Introduction
Lung cancer is still the leading cause of
cancer-related deaths due to the high diagnosis rate and mortality
rate (1). In the United States, it
is estimated that ~225,000 people are diagnosed with lung cancer
and ~160,000 people die of lung cancer each year (1). Histologically, non-small cell lung
cancer (NSCLC) is the main subtype of lung cancer, and lung
adenocarcinoma (LUAD) is the most diagnosed type of NSCLC (1). Although the molecular mechanisms of
LUAD have been elucidated and treatments for LUAD have improved,
the overall survival of patients with LUAD remains poor (2). For these patients, radiotherapy is
considered as a promising treatment strategy that can prolong
patient survival and improve the quality of life. However,
radiotherapy resistance is a critical issue limiting the efficacy
of radiation therapy (3). It is
therefore urgent to clarify the underlying mechanisms of tumor cell
radioresistance and increase the tumor sensitivity to
radiotherapy.
MicroRNAs (miRNA) represent a group of short
non-coding RNAs that regulate gene expression by complementary base
pairing with the 3′-UTR of mRNA and trigger translation repression
or RNA degradation (4). Over the
past decades, miRNAs have been demonstrated to serve critical roles
in numerous types of cancer, and their dysregulation is closely
associated with certain tumorigenic processes, such as
proliferation, apoptosis, cell cycle regulation and stress response
(5,6). In addition, evidence has shown that
miRNAs can modulate cancer cell radiosensitivity (7,8), which
indicates their potential for improving the efficacy of
radiotherapy. For example, miR-101-3p expression has been
demonstrated to be decreased in tumor tissues or cells and involved
in the regulation of various cancer activities (9–11). Wu
et al (9) reported that
miR-101-3p could target the serum response factor and inhibit HOX
transcript antisense RNA-mediated proliferation and invasion of
gastric carcinoma cells. In addition, Li et al (11) reported that miR-101-3p can enhance
the sensitivity of bladder urothelial carcinoma to cisplatin by
silencing the expression of Enhancer of zeste homolog 2. However,
the biological function and underlying mechanisms of miR-101-3p in
LUAD remain unclear.
Baculoviral IAP repeat containing 5 (BIRC5), also
known as survivin, belongs to the inhibitor of apoptosis protein
(IAP) family, and blocks apoptosis induced by various stimuli, such
as radiation and chemical drugs (12). The abnormal amplification of BIRC5
protein has been found in numerous malignancies, including breast
cancer, colon carcinomas and melanoma (13,14).
Furthermore, BIRC5 overexpression has been demonstrated to be
closely associated with tumor initiation and development,
radiotherapy and chemotherapy resistance and poor prognosis in
patients with cancer, such as glioblastoma and head and neck
squamous cell cancer (15,16). In the last decades, BIRC5 has
attracted considerable attention as a therapeutic target for
anticancer strategies due to its role in regulating the sensitivity
of cancer cells to radiotherapy and chemotherapy (17,18).
Therefore, it is crucial to elucidate the mechanism by which BIRC5
regulates human cancer sensitivity to radiotherapy and
chemotherapy.
The present study aimed to elucidate the effect of
miR-101-3p on the radiosensitivity of LUAD cells and to explore
whether miR-101-3p could affect this radiosensitivity by directly
regulating BIRC5. The findings from this study may provide some
future perspectives for the radio-sensitization of LUAD.
Materials and methods
Bioinformatics analysis
The RNA array datasets [GSE48414 (19) and GSE74190 (20)] were downloaded from the Genome
Expression Omnibus database (www.ncbi.nlm.nih.gov/gds). Furthermore, RNA seq data
and clinical survival data of LUAD patients extracted from The
Cancer Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov/) were used to
investigate the expression level of miR-101-3p and BIRC5 in LUAD
tissues and normal adjacent tissues and survival of LUAD patients.
The present study, used Starbase (http://starbase.sysu.edu.cn/) (21) to predict the target gene of
miR-101-3p. The online Kaplan–Meier analysis of the survival of all
patients with lung cancer which accepted radiotherapy with
different BIRC5 expression levels was assessed using the
Kaplan-Meier Plotter (http://kmplot.com/analysis/index.php?p=service&cancer=lung)
(22).
Cell culture
The LUAD cell lines Calu3, H1299 and H292 and the
normal lung epithelial cell line BEAS-2B were purchased from the
American Type Culture Collection. BEAS-2B cell line was cultured in
BEBM medium (Lonza Group, Ltd.) whereas Calu3, H1299 and H292 cell
lines were cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (HyClone; GE Healthcare
Life Sciences), 100 µg/ml streptomycin and 100 U/ml penicillin
(Gibco; Thermo Fisher Scientific, Inc.). All cell lines were placed
at 37°C in a humidified incubator containing 5% CO2.
Cell transfection
The plasmids pcDNA3.1-BIRC5 and pcDNA3.1-Vector and
the miR-101-3p mimics and negative control (NC) mimics were
purchased from Guangzhou RiboBio Co., Ltd. Calu3 and H292 cells
were seeded in 6-well plates (8×105 cells/well) and
transfected with 1.6 µg of pcDNA3.1-BIRC5/vector plasmids or 50 nM
mimics/NC mimics using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.). The media in each well was then
replaced with fresh medium 6 h following incubation with the
transfection mixture at 37°C. After 48 h, the cells were subjected
to subsequent analyses.
CCK-8 assay
Cells were seeded into 96-well plates at the density
of 2×103 cells per well and placed at 37°C in a
humidified incubator containing 5% CO2 for 12 h.
Subsequently, cells were treated with various doses of ionizing
radiation (IR; 0, 2, 4, 6 and 8 Gy). After another 72 h of
incubation post-irradiation, the cell survival rate was assessed
using Cell Counting Kit-8 (CCK-8; Signalway Antibody LLC) assay.
Cells were incubated with 20 µl CCK-8 reagent at 37°C for 4 h.
Absorbance was detected at 450 nm using a microplate reader.
