Introduction
Tumor-derived cytokines and growth factors have been
reported to alter the process of hematopoiesis, which regulates the
myeloid cell differentiation process, and to promote the
proliferation and expansion of cells with immunosuppressive
properties, namely myeloid-derived suppressor cells (MDSCs)
(1,2). MDSCs enhance tumor growth not only by
shaping immune responses towards tumor tolerance, but also by
supporting several processes required for the neoplastic
progression, including tumor angiogenesis and cancer metastasis
dissemination (3,4).
Murine MDSCs have been identified to exclusively
bear the markers CD11b (also known as integrin αM) and granulocyte
receptor-1 (Gr-1; the anti-Gr-1 monoclonal antibody recognizes
epitopes common to Ly6C and Ly6G) (5). Tumors have been reported to secrete a
large array of tumor-derived factors (TDFs), which work
synergistically to accelerate the accumulation of functional MDSCs
(6). The three major regulators of
the proliferation and differentiation of myeloid cells are
macrophage colony-stimulating factor (M-CSF), granulocyte
colony-stimulating factor (G-CSF) and granulocyte-macrophage
colony-stimulating factor (GM-CSF), and these are produced by tumor
cells and the tumor stroma (7).
GM-CSF exhibits a dual role in cancer, since it has been identified
to drive cell differentiation towards either an immunosuppressive
(involving MDSCs) or immunostimulatory (involving dendritic cells)
phenotype, depending on the strength of the stimulus and the
overall cytokine landscape (8,9). G-CSF
has been reported to promote the differentiation of myeloid
precursors to polymorphonuclear-MDSCs and to stimulate their
preferential recruitment towards tumors (10,11).
Therefore, GM-CSF and G-CSF may serve a crucial role in MDSC
activation and the progression of tumorigenesis, and altering their
levels with targeted compounds may represent a potential
therapeutic strategy.
Inflammation in solid tumors has been demonstrated
to enhance cancer growth and metastasis (12). Sustained inflammation is associated
with not only the activation of the NF-κB signaling pathway in
cancer cells, but also the recruitment of immature myeloid derived
cells (such as MDSCs) (13). In
cancer cells, overactive NF-κB has been demonstrated to promote the
excessive production of pro-inflammatory cytokines, such as IL-6,
IL-1β and TNF-α, which establishes an inflammatory microenvironment
and recruits MDSCs (14). Recruited
MDSCs have been demonstrated to further secrete pro-inflammatory
cytokines, causing a vicious circle, while also participating in
angiogenesis and immune tolerance (15–17).
Toll-like receptors (TLRs) have crucial roles in inflammation and
cancer, and proteins downstream of the receptors, such as myeloid
differentiation primary response 88 (MyD88) and NF-κB, enable
cancer cells to escape from the immune system, which contributes to
cancer progression (18). Notably,
the inhibition of the NF-κB-mediated secretion of pro-inflammatory
cytokines, including TNF-α, IL-6, IL-8, IL-1β and IL-4, has been
revealed to decrease the volume of xenograft tumors and the
accumulation of MDSCs (19).
Therefore, blocking the TLR4/NF-κB signaling pathway and inhibiting
MDSC recruitment may be an effective strategy for the treatment of
numerous types of cancer.
Curcumin, a diketone compound, can be isolated from
the rhizomes of the plant Curcuma longa. The anticancer
effects of curcumin have been established through multiple in
vivo and in vitro studies (20,21).
Curcumin exhibits pharmacological activities and has been
demonstrated to exert beneficial health effects through its ability
to regulate cancer cell proliferation (20), tumor growth (21), apoptosis (22), migration, invasion, angiogenesis,
metastasis, bioenergetics (23),
oxidation (24) and the inflammatory
environment (25,26). Previous studies have demonstrated
that curcumin prevents cancer progression through its antioxidant,
anti-inflammatory and immunoregulatory properties (27–30).
Although the anticancer properties of curcumin have been
extensively investigated, whether its effects are mediated by
modulating MDSCs and the mechanism by which MDSCs are regulated
remains elusive. Based on the aforementioned findings, it was
hypothesized that curcumin may inhibit MDSC-mediated angiogenesis
and immune tolerance in liver cancer by attenuating the
TLR4/NF-κB-induced inflammatory microenvironment and reducing the
levels of GM-CSF and G-CSF.
Materials and methods
Drugs and reagents
Curcumin (batch number, SLBN7214V; molecular weight,
368.39 kDa) was purchased from Sigma-Aldrich; Merck KGaA.
Fluorouracil (5-Fu; batch number, FA170415) was obtained from
Shanghai Xudong Haipu Pharmaceutical Co., Ltd., and cisplatin (DDP;
batch number, 8A0075B02) was purchased from Qilu Pharmaceutical
Co., Ltd. The anti-CD11b-PE/Cy7 (cat. no. ab218786) and
anti-Gr-1-FITC (cat. no. ab25024) antibodies were purchased from
Abcam. PBS, DMEM and BSA were purchased from Sigma-Aldrich; Merck
KGaA. HRP-conjugated goat anti-mouse IgG antibody (cat. no.
SA00001-1), HRP-conjugated goat anti-rabbit IgG antibody (cat. no.
SA00001-2) and HRP-conjugated donkey anti-goat IgG antibody (cat.
no. SA00001-3) were purchased from ProteinTech Group, Inc. Protease
inhibitors and protein phosphatase inhibitors were purchased from
Roche Diagnostics.
Cell culture
The human liver cancer cell lines (HepG2, Huh-7 and
MHCC-97H) were purchased from The Cell Bank of Type Culture
Collection of The Chinese Academy of Sciences and stored at the
Cell Center of Xiangya Medical College of Central South University
(Changsha, China). The cells were cultured in DMEM supplemented
with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1X
penicillin/streptomycin, and maintained in a humified atmosphere at
37°C with 5% CO2. Curcumin was dissolved in DMSO to make
a 9.6 mg/ml stock solution. HepG2, Huh-7 and MHCC-97H cells were
co-incubated with curcumin (1.2, 2.4, 4.8 or 9.6 µg/ml) for 24 and
48 h at 37°C. In addition, HepG2 cells in the exponential growth
phase were transplanted into mice to generate tumors as described
subsequently.
Cell Counting Kit-8 (CCK-8) assay
Huh-7, MHCC-97H and HepG2 cells were seeded into
96-well plates at a density of 4,000 cells/well. Once attached, the
cells were treated with 1.2, 2.4, 4.8 or 9.6 µg/ml curcumin for 24
or 48 h at 37°C. Cell viability was analyzed using CCK-8 reagent
(cat. no. C0038; Beyotime Institute of Biotechnology) according to
the manufacturer's protocol. Briefly, 10 µl CCK-8 reagent was added
into each well, and cells were incubated for 1 h in the dark.
Subsequently, the absorbance was measured at a wavelength of 450 nm
using a microplate reader (BioTek Instruments, Inc.).
Flow cytometry
The cells were collected by trypsinization and
adjusted to a density of 3×105 cells/ml. The cell
samples were washed twice with PBS via centrifugation for 10 min at
1,000 × g at 4°C, and resuspended in 1 µg anti-CD11b-PE/Cy7
(dilution, 1:100) and anti-Gr-1-FITC (dilution, 1:100) antibodies.
Following 30 min of incubation at 37°C in the dark, the samples
were performed on a Gallios flow cytometer (Beckman Coulter, Inc.)
and analyzed using FlowJo V.10 software (Tree Star, Inc.). MDSCs
were identified based on double positivity for CD11b and Gr-1.
