Introduction
Chronic lymphocytic leukemia (CLL) is a
heterogeneous disease with a number of markers used for patient
risk stratification. The most commonly utilized in clinical
practice are: i) deletion of chromosome arms17p and 11q, ii)
TP53 and immunoglobulin heavy chain variable region
(IGHV) genes mutations as well as iii) protein expression of
the zeta chain associated protein-70 (ZAP-70) and CD38 (1–3).
Since CLL is a malignancy of B lymphocytes, the role
of signaling through B-cell receptor (BCR) and microenvironment
stimulation seems to be crucial in the pathogenesis of the disease
(4,5). The inhibition of BCR signaling by
targeting Bruton's tyrosine kinase (BTK) or phosphatidylinositol 3
kinase (PI3K) led to durable remissions and prolonged
progression-free and overall survival even in CLL patients with
negative prognostic features. Despite those advances in CLL
therapy, there is still a group of relapsed and/or refractory
patients, therefore, the need to investigate novel treatment
options still exists (6). CLL cell
survival is driven by overexpression and abnormal activity of
several non-receptor tyrosine kinases, members of Src family kinase
(SFK)-LYN, SYK, and c-ABL tyrosine kinase (4,7,8). It was proven that inhibition of c-ABL
or LYN/SYK kinases with specific tyrosine kinase inhibitors (TKI)
induces apoptosis of CLL cells. Thus targeting particular kinases
might be a promising option for CLL therapy (4,9–12).
Dasatinib is a second generation inhibitor of
breakpoint cluster region-Abelson murine leukemia 1 (BCR-ABL1)
kinase approved in 2006 by the Food and Drug Administration in the
treatment of chronic myeloid leukemia (CML) and chromosome
Philadelphia-positive acute lymphoblastic leukemia (ALL) patients
(13). Dasatinib is a multiple
kinases inhibitor with significant activity against tyrosine
kinases that are known to be important in the pathogenesis of
several hematological malignancies and solid tumors. Low
concentrations of dasatinib have been shown to inhibit not only
BCR-ABL1, but also c-KIT, platelet-derived growth factor receptor
(PDGFR), BTK, TEC, LYN, and other SFK. In CLL, increased SFK and
c-ABL kinases activity was reported, giving thereby a rationale to
evaluate dasatinib antileukemic activity in CLL (6,14,15).
In vitro studies showed that dasatinib also inhibits
anti-apoptotic proteins that are overexpressed in CLL, namely
Bcl-xL, Bf1/A1 and Mcl-1 (16).
Furthermore, this inhibitor contributes to a reduction of
cytoskeletal activity by its interaction in the LYN/HS1 pathways
(17,18). Moreover, we found earier, that
dasatinib might target similar genes as thalidomide-antiangiogenic
agent with proven antileukemic activity in CLL (19).
It was previously reported that dasatinib at a
concentration of 5 µM was able to induce apoptosis in ZAP-70
positive CLL patients with unmutated IGHV genes (4). Moreover, Amrein et al (15) showed that CLL lymphocytes with del17
were highly sensitive to dasatinib when compared with CLL
lymphocytes without del17 (median IC50 values of 0.1 and
34 µM, respectively). Meanwhile, other groups demonstrated that
dasatinib in nanomolar concentrations induced 50% inhibition of SFK
kinases causing significant apoptosis of CLL cells disregard of
prognostic markers (8,9,20–22).
Dasatinib activity in CLL remains unclear. Moreover,
there is a lack of consistent data regarding which group of
patients could benefit most from therapy with this TKI. Therefore,
we aimed to evaluate the effect of dasatinib in vitro in a
cohort of 53 CLL patients. Moreover, as a proof of concept, we
present the detailed ex vivo and in vivo analyses of
dasatinib activity in a patient with concomitant CML and CLL who is
being successfully treated with dasatinib until date.
Materials and methods
Patients
Peripheral blood and bone marrow samples were
obtained from 53 patients diagnosed with CLL and a patient
diagnosed with CLL and CML at the Department of Hematooncology and
Bone Marrow Transplantation, Medical University of Lublin, Poland.
Table I summarizes the clinical
characteristics of CLL patients. The study was approved by the
Ethics Committee of the Medical University of Lublin (nos.
KE-0254/269/2019 and KE-0254/305/2019).
 | Table I.Clinical characteristics of
patients. |
Table I.
Clinical characteristics of
patients.
| Characteristic | N (%) |
|---|
| Age (years) |
|
|
Median | 65 |
|
Range | 47–84 |
| Sex |
|
|
Female | 33 (62.26) |
|
Male | 20 (37.74) |
| Rai stage |
|
| 0 | 22 (41.51) |
| I | 13 (24.53) |
| II | 8 (15.09) |
|
III | 3 (5.66) |
| IV | 3 (5.66) |
| Not
available | 4 (7.55) |
| WBC
(×109/l) |
|
|
Median | 28.8 |
|
Range | 3.70–144.00 |
| LDH (IU/l) |
|
|
Median | 384 |
|
Range | 258–961 |
| B2M (mg/l) |
|
|
Median | 2.76 |
|
Range | 1.61–9.49 |
| ZAP-70 (cut-off
20%) |
|
|
Positive | 30 (56.60) |
|
Negative | 12 (22.64) |
| Not
available | 11 (20.76) |
| CD38 (cut-off
30%) |
|
|
Positive | 11 (20.75) |
|
Negative | 35 (66.04) |
| Not
available | 7 (13.21) |
| IGHV
mutational status |
|
|
Mutated | 15 (28.30) |
|
Unmutated | 15 (28.30) |
| Not
available | 23 (43.40) |
| Cytogenetics |
|
|
del11q | 2 (3.77) |
|
del13q | 8 (15.10) |
|
del17p | 2 (3.77) |
| Normal
karyotype | 12 (22.64) |
| Not
available | 29 (54.72) |
| NOTCH1
mutational status |
|
|
Mutated | 2 (3.77) |
|
Unmutated | 20 (37.74) |
| Not
available | 31 (58.49) |
| SF3B1
mutational status |
|
|
Mutated | 0
(0.00) |
|
Unmutated | 28 (52.83) |
| Not
available | 25 (47.17) |
| MYD88 L265P
mutational status |
|
|
Mutated | 0 (0.00) |
|
Unmutated | 24 (45.28) |
| Not
available | 29 (54.72) |
Cell isolation
Peripheral blood mononuclear cells (PBMCs) and bone
marrow mononuclear cells (BMMCs) were isolated by Ficoll (Biochrom
AG) density gradient centrifugation. The viability of cells was
evaluated by Trypan blue staining (Sigma-Aldrich; Merck KGaA) and
quantified in a Neubauer chamber (Zeiss GmbH).
