Introduction
Human papillomavirus positive (HPV+) oropharyngeal
squamous cell carcinomas (OPSCC) are increasing in incidence and
patients with HPV+ OPSCC have a much better clinical outcome, as
compared to patients with HPV negative (HPV-) OPSCC. Therefore,
today, HPV+ and HPV- OPSCC are considered as two separate entities
and are staged separately according the new AJCC/IUCC staging
manual (TNM-8). Over the last years, it has also been debated if
oncological treatment of patients with HPV+ OPSCC can be tapered,
but previous attempts have not been successful (1). Notably, however, accumulated recent
data advocate that HPV+ OPSCC should be divided into its sub-sites,
when categorizing by HPV status and prognosis, more specifically
tonsillar squamous cell carcinoma (TSCC), base of tongue squamous
cell carcinoma (BOTSCC) and squamous cell carcinoma of the soft
palate and the pharyngeal walls (other OPSCC) (2). Data from others and us clearly indicate
that patients with HPV+ TSCC/BOTSCC have a better survival than
patients with HPV- TSCC/BOTSCC, but this survival benefit of having
HPV was not observed in patients with other OPSCC (3–7).
Nevertheless, although the survival in general is favorable in
patients with HPV+ TSCC/BOTSCC, prognostic markers are still needed
to identify those few patients with a poor clinical outcome, before
treatment can be tapered (8).
Psoriasin, or s100A7, is a protein part of the S100
family containing calcium-binding motifs and is an important cell
mediator for e.g. cell survival and maturation. Increased
expression of the protein has been reported in malignant and
premalignant lesions and overexpression has also been correlated
with clinical outcome (9–14). In a previous study by Tripathi et
al (15), the expression of
psoriasin was correlated to a worse prognosis in patients with head
and neck carcinomas (HNSCC), but neither HPV status nor sub-site
was considered in that study.
Hence, in this study, we wanted to examine the
previously reported prognostic significance of psoriasin staining
obtained in a heterogenous HNSCC patient cohort, in a homogenous
pilot cohort of only BOTSCC. This specific cohort had also
previously been tested for the presence or absence of HPV DNA and
p16 overexpression (16,17) and was therefore useful for examining
psoriasin expression in correlation to HPV status, as well as to
clinical outcome.
Materials and methods
Patients and tumors
Patients diagnosed with BOTSCC 2000–2007 in the
County of Stockholm, previously tested for HPV DNA by Luminex
Multiplex PCR and p16INK4a (p16) overexpression (>70%
cytoplasmatic and nuclear tumor expression) by
immunohistochemistry, were identified (16,17).
Available formalin-fixed paraffin-embedded (FFPE) pre-treatment
biopsies (obtained through an ENT forceps biopsy) were collected
from the Department of Clinical Pathology, Karolinska University
Hospital. Patient data were collected from patients records. The
study was conducted according to ethical permissions 2009/1278-31/4
and 2017/1035-31/2, Karolinska Institutet.
Immunohistochemistry and staining
evaluation
Tumor sections (4 µm) were cut and deparaffinized in
Xylene and rehydrated in graded ethanol. Heat-induced antigen
retrieval was performed with citric acid buffer (pH6) using a
microwave oven for 10 min, which was followed by
H2O2 treatment in order to block endogenous
peroxidase. The slides were then incubated with horse serum,
followed by incubation with primary antibody (mouse mAb S100A7
dilution 1:100, clone 47C1068; Santa Cruz Biotechnology) overnight.
Secondary anti-mouse antibody (1:200; Vector Laboratories, Inc.)
was then added followed by the ABC kit (Vectastain; Vector
Laboratories, Inc.). The staining was developed in DAB followed by
hematoxylin counterstaining. The staining was evaluated by three
researchers blinded for clinical outcome (LH, DL and AN). The
percentage of positive tumor cells per total tumor cells was
evaluated for each section and a cut-off value of 30% (more
specifically, the proportion of immunostained cells, irrespective
of whether the staining was cytoplasmic or nuclear) was applied as
previously described, and since we here wanted to compare out data
to such studies by others (15).