Colony formation assay
Colony formation assay was performed to evaluate
cell sensitivity to radiation. Briefly, following transfection for
48 h, cells were seeded in 6-well plates at an appropriate number
of cells (200, 400, 800, 1,500 and 3,000 cells/well) and cultured
for 12 h. Subsequently, cells were treated with various doses of IR
(0, 2, 4, 6 and 8 Gy) and cultured for 12 days. Cells were then
fixed with 100% methanol at room temperature for 10 min and stained
with 0.5% crystal violet at room temperature for 15 min. Colonies
>50 cells were subsequently imaged using a light microscope and
data were analyzed using SPSS (SPSS, Inc.; version 13.0).
Caspase-3 activity
Following transfection for 48 h, cells were treated
with a dose of 0 or 6 Gy IR. After 48 h, the activity of caspase-3
was assessed in cells using the Caspase-3 Assay kit (Sigma-Aldrich;
Merck KGaA) according to the manufacturers' instructions.
RNA extraction and reverse
transcription-quantitative (RT-q)PCR
Total RNA was isolated from cultured cells using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturers' instructions. The cDNAs were
obtained using a Reverse Transcription kit (Takara Biotechnology
Co., Ltd.). The temperature protocol for the reverse transcription
reaction consisted of cDNA synthesis at 37°C for 60 min and
termination at 80°C for 2 min. RT-qPCR reactions were performed on
the ABI Prism 7900 system (Thermo Fisher Scientific, Inc.) using
SYBR-Green PCR kit (Thermo Fisher Scientific, Inc.). The
thermocycling conditions were as follows: Initial denaturation at
95°C for 2 min, followed by 39 cycles at 94°C for 20 sec and 60°C
for 30 sec, and final extension at 72°C for 30 sec. U6 and GAPDH
were used as the internal controls. The sequences of the primers
were as follows: miR-101-3p, forward 5′-ACGGGCGAGCTACAGTACTGTG-3′,
reverse 5′-CCAGTGCAGGGTCCGAGGTA-3′; BIRC5, forward
5′-AGGACCACCGCATCTCTACAT−3′, reverse 5′-AAGTCTGGCTCGTTCTCAGTG−3′;
U6, forward 5′-TGCGTTCCCTTTGTCATCCT-3′, reverse
5′-AACGCTTCACGAATTTGCGT-3′; and GAPDH, forward
5′-AATCCCATCACCATCTTC−3′ and 5′-AGGCTGTTGTCATACTTC−3′. The relative
expression levels were normalized to endogenous controls and were
expressed as 2−ΔΔCq (23).
Immunofluorescence staining
After Calu3 and H292 cells (4×104 cells)
were incubated on 24-well coverslips (Thermo Fisher Scientific,
Inc.) overnight at 37°C, cells were treated with 6 Gy IR. After 24
h, cells were fixed with 4% paraformaldehyde at room temperature
for 1 min and with ice-cold methanol at room temperature for 10
min. Permeabilization was performed using 0.2% Triton X-100 in
PBS-T (0.05% Tween-20 in PBS) for 10 min followed by blocking with
5% bovine serum albumin (Gibco; Thermo Fisher Scientific, Inc.) in
PBS-T at room temperature for 1 h. Cells were then incubated with
the primary antibody against γ-H2A histone family member X [γ-H2AX,
a biomarker for DNA double-strand breaks (DSBs) (24); 1:500; cat. no. 9718; Cell Signaling
Technology, Inc.] overnight at 4°C, followed by the appropriate
Alexa fluor®-labeled secondary antibodies (1:400; cat.
no. 4412; Cell Signaling Technology, Inc.) at room temperature for
1 h. Coverslips were mounted with ProLong Diamond Anti-fade reagent
and DAPI (Invitrogen; Thermo Fisher Scientific, Inc.). Cells were
imaged using a laser scanning microscope (Axio Imager.Z2; Zeiss
GmbH).
Western blotting
The LUAD cell lines were lysed using RIPA lysis
buffer (Thermo Fisher Scientific, Inc.) and protein concentration
was determined with the BCA assay kit (Beyotime Institute of
Biotechnology). Proteins (40 µg/well) were separated by 12%
SDS-PAGE and transferred onto PVDF membranes (EMD Millipore).
Membranes were blocked with 5% skimmed milk at room temperature for
1 h and were incubated with primary antibodies against γ-H2AX
(1:1,000; cat. no. 9718; Cell Signaling Technology, Inc.), Bax
(1:2,000; cat. no. 60267-1-Ig; ProteinTech Group, Inc.), Bcl2
(1:1,000; cat. no. 15071; Cell Signaling Technology, Inc.), GAPDH
(1:2,000; cat. no. 5174; Cell Signaling Technology, Inc.) and BIRC5
(1:2,000; cat. no. ab469; Abcam) overnight at 4°C. Membranes were
then incubated with a horseradish peroxidase-conjugated goat
anti-rabbit secondary antibody (1:5,000; cat. no. ab6721; Abcam) at
room temperature for 1 h. Enhanced chemiluminescence (ECL, Abcam)
reagent was used to detect the signal on the membrane. The data
were analyzed via densitometry using ImageJ software version 1.41;
(National Institutes of Health) and normalized to expression of the
internal control GAPDH.
Luciferase reporter assay
A luciferase reporter assay was performed to verify
whether BIRC5 was a direct target of miR-101-3p. Cells were
co-transfected with luciferase reporter plasmid
PGL3-WT-BIRC5/PGL3-MUT-BIRC5 (Shanghai GenePharma Co., Ltd.) and
miR-101-3p mimic (5′-UACAGUACUGUGAUAAUCGAA−3′) and miR-NC
(5′-CAGUACUUUUGUGUAGUACAA-3′) using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific Inc.) at 37°C for 48 h. The
luciferase and Renilla signals were then measured using the Dual
Luciferase Reporter Assay kit (Promega Corporation) according to
the manufacturer's instructions. The luciferase activity was
normalized using Renilla activity. The automatic microplate
reader (Molecular Devices, LLC) was used in luciferase assays
detection.