RNA extraction and reverse
transcription
Total RNA was extracted using QIAamp RNA BloodMini
Kit (Qiagen) according to the manufacturer's instructions. The
concentration and purity of isolated RNA were determined using
spectrophotometer BioSpec-nano Micro-volume UV–Vis (Shimadzu). From
each sample, 2 µg of total RNA was reverse transcribed to cDNA
using SuperScript III First-Strand Synthesis System for RT-PCR
(Thermo Fisher Scientific, Inc.) and Veriti Dx 96-Well Thermal
Cycler (Thermo Fisher Scientific, Inc.).
Quantitative reverse transcriptase
polymerase chain reaction (qRT-PCR) for BCR-ABL1
Quantitative analysis of BCR-ABL1 gene
expression was performed according to European Leukemia Net and
EUTOS standards for monitoring a TKI therapy in CML (23). Two separate reaction mixtures
providing amplification of BCR-ABL1 fusion gene and Abelson
(ABL) control gene were prepared. The following primers and
probes were used: 5′-TCCGCTGACCATCAATAAGGA-3′, forward primer
BCR-ABL1; 5′-CACTCAGACCCTGAGGCTCAA-3′, reverse primer
BCR-ABL1; 5′FAM-CCCTTCAGCGGCCAGTAGCATC TGA-3′TAMRA, probe
BCR-ABL1; 5′-TGGAGATAACACTCTAAGCATAACTAAAGG-3′, forward
primer ABL; 5′-GATGTAGTTCTTGGGACCCA-3′, reverse primer
ABL; 5′FAM-CCATTT TTGGTTTGGGCTTCACACCATT-3′ TAMRA, probe
ABL. The qRT-PCR reaction was performed on the 7500 Fast Dx
Real-Time PCR instrument (ThermoFisher Scientific, Inc.), using 5
µl cDNA, gene-specific primers, molecular probe and
TaqMan® Universal PCR Master Mix (ThermoFisher
Scientific, Inc.) in 25 µl end volume. Thermocycling program was
set for 50 cycles of 2 min at 50°C, 10 min at 95°C, 15 sec at 95°C,
60 sec at 60°C. Quantitative analysis of BCR-ABL1 and
ABL gene expression was performed using a calibration curve
prepared by serial dilutions of BCR-ABL1 Mbcr Standards and
ABL Control Gene 3 Standards for a known BCR-ABL1 and
ABL copy number. BCR-ABL1 copy number was normalized
to ABL. BCR-ABL1/ABL ratio expressed as a
percentage (%) was calculated. The result was multiplied by
laboratory-specific conversion factor (CF) and expressed using
International Scale (IS).
Analyses of SF3B1, NOTCH1, MYD88 L265P
and IGHV mutations
Genomic DNA was isolated from PBMCs using QIAamp DNA
BloodMini Kit (Qiagen) according to the manufacturer's
recommendation. Detection of SF3B1, NOTCH1 and MYD88
L265P mutation, and IGHV mutation status were assessed as
described earlier in detail (24). A
cutoff of 98% germline homology was used to assess IGHV
mutation status. The sequences with a germline homology of 98% or
higher were considered unmutated and those with a homology <98%
were considered mutated.
Immunophenotypic analysis
The immunophenotypic analysis was performed by flow
cytometry. In our study, the standard diagnostic flow cytometric
analysis included monoclonal antibodies (MoAbs)
anti-CD5-FITC/CD19-PE, anti-CD19-FITC and anti-CD23-PE (BD
Biosciences). A standard, whole-blood assay with erythrocyte cell
lysis was used for preparing the peripheral blood specimens. The
samples were analyzed by flow cytometry directly after preparation.
For data acquisition and analysis, a FACSCalibur instrument
(Becton-Dickinson and Company) with CellQuest software (Becton
Dickinson and Company) was used. For each analysis, 10,000 events
were acquired and analyzed. The percentage of positive cells was
measured from a cut-off set using isotype-matched nonspecific
control antibody. Evaluation of ZAP-70 expression in
CD19+/CD5+ leukemic cells in CLL samples was
performed as previously described (25). Flow cytometric analysis of CD38 on
leukemic cells was performed on PB samples using monoclonal
antibodies: anti-CD19 FITC, anti-CD38 PE and anti-CD5 PE-Cy5 (BD
Pharmingen). The cut-off for positivity of leukemia cells for
ZAP-70 expression was ≥20%, while for CD38 was ≥30%.
Routine laboratory results such as white blood cell
count (WBC), lactate dehydrogenase level (LDH) and B2M
(β2-mikroglobulin), and cytogenetic and FISH analyses of CLL
patients were accessed from the hospital laboratory at the first
admission.
In vitro cytotoxicity assay with XTT
dye
The cytotoxic effect of dasatinib was measured using
in vitro
2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide
(XTT)-based method (Sigma-Aldrich; Merck KGaA). PBMCs were
suspended in X–VIVO w/o Phenol red and Genatamycin (Lonza) and
added on a 96-well plate at a concentration of 5×105
cells/100 µl/well. Dasatinib at a final concentration of 180 nM,
reflecting plasma drug concentrations observed in a clinical
setting, was added to experimental wells. As a negative control,
only live cells were used, positive control consisted of cells
treated with 0.1% Triton X-100 (Sigma-Aldrich; Merck KGaA).
Twenty-five microliters of XTT reagent was added to all samples.
Plates were incubated for 24 h in a humidified atmosphere with 5%
CO2 at 37°C. Optical densities (OD) were measured at 450
nm with a VICTOR3 1420 multilabel counter (PerkinElmer, Inc.), as a
background wavelength at 690 nm was used. Each sample was performed
in triplicates. Cytotoxic effect was calculated as below:
cytotoxicity =
[1-(ODs-ODb)/(ODc-ODb)]
× 100%, where: ODs is an OD of assayed sample, ODb is an OD of
positive control and ODc is an OD of live cells (negative
control).
Cell culture
The PBMCs of a patient diagnosed with CLL and CML
were incubated at a concentration of 2×106 cells/ml for
24 h with 180 nM of dasatinib and without dasatinib in a control
sample. Cells were incubated in a standard medium consisting of
RPMI-1640 (Biochrom, Berlin, Germany) supplemented with 50 U/ml
penicillin, 50 µg/ml streptomycin, 100 µg/ml neomycin and 10%
heat-inactivated fetal bovine serum at 37°C in a humidified
atmosphere of 5% CO2. After cell culture, the percentage
of apoptotic cells within CD19+ cells was measured using
flow cytometry.
