Introduction
Cervical cancer is the second most common
gynecological malignancy, with an estimated 570,000 cases and
311,000 deaths in 2018 worldwide (1). Although several studies have revealed
that the human papillomavirus is the most important cause of
cervical cancer, other factors are required for malignant
transformation of cervical cell (2).
Genetic factors are also involved in the development of cervical
cancer (3). Thus, innovative
biomarkers and related molecular mechanisms are essential for the
diagnosis, prognosis and treatment of cervical cancer.
DNA methylation is one of the predominant epigenetic
modifications in mammals, which performs a critical function in
regulating gene expression (4).
Aberrant DNA methylation, particularly methylation of CpG islands
in gene promoter regions, often occurs in different types of
cancer, incuding cervical cancer and is an early event of malignant
transformation (5,6). P16 is a common studied tumor suppressor
gene (7). The promoter regions of
p16 are often methylated, which decreases the levels of p16 in
cervical cancer (8). Previous
studies have demonstrated that p16 promoter methylation is closely
associated with the development and progression of cervical cancer,
so it is considered a potential diagnostic and therapeutic target
(9,10). The changes involved in DNA
methylation are controlled by DNA methyltransferases (DNMTs)
(11). A total of three
catalytically active DNMTs (DNMT1, DNMT3A and DNMT3B) have been
identified in mammals (4). DNMT1
maintains methylation pattern, while DNMT3A and DNMT3B are
responsible for de novo DNA methylation (12). Previous studies have reported
elevated levels of DNMT1, DNMT3A and DNMT3B in various tumors,
including hepatic, prostate, colorectal and breast cancers
(13–16). Recently, high DNMT1 protein
expression was reported in cervical cancer, and is associated with
poor survival outcome (17).
Inhibition of DNMTs can reactivate the expression of
methylation-silenced tumor suppressor genes in human cervical
cancer cells (18–20).
MicroRNAs (miRNAs/miRs) are a class of short (20–24
nucleotides) non-coding RNA molecules that negatively regulate gene
expression by translational inhibition or destabilization of
targets through binding to the 3′-untranslated region (UTR) of
mRNAs (21). miRNAs play important
roles in several biological processes, such as cell proliferation,
apoptosis, differentiation and cell cycle (22). Thus, abnormal expression or
dysfunction of miRNAs are associated with the development of
diseases, including cancer (23).
miR-29a is a tumor suppressor gene that can inhibit the malignant
proliferation, invasion and metastasis of several human cancer
cells (24–26). Furthermore, miR-29a suppresses cell
proliferation by targeting SIRT1 in cervical and hepatocellular
carcinomas (27,28). miR-29a also inhibits cell
proliferation, migration and invasion by directly targeting CDC42
in cervical cancer, osteosarcoma, gliomas and breast cancer
(29–32). In addition, miR-29a inhibits cancer
cell migration and invasion by targeting HSP47 in cervical squamous
cell carcinoma (33). Increasing
evidence suggests that miR-29a regulates DNA methylation with the
suppression of DNMTs in lung cancer (34), hepatocellular carcinoma (35,36),
Burkitt lymphoma cells (37) and
T-cell acute lymphoblastic leukemia (38).
Thus, the present study aimed to investigate the
role of miR-29a and whether miR-29a regulates the methylated status
of p16 promoter by modulation of DNMT3s in cervical cancer.
Materials and methods
Tissue samples
In the present study, 40 patients who underwent
cervical cancer surgery at the Affiliated Hospital of Qinghai
University between January 2017 and December 2018 were recruited.
All participants were female, with a median age of 55 years (age
range, 32–68 years). Cervical cancer tissues and paired adjacent
normal tissues were collected in surgery, and the distance between
adjacent normal and cancer tissue boundary was ~1–2 cm. The present
study was approved by the Ethics Committee of the Affiliated
Hospital of Qinghai University (Xining, China; approval no.
SL-2018016) and written informed consent was provided by all
patients prior to the study start. Tissue samples were obtained
during surgical resection and immediately snap-frozen in liquid
nitrogen, and stored at −80°C until subsequent experimentation.
Diagnosis was independently confirmed via two pathologists from the
Affiliated Hospital of Qinghai University.
Cell culture
The human cervical cancer cell lines, HeLa and
C-33A, were purchased from The Cell Bank of the Chinese Academy of
Sciences, while the ectocervical epithelial cell line, ECT1/E6E7,
was purchased from the American Type Culture Collection. The
cervical cell lines were maintained in DMEM supplemented with 10%
fetal bovine serum (both purchased from Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc.), at 37°C with 5%
CO2.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from cervical cancer cells
and tissues using TRIzol® reagent (Invitrogen, Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
RT was performed using PrimeScript 1st Strand cDNA synthesis kit
(cat. no. 6110A; Takara Bio, Inc.) at 37°C for 15 min. qPCR was
subsequently performed using the SYBR Green PCR Master Mix (Takara
Bio, Inc.). The following conditions were used for all RT-PCR
assays: 95°C for 30 sec, followed by 40 cycles of 95°C for 15 sec
and 60°C for 35 sec. After the PCR run, a melting curve analysis
was performed at a melting rate of 0.1°C/sec, and data were
collected every 0.23°C from 6–95°C (LineGene9600 version 1, Bioer
Technology). Relative expression levels were calculated using the
2−ΔΔCq method (39) and
all experiments were performed in triplicate. miR-29a expression
was assessed via the stem-loop RT primer assay and U6 was used as
the internal control. DNMT3A and DNMT3B mRNA expression was
standardized to control values of GAPDH. The primers sequences used
for qPCR are listed in Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Sequence
(5′-3′) |
|---|
| miR-29a | F:
ACACTCCAGCTGGGTTTGGAGTCT |
|
| R:
CTCAACTGGTGTCGTGGA |
| U6 | F:
CTCGCTTCGGCAGCACA |
|
| R:
AACGCTTCACGAATTTGCGT |
| DNMT3A | F:
GCTGCACCTGGCCTTATG |
|
| R:
GGCTTTCTTCTCAGCCGTATC |
| DNMT3B | F:
CCAATCCTGGAGGCTATCCG |
|
| R:
CCGTCTCAGGGACTGTGTGT |
| GAPDH | F:
ATGACATCAAGAAGGTGGT |
|
| R:
GCGTCAAAGGTGGAGGA |
| P16 [U] |
TTATTAGAGGGTGGGGTGGATTGT |
|
|
CAACCCCAAACCACAACCATAA |
| P16 [M] |
TTATTAGAGGGTGGGGCGGATCGC |
|
|
GACCCCGAACCGCGACCGTA |
| DNMT3A siRNA |
GCGUCACACAGAAGCAUAUTT |
| DNMT3B siRNA |
UUGUUGUUGGCAACAUCUGAA |
| siRNA-NC |
CAGAUGUUGCCAACAACAAGA |
| miR-29a mimics |
ACCCCTTAGAGGATGACTGATTTCTTTTGGTGTTCAG |
|
|
AGTCAATAGAATTTTCTAGCACCA |
|
|
TCTGAAATCGGTTATAATGATTGGGGA |
Methylation-specific PCR (MSP)
Genomic DNA was bisulphite converted using the EZ
DNA Methylation Gold bisulphite conversion kit (cat. no. D5008,
Zymo Research Corp. Irvine, CA) and diluted to a final
concentration of 20 ng/µl. MS-PCR primers [targeting methylated
sequence (M) and unmethylated sequence (U)] were designed using
MethPrimer 2.0 to span the CpG island of the p16 promoter region.