Statistical analysis
Statistical analyses were performed using SPSS (IBM
Corp.; version 20.0). Data are presented as the mean ± standard
deviation of three independent experiments. Log-rank test was used
to determine the statistical significance of Kaplan-Meier overall
survival data of patients with LUAD. An unpaired t-test was used to
compare data between two groups whereas one-way ANOVA followed by
Tukey's or Bonferroni's post hoc test was used to compare data
between three groups or more. The gene expression correlation
between miR-101-3p and BIRC5 was assessed using the Pearson's
correlation test. P<0.05 was considered to indicate a
statistically significant difference.
Results
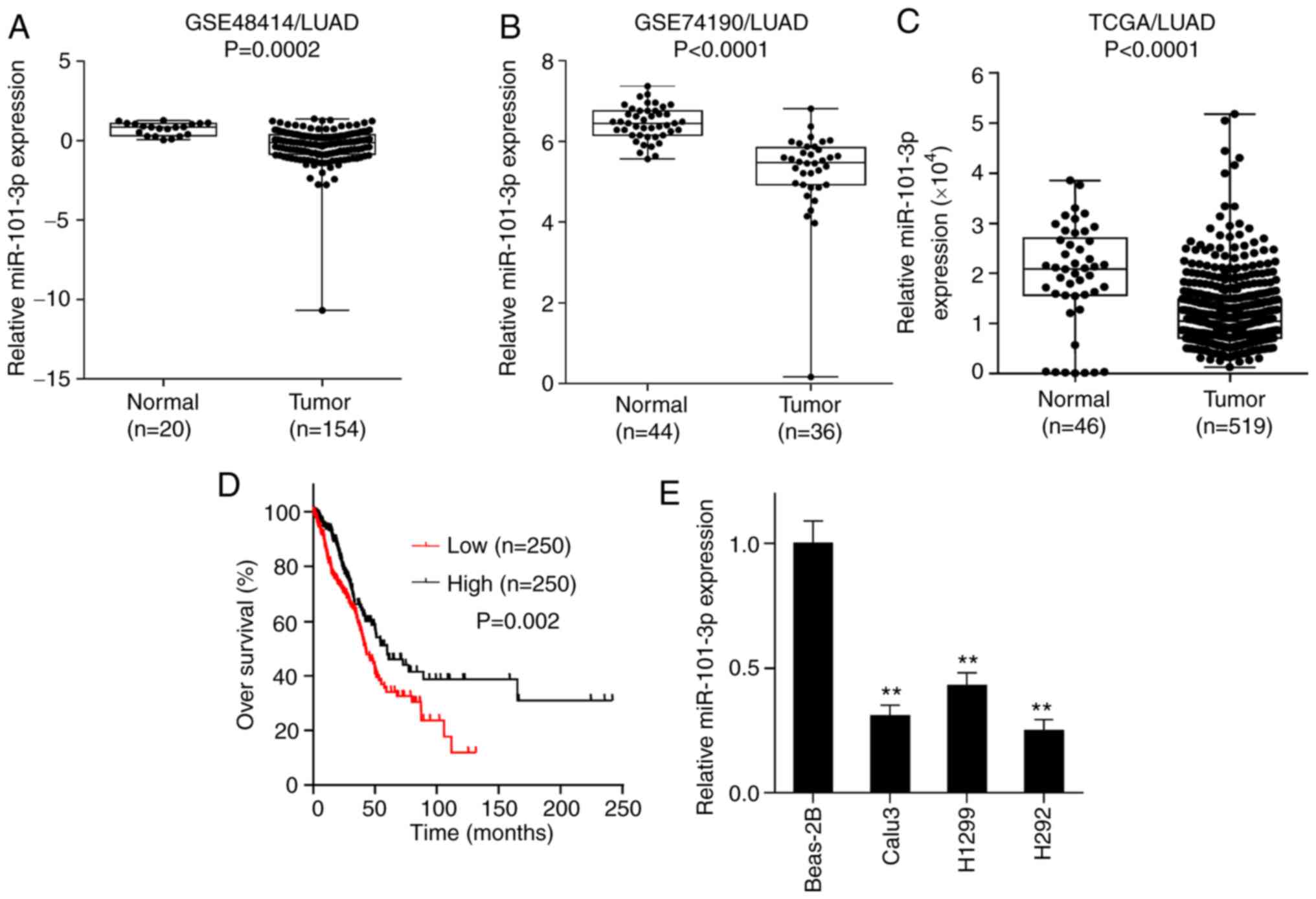
miR-101-3p expression is lower in
patients with LUAD and associated with poor prognosis
The expression of miR-101-3p was investigated in
LUAD and adjacent tissues using NCBI/GEO data mining and TCGA
database analysis. As presented in Fig.
1A-C, miR-101-3p expression was significantly lower in LUAD
tissues compared with adjacent tissues. Furthermore, all TCGA/LUAD
patients were separated into low and high expression groups based
on median value of miR-101-3p (10468.29) expression, and it was
revealed that patients with low expression of miR-101-3p in
TCGA/LUAD had a poorer prognosis compared with patients with high
miR-101-3p expression (Fig. 1D). In
addition, miR-101-3p expression was determined by RT-qPCR in the
normal lung epithelial cell line BEAS-2B and the three LUAD cell
lines Calu3, H1299 and H292. As shown in Fig. 1E, the expression of miR-101-3p in the
three LUAD cell lines was significantly lower compared with the
BEAS-2B cell line. These results suggested that miR-101-3p
expression was lower in LUAD tissues compared with normal lung
tissues and was associated with poor prognosis of patients with
LUAD.
miR-101-3p overexpression sensitizes
LUAD cells to IR
The two LUAD cell lines Calu3 and H292 were chosen
for subsequent experiments, as they exhibited the lowest expression
of miR-101-3p, and were transfected with miR-101-3p mimic or
miR-NC. As presented in Fig. 2A, the
expression of miR-101-3p in both cell lines was significantly
increased compared with the control group 48 h following
transfection. Subsequently, the role of miR-101-3p on the
radiosensitivity was examined. The results from CCK-8 assay
demonstrated a decreased proliferation LUAD cells overexpressing
miR-101-3p (Fig. 2B and C). In
addition, the results from colony formation assay showed that
miR-101-3p overexpression significantly enhanced the
radiosensitivity of LUAD cells (Fig.
