Introduction
In recent years, malignant tumors have become a
major public health burden that significantly impact the health of
individuals worldwide. Gastric cancer (GC) is a cancer of the
gastrointestinal tract that currently ranks as the 5th most common
type of cancer worldwide, accounting for an estimated 1,033,000 new
cases and 783,000 deaths in 2018 (1). Due to the low early diagnosis rate of
GC and the poor therapeutic effects of traditional treatment
regimens, which usually combine surgery and chemotherapy, the
prognosis of patients with GC remains poor. The
Tumor-Node-Metastasis (TNM) classification, which classifies tumors
according to the pathological examination of resected specimens,
remains the most reliable tool for predicting the clinical outcome
of patients with GC (2). However,
substantial differences in survival are observed for patients at
the same clinical stage (2,3). Although numerous molecules have been
reported as potential biomarkers for predicting clinical outcomes
in GC, such as upregulated expression levels of SWI/SNF related,
matrix associated, actin dependent regulator of chromatin,
subfamily e, member 1 and chemokine receptor 6, which were
discovered to be associated with a poor prognosis in patients with
GC (4,5), none of the identified molecules have
been clinically evaluated for the disease to date. Therefore,
further studies are required to determine specific biomarkers that
may help distinguish patients with different prognoses more
accurately.
It is well known that cancer cells prefer to
metabolize glucose via glycolysis rather than mitochondrial
oxidative phosphorylation, even in oxygen-rich conditions, and this
phenomenon is referred to as the Warburg effect or aerobic
glycolysis (6).
Phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 (PFKFB4) is one
of the four subtypes of the bifunctional enzyme PFKFB (7). By regulating the levels of
fructose-2,6-bisphosphate, the strongest allosteric activator of
phosphoric acid fructose kinase-1, PFKFB4 serves an important role
in modulating the glycolytic and pentose phosphate pathway flux, in
addition to the production of ATP, which affects the growth of
tumors (8,9). Recently, a previous study conducted in
prostate cancer revealed that CD44 could modulate the aggressive
phenotype of prostate cancer cells by regulating the expression
levels of pyruvate dehydrogenase kinase, isozyme 1 and PFKFB4
(10). In addition, another previous
study reported that PFKFB4 enhanced the migration and invasion of
GC cells by downregulating the expression levels of the tumor
suppressor gene liver kinase B1 (LKB1) (11), indicating an association between
PFKFB4 and cancer development. However, to the best of our
knowledge, the expression levels and clinical significance of
PFKFB4 in GC remain to be elucidated.
The present study analysed PFKFB4 expression in GC
and adjacent normal tissues using immunohistochemistry (IHC), and
further determined the association between PFKFB4 expression levels
and the clinicopathological characteristics of patients with GC. In
addition, the association between PFKFB4 expression levels and the
survival of patients with GC was investigated using the
Kaplan-Meier (KM) plotter database.
Materials and methods
Patients and specimens
A total of 148 GC tissues were collected from
patients diagnosed with GC at the General Hospital of Ningxia
Medical University (Yinchuan, China) between September 2012 and
December 2013. There were only 46 GC tissues which contained
matched adjacent normal tissues (2 cm from the edge of tumors) due
to the process of generating the pathological sections.
Chemotherapy, radiotherapy and other antitumor treatments were not
administered to the patients before surgery. Oral informed consent
was obtained from all patients prior to collecting the specimens.
The study was approved by the Medical Research Ethics Review
Committee of The General Hospital of Ningxia Medical University.
The specimens were fixed with 4% paraformaldehyde for 24 h at room
temperature, embedded in paraffin and cut into 5-µm-thick sections
for IHC analysis. The pathological diagnosis was performed by two
senior pathologists according to the hematoxylin and eosin staining
results. TNM stage evaluation was determined according to the
criteria indicated in the staging procedures of the International
Association for the study of GC (12). The clinicopathological data of the
patients are summarized in Table I.
It should be noted that 2/148 GC cases had no information regarding
the tumor differentiation degree.
 | Table I.PFKFB4 expression and
clinicopathological characteristics of patients with gastric cancer
(n=148). |
Table I.
PFKFB4 expression and
clinicopathological characteristics of patients with gastric cancer
(n=148).