Statistical analysis
Differences in continuous and categorical variables
were assessed with double-sided t-test and Chi-square test,
respectively. Three-year overall survival (3-year OS) was defined
as days from diagnosis until death. All patients were censored
after three years. Three-year disease-free survival (3-year DFS)
was defined as time from diagnosis until a relapse in disease.
Patients that never became tumor free were censored at day 0. All
patients were censored after 3 years. Differences in survival were
estimated with the log-rank test and visualized with the
Kaplan-Meier method. The Cox proportional hazards regression
analysis was used for the calculation of hazard ratios (HR) with
95% confidence intervals (95% CI) in the univariable and
multivariable analysis. Besides S100A7 expression, established
prognostic markers in OPSCC (age, TNM-status, smoking status and
treatment) were included. P-values <0.05 were considered
significant. All calculations and analyses were performed using IBM
SPSS Statistics, (version 25.0; IBM. Corp.)
Results
Patients, tumors and psoriasin
expression
In total, 76 patients diagnosed with BOTSCC between
2000–2007 in Stockholm, treated with curative intent and with
tumors tested for possible presence of HPV DNA (HPV DNA positive by
PCR) and overexpression of p16 were identified from previous
publications (16,17). Tumor slides, available from 72 of
these 76 patients, were subsequently stained for S100A7 (psoriasin)
expression. Patients and tumor characteristics of these 72 patients
are depicted in Table I.
 | Table I.Patient and tumor characteristics. |
Table I.
Patient and tumor characteristics.
| Characteristics | Low psoriasin