Xenograft experiments
BALB/c-nu nude mice [specific pathogen-free (SPF)
grade; male; weight, 18–22 g; age, 21–28 days] were provided by
Hunan SJA Laboratory Animal Co., Ltd. Mice were raised in an animal
room (SPF grade) at Xiangya Medical College (Changsha, China). The
animal experiment protocols were performed in accordance with the
National Institutes of Health Guide for the Care and Use of
Laboratory Animals (2011) (31) and
approved by the Animal Research Ethics Committee of Central South
University (approval no. 2018SYNKW0181; Changsha, China). A total
of 28 mice were maintained at 25.0±2.0°C and 55.0±5.0% relative
humidity with a 12-h light/dark cycle, and ad libitum access
to food and water. Following acclimation for 1 week,
1×107 HepG2 cells in 0.1 ml saline were injected into
the right flank from the back of the mice to form subcutaneous
xenograft tumors. Mice were divided into the following 4 groups
(n=7/group) on the 5th day after injection when the tumor volume of
HepG2 cells reached ~50 mm3: i) Model group (injected
with cancer cells and administered intragastrically with the
equivalent volume of 0.5% carboxymethylcellulose sodium, served as
the negative control); ii) curcumin low dose group (Cur L; injected
with cancer cells and administered intragastrically with 120 mg/kg
curcumin daily); iii) curcumin high dose group (Cur H; injected
with cancer cells and administered intragastrically with 240 mg/kg
curcumin daily); and iv) chemotherapy group (injected with cancer
cells and intraperitoneally injected with 50 mg/kg 5-Fu + 5 mg/kg
DDP once a week; positive control). The regimens of 5-Fu+DDP and
curcumin were based on their clinical application and a previous
study, respectively (32,33). Furthermore, 240 mg/kg curcumin used
in mice is equivalent to ~1.3 g/day in humans based on the body
surface area (m2/kg) conversion between humans and mice,
with a postulated adult body weight of 75 kg (human dosage=(240
mg/kg/14.16) ×75 kg), which was considered safe for humans
according to a phase I clinical trial (34). The body weight and tumor volume of
each mouse were measured every 3 days over a period of 15 days. The
tumor volume was calculated using the following equation: Tumor
volume=length × width × width/2. Animals were euthanized when
>20% of weight loss was recorded, tumors were 1.0–1.5 cm in
diameter or severe disease signs (e.g., difficulty breathing or
paralysis) were observed. On day 15, a total of 28 mice (n=7/group)
were sacrificed by intraperitoneal injection with an overdose of
sodium pentobarbital (200 mg/kg), followed by cervical dislocation
and rigor mortis as confirmation of death. The duration between
injection and final tumor growth measurement was 20 days. The tumor
tissues were removed, weighed and immediately stored at −80°C for
further analysis. The maximum tumor diameter and volume observed in
the present study was 15.83 mm and 516.32 mm3,
respectively.
Western blotting
Total protein was extracted from tissues using 200
µl RIPA lysis buffer (Beyotime Institute of Biotechnology). Total
protein was quantified by BCA and proteins (50 µg/lane) were
separated via 10–12% SDS-PAGE and distinguished according to their
molecular weights: TLR4, 75 kDa; TNF-α, 17 kDa; nuclear factor-κB
kinase α (IKKα), 88 kDa; nuclear factor-κB kinase β (IKKβ), 85 kDa;
phosphorylated (p-)IKKα, 88 kDa; p-IKKβ, 85 kDa; NF-κB, 65 kDa;
MyD88, 33 kDa; IL-6, 24 kDa; IL-1β, 35 kDa; prostaglandin E2
(PGE2), 53 kDa; cyclooxygenase-2 (COX-2), 74 kDa; vascular
endothelial growth factor (VEGF), 25 kDa; and β-actin, 42 kDa. The
separated proteins were subsequently transferred onto
nitrocellulose membranes, which were blocked with 5% BSA at room
temperature for 2 h and incubated with the following primary
antibodies at 4°C overnight: Anti-TLR4 (dilution, 1:700; cat. no.
ab13556; Abcam), anti-TNF-α (dilution, 1:2,000; cat. no.
17590–1-AP; ProteinTech Group, Inc.), anti-IKKα (dilution,
1:10,000; cat. no. ab32041; Abcam), anti-IKKβ (dilution, 1:200;
cat. no. 15649-1-AP; ProteinTech Group, Inc.), anti-p-IKKα
(dilution, 1:500; cat. no. ab38515; Abcam), anti-p-IKKβ (dilution,
1:500; cat. no. ab59195; Abcam), anti-NF-κB (dilution, 1:500; cat.
no. 10745-1-AP; ProteinTech Group, Inc.), anti-MyD88 (dilution,
1:500; cat. no. 23230-1-AP; ProteinTech Group, Inc.), anti-IL-6
(dilution, 1:500; cat. no. 21865-1-AP; ProteinTech Group, Inc.),
anti-IL-1β (dilution, 1:200; cat. no. 16806-1-AP; ProteinTech
Group, Inc.), anti-PGE2 (dilution, 1:1,000; cat. no. ab2318;
Abcam), anti-COX-2 (dilution, 1:500; cat. no. 12375-1-AP;
ProteinTech Group, Inc.), anti-VEGF (dilution, 1:500; cat. no.
19003-1-AP; ProteinTech Group, Inc.) and β-actin (dilution,
1:5,000; cat. no. 60008-1-Ig; ProteinTech Group, Inc.). Following
the primary antibody incubation, the membranes were incubated with
anti-rabbit secondary antibody (dilution, 1:6,000; cat. no. A0208;
Beyotime Institute of Biotechnology) or HRP-conjugated goat
anti-mouse IgG antibody (dilution, 1:500; cat. no. SA00001-1;
ProteinTech Group, Inc.) for 1 h at room temperature. The protein
bands were visualized using an ECL Western Blotting Substrate kit
(cat. no. P0018FS; Beyotime Institute of Biotechnology). Bands were
semi-quantified using ImageJ software (ImageJ 1.8.0; National
Institutes of Health).
ELIAS assay
Mouse Granulocyte Colony Stimulating Factor and
mouse Granulocyte Macrophage Colony Stimulating Factor ELISA kits
were respectively used to determine G-CSF (cat. no. CSB-E04564m;
Cusabio Biotech Co., Ltd.) and GM-CSF levels (cat. no. CSB-E04569m;
Cusabio Biotech Co., Ltd.) in tumor tissues according to the
manufacturer's protocols.
Immunohistochemistry (IHC)
staining
The tumor tissues were fixed in 10% neutral buffered
formalin for 24 h at room temperature, and then embedded in
paraffin. The paraffin-embedded tumor tissue sections (thickness, 5
µm) were deparaffinized in xylene, rehydrated using an ascending
series of alcohol in the following sequence: 100% ethanol for 3
min, 90% ethanol for 3 min, 80% ethanol for 3 min and 70% ethanol
for 3 min and subsequently flushed with distilled water, followed
by washing thoroughly with 0.01 M PBS (pH 7.2–7.6). For antigen
retrieval, trypsin antigen retrieval solution was directly pipetted
onto the tissue on the slide for 10 min at 37°C. Subsequently,
endogenous peroxidase activity was quenched by a 30-min incubation
in methanolic hydrogen peroxide (2.5%). After being sealed with 5%
BSA for 1 h at room temperature, the slides were incubated
overnight at 4°C with the following primary antibodies: Anti-CD31
(dilution, 1:500; cat. no. 11265-1-AP; ProteinTech Group, Inc.),
anti-α-smooth muscle actin (αSMC; dilution, 1:300; cat. no.
ab21027; Abcam) and anti-VEGF (dilution, 1:500; cat. no.
19003-1-AP; ProteinTech Group, Inc.). Following the primary
antibody incubation, the sections were incubated with the
corresponding HRP-conjugated anti-rabbit secondary antibody
(dilution, 1:500; cat. no. A0208; Beyotime Institute of
Biotechnology) or HRP-conjugated donkey anti-goat IgG antibody
(dilution, 1:500; cat. no. SA00001-3; ProteinTech Group, Inc.) at
37°C for 30 min. Subsequently, the slides were stained with
3,3′-diaminobenzidine at 25°C for 5 min. All sections were observed
under a light or fluorescence microscope (magnification, ×400) and
analyzed using HistoQuest 4.0 software (TissueGnostics GmbH) as
previously described (35).
Statistical analysis
Statistical analysis was performed using SPSS
version 21.0 software (IBM Corp.) and the results are presented as
the mean ± SD, except cell viability assay data which are presented
as the mean ± SEM. All data were from at least three independent
triplicate experiments. Statistical differences among groups were
analyzed using one-way ANOVA followed by Tukey's multiple
comparisons test and the normality of data was determined using a
Kolmogorov-Smirnov test. P<0.05 was considered to indicate a
statistically significant difference.