Apoptosis analyses
The percentage of apoptotic cells was measured after
24 h incubation with 180 nM of dasatinib and compared to
non-treated samples. Two methods were used for apoptosis
assessment:
i) Annexin V staining of CLL samples and CLL/CML
patient. The analysis was performed using Annexin V-FITC Apoptosis
Detection Kit (Sigma-Aldrich; Merck KGaA) according to the
manufacturer's instructions. PBMCs were washed with PBS (Biochrom
AG), suspended in a binding buffer provided and stained with 5 µl
of Annexin V-FITC. The PBMCs were incubated for 10 min in the
darkness and immediately analyzed on a FACSCalibur (BD
Biosciences).
ii) Mito Tracker Red CMXRos (Molecular Probes)
technique used on CLL/CML patient. Chloromethyl-X-rosamine (CMXRos)
is a cationic lipophilic fluorochrome that does not accumulate in
depolarised mitochondria and thus can be used to detect disruptions
in the mitochondrial membrane potential (ΔΨm). PBMCs were incubated
with CMXRos for 30 min at 37°C and analyzed on a FACSCalibur. Cells
considered to be apoptotic displayed a decrease in mitochondrial
membrane potential in CMXRos staining (ΔΨmlow).
The collapse of ΔΨm is a marker of early apoptosis,
preceding other hallmarks of cell death, such as DNA fragmentation
or phosphatidylserine externalisation (detected by Annexin V).
Statistical analysis
Statistical analysis was performed using STATISTICA
12 program (StatSoft Polska Sp. z o. o.). All results are presented
as median values with range. The Mann-Whitney U test was used to
evaluate the differences between subgroups of patients.
Correlations of variables were calculated with the Spearman rank
correlation coefficient.
Results
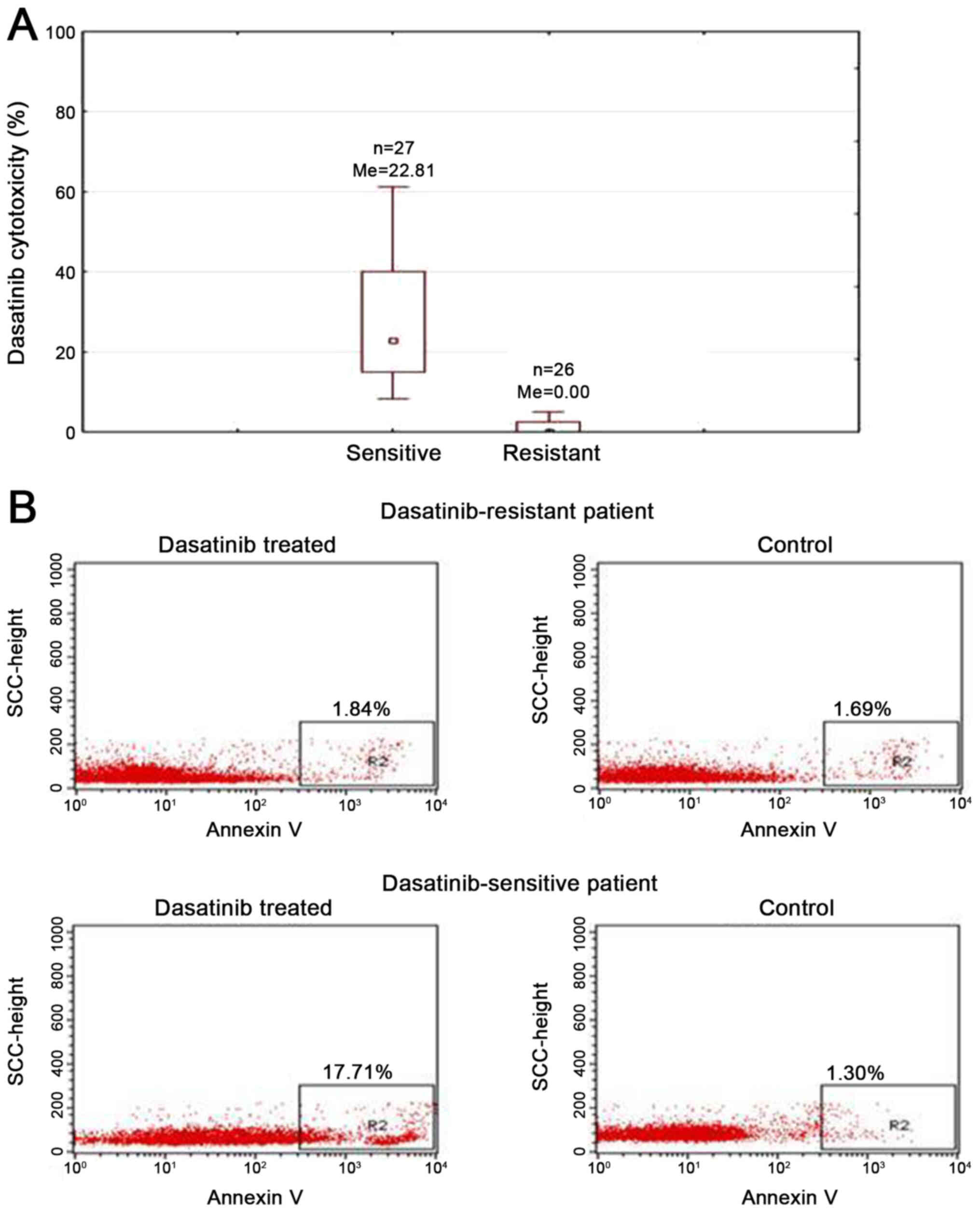
Dasatinib induces cytotoxic effect of
CLL cells
To assess the cytotoxic effect of dasatinib on
primary CLL cells, we performed XTT test. Freshly isolated PBMCs of
53 CLL patients were incubated with 180 nM of dasatinib for 24 h.
Observed median cytotoxicity of dasatinib was 8.3% and it varied
between individual cases (range: 0–77.89%). In 10 patients (18.87%)
obtained dasatinib cytotoxicity was exceeding 30%. The cytotoxicity
between 20–30% was observed in 7 patients (13.21%). In 9 patients
(16.99%) dasatinib cytotoxicity ranged from 10 to 20%. In 27 cases
(50.93%) the cytotoxicity was <10%. Due to high dispersion of
dasatinib activity, we have divided analyzed group into sensitive
(n=27, 50.94%, median cytotoxicity: 22.81%) and resistant patients
(n=26, 49.06%, median cytotoxicity: 0%) using median cytotoxicity
of 8.3% as a cut off value (Fig.
1A).
Dasatinib cytotoxicity correlates with
β2-microglobulin levels
To identify patients who will benefit most from
dasatinib therapy, we correlated the dasatinib activity with CLL
prognostic factors. Statistical analysis revealed a positive
correlation of dasatinib cytotoxicity with β2-microglobulin serum
levels (R=0.317; P=0.041). We observed no correlations between
dasatinib activity and white blood cell count, lactate
dehydrogenase level or age of patients.