expression | High psoriasin
expression | All patients | P-value |
|---|
| Age at diagnosis,
years (mean) | 62 | 60 | 61 | 0.5 |
| Sex, n (%) |
|
Female | 19 (35) | 4 (22) | 23 (32) | 0.4 |
| Male | 35 (65) | 14 (78) | 49 (68) |
|
| Stage (TNM-8), n
(%) |
| I | 21 (39) | 6 (33) | 27 (38) | 0.4 |
| II | 11 (20) | 2 (11) | 13 (18) |
|
| III | 14 (26) | 4 (22) | 18 (25) |
|
| IV | 8 (15) | 6 (33) | 14 (19) |
|
|
Treatmenta,
n (%) |
| RT | 25 (46) | 14 (78) | 39 (54) | 0.03 |
| CRT | 29 (54) | 4 (22) | 33 (46) |
|
|
Radiotherapyb, n (%) |
|
Conventional | 39 (72) | 12 (67) | 51 (71) | 0.8 |
|
Accelerated | 15 (18) | 6 (33) | 21 (29) |
|
| Cetuximab
treatmentc, n (%) |
| No | 52 (96) | 18 (100) | 70 (97) | >0.9 |
|
Yes | 2 (4) | 0 (0) | 2 (3) |
|
| Current
smokerd, n (%) |
| No | 42 (78) | 8 (44) | 50 (69) | 0.02 |
|
Yes | 12 (22) | 10 (56) | 22 (31) |
|
| HPV DNA
statuse, n (%) |
|
Negative | 6 (11) | 10 (56) | 16 (22) | <0.001 |
|
Positive | 48 (89) | 8 (44) | 56 (78) |
|
| p16
upregulatione, n
(%) |
|
Negative | 9 (17) | 11 (61) | 20 (28) | <0.001 |
|
Positive | 45 (83) | 7 (39) | 52 (72) |
|
| HPV DNA and p16
upregulatione, n
(%) |
|
Negative | 10 (19) | 10 (56) | 20 (28) | 0.005 |
|
Positive | 44 (81) | 8 (44) | 52 (72) |
|
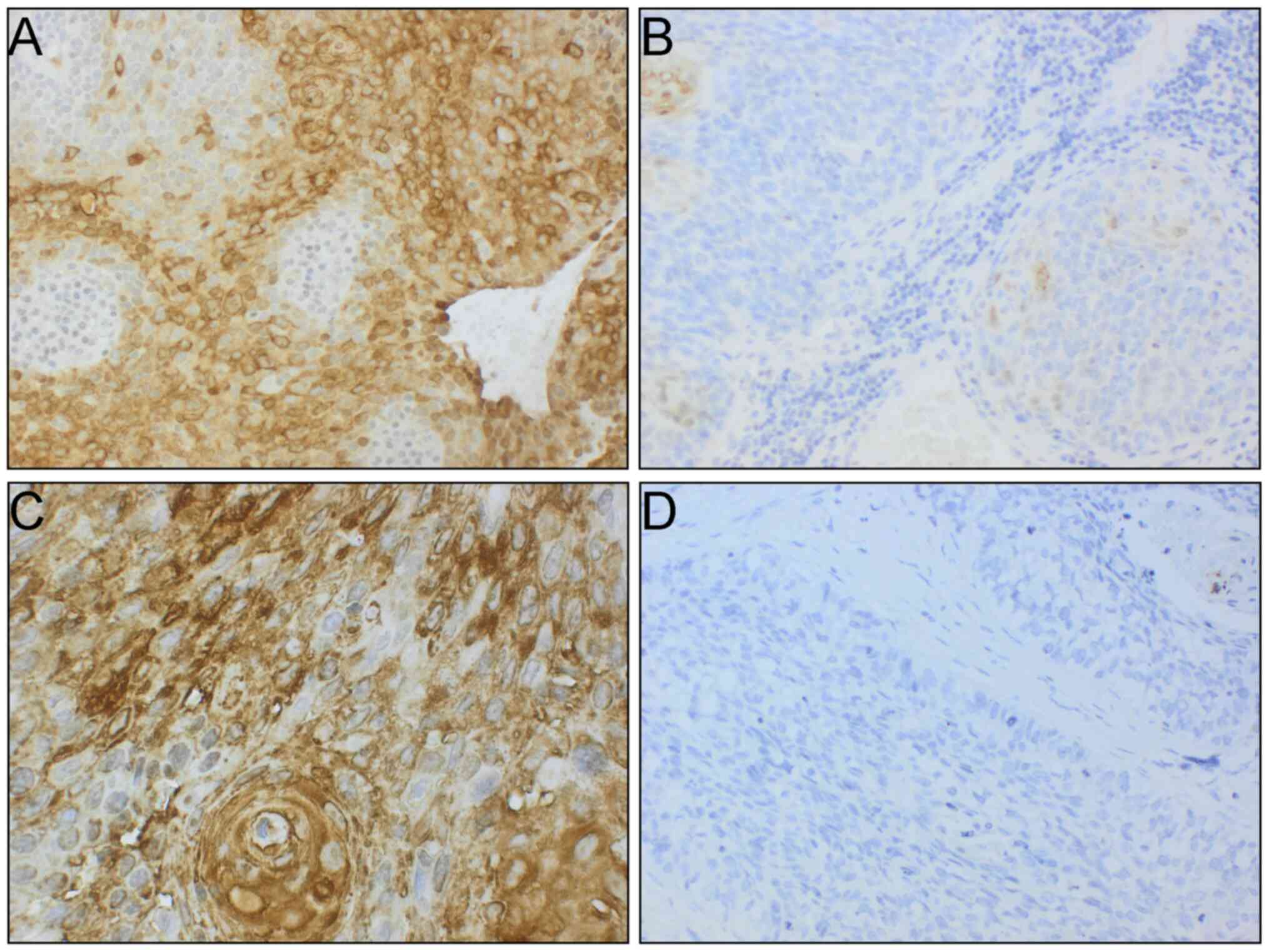
Psoriasin expression varied greatly in the invasive
tumor areas, ranging from no staining to 100% stained tumor cells.
The majority of tumors (n=36) had no (absent) psoriasin staining
(0%) in invasive BOTSCC. When a cut-off of 30% positivity was
applied [as previously suggested by others (15)], 18 tumors were positive in their
invasive tumor component and were defined as having high psoriasin
expression (Fig. 1A and C).
