Introduction
Breast cancer is the leading cause of
cancer-associated mortality (17–20%) among women worldwide
(1). Patients with breast cancer
have a 41% 5-year survival rate, particularly those with metastatic
disease (1). Despite improvement in
the understanding of the pathogenic mechanisms involved in breast
cancer, considerable challenges remain in the prevention and
treatment of breast cancer.
MicroRNAs (miRNAs/miRs) are small non-coding RNA
molecules that are 20–22 nucleotides in length (2). miRNAs regulate various biological
processes, including development, differentiation, apoptosis and
proliferation, through imperfect pairing with target mRNAs of
protein-coding genes and transcriptional or post-transcriptional
regulation of their expression (3).
miRNAs have been proposed to contribute to
oncogenesis since they can function as either tumor suppressors or
oncogenes (3). For example, miR-124
functions as a tumor suppressor in the development of different
tumors, such as glioblastoma (4),
prostate cancer (5) gastric cancer
(6) and lung cancer (7). In addition, miR-124 has been reported
to inhibit the prognosis of patients with breast cancer by
targeting several genes (8–16). Shi et al (8) demonstrated that STAT3 is a downstream
target of miR-124, and STAT3 mRNA and protein downregulation was
observed in breast cancer cells with upregulated miR-124
expression. STAT3 expression is downregulated following
overexpression of miR-124, thereby inhibiting the proliferation and
invasion of triple-negative breast cancer cells (8). Another study demonstrated that miR-124
can inhibit epithelial-to-mesenchymal transition (EMT) and
metastasis of triple negative breast cancer cells by downregulating
zinc finger E-box binding homeobox 2 expression (9). In addition, miR-124 can significantly
inhibit the proliferation of breast cancer cells via cell cycle
arrest, but does not induce apoptosis, by suppressing CD151
expression (10). Yan et al
(11) identified
α-1,6-mannosylglycoprotein 6-β-N-acetylglucosaminyltransferase
(MGAT5) as a target of miR-124, whereby miR-124 can suppress the
proliferation and metastasis of breast cancer cells by regulating
MGAT5 expression. In addition, several target genes of miR-124 have
been identified in breast cancer, including snail family
transcriptional repressor 2 (12),
CBL (13), cyclin-dependent kinase 4
(14), flotillin-1 (15) and Ets-1 (16).
It has been reported that miR-124 regulates the
expression levels of the aforementioned genes, resulting in
inhibition proliferation and migration of breast cancer. In
addition, clinical data have demonstrated that patients with breast
cancer, with bone metastasis and high miR-124 expression have a
longer survival time (17), whereas
in vitro experiments suggest that cancer cell-derived
miR-124 may inhibit the survival and differentiation of osteoclast
progenitor cell by targeting interleukin 11 (17).
Taken together, these findings suggest that miR-124
acts as both a key regulator of several oncogenes and a potential
tumor suppressor in breast cancer. In addition, miR-124 is widely
accepted as a negative regulator and inhibitor of tumor-derived
cytokines, and abnormal miR-124 expression is associated with
breast cancer progression (8–16).
However, the molecular mechanism underlying the role of miR-124 in
breast cancer, notably in Her2-positive/triple-negative breast
cancer, is yet to be fully elucidated.
In the present study, miR-124 was overexpressed in
Her2-positive breast cancer SKBR3 cells using lentiviral
transduction. Subsequently, RNA sequencing was performed to
investigate changes in gene expression profiles following
overexpression of miR-124. The novel candidate miR-124 target genes
were screened using bioinformatics analysis and the corresponding
roles of these genes were investigated through proliferation and
migration experiments. Thus, the present study aimed to improve our
understanding of the potential role of miR-124 in breast cancer and
to provide potential strategies of therapeutic intervention.
Materials and methods
Construction of the vector and stable
cell line
The lentivirus with EGFP-expression miR-124
(lenti-miR-124) and negative control (lenti-NC) were purchased from
Sangon Biotech, Co., Ltd. The SKBR3 breast cancer cell line was
purchased from the American Type Culture Collection. A total of
2×10−5 SKBR3 cells were seeded into 6-well plates and
cultured in the DMEM (Thermo Fisher Scientific, Inc.) medium
containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) at 37°C
in the presence of 5% CO2 for 12 h. miR-124 precursor
sequences were amplified and cloned into the lentiviral vector
pCDH-CMV-MCS-EF1-copGFP (System Biosciences, LLC). A lentiviral
vector that expressed GFP alone was used as a control. pCDH-miR-124
or control vectors (6 µg) were co-transfected with the packaging
plasmids psPAX2 (4.5 µg) (System Biosciences, LLC) and pMD (1.5 µg)
(System Biosciences, LLC) into 3rd generation 293T cells
(3×106) using Lipofectamine 2000®
(Invitrogen; Thermo Fisher Scientific, Inc.). After 48 h in 5%
CO2 at 37°C, the virus-containing medium was harvested
and subsequently pre-cleaned with a 3,000 × g centrifugation step
and a 0.45 µm filtration (Millipore; Merck KGaA). The
virus-containing medium was overlaid on a sucrose-containing buffer
[50 mM Tris-HCl, pH 7.4,100 mM NaCl, 0.5 mM ethylene diamine tetra
acetic acid (EDTA)] at a 4:1v/v ratio and centrifuged at the
indicated 9,000 × g at 4°C. After centrifugation, the supernatant
was carefully removed and the tube was left standing for 3 min.
Phosphate buffered saline (PBS) was added to the semi-dried tube
for re-suspension and then the tube was kept at −80°C until further
use. SKBR3 cells were subsequently infected with lenti-miR-124 or
lenti-NC with MOI=1×10−6 in the presence of 8 µg/ml
polybrene (Chemicon International; Thermo Fisher Scientific, Inc.)
Following transduction for 96 h, EGFP positive SKBR3 cells were
selected using a FACS instrument (Celesta; BD Biosciences). The
target cells were harvested for subsequent experimentation.
Total RNA extraction and
sequencing
Total RNA was extracted from SKBR3 cells using the
RNeasy Mini kit (cat. no. 74104; Qiagen, Inc.), according to the
manufacturer's instructions. RNA Integrity Number (RIN) was
measured using Agilent 2100 bio-analyzer. An RNA sequencing (seq)
library was constructed using the Hieff NGSR MaxUP II DNA Library
Prep kit for Illumina (cat. no. 12200E; Shanghai Yeasen
Biotechnology Co., Ltd.), according to the manufacturer's
instructions. Sequencing was performed by Novogene Biotech
(www.novogene.com), using the HiSeq X Ten system
(Illumina, Inc.) with HiSeq X Ten Reagent kit v.2.5 for 2×150
cycles (paired-end read length of 150 bp), 300 pM DNA was the
loading concentration. Quality control was performed using Fastp
and clean reads (reads contaminated by adaptors were removed, reads
with Phred quality score <5 accounting for >50% were removed,
and reads with N content >10% were removed) were mapped to the
human genome (hg19) using TopHat software (v2.1.0) (18).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from SKBR3 cells using the
RNeasy Mini kit (cat. no. 74104 Qiagen, Inc.). RT of miR was
performed using the One Step miR cDNA Synthesis kit (cat. no.