2D-F).
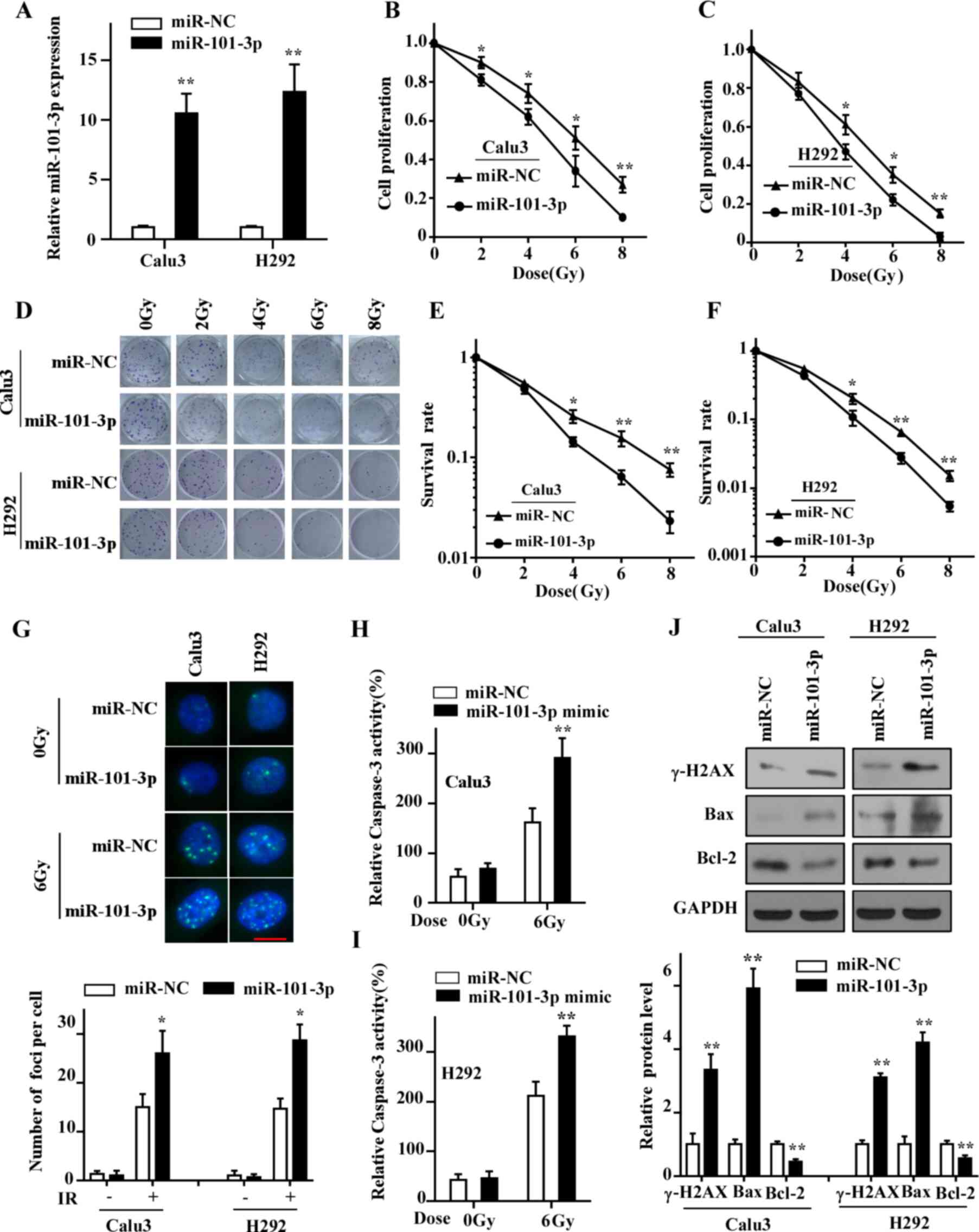 | Figure 2.Overexpression of miR-101-3p
sensitizes LUAD cells to IR. (A) Expression of miR-101-3p in cells
transfected with miR-101-3p mimics assessed by reverse
transcription quantitative PCR. (B and C) Proliferation of LUAD
cells treated with 0, 2, 4, 6 and 8 Gy of IR was determined by Cell
Counting Kit-8 assay. (D-F) Colony forming assay indicated that
transfection of miR-101-3p mimics enhanced radiosensitivity of LUAD
cells compared with control group cells after treatment with 0, 2,
4, 6 and 8 Gy of IR. (G) Cells were treated with 6 Gy IR and
stained with antibody against γ-H2AX (scale bar =10 µm). (H and I)
Caspase-3 activity was detected in cells exposed to IR. (J) Protein
expression of γ-H2AX, Bax, Bcl-2 and GAPDH in LUAD cells determined
by western blotting. *P<0.05 and **P<0.01 vs. miR-NC. γ-H2AX,
γ-H2A histone family member X; LUAD, lung adenocarcinoma; miR,
microRNA; IR, ionizing radiation; NC, negative control. |
The effect of IR on cancer cell death mainly depends
on DNA double-strand breaks (DSBs), and repair of this DNA damage
determines the radiosensitivity of cancer cells (25). To investigate whether miR-101-3p
could affect DNA damage repair ability, the expression of γ-H2AX,
which is a biomarker for DSBs, was determined. The results from
immunofluorescence staining demonstrated that more foci of γ-H2AX
were observed in cells overexpressing miR-101-3p compared with the
control group cells at 24 h following irradiation (Fig. 2G). Furthermore, the results from
western blotting indicated that γ-H2AX expression in cells
overexpressing miR-101-3p was significantly increased compared with
the control group at 24 h following irradiation (Fig. 2J). In addition, to evaluate the
effect of miR-101-3p on cell apoptosis, a caspase-3 assay was
performed to detect changes in caspase-3 activity after irradiation
treatment. As presented in Fig. 2H and
I, the activity of caspase-3 in cells overexpressing miR-101-3p
was significantly higher compared with the control group cells at
48 h after irradiation. The expression of the pro-apoptotic protein
Bax was increased and that of the anti-apoptotic Bcl-2 was
decreased in cells overexpressing miR-101-3p compared with control
cells (Fig. 2J). These findings
indicated that miR-101-3p may promote the radiation-induced cell
apoptosis by increasing the expression of Bax and decreasing the
expression of Bcl-2 in LUAD cells. Taken together, these results
suggested that miR-101-3p may enhance the radiosensitivity of LUAD
cells by attenuating the DNA damage repair ability and regulating
the expression of apoptosis-associated proteins.