Results
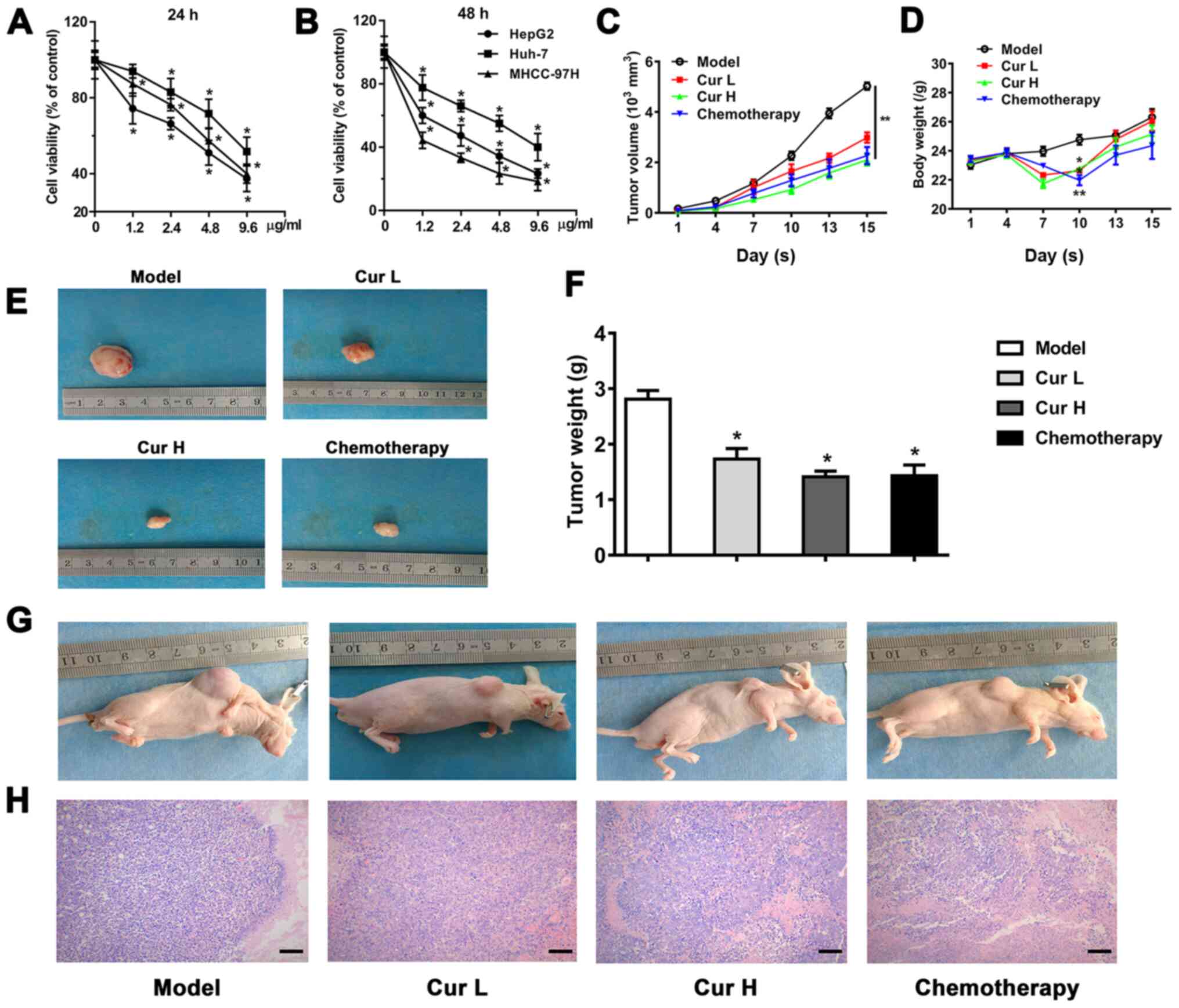
Curcumin inhibits the viability of
liver cancer cells
To investigate the role of curcumin in regulating
liver cancer cell proliferation, the effects of treatment with
1.2–9.6 µg/ml curcumin on the viability of Huh-7, MHCC-97H and
HepG2 cells were determined. The results revealed a dose-dependent
reduction in cell viability following 24 and 48 h of treatment
(Fig. 1A and B). To verify the
results in vivo, a HepG2 ×enograft nude mouse model was
used. Similarly, 120 and 240 mg/kg/day curcumin progressively
decreased the tumor volume compared with the model group, which was
measured for 15 days (Fig. 1C).
Treatment with curcumin was observed to cause a decrease in body
weight on the 10th day; however, the body weight subsequently
increased and was not affected by curcumin treatment by the 15th
day (Fig. 1D). As expected, the
curcumin-induced decline in tumor volume was also associated with a
marked decrease in tumor weight, revealing comparable tumor growth
inhibition efficacy between curcumin and chemotherapy treatments,
which indicated the therapeutic potential of curcumin in liver
cancer (Fig. 1F). The evaluation of
tumor histology using hematoxylin and eosin staining indicated that
the groups treated with curcumin (120 and 240 mg/kg/day) and the
chemotherapy treatment group exhibited necrosis and lower
cellularity compared with the model group (Fig. 1H). As shown in Fig. 1E and G, the in vivo and ex
vivo images of the tumors further indicated the
tumor-suppressive effects of curcumin.
Curcumin suppresses MDSCs and the
secretion of modulatory-related factors GM-CSF and G-CSF
MDSCs serve a crucial role in the immunology of the
majority of tumors, and are responsible for inhibiting the immune
function of T cells and promoting angiogenesis (36). It was hypothesized that curcumin may
inhibit the expansion of MDSCs, which would subsequently enhance
immune function. To test this hypothesis, flow cytometry was used
to determine CD11b+Gr-1+ expression in mouse
xenograft tumor tissues. As shown in Fig. 2A and B, compared with the model
group, treatment with 120 or 240 mg/kg curcumin or chemotherapy
significantly reduced the percentage of MDSCs (in 7 mice). Notably,
the treatment with curcumin at both a low (120 mg/kg) and high (240
mg/kg) dose exhibited a comparable inhibitory effect on MDSCs,
suggesting that there were no dose-dependent effects when comparing
low and high doses of curcumin. These results suggested that
curcumin may inhibit the expansion of MDSCs in mice xenograft tumor
tissues, but not in a dose-dependent manner.
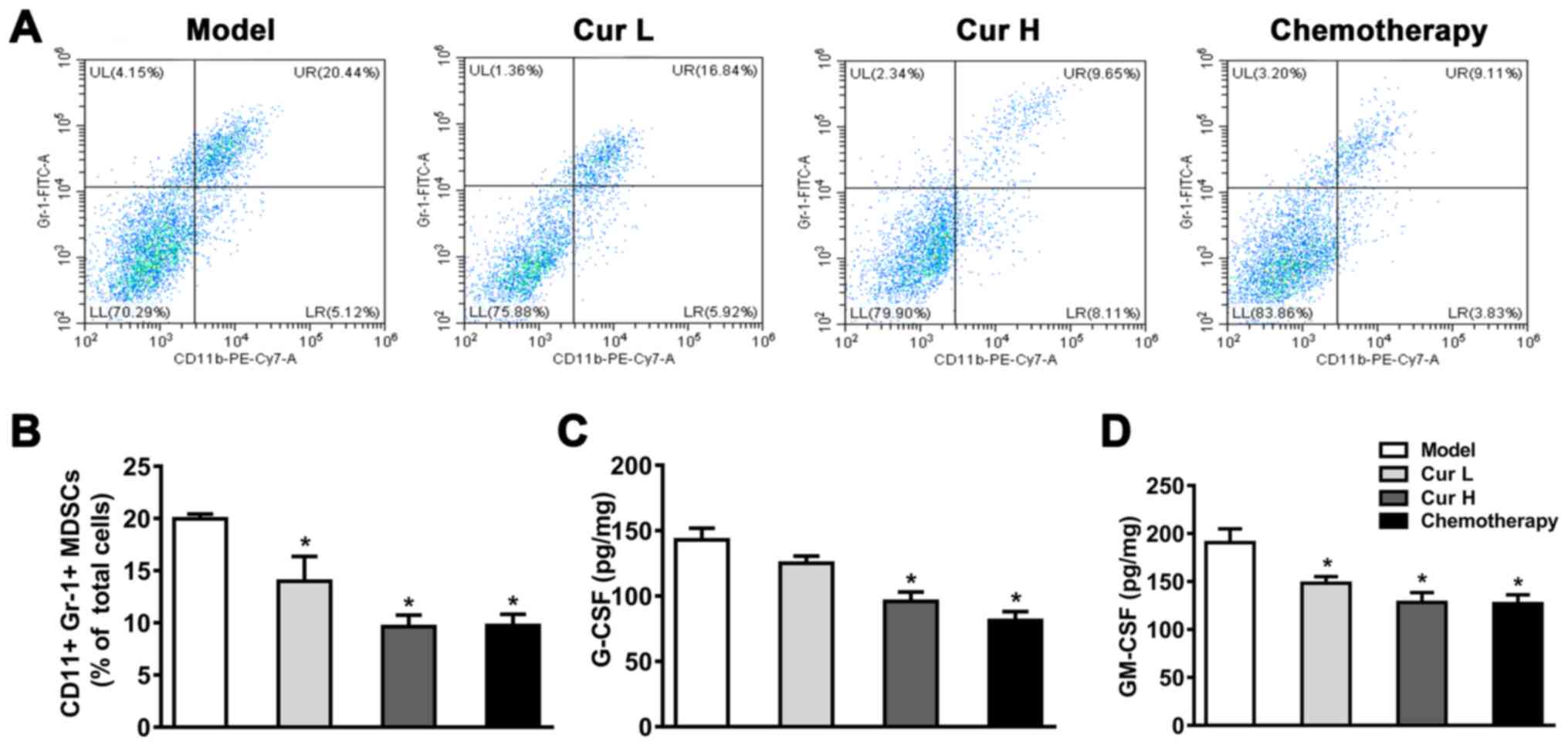 | Figure 2.Curcumin inhibits MDSCs in tumor
tissues. (A) Flow cytometric analysis of
CD11b+/Gr-1+ cells in murine tumor tissues.