D1801; Xinhai Gene Testing Co., Ltd.), and U6 was used as the
internal reference for miR-124. The RT steps used were as follows:
Initial denaturation step at 95°C for 30 sec, followed by 40 cycles
of amplification at 95°C for 5 sec and 60°C for 30 sec using SYBR
Green qPCR kit (cat. no. A2202A; Xinhai Gene Testing Co., Ltd.,).
The relative transcript levels were quantified using the
2−ΔΔCq method (19) on
the ABI 7500 (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The following primer sequences were used for qPCR: miR-124 forward,
5′-TAAGGCACGCGGTGAATGCC-3′ and reverse,
5′-CAGGTCCAGTTTTTTTTTTTTTTTVN-3′; U6 forward,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′. β-actin forward,
5′-AAAGACCTGTACGCCAACAC-3′ and reverse,
5′-GTCATACTCCTGCTTGCTGAT-3′; BCL6 forward,
5′-GACTCTGAAGAGCCACCTG-3′ and reverse, 5′-CTGGCTTTTGTGACGGAAAT-3′;
IFR1 forward, 5′-ATGGCGACTAAGAAGCACAC-3′ and reverse,
5′-CGAAGCCTGCTCATTGTAGT-3′; Mxd1 forward,
5′-TGAACATGGTTATGCCTCCA-3′ and reverse, 5′-ACTTGATTCGGGTCCAAGTG-3′;
LIF forward, 5′-TCTTGGCGGCAGGAGTTGTG-3′ and reverse,
5′-CTTCTCCGTGCCGTTGGCGT-3′; and TFAP4 forward,
5′-GCAGGCAATCCAGCACAT-3′ and reverse, 5′-GGAGGCGGTGTCAGAGGT-3′.
The following thermocycling conditions were used:
Initial denaturation at 50°C for 2 min and 95°C for 5 min, followed
by 40 amplification cycles of 95°C for 20 sec, 65°C for 10 sec and
72°C for 30 sec in the ABI instrument (ABI).
Western blotting
SKBR3 were lysed in NP-40 Lysis-Buffer [150 mM NaCl,
1% NP-40 and 50 mM Tris (pH 8.0)] and analyzed via BCA protein
assay (cat. no. P0011; Beyotime Institute of Biotechnology).
Proteins were denatured by heating for 5 min at 85°C, separated via
10% SDS-PAGE (50 µg protein/lane) and transferred to PVDF membranes
(Merck KGaA). Membranes were blocked with 5% bovine serum albumin
for 2 h at room temperature, and subsequently incubated with
anti-TFAP4 antibody (1:1,000; cat. no. ab223771; Abcam) or
anti-GAPDH (1:5,000; cat. no. bsm-33033M; BIOSS) overnight at 4°C.
After washing 3 times with PBS-Tween (0.1% Tween-20), membranes
were incubated with HRP-conjugated goat anti-rabbit IgG (1:10,000;
cat. no. bs-0295G-HRP; BIOSS) at room temperature for 2 h. Protein
bands were visualized using the chemiluminescence kit (Thermo
Fisher Scientific, Inc.).
Gene knockdown assay
Cells were seeded into 6-well plates and grown until
60% confluence. Cells were subsequently transfected with 50 nM
small interfering (si) RNA using Hiperfect reagent (Takara Bio,
Inc.) in Opti-MEM medium (Thermo Fisher Scientific, Inc.).
Transfected cells were cultured at 37°C in the presence of 5%
CO2 for 48 h, after which subsequent experiments were
performed. The TFAP4 targeting siRNA and non-target scramble
controls were provided by Sangon Biotech Co., Ltd. (TFAP4 siRNA,
5′-GUGAUAGGAGGGCUCUGUAG-3′; and control
5′-GUAUCGGCUUAUCAGUCCGAGUAATT-3′).
Report gene assay
The dual-luciferase reporter assay was performed,
according to the manufacturer's instructions (cat. no. E1910;
Promega Corporation). Briefly, the sequence wild type and mutated
3′-untranslated region (UTR) of TFAP4 was synthesized by Sangon
Biotech, Co., Ltd., and subcloned downstream into the luciferase
reporter gene pmirGLO vector (Promega Corporation).
pmirGLO-3′-UTR-TFAP4 and the sequence of precursor miR-124 were
subcloned into pSuper (OligoEngine), and the resulting
pSuper-miR124, together with the reporter plasmid, were
co-transfected into 293T cells using Lipofectamine® 3000
(Thermo Fisher Scientific, Inc.). Following transfection and
cultured at 37°C in the presence of 5% CO2 for 48 h,
cells were harvested and lysed with 200 µl Reporter Lysis Buffer
(Promega Corporation). Luciferase activities were detected using
the Luciferase Assay System (Promega Corporation). In this assay,
the activity of firefly represented the experimental results and
Renilla luciferase activity was used to normalize the data.
The experiment was set up in triplicate and the experiment was
carried out three times.
Cell invasion assay
The Transwell Matrigel™ assay was performed to
assess cell invasion. Briefly, each well was coated with 60 µl
Matrigel at 37°C for 1 h. A total of 200 µl of DMEM medium without
serum (Thermo Fisher Scientific, Inc.) containing 2×105
cells were plated in the upper chambers of 24-well Transwell plates
with polycarbonate filters of 8-µm pores (Corning, Inc.), while 600
µl of DMEM medium (Thermo Fisher Scientific, Inc.) with 20% FBS was
plated in the lower chambers. Following incubation at 37°C for 24
h, cells in the upper chambers were removed using a cotton swab,
and the migratory cells were fixed with 4% paraformaldehyde at 4°C
for 1 h and stained with 2.5% crystal violet at room temperature
for 1 h. Stained cells were counted in six randomly selected fields
using a light microscope (magnification, ×100).
Cell proliferation assay
The Cell Counting Kit-8 (CCK-8) assay (Takara Bio,
Inc.) was performed to assess cell proliferation. For the CCK-8
assay, 5,000 cells were seeded into 96-well plates. At the 24, 48,
72 and 96 h, 10 µl CCK-8 solution was added to each well and
incubated for 4 h at 37°C. Following incubation, cell proliferation
was measured at a wavelength of 450 nm.
