Introduction
Soft tissue tumors in the extremities and the trunk
can exhibit a variety of pathological features, making them
potentially difficult to diagnose (1). Although magnetic resonance imaging
(MRI) results are typically used for diagnosis, the examination
frequently lacks specific findings. Even radiological
diagnosticians and medical specialists of bone/soft tissue tumors
often struggle to distinguish between benign and malignant tumors
based on such imaging data (2).
Since inappropriate primary care adversely affects patients'
prognoses, a clear distinction between benign and malignant tumors
upon the initial diagnosis is essential (3,4).
Ultrasonography (US) is a simple imaging diagnostic
tool with low invasiveness that has improved through technical
advancements and is increasingly being utilized in orthopedic cases
(5). Vascularity evaluation using
color Doppler and power Doppler is also useful in distinguishing
between benign and malignant tumors when diagnosing soft tissue
tumors (6–8). The Giovagnorio classification (6) divides the vascular distribution into 4
types and has been used in various studies (8–10).
Furthermore, a scoring system (SS) evaluating vascularity, tumor
size, echogenicity, and internal structure findings can improve the
accuracy of benign and malignant tumor distinction (9,10).
Strain elastography (SE) is a method for elasticity evaluation
based on the strain on the tissue upon the application of pressure
via probes. Using SE to evaluate the elasticity can also be useful
in the diagnosis of soft tissue tumors (11–16).
Among the newly improved evaluation techniques for
US, superb microvascular imaging (SMI) facilitates the assessment
of lower flow vascularity better than color Doppler and power
Doppler (17,18). In addition, shear wave elastography
(SWE) is useful to evaluate tissue elasticity by calculating the
velocity of the shear waves passing through the tissue (19–22).
Unlike SE, SWE is a simple method because it does not require the
application of pressure via probes and provides quantitative data
on the stiffness of the tissue. These techniques have been applied
to other anatomical regions such as the thyroid gland, mammary
gland, liver, and prostate gland (17–22).
However, the usefulness of SMI and SWE in the diagnosis of soft
tissue tumors remains unclear.
This study aimed to determine: i) the usefulness of
vascularity and elasticity evaluation, using SMI and SWE,
respectively, to distinguish between benign and malignant soft
tissue tumors, and ii) the diagnostic accuracy of soft tissue
tumors by establishing an original SS based on vascularity and
elasticity assessments.
Materials and methods
Patient characteristics
This retrospective study was approved by the Human
Research Ethics Committee of the Hirosaki University Graduate
School of Medicine (Aomori, Japan; reference no. 2019-1023), and
the requirement for informed consent was waived because of the
anonymous nature of the data. This study targeted consecutive cases
that were examined by US prior to biopsy, surgery, and pathological
tissue diagnosis at our hospital from April 2016 to September 2018.
Medical records, US data, and MRI data were investigated in April
2019, and the cases without sufficient data were excluded. A total
of 167 lesions in 164 cases (86 male, 78 female) was enrolled in
the present study. The mean age was 56 years (range: 5 to 92).
Pediatric cases were only eight (5 to 16 years). There were 47
targeted lesions in the upper extremities, 76 in the lower