Dasatinib cytotoxicity in different
prognostic subgroups
We observed a lack of differences in dasatinib
cytotoxicity in different stages of CLL, according to Rai
classification (P=0.823). Rai stage 0 patients displayed the median
cytotoxicity of dasatinib of 14.87% (range: 0–59.18%, n=22). In Rai
stage I the median cytotoxicity of dasatinib was 7.46% (range:
0–36.90%, n=13). A similar result was observed in cases with Rai
stage II-median: 7.36% (n=8; range: 0–77.89%). In groups with Rai
stage III (n=3) and IV (n=3) the median dasatinib cytotoxicities
were 10.92% (range: 0–46.22%, n=3) and 10.59% (range: 0.51–49.31%,
n=3), respectively. We also found no significant differences of
dasatinib activity depending on the sex of patients: Among women
dasatinib cytotoxicity was 14.73% (range: 0–61.14%, n=33), while in
group of men 6.61% (range: 0–77.89%; n=20, P=0.442). The effect of
dasatinib on CLL primary cells was independent of CD38 (P=0.837) as
well as ZAP-70 expression (P=0.404). Among CD38 and ZAP-70 positive
cases median TKI cytotoxicity was 10.59% (range: 0–77.89%, n=11)
and 18.93% (range: 0–77.89%, n=12), in cohorts assigned as CD38 and
cases ZAP-70 negative we observed median cytotoxicity of 14.27%
(range: 0–61.14%, n=35) and 9.58% (range: 0–61.14%, n=30),
respectively. We also observed no differences in dasatinib response
depending on the mutation status of IGHV genes (P=0.901). In
the group with unmutated IGHV genes median dasatinib
cytotoxicity was 17.76% (range: 0–77.89%, n=15), likewise to
patients with mutated IGHV genes the median of 15.02%
(range: 0–61.14%, n=15). Similarly, there were no statistical
differences in the dasatinib effect in subgroups with different
cytogenetic abnormalities (P=0.826). Mean cytotoxicity in cases
with del11q was 4.29% (range: 4.13–4.46%, n=2) and in patients with
del17p was 2.66% (range: 0–4.13%, n=2). In patients with del13q
median cytotoxicity of dasatinib was 10.05% (range: 0–61.14%, n=8).
Among patients with normal karyotype dasatinib cytotoxicity was
5.68% (range: 0–77.89%, n=12). Moreover, we analyzed dasatinb
response in regard to the new prognostic factors of CLL, namely
mutations in NOTCH1, SF3B1 and MYD88 genes. All
tested samples did not have SF3B1 (n=28) nor MYD88
mutation (n=24). Of 22 examined cases 2 had mutation in
NOTCH1 and 20 were unmutated with median cytotoxicity of
29.85 and 2.74%, respectively, but the difference in dasatanib
cytotoxicity between these subgroups was not of statistical
significance (P=0.074).
Dasatinib induces apoptosis of CLL
cells in vitro
To analyze induction of apoptosis after 24 h
incubation with dasatinib Annexin V staining by flow cytometry on
the representative sensitive or resistant to dasatinib cases (based
on XTT results) was performed. We observed 17.71 and 1.84% of
apoptotic cells, respectively for sensitive or resistant samples
(Fig. 1B). In non-treated control
cells the percentage of Annexin V positive cells was 1.30 and
1.69%, respectively (Fig. 1B).
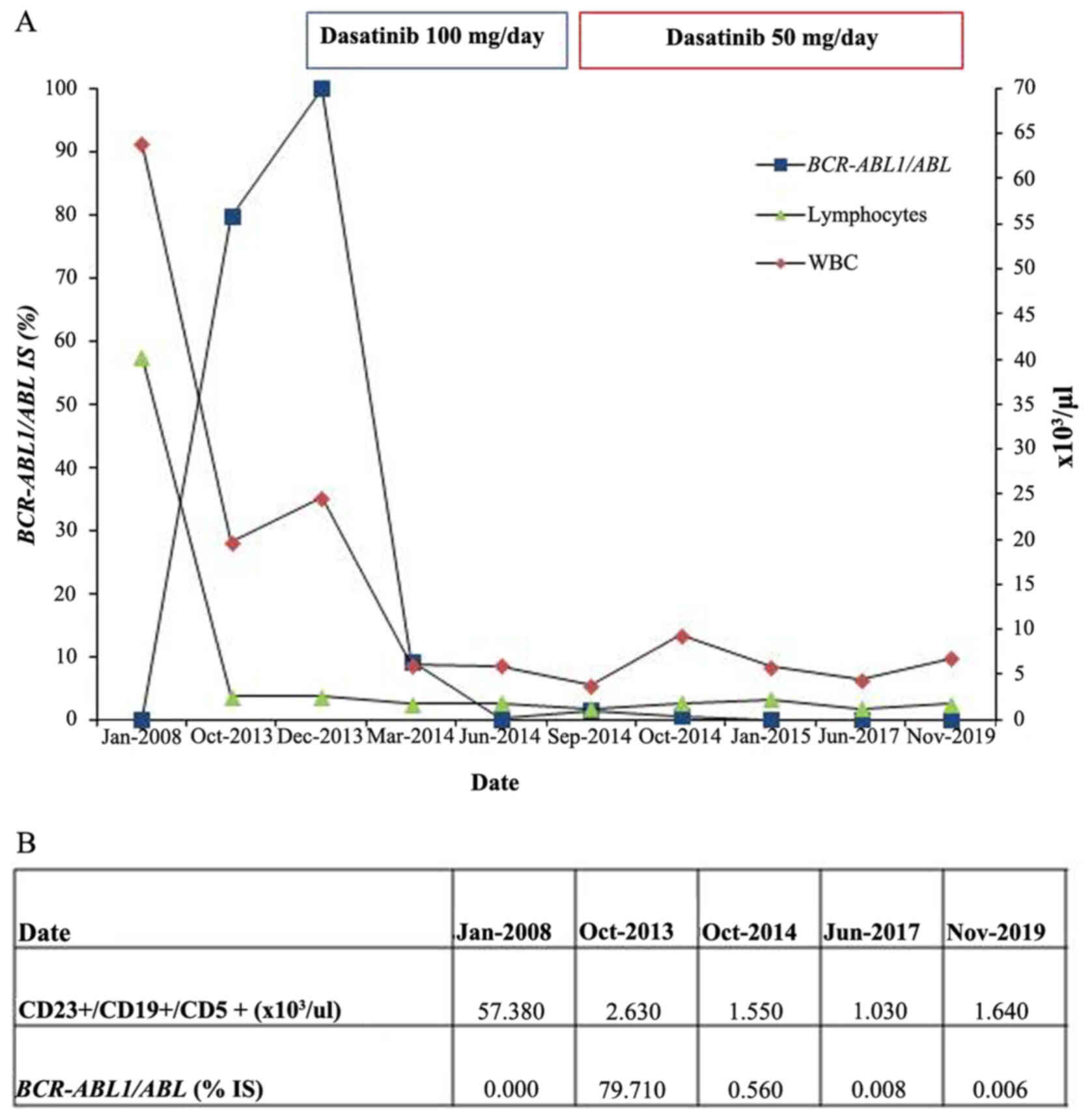
Case report
In January 2008, a 76-year-old man was diagnosed
with CLL in Rai stage II. Bone marrow aspiration showed 70–90%
infiltration by small mature-appearing lymphocytes carrying a
clonal immunophenotype of
CD19+/CD20+/CD23+/CD5+.
