Introduction
Atherosclerosis is the leading cause of acute
myocardial infarction and stroke, resulting in large global health
and economic burdens (1). It has
been reported that atherosclerosis is a chronic vascular
wall-related inflammatory disease that occurs within the arterial
wall (2). Inflammatory factors are
mainly divided into three categories: i) Chemokines, including
monocyte chemoattractant protein-1 (MCP-1), fractalkine/CX3CR1 and
macrophage colony stimulating factor, whose effects are inhibited
by macrophage migration inhibition factor (MIF); ii)
pro-inflammatory factors, including C-reactive protein, IL-6, IL-1
and TNF-α; and iii) anti-inflammatory factors, including IL-10 and
TGF-β (3). Pharmacological studies
have reported that the imbalances between the pro-atherogenic
inflammatory response and atheroprotective anti-inflammatory
responses serve a key role in the initiation and progression of
atherosclerosis (4). Previous in
vivo and in vitro experimental data have revealed the
key signaling pathways, such as programmed death
ligand-1/programmed cell death protein 1 axis, brain-derived
neurotrophic factor/tyrosine kinase B signaling pathway (5), Nod-like receptor protein 3
inflammasome/IL-1/IL-18/IL-6 pathway (6) and Toll-like receptor pathways (7), that mediate the inflammatory response,
which may be research hotspots and provide potential preventive
targets for atherosclerosis. Therefore, the treatment of
atherosclerosis using anti-inflammatory drugs may be an attractive
strategy (8–10).
It has been reported that the Ras homolog gene
family, member A (RhoA)/Rho kinases (ROCK) pathway is an important
signal transduction system involved in cell proliferation,
endothelial dysfunction, oxidative stress, inflammation, vascular
remodeling and atherosclerosis (11–13).
Previous studies have suggested that various multiple risk factors
and pathological mediators of atherosclerosis can activate the
RhoA/ROCK pathway to different degrees (14–16), and
the inhibition of the RhoA/ROCK pathway could be a therapeutic
potential target in the treatment of atherosclerosis (16). Furthermore, the regulatory effect of
the RhoA/ROCK pathway on the inflammatory response of the
atherosclerotic process has been confirmed by previous finding
(17,18). The study from Shimada and Rajagopalan
(19) revealed that ROCK mediates
lysophosphatidic acid (an inflammatory mediator that is elevated in
multiple inflammatory diseases)-induced IL-8 and MCP-1 production
in human endothelial cells. The ROCK pathway also contributes to
hyperglycemia-activated macrophages, which results in a
pro-inflammatory phenotype and eventually contributes to
atherosclerosis (17). However, the
underlying potential RhoA/ROCK-regulated signaling pathways in
inflammatory response under atherosclerosis remain to be
elucidated.
Urotensin II (UII), a vasoactive cyclic peptide, and
its high-affinity G-protein-coupled receptor UT are both highly
expressed in the human cardiovascular system, and UII is involved
in the development of cardiovascular homeostasis disease (20,21).
Clinical and experimental studies have identified a positive
correlation between increased UII levels and the development of
atherosclerosis (22–24). UII can enhance the development of
aortic atherosclerotic lesions and destabilizes atherosclerotic
plaques (25). UII also exerts a
pro-inflammatory effect on vascular wall cells in atherosclerosis
(24,26). Previous studies have shown that the
RhoA/ROCK pathway mediates UII-induced migration of endothelial
progenitor cells and the formation of macrophage derived foam
cells, suggesting that the RhoA/ROCK pathway may contribute to the
UII-induced inflammatory response (27). However, to the best of our knowledge,
there are no previous reports on the roles of the RhoA/ROCK pathway
in UII-derived inflammatory effects.
Salidroside and isorhamnetin are two early used
antioxidant Tibetan drugs, possessing a variety of biological
activities such as anti-apoptosis, anti-oxidative stress and
anti-inflammation effects (28,29). It
has also been reported that salidroside and isorhamnetin exert a
cardioprotective effect on the development of atherosclerosis,
which is partly dependent on their anti-inflammatory ability
(26,30,31).
However, the anti-inflammatory or protective properties of
salidroside and isorhamnetin against UII-derived inflammatory
effects in atherosclerosis, as well as the underlying molecular
mechanisms, are yet not fully understood.
Therefore, the present study aimed to investigate
the role of the RhoA/ROCK II pathway in the UII-induced
inflammatory response, as well as to identify the effects of
salidroside and isorhamnetin treatment on the UII-induced
inflammatory response and their potential mechanism in vivo
and in vitro.
Materials and methods
Chemicals and reagents
UII (cat. no. U4753), salidroside (cat. no.
05410590) and isorhamnetin (cat. no. 17794) were purchased from
Sigma-Aldrich (Merck KGaA). DMEM (cat. no. 11965092) and FBS (cat.
no. 16140071) were purchased from Gibco (Thermo Fisher Scientific,
Inc.). Anti-RhoA (cat. no. 2117s), anti-ROCK II (cat. no. 9029s)
and anti-GAPDH (cat. no. 5174) antibodies were purchased from Cell
Signaling Technology, Inc.
Rat modeling and animal treatment
All the experimental procedures were performed in
accordance with the National Institutes of Health Guide for the
Care and Use of Laboratory Animals (32), and were approved by the Experimental
Animal Administration Committee of the School of Basic Medical
Sciences, Northwest Minzu University Health Science Center
(approval no. XBMZ-YX20130101; March 1, 2013). In total, 120
healthy male Wistar rats (weight, 180–200 g; age, 8 weeks) were
provided by Jiangning Qinglongshan Animal Cultivation Farm and were
housed under laboratory conditions (temperature 22±2°C with a
relative humidity of 40–50% and natural light-dark cycle time of
12/12 h) with free access to food and water.
For the preparation of the rat model, rats were
anaesthetized with pentobarbital sodium [60 mg/kg; intraperitoneal
(i.p.)] and osmotic mini-pumps (Alzet Model 2006D; Durect
Corporation) were loaded with either UII or saline alone (vehicle).
The rats were randomly divided into eight groups: i) Normal control
(saline alone, equal amount as the UII group, n=15); ii) UII group
[rats were subcutaneously injected with UII (10 ng/kg/min) for 7
consecutive days, n=15]; ii) Salidroside (12 mg/kg) + UII group,
(n=15); iv) Salidroside (24 mg/kg) + UII group, (n=15); v)
Isorhamnetin (12 mg/kg) + UII group, (n=15); vi) Isorhamnetin (24
mg/kg) + UII group, (n=15); vii) Salidroside + isorhamnetin (Both,
12 mg/kg) + UII group, (n=15); and viii) Salidroside + isorhamnetin
(Both, 24 mg/kg) + UII group, (n=15). The doses of salidroside and
isorhamnetin in vivo and in vitro were chosen in
accordance with the previous literatures (33–36).
Isolation and identification of
primary vascular smooth muscle cells (VSMCs)
A total of 8 adult male Wistar rats (age, 10 weeks;
weight, 250–350 g) housed under the same aforementioned laboratory
conditions were heparinized (4 IU/g; i.p.) and then euthanatized
via pentobarbital sodium (100 mg/kg; i.p.) administration. The
procedure for VSMCs isolation was performed in accordance with a
previously described protocol (31).