(B) Quantitative analysis of MDSCs in murine tumor tissue.
Secretory levels of (C) G-CSF and (D) GM-CSF in the tumor tissues
were measured using ELISAs. Tumor tissue samples were collected
within 24 h following the attenuation of treatment with all drugs.
Data are presented as the mean ± SD (n=7). *P<0.05 vs. model
group. MDSCs, myeloid-derived suppressor cells; GM-CSF,
granulocyte-macrophage colony-stimulating factor; G-CSF,
granulocyte-colony-stimulating factor; Cur L, curcumin low dose
group (120 mg/kg); Cur H, curcumin high dose group (240 mg/kg);
Gr-1, granulocyte receptor-1; UL, upper left; UR, upper right; LL,
lower left; LR, lower right. |
To investigate whether the inhibitory effect of
curcumin on MDSCs could be attributed to M-CSF and GM-CSF
secretion, ELISAs were used to determine the secretory levels of
GM-CSF and G-CSF. As illustrated in Fig.
2C and D, treatment with Cur H and chemotherapy notably reduced
G-CSF and GM-CSF levels compared with the model group, whereas Cur
L only significantly decreased GM-CSF levels. These data indicated
that curcumin may suppress M-CSF and GM-CSF secretion, which could
lead to the inactivation of MDSCs, and thereby restrict cancer cell
proliferation.
Alterations in the expression levels
of TLR4/NF-κB signaling pathway-related proteins account for the
inhibition of MDSCs
To investigate the latent molecular mechanism
underlying the curcumin-modulated activity of MDSCs, the expression
levels of proteins associated with the TLR4/NF-κB signaling pathway
were analyzed using western blotting. The results revealed that
both curcumin and chemotherapy treatment significantly decreased
TLR4, MyD88, p-IKKα/IKKα, p-IKKβ/IKKβ and NF-κB expression compared
with the model group. Notably, high doses of curcumin (Cur H)
exhibited greater effects than the treatment with chemotherapy in
terms of the inhibition of the TLR4/NF-κB signaling pathway, except
the ratios of p-IKKα/IKKα and p-IKKβ/IKKβ (Fig. 3A-E). Considering that the TLR4/NF-κB
signaling pathway is the major inflammatory signaling pathway that
mediates the expression levels of numerous inflammatory cytokines
in cancer (37), and that
inflammation serves an important role in cancer progression and
MDSC recruitment and activation (38), the expression levels of TNF-α, IL-6,
IL-1β, PGE2 and COX-2 in xenograft tumor tissues were analyzed. As
shown in Fig. 3, treatment with
curcumin at two doses (Cur L and Cur H) and chemotherapy
significantly blocked the increased expression of TNF-α, IL-6,
IL-1β, PGE2 and COX-2 compared with the model group (Fig. 3F-I). Notably, Cur H was at least
comparable or superior to the chemotherapy treatment in terms of
its ability to downregulate the expression levels of the proteins.
These data indicated that curcumin may suppress the TLR4/NF-κB
signaling pathway and control the inflammatory response, which may
be the underlying mechanism inhibiting MDSC activity and thus,
inhibiting cancer progression.
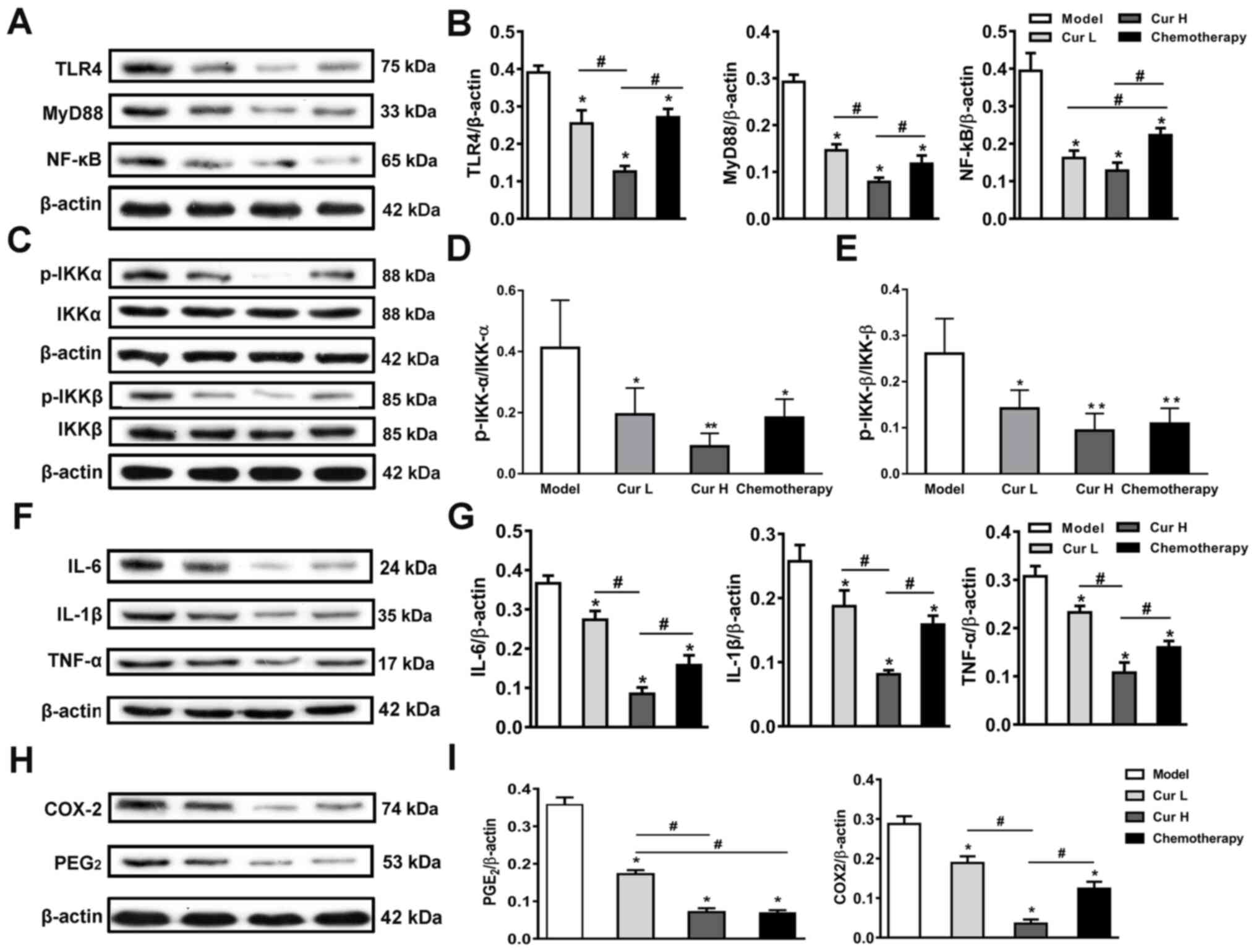 | Figure 3.Inhibition of the TLR4/NF-κB
signaling pathway-mediated inflammatory response. (A) Western
blotting was used to analyze and (B) semi-quantify the expression
levels of TLR4, MyD88 and NF-κB in murine tumor tissues. (C)
Expression levels and semi-quantitative analysis of (D) IKK-α,
p-IKK-α, (E) IKK-β and p-IKK-β in murine tumor tissues. (F)
Expression levels and (G) semi-quantitative analysis of IL-6, IL-1β
and TNF-α in murine tumor tissues. (H) Expression levels and (I)