The following thermocycling conditions were used: Initial
denaturation at 95°C for 2 min, followed by 35 cycles of
denaturation at 94°C for 30 sec, annealing at 55°C for 30 sec,
extension at 72°C for 60 sec and a final extension at 72°C for 4
min. The methylation-specific primers for p16 are presented in
Table I. The PCR products were
stained with ethidium bromide for 2 min at 37°C, analyzed on 2%
agarose gels and subsequently visualized via UV illumination. The
presence of specific bands in (M) or both (M) and (U) were
considered positive for methylation. However, the presence of
specific bands only observed in (U) but not in (M) were considered
unmethylated.
Western blotting
Total protein was extracted from cervical cancer
cells using RIPA lysis buffer (Sigma-Aldrich; Merck KGaA) and
qualified using the BCA detecting kit (cat. no. P0006, Beyotime
Institute of Biotechnology), according to the manufacturer's
instructions. A total of 50 µg protein/lane was separated by 10%
SDS-PAGE, transferred onto PVDF membranes (EMD Millipore) and
blocked with 5% dry milk blocking buffer for 2 h at room
temperature. The membranes were incubated with primary antibodies
against DNMT3A (1:1,000; cat. no. ab188470; Abcam), DNMT3B
(1:1,000; cat. no. ab79822; Abcam) and tubulin (1:2,000; cat. no.
ab7291; Abcam) overnight at 4°C. Following the primary incubation,
membranes were incubated with HRP-conjugated goat anti-rabbit lgG
(H+L) (1:2,000; cat. no. ab205718; Abcam) at room temperature for
40 min. Protein bands were visualized using an enhanced
chemiluminescence detection system (Pierce; Thermo Fisher
Scientific, Inc.) and intensities of bands were quantified using
Image Lab™ software (Bio-Rad Laboratories, Inc.).
Cell transfection
miR-29a mimics (miR-29a) and scrambled miRNA
(Scrambled), siRNA for DNMT3A (si-DNMT3A), siRNA for DNMT3B
(si-DNMT3B) and negative control (siRNA-NC) were purchased from
Shanghai GenePharma Co., Ltd., and the sequences are presented in
Table I. HeLa and C-33A cells were
seeded into 6-well plates at a density of 2×105
cells/well and cultured at 37°C for 24 h, prior to transfection
using the Lipofectamine® 2000 kit (cat. no. 11668027;
Invitrogen, Thermo Fisher Scientific, Inc.) at 37°C for 48 h,
according to the manufacturer's protocol. The final concentrations
of miR-29a mimics and siRNAs were 50 nM and 80 nM, respectively.
Cells were harvested 24 h post-transfection.
Colony formation assay
Hela and C-33A cells were seeded into 6-well plates,
incubated at 37°C for 14 days and fixed with 4% paraformaldehyde
for 30 min at room temperature. Cells were subsequently stained
with 0.1% crystal violet for 2 h at room temperature. Colonies
(>50 cells) were observed under a light microscope
(magnification, ×100). Colony forming efficiency = number of
colonies/number of seeded cells.
Cell cycle analysis
Transfected cells were digested with trypsin and
fixed with 70% ice-cold ethanol overnight at −20°C. Cells were
subsequently stained with propidium iodide (50 µg/ml) and RNAse A
(0.1 mg/ml) for 30 min at 37°C (both purchased from Sigma-Aldrich;
Merck KGaA), and analyzed using the FACS Calibur flow cytometer (BD
Biosciences). All experiments were performed in triplicate.
miRNA target prediction
Potential miR-29a binding sites in the 3′-UTR
regions of DNMT3A and DNMT3B mRNA were predicted using the
TargetScanHuman 7.2 database (www.targetscan.org). Position 862–868 for DNMT3A and
position 1206–1213 for DNMT3B were identified as putative conserved
binding sites for miR-29a.
Dual-luciferase reporter assay
The 3′-UTR regions of DNMT3A and DNMT3B mRNA
harboring the predicted miR-29a binding sites [wild-type
(wt)-DNMT3A and wt-DNMT3B] or the corresponding mutants
[(mut)-DNAMT3A and mut-DNMT3B] were synthesized by Beijing Genomics
Institute (https://www.genomics.cn) and
subsequently inserted into the pmiRGLO vector (Promega
Corporation). HeLa and C-33A cells were transfected with wt-DNMT3A
or mut-DNAMT3A, as well as wt-DNMT3B or mut-DNMT3B, followed by
transfection with miR-29a mimics or scrambled miRNA using the
Lipofectamine® 2000 kit (cat. no. 11668027; Invitrogen,
Thermo Fisher Scientific, Inc.) at 37°C for 48 h. Finally,
luciferase activities were detected using a dual-luciferase
reporter assay system (Promega Corporation), according to the
manufacturer's protocol. The luciferase activity was expressed as
fold change compared with the non-treated controls, both as
normalized Firefly/Renilla readouts and single luciferase
read-outs.
LinkedOmics database
The LinkedOmics database (http://www.linkedomics.org) contains multi-omics data
and clinical data for 32 cancer types and a total of 11,158
patients from The Cancer Genome Atlas (TCGA) project (40). LinkedOmics has three data analysis
modules: LinkFinder, LinkCompare and LinkInterpreter. The
LinkFinder module was used to calculate the association between
miR-29a expression and DNMT3A or DNMT3B mRNA expression, and
association between p16 expression and DNMT3A or DNMT3B mRNA
expression in the TCGA cervical and endocervical cancers (CESC)
cohort (n=304), using Pearson's correlation coefficient.
Statistical analysis
Statistical analysis was performed using SPSS v21.0
software (SPSS, Inc.) and the Prism statistical v8.0 software
package (GraphPad Software, Inc.). All experiments were performed
in triplicate and data are presented as the mean ± standard
deviation. Unpaired Student's t-test was used to compare
differences between two groups, while one-way analysis of variance
and Tukey's post hoc test were used to compare multiple groups.
Pearson's correlation coefficient was used to assess linear
correlation between miR-29a and DNMT3A or DNMT3B, and the
correlation between p16 and DNMT3A or DNMT3B. P<0.05 was
considered to indicate a statistically significant difference.