extremities, and 44 in the trunk. The pathological tissue diagnosis
was based on the World Health Organization classification 2013
(1), and was divided into 3 groups:
benign, intermediate, and malignant (Table I). Non-tumoral benign lesion types,
such as ganglion, atheroma, and hematoma were also targeted. One
case of an intramuscular glomus tumor, which was a non-typical case
that was diagnosed with uncertain malignant potential, was
classified into the intermediate group in this study.
 | Table I.Number of patients diagnosed with
different forms of pathological tumor. |
Table I.
Number of patients diagnosed with
different forms of pathological tumor.
| Tumor type | Number of
patients |
|---|
| Benign soft tissue
tumors (n=99) |
|
|
Lipoma | 35 |
|
Schwannoma | 23 |
| Vascular
malformation | 9 |
| Epidermal
cyst | 9 |
| Spindle
cell lipoma | 5 |
|
Tenosynovial giant cell tumor
(localized type) | 3 |
|
Intramuscular myxoma | 2 |
| Chronic
expanding hematoma | 2 |
| Fibroma
of tendon sheath | 2 |
|
Ganglion | 2 |
|
Elastofibroma | 1 |
|
Hematoma | 1 |
|
Desmoplastic
fibroblastoma | 1 |
|
Neurofibroma | 1 |
|
Hibernoma | 1 |
|
Angiolipoma | 1 |
| Nodular
fasciitis | 1 |
| Intermediate soft
tissue tumors (n=21) |
|
|
Atypical lipomatous tumor | 8 |
| Desmoid
type fibromatosis | 5 |
| Plantar
fibromatosis | 4 |
|
Dermatofibrosarcoma
protuberans | 2 |
| Soft
tissue recurrence of bone GCTs | 1 |
| Glomus
tumor (uncertain malignant potential) | 1 |
| Malignant soft
tissue tumors (n=47) |
|
|
Myxofibrosarcoma | 14 |
| Myxoid
liposarcoma | 5 |
|
Undifferentiated pleomorphic
sarcoma | 4 |
| Soft
tissue metastasis of carcinoma | 4 |
|
Rhabdomyosarcoma | 3 |
|
Leiomyosarcoma | 3 |
|
Extraskeletal myxoid
chondrosarcoma | 2 |
|
Extraskeletal
osteosarcoma | 2 |
|
Synovial sarcoma | 2 |
|
Dedifferentiated
liposarcoma | 2 |
| High
grade sarcoma | 2 |
|
Malignant lymphoma | 2 |
|
Low-grade fibromyxoid
sarcoma | 1 |
| Clear
cell sarcoma | 1 |
Evaluation of image findings on US and
MRI
The 4 items evaluated were as follows: vascularity
index (VI) for SMI, maximal shear velocity (MSV) for SWE, and tumor
size and tumor depth on MRI. All US examinations and MRI reviews
were conducted by a single musculoskeletal oncologist with 19 years
of experience. The Aplio 500 (Toshiba Medical Systems Corporation)
was used as the US diagnostic device and a linear-type probe
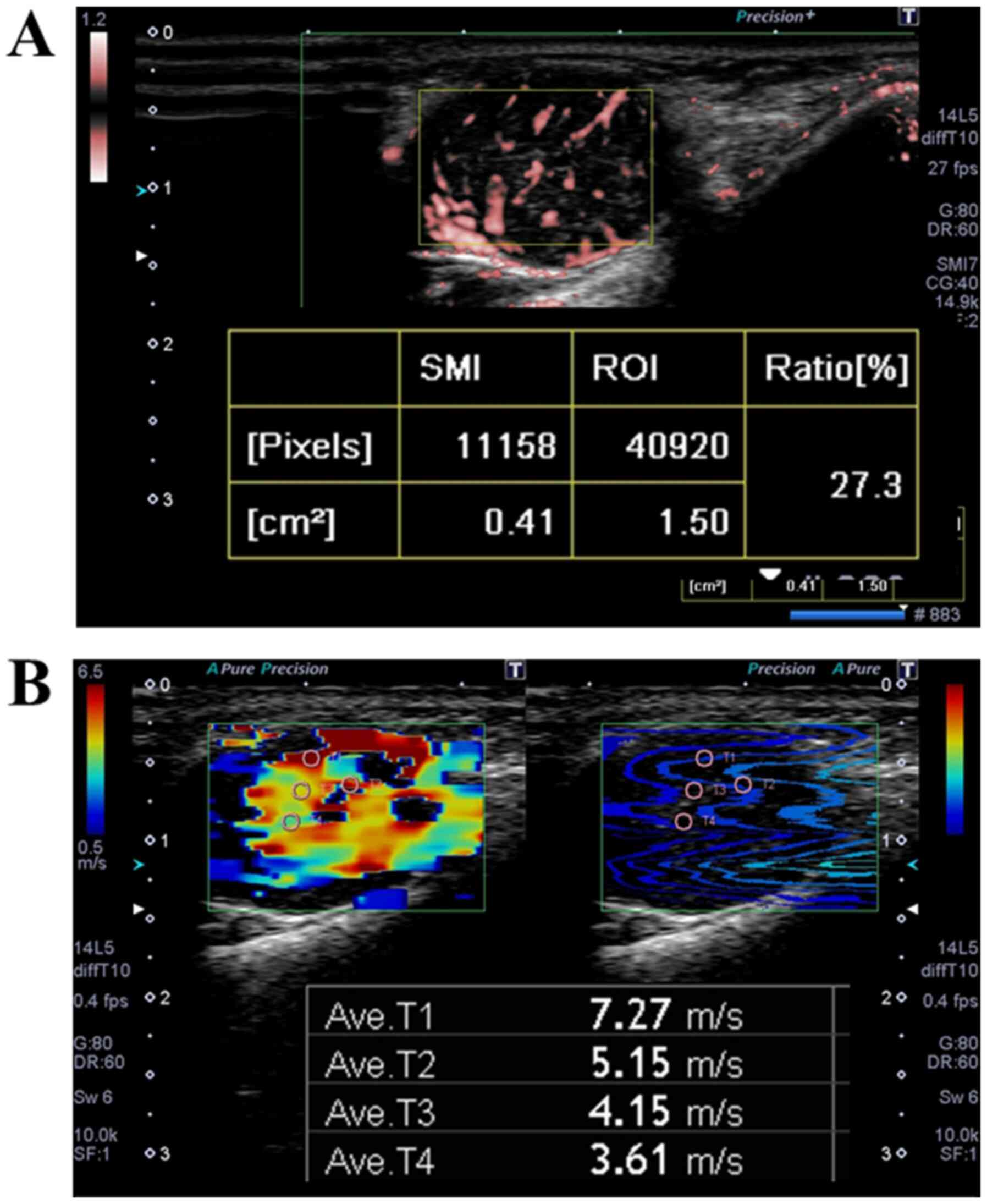
PLT-1005BT was used for the evaluations. The vascularity of the
tumors was evaluated by SMI (Fig.
1A). The vascular ratio in the region of interest (ROI) was
quantified. The mean vascularity ratio at three measured positions
was defined as the VI. The elasticity of the tumors was evaluated
by SWE in the same intratumoral areas that were measured by SMI.
Small circular ROIs with a diameter of 1 mm were placed in the
rectangular ROI of SWE to calculate shear velocity (Fig. 1B). The maximal value among shear
velocity values was defined as the MSV. The size of the ROI was
equal to the tumor size when the entire tumor fit within the width
of the probe. When the tumor was larger than the width of the
probe, the ROI was adjusted to evaluate the tumor from the shallow
to central parts. The maximal length of the tumor visible via MRI
was used as the tumor size for all cases because most tumors were
large and unmeasurable by the US device. The depth of the tumor was
also evaluated by MRI for all cases. Tumors were divided into
superficial or deep categories based on their location relative to
the fascia.