The aorta was immediately collected, placed into 75% (v/v) alcohol,
dissected into sections (length, 3 cm) and subsequently placed in
PBS. After removing fibroblasts that were present in the tunica
externa (the external third of the vessel wall thickness) using
forceps, the residual vessels were longitudinally cut and the
tunica interna was scraped off, leaving the tunica media. Then, the
tunica media was washed with DMEM, cut into sections (1
mm3) and maintained in DMEM containing 10% (v/v) FBS at
37°C in a Heraeus 5% CO2 incubator (Thermo Fisher
Scientific, Inc.). The morphology of cultured VSMCs was observed by
phase contrast microscopy at passages 3–8 at room temperature. The
identification of VSMCs was performed using a
Histostain-streptavidin-peroxidase kit (rabbit; cat. no. SP-0023;
Beijing Biosynthesis Biotechnology Co., Ltd.) according to the
manufacturer's protocol. Briefly, the third generation of VSMCs was
inoculated into a 100-mm culture. When the cells grew to near
fusion state, the cover glass was removed and the cells were fixed
with 4% freshly prepared cooled neutral paraformaldehyde buffer at
room temperature for 15 min and washed with PBS. Subsequently,
after blocking non-specific binding sites with 5% BSA Blocking
Reagent included in the aforementioned immunohistochemical kit at
37°C for 30 min, rabbit anti-a smooth muscle actin polyclonal
antibodies (cat. no. bs-0189R; Beijing Biosynthesis Biotechnology
Co., Ltd.) were incubated with the cells overnight at 4°C. Next,
the slides were incubated with secondary antibodies for 1 h at room
temperature and subsequently stained with a-smooth muscle actin
antibody included in the immunohistochemical kit for 10 min at room
temperature. The cells were visualized using a laser confocal
microscope (Leica Microsystems GmbH; magnification, ×400).
Cell culture and treatment
VSMCs were cultured in DMEM supplemented with 10%
FBS in a humidified atmosphere with 5% CO2 at 37°C. To
investigate the effects of UII on VSMCs, cells were treated with
different concentration of UII (10−9, 10−8,
10−7 and 10−6 mol/l) for 24 hat 37°C. To
demonstrate the impacts of salidroside and isorhamnetin on VSMCs
exposed to UII, cells were pretreated with salidroside (1, 3 or 10
µM), isorhamnetin (1, 3 or 10 µM) or both salidroside (3 or 10 µM)
and isorhamnetin (3 or 10 µM) for 1 h, followed by treatment with
UII (10−6 mol/l) for 24 hat 37°C.
Measurement of inflammatory markers in
the culture supernatant using ELISA
VSMCs were seeded into a 6-well plate at a density
of 1×106 cells/ml. After incubation for 24 h as
aforementioned, the culture supernatants were collected,
centrifuged at 1,000 × g for 5 min at room temperature, and used to
assess the levels of TNF-α (Nanjing Jiancheng Bioengineering
Research Institute; cat. no. H052), IL-1β (Nanjing Jiancheng
Bioengineering Research Institute; cat. no. H002), IL-10 (Nanjing
Jiancheng Bioengineering Research Institute; cat. no. H009) and MIF
(BioLegend® Legend Max TM Human Active MIF; cat. no.
438408; BioLegend, Inc.) with ELISA kits, according to
manufacturer's instructions. The supernatants (500 µM) were seeded
into enzyme labelling 96-well plate and anti-TNF-α, IL-1β, IL-10
and MIF antibodies (200 µM) included in the kits were added,
respectively. Following incubation for 2 h at room temperature,
horseradish peroxidase (HPR)-labelled secondary antibody included
in the kit (200 µM) was added to for 1 h at room temperature. The
absorbance value at 450 nm was determined using a Multiskan
Microplate reader (Thermo Fisher Scientific, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR) assay
The total RNA in each group was extracted with
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.; cat. no. 15596018), and reverse-transcribed into cDNA using a
PrimeScript RT Reagent kit (Takara Bio, Inc.; cat. no. RR037B). The
RT conditions were 10 min at 25°C, 45 min at 48°C and a final step
for 6 min at 95°C. RT-qPCR was performed using Platinum SYBR Green
qPCR SuperMix (Invitrogen; Thermo Fisher Scientific, Inc.; cat. no.
11744100) on an ABI Prism 7500 system (MP Biomedicals) according to
the manufacturer's instructions. The amplification conditions were
as follows: Initial denaturation at 95°C for 1 min, followed by 40
cycles of 95°C for 5 sec, 60°C for 15 sec and 72°C for 30 sec.
GAPDH was used as the endogenous control. The mRNA expression
levels of RhoA and ROCK II were normalized to GAPDH using the
2−ΔΔCq equation (37).
The primer sequences used were as follows: RhoA forward,
5′-TCGGAATGATGAGCACACAA-3′ and reverse, 5′-GCTTCACAAGATGAGGCAC-3′;
ROCK II forward, 5′-CAGCAACTTTGACGACATTGAG-3′ and reverse,
5′-AGATTTGCACTTCTGTTCCAGC-3′; and GAPDH forward,
5′-ACGGCAAGTTCAACGGCACAG-3′ and reverse,
5′-GACGCCAGTAGACTCCACGACA-3′.
Western blot analysis
After the indicated treatments, VSMCs were
harvested, lysed in RIPA buffer (Beyotime Institute of
Biotechnology; cat. no. P0013K) containing 1% (V/V) PMSF (Beyotime
Institute of Biotechnology; cat. no. ST506) on ice for 30 min, and
then centrifuged at 13,000 × g for 10 min at 4°C. The protein
concentration was confirmed using a BCA Protein Assay kit (Beyotime
Institute of Biotechnology; cat. no. P0012). Equal amounts of
protein (30 µg/lane) were separated on 12% SDS-PAGE, transferred
onto PVDF membranes (EMD Millipore; cat. no. IPVH00010) and blocked
with blocking buffer [0.1% Tween-20 in TBS (TBS-T) supplemented
with 5% fat-free milk] for 2 h at room temperature. After washing
with TBS-T buffer, the membranes were incubated with anti-RhoA,
anti-ROCK II and anti-GAPDH antibodies (1:2,000) overnight at 4°C.
GAPDH was used as a loading control. Then, the membranes were
incubated with HRP-conjugated secondary antibody (1:5,000; cat. no.
7077; Cell Signaling Technology, Inc.) for 2 h at room temperature.
The immunoreactive bands were visualized using a chemiluminescence
imaging analysis system (cat. no. 32106; Pierce; Thermo Fisher
Scientific, Inc.). The densities of protein expression were
semi-quantified using Bio-Rad ChemiDoc XRS (version 4.3.0;Bio-Rad
Laboratories, Inc.).