semi-quantitative analysis of PGE2 and COX-2 in murine tumor
tissues. Data are presented as the mean ± SD (n=7). *P<0.05 and
**P<0.01 vs. model group; #P<0.05, as indicated.
TLR4, toll-like receptor 4; MyD88, myeloid differentiation primary
response 88; IKKα, nuclear factor-κB kinase α; IKKβ, nuclear
factor-κB kinase β; PGE2, prostaglandin E2; COX-2,
cyclooxygenase-2; p-, phosphorylated; Cur L, curcumin low dose
group (120 mg/kg); Cur H, curcumin high dose group (240 mg/kg). |
Curcumin suppresses tumor
angiogenesis
The angiogenic programming of neoplastic tissue is a
multidimensional process that is regulated by cancer cells in
concert with a variety of tumor-associated stromal cells and their
bioactive products. MDSCs promote tumor angiogenesis by producing
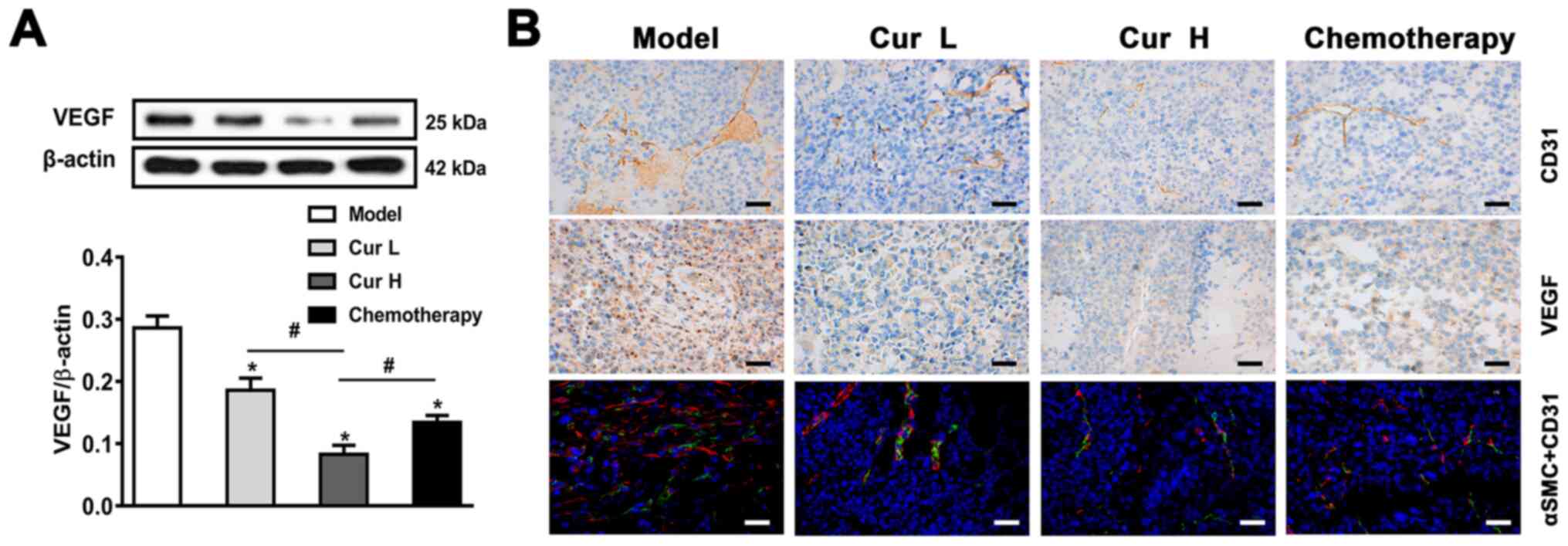
pro-angiogenic growth factors, such as VEGF (39). The results of the western blot
analysis demonstrated that 120 and 240 mg/kg curcumin, particularly
Cur H, significantly downregulated VEGF expression in vivo
compared with that in the model group (Fig. 4A). To further verify the
anti-angiogenic effect of curcumin in vivo, the excised
tumor tissues were analyzed using IHC for CD31 expression (a
platelet endothelial cell adhesion molecule) and VEGF, and by
immunofluorescence using anti-αSMC (an arterial smooth muscle cell
marker) and anti-CD31 antibodies. The observed downregulated
expression levels of VEGF, CD31 and αSMC in the tumor tissues
indicated that angiogenesis may be suppressed by curcumin treatment
(Fig. 4B). Overall, these findings
indicated that curcumin may inhibit VEGF and CD31-induced
angiogenesis in vivo.
Discussion
Curcumin has been reported to be a promising
anticancer agent in various types of cancer. In the present study,
data obtained from the xenograft nude mouse model provided further
evidence that curcumin effectively suppressed the growth of liver
cancer. Similarly, numerous previous in vitro and in
vivo studies have demonstrated that curcumin inhibits the
growth cancer in both animals and humans (40–42),
including hepatocellular carcinoma (43). Several reports have suggested that
curcumin or its derivative alone or in combination with other drugs
exhibit favorable anti-liver cancer activity (44–46).
Shao et al (47) reported
that bisdemethoxycurcumin, a natural dimethoxy derivative of
curcumin, decreases the number of tumor-infiltrating MDSCs in MB49
metastasized bladder cancer cells. In addition, curcumin could
suppress MKN-45 human gastric cancer cell and CT26 mouse colon
cancer cell proliferation by blocking the STAT3 and NF-κB signaling
pathway-mediated activation and expansion of MDSCs, in addition to
the interaction of cancer cells with MDSCs (48). However, to the best of our knowledge,
no previous study has investigated the anti-liver cancer activity
of curcumin, and in particular the association among inflammation,
MDSCs and angiogenesis in tumor tissues. Therefore, the present
study was the first to report that the anticancer activity of
curcumin may be due to the inactivation of MDSCs, which was
associated with the ability of curcumin to inhibit
TLR4/NF-κB-mediated inflammation and decrease GM-CSF and G-CSF
secretion, which subsequently repressed angiogenesis and tumor
growth.
In-depth research on the pathogenesis of cancer has
revealed that inflammation and the immune microenvironment are key
players in cancer growth, spread and metastasis (49). Defined immune cell subsets have been
reported to dictate cancer fate by their expansion and recruitment
within the loco-regional tumor microenvironment (50). High numbers of myeloid cells,
particularly MDSCs, have been associated with tumor promotion,
metastasis and a poor prognosis (2).
MDSCs are found in the peripheral blood and solid tumors, where
they are known to maintain an immunosuppressive network in the
tumor microenvironment (50,51). In particular, they constitute a key
checkpoint that prevents an immune response against tumors
(51). Previous studies have
reported that the inhibition of MDSCs in the tumor may weaken the
tumor defense mechanisms and inhibit tumor progression, including
in lung cancer (52,53), malignant melanoma (54) and liver cancer (55). Previous evidence has indicated that
curcumin suppresses and prevents carcinogenesis via multifaceted
molecular targets; however, to the best of our knowledge, there are
few studies reporting the role of curcumin in regulating MDSCs
(48), particularly in liver
cancer.
TDFs, which are mainly cytokines, chemokines and
metabolic soluble mediators, promote and sustain the expansion of
the heterogeneous population of myeloid cells, skew myeloid cells
towards an immunosuppressive phenotype and endow them with
regulatory functions (2). TDFs, such
as GM-CSF, G-CSF and inflammatory factors (TNF-α, IL-1β and IL-6),
are crucial mediators in MDSC modulation (4). G-CSF and GM-CSF have been discovered to
be the main regulators of the proliferation and differentiation of
myeloid cells (56). A recent study
demonstrated that cancer cell-derived GM-CSF serves a key role in
cancer progression, and GM-CSF is indispensable for the tuning of
the tumor microenvironment and MDSCs (57). Furthermore, the inflammatory
microenvironment in cancer, which is established by multiple
inflammatory factors, recruits and activates MDSCs (58). The results of the present study
revealed that curcumin markedly suppressed the levels of M-CSF,
GM-CSF and numerous inflammatory factors, which provided evidence
for the inhibitory effect of curcumin on MDSCs and its subsequent
anti-liver cancer activity.
The immunosuppressive potential of MDSCs is mainly
dependent on the expression of inflammation-related proteins
(59). TLR4/NF-κB is a canonical
inflammatory signaling pathway that has a prominent role in
mediating the inflammatory response in multiple types of disease,
including cancer (12). Activated
TLR4 interacts with MyD88, and then induces IKK phosphorylation
(18). IKK phosphorylation triggers
the activation of NF-κB, which in turn promotes the expression of
inflammation- and immune-suppressive signaling pathway genes
(60). Several previous studies have
demonstrated that the TLR4 axis enhances the immunosuppressive
function of MDSCs (61) and that
curcumin inhibits liver cancer via the TLR4/NF-κB signaling pathway
(62). The present study
investigated whether curcumin alleviated the MDSC-mediated
immunosuppressive function via the TLR4/NF-κB signaling pathway.
The data revealed that curcumin treatment decreased the protein
expression levels of TLR4, MyD88, p-IKK and NF-κB. This indicated
that curcumin may inactivate the TLR4/NF-κB signaling pathway,
which may in turn result in a decreased inflammatory response.
In addition, MDSCs promote tumor angiogenesis by
producing pro-angiogenic growth factors, such as VEGF, and
endothelial cell adhesion molecules, such as CD31 (63). Additionally, the inhibition of
inflammatory factors, such as IL-6, has been associated with the
attenuation of angiogenesis (64).