Statistical analyses
The tumor size values were normally distributed,
those of VI and MSV were not normally distributed by the
Shapiro-Wilk test. Values of tumor size, VI, and MSV were presented
as medians with interquartile range, and medians among the 3 groups
(benign, intermediate, and malignant) were compared by the
Kruskal-Wallis test followed by Dunn's test as a post hoc analysis.
The depth (deep/superficial) was compared using a Fisher's exact
test. To investigate the cut-off values of the tumor size, VI, and
MSV, the receiver operating characteristic (ROC) analysis was
performed. In this analysis, the area under the curve (AUC), odds
ratio, and the respective cut-off values based on the malignancy
were calculated. Using the cut-off values of the tumor size
(large/small), VI (hyper/hypo vascularity) and MSV (hard/soft) from
the ROC analysis, multivariate logistic regression analysis was
performed, with the malignancy against the benign or intermediate
tumors as a dependent variable, and the presence of tumor size, VI,
MSV, and depth. Data input and data analysis were performed using
SPSS version 25.0J (SPSS Inc.), and the Excel statistical software
BellCurve for Excel (Version 3.0; Social Survey Research
Information Co., Ltd.). A P-value <0.05 was considered
statistically significant.
Establishment and evaluation of an
original SS
Based on the odds ratios of the multivariate
logistic regression analysis, an original SS was established. A
cut-off value of the SS score for malignancy was also determined by
a ROC analysis, and the sensitivity and specificity were
calculated. The median scores of the SS between the three groups
were compared using Kruskal-Wallis test followed by Dunn's test as
a post hoc analysis. In addition, the scores for each soft tissue
tumor, in which the number of lesions was 3 or more, were
evaluated.
Results
Evaluation of image findings on US and
MRI
Values of VI and MSV were significantly higher in
the malignant group than in the benign and intermediate group
(P<0.001, respectively) (Table
II). There were no significant differences in the values of VI
between the benign and intermediate groups (P=0.592). While the
tumor size in the intermediate and malignant groups was
significantly larger than that of the benign group (P<0.001),
there was no significant difference in tumor size between the
intermediate and malignant groups (P=0.109). Ratio of deep lesion
was higher in the intermediate and malignant groups (P=0.005).
 | Table II.Comparison of VI, MSV, tumor size and
tumor depth between each group. |
Table II.
Comparison of VI, MSV, tumor size and
tumor depth between each group.
| Method | Benign | Intermediate | Malignancy |
|---|
| Vascularity index
(%) |
|
Median | 2.4 | 3.1 | 10.0a,b |