Bioinformatics analysis
Cuffcompare v.0.8.3 was used to compare the
similarity of transcripts and assess the construction of
transcripts (20). Cuffmerge was
used to combine multiple transcript sets into a single transcript
set (20). Gene expression pattern
was analyzed using PlotPCA package v1.12.3 (21) to reduce the dimensionality and a
heatmap was constructed according to the gene expression using
Pheatmap package v.1.0.10, (https://www.rdocumentation.org/packages/pheatmap/versions/1.0.10)
in the R language. Cuffdiff was used to screen differentially
expressed genes (DEGs) (20) and
Ggplot2 package v.3.3.0 (https://www.rdocumentation.org/packages/ggplot2/versions/3.3.0)
was used to construct the volcano plot. The distribution of DEGs
was mapped on chromosomes using graphics function of R language.
Gene Ontology (GO) enrichment analysis of DEGs was performed using
Clusterprofiler package v.3.0.2, (https://www.rdocumentation.org/packages/clusterProfiler/versions/3.0.2)
of R language. The similarities between GO terms were calculated
using GOSemSim v.1.28.1, (https://www.rdocumentation.org/packages/GOSemSim/versions/1.28.1)
and GO terms cluster was labeled using Ggtree v.1.4.11, (https://www.rdocumentation.org/packages/ggtree/versions/1.4.11).
The potential downstream genes of miR-124 was predicted using the
TargetScan database v.7.2 (http://www.targetscan.org/vert_72/). Combined with the
DEGs from RNA-seq, the intersection was used for semantic
similarity analysis using GOSemSim. The Protein-protein interaction
network was analyzed using the Search Tool for the Retrieval of
Interacting Genes/Proteins (STRING) database (https://string-db.org). The threshold for screening
DEGs was adjusted P<0.05, and the multiple difference was
>2-fold. Adjusted P-value <0.05 and gene counts >5 were
used as thresholds to identify significant GO terms.
Ethics
Although human samples or patient data were not
included in the present study, CNSA requires ethics approval when
uploading sequencing data. The present study was approved by the
Ethics Committee of Inner Mongolia Autonomous Region People's
Hospital, (Hohhot, China) (approval no. 202001005L).
Statistical analysis
GraphPad Prism 8.0 (GraphPad Software Inc.) was used
for statistical analysis. All experiments were performed in
triplicate and data are presented as the mean ± standard deviation.
unpaired Student's t-test was used to compare differences between
two groups, while one-way ANOVA and Bonferroni post hoc test
(non-parametric test) were used to compare differences between
multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Overexpression of miR-124 suppresses
the migration and proliferation of SKBR3 cells
To determine the role of miR-124 in the pathogenesis
of Her2-positive breast cancer, miR-124 was overexpressed in SKBR3
cells using lentivirus and RT-qPCR analysis was performed to assess
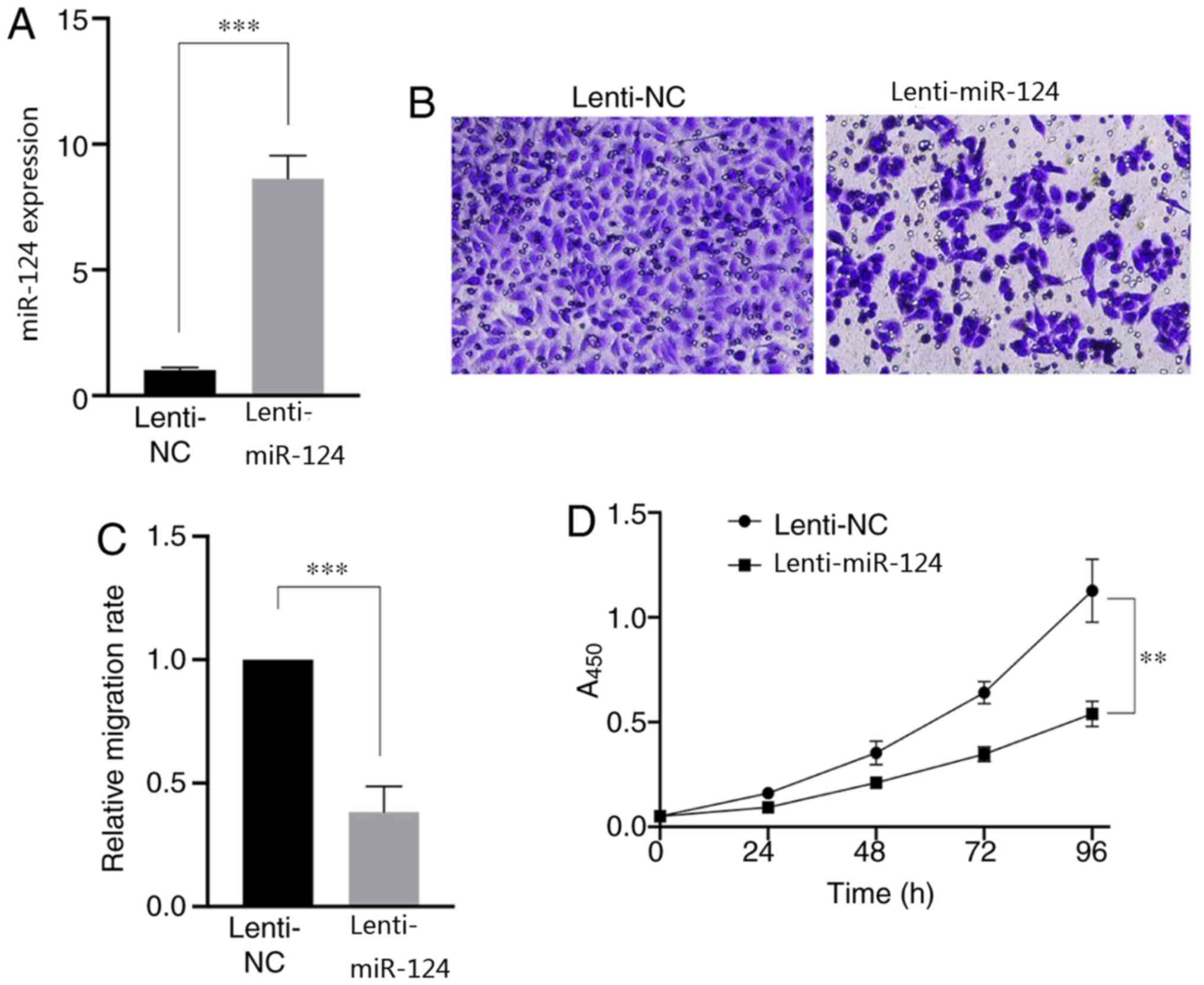
transfection efficiency (Fig. 1A).
Subsequently, cell migration and proliferation assays were
performed to assess the change in cell malignancy following
overexpression of miR-124. The results demonstrated that
overexpression of miR-124 significantly impaired cell migration
(P<0.001; Fig. 1B and C) and
suppressed cell proliferation (Fig.