|
|
| PFKFB4
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Variables | Cases, n | Low, n (%) | High, n (%) | χ2 | P-value |
|---|
| Age, years |
| ≥65 | 57 | 42 (73.7) | 15 (26.3) | 7.782 | 0.005a |
|
<65 | 91 | 46 (50.5) | 45 (49.5) |
|
|
| Sex |
| Male | 123 | 71 (57.7) | 52 (42.3) | 0.910 | 0.340 |
|
Female | 25 | 17 (68.0) | 8 (32.0) |
|
|
| Differentiation |
| Well | 21 | 10 (47.6) | 11 (52.4) | 1.940 | 0.379 |
|
Moderate | 22 | 15 (68.2) | 7 (31.8) |
|
|
| Poor | 103 | 62 (60.2) | 41 (39.8) |
|
|
| NA | 2 | 1 (50.0) | 1 (50.0) |
|
|
| Tumor size, cm |
| ≥4 | 88 | 46 (52.3) | 42 (47.7) | 4.651 | 0.031a |
|
<4 | 60 | 42 (70.0) | 18 (30.0) |
|
|
| pT stage |
| T1-2 | 39 | 31 (79.5) | 8 (20.5) | 8.812 | 0.003a |
|
T3-4 | 109 | 57 (52.3) | 52 (47.7) |
|
|
| pN stage |
| N0 | 59 | 40 (67.8) | 19 (32.2) | 2.829 | 0.093 |
|
N1-3 | 89 | 48 (53.9) | 41 (46.1) |
|
|
| pM stage |
| M0 | 141 | 85 (60.3) | 56 (39.7) |
| 0.442b |
| M1 | 7 | 3 (42.9) | 4 (57.1) |
|
|
| pTNM stage |
|
I+II | 45 | 33 (73.3) | 12 (26.7) | 5.163 | 0.023a |
|
III+IV | 103 | 55 (53.4) | 48 (46.6) |
|
|
IHC staining
The expression levels of PFKFB4 in GC tissues were
determined by IHC analysis. Briefly, the formalin-fixed and
paraffin-embedded tissue sections were heated at 65°C for 1 h, then
deparaffinized in xylene and rehydrated in a series of graded
ethanol. After washing 3 times with PBS, the sections were
incubated with 3% hydrogen peroxide at room temperature for 10 min
to block endogenous peroxidase activity. EDTA buffer (pH 8.0) was
then used for antigen retrieval. Subsequently, the sections were
blocked with 10% goat serum (cat. no. ZLI-9022; OriGene
Technologies) for 10 min at 37°C prior to incubation with an
anti-PFKFB4 antibody (1:100; cat. no. ab137785; Abcam) at 37°C for
1 h and then overnight at 4°C in a humidified chamber. Following
primary antibody incubation, the sections were washed 3 times with
PBS and then incubated with an HRP-conjugated peroxidase working
solution (cat. no. P0448; Dako; Agilent Technologies, Inc.) for 30
min at 37°C. The sections were incubated with the chromogen
substrate 3,3′-diaminobenzidine for 10 min at room temperature,
followed by haematoxylin counterstaining at room temperature for 1
min. Finally, the slides were dehydrated in a graded series of
alcohol and xylene, and sealed with neutral balsam and a glass
coverslip.
The expression levels of PFKFB4 were observed and
the images were captured using a light microscope at ×100 and ×400
magnification, and determined using a semi-quantitative method
(13). The immunoreactivity score
was evaluated blindly by two senior pathologists. The staining
intensity was scored as follows: 0, negative; 1+, light yellow; 2+,
yellowish brown; and 3+, brown. The number of stained cells was
also scored and divided into four groups according to the
percentage of positively stained cells: 0, no positively stained
cells; 1+, ≤10% positively stained cells; 2+, 11–50% positively
stained cells; 3+, 51–75% positively stained cells; and 4+, >75%
positively stained cells. The final score was the product of the
staining intensity score and the score for the percentage of
positively stained cells. The median immunoreactivity score was
used as the cut-off value to define high and low expression in the
samples.
Survival analysis
KM survival analysis for GC and the subsequent
log-rank test were performed using the KM plotter database
(http://kmplot.com/analysis/index.php?p=service&cancer=gastric).
Briefly, Affy ID 206246_at (PFKFB4) was selected for survival
analysis. A total of 875 patients with GC were included for overall
survival (OS; the survival time from diagnosis until death from any
cause) analysis, while 640 patients were used to analyze the first
progression survival (FPS; the survival time without progression of
the disease), and 498 patients were used to analyze the
post-progression survival (PPS; the survival time following
progressive disease during treatment) times. In addition, 241
patients with GC with pT2 stage disease, 204 patients with pT3
stage disease, 140 patients with stage II disease, 305 patients
with stage III disease and 148 patients with stage IV disease were
used to determine the association between PFKFB4 expression and
OS.
Statistical analysis
Statistical analyses were performed using SPSS
version 17.0 software (SPSS, Inc.). A Mann-Whitney U test was
performed to compare the differences in the expression levels of
PFKFB4 between the GC and adjacent normal tissues. The statistical
differences between the 46 pairs of GC and matched adjacent normal
tissues were determined using a Wilcoxon signed rank test.
Scatterplots showing the distribution of PFKFB4 staining scores in
GC and adjacent normal tissues were drawn using GraphPad Prism
V5.01 software (GraphPad Software, Inc.). The association between
PFKFB4 expression and the clinicopathological characteristics of
the patients was analysed using a χ2 test or Fisher's
exact test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinicopathological characteristics of
patients with GC
In total, 148 GC tissues, including 46 GC and
matched adjacent normal tissues, were collected to analyze the
expression levels of PFKFB4. Among these specimens, 123 cases were
men and 25 cases were women, with an age range of 23–96 years old
and a mean age of 61.80±14.11 years old. The detailed
clinicopathological characteristics of the patients, including
tumor differentiation, tumor size and TNM stage, are summarized in
Table I.