Results
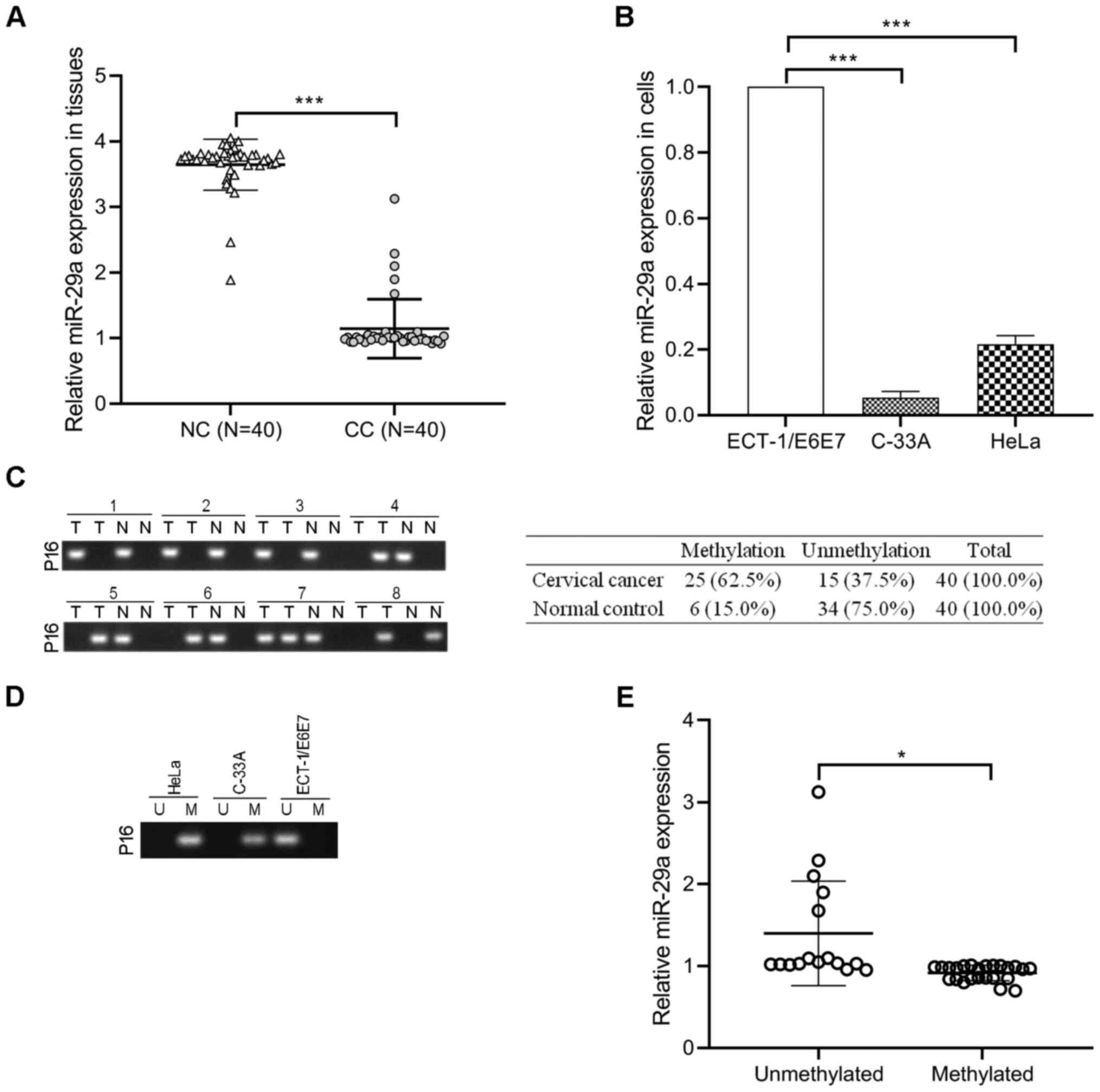
miR-29a levels decrease in cervical
cancer tissues and cells and are negatively correlated with p16
hypermethylation
RT-qPCR analysis was performed to detect miR-29a
expression in 40 cervical cancer tissues and paired normal cervical
tissues. The results demonstrated that miR-29a expression was
significantly decreased in cervical cancer tissues compared with
paired normal tissues (P<0.001; Fig.
1A). Consistently, miR-29a expression was lower in the cervical
cancer cell lines (HeLa and C-33A) compared with the normal
cervical cell line, ECT1/E6E7 (P<0.001; Fig. 1B). The methylation status of p16
promoter was assessed in 40 cervical cancer tissues and
paracancerous tissues. As presented in Fig. 1C, hypermethylation of p16 occurred in
62.5% (25/40) of cervical cancer tissues and 15.0% (6/40) of
paracancerous tissues. In addition, p16 was hypermethylated in HeLa
and C-33A cells compared with ECT1/E6E7 cells (Fig. 1D). Notably, the levels of miR-29a in
the unmethylated p16 group were higher than the methylated p16
group (P<0.05; Fig. 1E),
suggesting that miR-29a may be associated with the methylation
status of p16 in cervical cancer.