|
Interquartile range | (0.2–6.0) | (1.3–7.6) | (5.0–12.0) |
| Maximal shear
velocity (m/sec) |
|
Median | 6.1 | 7 | 8.3a,b |
|
Interquartile range | (3.4–7.7) | (5.9–7.6) | (7.9–8.7) |
| Tumor size
(cm) |
|
Median | 5.0 | 7.8 | 8.0a |
|
Interquartile range | (3.0–7.4) | (3.8–14.6) | (5.0–12.0) |
| Depth |
|
Superficial lesion (n) | 37 | 2 | 8 |
| Deep
lesion (n) | 62 | 19 | 39 |
| Ratio
of deep lesion (%) |
62.6 |
90.5 | 83.0c |
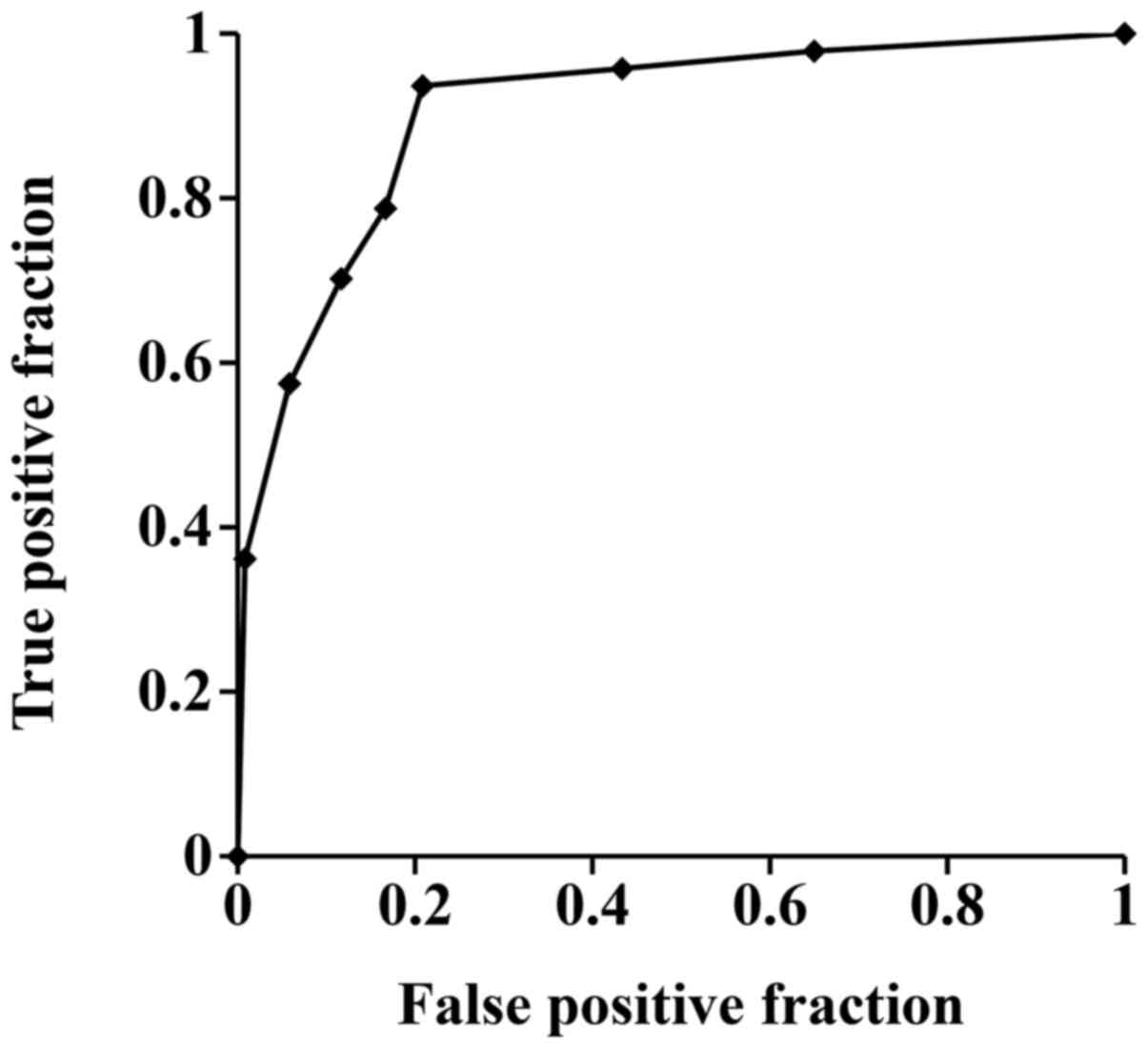
ROC analysis (Fig. 2)
indicated the cut-off value of tumor size for malignancy was 7 cm
(AUC: 0.69, P<0.001, odds: 4.0, 95% CI: 0.61–0.78). The cut-off
value of VI was 5.3% (AUC: 0.75, P<0.001, odds: 7.0, 95% CI:
0.68–0.83). The cut-off value of MSV was 7.9 m/sec (AUC: 0.84,
P<0.001, odds: 18.5, 95% CI: 0.77–0.91).
 | Figure 2.ROC analysis for tumor size, VI and
MSV. The area under the curve values for tumor size, VI and MSV
were 0.69, 0.75 and 0.84, respectively. The cut-off values with
respect to malignancy for tumor size, VI and MSV were 7 cm, 5.3%
and 7.9 m/sec, respectively. ROC, receiver operating
characteristic; VI, vascularity index; MSV, maximal shear
velocity. |
Establishment and evaluation of
original SS
All the 4 items (VI, MSV, tumor size and tumor
depth) were entered into the multivariate logistic regression
analysis, and the three values (VI, MSV and tumor size) were
selected as independent risk factors (Table III). The odds ratios for malignancy
against other groups were 8.74, 18.81 and 6.03 for VI, MSV and
tumor size, respectively. An original SS consisting of these 3
items (VI, MSV and tumor size) was established based on the odds
ratio for identifying the malignancy of the soft tissue tumors
(Table IV). The score of the 3
items summed for each lesion ranged from 0 to 5.5 points.
 | Table III.Multivariate logistic regression
analysis of each method. |
Table III.
Multivariate logistic regression
analysis of each method.
|
| Crude | Adjusted |
|---|
|
|
|
|
|---|
| Method | B | P-value | Odds ratio | 95% CI | B | P-value | Odds ratio | 95% CI |
|---|
| Vascularity
index | 1.80 | <0.001 | 6.06 | 2.8–12.9 | 2.17 | <0.001 | 8.74 | 3.0–25.2 |
| Maximal shear
velocity | 2.92 | <0.001 | 18.5 | 7.9–43.2 | 2.9 | <0.001 | 18.81 | 6.9–51.2 |
| Tumor size | 1.39 | <0.001 | 4.02 | 2.0–8.2 | 1.80 | 0.001 | 6.03 | 2.2–16.7 |
| Depth | 0.85 | 0.049 | 2.35 | 1.0–5.5 | −0.01 | 0.983 | 0.99 | 0.31–3.2 |
 | Table IV.An original scoring system based on
the odds ratio from the multivariate logistic regression analysis
for the identification of soft tissue malignancy. |
Table IV.
An original scoring system based on
the odds ratio from the multivariate logistic regression analysis
for the identification of soft tissue malignancy.
