Introduction
As a type of malignancy developing from mouth,
tongue and lips, oral cancer is one of the major subtypes of head
and neck cancer (1). According to
the latest GLOBOCAN statistics, oral cancer affected 354,864 new
cases (2.0% of all new cancer cases) and caused 177,384 deaths
(1.9% of all cancer deaths) (2).
Therefore, approximately 50% of oral cancer patients succumb to
this disease (3). Smoking, betel
quid consumption and human papillomavirus infections are major risk
factors for oral cancer (4), while
the molecular pathogenesis of this disease remains largely unknown
(5,6), leading to difficulties in the
development of anticancer treatment. In addition, the early
diagnostic rate of oral cancer is low, resulting in poor survival
(7).
Previous studies on the pathogenesis of oral cancer
have identified a considerable number of molecular pathways
involved in the development and progression of this disease
(8,9). In effect, some of the altered signaling
pathways involved in oral cancer have been proven to be potential
targets for the development of anti-cancer therapies (10). Long non-coding RNAs (lncRNAs, >200
nt) encode no proteins but participate in cancer biology by
regulating cancer-related gene expression (11,12).
Therefore, regulating the expression of certain lncRNAs may
indirectly regulate cancer-related gene expression. However, the
functions of most lncRNAs in cancer remain unclear. UASR1 has been
characterized as an oncogenic lnRNA in breast cancer (13); however, its roles in other types of
cancer remain unknown. Our preliminary bioinformatics analysis
revealed that UASR1 may interact with miR-375, which plays a tumor
suppressive role by targeting JAK2 (14). The present study was therefore
carried out to investigate the interactions of UASR1, miR-375 and
JAK2 in oral squamous cell carcinoma (OSCC), which is a major
subtype of oral cancer.
Materials and methods
Tissue collections
The present study was approved by the Ethics
Committee of Jinan Stomatological Hospital (Shandong, China). A
total of 62 OSCC patients (sex, 38 males and 24 females; age range,
41–69 years; mean age, 55.5±5.2 years) who were admitted at this
hospital between March 2012 and December 2014 were enrolled in this
study. Diagnosis of OSCC was performed by histopathological biopsy.
Patients complicated with other clinical disorders or ones with
initiated therapy were excluded from this study. Patients with a
history of malignancies were also excluded. During biopsy, paired
OSCC and adjacent non-tumor tissue samples (within 5 cm around
tumors) were collected from each patient. Fresh samples were stored
in liquid nitrogen prior to use. All patients signed the informed
consent.
Clinical stages and treatment
Based on AJCC staging system, the 62 patients
included 14, 18, 15 and 15 cases at stage I, II, III and IV,
respectively. Based on patients' clinical stages and their health
conditions, treatments, such as chemotherapy, radiotherapy,
surgical resections or the combination of different treatments,
were performed on the patients.
Follow-up
From the day of admission, the 62 patients were
followed up for 5 years to record their survival conditions.
Follow-up was performed in a monthly manner through telephone. All
patients completed the follow-up. Patients who succumbed to causes
unrelated to OSCC were excluded.
Cell lines and culture
Normal human oral keratinocyte cells (NHOK) and OSCC
cell lines (SCC25 and SCC154) were purchased from the American Type
Culture Collection. NHOK cells were grown in the oral keratinocyte
medium (ScienCell Research Laboratories, Inc.) and maintained at
37°C in a humidified chamber with 5% CO2. The two OSCC
cell lines were cultured in DMEM/F-12 (Sigma-Aldrich; Merck KGaA)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and 0.4 µg/ml hydrocortisone and placed at 37°C in a humidified
incubator containing 5% CO2.
Cell transfection
SCC25 and SCC154 cells were seeded at a density of
105 cells per well on a 6-well plate 24 h prior to
transfection. Once cells reached 70% confluence, they were
transfected with lentivirus expressing JAK2 (shJAK2) and matched
control (designated as shNC), or miR-375 mimics (designated as
miR-375 mimics) and its negative control (designated as NC mimics),
which were purchased from RiboBio. UASR1 expression plasmid
(designated as UASR1) was obtained from Sangon Biotech, as well as
the negative control (named as Vector). Lipofectamine 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to perform
cell transfection with siRNA or miRNA mimics at a dose of 50 nM and
with vectors at a dose of 15 nM. Cells were used for subsequent
experiments after 48 h transfection.
Dual-luciferase activity assay
Luciferase vector of UASR1 was constructed using
pGL3 vector (Promega Corporation) as backbone. To perform
Dual-luciferase activity assay, SCC25 and SCC154 cells were
co-transfected with UASR1 expression vector + miR-375 mimic
(miR-375 group) or UASR1 expression vector + NC miRNA (NC group).