Expression levels of PFKFB4 in GC and
adjacent normal tissues
The expression levels of PFKFB4 in GC tissues were
determined using IHC. Positive PFKFB4 expression was observed in
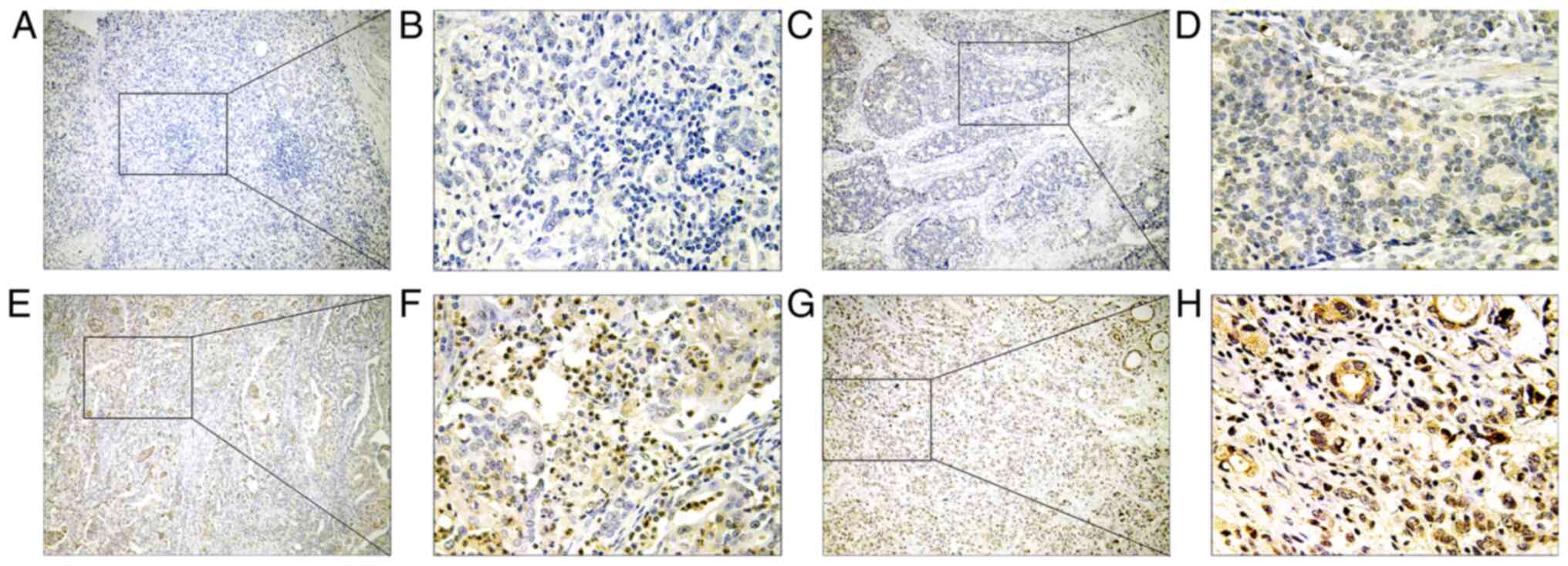
almost all the tumor and adjacent normal tissues. Fig. 1 shows the representative IHC staining
images of PFKFB4 expression in GC tissues. PFKFB4 was found to be
mainly expressed in the cytoplasm and nucleus of the cells, and
distributed diffusely throughout the tumor tissues. The intensity
and range of PFKFB4 expression was stronger in the cytoplasm
compared with the nucleus. However, the expression levels of PFKFB4
were markedly upregulated in the cell nuclei of tumor tissues with
high PFKFB4 expression. Following the semi-quantification, PFKFB4
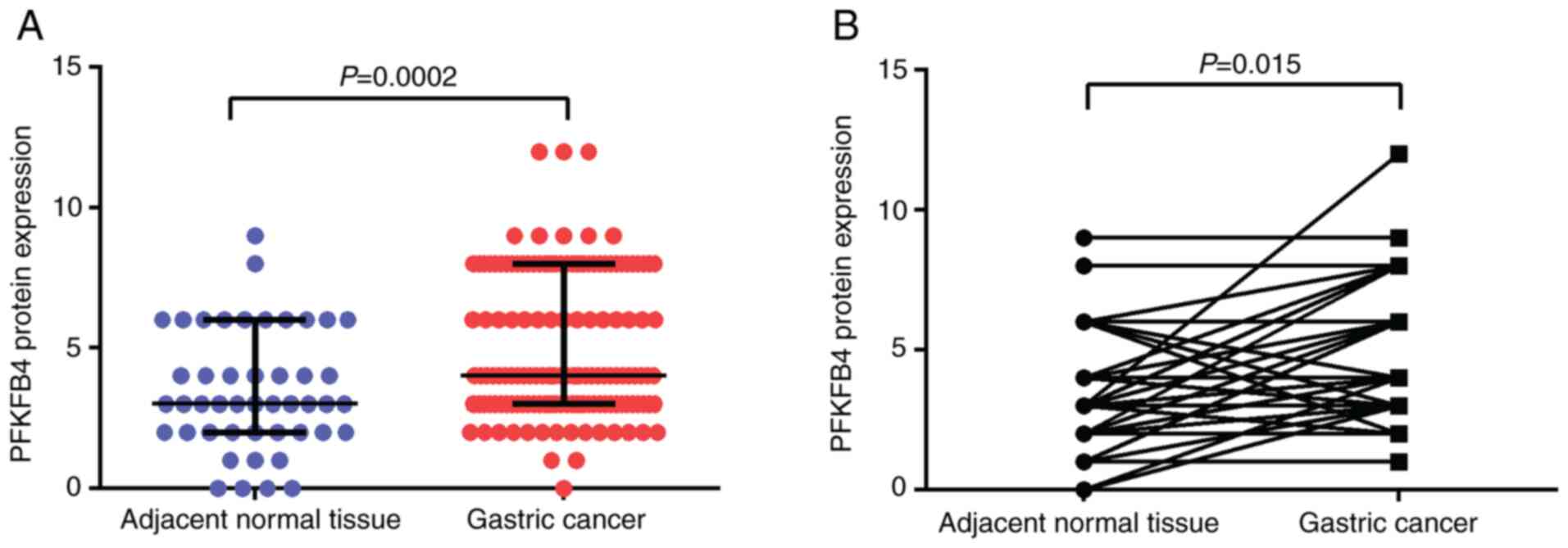
expression between GC and normal adjacent normal tissues was
compared. The results revealed that PFKFB4 expression was
significantly upregulated in the tumor tissues compared with in the
adjacent normal tissues (median, 4 vs. 3; P=0.0002; Fig. 2A). In addition, in the matched 46
pairs of GC and adjacent normal tissues, PFKFB4 expression was
significantly upregulated in the GC tissues compared with in the
normal tissues (P=0.015; Fig.