| Method | Points |
|---|
| Tumor size
(cm) |
|
|
<7 | 0.0 |
| ≥7 | 1.0 |
| Vascularity index
(%) |
|
|
<5.3 | 0.0 |
|
≥5.3 | 1.5 |
| Maximal shear
velocity (m/sec) |
|
|
<7.9 | 0.0 |
|
≥7.9 | 3.0 |
| Total | 0.0–5.5 |
ROC analysis (Fig. 3)
showed that the cut-off value of score of original SS for
malignancy was estimated as 2.5 points (AUC: 0.90, P<0.001,
odds: 55.7, 95% CI: 0.85–0.96). Based on this cut-off value,
original SS showed 93.6% sensitivity and 79.2% specificity to
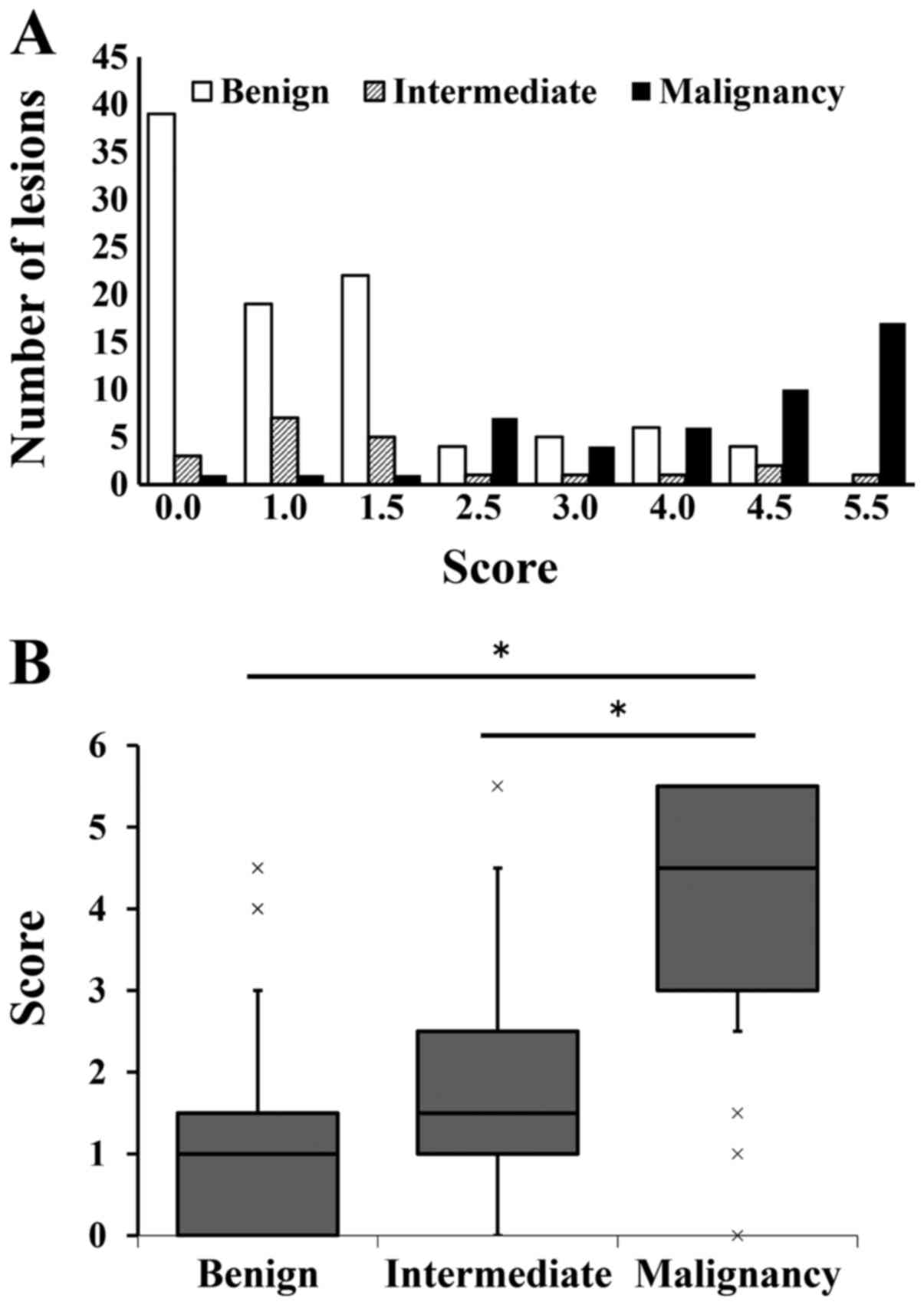
identify malignancy. In the score distribution by using the
original SS, as the score increased, the incidence of malignant
lesions increased (Fig. 4A). Three
(1 myxofibrosarcoma, 1 soft tissue metastasis of carcinoma, 1
malignant lymphoma) out of the 47 malignant lesions had scores
<2.5 points and the other 44 had scores ≥2.5 points. Nineteen of
the 99 benign lesions and 6 of the 21 intermediate lesions had
scores ≥2.5 points. The median and interquartile range of scores
for the benign, intermediate, and malignant groups were 1.0 (0–1.5)
(95% CI: 0.94–1.47), 1.5 (1.0–2.5) (95% CI: 1.12–2.55), and 4.5
(3.0–5.5) (95% CI: 3.73–4.55), respectively. The original SS score
of the malignant group was significantly higher than that of the
other groups (P<0.001) (Fig. 4B).
There was no significant difference between the benign and the
intermediate group (P=0.350). Representative scores of soft tissue
tumors were also calculated (Table
SI). In the benign group, schwannoma, vascular malformation,
and spindle cell lipoma showed relatively high scores compared to
the other benign lesions. In the intermediate group, desmoid type
fibromatosis showed a high score compared to the other lesions.
There were no significant differences between the three lipomatous
tumors, namely, lipoma, spindle cell lipoma, and atypical
lipomatous tumor. In the malignant group, all lesions showed a high
score, and there was no significant difference between them.
Discussion
In this study, our results revealed significantly
high VI and MSV values for malignant group and demonstrated the
usefulness of these techniques in distinguishing between benign or
intermediate and malignant group. Furthermore, the original SS
based on vascularity and elasticity assessments by US in
conjunction with tumor size on MRI offered high diagnostic accuracy
for malignant group. This study is, to the best of our knowledge,
the first report to demonstrate the usefulness of both vascularity
and elasticity to distinguish between benign or intermediate and
malignant soft tissue tumors.