In a previous study, curcumin inhibited hepatocellular carcinoma
growth by downregulating VEGF expression (65). Similarly, the present results
demonstrated that curcumin treatment suppressed angiogenesis by
downregulating VEGF and CD31 expression in the tumor tissues, which
was demonstrated using western blotting, IHC and immunofluorescence
staining experiments. These results might be attributed to the
inhibitory effect of curcumin on MDSCs and inflammation.
The doses of curcumin used in the present study were
selected based on our preliminary experiment and a pervious study
(33). In addition, 120 and 240
mg/kg curcumin used in mice is equivalent to ~0.65 and 1.3 g/day,
respectively, in humans, based on the mouse/human dose conversion
coefficient. Furthermore, a phase I clinical trial has demonstrated
that curcumin is safe even at high doses (12 g/day) in humans
(34). Therefore, from the
perspective of safety, the dosage is acceptable when it comes to
humans. In respect to efficacy, curcumin could efficaciously delay
liver cancer growth, almost equivalent to the chemotherapy group.
Even marketed anticancer drugs, such as sorafenib and lenvatinib,
cannot prevent the growth of liver cancer (66). Therefore, the anti-liver cancer
effect of curcumin revealed in the present study is of values.
Importantly, the present study revealed a novel mechanism in which
curcumin suppressed the growth of liver cancer by impairing MDSCs
in murine tumor tissues.
Although curcumin is deemed safe and nontoxic, even
at high doses (12 g/day), in humans, the low aqueous solubility,
poor absorption and rapid metabolism of curcumin severely impedes
its bioavailability and thus, prevents its application as a
therapeutic agent (34,67). To overcome this challenge, various
strategies have been explored, including the alteration of the
administration route of curcumin, blocking the metabolism of
curcumin with other agents, altering the structural modifications
of curcumin and investigating the possibility of oral delivery
systems (34,68). Among them, oral delivery systems, a
novel delivery strategy established in recent years, has offered
significant promise in enhancing the bioavailability of curcumin,
and this involves the use of nano and microparticles, polymeric
micelles, nanosuspensions or lipid-based nanocarriers (67,69).
Further studies should aim to introduce the aforementioned
bioavailable curcumin-based formulations to explore the efficacy of
the anti-liver cancer effects of curcumin through this delivery
method, with a specific focus on its ability to affect
inflammation, MDSCs and their interactions.
There are certain limitations of the present study:
i) The HepG2 cell line was not authenticated; ii) in vivo
imaging of tumors and Kaplan-Meier curves of mice were not
provided; iii) no information was provided regarding the
effectiveness of either curcumin or chemotherapy on tumor once the
treatment was discontinued; and iv) the effect of curcumin on the
TLR4/NF-κB signaling pathway in isolated MDSCs from xenografts was
not determined. Based on the aforementioned limitations, in future
research, authentication of HepG2 cells is warranted, Kaplan-Meier
curves, in vivo imaging of tumors, and a prolonged period of
time effectiveness of curcumin, particularly once the treatment is
discontinued, should be provided to comprehensively evaluate the
effect of curcumin on liver cancer. MDSCs should be isolated from
xenografts and IKK activator should be applied to examine whether
curcumin exerts its anti-liver cancer effect via blocking of the
TLR4/NF-κB signaling pathway in MDSCs.
In conclusion, the findings of the present study
indicated that curcumin may effectively prevent liver cancer by
impairing MDSCs in tumor tissues, which was demonstrated by the
inhibition of GM-CSF and G-CSF secretion, and TLR4/NF-κB-mediated
inflammation (Fig. 5). The present
study provided novel insights into the anti-liver cancer mechanism
of curcumin and a theoretical basis for anti-liver cancer drug
development targeting MDSCs.
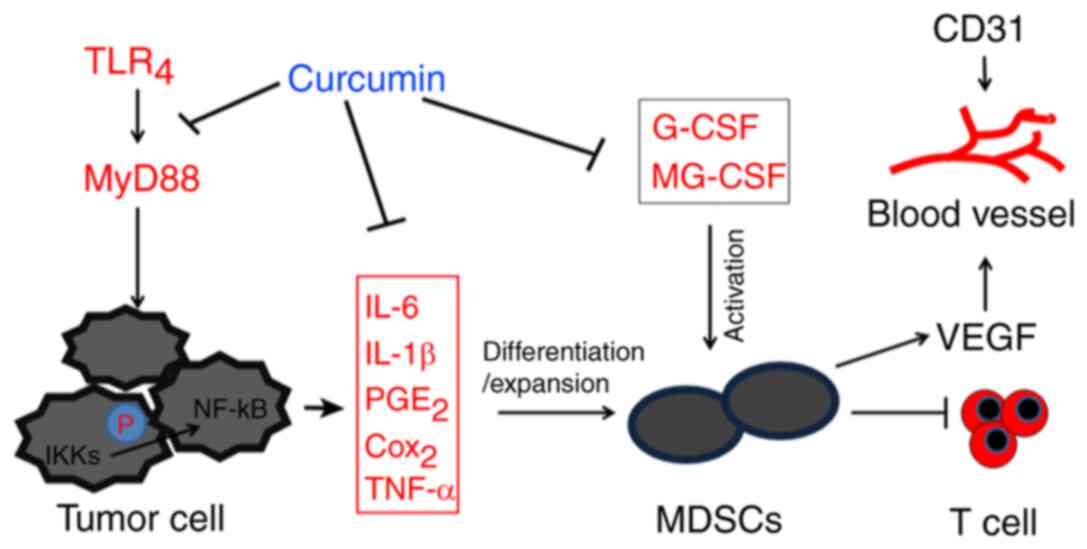 | Figure 5.Schematic diagram of the inhibitory
effect of curcumin on MDSCs and liver cancer. Curcumin exerted an
inhibitory effect on MDSCs by inhibiting the TLR4/NF-κB-mediated
inflammatory microenvironment and attenuating G-CSF and GM-CSF
secretion. This subsequently suppresses tumor angiogenesis and
ameliorates immune tolerance in liver cancer, thus exhibiting
anti-liver cancer effects. TLR4, toll-like receptor 4; MyD88,
myeloid differentiation primary response 88; IKK, nuclear factor-κB
kinase; PGE2, prostaglandin E2; COX-2, cyclooxygenase-2; MDSCs,
myeloid-derived suppressor cells; G-CSF, granulocyte-colony
stimulating factor; GM-CSF, granulocyte-macrophage
colony-stimulating factor; VEGF, vascular endothelial growth
factor. |
Acknowledgements
The authors would like to thank Dr Yiming Tao
(Xiangya Medical College, Central South University, Changsha,
China) for providing the reagents used in the present study and for
help revising the manuscript.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81473617),
the Science and Technology Department of Hunan Province (grant no.
2017SK50310), the Innovation Platform Open Foundation of Hunan
Educational office (grant no. 16K066) and Hunan Province ‘domestic
first-class cultivation discipline’ Integrated Traditional Chinese
and Western medicine open fund project (2018ZXYJH03,
2019ZXJH02).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XT conceived, designed and guided the study. ST and
LL performed the experiments. QZ, XH, PZ and YG analyzed the data
and generated the graphs. TD and XT helped analyze the experimental
results and provided technical guidance. XT and ST drafted the
manuscript. XT and ST are responsible for confirming the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Institute Review Board of Xiangya Medical College (Changsha,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Galon J, Costes A, Sanchez-Cabo F,
Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M,
Berger A, Wind P, et al: Type, density, and location of immune
cells within human colorectal tumors predict clinical outcome.
Science. 313:1960–1964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Condeelis J and Pollard JW: Macrophages:
Obligate partners for tumor cell migration, invasion, and
metastasis. Cell. 124:263–266. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Movahedi K, Guilliams M, Van den Bossche
J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P and Van
Ginderachter JA: Identification of discrete tumor-induced
myeloid-derived suppressor cell subpopulations with distinct T
cell-suppressive activity. Blood. 111:4233–4244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Sanctis F, Solito S, Ugel S, Molon B,
Bronte V and Marigo I: MDSCs in cancer: Conceiving new prognostic
and therapeutic targets. Biochim Biophys Acta. 1865:35–48.