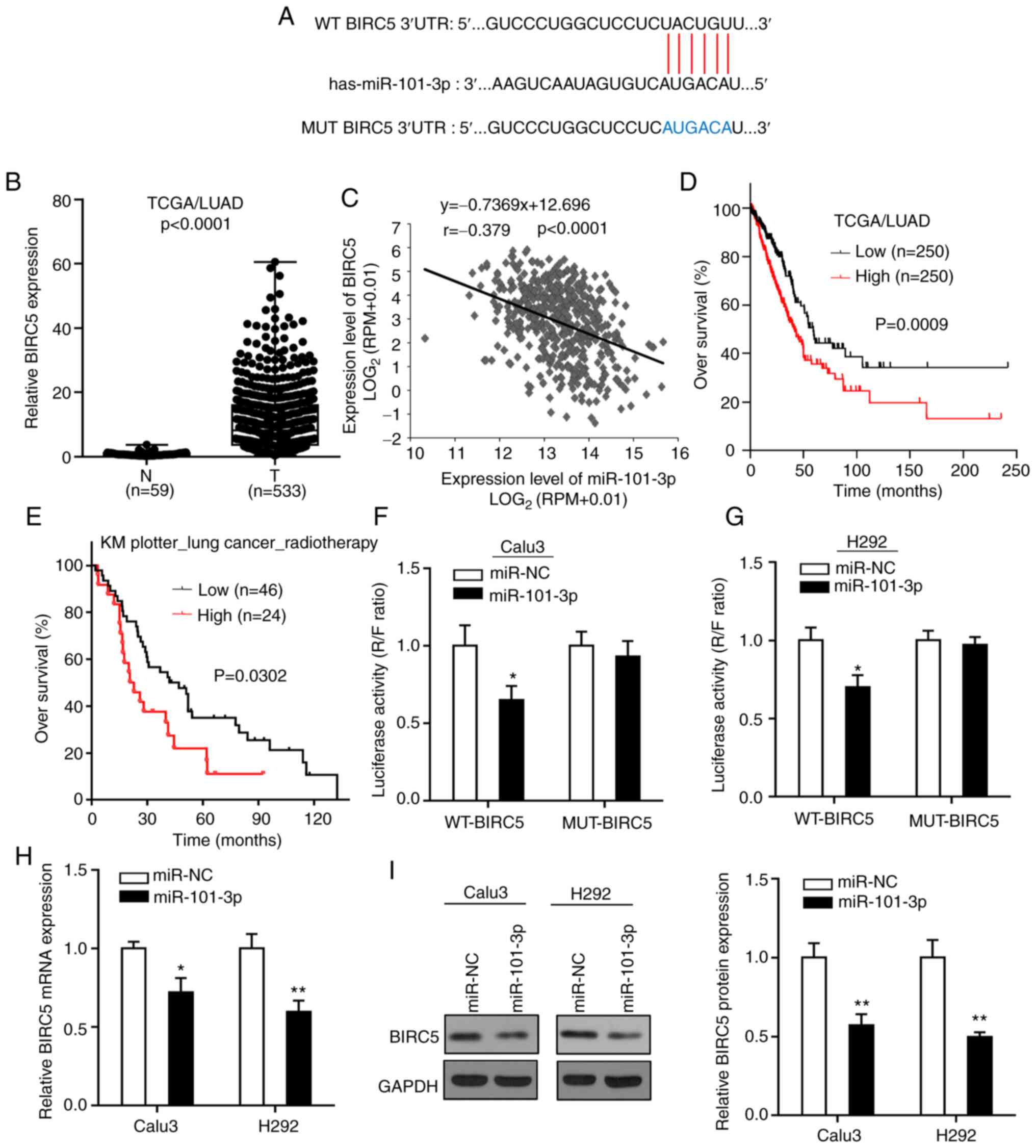
BIRC5 is a direct target of
miR-101-3p
To explore the regulatory mechanism of miR-101-3p on
the radiosensitivity of LUAD cells, the Starbase was used to
predict the possible targets of miR-101-3p. Among the predicted
genes, BIRC5 was selected as a candidate target for its essential
roles in regulating the radiosensitivity of cancer cells (26). Subsequently, analysis of the TCGA
database demonstrated that the expression of BIRC5 in LUAD tissues
was significantly higher than in normal lung tissue, and that
miR-101-3p expression in LUAD tissues was negatively correlated
with BIRC5 expression level (Fig. 3B and
C). Furthermore, all patients with TCGA/LUAD were separated
into low and high expression groups based on median value of BIRC5
expression (8.17), and it was demonstrated that in contrast to the
impact of miR-101-3p on patient survival, patients with LUAD with
high BIRC5 expression had a poorer prognosis compared with patients
with a low BIRC5 expression (Fig.
3D). In addition, analysis of patient overall survival
(Fig. 3E) by Kaplan-Meier Plotter
online tool indicated that BIRC5 high expression level was
significantly associated with a shorter overall survival of
patients with lung cancer who received radiotherapy.
To validate the prediction that miR-101-3p could
bind to the 3′-UTR of BIRC5 (Fig.
3A), a luciferase reporter assay was performed in LUAD cells.