2B).
Association between PFKFB4 expression
and the clinicopathological characteristics of patients with
GC
The association between PFKFB4 protein expression in
GC tissues and the clinicopathological characteristics of patients
was subsequently analyzed. According to the median immunoreactivity
score of PFKFB4 expression in the tumor tissues, which was 4,
specimens with an immunoreactivity score >4 were defined as
having high PFKFB4 expression, while those with an immunoreactivity
score <4 were defined as having low PFKFB4 expression. As shown
in Table I, 59.5% (88/148) and 40.5%
(60/148) of the cases were classified as low and high expression,
respectively. The percentage of specimens with high PFKFB4
expression was markedly increased in the <65 age group compared
with that in the ≥65 age group (49.5 vs. 26.3%; P=0.005). The
percentage of tumors with high PFKFB4 expression and a large tumor
size (≥4 cm) compared with a smaller tumor size (<4 cm) was also
increased (47.7 vs. 30%; P=0.031). Furthermore, in the advanced pT
stage (pT3 and 4) or pTNM stage groups, the percentage of specimens
with high PFKFB4 expression was markedly increased compared with
the early stage groups (P<0.05). The aforementioned results
indicated that there was a significant association between PFKFB4
expression level and the age of the patient, tumor size, pT stage,
and pTNM stage. However, no significant associations were
identified between PFKFB4 expression and the sex, tumor
differentiation or pathological N and M stages of the patients.
Association between PFKFB4 expression
and the survival of patients with GC
To further evaluate the clinical significance of
PFKFB4 in GC, the difference in the survival time between patients
with GC with high and low PFKFB4 expression was investigated using
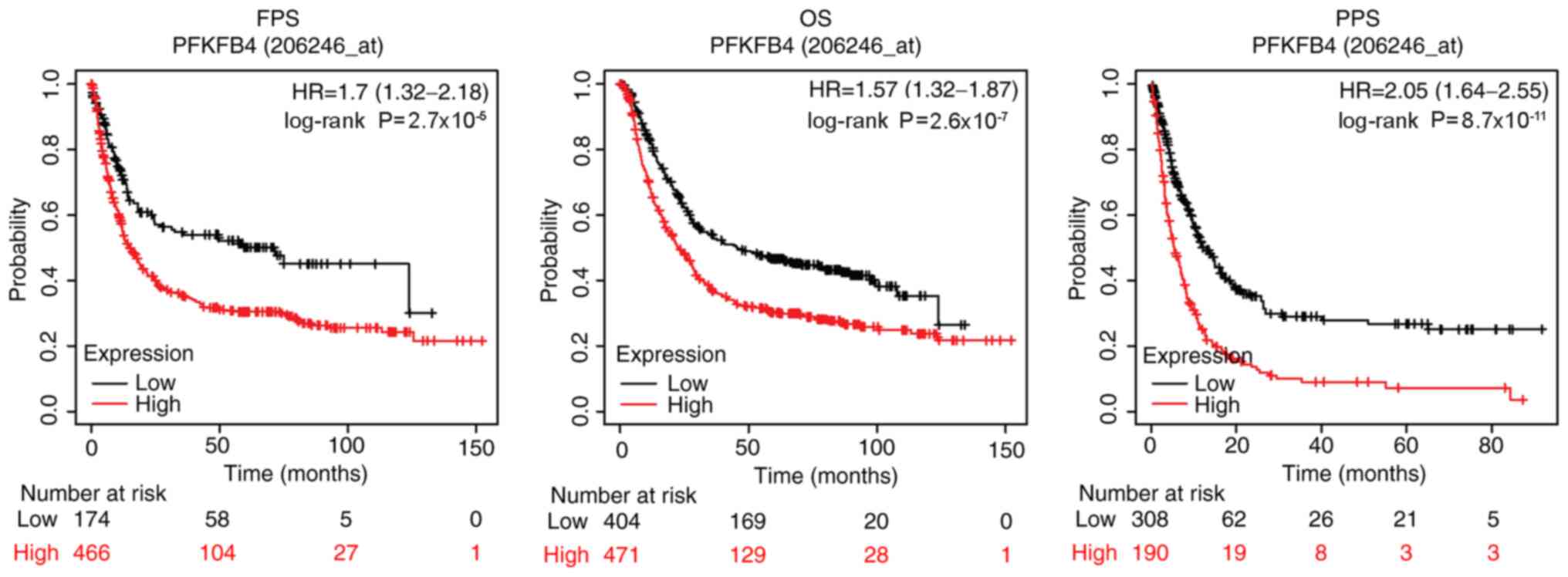
the online KM plotter database. As shown in Fig. 3, patients with high expression levels
of PFKFB4 had a higher probability of a shorter OS, FPS and PPS
compared with patients with low expression levels. The median
survival time for OS, FPS and PPS was 22.5, 15.1 and 5.4 months,
respectively, in the high PFKFB4 expression group, while it was
45.4, 71.7 and 12.1 months, respectively, in the low PFKFB4
expression group (Fig. 3).