Cells were cultivated at 37°C for 48 h, and the luciferase activity
was analyzed using Dual-Luciferase Reporter Assay System (Promega
Corporation). Renilla luciferase activity was used as the
control. Each sample was assessed in triplicate.
RNA preparations
Total RNAs were extracted from SCC25 and SCC154
cells and paired tissue samples using RNAzol reagent
(Sigma-Aldrich; Thermo Fisher Scientific, Inc.). RNA precipitation
and washing were performed using 85% ethanol. RNA samples were
digested with gDNA eraser (Takara) to remove genomic DNAs.
RT-qPCR assay
Digested RNA samples were subjected to reverse
transcription using Bio-Rad iScript cDNA Kit to prepare cDNA
samples (Bio-Rad Laboratories, Inc.). With cDNA samples as
template, qPCR reactions were prepared using SYBR-Green Master Mix
(Bio-Rad Laboratories, Inc.). The expression levels of UASR1 and
JAK2 mRNA were determined with GAPDH as the endogenous control. The
expression levels of mature miR-375 were detected using
All-in-One™ miRNA qRT-PCR Detection Kit (GeneCopoeia)
with U6 as the internal control. The sequences of the primers used
were as follows: UASR1, forward, 5′-CCCTCCTCAAACACACATCC-3′ and
reverse, 5′-TTAAGGAAATTAAAAATACC-3′; miR-375, forward,
5′-GGCTCTAGAGGGGACGAAGC-3′ and reverse,
5′-GGCAAGCTTTTTCCACACCTCAGCCTTG-3′; JAK2, forward,
5′-TCTATTTTATTATGGTTTCCCTTG-3′ and reverse,
5′-TTTTACTTATTTACCTCATTTCCC-3′; GAPDH, forward,
5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse, 5′-TGGTGAAGACGCCAGTGGA-3′;
and U6, forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′. PCR reactions were performed in
triplicates and Cq values were processed using the
2−ΔΔCq method (15).
Western blot assay
Total proteins were isolated from cells using RIPA
buffer (Invitrogen; Thermo Fisher Scientific, Inc.), followed by
BCA assay (Invitrogen; Thermo Fisher Scientific, Inc.) to measure
protein concentrations. Protein samples were first denatured at
95°C for 10 min. Proteins (20 µg) were then separated by 8–15%
SDS-PAGE. Proteins were transferred to polyvinylidene fluoride
(PVDF) membranes, followed by blocking in phosphate-buffered saline
(PBS) containing 5% non-fat milk for 2 h. Membranes were first
incubated with rabbit primary antibodies of JAK2 (product code
ab39636; Abcam) and GAPDH (product code ab9485; Abcam) at 4°C for
15 h, followed by incubation at room temperature for 2 h with HRP
IgG goat anti-rabbit (product code ab97051; Abcam). ECL solution
(Sigma-Aldrich; Merck KGaA) was dropped onto membranes to develop
signals. Enhanced chemiluminescence reagent (Amersham; Cytiva) was
used to detect the signal on the membrane. The data were analyzed
via densitometry using Image-Pro Plus software (64-bit Java
1.8.0_112; Media Cybernetics, Inc.) and normalized to expression of
the internal control GAPDH.
Cell proliferation assay
Cells harvested after transfections were counted,
and 4,000 cells in 0.1 ml medium were seeded in a 96-well plate.
Cells were cultured for 24, 48 or 72 h. Subsequently, 10 µl CCK-8
solution (Shanghai Yeasen Biotechnology Co., Ltd.) was added and
incubated for another 2 h at 37°C. The absorbance at 450 nm was
detected using a microplate reader.
Assay of colony formation
SCC25 and SCC154 cells were seeded into 96-well
plates at the density of 3×103 cells/well following
transfection with shNC, pcDN3.1, NC miRNA, shJAK2, UASR1, miR-375
and UASR1 + miR-375. Two weeks later, the cells were fixed with 4%
paraformaldehyde (Sigma-Aldrich; Merck KGaA), stained with 0.5%
crystal violet (Sigma-Aldrich; Merck KGaA) and then washed with PBS
(Sigma-Aldrich; Merck KGaA). The total number of colonies from
three separate transfections was counted. The average value was
used to evaluate colony formation ability.