Statistical analysis
Data are presented as the mean ± SD from ≥3
different experiments. Data were analyzed using SPSS.18 software
(SPSS, Inc.). Comparison among multiple relevant groups was
performed using a one-way ANOVA followed by Bonferroni's multiple
comparison test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Salidroside, isorhamnetin and both in
combination decrease the levels of serum inflammatory cytokines
after subacute infusion of UII in rats
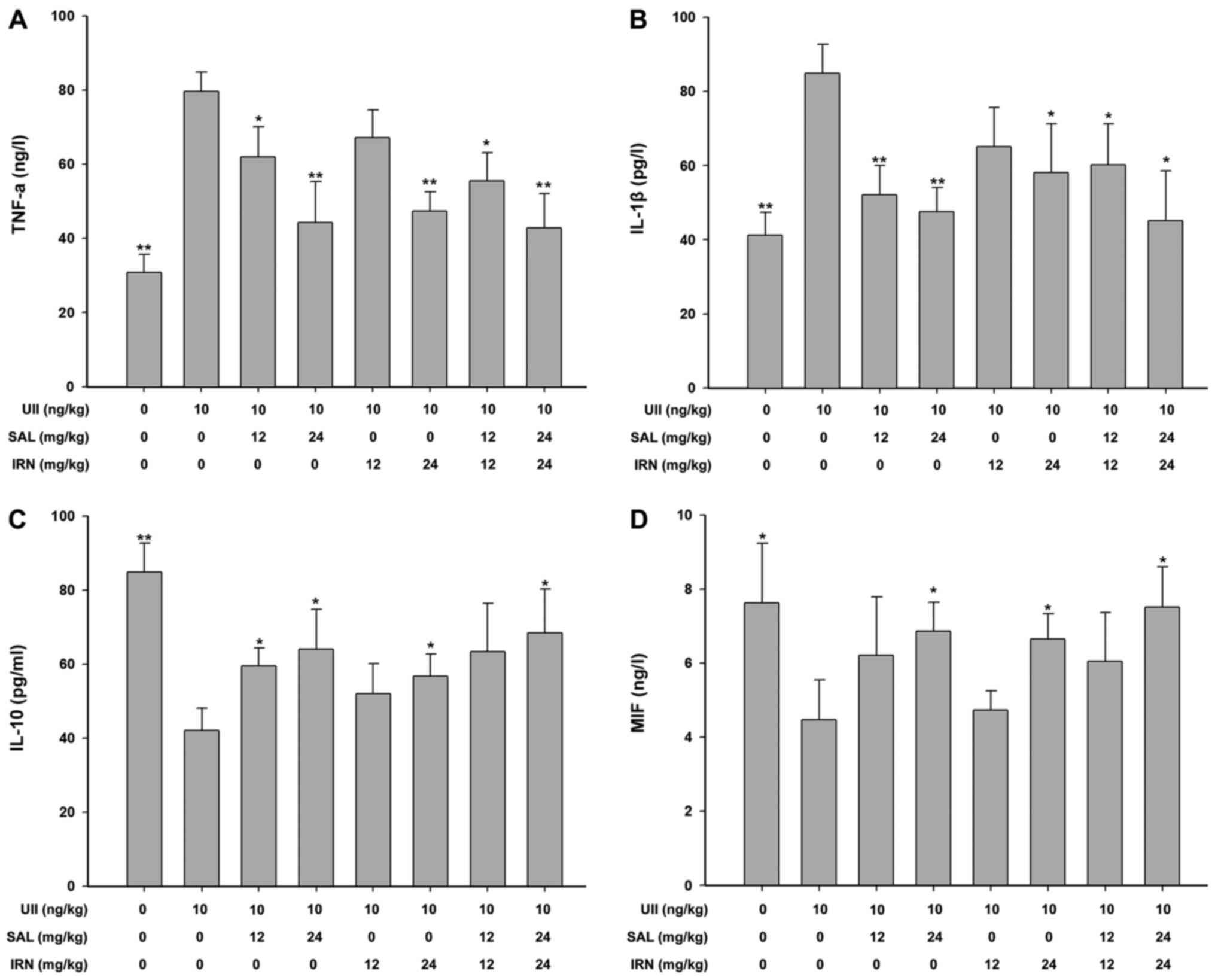
The results of ELISA suggested that compared with
the control group, after subacute infusion of UII (10 ng/kg/min)
for 7 days, the levels of pro-inflammatory cytokines TNF-α
(Fig. 1A) and IL-1β (Fig. 1B) were significantly upregulated,
while the levels of anti-inflammatory cytokine IL-10 (Fig. 1C) and MIF (Fig. 1D) were significantly downregulated in
rat serum. However, compared with UII group, salidroside (12 and 24
mg/kg), isorhamnetin (24 mg/kg) and the combination of salidroside
(12 or 24 mg/kg) and isorhamnetin (12 or 24 mg/kg) significantly
attenuated the levels of TNF-α (Fig.
1A) and IL-1β (Fig. 1B).
Moreover, salidroside (12 and 24 mg/kg), isorhamnetin (24 mg/kg)
and the combination of salidroside (24 mg/kg) and isorhamnetin (24
mg/kg) significantly increased the level of IL-10 compared with UII
group (Fig. 1C). It was also
identified that salidroside (24 mg/kg), isorhamnetin (24 mg/kg) and
the combination of salidroside (24 mg/kg) and isorhamnetin (24
mg/kg) significantly promoted the level of MIF compared with UII
group (Fig. 1D) in the serum of
rats. The combination of salidroside (24 mg/kg) and isorhamnetin
(24 mg/kg) did not exert a higher effect compared with salidroside
or isorhamnetin alone (one-way ANOVA followed by Bonferroni's
multiple comparison test). These results indicated that
salidroside, isorhamnetin and both in combination protected rats
against the UII-induced inflammatory response.
Salidroside, isorhamnetin and both in
combination inhibit the RhoA/ROCK II pathway in the thoracic aorta
of rats following subacute infusion of UII
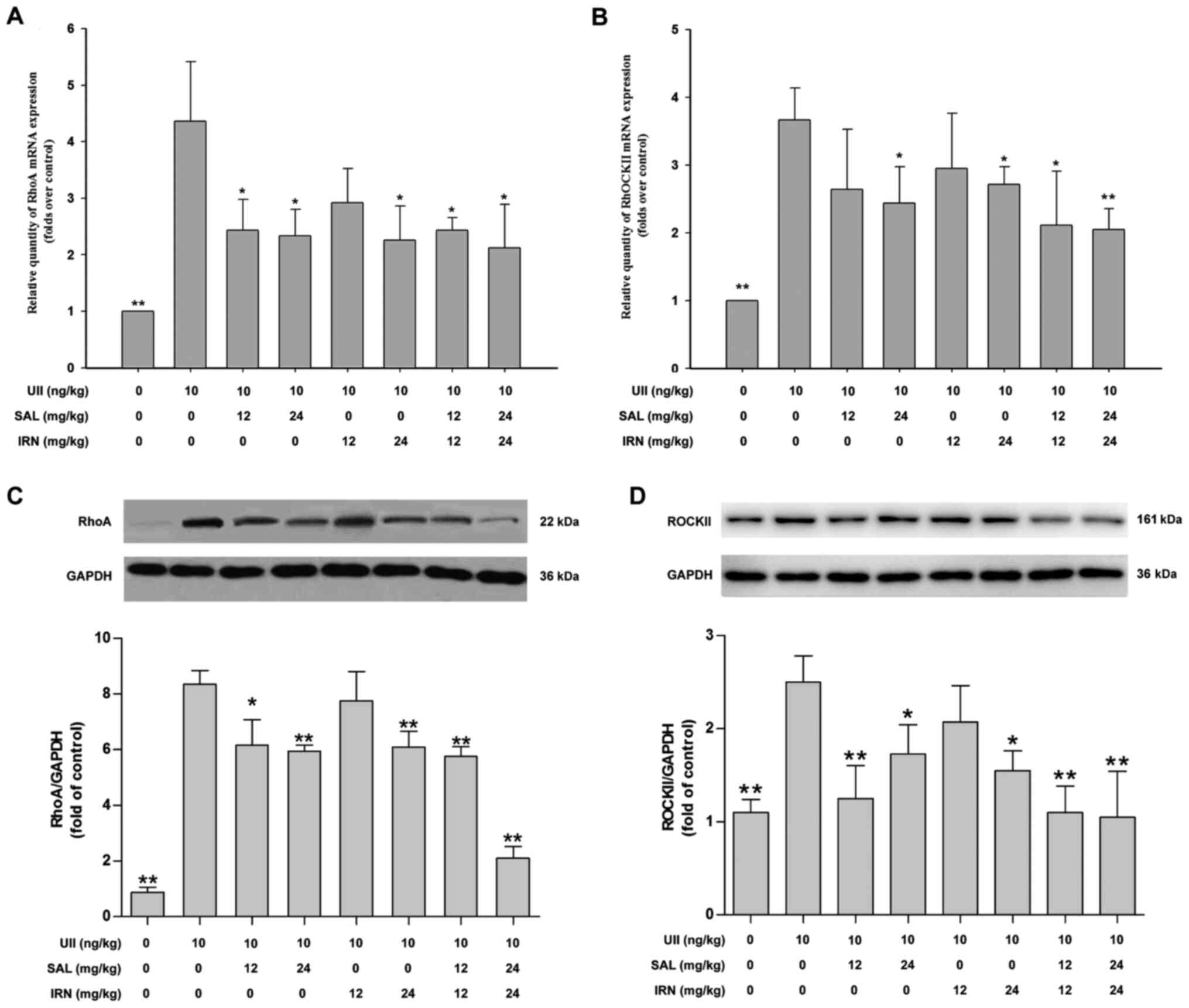
Previous studies have reported that the RhoA/ROCK
pathway can be activated by various atherosclerosis-related risk
factors to different degrees, and may participate in the
pathogenesis of atherosclerosis (11,38).