Remaining patients (n=18) had low psoriasin expression in their
invasive BOTSCC (Fig. 1B and D), and
were grouped with the patients with tumors not expressing psoriasin
(low psoriasin expression group).
Patients with BOTSCC and low psoriasin expression
were significantly more often treated with chemo-radiotherapy, were
significantly more often not a current smoker, and their tumors
were significantly more often HPV+ (Table I). Psoriasin expression was not
correlated to histological grade (data not shown).
Psoriasin expression and correlation
to prognosis in patients with HPV+ and HPV- BOTSCC
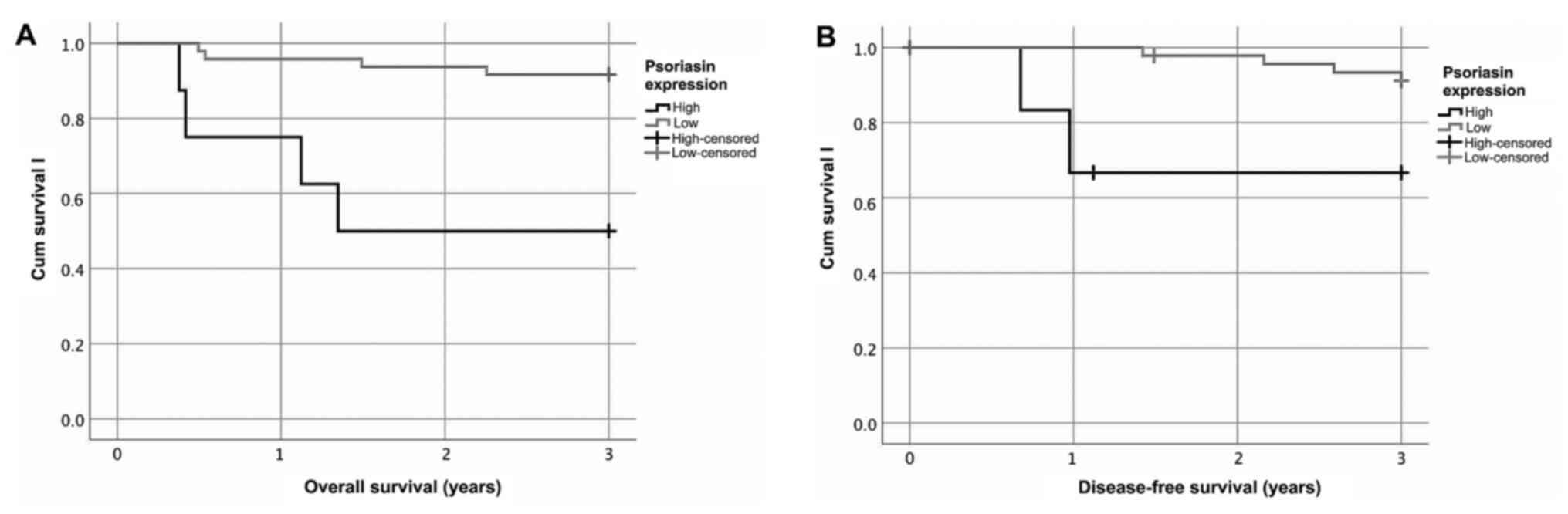
Patients with HPV+ BOTSCC (presence of HPV DNA) and
having high psoriasin expression (≥30%) had a significantly worse
overall survival (OS) and disease-free survival (DFS), as compared
to patients with HPV+ BOTSCC and having a low psoriasin expression
(log rank test: OS P<0.001; DFS P=0.02; Fig. 2). Similar results were obtained when
HPV status was defined as p16 overexpression (>70% p16 positive
tumor cells) alone (log rank test: OS P<0.001; DFS P=0.02; data
not shown) or when HPV DNA positive combined with over expression
of p16 (log rank test: OS P<0.001; DFS P=0.03; data not shown)
was tested. Moreover, high psoriasin expression was independently
correlated to a worse OS and DFS both in uni- and multivariable
analysis, including previously known prognostic factors (Table II).
 | Table II.Univariable and multivariable
analysis of OS and DFS in patients with human papillomavirus
DNA-positive base of tongue squamous cell carcinoma. |
Table II.