1D). Taken together, these results suggest that overexpression
of miR-124 can significantly influence the migratory and
proliferative abilities of SKBR3 cells (P<0.01). Thus, total RNA
was extracted from SKBR3 cells overexpressed with miR-124 for
sequencing.
Quality evaluation of sequencing
data
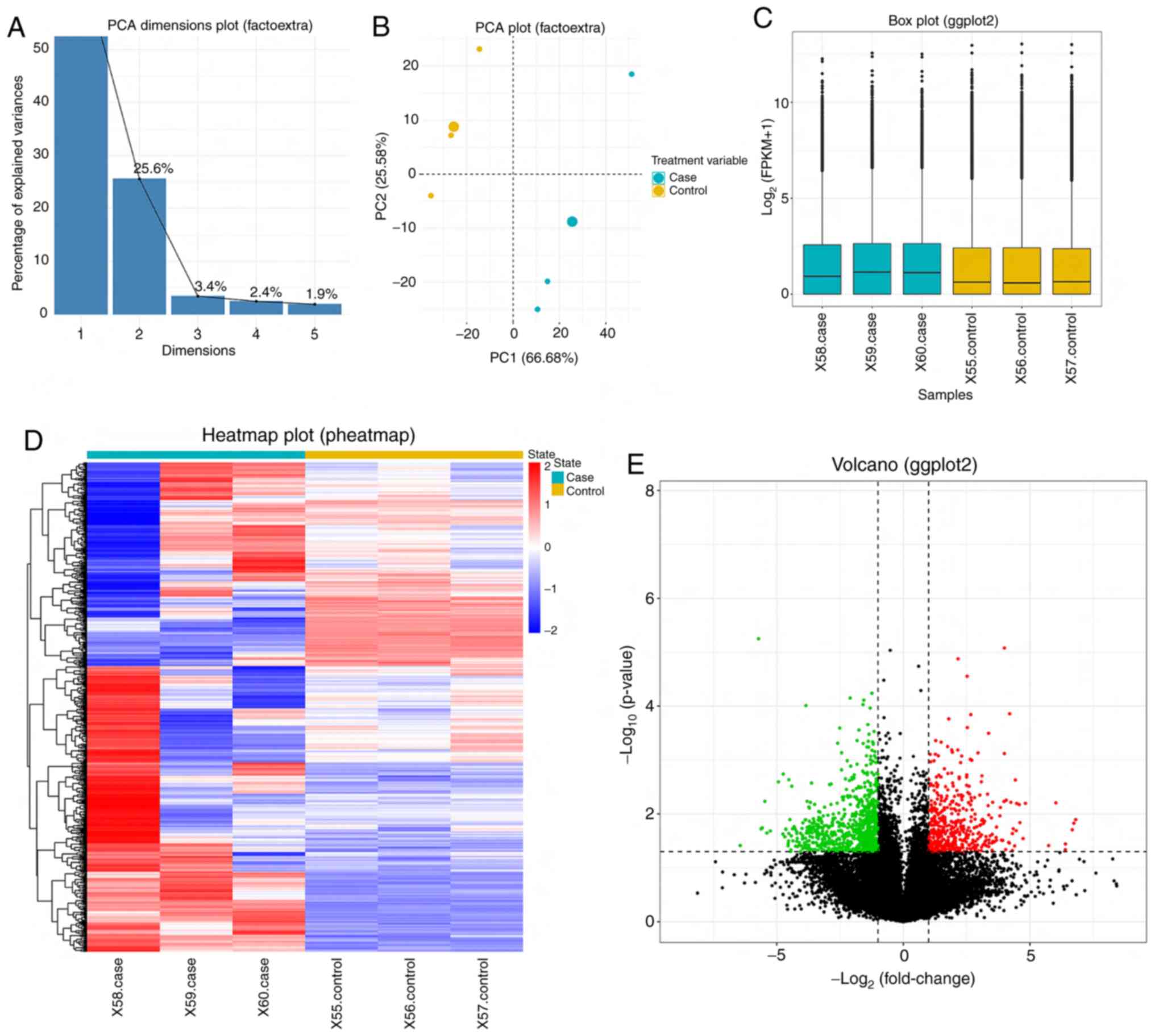
In the present study, the PlotPCA package was used
to assess the transcriptome sequencing data of SKBR3 cells
overexpressed with miR-124 and control cells. Following
dimensionality reduction, the two principal components, PC1 and
PC2, can explain >80% of the sample differences, which
demonstrated that PC1 and PC2 were sufficient for cluster analysis
of the differences in miR-124 expression (Fig. 2A). Since the transcriptome data of
the same processed samples should have had the same
characteristics, the principal components, PC1 and PC2, were used
to depict the expression characteristics of different samples. The
results demonstrated that both SKBR3 cells overexpressed with
miR-124 and control cells exhibited high similarity; however, the
expression characteristics of the two groups of samples were
different (Fig. 2B).
DEGs
Raw reads contaminated by adaptors were removed,
reads with Phred quality score <5 accounting for >50% were
removed, and reads with N content >10% were removed. Clean reads
were mapped to the reference to calculate the expression value o.
The expression value was normalized by sequencing depth to ensure
the expression levels of all genes were comparable across samples,
which indicated the quality of the original data (Fig. 2C). Hierarchical cluster analysis
exhibited the comprehensive DEG patterns of samples with miR-124
overexpression and miR-NC. The effect of miR-124 on DEGs in SKBR3
cells further supported the role of miR-124 in breast cancer
(Fig. 2D). Differential expression
analysis was performed with all the genes and 716 DEGs were
identified between the control group and the miR-124 overexpression
group. Thus, PCR analysis was subsequently performed to verify the
results following bioinformatic analysis. Among the 717 DEGs, 418
genes were upregulated and 299 genes were downregulated following
overexpression of miR-124 (Fig. 2E).