Thus, the present study investigated the effects of salidroside and
isorhamnetin on the RhoA/ROCK II pathway in UII-treated rats. The
results demonstrated that subacute infusion of UII significantly
increased the mRNA expression levels of RhoA (Fig. 2A) and ROCK II (Fig. 2B) in thoracic aorta compared with
control group. Furthermore, administration of salidroside (12 and
24 mg/kg), isorhamnetin (24 mg/kg) and the combination of
salidroside (12 or 24 mg/kg) and isorhamnetin (12 or 24 mg/kg)
significantly reduced the mRNA expression level of RhoA (Fig. 2A), while administration of
salidroside (24 mg/kg), isorhamnetin (24 mg/kg) and the combination
of salidroside (12 or 24 mg/kg) and isorhamnetin (12 or 24 mg/kg)
significantly decreased the mRNA expression level of ROCK II
(Fig. 2B), compared with the UII
group.
Western blot analysis results further indicated that
UII significantly increased the protein expression levels of RhoA
(Fig. 2C) and ROCK II (Fig. 2D) in thoracic aorta of rats, compared
with the control group. However, these impacts were mitigated by
salidroside (12 or 24 mg/kg), isorhamnetin (24 mg/kg), both
salidroside (12 mg/kg) and isorhamnetin (12 mg/kg), and both
salidroside (24 mg/kg) and isorhamnetin (24 mg/kg) in combination.
When the drug concentration was low, the combination of the two
drugs could inhibit the RhoA/ROCK II pathway under UII-induced
conditions. Moreover, the inhibitory effects of the combination of
salidroside and isorhamnetin on the RhoA/ROCK II pathway were not
more effective compared with salidroside or isorhamnetin alone
(one-way ANOVA followed by Bonferroni's multiple comparison test).
These results suggested that salidroside, isorhamnetin and both in
combination inhibited the RhoA/ROCK II pathway, which may lead to
the inhibition of UII-induced inflammation.
UII promotes the inflammatory response
and the RhoA/ROCK II pathway in primary VSMCs
VSMCs were isolated from male Wistar rats. Under a
phase contrast microscope, a small number of cells emerged from the
surrounding of the tissue block (rat thoracic aorta) after 4–5 days
of culture (Fig. 3A-1). After 10–12
days of culture, the cells in the local bundles were arranged in
parallel, and some cells overlapped in multiple layers,
demonstrating typical ups and downs of ‘peaks’ and ‘valleys’
(Fig. 3A-2). In the third generation
of VSMCs, actin immunocytochemical staining identified that >99%
of the cells were positive; the cytoplasm was brownish yellow and
the nucleus was not stained (Fig.
3A-3). These results indicated that VSMCs were successfully
extracted.
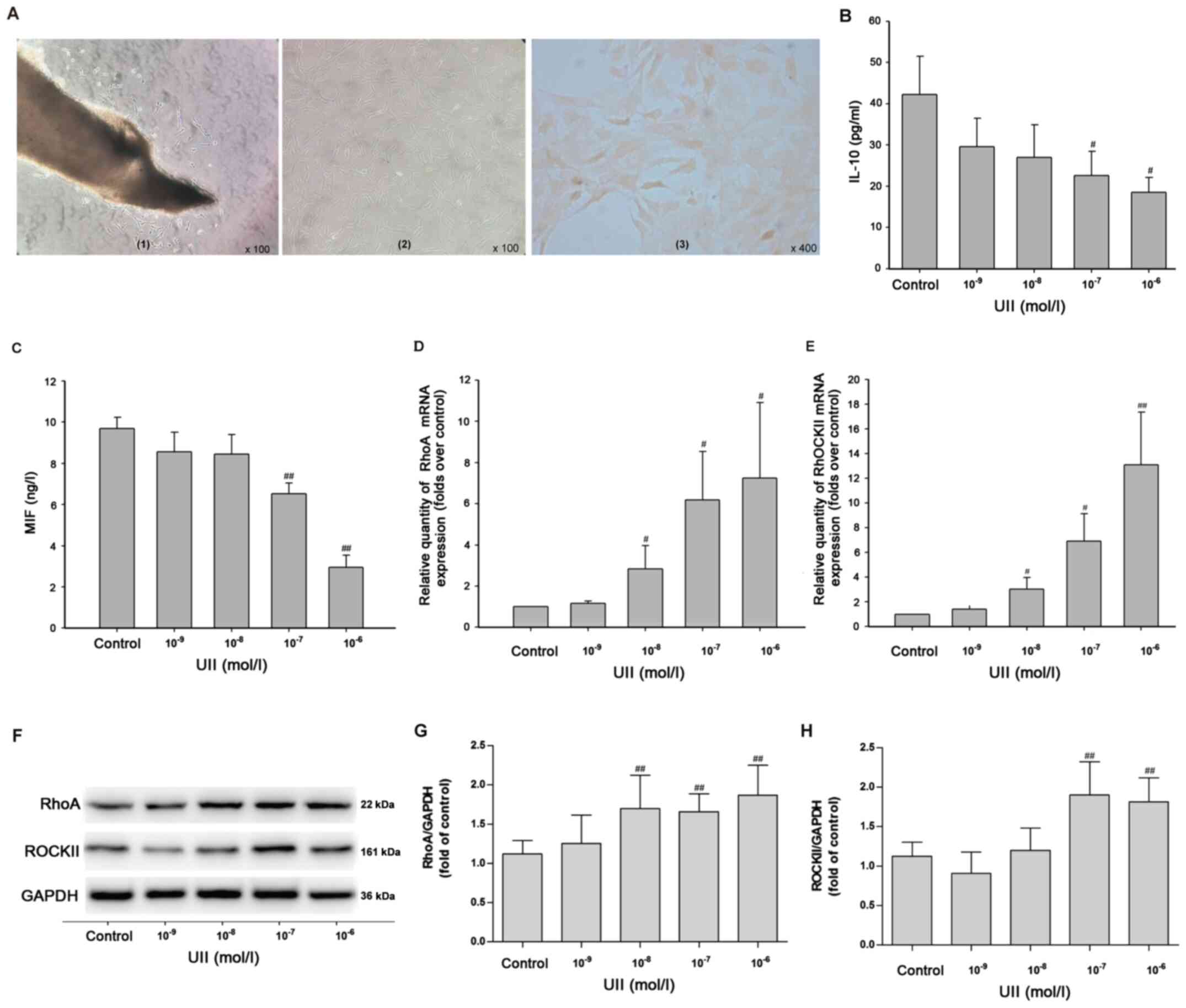 | Figure 3.Effects of UII on the inflammatory
response and the RhoA/ROCK II pathway in primary vascular smooth
muscle cells. (A) Observation of cell morphology. (A-1) After 4–5
days of culture, cell morphology was observed under the phase
contrast microscope (magnification, ×100). (A-2) After 10–12 days
of culture, cell morphology was observed under an invert microscope
(magnification, ×100). (A-3) α-actin immunocytochemical staining
(magnification, ×400). Inflammatory markers (B) IL-10 and (C) MIF
in the culture supernatant were measured via ELISA. mRNA expression
levels of (D) RhoA and (E) ROCK II were detected using reverse
transcription-quantitative PCR. Protein expression levels of (G)
RhoA and (H) ROCK II were detected using (F) western blot analysis.