Univariable and multivariable
analysis of OS and DFS in patients with human papillomavirus
DNA-positive base of tongue squamous cell carcinoma.
|
| OS | DFS |
|---|
|
|
|
|
|---|
|
| Univariable | Multivariable | Univariable | Multivariable |
|---|
|
|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| TNM-8 stage |
| I and II | 1 |
|
| 1 |
|
| 1 |
|
| 1 |
|
|
| III and IV | 4.7 | 0.94–23 | 0.06 | 3.4 | 0.60–19 | 0.2 | 0.82 | 0.15–4.5 | 0.8 | 0.15 | 0.010–2.3 | 0.2 |
| Agea | 1.0 | 0.96–1.1 | 0.5 | 1.1 | 0.97–1.2 | 0.2 | 1.1 | 0.97–1.2 | 0.2 | 1.3 | 1.0–1.6 | 0.03 |
| Treatment |
| CRT | 1 |
|
| 1 |
|
| 1 |
|
| 1 |
|
|
| RT | 0.59 | 0.14–2.5 | 0.5 | 1.1 | 0.23–5.0 | 0.9 | 0.46 | 0.084–2.5 | 0.4 | 0.28 | 0.022–3.6 | 0.3 |
| Current smoker |
| Yes | 1 |
|
| 1 |
|
| 1 |
|
| 1 |
|
|
| No | 0.32 | 0.075–1.4 | 0.1 | 0.45 | 0.093–2.2 | 0.3 | 0.17 | 0.034–0.84 | 0.03 | 0.0060 | 0-0.21 | 0.005 |
| Psoriasin |
| Low | 1 |
|
| 1 |
|
| 1 |
|
| 1 |
|
|
| High | 8.3 | 2.1–33 | 0.003 | 13 | 2.0–88 | 0.007 | 6.0 | 1.1–33 | 0.04 | 230 | 3.8–14000 | 0.01 |
In contrast, however, no differences in survival
were observed in patients with HPV DNA negative BOTSCC between low
and high psoriasin expression (log rank test: OS P=0.8; DFS P=0.9;
data not shown). Similarly, no differences were identified when HPV
negative status was defined as no p16 overexpression or as absence
of HPV DNA in combination with no p16 overexpression (data not
shown).
Finally, low psoriasin expression as compared to
having a high psoriasin expression in BOTSCC, irrespective of HPV
DNA or p16 status, correlated significantly to a better 3-year OS
and DFS in these patients (P=0.001 and P=0.007, respectively; data
not shown).
Discussion
In this short study, we demonstrate a prognostic
role of psoriasin in a pathological homogenous cohort of only
BOTSCC, and more specifically in HPV associated BOTSCC, suggesting
that psoriasin could potentially be used as a prognostic marker
also in HPV associated OPSCC.
Numerous previous studies have established the
prognostic role of HPV in OPSCC and there is a discussion if and
how treatment can be tapered in patients with HPV+ OPSCC (1,18).
Nevertheless, before such de-escalation may be introduced,
additional prognostic markers are needed to stratify these
patients, in order to avoid undertreatment. Many such markers have
been proposed in HPV+ OPSCC, but few have been validated in
separate cohorts/studies (8). In
this study, we confirm the previously reported prognostic role of
psoriasin expression in head and neck cancer (15) in a homogenous cohort of BOTSCC,
supporting that psoriasin expression correlates to an unfavorable
clinical outcome, especially in patients with HPV+ BOTSCC.
Furthermore, recent data indicate, several
prognostic markers could be assessed together in different
prognostic algorithms in order to better separate patients with
HPV+ OPSCC and a favorable clinical outcome from those with HPV+
OPSCC and a poor clinical outcome (19). It is possible that psoriasin also
should be included in such algorithm.