Evaluation of tumor vascularity using SMI
established the technique's efficacy in distinguishing between
benign or intermediate, and malignant soft tissue tumors. Malignant
solid tumors show rich vascularity since tumor angiogenesis is
enhanced in invasive and progressive malignant solid tumors
(23). Many examiners frequently
assess the distribution of intratumoral vascularity using the
Giovagnorio classification for evaluation in US (6,8–10). Oebisu et al reported color
Doppler US with a contrast medium increased the diagnostic accuracy
of malignant soft tissue tumors based on the Giovagnorio
classification, since the use of the contrast medium made
evaluation of the intratumoral vascularity possible for a longer
duration and in more detail (8). We
attempted the quantification of the intratumoral vascularity by SMI
instead of the vascular distribution by color Doppler, since VI
values were significantly higher for the malignant group than
benign or intermediate group. This study is, to the best of our
knowledge, the first report to demonstrate the usefulness of
quantification of the intratumoral vascularity by SMI to
distinguish between benign or intermediate and malignant soft
tissue tumors. SMI is a non-invasive and easy-to-use technique to
enable the visualization of low flow and fine vascularity without
contrast medium and could be used for initial diagnostic
distinction of soft tissue tumors.
SWE for the evaluation of tumor elasticity was also
useful for making a distinction between benign or intermediate, and
malignant soft tissue tumors. Malignant lesions in other organs,
such as the breast, prostate, and thyroid, showed high shear wave
velocity indicating stiffness on palpation (24–26).
Recent studies reported that SWE was not useful for making a
distinction between benign and malignant tumors and malignant
musculoskeletal lesions trended toward a lower shear wave velocity
compared to benign lesions (26–29),
indicating that malignant lesions are softer than benign lesions.
In our study, MSV values in SWE were significantly higher for the
malignant group than the benign group, signifying malignant lesions
are stiffer, which is in accordance with previous SE studies that
malignant soft tissue lesions tended to be stiffer compared to the
benign lesions (13,15,16). The
different results in SWE between previous studies (27–30) and
ours could be explained by the differences of implemented US
devices and evaluation methods. Previous studies used US devices
made in Euro-American companies such as Siemens Acuson and
LOGIQ-E9, and evaluated each in different ways such as mean of
multi-velocity readings and use of fixed sized SWE rectangular box.
We used the Aplio 500 made in Japan, and assessed the maximal
values among the shear velocity values calculated in our study.
Other reasons may be the differences in clinical characteristics of
targeted lesions such as size, depth, and pathological
heterogeneous nature.
Tumor size is a universal finding suggesting
malignant soft tissue tumors (31).
As malignant soft tissue tumors grow, they may become firmer
because intratumoral necrotic tissue enlarges due to hypoxia or
intratumoral pressure is increased due to compression between
surrounding tissues such as muscle and fascia. Our study had an
increased number of larger malignant soft tissue tumors that were
unmeasurable by the US device compared to previous SWE reports
(28–30). Our SWE results are similar to the
common clinical presentation of most soft tissue sarcomas, which
tend to be found on palpation with elastic to hard firmness. The
depth of the tumor is also an important finding suggesting
malignant soft tissue tumors. The depth of the malignant group was
significantly deeper compared to that of the benign group in
univariate analysis in our study. However, we had not include the
depth of the tumor in our SS while considering the results of
multivariate logistic regression analysis. Indeed, notation
considering the depth of the tumor was eliminated in the new TNM
classification of soft tissue sarcoma (31).
Our SS based on vascularity and elasticity evaluated
by US showed high diagnostic accuracy for malignant soft tissue
tumors. Nagano et al used an SS consisting of 4 items (i.e.,
tumor size, echogenicity, internal texture, and vascular
distribution with color Doppler) to report sarcoma diagnosis
capability with an AUC value of 0.88, 85.1% sensitivity, and 86.9%
specificity (9). Recently, Morii
et al used an SS consisting of tumor size, vascular
distribution with power Doppler, and tumor margins to report its
usefulness in sarcoma diagnoses with an AUC value of 0.85, 82.5%
sensitivity, and 73.2% specificity (10). Our SS showed higher AUC values and
sensitivity compared to previous reports, and also showed the
possibility to distinguish soft tissue tumors with same
differentiation such as lipomas, spindle cell lipomas, and atypical
lipomatous tumors. In our hospital, most of the patients were
referred to our department with MRI data without contrast
enhancement, and we had evaluated the intratumoral vascularity and
elasticity by SMI and SWE at the date of first visit. Furthermore,
we performed needle biopsy under US guidance for an early diagnosis
if soft tissue lesions of the referral cases had high vascularity
and elasticity. Evaluation of tumor size via MRI is an
uncomplicated approach for both non-medical and medical
specialists. US examination is also safe, low-cost, and easy to use
for a non-medical specialist. Our SS based on US evaluation
including tumor size via MRI may be universally suitable for
initial and early diagnostic distinction.
