
|
References
|
1
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fearon ER and Vogelstein B: A genetic
model for colorectal tumorigenesis. Cell. 61:759–767. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moon RT, Bowerman B, Boutros M and
Perrimon N: The promise and perils of Wnt signaling through
beta-catenin. Science. 296:1644–1646. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ashton-Rickardt PG, Dunlop MG, Nakamura Y,
Morris RG, Purdie CA, Steel CM, Evans HJ, Bird CC and Wyllie AH:
High frequency of APC loss in sporadic colorectal carcinoma due to
breaks clustered in 5q21-22. Oncogene. 4:1169–1174. 1989.PubMed/NCBI
|
|
5
|
Nishisho I, Nakamura Y, Miyoshi Y, Miki Y,
Ando H, Horii A, Koyama K, Utsunomiya J, Baba S and Hedge P:
Mutations of chromosome 5q21 genes in FAP and colorectal cancer
patients. Science. 253:665–669. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moser AR, Pitot HC and Dove WF: A dominant
mutation that predisposes to multiple intestinal neoplasia in the
mouse. Science. 247:322–324. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Coletta PL, Müller AM, Jones EA, Mühl B,
Holwell S, Clarke D, Meade JL, Cook GP, Hawcroft G, Ponchel F, et
al: Lymphodepletion in the ApcMin/+ mouse model of
intestinal tumorigenesis. Blood. 103:1050–1058. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lane SW, Sykes SM, Al-Shahrour F,
Shterental S, Paktinat M, Lo Celso C, Jesneck JL, Ebert BL,
Williams DA and Gilliland DG: The Apc(min) mouse has altered
hematopoietic stem cell function and provides a model for MPD/MDS.
Blood. 115:3489–3497. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang J, Fernald AA, Anastasi J, Le Beau MM
and Qian Z: Haploinsufficiency of Apc leads to ineffective
hematopoiesis. Blood. 115:3481–3488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marabelle A, Le DT, Ascierto PA, Di
Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M,
Penel N, Hansen AR, et al: Efficacy of pembrolizumab in patients
with noncolorectal high microsatellite instability/mismatch
repair-deficient cancer: Results from the phase II KEYNOTE-158
study. J Clin Oncol. 38:1–10. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Overman MJ, Lonardi S, Wong KYM, Lenz HJ,
Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill
A, et al: Durable clinical benefit with nivolumab plus ipilimumab
in DNA mismatch repair-deficient/microsatellite instability-high
metastatic colorectal cancer. J Clin Oncol. 36:773–779. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Toh JWT, de Souza P, Lim SH, Singh P, Chua
W, Ng W and Spring KJ: The potential value of immunotherapy in
colorectal cancers: Review of the evidence for programmed death-1
inhibitor therapy. Clin Colorectal Cancer. 15:285–291. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schumacher TN and Schreiber RD:
Neoantigens in cancer immunotherapy. Science. 348:69–74. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tumeh PC, Harview CL, Yearley JH, Shintaku
IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu
V, et al: PD-1 blockade induces responses by inhibiting adaptive
immune resistance. Nature. 515:568–571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Spranger S, Bao R and Gajewski TF:
Melanoma-intrinsic β-catenin signalling prevents anti-tumour
immunity. Nature. 523:231–235. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bain CC and Mowat AM: Macrophages in
intestinal homeostasis and inflammation. Immunol Rev. 260:102–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Faust N, Varas F, Kelly LM, Heck S and
Graf T: Insertion of enhanced green fluorescent protein into the
lysozyme gene creates mice with green fluorescent granulocytes and
macrophages. Blood. 96:719–726. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dietrich WF, Lander ES, Smith JS, Moser
AR, Gould KA, Luongo C, Borenstein N and Dove W: Genetic
identification of Mom-1, a major modifier locus affecting
Min-induced intestinal neoplasia in the mouse. Cell. 75:631–639.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Smith PG, Coletta PL, Markham AF and
Whitehouse A: In vivo episomal maintenance of a herpesvirus
saimiri-based gene delivery vector. Gene Ther. 8:1762–1769. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Walter I, Fleischmann M, Klein D, Müller
M, Salmons B, Günzburg WH, Renner M and Gelbmann W: Rapid and
sensitive detection of enhanced green fluorescent protein
expression in paraffin sections by confocal laser scanning
microscopy. Histochem J. 32:99–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Newberry RD, Stenson WF and Lorenz RG:
Cyclooxygenase-2-dependent arachidonic acid metabolites are
essential modulators of the intestinal immune response to dietary
antigen. Nat Med. 5:900–906. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hull MA, Faluyi OO, Ko CW, Holwell S,
Scott DJ, Cuthbert RJ, Poulsom R, Goodlad R, Bonifer C, Markham AF,
et al: Regulation of stromal cell cyclooxygenase-2 in the
ApcMin/+ mouse model of intestinal tumorigenesis.