There are some limitations in this study. No
prognostic effect of psoriasin expression could be observed in
patients with HPV DNA negative BOTSCC, however only 16 such
patients were included, and data on the HPV DNA negative BOTSCC
group should therefore be interpreted with great caution. In
addition, in this pilot study, patients with TSCC and otherOPSCC
were not included. In future studies, to assess the role of
psoriasin expression in OPSCC and its subgroups, it would be
beneficial to include larger patient groups, including more
patients with HPV associated and non-associated BOTSCC, TSCC and
otherOPSCC. Larger, studies would also allow for studies of a
potential correlation of the presence of high psoriasin expression
and smoking as well as other characteristics of the patients and
their tumors.
Finally, one could argue, that it could be possible
to use other cut-off values for psoriasin positivity, rather than a
cut-off of 30%, used here and by others (15). In this specific patient material, an
optimal cut-off value according to Youdens index would be 15% for
OS and 7.5% for DFS (data not shown). However, new cut-off levels
for evaluating psoriasin positivity and survival, would also be
needed to be validated in separate and larger cohorts. Therefore,
the present cut-off should be regarded as a validation of a similar
previously published cut-off (15).
Nevertheless, increased expression of S100A7 protein
has been described in various cancer types (e.g. breast, bladder
and head and neck carcinomas) and often with a correlation to poor
clinical outcome (9–14,20). In
addition, S100A7 has been shown to be functionally linked to
oncogenic properties in oral SCCs, as depletion of this gene
inhibited cell growth, invasion and migration (21). A handful of these prognostic studies
have utilized IHC to quantify psoriasin expression. However,
different quantification approaches and different cut-off levels
for psoriasin expression have been used (10,14,15). For
this reason, we here applied a cut-off value previously used in
HNSCC. Moreover, to our knowledge, the vast majorities of studies
examining psoriasin expression in correlation to prognosis have
shown a poor prognostic value of high psoriasin expression.
However, in a study by Tiveron et al (22), where psoriasin expression was
analyzed in correlation to prognosis in laryngeal carcinoma, an
increased expression did not correlate to prognosis. Therefore,
still, more studies are needed to verify the prognostic role of
psoriasin. Moreover, it may be possible, that examining possible
amplifications of mutations or methylation of the S100A7 gene in
cases where psoriasin expression is correlated to poor prognosis
could give more information. Nevertheless, interestingly is that it
seems that psoriasin more often is expressed in in situ tumor
component as compared to invasive tumor (14,23),
which also applies for HPV+ OPSCC (24). In line with that assumption, only a
fraction of our invasive tumors showed high psoriasin
expression.
Taken together, this short report confirms a
prognostic role of psoriasin and the results imply that psoriasin
may have a prognostic effect in HPV+ BOTSCC. In order to identify
patients with HPV+ BOTSCC and a favorable clinical outcome with
high sensitivity and specificity, it is possible that psoriasin
should be included in such prognostic algorithm.
In conclusion, high psoriasin expression was here
shown as an independent poor prognostic factor in a homogenous
cohort of patients with HPV+ BOTSCC.
Acknowledgements
The authors would like to thank Mrs. Anna Malmerfelt
(Department of Oncology-Pathology, Karolinska Institutet,
Stockholm, Sweden) for technical assistance.