This study, while emphasizing the importance of
elasticity and vascularity, has pitfalls of US such as the
potential for false negatives and false positives. Several mucinous
malignant tumors (4 myxofibrosarcomas, 2 myxoid liposarcomas, and 1
low grade fibromyxoid sarcoma) showed low MSV (<7.9 m/sec)
values as false negatives in our study. Two myxofibrosarcomas and 1
myxoid liposarcoma showed considerably low MSV (4.4, 2.2 and 4.9
m/sec). Evaluating the vascularity also enhances the likelihood of
acquiring false negatives for large malignant tumors with wide
range necrosis, which exhibit low values for VI. Meanwhile,
schwannomas and desmoid-type fibromatoses exhibited high values for
vascularity and elasticity and were frequently determined as false
positives. US provides information using a non-invasive technique
at a low cost and is an essential imaging examination tool for the
initial diagnosis of soft tissue tumors despite these pitfalls.
Our study had several limitations. First, all
examinations, and evaluations were performed by the first author, a
medical specialist for bone/soft tissue tumors. The retrospective
nature of the study is also a limitation. Poor inter-rater
reliability may be caused by the differences of US technique
between specialists and non-specialists, especially with regard to
location of the probe and selection of ROI. Thus, in the future,
prospective studies with examinations conducted by medical
specialists and non-medical specialists should be conducted to
determine the repeatability and reliability of the results.
Additionally, patient population was heterogeneous with regard to
tumor size, depth, and pathological diagnosis. It is favorable to
compare between benign and malignant soft tissue tumors with the
same sizes and depths, but malignant lesions are often larger and
deeper than benign ones in clinical practice. Sample size was too
small to compare between benign or intermediate, and malignant soft
tissue tumors under the same conditions. A multicenter study with
larger sample size is necessary to elucidate the US characteristics
such as tumor size and depth under similar conditions. A
multicenter study might also reveal novel US findings for specific
types of soft tissue tumors such as mucinous and lipomatous soft
tissue tumors. Other limitation of our study is the selection bias
due to the indication of the surgery. We included only cases in
which the pathological tissue diagnosis was proven. We should
consider that the cut-off values and the SS setting might have
changed if our study had included all benign and intermediate soft
tissue tumors that were evaluated by US but not resected.
In conclusion, evaluating vascularity by SMI and
elasticity by SWE is a useful technique to distinguish between
benign or intermediate and malignant tumors, even if the
evaluations are performed separately. Furthermore, our SS
established based on these evaluations including tumor size via MRI
showed high diagnostic accuracy for malignant soft tissue tumors.
Therefore, an SS based on the US evaluation of vascularity and
elasticity is a useful initial diagnostic tool for soft tissue
tumors.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Sasaki E
(Department of Orthopaedic Surgery, Hirosaki University Graduate
School of Medicine, Hirosaki, Japan) for his assistance in the
statistical analyses of the data.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SO, TS, TO, HO and YI conceived and designed the
current study. SO, TS, TO and HO treated and observed patients at
Hirosaki University. SO performed ultrasonography examinations and
MRI reviews, prepared the manuscript and acquired the data. TS and
TO confirmed the authenticity of all the raw data. YI supervised
the current study. All authors participated in the interpretation
of the results and have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or National Research Committee and with the 1964
Helsinki declaration and its later amendments or comparable ethical
standards. The study protocol was approved by Human Research Ethics
Committee of the Hirosaki University Graduate School of Medicine
(Aomori, Japan; reference no. 2019-1023). The present study was
performed using a retrospective design. The requirement for
informed consent was waived because of the anonymous nature of the
data. The opt-out approach was used.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AUC
|
area under the curve
|
|
MRI
|
magnetic resonance imaging
|
|
MSV
|
maximal shear velocity
|
|
ROI
|
region of interest
|
|
ROC
|
receiver operating characteristic
|
|
SE
|
strain elastography
|
|
SMI
|
superb microvascular imaging
|
|
SS
|
scoring system
|
|
SWE
|
shear wave elastography
|
|
US
|
ultrasonography
|
|
VI
|
vascularity index
|
References
|
1
|
Fletcher CD, Bridge JA, Hogendoorn PC and
Mertens F: World Health Organization classification of tumours of
soft tissue and bone. 4th edition. IARC Press; Lyon: 2013
|
|
2
|
Moulton JS, Blebea JS, Dunco DM, Braley
SE, Bisset GS III and Emery KH: MR imaging of soft-tissue masses:
Diagnostic efficacy and value of distinguishing between benign and
malignant lesions. AJR Am J Roentgenol. 164:1191–1199. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arai E, Nishida Y, Tsukushi S, Wasa J and
Ishiguro N: Clinical and treatment outcomes of planned and
unplanned excisions of soft tissue sarcomas. Clin Orthop Relat Res.
468:3028–3034. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pretell-Mazzini J, Barton MD Jr, Conway SA
and Temple HT: Unplanned excision of soft-tissue sarcomas: Current
concepts for management and prognosis. J Bone Joint Surg Am.
97:597–603. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blankstein A: Ultrasound in the diagnosis
of clinical orthopedics: The orthopedic stethoscope. World J
Orthop. 2:13–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giovagnorio F, Andreoli C and De Cicco ML:
Color Doppler sonography of focal lesions of the skin and
subcutaneous tissue. J Ultrasound Med. 18:89–93. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Futani H, Yamagiwa T, Yasojimat H,
Natsuaki M, Stugaard M and Maruo S: Distinction between
well-differentiated liposarcoma and intramuscular lipoma by power
Doppler ultrasonography. Anticancer Res. 23:1713–1718.