Carcinogenesis. 27:382–391. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Leenen PJ, de Bruijn MF, Voerman JS,
Campbell PA and van Ewijk W: Markers of mouse macrophage
development detected by monoclonal antibodies. J Immunol Methods.
174:5–19. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Scott DJ, Hull MA, Cartwright EJ, Lam WK,
Tisbury A, Poulsom R, Markham AF, Bonifer C and Coletta PL: Lack of
inducible nitric oxide synthase promotes intestinal tumorigenesis
in the Apc(Min/+) mouse. Gastroenterology. 121:889–899. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Keshav S, Chung P, Milon G and Gordon S:
Lysozyme is an inducible marker of macrophage activation in murine
tissues as demonstrated by in situ hybridization. J Exp Med.
174:1049–1058. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lelouard H, Fallet M, de Bovis B, Méresse
S and Gorvel JP: Peyer's patch dendritic cells sample antigens by
extending dendrites through M cell-specific transcellular pores.
Gastroenterology. 142:592–601.e3. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Joeris T, Müller-Luda K, Agace WW and
Mowat AM: Diversity and functions of intestinal mononuclear
phagocytes. Mucosal Immunol. 10:845–864. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Emile JF, Julié C, Le Malicot K, Lepage C,
Tabernero J, Mini E, Folprecht G, Van Laethem JL, Dimet S,
Boulagnon-Rombi C, et al PETACC8 Study Investigators; Austrian
Breast and Colorectal cancer Study Group (ABCSG); Belgian Group of
Digestive Oncology (BGDO); John Allen Bridgewater, : Prospective
validation of a lymphocyte infiltration prognostic test in stage
III colon cancer patients treated with adjuvant FOLFOX. Eur J
Cancer. 82:16–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bain CC, Bravo-Blas A, Scott CL,
Perdiguero EG, Geissmann F, Henri S, Malissen B, Osborne LC, Artis
D and Mowat AI: Constant replenishment from circulating monocytes
maintains the macrophage pool in the intestine of adult mice. Nat
Immunol. 15:929–937. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shaw TN, Houston SA, Wemyss K, Bridgeman
HM, Barbera TA, Zangerle-Murray T, Strangward P, Ridley AJL, Wang
P, Tamoutounour S, et al: Tissue-resident macrophages in the
intestine are long lived and defined by Tim-4 and CD4 expression. J
Exp Med. 215:1507–1518. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang D and DuBois RN: The Role of
Prostaglandin E(2) in Tumor-Associated Immunosuppression. Trends
Mol Med. 22:1–3. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Faluyi OO, Fitch P and Howie SE: An
increased CD25-positive intestinal regulatory T lymphocyte
population is dependent upon Cox-2 activity in the
Apcmin/+ model. Clin Exp Immunol. 191:32–41. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bain CC, Scott CL, Uronen-Hansson H,
Gudjonsson S, Jansson O, Grip O, Guilliams M, Malissen B, Agace WW
and Mowat AM: Resident and pro-inflammatory macrophages in the
colon represent alternative context-dependent fates of the same
Ly6Chi monocyte precursors. Nat Immunol. 15:929–937. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zelenay S, van der Veen AG, Böttcher JP,
Snelgrove KJ, Rogers N, Acton SE, Chakravarty P, Girotti MR, Marais
R, Quezada SA, et al: Cyclooxygenase-dependent tumor growth through
evasion of immunity. Cell. 162:1257–1270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Grainger JR, Askenase MH,
Guimont-Desrochers F, da Fonseca DM and Belkaid Y: Contextual
functions of antigen-presenting cells in the gastrointestinal
tract. Immunol Rev. 259:75–87. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
de Jong JP, Voerman JS, van der
Sluijs-Gelling AJ, Willemsen R and Ploemacher RE: A monoclonal
antibody (ER-HR3) against murine macrophages. I. Ontogeny,
distribution and enzyme histochemical characterization of
ER-HR3-positive cells. Cell Tissue Res. 275:567–576. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
de Jong JP, Leenen PJ, Voerman JS, van der
Sluijs-Gelling AJ and Ploemacher RE: A monoclonal antibody (ER-HR3)
against murine macrophages. II. Biochemical and functional aspects
of the ER-HR3 antigen. Cell Tissue Res. 275:577–585. 1994.
View Article : Google Scholar : PubMed/NCBI
|