The detailed list of gene expression is presented in Table SI.
miR-124 associated pathway and genes
analysis
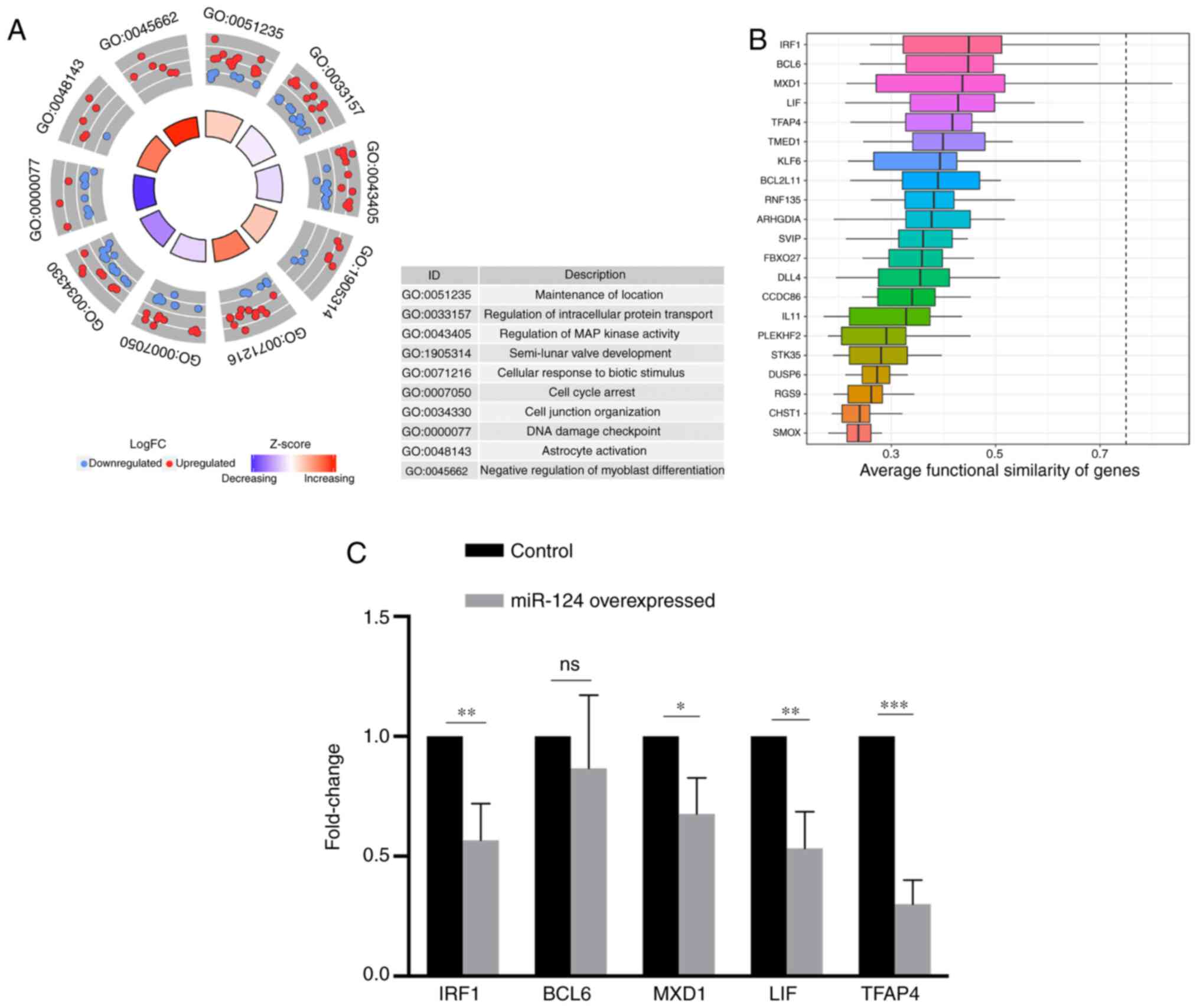
GO enrichment analysis was performed on the DEGs.
Using the corrected P-value as the threshold, a total of 42 GO
pathways were identified (Table
SII), of which the top ten pathways were ‘maintenance of
location’, ‘regulation of intracellular protein transport’,
‘regulation of MAP kinase activity’, ‘semi-lunar valve
development’, ‘cellular response to biotic stimulus’, ‘cell cycle
arrest’, ‘cell junction organization’, ‘DNA damage checkpoint’,
‘astrocyte activation’ and ‘negative regulation of myoblast
differentiation’ (Fig. 3A). Given
that miRNAs function to degrade the target gene RNA (3), genes with cells expressing low levels
of miR-124 and genes that overlapped with target genes regulated by
miR-124, which was predicted using the TargetScan database
(http://www.targetscan.org/vert_72/),
were selected. To determine the genes most closely associated with
miR-124 in SKBR3 cells in this list, the average functional
similarity associations of the genes were calculated. The results
demonstrated that the four genes with the highest scores were
interferon regulatory factor 1 (IRF1), B-cell lymphoma 6 (BCL6),
MXD1 and leukemia inhibitory factor (LIF) (Fig. 3B). Notably, although the negative
regulation of myoblast differentiation and astrocyte activation
pathways in the GO enrichment analysis had the largest proportion
of genes downregulated by miR-124, the two most important genes
identified in functional similarity analysis, IRF1 and LIF genes,
were mainly enriched in the ‘cell cycle arrest’ term.
TFAP4 expression is regulated by
miR-124
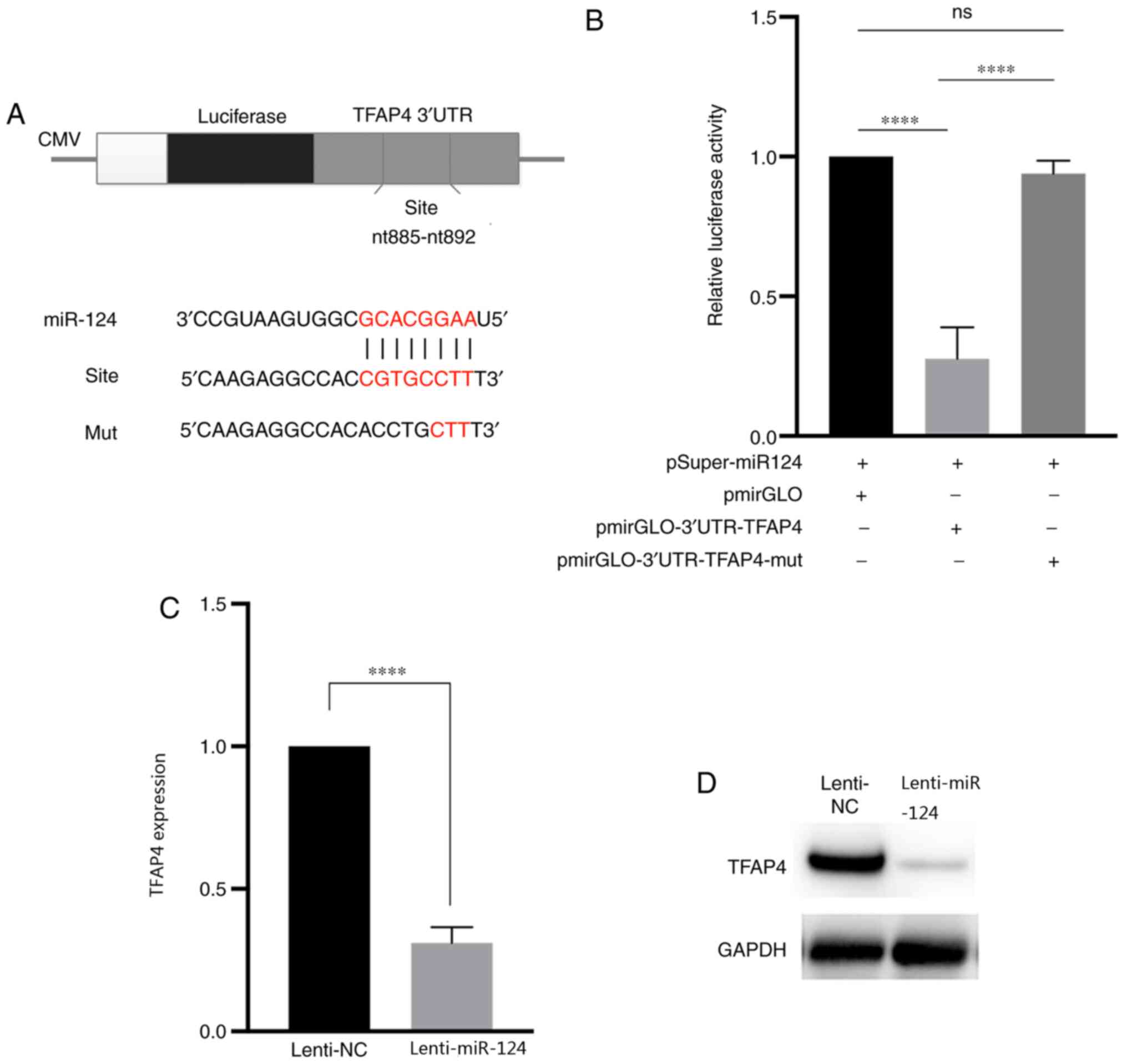
The dual-luciferase reporter assay was performed to