2003.PubMed/NCBI
|
|
8
|
Oebisu N, Hoshi M, Ieguchi M, Takada J,
Iwai T, Ohsawa M and Nakamura H: Contrast-enhanced color Doppler
ultrasonography increases diagnostic accuracy for soft tissue
tumors. Oncol Rep. 32:1654–1660. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nagano S, Yahiro Y, Yokouchi M, Setoguchi
T, Ishidou Y, Sasaki H, Shimada H, Kawamura I and Komiya S: Doppler
ultrasound for diagnosis of soft tissue sarcoma: Efficacy of
ultrasound-based screening score. Radiol Oncol. 49:135–140. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morii T, Kishino T, Shimamori N, Motohashi
M, Ohnishi H, Honya K, Aoyagi T, Tajima T and Ichimura S:
Differential diagnosis between benign and malignant soft tissue
tumors utilizing ultrasound parameters. J Med Ultrason 2001.
45:113–119. 2018.PubMed/NCBI
|
|
11
|
Lalitha P, Reddy MC and Reddy KJ:
Musculoskeletal applications of elastography: A pictorial essay of
our initial experience. Korean J Radiol. 12:365–375. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee YH, Song HT and Suh JS: Use of strain
ratio in evaluating superficial soft tissue tumors on ultrasonic
elastography. J Med Ultrason 2001. 41:319–323. 2014.PubMed/NCBI
|
|
13
|
Magarelli N, Carducci C, Bucalo C,
Filograna L, Rapisarda S, De Waure C, Dell'Atti C, Maccauro G,
Leone A and Bonomo L: Sonoelastography for qualitative and
quantitative evaluation of superficial soft tissue lesions: A
feasibility study. Eur Radiol. 24:566–573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim SJ, Park HJ and Lee SY: Usefulness of
strain elastography of the musculoskeletal system. Ultrasonography.
35:104–109. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park HJ, Lee SY, Lee SM, Kim WT, Lee S and
Ahn KS: Strain elastography features of epidermoid tumours in
superficial soft tissue: Differences from other benign soft-tissue
tumours and malignant tumours. Br J Radiol. 88:201407972015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hahn S, Lee YH, Lee SH and Suh JS: Value
of the strain ration on ultrasonic elastography for differentiation
of benign and malignant soft tissue tumors. J Ultrasound Med.
36:121–127. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Machado P, Segal S, Lyshchik A and
Forsberg F: A novel microvascular flow technique: Initial results
in thyroids. Ultrasound Q. 32:67–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ohno Y, Fujimoto T and Shibata Y: A new
era in diagnostic ultrasound, superb microvascular imaging:
Preliminary results in pediatric hepato-gastrointestinal disorders.
Eur J Pediatr Surg. 27:20–25. 2017.PubMed/NCBI
|
|
19
|
Lin P, Chen M, Liu B, Wang S and Li X:
Diagnostic performance of shear wave elastography in the
identification of malignant thyroid nodules: A meta-analysis. Eur
Radiol. 24:2729–2738. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu B, Zheng Y, Huang G, Lin M, Shan Q, Lu
Y, Tian W and Xie X: Breast lesions: Quantitative diagnosis using
ultrasound shear wave elastography - a systematic review and
meta-analysis. Ultrasound Med Biol. 42:835–847. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Woo S, Suh CH, Kim SY, Cho JY and Kim SH:
Shear-wave elastography for detection of prostate cancer: A
systematic review and diagnostic meta-analysis. AJR Am J
Roentgenol. 209:806–814. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiao Y, Dong F, Wang H, Zhang L, Xu J,
Zheng J, Fan H, Gan H, Chen L and Li M: Shear wave elastography
imaging for detecting malignant lesions of the liver: A systematic
review and pooled meta-analysis. Med Ultrason. 19:16–22. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gasparini G: Quantification of
intratumoral vascularization predicts metastasis in human invasive
solid tumors (Review). Oncol Rep. 1:7–12. 1994.PubMed/NCBI
|
|
24
|
Zhang FJ and Han RL: The value of acoustic
radiation force impulse (ARFI) in the differential diagnosis of
thyroid nodules. Eur J Radiol. 82:e686–e690. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Woo S, Kim SY, Cho JY and Kim SH: Shear
wave elastography for detection of prostate cancer: A preliminary
study. Korean J Radiol. 15:346–355. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barr RG and Zhang Z: Shear-wave
elastography of the breast: Value of a quality measure and
comparison with strain elastography. Radiology. 275:45–53. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pass B, Johnson M, Hensor EM, Gupta H and
Robinson P: Sonoelastography of musculoskeletal soft tissue masses:
A pilot study of quantitative evaluation. J Ultrasound Med.
35:2209–2216. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pass B, Jafari M, Rowbotham E, Hensor EM,
Gupta H and Robinson P: Do quantitative and qualitative shear wave
elastography have a role in evaluating musculoskeletal soft tissue
masses? Eur Radiol. 27:723–731. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tavare AN, Alfuraih AM, Hensor EMA,
Astrinakis E, Gupta H and Robinson P: Shear-wave elastography of
benign versus malignant musculoskeletal soft-tissue masses:
Comparison with conventional US and MRI. Radiology. 290:410–417.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Winn N, Baldwin J, Cassar-Pullicino V,
Cool P, Ockendon M, Tins B and Jaremko JL: Characterization of soft
tissue tumours with ultrasound, shear wave elastography and MRI.
Skeletal Radiol. 49:869–881. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Steffner RJ and Jang ES: Staging of bone
and soft-tissue sarcomas. J Am Acad Orthop Surg. 26:e269–e278.
2018. View Article : Google Scholar : PubMed/NCBI
|