A flow cytometry analysis of the patient's peripheral blood
revealed a monoclonal B-cell population (92%) positive for CD5,
CD19, CD23. CD38 and ZAP-70 expressions were classified as negative
(i.e. CD38 expression below cut-off value of 30% and ZAP-70 below
20%). The IGHV gene mutation status was mutated. No
chromosomal abnormalities were detected in the peripheral blood
cells. At that time, disease was not active with no indications to
start the treatment. In February 2009, the patient was referred to
our institution with symptoms of active disease and was qualified
to start therapy. He was treated with 6 cycles of fludarabine and
cyclophosphamide (FC) chemotherapy. After completion of therapy,
the patient had achieved a complete response.
In October 2013, leukocytosis with neutrocytosis was
detected in peripheral blood. The white blood cell count (WBC) was
19.62×109/l (61% of neutrophils, 6% of eosinophils, 1%
of monocytes, 2% of band cells, 19% of lymphocytes, 9% of
myeloblasts, 2% of promyelocytes), hemoglobin was 14.9 g/dl and
platelets were 163×109/l. Bone marrow examination
revealed hypercellularity, increased percentage of granulocytic
lineage and the percentage of lymphocytes was 5%. Karyotype was as
follows: 46,XY, t(9;22)(q34;q11.2) (19). The cytogenetic analysis showed a
Philadelphia chromosome in 100% of cells. Fluorescence in
situ hybridization (FISH) analysis was performed on the same
cytogenetic sample. It showed a standard BCR-ABL1
rearrangement in 96% of interphase nuclei. RT-PCR showed a
BCR-ABL1 b3a2 transcript. The level of transcript
[BCR-ABL1 × CF] was 80%. Based on these findings, the
diagnosis of the second malignancy-Ph+CML was made. In November
2013 imatinib at a dose of 400 mg/day was started. After two weeks,
imatinib was stopped due to muscle and osteoarticular system pains.
Based on the literature suggesting the effectiveness of dasatinib
in CLL, we proposed to replace imatinib with dasatinib in an
attempt to control both diseases with monotherapy. Dasatinib was
introduced at a dose of 100 mg/day in December 2013. After 2 weeks,
dasatinib treatment was suspended for 3 weeks due to pleural
effusion. Steroids and diuretics have been used for pleural
effusion management. After 1 month of dasatinib treatment,
normalization of the peripheral blood hematological values was
noticed. After seven months of therapy, the pleural effusion
occurred again. Dasatinib dose reduction (from 100 to 50 mg) was
required. In January 2015, after 12 months of dasatinib treatment,
the patient exhibited major molecular response. This response has
been maintained until the time of this report, with continued
dasatinib therapy. Patient required no subsequent therapy for CLL.
Fig. 2 displays changes in
BCR-ABL1 transcript, WBC and CLL cells
(CD23+/CD19+/CD5+) from January
2008 to November 2019 (last observation).
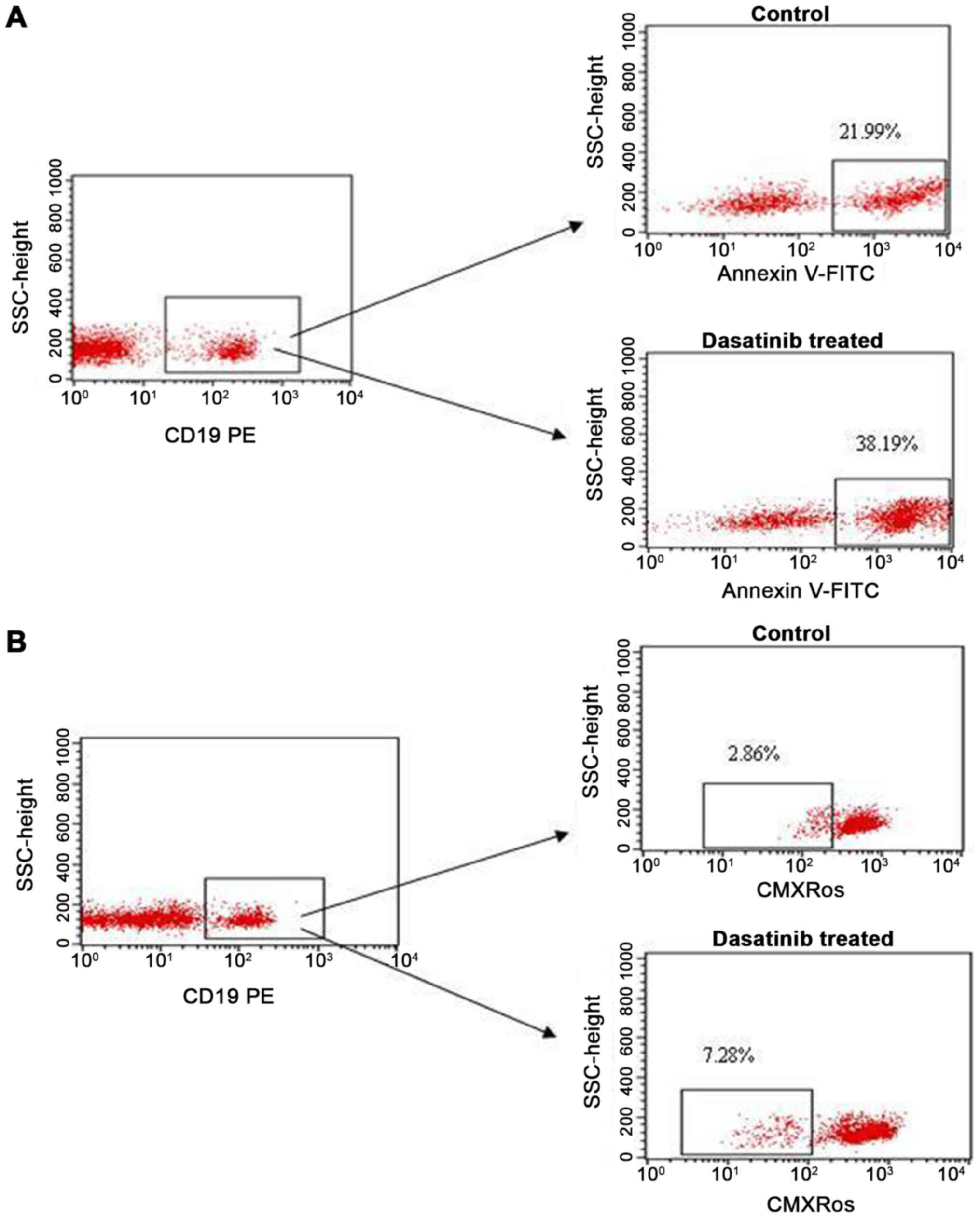
Antileukemic effect of dasatinib in
CLL
In this study, we assessed whether dasatinib could
induce apoptosis of leukemic cells ex vivo of the
abovementioned CLL/CML patient. To assess apoptosis, we used both
Annexin V and CMXRos stainings. In the present assay, the
mononuclear cells did not exhibit of BCR-ABL1
transcript.