2016.PubMed/NCBI
|
|
5
|
Watson GA, Fu YX and Lopez DM: Splenic
macrophages from tumor-bearing mice co-expressing MAC-1 and MAC-2
antigens exert immunoregulatory functions via two distinct
mechanisms. J Leukoc Biol. 49:126–138. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kidiyoor A, Schettini J, Besmer DM, Rego
SL, Nath S, Curry JM, Roy LD, Dréau D and Mukherjee P: Pancreatic
cancer cells isolated from Muc1-null tumors favor the generation of
a mature less suppressive MDSC population. Front Immunol. 5:672014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gutschalk CM, Yanamandra AK, Linde N,
Meides A, Depner S and Mueller MM: GM-CSF enhances tumor invasion
by elevated MMP-2, −9, and −26 expression. Cancer Med. 2:117–129.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kowanetz M, Wu X, Lee J, Tan M, Hagenbeek
T, Qu X, Yu L, Ross J, Korsisaari N, Cao T, et al:
Granulocyte-colony stimulating factor promotes lung metastasis
through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci
USA. 107:21248–21255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Quail DF, Olson OC, Bhardwaj P, Walsh LA,
Akkari L, Quick ML, Chen IC, Wendel N, Ben-Chetrit N, Walker J, et
al: Obesity alters the lung myeloid cell landscape to enhance
breast cancer metastasis through IL5 and GM-CSF. Nat Cell Biol.
19:974–987. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Serafini P, Carbley R, Noonan KA, Tan G,
Bronte V and Borrello I: High-dose granulocyte-macrophage
colony-stimulating factor-producing vaccines impair the immune
response through the recruitment of myeloid suppressor cells.
Cancer Res. 64:6337–6343. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dolcetti L, Peranzoni E, Ugel S, Marigo I,
Fernandez Gomez A, Mesa C, Geilich M, Winkels G, Traggiai E, Casati
A, et al: Hierarchy of immunosuppressive strength among
myeloid-derived suppressor cell subsets is determined by GM-CSF.
Eur J Immunol. 40:22–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yao D, Dong M, Dai C and Wu S:
Inflammation and inflammatory cytokine contribute to the initiation
and development of ulcerative colitis and its associated cancer.
Inflamm Bowel Dis. 25:1595–1602. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu J, Pei S, Wang Y, Liu J, Qian Y, Huang
M, Zhang Y and Xiao Y: Tpl2 protects against fulminant hepatitis
through mobilization of myeloid-derived suppressor cells. Front
Immunol. 10:19802019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li ZW, Sun B, Gong T, Guo S, Zhang J, Wang
J, Sugawara A, Jiang M, Yan J, Gurary A, et al: GNAI1 and GNAI3
reduce colitis-associated tumorigenesis in mice by blocking il6
signaling and down-regulating expression of GNAI2.
Gastroenterology. 156:2297–2312. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tariq M, Zhang J, Liang G, Ding L, He Q
and Yang B: Macrophage polarization: Anti-cancer strategies to
target tumor-associated macrophage in breast cancer. J Cell
Biochem. 118:2484–2501. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Musolino C, Allegra A, Pioggia G and
Gangemi S: Immature myeloid-derived suppressor cells: A bridge
between inflammation and cancer (Review). Oncol Rep. 37:671–683.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Perfilyeva YV, Abdolla N, Ostapchuk YO,
Tleulieva R, Krasnoshtanov VC, Perfilyeva AV and Belyaev NN:
Chronic inflammation contributes to tumor growth: Possible role of
l-selectin-expressing myeloid-derived suppressor cells (MDSCs).
Inflammation. 42:276–289. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guven Maiorov E, Keskin O, Gursoy A and
Nussinov R: The structural network of inflammation and cancer:
Merits and challenges. Semin Cancer Biol. 23:243–251. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang L, Niu Z, Wang X, Li Z, Liu Y, Luo F
and Yan X: PHD2 exerts anti-cancer and anti-inflammatory effects in
colon cancer xenografts mice via attenuating NF-κB activity. Life
Sci. 242:1171672020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Banerjee S, Ji C, Mayfield JE, Goel A,
Xiao J, Dixon JE and Guo X: Ancient drug curcumin impedes 26S
proteasome activity by direct inhibition of dual-specificity
tyrosine-regulated kinase 2. Proc Natl Acad Sci USA. 115:8155–8160.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Y, Wang X, Zeng S, Zhang X, Zhao J,
Zhang X, Chen X, Yang W, Yang Y, Dong Z, et al: The natural
polyphenol curcumin induces apoptosis by suppressing STAT3
signaling in esophageal squamous cell carcinoma. J Exp Clin Cancer
Res. 37:3032018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang P, Lai ZL, Chen HF, Zhang M, Wang A,
Jia T, Sun WQ, Zhu XM, Chen XF, Zhao Z and Zhang J: Curcumin
synergizes with 5-fluorouracil by impairing AMPK/ULK1-dependent
autophagy, AKT activity and enhancing apoptosis in colon cancer
cells with tumor growth inhibition in xenograft mice. J Exp Clin
Cancer Res. 36:1902017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang L, Chen X, Du Z, Li G, Chen M, Chen
X, Liang G and Chen T: Curcumin suppresses gastric tumor cell
growth via ROS-mediated DNA polymerase γ depletion disrupting
cellular bioenergetics. J Exp Clin Cancer Res. 36:472017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rana M, Maurya P, Reddy SS, Singh V, Ahmad
H, Dwivedi AK, Dikshit M and Barthwal MK: A standardized chemically
modified Curcuma longa extract modulates IRAK-MAPK signaling
in inflammation and potentiates cytotoxicity. Front Pharmacol.
7:2232016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uchio R, Higashi Y, Kohama Y, Kawasaki K,
Hirao T, Muroyama K and Murosaki S: A hot water extract of turmeric
(Curcuma longa) suppresses acute ethanol-induced liver
injury in mice by inhibiting hepatic oxidative stress and
inflammatory cytokine production. J Nutr Sci. 6:e32017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Farajzadeh R, Zarghami N, Serati-Nouri H,
Momeni-Javid Z, Farajzadeh T, Jalilzadeh-Tabrizi S, Sadeghi-Soureh
S, Naseri N and Pilehvar-Soltanahmadi Y: Macrophage repolarization
using CD44-targeting hyaluronic acid-polylactide nanoparticles
containing curcumin. Artif Cells Nanomed Biotechnol. 46:2013–2021.
2018.PubMed/NCBI
|
|
27
|
Illuri R, Bethapudi B, Anandakumar S,
Murugan S, Joseph JA, Mundkinajeddu D, Agarwal A and Chandrasekaran
CV: Anti-Inflammatory activity of polysaccharide fraction of
Curcuma longa extract (NR-INF-02). Antiinflamm Antiallergy
Agents Med Chem. 14:53–62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kocaadam B and Şanlier N: Curcumin, an
active component of turmeric (Curcuma longa), and its
effects on health. Crit Rev Food Sci Nutr. 57:2889–2895. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pulido-Moran M, Moreno-Fernandez J,
Ramirez-Tortosa C and Ramirez-Tortosa M: Curcumin and health.
Molecules. 21:2642016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Darvesh AS, Aggarwal BB and Bishayee A:
Curcumin and liver cancer: A review. Curr Pharm Biotechnol.
13:218–228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
National Research Council, . Guide for the
Care and Use of Laboratory Animals. The National Academies Press;
Washington DC, USA: eighth edition. pp. 11–154. 2011
|
|
32
|
Kolligs FT, Hoffmann RT, op den Winkel M,
Bruns CJ, Herrmann K, Jakobs TF, Lamerz R, Trumm C, Zech CJ,
Wilkowski R and Graeb C: Diagnosis and multimodal therapy for
hepatocellular carcinoma. Z Gastroenterol. 48:274–288. 2010.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hu P, Ke C, Guo X, Ren P, Tong Y, Luo S,
He Y, Wei Z, Cheng B, Li R, et al: Both glypican-3/Wnt/β-catenin
signaling pathway and autophagy contributed to the inhibitory
effect of curcumin on hepatocellular carcinoma. Dig Liver Dis.
51:120–126. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Anand P, Kunnumakkara AB, Newman RA and
Aggarwal BB: Bioavailability of curcumin: Problems and promises.
Mol Pharm. 4:807–818. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Karova K, Wainwright JV, Machova-Urdzikova
L, Pisal RV, Schmidt M, Jendelova P and Jhanwar-Uniyal M:
Transplantation of neural precursors generated from spinal
progenitor cells reduces inflammation in spinal cord injury via
NF-κB pathway inhibition. J Neuroinflammation. 16:122019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gabrilovich DI: Myeloid-derived suppressor
cells. Cancer Immunol Res. 5:3–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rafa H, Benkhelifa S, AitYounes S, Saoula
H, Belhadef S, Belkhelfa M, Boukercha A, Toumi R, Soufli I, Moralès
O, et al: All-Trans retinoic acid modulates TLR4/NF-κB signaling
pathway targeting TNF-α and nitric oxide synthase 2 expression in
colonic mucosa during ulcerative colitis and colitis associated
cancer. Mediators Inflamm. 2017:73532522017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cheng R, Billet S, Liu C, Haldar S,
Choudhury D, Tripathi M, Hav M, Merchant A, Hu T, Huang H, et al:
Periodontal inflammation recruits distant metastatic breast cancer
cells by increasing myeloid-derived suppressor cells. Oncogene.