Data are from ≥3 independent experiments and presented as the mean
± SD. #P<0.05, ##P<0.01 vs. control.
RhoA, Ras homolog gene family, member A; ROCK, Rho kinases; UII,
Urotensin II; MIF, macrophage migration inhibitory factor; SAL,
salidroside; IRN, isorhamnetin. |
Subsequently, the effects of UII on the inflammatory
response in VSMCs were detected via ELISA. With the increased
concentration of UII (10−9, 10−8,
10−7 and 10−6 mol/l) stimulation for 24 h,
the levels of the anti-inflammatory cytokines IL-10 (Fig. 3B) and MIF (Fig. 3C) were significantly decreased. In
addition, RT-qPCR results demonstrated that UII (10−8,
10−7 and 10−6 mol/l) treatment for 24 h
induced significant increases in the mRNA expression levels of RhoA
(Fig. 3D) and ROCK II (Fig. 3E) in VSMCs. Western blot analysis
(Fig. 3F) results identified that
UII (10−8, 10−7 and 10−6 mol/l)
stimulation for 24 h significantly increased the expression of RhoA
(Fig. 3G), and UII (10−7
and 10−6 mol/l) significantly increased the expression
of ROCK II (Fig. 3H) in VSMCs. As
UII (10−6 mol/l) treatment for 24 h significantly
upregulated the protein expression levels of RhoA and ROCK II in
VSMCs, this treatment regimen was selected for subsequent
experiments. Taken together, it was suggested that UII stimulation
promoted the inflammatory response by enhancing the RhoA/ROCK II
pathway in primary VSMCs.
Salidroside, isorhamnetin and both in
combination attenuate the UII-induced inflammatory response and
inhibit the RhoA/ROCK II pathway in VSMCs
Next, in order to demonstrate the inhibitory effects
of salidroside and isorhamnetin on inflammatory responses in VSMCs
stimulated with UII, VSMCs were pretreated with salidroside,
isorhamnetin or both in combination for 1 h followed by treatment
with UII (10−6 mol/l) for 24 h. Compared with the
control group. UII treatment significantly increased the TNF-α
(Fig. 4A) and IL-1β (Fig. 4B) levels, and decreased the IL-10
level (Fig. 4C). Moreover, compared
with the UII treatment group, pretreatment with salidroside (3 and
10 µM), isorhamnetin (3 and 10 µM) and both in combination reduced
TNF-α level (Fig. 4A), while
pretreatment with salidroside (10 µM), isorhamnetin (10 µM) and
both in combination reduced IL-1β level (Fig. 4B) in UII-treated VSMCs. It was also
found that pretreatment with salidroside (3 and 10 µM),
isorhamnetin (1, 3 and 10 µM) and both in combination increased the
IL-10 level (Fig. 4C) in UII-treated
VSMCs. The low concentration of salidroside (1 µM) had no effect on
the level of inflammatory-related factors in UII-treated VSMCs.
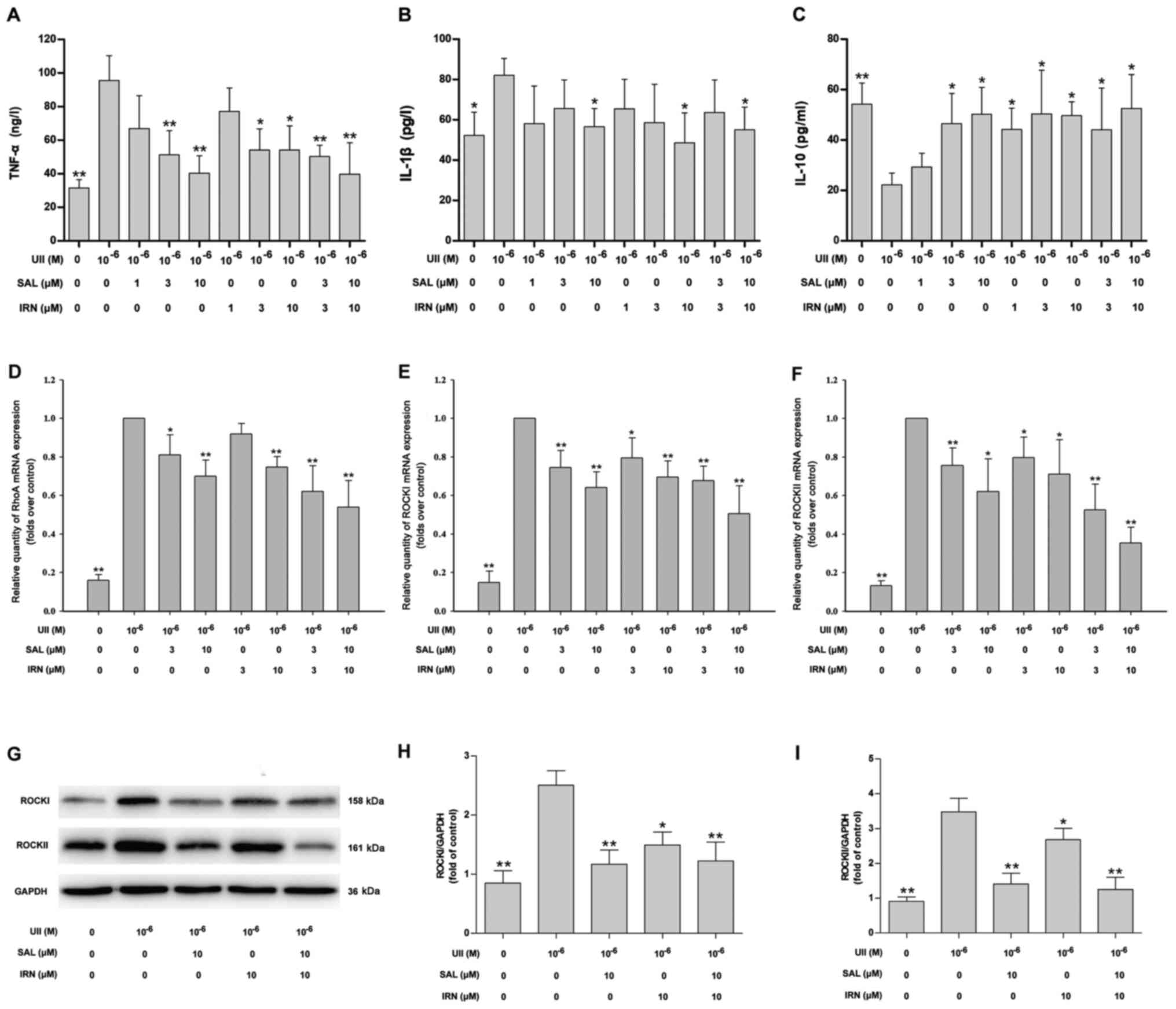 | Figure 4.Effects of SAL and IRN on
inflammatory response and the RhoA/ROCKII pathway in VSMCs
stimulated with UII. (A) VSMCs were pretreated with SAL or IRN (1,
3 and 10 µM) alone or SAL and IRN (3 and 10 µM) in combination for
1 h followed by treatment with UII (10−6 mol/l) for 24
h. Quantitative analysis of (A) TNF-α, (B) IL-1β and (C) IL-10
levels via ELISA. mRNA expression levels of (D) RhoA, (E) ROCK I
and (F) ROCK II were detected via reverse
transcription-quantitative PCR. (G) Western blot analysis of the
protein expression levels of (H) ROCK I and (I) ROCK II were
measured using. Data are from ≥3 independent experiments and
presented as the mean ± SD. *P<0.05, **P<0.01 vs. UII group.