Funding
The present study was supported by Åke Wibergs
Stiftelse, Jeanssons Stiftelser, Tore Nilsons Stiftelse för
Medicinsk Forskning, Stiftelsen Tornspiran, Magnus Bergvalls
Stiftelse, Stockholms Läns Landsting (SLL; grant no. FOUI954801),
Karolinska Institutet, The Swedish Cancer Foundation (grant nos. 20
0704 Pj and 20 0778 Pj) and Cancer och Allergifonden (grant nos.
194 and 10127). The funding bodies had no role in the study design,
data collection, analysis, interpretation of data or in writing the
manuscript.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors contributions
MZ, LH, TD and AN formulated the research question
and came up with the study design. Sample selection and collection
was performed by MZ, LH, LM and DL. LH and MZ performed
immunohistochemistry (IHC). LH, DL and AN evaluated the IHC
staining. LM and DL collected information from the patient case
reports regarding response to treatment, clinical performance and
survival. All raw data has been assessed by MZ, LM, TD and AN to
ensure its legitimacy. MZ, LM, TD and AN analyzed, summarized and
interpreted the data and wrote the manuscript, which was revised
and approved by all co-authors. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study, including patient information and
consent, was conducted according to ethical permissions
2009/1278-31/4 and 2017/1035-31/2 from the Ethics Committee at
Karolinska Institute, Stockholm, Sweden.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BOTSCC
|
base of tongue squamous cell
carcinoma
|
|
DFS
|
disease-free survival
|
|
FFPE
|
formalin-fixed paraffin-embedded
|
|
HNSCC
|
head and neck squamous cell
carcinoma
|
|
HPV
|
human papillomavirus
|
|
HPV-
|
human papillomavirus-negative
|
|
HPV+
|
human papillomavirus-positive
|
|
IHC
|
immunohistochemistry
|
|
OPSCC
|
oropharyngeal squamous cell
carcinoma
|
|
OS
|
overall survival
|
References
|
1
|
Näsman A, Du J and Dalianis T: A global
epidemic increase of an HPV-induced tonsil and tongue base cancer -
potential benefit from a pan-gender use of HPV vaccine. J Intern
Med. 287:134–152. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haeggblom L, Ramqvist T, Tommasino M,
Dalianis T and Näsman A: Time to change perspectives on HPV in
oropharyngeal cancer. A systematic review of HPV prevalence per
oropharyngeal sub-site the last 3 years. Papillomavirus Res.
4:1–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gelwan E, Malm IJ, Khararjian A, Fakhry C,
Bishop JA and Westra WH: Nonuniform distribution of high-risk human
papillomavirus in squamous cell carcinomas of the oropharynx:
rethinking the anatomic boundaries of oral and oropharyngeal
carcinoma from an oncologic HPV perspective. Am J Surg Pathol.
41:1722–1728. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ljøkjel B, Lybak S, Haave H, Olofsson J,
Vintermyr OK and Aarstad HJ: The impact of HPV infection on
survival in a geographically defined cohort of oropharynx squamous
cell carcinoma (OPSCC) patients in whom surgical treatment has been
one main treatment. Acta Otolaryngol. 134:636–645. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marklund L, Näsman A, Ramqvist T, Dalianis
T, Munck-Wikland E and Hammarstedt L: Prevalence of human
papillomavirus and survival in oropharyngeal cancer other than
tonsil or base of tongue cancer. Cancer Med. 1:82–88. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tham T, Wotman M, Roche A, Kraus D and
Costantino P: The prognostic effect of anatomic subsite in
HPV-positive oropharyngeal squamous cell carcinoma. Am J
Otolaryngol. 40:567–572. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marklund L, Holzhauser S, de Flon C,
Zupancic M, Landin D, Kolev A, Haeggblom L, Munck-Wikland E,
Hammarstedt-Nordenvall L, Dalianis T, et al: Survival of patients
with oropharyngeal squamous cell carcinomas (OPSCC) in relation to
TNM 8 - Risk of incorrect downstaging of HPV-mediated
non-tonsillar, non-base of tongue carcinomas. Eur J Cancer.
139:192–200. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Näsman A, Bersani C, Lindquist D, Du J,
Ramqvist T and Dalianis T: Human papillomavirus and potentially
relevant biomarkers in tonsillar and base of tongue squamous cell
carcinoma. Anticancer Res. 37:5319–5328. 2017.PubMed/NCBI
|
|
9
|
Ye L, Sun PH, Martin TA, Sanders AJ, Mason
MD and Jiang WG: Psoriasin (S100A7) is a positive regulator of
survival and invasion of prostate cancer cells. Urol Oncol.
31:1576–1583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu J, Zhao Z, Sun Z, Liu C, Cheng X, Ruge
F, Yang Y, Jiang WG and Ye L: Increased expression of psoriasin is
correlated with poor prognosis of bladder transitional cell
carcinoma by promoting invasion and proliferation. Oncol Rep.