Statistical analysis
Mean ± SD values were used to express data from 3
biological replicates. Paired t-test was used to compare
differences between paired tissues. Unpaired t-test was used to
compare differences between two independent groups. One-way
analysis of variance (ANOVA) and Tukey's test were used to compare
differences among multiple groups. The 62 OSCC patients were
divided into high and low UASR1 level groups (n=31) with the median
level of UASR1 expression in OSCC tissues as the cut-off value.
Survival curves were plotted for the two groups based on follow-up
data. Log-rank test was used to compare survival curves.
Chi-squared test was performed to explore the associations between
expression levels of UASR1 and patients' clinical data. P<0.05
was statistically significant.
Results
Upregulation of UASR1 predicted poor
survival of OSCC patients
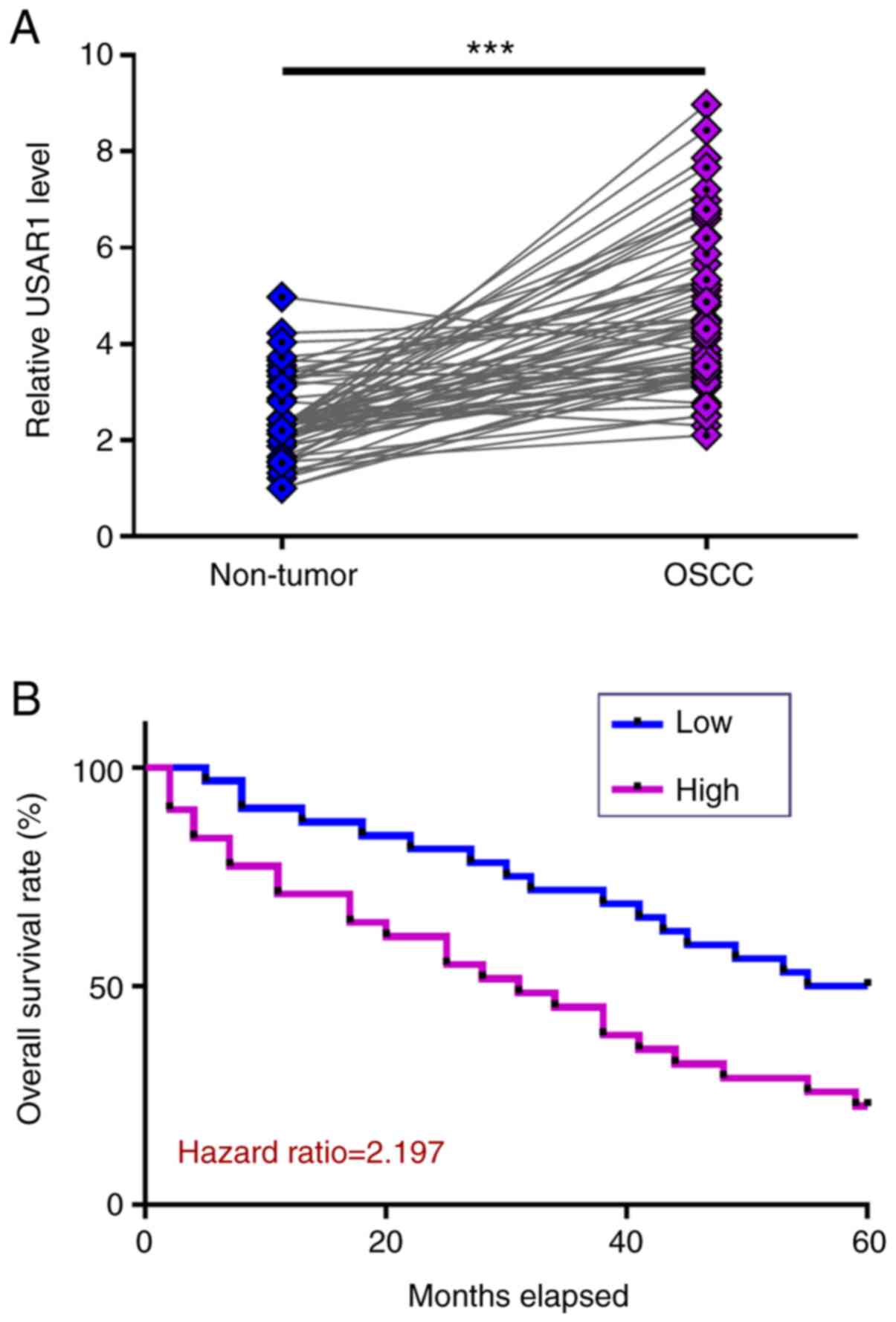
The differential expression of UASR1 in OSCC was
detected by measuring the expression level of UASR1 in paired OSCC
and non-tumor tissues collected from 62 OSCC patients in the
present study (Table I). Compared
with non-tumor tissues, expression levels of UASR1 were
significantly higher in OSCC tissues (Fig. 1A, P<0.001). Survival analysis
showed that patients in high UASR1 level group experienced higher
mortality rate during the 5-year follow-up (Fig. 1B). It is noteworthy that treatment
approaches and clinical stages were not significantly different
between high and low UASR1 groups. Thus, UASR1 is likely an
independent prognostic factor for OSCC. Chi-squared test showed
that the expression levels of UASR1 were not significantly
associated with patient age, sex, clinical stage, and smoking and
drinking habits.