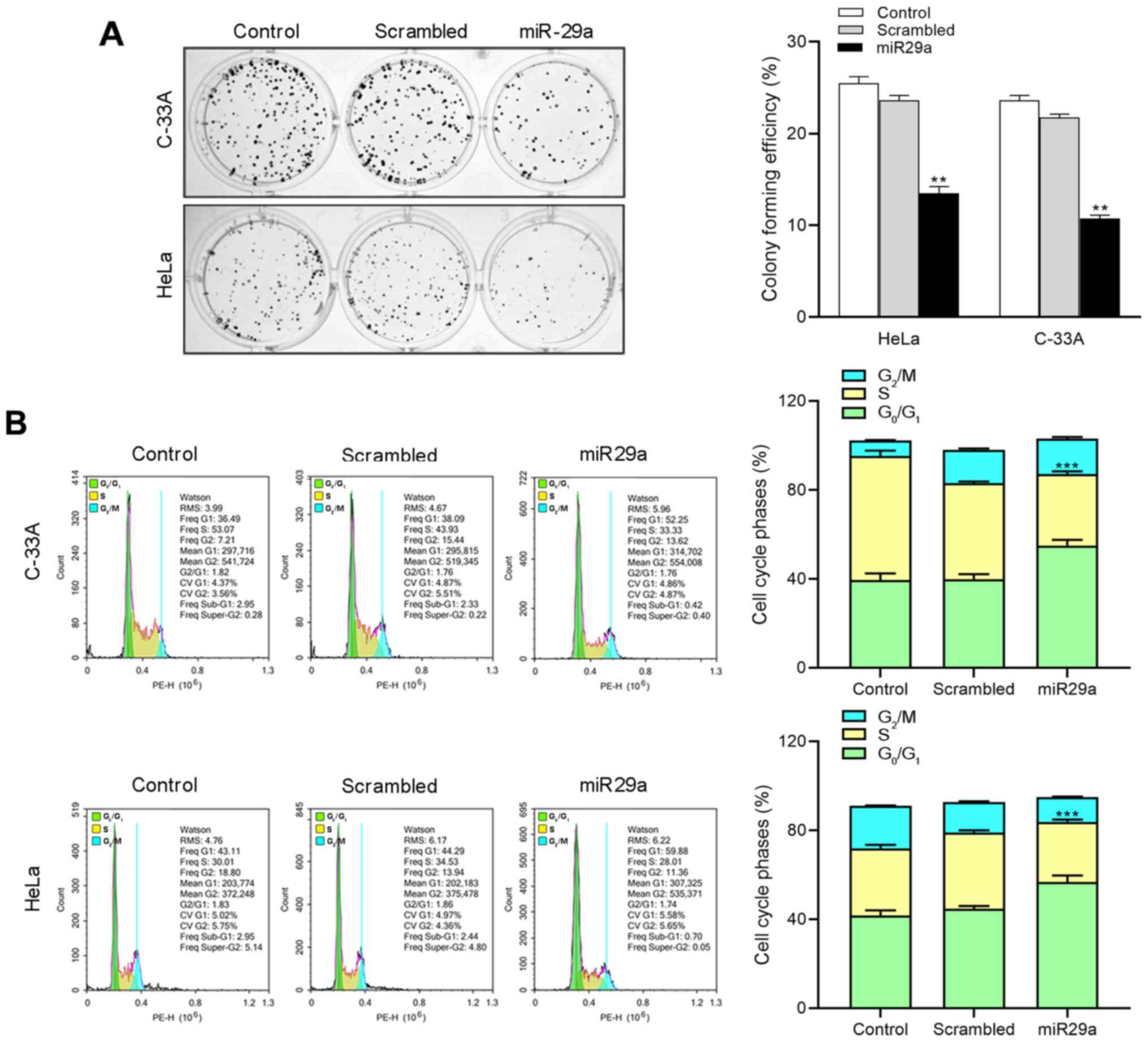
miR-29a suppresses cell proliferation
and induces cell cycle arrest of HeLa and C-33A cells
To assess the biological function of miR-29a in
cervical cancer cell lines, HeLa and C-33A cells were transfected
with miR-29a mimics or negative control, and subjected to the
colony formation assay and cell cycle analysis. The results
demonstrated that compared with the control groups, miR-29a mimics
effectively increased the expression of miR-29a in HeLa and C-33A
cells (P<0.001; Fig. S1).
Overexpression of miR-29a significantly decreased the colony
formation capacity in both HeLa and C-33A cells compared with the
negative control groups (P<0.01; Fig.
2A). Furthermore, cell cycle analysis demonstrated that
overexpression of miR-29a significantly promoted cell cycle arrest
at G0/G1 phase in both HeLa and C-33A cells
(P<0.001; Fig. 2B). Taken
together, these results suggest that miR-29a exerts an antitumor
effect in cervical cancer cells.
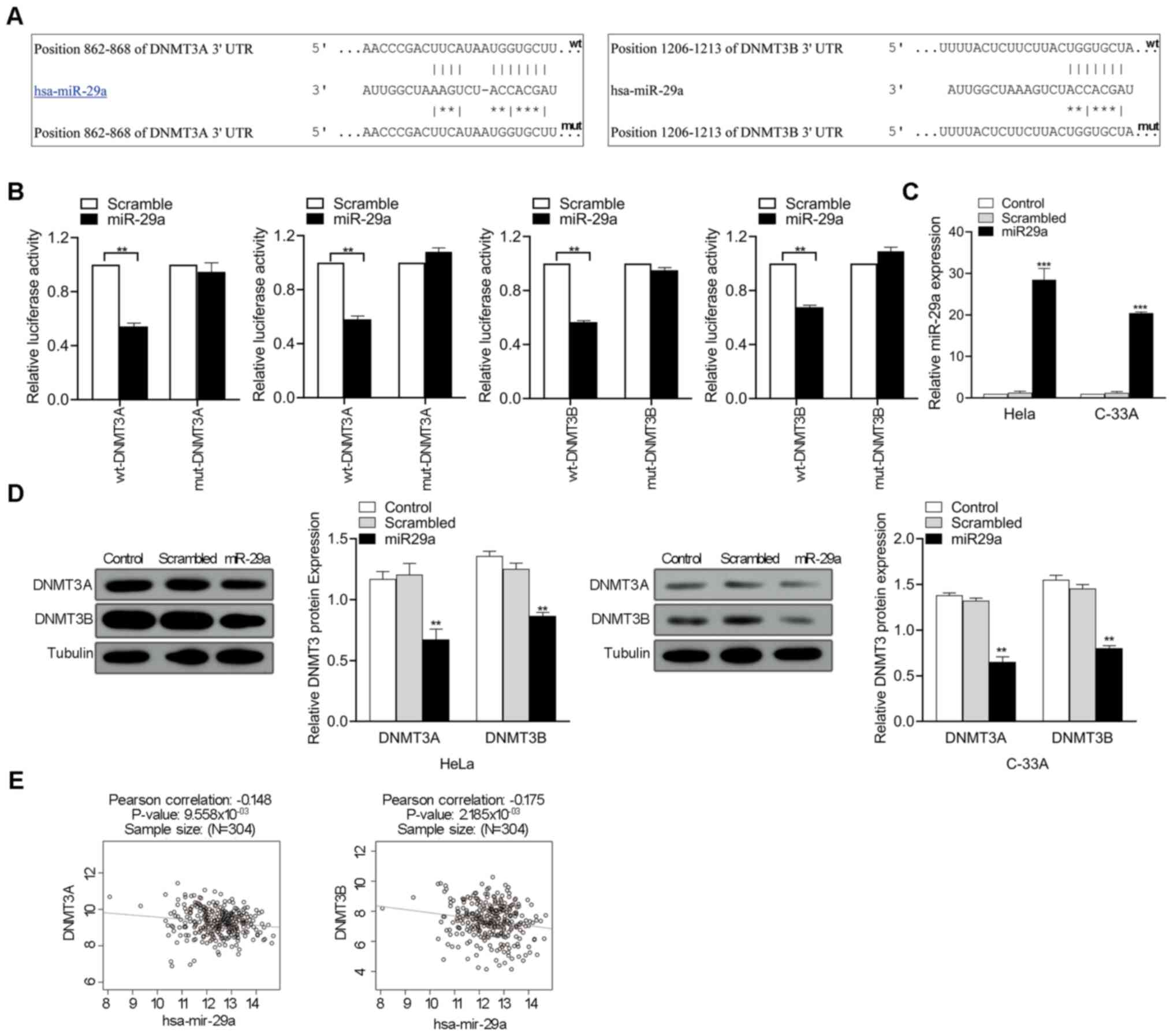
miR-29a inhibits DNMT3A and DNMT3B
expression by directly targeting their 3′-UTRs
To determine the association between miR-29a
expression and p16 methylation status, DNMT3A and DNMT3B were
selected as potential miR-29a targets for further experiments based
on literatures and bioinformatics analysis. As predicted by the
TargetScanHuman 7.2 database, miR-29a had intriguing
complementarity sites in the 3′-UTRs of the DNMT3A and DNMT3B genes
(Fig. 3A). To validate the
interaction between miR-29a and targets, the 3′-UTRs of DNMT3A and
DNMT3B were cloned into a modified pGL3 plasmid downstream of the
luciferase reporter gene. Corresponding mutant versions with the