determine whether TFAP4 was directly regulated by miR-124 (Fig. 4A). The overexpression of miR-124
significantly decreased the relative luciferase activity of cells
transfected with 3′-UTR of the TFAP4, the results of RT-qPCR and
western blot analyses demonstrated decreased mRNA and protein
levels of TFAP4 (Fig. 4B and C). In
addition, TFAP4 expression significantly decreased following
overexpression of miR-124 (Fig. 4D),
suggesting that miR-124 inversely regulates TFAP4 expression.
Downregulation of TFAP4 attenuates the
migratory ability of SKBR3 cells
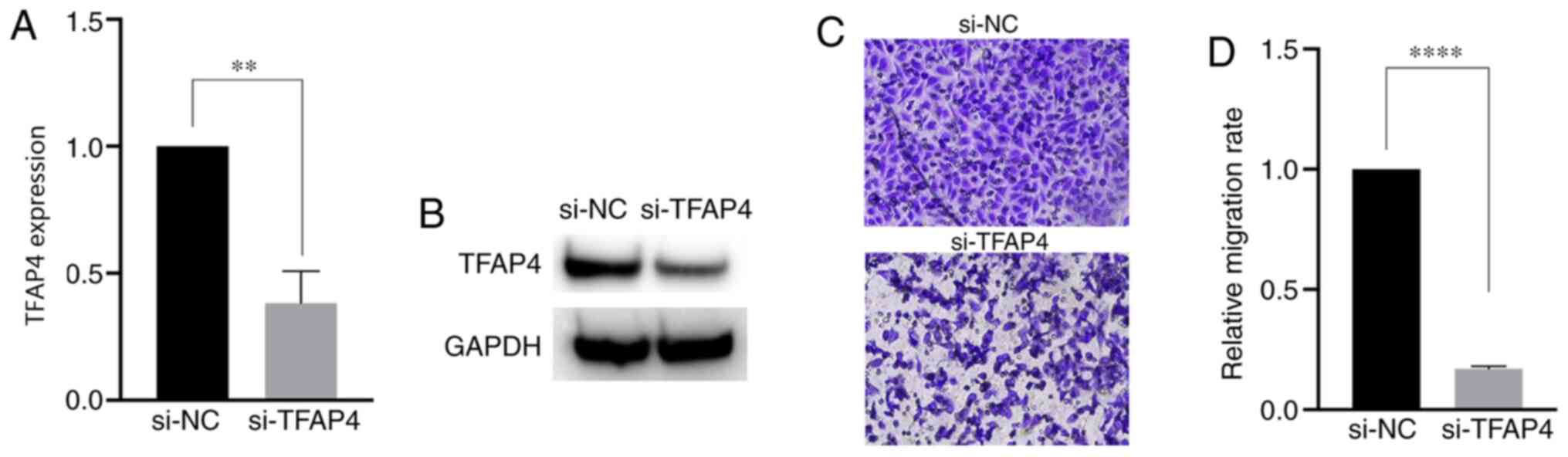
To further confirm that downregulation of TFAP4 can
affect the migratory ability of SKBR3 cells, cells were transfected
with si-TFAP4. As presented in Fig. 5A
and B, TFAP4 mRNA and protein expression levels were
significantly downregulated in SKBR3 cells following TFAP4
knockdown compared with the control. As presented in Fig. 5C and D, the migratory ability of
SKBR3 cells was significantly reduced following TFAP4 knockdown
compared with the control samples. Taken together, these results
suggest that downregulation of TFAP4 significantly impairs the
migratory ability of SKBR3 cells.
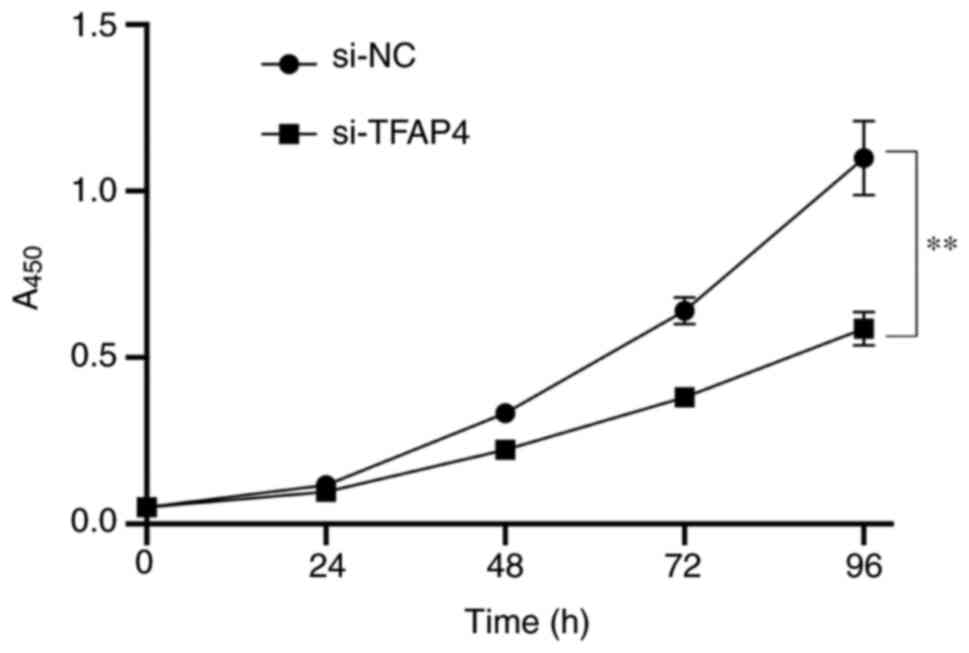
Downregulation of TFAP4 suppresses the
proliferation of SKBR3 cells
The CCK-8 assay was performed to assess the effect
of downregulating TFAP4 expression on the proliferation of SKBR3
cells. As presented in Fig. 6,
transfection with si-TFAP4 significantly decreased the
proliferative ability of SKBR3 cells compared with the control
group, which is consistent with the results following
overexpression of miR-124 in SKBR3 cell (Fig. 1D). Taken together, these results
suggest that TFAP4 knockdown significantly decreases the
proliferative ability of SKBR3 cells.