 | Table I.Associations between the expression
levels of UASR1 and patients' clinical data. |
Table I.
Associations between the expression
levels of UASR1 and patients' clinical data.
| Items | Cases | High | Low | χ2 | P-value |
|---|
| Age (years) |
|
>55 | 30 | 14 | 16 | 0.26 | 0.61 |
|
<55 | 32 | 17 | 15 | 1.63 |
|
| Sex |
| Male | 38 | 16 | 22 | 1.61 | 0.20 |
|
Female | 24 | 15 | 9 |
|
|
| Tumor stage |
| I | 14 | 6 | 8 | 1.17 | 0.76 |
| II | 18 | 8 | 10 |
|
|
| III | 15 | 9 | 6 |
|
|
| IV | 15 | 8 | 7 |
|
|
| Smoking |
| Yes | 35 | 16 | 9 | 0.59 | 0.44 |
| No | 37 | 15 | 12 |
|
|
| Drinking |
| Yes | 41 | 18 | 23 | 1.80 | 0.18 |
| No | 21 | 13 | 5 |
|
|
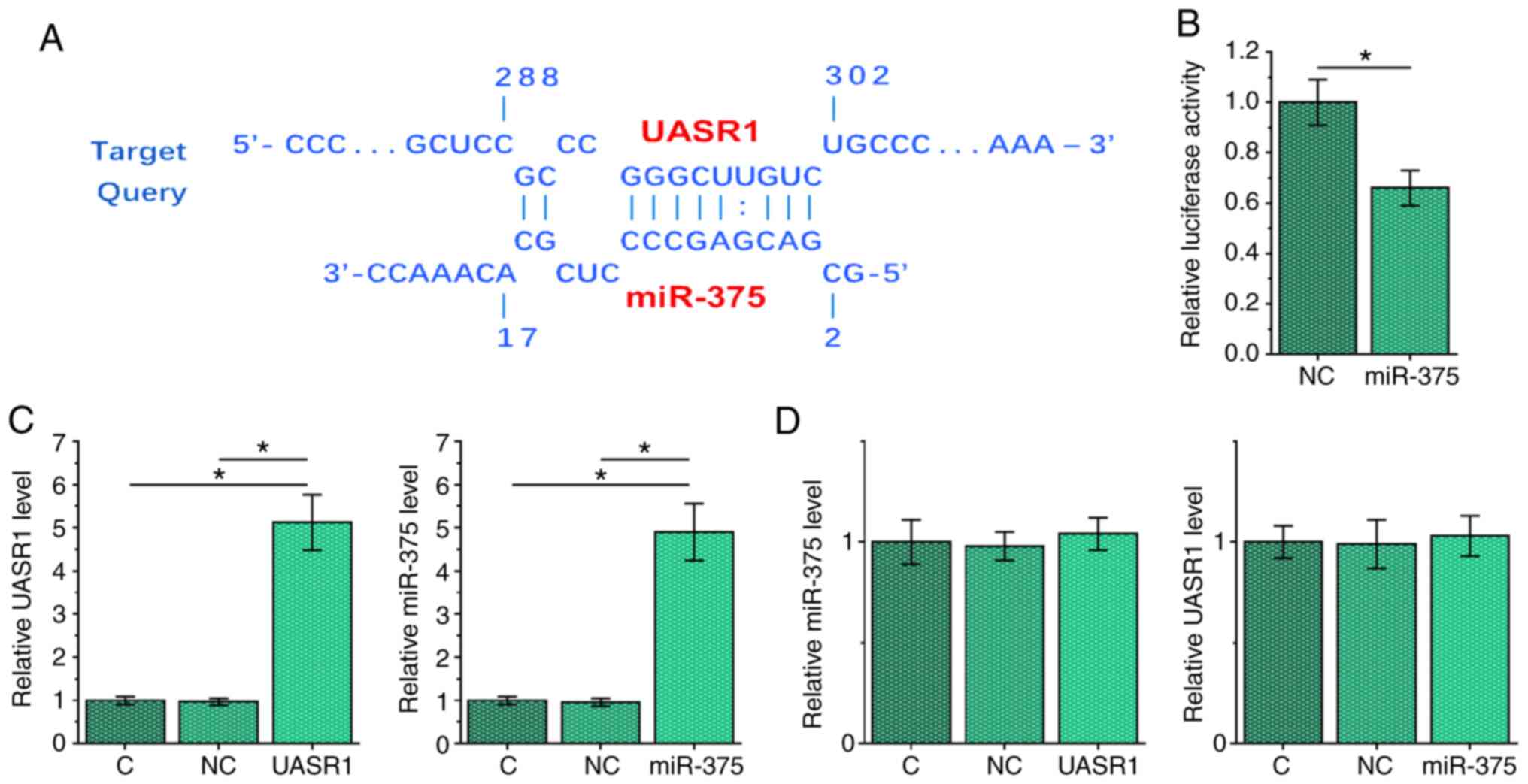
UASR1 and miR-375 interacted with each
other but did not regulate the expression of each other in SCC25
cells
IntaRNA (http://rna.informatik.uni-freiburg.de/IntaRNA/Input.jsp)
was used to predict the interaction between UASR1 and miR-375. It
showed that UASR1 and miR-375 may form strong base pairs (Fig. 2A). To perform Dual-luciferase
activity assay, SCC25 cells were co-transfected with UASR1
expression vector + miR-375 mimic (miR-375 group) or UASR1
expression vector + NC miRNA (NC group). Compared with the NC
group, the relative luciferase activity of miR-375 group was
relatively lower at 48 h post-transfection, indicating the direct
interaction between them (Fig. 2B,