binding site mutagenesis were also constructed, and subsequently
co-transfected with miR-29a mimics in HeLa cell. The results
demonstrated that miR-29a significantly decreased the luciferase
activities in the wt-DNMT3A and wt-DNMT3B groups compared with the
scrambled oligonucleotide (P<0.01; Fig. 3B). miR-29a mimics was transfected
into HeLa and C-33A cells to assess whether miR-29a regulates
DNMT3A and DNMT3B expression. As presented in Fig. 3C, transfection with miR-29a mimics
significantly increased miR-29a expression in HeLa and C-33A
(P<0.001). Furthermore, DNMT3A and DNMT3B expression in HeLa and
C-33A cells significantly decreased following overexpression of
miR-29a (P<0.01; Fig. 3D). The
association between DNMT3A or DNMT3B and miR-29a expression levels
in cervical cancer tissues was determined using LinkedOmics
(http://www.linkedomics.org). Pearson's
correlation analysis demonstrated that miR-29a expression was
negatively correlated with DNMT3A and DNMT3B expression (P<0.01;
Fig. 3E). Collectively, these
results suggest that miR-29a decreases mRNA DNMT3A and DNMT3B
expression by directly targeting to their 3′-UTRs.
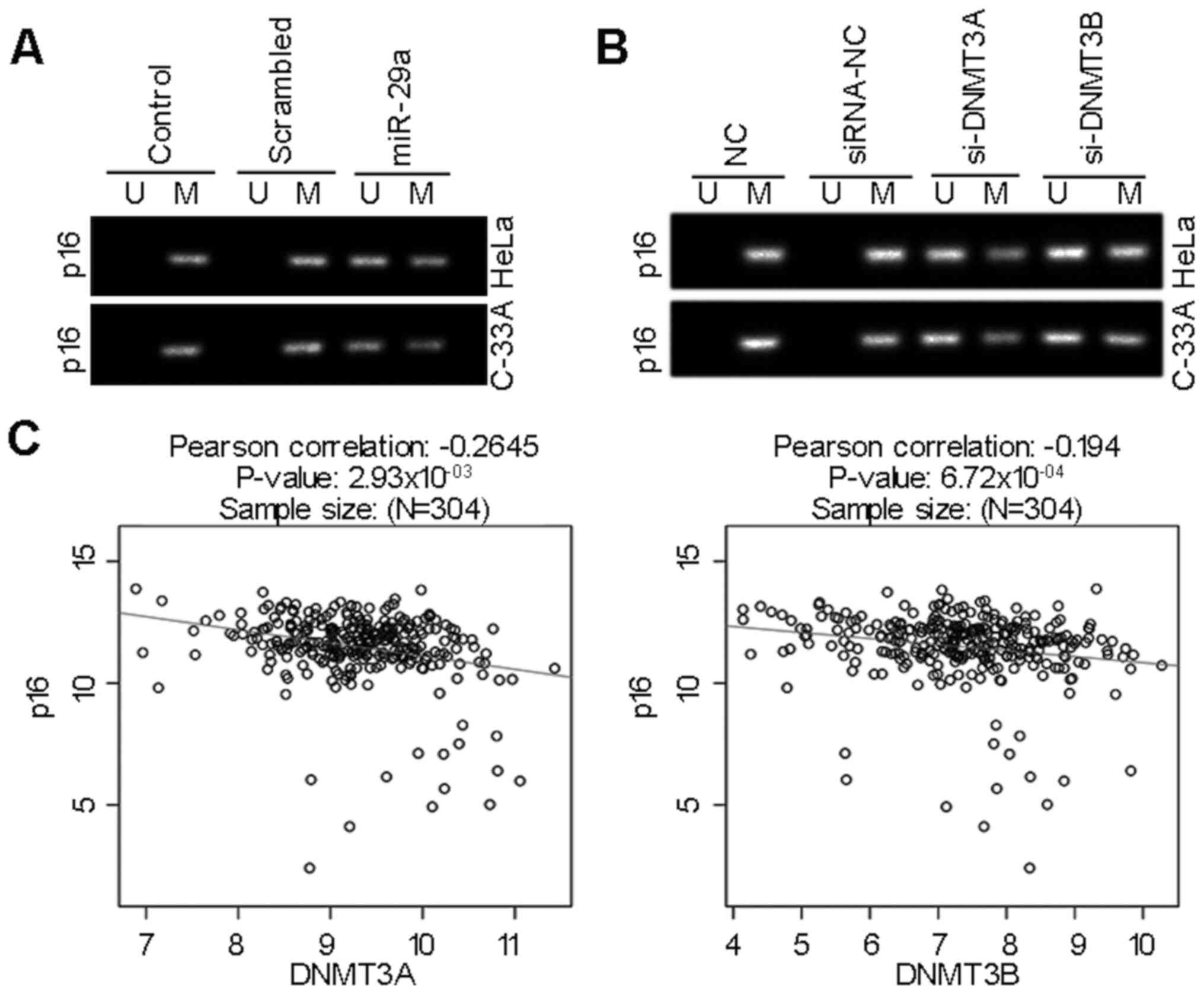
miR-29a inhibits p16 gene methylation
via modulation of DNMT3A and DNMT3B
To assess the potential effect and molecular
mechanism of miR-29a on the methylation pattern of p16 gene, HeLa
and C-33A cells were transfected with miR-29a mimics, or siRNAs for
DNMT3A and DNMT3B. The levels of DNMT3A and DNMT3B significantly
decreased in cells transfected with their specific siRNAs compared
with their corresponding control groups (P<0.001; Fig. S2A and B). The MSP results
demonstrated that miR-29a attenuated the methylation status of p16
in HeLa and C-33A cells (Fig. 4A).