MIF, macrophage migration inhibitory factor; UII, Urotensin II;
RhoA, Ras homolog gene family, member A; ROCK, Rho kinases; SAL,
salidroside; IRN, isorhamnetin; VSMCs, vascular smooth muscle
cells. |
The results suggested that pretreatment with
salidroside (3 and 10 µM), isorhamnetin (10 µM) and a combination
of salidroside and isorhamnetin attenuated the UII-induced
increases in the mRNA expression level of RhoA (Fig. 4D). In addition, pretreatment with
salidroside (3 and 10 µM), isorhamnetin (3 and 10 µM) and both in
combination attenuated the UII-induced the increases in the mRNA
expression levels of ROCK I (Fig.
4E) and ROCK II (Fig. 4F).
Western blotting results (Fig. 4G)
also identified that salidroside (10 µM), isorhamnetin (10 µM) and
both in combination reversed UII-induced upregulation of ROCK I
(Fig. 4H) and ROCK II (Fig. 4I) protein expression levels in VSMCs.
There were no statistically significant differences between the
combination of drugs and with salidroside or isorhamnetin alone
(one-way ANOVA followed by Bonferroni's multiple comparison test).
Collectively, it was indicated that salidroside, isorhamnetin and
both in combination mitigated the UII-induced inflammatory response
in VSMCs partly by inhibiting the RhoA/ROCK pathway.
Discussion
The present findings indicated that UII stimulation
resulted in an inflammatory response along with an increase in the
RhoA/ROCK pathway activation in vivo and in vitro. It
was also demonstrated that salidroside, isorhamnetin and both in
combination could elicit inhibitory effects on the UII-induced
inflammatory response, at least partially by attenuating the
RhoA/ROCK pathway.
Previous studies have reported that UII exerts
vasculopathic and vasculoprotective effects, and contributes to the
pathogenesis of atherosclerosis (26,39,40). UII
promotes VSMCs proliferation by activating reactive oxygen species
(ROS) and MAPK signaling pathways, which results in vascular
remodeling (41). UII also
participates in macrophage activation via UII receptor/ROS/Akt
pathways in RAW264.7 macrophages (26). Furthermore, UII can promote NAD(P)H
oxidase-induced reactive oxygen production from a variety of
inflammatory cells, resulting in activated NF-κB (42,43),
which is the most important downstream event involved in the signal
transduction pathway of various inflammatory factors in the process
of vascular injury and atherosclerosis (44–46).
These results provide evidence for the inflammatory effect of UII
in cardiovascular disease. Notably, to the best of our knowledge,
the present results were the first to demonstrate that UII
stimulation results in an inflammatory response, as shown by the
increases in the levels of pro-inflammatory cytokines (TNF-α and
IL-1β) and the decreases in the levels of anti-inflammatory
cytokines (IL-10 and MIF) in the serum of rats and the culture
supernatant of VSMCs. Therefore, it was indicated that inflammatory
factors induced by UII led to an inflammatory response that is
involved in the pathological process of atherosclerosis.
RhoA and its downstream effector ROCK, which exists
in two isoforms, ROCK1 and ROCK2, serve significant roles in
multiple cellular processes, such as proliferation, apoptosis and
migration (47). Abnormal activation
of the RhoA/ROCK pathway has been reported to be involved in
various types of diseases including diabetes, osteoarthritis,
cardiovascular and cerebrovascular diseases (11–13). In
recent years, the regulatory effect of the RhoA/ROCK pathway on the
inflammatory process of atherosclerosis has been revealed. For
instance, upregulation of the RhoA/ROCK signaling cascade has been
identified in atherosclerosis (11).
Moreover, RhoA-mediated NF-κB signaling pathways lead to vascular
endothelial dysfunction in diabetes (48). ROCK pathways also contribute to
hyperglycemia-activated macrophages, which result in a more
pro-inflammatory phenotype and eventually lead to atherosclerosis
(17). Previous studies have shown
that ROCK I is predominantly increased in the process of macrophage
adherence, and ROCK1-deficiency decreases atherosclerosis in bone
marrow-derived cells (49),
indicating that ROCK I serves an important role in the development
of atherosclerosis. However, whether ROCK II inhibition could also
be beneficial in attenuating atherosclerosis remain to be
investigated. In line with these previous results, the present
findings suggested that UII stimulation promoted the RhoA/ROCK
pathway in rats and VSMCs, implying the involvement of the
RhoA/ROCK II pathway in UII-induced inflammatory response.
It has been revealed that salidroside, an early
antioxidant Tibetan medicine, has anti-inflammatory effects in
atherosclerosis (50,51). Li et al (50) reported that salidroside can decrease
the generation of inflammatory cytokines, such as IL-6, IL-1β and
MCP-1, in TNF-α-induced cardiac microvascular endothelial cells,
alleviating vascular inflammation and atherosclerosis. Another
previous study revealed that salidroside attenuated endothelial
cellular senescence via reducing the expression of inflammatory
cytokines, thus mitigating the pathogenesis of atherosclerosis
(51). In addition, isorhamnetin, a
flavonoid monomer extracted from seabuckthorn fruit, has been shown
to possess anti-cancer, anti-oxidant, anti-inflammatory and
anti-atherosclerotic activities (34,52,53).
However, there are few studies on the anti-inflammatory and
anti-atherosclerotic effects of salidroside, isorhamnetin and both
in combination.
To the best of our knowledge, the present study was
the first to demonstrate that salidroside, isorhamnetin and both in
combination decreased TNF-α and IL-1β levels, and increased IL-10
level in the serum of UII-treated rats and in the culture
supernatant of UII-stimulated VSMCs, at concentrations
corresponding to human therapeutic blood plasma concentrations,
thus eliminating the UII-induced inflammatory response. High
concentrations of salidroside and isorhamnetin both reversed the
UII-induced inflammatory response in vivo and in
vitro. However, the inhibitory effects of low concentration of
salidroside and isorhamnetin on inflammatory response were
inconsistent. The combination of low and high concentrations of
salidroside and isorhamnetin both eliminated the UII-induced
inflammatory response in vivo and in vitro.
Furthermore, the anti-inflammatory effect of the combination of
salidroside and isorhamnetin was not significantly different
compared with the single drug alone during UII conditions in
atherosclerosis.
Subsequently, based on the role of the RhoA/ROCK
pathway in UII-induced inflammatory response, the present study
further investigated the effects of salidroside, isorhamnetin and
both in combination on the RhoA/ROCK pathway. It was found that
salidroside, isorhamnetin and both in combination inhibited the
RhoA/ROCK pathway in the thoracic aorta of rats following subacute
infusion of UII and in UII-stimulated VSMCs. Consistent with the
in vivo results, the inhibitory effects of low
concentrations of salidroside and isorhamnetin on the RhoA/ROCK
pathway were inconsistent, while the high concentrations of
salidroside and isorhamnetin both reversed UII-induced the
enhancement of the RhoA/ROCK pathway in vivo and in
vitro. The combination of low and high concentrations of
salidroside and isorhamnetin both attenuated the RhoA/ROCK pathway
under UII in vivo and in vitro. There was no
significant difference between salidroside and isorhamnetin in
isolation and in combination. Thus, the results suggested that, to
a certain extent, the RhoA/ROCK pathway contributed to the
anti-inflammatory effects of salidroside, isorhamnetin and both in
combination under UII simulation in atherosclerosis.
However, there are a few limitations to the present
study. First, gain-of-function and loss-of function experiments
were not used to investigate the role of the RhoA/ROCK pathway in
UII-induced inflammatory or the anti-inflammatory effects of
salidroside, isorhamnetin and both in combination. This will
require further examination in future studies. In addition, the
potential mechanism of salidroside, isorhamnetin and both in
combination in the inhibition of the RhoA/ROCK pathway under UII
has not yet been elucidated. The present study also did not examine
the comparison of the two drugs with currently, widely-used
anti-atherosclerosis drugs and other reported anti-inflammatory
drugs in atherosclerosis, which may be interesting to evaluate in
the subsequent experiment plan.