The results demonstrated that the luciferase activity in LUAD cells
co-transfected with miR-101-3p and WT-BIRC5 was significantly
decreased compared with control group, whereas no change was
observed in LUAD cells co-transfected with miR-101-3p and MUT-BIRC5
(Fig. 3F and G). In addition, the
mRNA and protein expression of BIRC5 in LUAD cells following
transfection with miR-101-3p or miR-NC was detected, and the
results demonstrated that miR-101-3p overexpression significantly
decreased the expression of BIRC5 at mRNA and protein levels in
LUAD cells (Fig. 3H and I). Taken
together, these findings suggested that BIRC5 may be a direct
target of miR-101-3p.
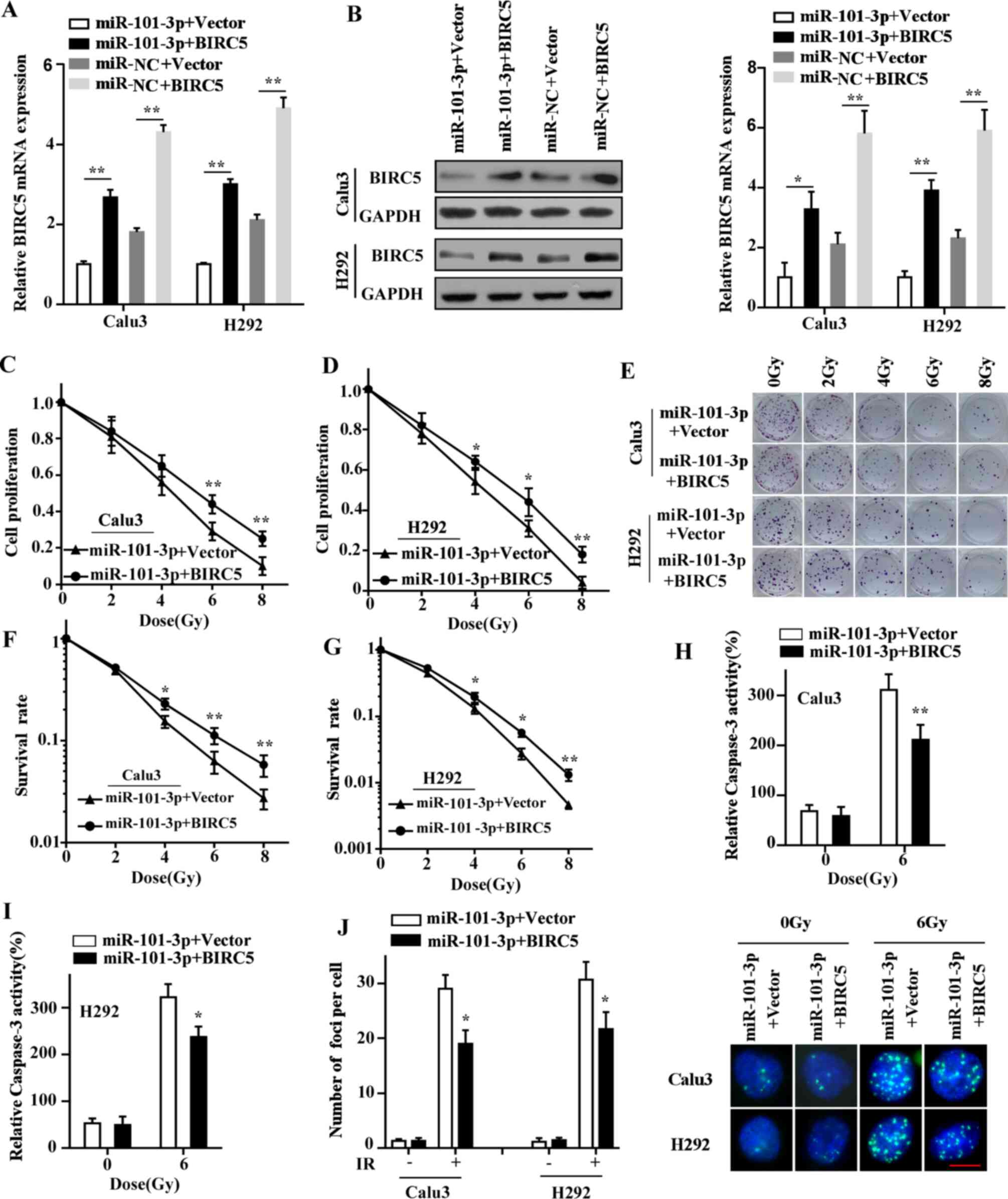
BIRC5 has a role in the
miR-101-3p-mediated radiosensitivity of LUAD cells
As BIRC5 was demonstrated as a downstream target of
miR-101-3p, we hypothesized that BIRC5 might be responsible for
miR-101-3p-mediated radiosensitivity of LUAD cells. Rescue assays
were therefore conducted to verify this hypothesis. First, the
expression efficiency of BIRC5 plasmid in LUAD cells was verified
by RT-qPCR (Fig. S1). Then, the
results from RT-qPCR and western blotting indicated that BIRC5 was
significantly upregulated at both mRNA and protein levels in LUAD
cells co-transfected with miR-101-3p and BIRC5 compared with
miR-101-3p and vector co-transfection (Fig. 4A and B). Furthermore, CCK-8 and
colony formation assays demonstrated that restoration of BIRC5
markedly alleviated miR-101-3p-mediated radiosensitivity of LUAD
cells (Fig. 4C-G). BIRC5
overexpression also significantly attenuated the effects on
caspase-3 activity following miR-101-3p overexpression in LUAD
cells after irradiation (Fig. 4H and
I). In addition, the DNA repair ability of LUAD cells was also
restored after BIRC5 overexpression (Fig. 4J). Taken together, these findings
supported the hypothesis that BIRC5 may serve a crucial role in the
miR-101-3p-mediated radiosensitivity of LUAD cells.
Discussion
Radiotherapy is an important treatment for LUAD;
however, radioresistance has impacted the survival of patients
(27). The abnormal proliferation,
anti-apoptotic process and DNA damage repair in cancer cells are
considered to be the main mechanisms of radioresistance (28). Previous studies have indicated that
miRNAs can serve as important regulators in radiosensitivity by
interacting with cancer-related genes (29,30).