Furthermore, silencing of DNMT3A or DNMT3B, two key enzymes
involved in DNA methylation (41),
normalized aberrant methylation pattern of p16 in cervical cancer
(Fig. 4B). In addition, LinkedOmics
analysis demonstrated that p16 (also known as CDKN2A) mRNA
expression was inversely correlated with DNMT3A or DNMT3B mRNA
levels in cervical cancer tissues (P<0.001; Fig. 4C). Thus, miR-29a inhibits aberrant
methylation of tumor suppressor gene p16 by regulating the levels
of DNMT3A and DNMT3B.
Discussion
miRNAs exhibit abnormal expression in different
types of cancer, and exert tumor suppression or promotion effects
by regulating the expression of target genes (42). For example, Chen et al
(43) reported that miR-132
expression is significantly downregulated in thyroid cancer tissues
and overexpression of miR-132 exerts tumor-suppressing functions
through targeting FOXA1. Previous studies have demonstrated that
miRNAs play a crucial regulatory role in cervical cancer. These
studies contribute to a profound understanding of the molecular
mechanism involved in the cervical cancer (44,45).
miR-29a has been reported to exert an antitumor effect in different
types of cancer, and the disorder of miR-29a is associated with the
development and progression of cancer (26).
The results of the present study demonstrated that
miR-29a expression was downregulated in cervical cancer tissues and
cell lines compared with normal cervical tissues and cells. In
addition, overexpression of miR-29a inhibited the proliferation and
induced cell cycle arrest in cervical cancer cells, which was
similarly observed in previous studies. It has been demonstrated
that miR-29a expression is downregulated in papillary thyroid
cancer (46), oral squamous cell
carcinoma (47), lung cancer
(48) and retinoblastoma (48), as well as cervical cancer (49,50), and
ectopic miR-29a expression significantly inhibits proliferation and
invasion. Taken together, these results confirm that miR-29a is a
tumor suppressor (51).
The results of the present study demonstrated a
significant correlation between miR-29a expression and methylation
patterns of p16, and that overexpression of miR-29a normalized
aberrant methylation status of the p16 gene. The p16 gene is a
well-known tumor suppressor gene that blocks the G1-S
phase of the cell cycle and inhibits abnormal proliferation of
cancer cells (52). In addition, p16
protein inhibits the activation of cyclin-dependent kinase 4 and
the phosphorylation of pRb, and further blocks the cell cycle
(10). Mutation, deletion and
abnormal methylation of the p16 gene are frequently observed, which
inactivates the p16 protein in different types of cancer and is
closely associated with the development and progression of cancers
(53,54). Methylation of the p16 gene promoter
inactivates p16, significantly decreasing its expression (55,56).
This results in the loss of the tumor suppressor function of p16,
which promotes the development of cervical cancer (8). However, the regulatory mechanism of p16
promoter methylation remains unknown. Thus, to investigate the
molecular mechanism by which miR-29a regulates the methylation
status of p16 promoter, DNMT3A and DNMT3B were identified and
confirmed as new direct targets for miR-29a via bioinformatics
analysis and the dual-luciferase reporter assay. DNMT3A and DNMT3B
are key DNA methyltransferases for de novo methylation,
which are essential for the establishment of DNA methylation
patterns during development (57).
Abnormal expression of DNMTs and disruption of DNA methylation
patterns are closely associated with the development of different
tumors (58). Increasing evidence
suggests that dysregulated DNMT3A and DNMT3B contributes to tumor
progression by modulating the methylation of targets or the global
DNA (59,60). The present study demonstrated the
associations between miR-29a, DNMT3A, DNMT3B and p16, and indicated
that miR-19a suppressed cell proliferation and induced cell cell
cycle arrest in cervical cancer cells by restoring DNMT-3s-induced
methylation status of p16.
The results of the present study confirmed that
miR-29a is involved in methylation modification of the tumor
suppressor gene, p16, by directly targeting DNMT3s. The results
demonstrated the underlying molecular mechanism by which miR-29a
inhibits cell proliferation and arrests the cell cycle in cervical
cancer. Taken together, these results provide a novel perspective
for the biological significance of miR-29a in regulating
methylation modification with potential diagnostic and therapeutic
biomarkers for clinical cervical cancer management.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Qinghai Basic
Research Program of China (grant no. 2018-0301-ZJC-0101).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
AW, QX and GG were involved in the study concept and
design, analysis and interpretation of data and drafting the
initial manuscript. RS, TB and XX were involved in the acquisition
of data and analysis. All authors performed the experiments,
revised and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Affiliated Hospital of Qinghai University (Xining,
China; approval no. SL-2018016), and written informed consent was
provided by all patients prior to the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
miRNA/miR
|
microRNA
|
|
DNMTs
|
DNA methyltransferases
|
References
|
1
|
Freddie B, Ferlay J, Soerjomataram I,
Siegel RL, Torre LA and Jemal A: Global cancer statistics 2018:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 68:394–424. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Razavi ZS, Tajiknia V, Majidi S, Ghandali
M, Mirzaei HR, Rahimian N, Hamblin MR and Mirzaei H: Gynecologic
cancers and non-coding RNAs: Epigenetic regulators with emerging
roles. Crit Rev Oncol Hematol. 157:1031922020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Burd EM: Human papillomavirus and cervical
cancer. Clin Microbiol Rev. 16:1–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Menezo Y, Clement P, Clement A and Elder
K: Methylation: An ineluctable biochemical and physiological
process essential to the transmission of life. Int J Mol Sci.
21:93112020. View Article : Google Scholar
|
|
5
|
Zhu H, Zhu H, Tian M, Wang D, He J and Xu
T: DNA methylation and hydroxymethylation in cervical cancer:
Diagnosis, prognosis and treatment. Front Genet. 9:3472020.
View Article : Google Scholar
|
|
6
|
Urbano A, Smith J, Weeks RJ and Chatterjee
A: Gene-Specific targeting of DNA methylation in the mammalian
genome. Cancers (Basel). 11:15152019. View Article : Google Scholar
|
|
7
|
Jiao Y, Feng Y and Wang X: Regulation of
tumor suppressor gene CDKN2A and Encoded p16-INK4a protein by
covalent modifications. Biochemistry (Mosc). 83:1289–1298. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang FL, Yang Y, Liu ZY, Qin Y and Jin T:
Correlation between methylation of the p16 promoter and cervical
cancer incidence. Eur Rev Med Pharmacol Sci. 21:2351–2356.
2017.PubMed/NCBI
|
|
9
|
O'Neill CJ and McCluggage WG: p16
expression in the female genital tract and its value in diagnosis.
Adv Anat Pathol. 13:8–15. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han YD, Wang XB, Cui NH, Zhang S, Wang C
and Zheng F: Associations of P16INK4a promoter hypermethylation
with squamous intra-epithelial lesion, cervical cancer and their
clinicopathological features: A meta-analysis. Oncotarget.