In conclusion, the present findings indicated that
UII stimulation resulted in an inflammatory response, accompanied
by the enhancement of the RhoA/ROCK pathway in vivo and
in vitro. In addition, to the best of our knowledge, the
present results provided the first evidence that salidroside,
isorhamnetin and both in combination attenuated the UII-induced
inflammatory response, which was partly dependent on inhibition of
the RhoA/ROCK pathway. Moreover, there is no significant difference
between salidroside and isorhamnetin, both in isolation or in
combination. The present study may provide a novel theoretical
basis for the separate used of the two drugs or their combination
in the treatment against atherosclerosis. Furthermore, the present
results may provide additional theoretical guidance for the
clinical combined use of the Tibetan medicines salidroside and
isorhamnetin in the prevention against atherosclerosis-related
cardiovascular diseases.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from
National Natural Science Foundation of China (grant no. 81360490),
research funds for Institutions of Higher Learning of Gansu
Province (grant no. 2017B-81), the Fundamental Research Funds for
the Central Universities (grant nos. 31920180023 and 31920190106)
and the Natural Science Foundation of Gansu Province, China (grant
no. 20JR5RA503).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CW and XN participated in the design of the study
and conducted the experiment. CW, SP and YZ wrote the manuscript
and analyzed data. XW, SM and GM collaborated in the analysis and
interpretation of data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experimental procedures were performed in
accordance with the National Institutes of Health Guide for the
Care and Use of Laboratory Animals, and were approved by the
Experimental Animal Administration Committee of the School of Basic
Medical Sciences, Northwest Minzu University Health Science Center
(approval no. XBMZ-YX20130101; March 1, 2013).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Libby P, Bornfeldt KE and Tall AR:
Atherosclerosis: Successes, surprises, and future challenges. Circ
Res. 118:531–534. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chistiakov DA, Kashirskikh DA, Khotina VA,
Grechko AV and Orekhov AN: Immune-inflammatory responses in
atherosclerosis: The role of myeloid cells. J Clin Med. 8:17982019.
View Article : Google Scholar
|
|
3
|
Hanna A and Frangogiannis NG: Inflammatory
cytokines and chemokines as therapeutic targets in heart failure.
Cardiovasc Drugs Ther. 34:849–863. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Taleb S: Inflammation in atherosclerosis.
Arch Cardiovasc Dis. 109:708–715. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Veluswamy P, Wacker M, Scherner M and
Wippermann J: Delicate role of PD-L1/PD-1 axis in blood vessel
inflammatory diseases: Current insight and future significance. Int
J Mol Sci. 21:81592020. View Article : Google Scholar
|
|
6
|
Sethwala AM, Goh I and Amerena JV:
Combatting inflammation in cardiovascular disease. Heart Lung Circ.
30:197–206. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koushki K, Shahbaz SK, Mashayekhi K,
Sadeghi M, Zayeri ZD, Taba MY, Banach M, Al-Rasadi K, Johnston TP
and Sahebkar A: Anti-inflammatory action of statins in
cardiovascular disease: The role of inflammasome and toll-like
receptor pathways. Clin Rev Allergy Immunol. May 6–2020.(Epub ahead
of print). doi: 10.1007/s12016-020-08791-9. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luque-Martin R, Van den Bossche J, Furze
RC, Neele AE, van der Velden S, Gijbels MJJ, van Roomen CPPA,
Bernard SG, de Jonge WJ, Rioja I, et al: Targeting histone
deacetylases in myeloid cells inhibits their maturation and
inflammatory function with limited effects on atherosclerosis.
Front Pharmacol. 10:12422019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mog B, Asase C, Chaplin A, Gao H,
Rajagopalan S and Maiseyeu A: Nano-antagonist alleviates
inflammation and allows for MRI of atherosclerosis.
Nanotheranostics. 3:342–355. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang G, Zhuo J, Lin Y, Zhang M, Liu L,
Chen X and Gao R: Ginsenoside Rb1 prevents dysfunction of
endothelial cells by suppressing inflammatory response and
apoptosis in the high-fat diet plus balloon catheter-injured rabbit
model via the G protein-coupled estrogen receptor-mediated
phosphatidylinositol 3-kinases (PI3K)/akt pathway. Med Sci Monit.
25:7407–7417. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cai A, Li L and Zhou Y: Pathophysiological
effects of RhoA and Rho-associated kinase on cardiovascular system.
J Hypertens. 34:3–10. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deng Z, Jia Y, Liu H, He M, Yang Y, Xiao W
and Li Y: RhoA/ROCK pathway: Implication in osteoarthritis and
therapeutic targets. Am J Transl Res. 11:5324–5331. 2019.PubMed/NCBI
|
|
13
|
Strzelecka-Kiliszek A, Mebarek S,
Roszkowska M, Buchet R, Magne D and Pikula S: Functions of Rho
family of small GTPases and Rho-associated coiled-coil kinases in
bone cells during differentiation and mineralization. Biochim
Biophys Acta Gen Subj. 1861:1009–1023. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li N, Chen J, Zhao J and Wang T:
MicroRNA-3188 targets ETS-domain protein 4 and participates in
RhoA/ROCK pathway to regulate the development of atherosclerosis.
Pharmazie. 72:687–693. 2017.PubMed/NCBI
|
|
15
|
Surma M, Wei L and Shi J: Rho kinase as a
therapeutic target in cardiovascular disease. Future Cardiol.
7:657–671. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou Q, Gensch C and Liao JK:
Rho-associated coiled-coil-forming kinases (ROCKs): Potential
targets for the treatment of atherosclerosis and vascular disease.
Trends Pharmacol Sci. 32:167–173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng CI, Chen PH, Lin YC and Kao YH: High
glucose activates Raw264.7 macrophages through RhoA kinase-mediated
signaling pathway. Cell Signal. 27:283–292. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Loirand G, Guerin P and Pacaud P: Rho
kinases in cardiovascular physiology and pathophysiology. Circ Res.
98:322–334. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shimada H and Rajagopalan LE: Rho-kinase
mediates lysophosphatidic acid-induced IL-8 and MCP-1 production
via p38 and JNK pathways in human endothelial cells. FEBS Lett.
584:2827–2832. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Desche P, Couderc LJ and Epardeau B:
Sjogren's syndrome and pulmonary lymphangiomyomatosis. Chest.
94:8981988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Douglas SA and Ohlstein EH: Human
urotensin-II, the most potent mammalian vasoconstrictor identified
to date, as a therapeutic target for the management of
cardiovascular disease. Trends Cardiovasc Med. 10:229–237. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Albanese I, Daskalopoulou SS, Yu B, You Z,
Genest J, Alsheikh-Ali A and Schwertani AG: The urotensin II system
and carotid atherosclerosis: A role in vascular calcification.
Front Pharmacol. 7:1492016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Demirpence M, Guler A, Yilmaz H, Sayin A,
Pekcevik Y, Turkon H, Colak A, Ari EM, Aslanipour B, Kocabas GU and
Calan M: Is elevated urotensin II level a predictor for increased
cardiovascular risk in subjects with acromegaly? J Endocrinol
Invest. 42:207–215. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Şatıroğlu Ö, Durakoğlugil ME, Çetin M,
Çiçek Y, Erdoğan T and Duman H: The role of urotensin II and
atherosclerotic risk factors in patients with slow coronary flow.