43:562–570. 2020.PubMed/NCBI
|
|
11
|
Hattinger E, Zwicker S, Ruzicka T, Yuspa
SH and Wolf R: Opposing functions of psoriasin (S100A7) and
koebnerisin (S100A15) in epithelial carcinogenesis. Curr Opin
Pharmacol. 13:588–594. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Y, Bunston C, Hodson N, Resaul J, Sun
PH, Cai S, Chen G, Gu Y, Satherley LK, Bosanquet DC, et al:
Psoriasin promotes invasion, aggregation and survival of pancreatic
cancer cells; association with disease progression. Int J Oncol.
50:1491–1500. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Al-Haddad S, Zhang Z, Leygue E, Snell L,
Huang A, Niu Y, Hiller-Hitchcock T, Hole K, Murphy LC and Watson
PH: Psoriasin (S100A7) expression and invasive breast cancer. Am J
Pathol. 155:2057–2066. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Emberley ED, Murphy LC and Watson PH:
S100A7 and the progression of breast cancer. Breast Cancer Res.
6:153–159. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tripathi SC, Matta A, Kaur J, Grigull J,
Chauhan SS, Thakar A, Shukla NK, Duggal R, DattaGupta S, Ralhan R,
et al: Nuclear S100A7 is associated with poor prognosis in head and
neck cancer. PLoS One. 5:e119392010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Attner P, Du J, Näsman A, Hammarstedt L,
Ramqvist T, Lindholm J, Marklund L, Dalianis T and Munck-Wikland E:
The role of human papillomavirus in the increased incidence of base
of tongue cancer. Int J Cancer. 126:2879–2884. 2010.PubMed/NCBI
|
|
17
|
Näsman A, Andersson E, Marklund L,
Tertipis N, Hammarstedt-Nordenvall L, Attner P, Nyberg T, Masucci
GV, Munck-Wikland E, Ramqvist T, et al: HLA class I and II
expression in oropharyngeal squamous cell carcinoma in relation to
tumor HPV status and clinical outcome. PLoS One. 8:e770252013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ang KK, Harris J, Wheeler R, Weber R,
Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C,
et al: Human papillomavirus and survival of patients with
oropharyngeal cancer. N Engl J Med. 363:24–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tertipis N, Hammar U, Näsman A, Vlastos A,
Nordfors C, Grün N, Ährlund-Richter A, Sivars L, Haeggblom L,
Marklund L, et al: A model for predicting clinical outcome in
patients with human papillomavirus-positive tonsillar and base of
tongue cancer. Eur J Cancer. 51:1580–1587. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mayama A, Takagi K, Suzuki H, Sato A,
Onodera Y, Miki Y, Sakurai M, Watanabe T, Sakamoto K, Yoshida R, et
al: OLFM4, LY6D and S100A7 as potent markers for distant metastasis
in estrogen receptor-positive breast carcinoma. Cancer Sci.
109:3350–3359. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dey KK, Bharti R, Dey G, Pal I, Rajesh Y,
Chavan S, Das S, Das CK, Jena BC, Halder P, et al: S100A7 has an
oncogenic role in oral squamous cell carcinoma by activating
p38/MAPK and RAB2A signaling pathway. Cancer Gene Ther. 23:382–391.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tiveron RC, de Freitas LC, Figueiredo DL,
Serafini LN, Mamede RC and Zago MA: Expression of calcium binding
protein S100 A7 (psoriasin) in laryngeal carcinoma. Rev Bras
Otorrinolaringol (Engl Ed). 78:59–65. 2012.
|
|
23
|
Alowami S, Qing G, Emberley E, Snell L and
Watson PH: Psoriasin (S100A7) expression is altered during skin
tumorigenesis. BMC Dermatol. 3:12003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Haeggblom L, Ährlund-Richter A, Mirzaie L,
Farrajota Neves da Silva P, Ursu RG, Ramqvist T and Näsman A:
Differences in gene expression between high-grade dysplasia and
invasive HPV+ and HPV- tonsillar and base of tongue cancer. Cancer
Med. 8:6221–6232. 2019. View Article : Google Scholar : PubMed/NCBI
|