8:1871–1883. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kohler F and Rodríguez-Paredes M: DNA
methylation in epidermal differentiation, aging, and cancer. J
Invest Dermatol. 140:38–47. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gujar H, Weisenberger DJ and Liang G: The
roles of human DNA methyltransferases and their isoforms in shaping
the epigenome. Genes (Basel). 10:1722019. View Article : Google Scholar
|
|
13
|
Hassouna MM, Naguib M, Radwan EM,
Abdel-Samiee M, Estaphan S and Abdelsameea E: DNA
methyltransferases as potential biomarkers for HCV related
hepatocellular carcinoma. Asian Pac J Cancer Prev. 1:3357–3363.
2020. View Article : Google Scholar
|
|
14
|
Zhu A, Hopkins KM, Friedman RA, Bernstock
JD, Broustas CG and Lieberman HB: DNMT1 and DNMT3B regulate
tumorigenicity of human prostate cancer cells by controlling RAD9
expression through targeted methylation. Carcinogenesis.
11:bgaa0882020. View Article : Google Scholar
|
|
15
|
Cervena K, Siskova A, Buchler T, Vodicka P
and Vymetalkova V: Methylation-based therapies for colorectal
cancer. Cells. 24:15402020. View Article : Google Scholar
|
|
16
|
Liu H, Song Y, Qiu H, Liu Y, Luo K, Yi Y,
Jiang G, Lu M, Zhang Z, Yin J, et al: Downregulation of FOXO3a by
DNMT1 promotes breast cancer stem cell properties and
tumorigenesis. Cell Death Differ. 27:966–983. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Piyathilake CJ, Badiga S, Borak SG,
Weragoda J, Bae S, Matthews R, Bell WC and Partridge EE: A higher
degree of expression of DNA methyl transferase 1 in cervical cancer
is associated with poor survival outcome. Int J Womens Health.
9:413–420. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sundaram MK, Hussain A, Haque S, Raina R
and Afroze N: Quercetin modifies 5′CpG promoter methylation and
reactivates various tumor suppressor genes by modulating epigenetic
marks in human cervical cancer cells. J Cell Biochem.
120:18357–18369. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sundaram MK, Ansari MZ, Al Mutery A,
Ashraf M, Nasab R, Rai S, Rais N and Hussain A: Genistein induces
alterations of epigenetic modulatory signatures in human cervical
cancer cells. Anticancer Agents Med Chem. 18:412–421. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khan MA, Sundaram MK, Hamza A, Quraishi U,
Gunasekera D, Ramesh L, Goala P, Al Alami U, Ansari MZ, Rizvi TA,
et al: Sulforaphane reverses the expression of various tumor
suppressor genes by targeting DNMT3B and HDAC1 in human cervical
cancer cells. Evid Based Complement Alternat Med.
2015:4121492015.PubMed/NCBI
|
|
21
|
Lu TX and Rothenberg ME: MicroRNA. J
Allergy Clin Immunol. 141:1202–1207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kabekkodu SP, Shukla V, Varghese VK,
D'Souza J, Chakrabarty S and Satyamoorthy K: Clustered miRNAs and
their role in biological functions and diseases. Biol Rev Camb
Philos Soc. 93:1955–1986. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Condrat CE, Thompson DC, Barbu MG, Bugnar
OL, Boboc A, Cretoiu D, Suciu N, Cretoiu SM and Voinea SC: miRNAs
as biomarkers in disease: Latest findings regarding their role in
diagnosis and prognosis. Cells. 9:2762020. View Article : Google Scholar
|
|
24
|
Yang Y, Dodbele S, Park T, Glass R, Bhat
K, Sulman EP, Zhang Y and Abounader R: MicroRNA-29a inhibits
glioblastoma stem cells and tumor growth by regulating the PDGF
pathway. J Neurooncol. 145:23–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu YB, Wang Y, Zhang MD, Yue W and Sun
CN: MicroRNA-29a functions as a tumor suppressor through targeting
STAT3 in laryngeal squamous cell carcinoma. Exp Mol Pathol.
116:1045212020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gong HL, Tao Y, Mao XZ, Song DY, You D and
Ni JD: MicroRNA-29a suppresses the invasion and migration of
osteosarcoma cells by regulating the SOCS1/NF-κB signalling pathway
through negatively targeting DNMT3B. Int J Mol Med. 44:1219–1232.
2019.PubMed/NCBI
|
|
27
|
Nan P, Niu Y, Wang X and Li Q: miR-29a
function as tumor suppressor in cervical cancer by targeting SIRT1
and predict patient prognosis. Onco Targets Ther. 26:6917–6925.
2019. View Article : Google Scholar
|
|
28
|
Zhang Y, Yang L, Wang S, Liu Z and Xiu M:
miR-29a suppresses cell proliferation by targeting SIRT1 in
hepatocellular carcinoma. Cancer Biomark. 22:151–159. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu ZJ, Chen SG, Yang YZ, Lu SJ, Zhao XM,
Hu B and Zhang L: miR-29a inhibits adhesion, migration, and
invasion of osteosarcoma cells by suppressing CDC42. Int J Clin Exp
Pathol. 12:4171–4180. 2019.PubMed/NCBI
|
|
30
|
Chen R and Zhang L: miR-29a inhibits cell
proliferation and migration by targeting the CDC42/PAK1 signaling
pathway in cervical cancer. Anticancer Drugs. 30:579–587. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shi C, Ren L, Sun C, Yu L, Bian X, Zhou X,
Wen Y, Hua D, Zhao S, Luo W, et al: miR-29a/b/c function as
invasion suppressors for gliomas by targeting CDC42 and predict the
prognosis of patients. Br J Cancer. 117:1036–1047. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang M, Guo W, Qian J and Wang B:
Negative regulation of CDC42 expression and cell cycle progression
by miR-29a in breast cancer. Open Med (Wars). 11:78–82. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamamoto N, Kinoshita T, Nohata N, Yoshino
H, Itesako T, Fujimura L, Mitsuhashi A, Usui H, Enokida H, Nakagawa
M, et al: Tumor-suppressive microRNA-29a inhibits cancer cell
migration and invasion via targeting HSP47 in cervical squamous
cell carcinoma. Int J Oncol. 43:1855–1863. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bibaki E, Tsitoura E, Vasarmidi E,
Margaritopoulos G, Trachalaki A, Koutoulaki C, Georgopoulou T,
Spandidos DA, Tzanakis N and Antoniou KM: miR-185 and miR-29a are
similarly expressed in the bronchoalveolar lavage cells in IPF and
lung cancer but common targets DNMT1 and COL1A1 show disease
specific patterns. Mol Med Rep. 17:7105–7112. 2018.PubMed/NCBI
|
|
35
|
Kogure T, Kondo Y, Kakazu E, Ninomiya M,
Kimura O and Shimosegawa T: Involvement of miRNA-29a in epigenetic
regulation of transforming growth factor-beta-induced
epithelial-mesenchymal transition in hepatocellular carcinoma.