Considering that the expression levels of PFKFB4 were upregulated
in advanced stage tumor tissues, the association between PFKFB4
expression and the OS of patients with different tumor stages was
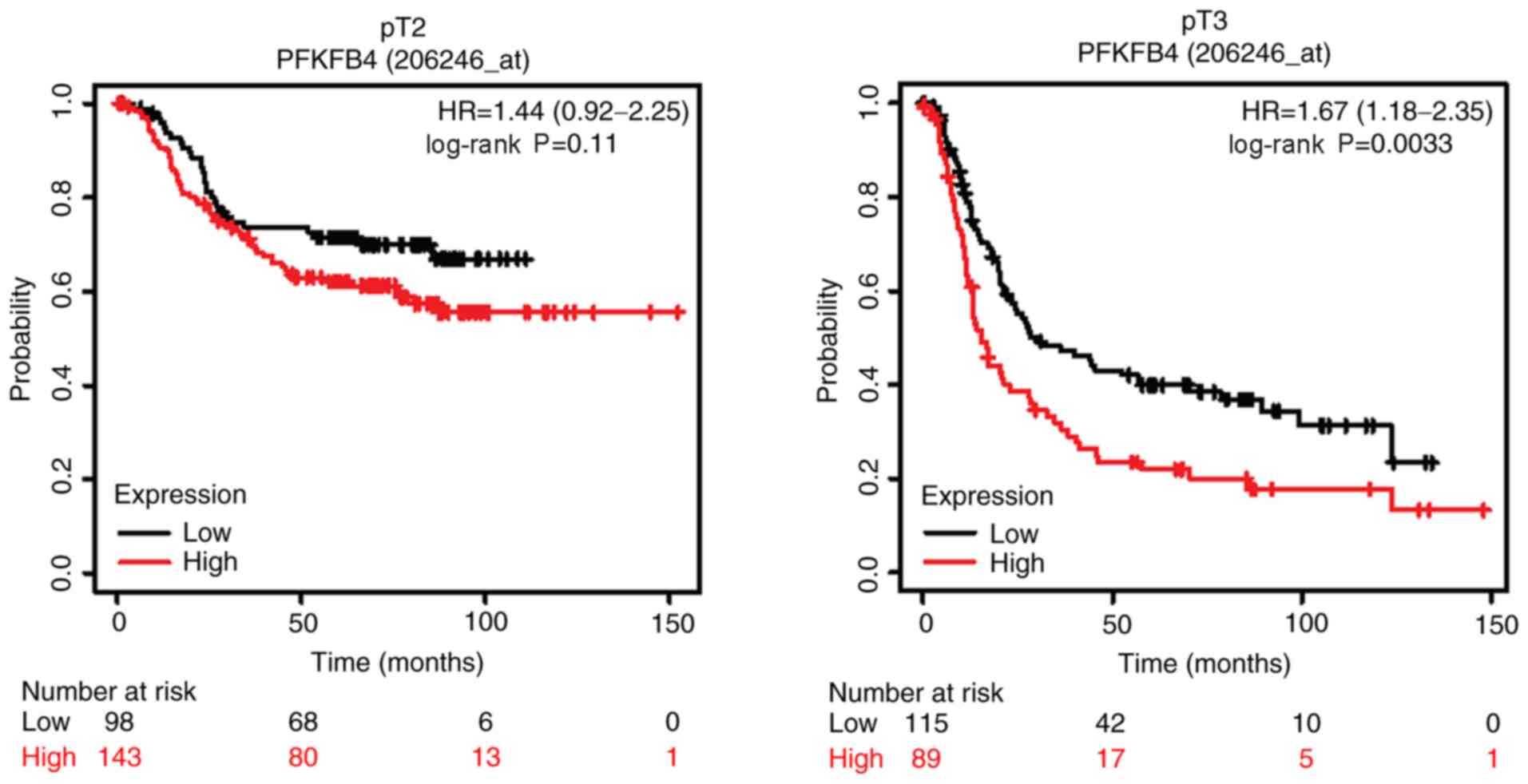
further analysed. The results revealed that PFKFB4 expression was
not significantly associated with OS in patients with an early pT
stage (pT2) or TNM stage (stage II) disease. The median OS times
for patients with low and high PFKFB4 expression at pT2 disease
stage was 30.9 and 26.4 months respectively, while the OS time in
patients with low and high PFKFB4 expression at stage II disease
was 78.6 and 35.2 months respectively. However, at the advanced
stages (pT3 and TNM stages III and IV), high expression levels of
PFKFB4 were significantly associated with a poor survival in
patients with GC (Figs. 4 and
5). The median OS time for patients
with low and high PFKFB4 expression at pT3 disease stage was 30.4
and 15.3 months respectively. Furthermore, OS time was 52.6 and
25.17 months, respectively for patients with low and high
expression of PFKFB4 at stage III disease, and 20.03 and 11.47
months for patients at stage IV disease, respectively.