Therefore, the identification of miRNAs and their target genes
associated with radioresistance is crucial in cancer treatment. The
results from the present study demonstrated that miR-101-3p may
serve an important role in regulating LUAD cell radiosensitivity.
In particular, BIRC5 was predicted and confirmed to be a direct
target gene of miR-101-3p, which is involved in the
radiosensitivity of LUAD cells.
miR-101-3p has been reported to be frequently
downregulated and to exhibit antitumorigenic properties in various
types of cancer (27,31,32). The
results from the present study demonstrated that miR-101-3p
expression was lower in LUAD tissues compared with normal lung
tissues and considered as a poor prognostic factor for patients,
according to analysis of GEO and TCGA data. These findings
suggested that miR-101-3p may function as a tumor suppressor in
LUAD. Subsequently, we hypothesized that miR-101-3p might
participate in the regulation of LUAD treatment sensitivity.
miR-101-3p expression has been reported to be negatively correlated
with chemoresistance in several types of malignancy, such as
gastric cancer and colon cancer (33–35).
However, the role of miR-101-3p in LUAD radiosensitivity remains
unknown. The present study demonstrated that miR-101-3p could
sensitize LUAD cells to irradiation via targeting BIRC5, suggesting
that the regulation of miR-101-3p expression and its target BIRC5
may help the development novel radio-sensitizers.
BIRC5 is the smallest member of the IAP family and
has attracted attention due to its abnormally increased expression
in a variety of human cancers, its prognostic relevance and its
prominent role in the regulation of cancer cell apoptosis and
proliferation (36,37). BIRC5 has also been reported to
participate in the repair of radiation-induced DNA damage, thereby
affecting cancer cell sensitivity to radiotherapy and chemotherapy.
For example, overexpression of nuclear BIRC5 can enhance DNA repair
in glioblastoma cells, leading to radioresistance in glioblastoma
(15). Furthermore, BIRC5 depletion
decreases the expression of DNA damage repair-related genes,
sensitizing therefore cancer cells to PARP inhibition (26). In NSCLC, BIRC5 has also been
demonstrated to promote DNA damage response and radiotherapy
resistance (38).
In the present study, BIRC5 was predicted to be a
target of miR-101-3p. Subsequently, the results demonstrated that
BIRC5 was highly expressed in LUAD tissues compared with normal
lung tissues and was considered as a poor prognostic factor in
patients, according to TCGA data analysis. Furthermore, BIRC5 and
miR-101-3p expression levels were negatively correlated, and their
expression had opposite effects on patient survival. In addition,
analysis of the overall survival of patients with LUAD using
Kaplan-Meier Plotter online database indicated that BIRC5
expression level was associated with the overall survival of
patients who received radiotherapy. Taken together, these findings
suggested that miR-101-3p may likely affect patients survival
prognosis and radiotherapy efficacy by targeting BIRC5. Indeed,
this study demonstrated that BIRC5 was a direct target site for
miR-101-3p, and that restoring BIRC5 significantly inhibited
miR-101-3p-mediated decline in proliferation, increased apoptosis
and impaired DNA repair capacity in LUAD cells following radiation
exposure. BIRC5 may therefore serve a crucial role in
miR-101-3p-mediated radiosensitivity of LUAD cells.
In summary, the present study demonstrated that
miR-101-3p could sensitize LUAD cells to irradiation via targeting
BIRC5. These findings may provide a new mechanism for
radioresistance and help the development of novel strategies to
treat patients with LUAD.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XM and YS conducted the cell culture, cell
transfection, CCK-8 assay, colony formation assay, caspase-3
activity assay, and were major contributors in writing the
manuscript. XM and YM conducted the RT-qPCR, western blot,
immunofluorescence staining, luciferase reporter assay and
performed the statistical analyses. SL performed public platform
database analysis and revised the manuscript. YS contributed to the
study design. XM and YM confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen J, Xu Y, Tao L, Pan Y, Zhang K, Wang
R, Chen LB and Chu X: miRNA-26a contributes to the acquisition of
malignant behaviors of doctaxel-resistant lung adenocarcinoma cells
through targeting EZH2. Cell Physiol Biochem. 41:583–597. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jonas S and Izaurralde E: Towards a
molecular understanding of microRNA-mediated gene silencing. Nat
Rev Genet. 16:421–433. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Di Leva G, Garofalo M and Croce CM:
MicroRNAs in cancer. Annu Rev Pathol. 9:287–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang T, Yin L, Wu J, Gu JJ, Wu JZ, Chen
D, Yu HL, Ding K, Zhang N, Du MY, et al: MicroRNA-19b-3p regulates
nasopharyngeal carcinoma radiosensitivity by targeting
TNFAIP3/NF-κB axis. J Exp Clin Cancer Res. 35:1882016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen W, Song J, Bian H, Yang X, Xie X, Zhu
Q, Qin C and Qi J: The functions and targets of miR-212 as a
potential biomarker of cancer diagnosis and therapy. J Cell Mol
Med. 24:2392–2401. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu X, Zhou J, Wu Z, Chen C, Liu J, Wu G,
Zhai J, Liu F and Li G: miR-101-3p suppresses HOX transcript
antisense RNA (HOTAIR)-induced proliferation and invasion through
directly targeting SRF in gastric carcinoma cells. Oncol Res.
25:1383–1390. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hou Y, Li L, Ju Y, Lu Y, Chang L and Xiang
X: miR-101-3p regulates the viability of lung squamous carcinoma
cells via targeting EZH2. J Cell Biochem. 118:3142–3149. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li B, Xie D and Zhang H: MicroRNA-101-3p
advances cisplatin sensitivity in bladder urothelial carcinoma
through targeted silencing EZH2. J Cancer. 10:2628–2634. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamamoto H, Ngan CY and Monden M: Cancer
cells survive with survivin. Cancer Sci. 99:1709–1714. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pennati M, Folini M and Zaffaroni N:
Targeting survivin in cancer therapy. Expert Opin Ther Targets.