39:1543–1556. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ching MM, Reader J and Fulton AM:
Eicosanoids in cancer: Prostaglandin E(2) receptor 4 in cancer
therapeutics and immunotherapy. Front Pharmacol. 11:8192020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Q, Yuan JW, Zhang F, Qiao F, Sui XF
and Liu CH: Curcumin protects rat H9C2 cardiomyocytes against
doxorubicin toxicity by modulating oxidative stress and apoptosis.
J Biol Regul Homeost Agents. 33:1849–1854. 2019.PubMed/NCBI
|
|
41
|
Flores-Pérez JA, de la Rosa Oliva F,
Argenes Y and Meneses-Garcia A: Nutrition, cancer and personalized
medicine. Adv Exp Med Biol. 1168:157–168. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Willenbacher E, Khan SZ, Mujica SCA,
Trapani D, Hussain S, Wolf D, Willenbacher W, Spizzo G and Seeber
A: Curcumin: New insights into an ancient ingredient against
cancer. Int J Mol Sci. 20:18082019. View Article : Google Scholar
|
|
43
|
Kong ZL, Kuo HP, Johnson A, Wu LC and
Chang KLB: Curcumin-loaded mesoporous silica nanoparticles markedly
enhanced cytotoxicity in hepatocellular carcinoma cells. Int J Mol
Sci. 20:29182019. View Article : Google Scholar
|
|
44
|
Wang L, Zhu Z, Han L, Zhao L, Weng J, Yang
H, Wu S, Chen K, Wu L and Chen T: A curcumin derivative, WZ35,
suppresses hepatocellular cancer cell growth via downregulating
YAP-mediated autophagy. Food Funct. 10:3748–3757. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shao S, Duan W, Xu Q, Li X, Han L, Li W,
Zhang D, Wang Z and Lei J: Curcumin suppresses hepatic stellate
cell-induced hepatocarcinoma angiogenesis and invasion through
downregulating CTGF. Oxid Med Cell Longev. 2019:81485102019.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tang D, Zhang S, Shi X, Wu J, Yin G, Tan
X, Liu F, Wu X and Du X: Combination of astragali polysaccharide
and curcumin improves the morphological structure of tumor vessels
and induces tumor vascular normalization to inhibit the growth of
hepatocellular carcinoma. Integr Cancer Ther.
18:15347354188244082019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shao Y, Zhu W, Da J, Xu M, Wang Y, Zhou J
and Wang Z: Bisdemethoxycurcumin in combination with α-PD-L1
antibody boosts immune response against bladder cancer. Onco
Targets Ther. 10:2675–2683. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tu SP, Jin H, Shi JD, Zhu LM, Suo Y, Lu G,
Liu A, Wang TC and Yang CS: Curcumin induces the differentiation of
myeloid-derived suppressor cells and inhibits their interaction
with cancer cells and related tumor growth. Cancer Prev Res
(Phila). 5:205–215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Keren L, Bosse M, Marquez D, Angoshtari R,
Jain S, Varma S, Yang SR, Kurian A, Van Valen D, West R, et al: A
structured tumor-immune microenvironment in triple negative breast
cancer revealed by multiplexed ion beam imaging. Cell.
174:1373–1387. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lazăr DC, Avram MF, Romoșan I, Cornianu M,
Tăban S and Goldiș A: Prognostic significance of tumor immune
microenvironment and immunotherapy: Novel insights and future
perspectives in gastric cancer. World J Gastroenterol.
24:3583–3616. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Salminen A: Activation of
immunosuppressive network in the aging process. Ageing Res Rev.
57:1009982020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liu D, You M, Xu Y, Li F, Zhang D, Li X
and Hou Y: Inhibition of curcumin on myeloid-derived suppressor
cells is requisite for controlling lung cancer. Int
Immunopharmacol. 39:265–272. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li YD, Lamano JB, Lamano JB, Quaggin-Smith
J, Veliceasa D, Kaur G, Biyashev D, Unruh D and Bloch O:
Tumor-induced peripheral immunosuppression promotes brain
metastasis in patients with non-small cell lung cancer. Cancer
Immunol Immunother. 68:1501–1513. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hutchison S, Sahay B, de Mello SC, Sayour
EJ, Lejeune A, Szivek A, Livaccari AM, Fox-Alvarez S, Salute M,
Powers L and Milner RJ: Characterization of myeloid-derived
suppressor cells and cytokines GM-CSF, IL-10 and MCP-1 in dogs with
malignant melanoma receiving a GD3-based immunotherapy. Vet Immunol
Immunopathol. 216:1099122019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xu Y, Fang F, Jiao H, Zheng X, Huang L, Yi
X and Zhao W: Activated hepatic stellate cells regulate MDSC
migration through the SDF-1/CXCR4 axis in an orthotopic mouse model
of hepatocellular carcinoma. Cancer Immunol Immunother.
68:1959–1969. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Al Sayed MF, Amrein MA, Bührer ED,
Huguenin AL, Radpour R, Riether C and Ochsenbein AF:
T-cell-secreted TNFα induces emergency myelopoiesis and
myeloid-derived suppressor cell differentiation in cancer. Cancer
Res. 79:346–359. 2019. View Article : Google Scholar
|
|
57
|
Yoshimura T, Nakamura K, Li C, Fujisawa M,
Shiina T, Imamura M, Li T, Mukaida N and Matsukawa A: Cancer
cell-derived granulocyte-macrophage colony-stimulating factor is
dispensable for the progression of 4t1 murine breast cancer. Int J
Mol Sci. 20:63242019. View Article : Google Scholar
|
|
58
|
Chen L, Huang CF, Li YC, Deng WW, Mao L,
Wu L, Zhang WF, Zhang L and Sun ZJ: Blockage of the NLRP3
inflammasome by MCC950 improves anti-tumor immune responses in head
and neck squamous cell carcinoma. Cell Mol Life Sci. 75:2045–2058.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bronte V, Brandau S, Chen SH, Colombo MP,
Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A,
Ostrand-Rosenberg S, et al: Recommendations for myeloid-derived
suppressor cell nomenclature and characterization standards. Nat
Commun. 7:121502016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lei R, Li J, Liu F, Li W, Zhang S, Wang Y,
Chu X and Xu J: HIF-1α promotes the keloid development through the
activation of TGF-β/Smad and TLR4/MyD88/NF-κB pathways. Cell Cycle.
18:3239–3250. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tachibana M: The immunosuppressive
function of myeloid-derived suppressor cells is regulated by the
HMGB1-TLR4 axis. Yakugaku Zasshi. 138:143–148. 2018.(In Japanese).
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ren B, Luo S, Tian X, Jiang Z, Zou G, Xu
F, Yin T, Huang Y and Liu J: Curcumin inhibits liver cancer by
inhibiting DAMP molecule HSP70 and TLR4 signaling. Oncol Rep.
40:895–901. 2018.PubMed/NCBI
|
|
63
|
Cassino TR, Drowley L, Okada M, Beckman
SA, Keller B, Tobita K, Leduc PR and Huard J: Mechanical loading of
stem cells for improvement of transplantation outcome in a model of
acute myocardial infarction: The role of loading history. Tissue
Eng Part A. 18:1101–1108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tawara K, Scott H, Emathinger J, Ide A,
Fox R, Greiner D, LaJoie D, Hedeen D, Nandakumar M, Oler AJ, et al:
Co-expression of VEGF and IL-6 family cytokines is associated with
decreased survival in HER2 negative breast cancer patients:
Subtype-specific IL-6 family cytokine-mediated VEGF secretion.
Transl Oncol. 12:245–255. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Pan Z, Zhuang J, Ji C, Cai Z, Liao W and
Huang Z: Curcumin inhibits hepatocellular carcinoma growth by
targeting VEGF expression. Oncol Lett. 15:4821–4826.
2018.PubMed/NCBI
|
|
66
|
Forner A, Reig M and Bruix J:
Hepatocellular carcinoma. Lancet. 391:1301–1314. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ma Z, Wang N, He H and Tang X:
Pharmaceutical strategies of improving oral systemic
bioavailability of curcumin for clinical application. J Control
Release. 316:359–380. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Liu W, Zhai Y, Heng X, Che FY, Chen W, Sun
D and Zhai G: Oral bioavailability of curcumin: Problems and
advancements. J Drug Target. 24:694–702. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Salehi M, Movahedpour A, Tayarani A,
Shabaninejad Z, Pourhanifeh MH, Mortezapour E, Nickdasti A,
Mottaghi R, Davoodabadi A, Khan H, et al: Therapeutic potentials of
curcumin in the treatment of non-small-cell lung carcinoma.
Phytother Res. 34:2557–2576. 2020. View Article : Google Scholar : PubMed/NCBI
|