P<0.05). To further explore the interaction between UASR1 and
miR-375, SCC25 cells were transfected with either UASR1 expression
vector or miR-375 mimic, and the overexpression of UASR1 and
miR-375, respectively, was confirmed by RT-qPCR (Fig. 2C, P<0.05). Compared with C and NC
groups, overexpression of UASR1 and miR-375 did not affect the
expression of each other (Fig.
2D).
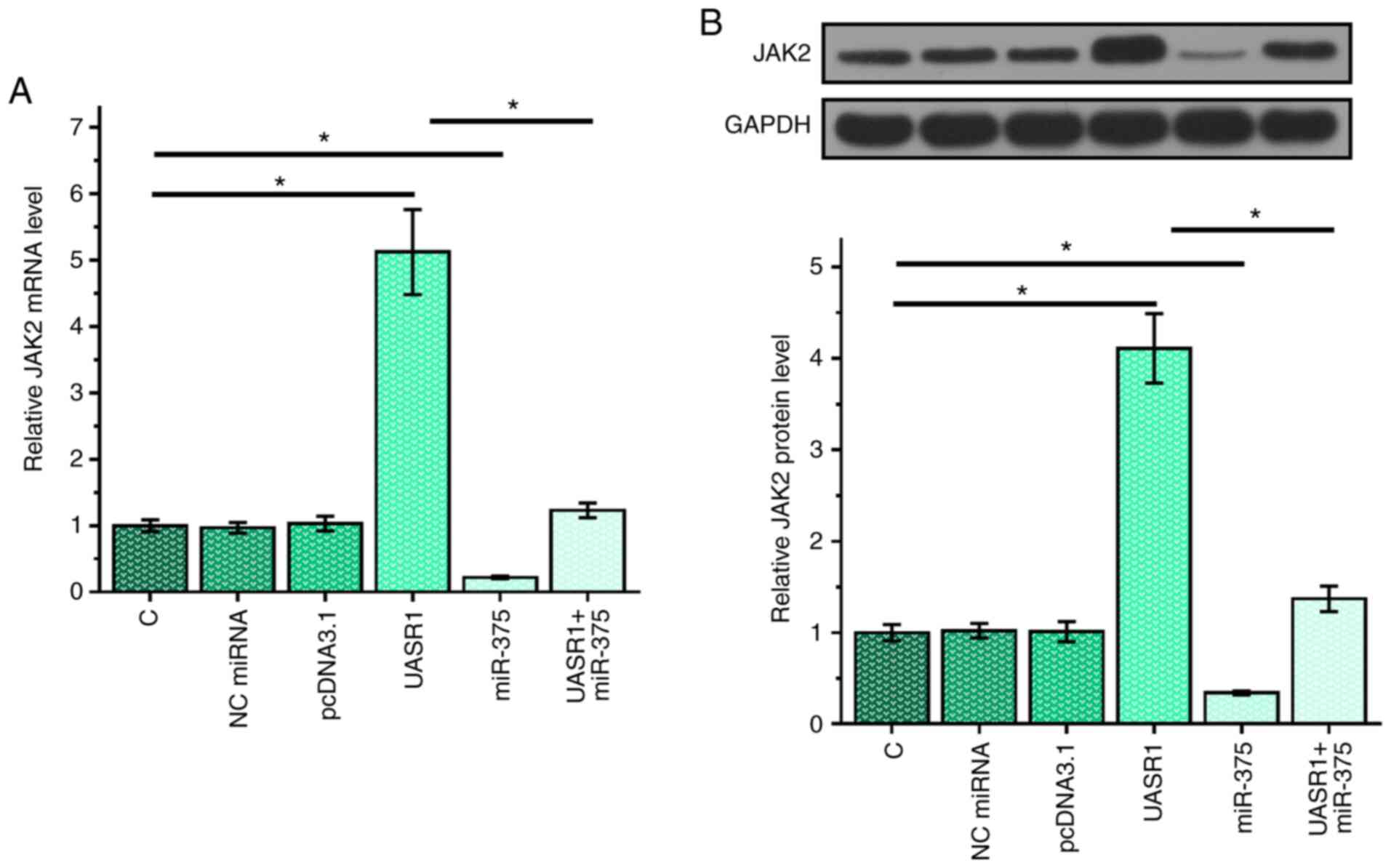
Overexpression of UASR1 in SCC25 cells
resulted in upregulation of JAK2 in SCC25 cells
To test the possibility that UASR1 can serve as the
endogenous control of miR-375, the effects of overexpressing UASR1
and miR-375 on the expression of JAK2, a target of miR-375, were
assessed by RT-qPCR (Fig. 3A) and
western blots (Fig. 3B). It showed
that overexpression of miR-375 led to downregulated expression of
JAK2, further confirming the targeting of JAK2 by miR-375
(P<0.05). Overexpression of UASR1 played an opposite role and
reduced the effects of overexpressing miR-375 (P<0.0.5). These
data supported the role of UASR1 as an internal control of
miR-375.
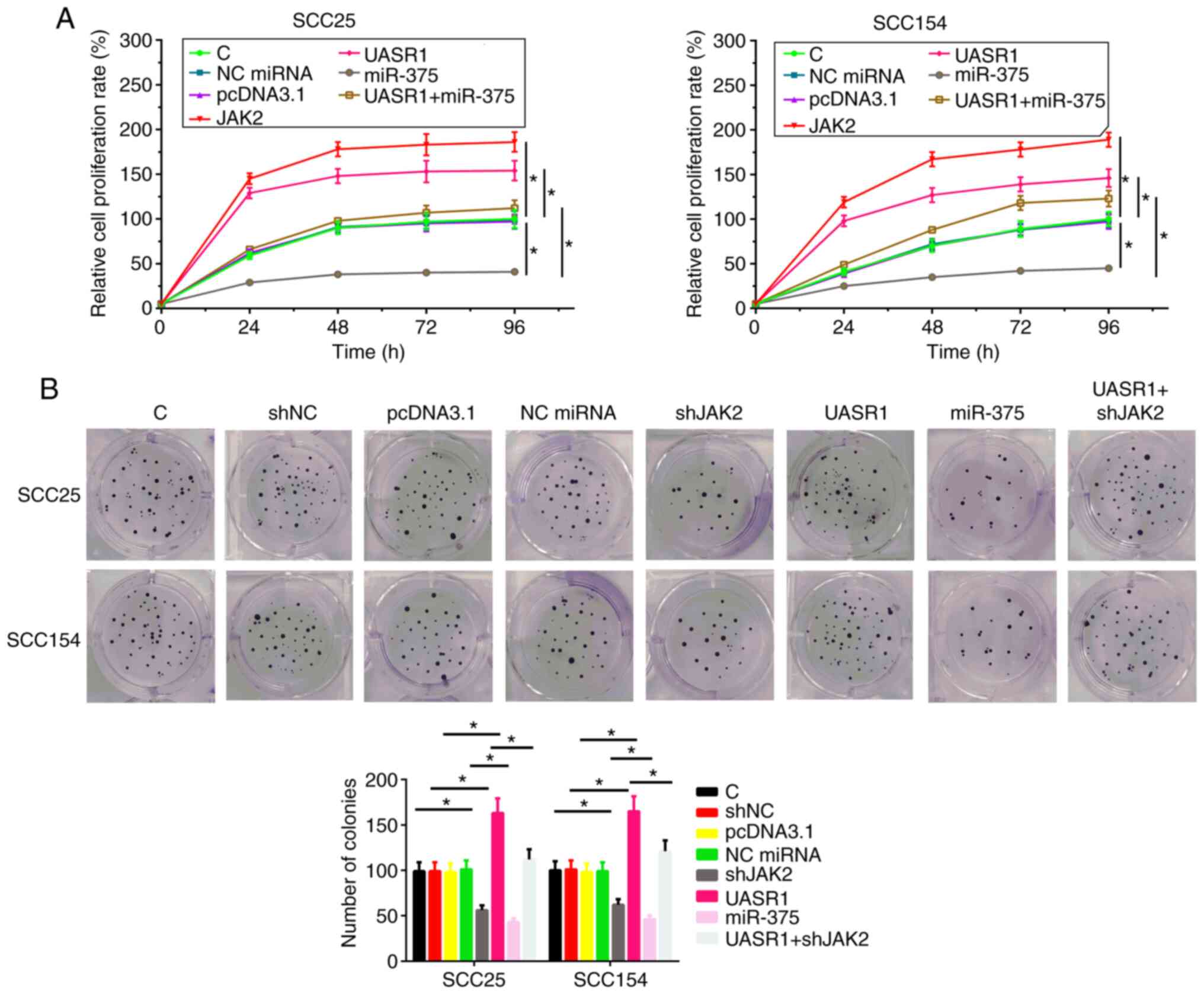
UASR1 promoted cell proliferation in
both SCC25 and SCC154 cell lines through miR-375/JAK2 axis
The effects of overexpressing UASR1, miR-375 and
JAK2 on the proliferation of SCC25 and SCC154 cells were assessed
by cell proliferation assay. Compared with C group, overexpression