Interv Med Appl Sci. 8:158–163. 2016.PubMed/NCBI
|
|
25
|
Li Y, Zhao S, Wang Y, Chen Y, Lin Y, Zhu
N, Zheng H, Wu M, Cheng D, Li Y, et al: Urotensin II promotes
atherosclerosis in cholesterol-fed rabbits. PLoS One. 9:e950892014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu D, Peng F, Li J, Zhao J, Ye X, Li B and
Ding W: Urotensin II promotes secretion of LTB4 through
5-lipoxygenase via the UT-ROS-Akt pathway in RAW264.7 macrophages.
Arch Med Sci. 15:1065–1072. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu S, Jiang H, Wu B, Yang J and Chen S:
Urotensin II induces migration of endothelial progenitor cells via
activation of the RhoA/Rho kinase pathway. Tohoku J Exp Med.
219:283–288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saito K, Yonekura-Sakakibara K,
Nakabayashi R, Higashi Y, Yamazaki M, Tohge T and Fernie AR: The
flavonoid biosynthetic pathway in arabidopsis: Structural and
genetic diversity. Plant Physiol Biochem. 72:21–34. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhong Z, Han J, Zhang J, Xiao Q, Hu J and
Chen L: Pharmacological activities, mechanisms of action, and
safety of salidroside in the central nervous system. Drug Des Devel
Ther. 12:1479–1489. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Boesch-Saadatmandi C, Loboda A, Wagner AE,
Stachurska A, Jozkowicz A, Dulak J, Döring F, Wolffram S and
Rimbach G: Effect of quercetin and its metabolites isorhamnetin and
quercetin-3-glucuronide on inflammatory gene expression: Role of
miR-155. J Nutr Biochem. 22:293–299. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lodi F, Jimenez R, Moreno L, Kroon PA,
Needs PW, Hughes DA, Santos-Buelga C, Gonzalez-Paramas A, Cogolludo
A, Lopez-Sepulveda R, et al: Glucuronidated and sulfated
metabolites of the flavonoid quercetin prevent endothelial
dysfunction but lack direct vasorelaxant effects in rat aorta.
Atherosclerosis. 204:34–39. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
National Research Council (US): Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press; Washington, DC: 2011
|
|
33
|
Chen L, Liu P, Feng X and Ma C:
Salidroside suppressing LPS-induced myocardial injury by inhibiting
ROS-mediated PI3K/Akt/mTOR pathway in vitro and in vivo. J Cell Mol
Med. 21:3178–3189. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jamali-Raeufy N, Baluchnejadmojarad T,
Roghani M, Keimasi S and Goudarzi M: Isorhamnetin exerts
neuroprotective effects in STZ-induced diabetic rats via
attenuation of oxidative stress, inflammation and apoptosis. J Chem
Neuroanat. 102:1017092019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang N, Pei F, Wei H, Zhang T and Yang C,
Ma G and Yang C: Isorhamnetin protects rat ventricular myocytes
from ischemia and reperfusion injury. Exp Toxicol Pathol. 63:33–38.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hwang SM, Lee YJ, Lee YP, Yoon JJ, Lee SM,
Cha JD, Choi KM, Kang DG and Lee HS: Anti-proliferative effect of
an aqueous extract of prunella vulgaris in vascular smooth muscle
cells. Evid Based Complement Alternat Med. 2013:9364632013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou J, Yin G, Yu T, Zhang Y, Tian X, Xia
D and Shi L: Rosuvastatin reduces expression of tissue factor
through inhibiting RhoA/ROCK pathway to ameliorate atherosclerosis.
Panminerva Med. Sep 24–2019.(Epub ahead of print). doi:
10.23736/S0031-0808.19.03761-3.
|
|
39
|
Pereira-Castro J, Bras-Silva C and
Fontes-Sousa AP: Novel insights into the role of urotensin II in
cardiovascular disease. Drug Discov Today. 24:2170–2180. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yu QQ, Cheng DX, Xu LR, Li YK, Zheng XY,
Liu Y, Li YF, Liu HL, Bai L, Wang R, et al: Urotensin II and
urantide exert opposite effects on the cellular components of
atherosclerotic plaque in hypercholesterolemic rabbits. Acta
Pharmacol Sin. 41:546–553. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tsai CS, Loh SH, Liu JC, Lin JW, Chen YL,
Chen CH and Cheng TH: Urotensin II-induced endothelin-1 expression
and cell proliferation via epidermal growth factor receptor
transactivation in rat aortic smooth muscle cells. Atherosclerosis.
206:86–94. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Diebold I, Petry A, Burger M, Hess J and
Görlach A: NOX4 mediates activation of FoxO3a and matrix
metalloproteinase-2 expression by urotensin-II. Mol Biol Cell.
22:4424–4434. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang Y, Zhang J, Chen X, Wu T, Xu X, Cao
G, Li H and Li Y: UII/GPR14 is involved in NF-κB-mediated colonic
inflammation in vivo and in vitro. Oncol Rep. 36:2800–2806. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fiordelisi A, Iaccarino G, Morisco C,
Coscioni E and Sorriento D: NFkappaB is a key player in the
crosstalk between inflammation and cardiovascular diseases. Int J
Mol Sci. 20:15992019. View Article : Google Scholar
|
|
45
|
Li Q, Zhao W, Zeng X and Hao Z: Ursolic
acid attenuates atherosclerosis in apoE(−/-) mice: Role of LOX-1
mediated by ROS/NF-κB pathway. Molecules. 23:1101–1108. 2018.
View Article : Google Scholar
|
|
46
|
Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu
Y and Dong W: ROS and ROS-mediated cellular signaling. Oxid Med
Cell Longev. 2016:43509652016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bustelo XR, Sauzeau V and Berenjeno IM:
GTP-binding proteins of the Rho/Racfamily: Regulation, effectors
and functions in vivo. Bioessays. 29:356–370. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tang ST, Zhang Q, Tang HQ, Wang CJ, Su H,
Zhou Q, Wei W, Zhu HQ and Wang Y: Effects of glucagon-like
peptide-1 on advanced glycation endproduct-induced aortic
endothelial dysfunction in streptozotocin-induced diabetic rats:
Possible roles of Rho kinase- and AMP kinase-mediated nuclear
factor κB signaling pathways. Endocrine. 53:107–116. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang HW, Liu PY, Oyama N, Rikitake Y,
Kitamoto S, Gitlin J, Liao JK and Boisvert WA: Deficiency of ROCK1
in bone marrow-derived cells protects against atherosclerosis in
LDLR-/-mice. FASEB J. 22:3561–3570. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li R, Dong Z, Zhuang X, Liu R, Yan F, Chen
Y, Gao X and Shi H: Salidroside prevents tumor necrosis
factor-α-induced vascular inflammation by blocking
mitogen-activated protein kinase and NF-κB signaling activation.
Exp Ther Med. 18:4137–4143. 2019.PubMed/NCBI
|
|
51
|
Xing SS, Li J, Chen L, Yang YF, He PL, Li
J and Yang J: Salidroside attenuates endothelial cellular
senescence via decreasing the expression of inflammatory cytokines
and increasing the expression of SIRT3. Mech Ageing Dev. 175:1–6.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Luo Y, Sun G, Dong X, Wang M, Qin M, Yu Y
and Sun X: Isorhamnetin attenuates atherosclerosis by inhibiting
macrophage apoptosis via PI3K/AKT activation and HO-1 induction.
PLoS One. 10:e1202592015.
|
|
53
|
Park C, Cha HJ, Choi EO, Lee H, Hwang-Bo
H, Ji SY, Kim MY, Kim SY, Hong SH, Cheong JH, et al: Isorhamnetin
induces cell cycle arrest and apoptosis via reactive oxygen
species-mediated AMP-activated protein kinase signaling pathway
activation in human bladder cancer cells. Cancers (Basel).
11:14942019. View Article : Google Scholar
|