12:463–476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Greve B, Sheikh-Mounessi F, Kemper B,
Ernst I, Götte M and Eich HT: Survivin, a target to modulate the
radiosensitivity of Ewing's sarcoma. Strahlenther Onkol.
188:1038–1047. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reichert S, Rödel C, Mirsch J, Harter PN,
Tomicic MT, Mittelbronn M, Kaina B and Rödel F: Survivin inhibition
and DNA double-strand break repair: A molecular mechanism to
overcome radioresistance in glioblastoma. Radiother Oncol.
101:51–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Erpolat OP, Gocun PU, Akmansu M, Karakus E
and Akyol G: High expression of nuclear survivin and Aurora B
predicts poor overall survival in patients with head and neck
squamous cell cancer. Strahlenther Onkol. 188:248–254. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Romagnoli M, Séveno C, Bataille R and
Barillé-Nion S: Survivin in cancerology: Molecular aspects and
therapeutic applications. Med Sci (Paris). 24:821–827. 2008.(In
French). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sprenger T, Rödel F, Beissbarth T, Conradi
LC, Rothe H, Homayounfar K, Wolff HA, Ghadimi BM, Yildirim M,
Becker H, et al: Failure of downregulation of survivin following
neoadjuvant radiochemotherapy in rectal cancer is associated with
distant metastases and shortened survival. Clin Cancer Res.
17:1623–1631. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bjaanaes MM, Halvorsen AR, Solberg S,
Jørgensen L, Dragani TA, Galvan A, Colombo F, Anderlini M,
Pastorino U, Kure E, et al: Unique microRNA-profiles in
EGFR-mutated lung adenocarcinomas. Int J Cancer. 135:1812–1821.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu KH, Zhou B, Lu SH, Feng B, Yang SG, Du
WT, Gu DS, Han ZC and Liu YL: In vitro and in vivo differentiation
of human umbilical cord derived stem cells into endothelial cells.
J Cell Biochem. 100:608–616. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang JH, Li JH, Shao P, Zhou H, Chen YQ
and Qu LH: StarBase: A database for exploring microRNA-mRNA
interaction maps from Argonaute CLIP-Seq and Degradome-Seq data.
Nucleic Acids Res. 39((Database Issue)): D202–D209. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Menyhárt O, Nagy Á and Győrffy B:
Determining consistent prognostic biomarkers of overall survival
and vascular invasion in hepatocellular carcinoma. R Soc Open Sci.
5:1810062018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mariotti LG, Pirovano G, Savage KI, Ghita
M, Ottolenghi A, Prise KM and Schettino G: Use of the γ-H2AX assay
to investigate DNA repair dynamics following multiple radiation
exposures. PLoS One. 8:e795412013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Shen L and Sun LQ: The regulation
of radiosensitivity by p53 and its acetylation. Cancer Lett.
363:108–118. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Véquaud E, Desplanques G, Jézéquel P, Juin
P and Barillé-Nion S: Survivin contributes to DNA repair by
homologous recombination in breast cancer cells. Breast Cancer Res.
Treat. 155:53–63. 2016.
|
|
27
|
Dong Y, Zhang D, Cai M, Luo Z, Zhu Y, Gong
L, Lei Y, Tan X, Zhu Q and Han S: SPOP regulates the DNA damage
response and lung adenocarcinoma cell response to radiation. Am J
Cancer Res. 9:1469–1483. 2019.PubMed/NCBI
|
|
28
|
Yang S, Chen J, Guo Y, Lin H, Zhang Z,
Feng G, Hao Y, Cheng J, Liang P, Chen K, et al: Identification of
prognostic biomarkers for response to radiotherapy by DNA
microarray in nasopharyngeal carcinoma patients. Int J Oncol.
40:1590–1600. 2012.PubMed/NCBI
|
|
29
|
Salim H, Akbar NS, Zong D, Vaculova AH,
Lewensohn R, Moshfegh A, Viktorsson K and Zhivotovsky B: miRNA-214
modulates radiotherapy response of non-small cell lung cancer cells
through regulation of p38MAPK, apoptosis and senescence. Br J
Cancer. 107:1361–1373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fang H, Xie J, Zhang M, Zhao Z, Wan Y and
Yao Y: miRNA-21 promotes proliferation and invasion of
triple-negative breast cancer cells through targeting PTEN. Am J
Transl Res. 9:953–961. 2017.PubMed/NCBI
|
|
31
|
Thu KL, Chari R, Lockwood WW, Lam S and
Lam WL: miR-101 DNA copy loss is a prominent subtype specific event
in lung cancer. J Thorac Oncol. 6:1594–1598. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li L, Shao MY, Zou SC, Xiao ZF and Chen
ZC: miR-101-3p inhibits EMT to attenuate proliferation and
metastasis in glioblastoma by targeting TRIM44. J Neurooncol.
141:19–30. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bao J, Xu Y, Wang Q, Zhang J, Li Z, Li D
and Li J: miR-101 alleviates chemoresistance of gastric cancer
cells by targeting ANXA2. Biomed Pharmacother. 92:1030–1037. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen LG, Xia YJ and Cui Y: Upregulation of
miR-101 enhances the cytotoxic effect of anticancer drugs through
inhibition of colon cancer cell proliferation. Oncol Rep.
38:100–108. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu F, Liao JZ, Xiang GY, Zhao PX, Ye F,
Zhao Q and He XX: miR-101 and doxorubicin codelivered by liposomes
suppressing malignant properties of hepatocellular carcinoma.
Cancer Med. 6:651–661. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Altieri DC: Molecular circuits of
apoptosis regulation and cell division control: The survivin
paradigm. J Cell Biochem. 92:656–663. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Altieri DC: Survivin, cancer networks and
pathway-directed drug discovery. Nat Rev Cancer. 8:61–70. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hu S, Qu Y, Xu X, Xu Q, Geng J and Xu J:
Nuclear survivin and its relationship to DNA damage repair genes in
non-small cell lung cancer investigated using tissue array. PLoS
One. 8:e741612013. View Article : Google Scholar : PubMed/NCBI
|