Discussion
miR-124 targets several genes, such as STAT3, CD151
and MGAT5 (α-1,6-mannosylglycoprotein
6-β-N-acetylglucosaminyltransferase) (8–16); thus,
the present study aimed to investigate changes in malignancy in
SKBR3 cells and screen potential novel target genes for miR-124.
Thus, miR-124 was overexpressed in SKBR3 cells. By sequencing the
transcriptome of the transfected cells, it was determined that
overexpression of miR-124 significantly changed gene expression in
SKBR3 cells compared with the control group, and ultimately
decreased the malignancy of tumor cells, which was consistent with
the findings by Lin et al (22). In addition, the results of the
present study demonstrated that miR-124 negatively regulated the
expression of the TFAP4 gene, and downregulation of TFAP4
expression significantly impaired the migratory and proliferative
abilities of SKBR3 cells.
The TFAP4 gene is a member basic helix-loop-helix
leucine-zipper domain family (23)
and participates in the regulation of cell proliferation and
differentiation, metastasis, angiogenesis, as well as other
biological functions in tumors (24). Overexpression of TFAP4 is associated
with unfavorable prognosis of patients with gastric cancer
(25), colorectal cancer (24), prostate cancer (26) and non-small cell lung carcinoma
(27). Mechanistically, Huang et
al (28) demonstrated that TFAP4
can promote hepatocellular carcinoma invasion and metastasis by
activating the PI3K/AKT signaling pathway (28), EMT (29) and the Wnt/β-catenin pathway (30). It has also been reported that
abnormal degradation of TFAP4 can block cell mitosis, leading to
the activation of the DNA damage response (31). This finding is consistent with the
results of the present study, where TFAP4 knockdown blocked the
proliferation of SKBR3 cells, not only inducing cell cycle arrest,
but also leading to cell apoptosis (27) and significantly impairing the
migratory and proliferative abilities of SKBR3 tumor cells.
Previous studies have reported that TFAP4 is
regulated by other miRNAs. For example, miR-302c suppresses EMT and
metastasis by targeting TFAP4 in colorectal cancer (32). In addition, TFAP4 is a direct target
of miR-15a/16-1, which is induced by p53 (33). In the present study, TFAP4 was
identified as a direct target of miR-124 in SKBR3 cells.
Overexpression of miR-124 significantly attenuated the migratory
and proliferative abilities of SKBR3 cells by downregulating TFAP4
expression. Taken together, these results suggest that miR-124
exerts anti-metastatic and anti-proliferative roles in SKBR3 cells
by downregulating TFAP4 expression.
The present study had limitations. The present study
only evaluated the function of miR124/TFAP4 axis in
Her2+ breast cancer cell line SKBR3. The function of
this regulatory mechanism in HER 2− breast cancer will
be investigated in future studies. In addition, the results of the
present study were not verified in clinical samples. Future studies
need to perform experiments in clinical samples to verify the
findings of the present study.
In conclusion, the results of the present study
suggested that miR-124 can attenuate the migration and
proliferation of SKBR3 cells by directly downregulating TFAP4
expression. This confirms the anti-metastatic role of miR-124,
which may represent a potential candidate for effective treatment
of breast cancer.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Sciences Foundation of Inner Mongolia (grant no. 2018MS08010).
Availability of data and materials
The data that support the findings of the present
study are available in the CNSA (https://db.cngb.org/cnsa) of CNGBdb, with accession
number CNP0001017. The data will be available from this repository
one year after initially submission (April 24th, 2021).
Authors' contributions
NC performed the cell experiments, prepared the
figures and wrote the manuscript. BJ and YH performed the qPCR and
western blot analysis and data interpretation. WL, XH and WG
performed the bioinformatics analysis. YG and WB designed the study
and revised the manuscript. NC and YG confirm the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Although human samples or patient data were not
included in the present study, CNSA requires ethics approval when
uploading sequencing data. The present study was approved by the
Ethics Committee of Inner Mongolia Autonomous Region People's
Hospital (Hohhot, China) (approval no. 202001005L).
Patient consent for publication
Not applicable.
Competing interests
These authors declare that they have no competing
interests.
References
|
1
|
Azamjah N, Soltan-Zadeh Y and Zayeri F:
Global trend of breast cancer mortality rate: A 25-year study.
Asian Pac J Cancer Prev. 20:2015–2020. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li M, Marin-Muller C, Bharadwaj U, Chow
KH, Yao QZ and Chen CY: MicroRNAs: Control and loss of control in
human physiology and disease. World J Surg. 33:667–684. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oliveto S, Mancino M, Manfrini N and Biffo
S: Role of microRNAs in translation regulation and cancer. World J
Biol Chem. 8:45–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Silber J, Lim DA, Petritsch C, Persson AI,
Maunakea AK, Yu M, Vandenberg SR, Ginzinger DG, James CD, Costello
JF, et al: miR-124 and miR-137 inhibit proliferation of
glioblastoma multiforme cells and induce differentiation of brain
tumor stem cells. BMC Med. 6:142008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen J, Xiao H, Huang Z, Hu Z, Qi T, Zhang
B, Tao X and Liu SH: MicroRNA124 regulate cell growth of prostate
cancer cells by targeting iASPP. Int J Clin Exp Pathol.