Hepatol Res. 44:907–919. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cicchini C, de Nonno V, Battistelli C,
Cozzolino AM, De Santis Puzzonia M, Ciafrè SA, Brocker C, Gonzalez
FJ, Amicone L and Tripodi M: Epigenetic control of EMT/MET
dynamics: HNF4α impacts DNMT3s through miRs-29. Biochim Biophys
Acta. 1849:919–929. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Robaina MC, Mazzoccoli L, Arruda VO, de
Souza Reis FD, Apa AG, de Rezende LM and Klumb CE: Deregulation of
DNMT1, DNMT3B and miR-29s in burkitt lymphoma suggests novel
contribution for disease pathogenesis. Exp Mol Pathol. 98:200–207.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Oliveira LH, Schiavinato JL, Fráguas MS,
Lucena-Araujo AR, Haddad R, Araújo AG, Dalmazzo LF, Rego EM, Covas
DT, Zago MA and Panepucci RA: Potential roles of microRNA-29a in
the molecular pathophysiology of T-cell acute lymphoblastic
leukemia. Cancer Sci. 106:1264–1277. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou X, Zhao F, Wang ZN, Song YX, Chang H,
Chiang Y and Xu HM: Altered expression of miR-152 and miR-148a in
ovarian cancer is related to cell proliferation. Oncol Rep.
27:447–454. 2012.PubMed/NCBI
|
|
40
|
Vasaikar SV, Straub P, Wang J and Zhang B:
LinkedOmics: Analyzing multi-omics data within and across 32 cancer
types. Nucleic Acids Res. 46:D956–D963. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gao L, Anteneh H and Song J: Dissect the
DNMT3A- and DNMT3B-mediated DNA Co-methylation through a covalent
complex approach. J Mol Biol. 17:569–575. 2020. View Article : Google Scholar
|
|
42
|
Di Leva G, Garofalo M and Croce CM:
MicroRNAs in cancer. Annu Rev Pathol. 9:287–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen X, Li M, Zhou H and Zhang L: miR-132
Targets FOXA1 and exerts tumor-suppressing functions in thyroid
cancer. Oncol Res. 29:431–437. 2019. View Article : Google Scholar
|
|
44
|
Wang JY and Chen LJ: The role of miRNAs in
the invasion and metastasis of cervical cancer. Biosci Rep.
15:BSR201813772019. View Article : Google Scholar
|
|
45
|
Li J, Liu Q, Clark LH, Qiu H, Bae-Jump VL
and Zhou C: Deregulated miRNAs in human cervical cancer: Functional
importance and potential clinical use. Future Oncol. 13:743–753.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang Y, Han J, Lv Y and Zhang G: miR-29a
inhibits proliferation, invasion, and migration of papillary
thyroid cancer by targeting DPP4. Onco Targets Ther. 12:4225–4233.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Huang C, Wang L, Song H and Wu C: miR-29a
inhibits the progression of oral squamous cell carcinoma by
targeting wnt/β-catenin signalling pathway. Artif Cells Nanomed
Biotechnol. 47:3037–3042. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu X, Lv X, Yang Q, Jin H, Zhou W and Fan
Q: MicroRNA-29a functions as a tumor suppressor and increases
cisplatin sensitivity by targeting NRAS in lung cancer. Technol
Cancer Res Treat. 17:15330338187589052018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li Y, Wang F, Xu J, Ye F, Shen Y, Zhou J,
Lu W, Wan X, Ma D and Xie X: Progressive miRNA expression profiles
in cervical carcinogenesis and identification of HPV-related target
genes for miR-29. J Pathol. 224:484–495. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhu Y, Huang Y, Liu M, Yan Q, Zhao W, Yang
P, Gao Q, Wei J, Zhao W and Ma L: Epigallocatechin gallate inhibits
cell growth and regulates miRNA expression in cervical carcinoma
cell lines infected with different high-risk human papillomavirus
subtypes. Exp Ther Med. 17:1742–1748. 2019.PubMed/NCBI
|
|
51
|
Gong Y, Wan JH, Zou W, Lian GY, Qin JL and
Wang QM: miR-29a inhibits invasion and metastasis of cervical
cancer via modulating methylation of tumor suppressor SOCS1. Future
Oncol. 15:1729–1744. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang CY, Bao W and Wang LH:
Downregulation of p16(ink4a) inhibits cell proliferation and
induces G1 cell cycle arrest in cervical cancer cells. Int J Mol
Med. 33:1577–1585. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Suzuki N, Onda T, Yamamoto N, Katakura A,
Mizoe Je and Shibahara T: Mutation of the p16/CDKN2 gene and loss
of heterozygosity in malignant mucosal melanoma and adenoid cystic
carcinoma of the head and neck. Int J Oncol. 31:1061–1067.
2007.PubMed/NCBI
|
|
54
|
Aesif SW, Aubry MC, Yi ES, Kloft-Nelson
SM, Jenkins SM, Spears GM, Greipp PT, Sukov WR and Roden AC: Loss
of p16INK4A expression and homozygous CDKN2A deletion are
associated with worse outcome and younger age in thymic carcinomas.
J Thorac Oncol. 12:860–871. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Demokan S, Chuang A, Suoğlu Y, Ulusan M,
Yalnız Z, Califano JA and Dalay N: Promoter methylation and loss of
p16(INK4a) gene expression in head and neck cancer. Head Neck.
34:1470–1475. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Allameh A, Moazeni-Roodi A, Harirchi I,
Ravanshad M, Motiee-Langroudi M, Garajei A, Hamidavi A and
Mesbah-Namin SA: Promoter DNA methylation and mRNA expression level
of p16 gene in oral squamous cell carcinoma: Correlation with
clinicopathological characteristics. Pathol Oncol Res.
25:1535–1543. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang ZM, Lu R, Wang P, Yu Y, Chen D, Gao
L, Liu S, Ji D, Rothbart SB, Wang Y, et al: Structural basis for
DNMT3A-mediated de novo DNA methylation. Nature. 554:387–391. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jin B and Robertson KD: DNA
methyltransferases, DNA damage repair, and cancer. Adv Exp Med
Biol. 754:3–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liu D, Wu K, Yang Y, Zhu D, Zhang C and
Zhao S: Long noncoding RNA ADAMTS9-AS2 suppresses the progression
of esophageal cancer by mediating CDH3 promoter methylation. Mol
Carcinog. 59:32–44. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
de Silanes IL, Gorospe M, Taniguchi H,
Abdelmohsen K, Srikantan S, Alaminos M, Berdasco M, Urdinguio RG,
Fraga MF, Jacinto FV and Esteller M: The RNA-binding protein HuR
regulates DNA methylation through stabilization of DNMT3b mRNA.
Nucleic Acids Res. 37:2658–2671. 2009. View Article : Google Scholar : PubMed/NCBI
|