Collectively, these results indicated that PFKFB4 may be a
predictor for a poor prognosis in patients with GC.
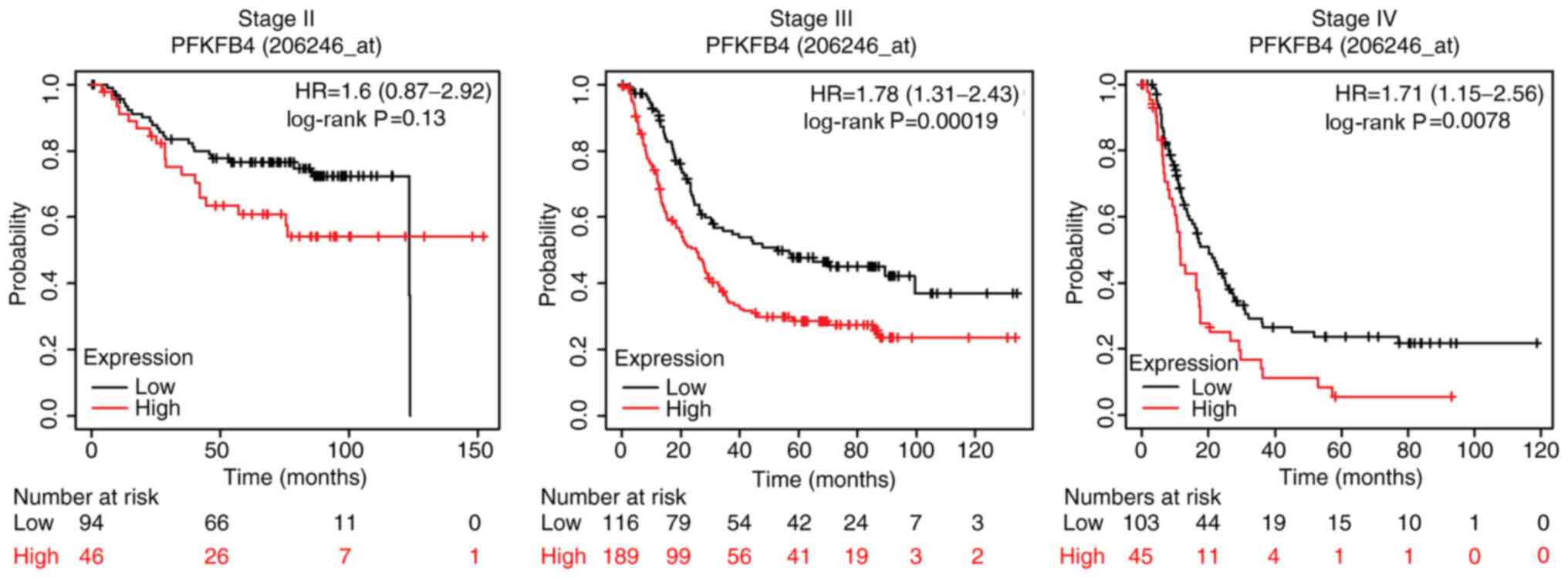 | Figure 5.High PFKFB4 expression is associated
with the OS of patients with GC with a high pTNM stage disease.
Association between PFKFB4 expression and the OS of 140 patients
with GC with stage II disease, 305 patients with stage III disease
and 148 patients with stage IV disease were analyzed using the
Kaplan-Meier plotter database. The median OS time for patients with
low and high PFKFB4 expression at stage II disease was 78.6 and
35.2 months, respectively. The median OS time for patients with low
and high expression of PFKFB4 at stage III disease was 52.6 and
25.17 months, respectively. The median OS time for patients with
low and high PFKFB4 expression at stage IV disease was 20.03 and
11.47 months, respectively. OS, overall survival; pTNM,
pathological Tumor-Node-Metastasis; PFKFB4,
phosphofructo-2-kinase/fructose-2,6-biphosphatase 4; GC, gastric
cancer; HR, hazard ratio. |
Discussion
Several previous studies have reported that PFKFB4
functions as an oncoprotein in various types of cancer, including
breast (14), prostate (15), bladder (16), hepatocellular carcinoma and thyroid
cancer (17). In addition, a
previous study demonstrated that 5-(n-(8-methoxy-4-quinolyl)amino)
pentyl nitrate, a selective inhibitor of PFKFB4, suppressed the
glycolysis and proliferation of multiple human cancer cell lines
(18). PFKFB4 is a bi-functional
metabolic enzyme that synthesizes a potent allosteric activator for
one of the rate-limiting enzymes of glycolysis. Thus, PFKFB4 is
able to modulate glucose metabolism, which is reprogrammed in
cancer cells (19). One of the
mechanisms underlying the tumor-promoting effect of PFKFB4 is its
ability to regulate the glycolytic flux under both normoxic and
hypoxic conditions (10,16,20–23). In
addition, PFKFB4 has been discovered to promote tumor progression
through glycolysis-independent mechanisms (14,17,24,25). For
example, it has been previously reported that PFKFB4 modulates the
chemoresistance of small-cell lung cancer by regulating autophagy
(24). PFKFB4 promotes breast cancer
tumorigenesis by phosphorylating and activating steroid receptor
coactivator-3, a crucial transcriptional coactivator of the
estrogen receptor (25). PFKFB4 also
induces the production of hyaluronan by activating p38 signaling
(14). However, whether PFKFB4 is
implicated in the tumorigenesis of GC remains unclear. Only one
study demonstrated that PFKFB4 promotes the migration and invasion
of GC cells by phosphorylating SRC-2 and inhibiting LKB1 expression
(11).