7:2283–2290. 2014.PubMed/NCBI
|
|
6
|
Hu CB, Li QL, Hu JF, Zhang Q, Xie JP and
Deng L: miR-124 inhibits growth and invasion of gastric cancer by
targeting ROCK1. Asian Pac J Cancer Prev. 15:6543–6546. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma T, Zhao Y, Wei K, Yao G, Pan C, Liu B,
Xia Y, He Z, Qi X, Li Z, et al: MicroRNA-124 functions as a tumor
suppressor by regulating CDH2 and epithelial-mesenchymal transition
in non-small cell lung cancer. Cell Physiol Biochem. 38:1563–1574.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi P, Chen C, Li X, Wei Z, Liu Z and Liu
Y: MicroRNA124 suppresses cell proliferation and invasion of triple
negative breast cancer cells by targeting STAT3. Mol Med Rep.
19:3667–3675. 2019.PubMed/NCBI
|
|
9
|
Ji H, Sang M, Liu F, Ai N and Geng C:
miR-124 regulates EMT based on ZEB2 target to inhibit invasion and
metastasis in triple-negative breast cancer. Pathol Res Pract.
215:697–704. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han ZB, Yang Z, Chi Y, Zhang L, Wang Y, Ji
Y, Wang J, Zhao H and Han ZC: MicroRNA-124 suppresses breast cancer
cell growth and motility by targeting CD151. Cell Physiol Biochem.
31:823–832. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yan G, Li Y, Zhan L, Sun S, Yuan J, Wang
T, Yin Y, Dai Z, Zhu Y, Jiang Z, et al: Decreased miR-124-3p
promoted breast cancer proliferation and metastasis by targeting
MGAT5. Am J Cancer Res. 9:585–596. 2019.PubMed/NCBI
|
|
12
|
Du S, Li H, Sun X, Li D, Yang Y, Tao Z, Li
Q and Liu K: MicroRNA-124 inhibits cell proliferation and migration
by regulating SNAI2 in breast cancer. Oncol Rep. 36:3259–3266.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Chen L, Wu Z, Wang M, Jin F, Wang
N, Hu X, Liu Z, Zhang CY, Zen K, et al: miR-124-3p functions as a
tumor suppressor in breast cancer by targeting CBL. BMC Cancer.
16:8262016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feng T, Xu D, Tu C, Li W, Ning Y, Ding J,
Wang S, Yuan L, Xu N, Qian K, et al: miR-124 inhibits cell
proliferation in breast cancer through downregulation of CDK4.
Tumour Biol. 36:5987–5997. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li L, Luo J, Wang B, Wang D, Xie X, Yuan
L, Guo J, Xi S, Gao J, Lin X, et al: Microrna-124 targets
flotillin-1 to regulate proliferation and migration in breast
cancer. Mol Cancer. 12:1632013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li W, Zang W, Liu P, Wang Y, Du Y, Chen X,
Deng M, Sun W, Wang L, Zhao G and Zhai B: MicroRNA-124 inhibits
cellular proliferation and invasion by targeting Ets-1 in breast
cancer. Tumour Biol. 35:10897–10904. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cai WL, Huang WD, Li B, Chen TR, Li ZX,
Zhao CL, Li HY, Wu YM, Yan WJ and Xiao JR: microRNA-124 inhibits
bone metastasis of breast cancer by repressing Interleukin-11. Mol
Cancer. 17:92018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Trapnell C, Roberts A, Goff L, Pertea G,
Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL and Pachter L:
Differential gene and transcript expression analysis of RNA-seq
experiments with TopHat and Cufflinks. Nat Protoc. 7:562–578. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Trapnell C, Williams BA, Pertea G,
Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ and Pachter
L: Transcript assembly and quantification by RNA-Seq reveals
unannotated transcripts and isoform switching during cell
differentiation. Nat Biotechnol. 28:511–515. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Love MI, Huber W and Anders S: Moderated
estimation of fold change and dispersion for RNA-seq data with
DESeq2. Genome Biol. 15:5502014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin J, Wen X, Zhang X, Sun X, Yunzhi L,
Peng R, Zhu M, Wang M, Zhang Y, Luo W, et al: miR-135a-5p and
miR-124-3p Inhibit Malignancy of Glioblastoma by Downregulation of
Syndecan Binding Protein. J Biomed Nanotechnol. 14:1317–1329. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee SU, Song HO, Lee W, Singaravelu G, Yu
JR and Park WY: Identification and characterization of a putative
basic helix-loop-helix (bHLH) transcription factor interacting with
calcineurin in C. elegans. Mol Cells. 28:455–461. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li X, Liang L, Huang L, Ma X, Li D and Cai
S: High expression of protein phosphatase 4 is associated with the
aggressive malignant behavior of colorectal carcinoma. Mol Cancer.
14:952015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu X, Zhang B, Guo Y, Liang Q, Wu C, Wu
L, Tao K, Wang G and Chen J: Down-regulation of AP-4 inhibits
proliferation, induces cell cycle arrest and promotes apoptosis in
human gastric cancer cells. PLoS One. 7:e370962012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cancer Genome Atlas Network: Comprehensive
molecular characterization of human colon and rectal cancer.
Nature. 487:330–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang YF, Ao X, Liu Y, Ding D, Jiao WJ, Yu
Z, Zhai WX, Dong SH, He YQ, Guo H and Wang JX: MicroRNA-608
promotes apoptosis in non-small cell lung cancer cells treated with
doxorubicin through the inhibition of TFAP4. Front Genet.
10:8092019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang T, Chen QF, Chang BY, Shen LJ, Li W,
Wu PH and Fan WJ: TFAP4 promotes hepatocellular carcinoma invasion
and metastasis via activating the PI3K/AKT signaling pathway. Dis
Markers. 2019:71292142019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song J, Xie C, Jiang L, Wu G, Zhu J, Zhang
S, Tang M, Song L and Li J: Transcription factor AP-4 promotes
tumorigenic capability and activates the Wnt/β-catenin pathway in
hepatocellular carcinoma. Theranostics. 8:3571–3583. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
D'Annibale S, Kim J, Magliozzi R, Low TY,
Mohammed S, Heck AJ and Guardavaccaro D: Proteasome-dependent
degradation of transcription factor activating enhancer-binding
protein 4 (TFAP4) controls mitotic division. J Biol Chem.
289:7730–7737. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ma W, Liu B, Li J, Jiang J, Zhou R, Huang
L, Li X, He X and Zhou Q: MicroRNA-302c represses
epithelial-mesenchymal transition and metastasis by targeting
transcription factor AP-4 in colorectal cancer. Biomed
Pharmacother. 105:670–676. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shi L, Jackstadt R, Siemens H, Li H,
Kirchner T and Hermeking H: p53-induced miR-15a/16-1 and AP4 form a
double-negative feedback loop to regulate epithelial-mesenchymal
transition and metastasis in colorectal cancer. Cancer Res.
74:532–542. 2014. View Article : Google Scholar : PubMed/NCBI
|