We found that the percentage of apoptotic cells
(CD19+/Annexin V+) after in vitro
incubation with dasatinib in a 24 h culture was higher than that in
the culture without dasatinib (38.19 vs. 21.99%, respectively). Dot
plots, illustrating the analysis method for the identification of
CD19+/Annexin V+ cells are shown in Fig. 3A. Similarly, the percentage of
apoptotic cells (ΔΨmlow/CD19+) measured by
CMXRos was higher after incubation with dasatinib (7.28%) than in
negative control (2.86%) (Fig.
3B).
Discussion
Over the past years, highly active novel therapies,
including kinase inhibitors targeting BTK or PI3K, were implemented
in the treatment of CLL patients. This approach is of high
specificity, although inhibition of these kinases affects the whole
signaling via BCR and, in consequence, multiple cellular processes
(26).
The mechanism underlying CLL development remains
unclear, but it is thought that some stimulus (unknown antigen:
exogenous, autologous or antigen-independent cell-autonomous
signaling) of BCR in combination with genetic, cytogenetic and
epigenetic abnormalities leads to abnormal activation of multiple
signaling pathways (27). In
vitro activity of dasatinib on the apoptosis of CLL cells has
been reported in patients who were ZAP-70 positive, with unmutated
IGVH genes, as well as with del17p or del11q (9,28,29). Our
in vitro results point to the preferential effectiveness of
dasatinib in CLL cells, regardless of theses prognostic factors,
though its cytotoxicity correlates with β2-microglobulin serum
levels. Moreover, Amrein et al (9) showed that addition of dasatinib at a
concentration of 0.1 µmol/l sensitizes CLL cells to chlorambucil
and fludarabine. While preclinical reports were encouraging, in
phase 2 clinical trials dasatinib demonstrated moderate activity in
relapsed CLL. Of 13 relapsed CLL patients, who received 50 mg of
dasatinib twice daily in phase 2 clinical study, 3 presented a
decrease of lymphocyte count and regression of nodal disease
(30). In another trial dasatinib
was administered in a dose of 150 mg once a day to patients
refractory to fludarabine-based therapies, partial responses were
observed in 3 out of 15 cases and 9 patients showed a nodal
response. These findings confirmed the modest activity of dasatinib
in monotherapy with acceptable toxicity (22). In a phase 2 study combining
fludarabine (40 mg/m2/day, days 1–3 every 28 day) and
dasatinib (100 mg/day, days 1–28) in fludarabine-refractory CLL
patients, the overall response rate was 18%, but the clinical
outcome of patients was significantly improved. Interestingly,
temporary lymphocytosis in 61% of patients was observed early after
introducing dasatinib (17). It is a
similar phenomenon observed also after therapy with BTK and PI3K
inhibitors, suggesting their shared mechanism of action. Kater
et al (17) speculated that
lymphocytosis is a result of dasatinib influence on BCR signaling
and chemokine-controlled integrin-mediated retention and homing of
malignant B cells in lymph node, what explains nodal responses of
CLL patients after treatment. The preclinical efficacy of dasatinib
is due to inhibition of LYN and SFK kinases in CLL cells,
overcoming pro-survival signals from BCR and leading to apoptosis
(8,20,31,32).
Moreover, several SFK kinases contribute to the regulation of NK
and NK/T cells. In CLL/CML patients responding to dasatinib,
cytotoxic T and NK large granular lymphocytes were increased
(33–35). Although the apoptotic effect of this
inhibitor on CLL cells might be diminished by the presence of
stromal cells and blood-derived ‘nurse-like cells’ (16,28).
CLL patients have more than twice the risk of second
malignancies development, regardless of treatment status, when
compared to the general population (36). In an analysis of patients treated
with fludarabine-based protocols in frontline therapy, the risk of
other cancers was 2.38 times higher than expected in the general
population. Alkylating agents, like cyclophosphamide, did not show
evidence of higher risk of second malignancies. Although
cyclophosphamide demonstrates weaker carcinogenicity than other
alkylating drugs, high doses of this agent might increase the risk
of second cancers in CLL and Non-Hodgkin's lymphoma patients
(37).
CML coexisting with CLL is a very rare phenomenon,
reported in over 20 cases until date. Usually, as in the patient
presented here, CML diagnosis follows CLL. Rarely the opposite
situation or simultaneous occurrence of CLL and CML was reported.
Until date, there is no consensus on the treatment approach for
these patients (38). Typically, if
CLL was in remission the treatment targeted against CML was
introduced. Here, we reported on the patient initially treated with
FC, who at the onset of CML was in remission for CLL. Although the
patient was in a good prognostic group as characterized by
IGVH mutational status, he was not treated optimally with
immunochemotherapy including rituximab, we, therefore, might
anticipate relapse within next months. In CLL8 clinical trail of
German CLL Study Group, patients treated with FC, as compared to
those with FCR, have significantly shorter progression-free
survival (PFS) of 32.0 vs. 56.8 months (39).
Tecchio et al (40) reported a case of previously untreated
CLL patient with trisomy of chromosome 12, a mutated IGVH
gene and negative expression of ZAP-70 who developed CML nine years
after CLL diagnosis. This patient received 400 mg/day of imatinib.
After 2 months, imatinib was discontinued due to skin toxicity and
treatment with dasatinib at 100 mg daily was started. Both diseases
were monitored after 3 months of therapy, showing a complete
hematologic remission and deep molecular response for
BCR-ABL1. Similar data concerning a case of CLL patient with
a del13q with coexisting CML was published by Nagao et al
(41). After 3 months of treatment
with dasatinib at a dose of 80 mg/day as the first-line therapy,
the patient demonstrated a major molecular response for CML and a
partial response for CLL characterized by the significant reduction
of lymphocyte count (41).