of UASR1 and JAK2 led to increased cell proliferation rate
(Fig. 4A, P<0.05). In contrast,
miR-375 led to decreased cell proliferation rate (P<0.05).
Moreover, overexpression of UASR1 reduced the effects of
overexpressing miR-375 on cell proliferation (P<0.05). The
effects of overexpressing UASR1, miR-375 and JAK2 on the
proliferation of SCC25 and SCC154 cells were assessed by colony
formation. Compared with C group, overexpression of UASR1 led to
increased cell proliferation rate. In contrast, shJAK2 and miR-375
led to decreased cell proliferation rate (P<0.05). Moreover,
shJAK2 reduced the effects of overexpressing UASR1 on cell
proliferation (Fig. 4B).
Discussion
The aim of the present study was to investigate the
interactions of UASR1, miR-375 and JAK2 in OSCC. We found that
UASR1 was upregulated in OSCC and it may sponge miR-375 to
upregulate JAK2, thereby promoting cancer cell proliferation.
The functionality of UASR1 has only been
investigated in breast cancer (13).
In breast cancer, UASR1 is overexpressed and interacts with the
AKT/mTOR pathway to promote the migration and proliferation of
cancer cells (13). To the best of
our knowledge, this study is the first to report the upregulation
of UASR1 in OSCC. In addition, overexpression of UASR1 led to
increased proliferation rate of OSCC cells. Therefore, UASR1 may
play an oncogenic role in OSCC.
The current survival of OSCC patients with
appropriate treatment is still poor (14,16) and
the early diagnosis of OSCC is still limited by the lack of
sensitive early detection markers (13). As an alternative approach, accurate
prognosis of OSCC may improve the survival of patients by guiding
the determination of treatment approaches. In this study, we showed
that high expression levels of UASR1 in OSCC tissues predicted poor
survival of OSCC patients. However, the reliability of UASR1 as a
prognostic biomarker for OSCC needs to be further confirmed by
future studies with a larger sample size.
miR-375 plays tumor suppressive roles in several
types of cancer including oral cancer (17). In oral cancer, miR-375 targets
SLC7A11 to suppress cancer cell proliferation (18). However, the upstream regulator of
miR-375 in oral cancer remains unclear. Our study further confirmed
the suppressive effects of miR-375 on the proliferation of OSCC
cells. In addition, we showed that UASR1 could serve as an internal
sponge of miR-375 in OSCC cells to regulate cell behaviors. Another
study reported that miR-375 could target JARK2 to suppress breast
cancer cell proliferation (14). In
the present study, we showed that miR-375 could also target JAK2 in
OSCC to inhibit cell proliferation. Therefore, miR-375 may target
multiple oncogenes in oral cancer to suppress cell proliferation.