The present study provided evidence to suggest an
association between PFKFB4 expression and GC in clinical samples.
The current results revealed that PFKFB4 protein expression was
upregulated in GC tissues compared with in adjacent normal tissues.
In addition, the expression levels of PFKFB4 were upregulated in
the tumor tissues from patients who were <65 years old compared
with tissues in patients who are ≥65 years old. Since PFKFB4 is a
crucial enzyme involved in glucose metabolism, one of the
explanations for this phenomenon may be that the metabolism in
younger people is more active compared with that in older people.
Moreover, patients with a tumor size >4 cm or those with a high
pathological stage had higher expression levels of PFKFB4,
indicating PFKFB4 may be involved in the malignant progression of
GC.
Next, the association between PFKFB4 expression and
the prognosis of patients with GC was investigated. By comparing
the differences in OS, FPS and PPS between patients with low and
high PFKFB4 expression, it was discovered that high expression
levels of PFKFB4 were positively associated with a decreased OS,
FPS and PPS compared with low expression levels, which is
consistent with the results from previous studies conducted in
other types of cancer (14–16). These results suggested that PFKFB4
may be a poor prognostic indicator for patients with cancer
(26,27).
After determining that PFKFB4 expression was
associated with a higher pathological stage in GC, the association
between PFKFB4 expression and the OS of patients with GC with
different pT or pTNM stages was further investigated. No
significant differences were observed in the OS between patients
with early stage GC and high or low PFKFB4 expression (pT2 or pTNM
II); however, patients with high expression levels of PFKFB4 in the
advanced stage GC group had a significantly worse OS compared with
patients with low expression levels.
In conclusion, the present study revealed the
expression profile of PFKFB4 in GC and analyzed its clinical
significance. Since PFKFB4 expression was upregulated in GC
tissues, and higher PFKFB4 expression was positively associated
with a shorter survival time for patients, the current results
suggested that PFKFB4 may serve as a useful poor prognostic marker
for GC, as well as an important therapeutic target for the
prevention of GC progression. To the best of our knowledge, this
was the first study to investigate the role of PFKFB4 in GC
progression and prognosis. However, a potential limitation of the
present study is the lack of follow-up of the patients in the
current cohort, and the absence of survival data including these
patients. Most of the patients in the cohort were from the mountain
regions of western China, a vast under-populated land with
relatively low levels of economic development, making it difficult
to follow up these patients. In the present study, only the KM
plotter database was used to collect information on the clinical
significance of PFKFB4. In addition, further studies are required
to elucidate the function and underlying mechanism of PFKFB4 in GC
using in vitro and/or in vivo experiments.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Ningxia Natural Science Foundation (grant no. NZ15150) and the
National Natural Science Foundation of China (grant nos. 81660486,
81872395 and 81860442).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FW and YG participated in the study design. XW, YL
and CZ assisted in collecting the specimens and IHC staining. FW,
XC, YG and YL performed the data analysis. YG and XW drafted the
manuscript. The authenticity of all the raw data was assessed by FW
and YG to ensure its legitimacy. All authors provided critical
review of the manuscript and read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Medical Research
Ethics Review Committee of the General Hospital of Ningxia Medical
University (Yinchuan, China). Oral informed consent from patients
was obtained before collecting specimens.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheng C, Zhang Q, Zhuang L and Sun J:
Prognostic value of lymphocyte-to-C-reactive protein ratio in
patients with gastric cancer after surgery: A multicentre study.
Jpn J Clin Oncol. 50:1141–1149. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maeda K, Shibutani M, Otani H, Nagahara H,
Ikeya T, Iseki Y, Tanaka H, Muguruma K and Hirakawa K:
Inflammation-based factors and prognosis in patients with
colorectal cancer. World J Gastrointest Oncol. 7:111–117. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu H, Zhao YR, Chen B, Ge Z and Huang JS:
High expression of SMARCE1 predicts poor prognosis and promotes
cell growth and metastasis in gastric cancer. Cancer Manag Res.
11:3493–3509. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang XG, Song BT, Liu FJ, Sun D, Wang KX
and Qu H: CCR6 overexpression predicted advanced biological
behaviors and poor prognosis in patients with gastric cancer. Clin
Transl Oncol. 18:700–707. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koppenol WH, Bounds PL and Dang CV: Otto
Warburg's contributions to current concepts of cancer metabolism.