Similarly, favorable outcome was observed in our patient with
concomitant CML and CLL after 12 months of dasatinib treatment, as
the patient exhibited major molecular response. We also observed a
reduction of circulating CLL cells pointing to in vivo
anti-CLL activity of dasatinib. In 2010, Serpa et al
(33) documented a case of CML
patient who developed CLL with del11q and positive expression of
CD38 in 4th month of imatinib treatment. Based on the literature
data suggesting the effectiveness of dasatinib on CLL, the authors
changed imatinib to dasatinib at a dose of 100 mg/day. After 6
months, the patient obtained a partial response, with a reduction
of lymphocytosis, the disappearance of enlarged lymph nodes, and
the maintenance of major molecular responses. Moreover, Pitini
et al (29) published case of
CLL responsive to treatment with dasatinib at a dose of 140 mg
daily in a patient coexisting with relapsed gastrointestinal
stromal tumor (GIST).
Results of our study, extended clinical observation
with functional analyses of dasatinib induced apoptosis.
In conclusion, dasatinib proved to induce durable
antileukemic effects against CML and CLL cells. Its activity could
be found in some sensitive CLL cases. Results of this study prove
that dasatinib might induce apoptosis in vivo, ex vivo and
in vitro in CLL and should represent a preferential
therapeutic option for CML/CLL cases.
Acknowledgements
Not applicable.
Funding
This work was supported by the Polish National
Science Center (grant nos. 2018/31/B/NZ6/03361 and N402 676540) and
the Medical University of Lublin (grant no. DS 462).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KG conceived the current study, discussed results
and wrote the manuscript. AK and MKa performed the experiments,
analyzed and interpreted the data, and wrote the manuscript. ABJ,
KK and MM performed the experiments. MKo, WT and MH recruited
patients and provided clinical data. TS designed the research and
discussed results. KG, AK and MKa confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of the Medical University of Lublin (Lublin, Poland;
approval nos. KE-0254/269/2019 and KE-0254/305/2019). All patients
enrolled in the present study provided their signed informed
consent. All forms together with medical history are enclosed in
the Experimental Hematooncology Department's database.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
References
|
1
|
Ghia P, Ferreri AM and Caligaris-Cappio F:
Chronic lymphocytic leukemia. Crit Rev Oncol Hematol. 64:234–246.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giannopoulos K: Biologia i rokowanie w
przewlekłej białaczce limfocytowej. Acta Haematol Pol. 41:433–440.
2010.
|
|
3
|
Zenz T, Mertens D, Döhner H and
Stilgenbauer S: Importance of genetics in chronic lymphocytic
leukemia. Blood Rev. 25:131–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuckertz M, Patz M, Veldurthy A, Gehrke I,
Claasen J, Frenzel LP, Wendtner CM, Hallek M and Krause G:
Comparison of the effects of two kinase inhibitors, sorafenib and
dasatinib, on chronic lymphocytic leukemia cells. Onkologie.
35:420–426. 2012.PubMed/NCBI
|
|
5
|
Stevenson FK and Caligaris-Cappio F:
Chronic lymphocytic leukemia: Revelations from the B-cell receptor.
Blood. 103:4389–4395. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Amrein PC: The potential for dasatinib in
treating chronic lymphocytic leukemia, acute myeloid leukemia, and
myeloproliferative neoplasms. Leuk Lymphoma. 52:754–763. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Winiarska M, Bojarczuk K, Pyrzynska B, Bil
J, Siernicka M, Dwojak M, Bobrowicz M, Miazek N, Zapala P,
Zagozdzon A, et al: Inhibitors of SRC kinases impair antitumor
activity of anti-CD20 monoclonal antibodies. MAbs. 6:1300–1313.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McCaig AM, Cosimo E, Leach MT and Michie
AM: Dasatinib inhibits B cell receptor signalling in chronic
lymphocytic leukaemia but novel combination approaches are required
to overcome additional pro-survival microenvironmental signals. Br
J Haematol. 153:199–211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Amrein L, Hernandez TA, Ferrario C,
Johnston J, Gibson SB, Panasci L and Aloyz R: Dasatinib sensitizes
primary chronic lymphocytic leukaemia lymphocytes to chlorambucil
and fludarabine in vitro. Br J Haematol. 143:698–706. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Contri A, Brunati AM, Trentin L, Cabrelle
A, Miorin M, Cesaro L, Pinna LA, Zambello R, Semenzato G and
Donella-Deana A: Chronic lymphocytic leukemia B cells contain
anomalous Lyn tyrosine kinase, a putative contribution to defective
apoptosis. J Clin Invest. 115:369–378. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gobessi S, Laurenti L, Longo PG, Sica S,
Leone G and Efremov DG: ZAP-70 enhances B-cell-receptor signaling
despite absent or inefficient tyrosine kinase activation in chronic
lymphocytic leukemia and lymphoma B cells. Blood. 109:2032–2039.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin K, Glenn MA, Harris RJ, Duckworth AD,
Dennett S, Cawley JC, Zuzel M and Slupsky JR: c-Abl expression in
chronic lymphocytic leukemia cells: Clinical and therapeutic
implications. Cancer Res. 66:7801–7809. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hochhaus A, Kantarjian HM, Baccarani M,
Lipton JH, Apperley JF, Druker BJ, Facon T, Goldberg SL, Cervantes
F, Niederwieser D, et al: Dasatinib induces notable hematologic and
cytogenetic responses in chronic-phase chronic myeloid leukemia
after failure of imatinib therapy. Blood. 109:2303–2309. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McCaig AM, Cosimo E, Leach MT and Michie
AM: Dasatinib inhibits CXCR4 signaling in chronic lymphocytic
leukaemia cells and impairs migration towards CXCL12. PLoS One.
7:e489292012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Amrein L, Soulières D, Johnston JB and
Aloyz R: p53 and autophagy contribute to dasatinib resistance in
primary CLL lymphocytes. Leuk Res. 35:99–102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hallaert DYH, Jaspers A, van Noesel CJ,
van Oers MH, Kater AP and Eldering E: c-Abl kinase inhibitors
overcome CD40-mediated drug resistance in CLL: Implications for
therapeutic targeting of chemoresistant niches. Blood.