Additionally, inhibition of JAK2 significantly attenuated the
effects of overexpressing UASR1 on cell proliferation, meaning that
JAK2 was involved in the downstream of UASR1/miR-375 axis. However,
more downstream oncogenes may serve similar roles, and need to be
further investigated in future studies. Moreover, we did not find
that UASR1 plays roles on the migration, invasion and apoptosis of
OSCC cells, which may be further verified in other OSCC cell lines.
In vivo studies should also be performed in future to verify
the findings of the present study. In conclusion, UASR1 is
upregulated in OSCC and predicts poor survival. UASR1 may sponge
miR-375 to upregulate JAK2, thereby promoting cancer cell
proliferation.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YS designed the study. YS, LC and LP organized the
data, performed the data analyses and produced the initial draft of
the manuscript. YS, LC and LP performed the experiments and
generated the figures. All authors have read and approved the final
version.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Jinan Stomatological Hospital (Shandong, China). All
patients signed informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lydiatt WM, Patel SG, O'Sullivan B,
Brandwein MS, Ridge JA, Migliacci JC, Loomis AM and Shah JP: Head
and neck cancers-major changes in the American joint committee on
cancer eighth edition cancer staging manual. CA Cancer J Clin.
67:122–137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kumar M, Nanavati R, Modi TG and Dobariya
C: Oral cancer: Etiology and risk factors: A review. J Cancer Res
Ther. 12:458–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ghantous Y and Abu Elnaaj I: Global
incidence and risk factors of oral cancer. Harefuah. 156:645–649.
2017.(In Hebrew). PubMed/NCBI
|
|
5
|
Rivera C: Essentials of oral cancer. Int J
Clin Exp Pathol. 8:11884–11894. 2015.PubMed/NCBI
|
|
6
|
Ram H, Sarkar J, Kumar H, Konwar R, Bhatt
ML and Mohammad S: Oral cancer: Risk factors and molecular
pathogenesis. J Maxillofac Oral Surg. 10:132–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brocklehurst P, Kujan O, O'Malley LA,
Ogden G, Shepherd S and Glenny AM: Screening programmes for the
early detection and prevention of oral cancer. Cochrane Database
Syst Rev. CD0041502013.PubMed/NCBI
|
|
8
|
Mishra R: Glycogen synthase kinase 3 beta:
Can it be a target for oral cancer. Mol Cancer. 9:1442010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Molinolo AA, Marsh C, El Dinali M, Gangane
N, Jennison K, Hewitt S, Patel V, Seiwert TY and Gutkind JS: mTOR
as a molecular target in HPV-associated oral and cervical squamous
carcinomas. Clin Cancer Res. 18:2558–2568. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ohnishi Y, Yasui H, Nozaki M and Nakajima
M: Molecularly-targeted therapy for the oral cancer stem cells. Jpn
Dent Sci Rev. 54:88–103. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sanchez Calle A, Kawamura Y, Yamamoto Y,
Takeshita F and Ochiya T: Emerging roles of long non-coding RNA in
cancer. Cancer Sci. 109:2093–2100. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gutschner T and Diederichs S: The
hallmarks of cancer: A long non-coding RNA point of view. RNA Biol.
9:703–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao Z, Wu P, Su M, Ling H, Khoshaba R,
Huang C, Gao H, Zhao Y, Chen J, Liao Q, et al: Long non-coding RNA
UASR1 promotes proliferation and migration of breast cancer cells
through the AKT/mTOR pathway. J Cancer. 10:2025–2034. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ding L, Xu Y, Zhang W, Deng Y, Si M, Du Y,
Yao H, Liu X, Ke Y, Si J and Zhou T: MiR-375 frequently
downregulated in gastric cancer inhibits cell proliferation by
targeting JAK2. Cell Res. 20:784–793. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tirelli G, Gatto A, Boscolo Nata F,
Bussani R, Piccinato A, Marcuzzo AV and Tofanelli M: Prognosis of
oral cancer: A comparison of the staging systems given in the 7th
and 8th editions of the American Joint Committee on Cancer Staging
Manual. Br J Oral Maxillofac Surg. 56:8–13. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jairajpuri ZS, Rana S, Hajela A and Jetley
S: Toward early diagnosis of oral cancer: Diagnostic utility of
cytomorphological features, a pilot study. Natl J Maxillofac Surg.
10:20–26. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu Y, Sun X, Song B, Qiu X and Zhao J:
MiR-375/SLC7A11 axis regulates oral squamous cell carcinoma
proliferation and invasion. Cancer Med. 6:1686–1697. 2017.
View Article : Google Scholar : PubMed/NCBI
|