Nat Rev Cancer. 11:325–327. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lai W, Wang Z and Liang HJ: Expression of
6-phosphofructokinase-2 in colorectal cancer and its clinical
significance. J Third Military Med University (Chinese).
39:983–989. 2017.
|
|
8
|
Chesney J, Clark J, Klarer AC,
Imbert-Fernandez Y, Lane AN and Telang S: Fructose-2,6-bisphosphate
synthesis by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 4
(PFKFB4) is required for the glycolytic response to hypoxia and
tumor growth. Oncotarget. 5:6670–6686. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li XM and Li W: Progress in the study on
the role of PFKFB4 and potential mechanism in tumor glucose
metabolism. Chin J N Clin Med. 11:1044–1048. 2018.
|
|
10
|
Li W, Qian L, Lin J, Huang G, Hao N, Wei
X, Wang W and Liang J: CD44 regulates prostate cancer
proliferation, invasion and migration via PDK1 and PFKFB4.
Oncotarget. 8:65143–65151. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang M and Sun TY: Effects of PFKFB4 on
invasion and migration of gastric cancer cells and its mechanism. J
Pract Oncol (Chinese). 34:229–234. 2020.
|
|
12
|
Washington K: 7th Edition of the AJCC
cancer staging manual: Stomach. Ann Surg Oncol. 17:3077–3079. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu L and Yang WT: The judgement standard
of immunohistochemical reaction results. Chin Oncol. 6:229–231.
1996.
|
|
14
|
Gao R, Liu Y, Li D, Xun J, Zhou W, Wang P,
Liu C, Li X, Shen W, Su W, et al: PFKFB4 promotes breast cancer
metastasis via induction of hyaluronan production in a
p38-Dependent manner. Cell Physiol Biochem. 50:2108–2123. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li X, Chen Z, Li Z, Huang G, Lin J, Wei Q,
Liang J and Li W: The metabolic role of PFKFB4 in
androgen-independent growth in vitro and PFKFB4 expression in human
prostate cancer tissue. BMC Urol. 20:612020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang H, Lu C, Fang M, Yan W, Chen M, Ji
Y, He S, Liu T, Chen T and Xiao J: HIF-1α activates hypoxia-induced
PFKFB4 expression in human bladder cancer cells. Biochem Biophys
Res Commun. 476:146–152. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu H, Chen S, You Z, Xie C, Huang S and Hu
X: PFKFB4 negatively regulated the expression of histone
acetyltransferase GCN5 to mediate the tumorigenesis of thyroid
cancer. Dev Growth Differ. 62:129–138. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chesney J, Clark J, Lanceta L, Trent JO
and Telang S: Targeting the sugar metabolism of tumors with a
first-in-class 6-phosphofructo-2-kinase (PFKFB4) inhibitor.
Oncotarget. 6:18001–18011. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Minchenko OH, Tsuchihara K, Minchenko DO,
Bikfalvi A and Esumi H: Mechanisms of regulation of PFKFB
expression in pancreatic and gastric cancer cells. World J
Gastroenterol. 20:13705–13717. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ros S, Floter J, Kaymak I, Da Costa C,
Houddane A, Dubuis S, Griffiths B, Mitter R, Walz S, Blake S, et
al: 6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 is
essential for p53-null cancer cells. Oncogene. 36:3287–3299. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shu Y, Lu Y, Pang X, Zheng W, Huang Y, Li
J, Ji J, Zhang C and Shen P: Phosphorylation of PPARg at Ser84
promotes glycolysis and cell proliferation in hepatocellular
carcinoma by targeting PFKFB4. Oncotarget. 7:76984–76994. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao R, Li D, Xun J, Zhou W, Li J, Wang J,
Liu C, Li X, Shen W, Qiao H, et al: CD44ICD promotes breast cancer
stemness via PFKFB4-mediated glucose metabolism. Theranostics.
8:6248–6262. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Trojan SE, Piwowar M, Ostrowska B, Laidler
P and Kocemba-Pilarczyk KA: Analysis of malignant melanoma cell
lines exposed to hypoxia reveals the importance of PFKFB4
overexpression for disease progression. Anticancer Res.
38:6745–6752. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Q, Zeng F, Sun Y, Qiu Q, Zhang J,
Huang W, Huang J, Huang X and Guo L: Etk interaction with PFKFB4
modulates chemoresistance of small-cell lung cancer by regulating
autophagy. Clin Cancer Res. 24:950–962. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dasgupta S, Rajapakshe K, Zhu B, Nikolai
BC, Yi P, Putluri N, Choi JM, Jung SY, Coarfa C, Westbrook TF, et
al: Metabolic enzyme PFKFB4 activates transcriptional coactivator
SRC-3 to drive breast cancer. Nature. 556:249–254. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yao L, Wang L, Cao ZG, Hu X and Shao ZM:
High expression of metabolic enzyme PFKFB4 is associated with poor
prognosis of operable breast cancer. Cancer Cell Int. 19:1652019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yun SJ, Jo SW, Ha YS, Lee OJ, Kim WT, Kim
YJ, Lee SC and Kim WJ: PFKFB4 as a prognostic marker in
non-muscle-invasive bladder cancer. Urol Oncol. 30:893–899. 2012.
View Article : Google Scholar : PubMed/NCBI
|