112:5141–5149. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kater AP, Spiering M, Liu RD, Doreen Te
Raa G, Slinger E, Tonino SH, Beckers MM, Daenen S, Doorduijn JK,
Lankheet NA, et al: Dasatinib in combination with fludarabine in
patients with refractory chronic lymphocytic leukemia: A
multicenter phase 2 study. Leuk Res. 38:34–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
ten Hacken E, Scielzo C, Bertilaccio MTS,
Scarfò L, Apollonio B, Barbaglio F, Stamatopoulos K, Ponzoni M,
Ghia P and Caligaris-Cappio F: Targeting the LYN/HS1 signaling axis
in chronic lymphocytic leukemia. Blood. 121:2264–2273. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Giannopoulos K, Dmoszynska A, Kowal M,
Wasik-Szczepanek E, Bojarska-Junak A, Rolinski J, Döhner H,
Stilgenbauer S and Bullinger L: Thalidomide exerts distinct
molecular antileukemic effects and combined thalidomide/fludarabine
therapy is clinically effective in high-risk chronic lymphocytic
leukemia. Leukemia. 23:1771–1778. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lombardo LJ, Lee FY, Chen P, Norris D,
Barrish JC, Behnia K, Castaneda S, Cornelius LAM, Das J, Doweyko
AM, et al: Discovery of
N-(2-chloro-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide
(BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor
activity in preclinical assays. J Med Chem. 47:6658–6661. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martinez Marignac VL, Smith S, Toban N,
Bazile M and Aloyz R: Resistance to Dasatinib in primary chronic
lymphocytic leukemia lymphocytes involves AMPK-mediated energetic
re-programming. Oncotarget. 4:2550–2566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Amrein PC, Attar EC, Takvorian T, Hochberg
EP, Ballen KK, Leahy KM, Fisher DC, Lacasce AS, Jacobsen ED, Armand
P, et al: Phase II study of dasatinib in relapsed or refractory
chronic lymphocytic leukemia. Clin Cancer Res. 17:2977–2986. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hochhaus A, Baccarani M, Silver RT,
Schiffer C, Apperley JF, Cervantes F, Clark RE, Cortes JE,
Deininger MW, Guilhot F, et al: European LeukemiaNet 2020
recommendations for treating chronic myeloid leukemia. Leukemia.
34:966–984. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Putowski M, Podgórniak M, Piróg M, Knap J,
Zaleska J, Purkot J, Zawiślak J, Zakrzewska E, Karczmarczyk A,
Własiuk P, et al: Prognostic impact of NOTCH1, MYD88, and SF3B1
mutations in Polish patients with chronic lymphocytic leukemia. Pol
Arch Intern Med. 127:238–244. 2017.PubMed/NCBI
|
|
25
|
Bojarska-Junak A, Giannopoulos K, Kowal M,
Dmoszyńska A and Roliński J: Comparison of methods for determining
zeta-chain associated protein-70 (ZAP-70) expression in patients
with B-cell chronic lymphocytic leukemia (B-CLL). Cytometry B Clin
Cytom. 70:293–301. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Burger JA and O'Brien S: Evolution of CLL
treatment-from chemoimmunotherapy to targeted and individualized
therapy. Nat Rev Clin Oncol. 15:510–527. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dühren-von Minden M, Übelhart R, Schneider
D, Wossning T, Bach MP, Buchner M, Hofmann D, Surova E, Follo M,
Köhler F, et al: Chronic lymphocytic leukaemia is driven by
antigen-independent cell-autonomous signalling. Nature.
489:309–312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Veldurthy A, Patz M, Hagist S, Pallasch
CP, Wendtner CM, Hallek M and Krause G: The kinase inhibitor
dasatinib induces apoptosis in chronic lymphocytic leukemia cells
in vitro with preference for a subgroup of patients with unmutated
IgVH genes. Blood. 112:1443–1452. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pitini V, Arrigo C and Altavilla G:
Dasatinib induces a response in chronic lymphocytic leukemia.
Blood. 113:4982009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Garg RJ, Wierda W, Fayad L, Estrov Z,
Bickel SM and O'Brien S: Phase II study of dasatinib in patients
with relapsed CLL. Blood. 112:4197a2008. View Article : Google Scholar
|
|
31
|
Song Z, Lu P, Furman RR, Leonard JP,
Martin P, Tyrell L, Lee FY, Knowles DM, Coleman M and Wang YL:
Activities of SYK and PLCgamma2 predict apoptotic response of CLL
cells to SRC tyrosine kinase inhibitor dasatinib. Clin Cancer Res.
16:587–599. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hantschel O, Rix U, Schmidt U,
Bürckstümmer T, Kneidinger M, Schütze G, Colinge J, Bennett KL,
Ellmeier W, Valent P, et al: The Btk tyrosine kinase is a major
target of the Bcr-Abl inhibitor dasatinib. Proc Natl Acad Sci USA.
104:13283–13288. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Serpa M, Bendit I, Seguro F, Xavier F,
Cavalcante M, Steinbaum D, Nardinelli L, Aldred VL, de Paula HM and
Dorlhiac-Llacer PE: Response to dasatinib in a patient with
concomitant chronic myeloid leukemia and chronic lymphocytic
leukemia. Acta Haematol. 124:105–109. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim DH, Kamel-Reid S, Chang H, Sutherland
R, Jung CW, Kim HJ, Lee JJ and Lipton JH: Natural killer or natural
killer/T cell lineage large granular lymphocytosis associated with
dasatinib therapy for Philadelphia chromosome positive leukemia.
Haematologica. 94:135–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mustjoki S, Ekblom M, Arstila TP, Dybedal
I, Epling-Burnette PK, Guilhot F, Hjorth-Hansen H, Höglund M,
Kovanen P, Laurinolli T, et al: Clonal expansion of T/NK-cells
during tyrosine kinase inhibitor dasatinib therapy. Leukemia.
23:1398–1405. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tsimberidou AM, Wen S, McLaughlin P,
O'Brien S, Wierda WG, Lerner S, Strom S, Freireich EJ, Medeiros LJ,
Kantarjian HM, et al: Other malignancies in chronic lymphocytic
leukemia/small lymphocytic lymphoma. J Clin Oncol. 27:904–910.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Benjamini O, Jain P, Trinh L, Qiao W,
Strom SS, Lerner S, Wang X, Burger J, Ferrajoli A, Kantarjian H, et
al: Second cancers in patients with chronic lymphocytic leukemia
who received frontline fludarabine, cyclophosphamide and rituximab
therapy: Distribution and clinical outcomes. Leuk Lymphoma.
56:1643–1650. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Boddu P, Gibbons J, Burger J, Sivina M,
Thakral B, Kanagal-Shamanna R and Ferrajoli A: Co-occurrence of
chronic myeloid leukemia with chronic lymphocytic leukemia: A
report of two cases. Leuk Lymphoma. 60:1568–1571. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fischer K, Bahlo J, Fink AM, Goede V,
Herling CD, Cramer P, Langerbeins P, von Tresckow J, Engelke A,
Maurer C, et al: Long-term remissions after FCR chemoimmunotherapy
in previously untreated patients with CLL: Updated results of the
CLL8 trial. Blood. 127:208–215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tecchio C, Nichele I, Todeschini G,
Pizzolo G and Ambrosetti A: Dasatinib-induced response in a rare
case of chronic lymphocytic leukaemia associated with chronic
myeloid leukaemia. Br J Haematol. 146:222–223. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nagao T, Takahashi N, Kameoka Y, Noguchi
S, Shinohara Y, Ohyagi H, Kume M and Sawada K: Dasatinib-responsive
chronic lymphocytic leukemia in a patient treated for coexisting
chronic myeloid leukemia. Intern Med. 52:2567–2571. 2013.
View Article : Google Scholar : PubMed/NCBI
|