Introduction
Pancreatic cancer (PC) is a digestive system
malignancy that mainly arises from ductal epithelial and acinar
cells (1). PC has a poor prognosis,
with a 5-year survival rate <5%, and patients with advanced PC
have a shorter survival time of 3–6 months (1). There were 432,242 new mortalities
associated with PC worldwide in 2018 due to treatment delays caused
by difficulties in the early diagnosis of PC (1). In addition, PC ranks 13th in cancer
prevalence worldwide, with 458,918 new cases in 2018, and the
incidence rates in South Europe was 14% and North America was 13.5%
(2). PC cases are mainly ductal cell
carcinoma, with a few cases of acinar cell carcinoma,
acanthocutaneous carcinoma of the pancreas and cystadenocarcinoma.
Due to the insensitivity of PC to chemotherapy, surgical resection
remains the mainstay of treatment (3). However, PC is curable in only a
minority of patients with locally resectable tumours, accounting
for only 5–10% of patients, and the survival rate more than 5 years
after surgery is only 10–20%. The poor prognosis of PC is mainly
due to its strong invasive ability (3), and the biological molecular mechanism
underlying its malignancy has not yet been determined.
Exosomes, which contain proteins [cytoskeletal
proteins, transmembrane proteins and heat shock proteins (HSPs)],
nucleic acids (DNA, mRNA, miRNA, and long and short non-coding
RNAs) and enzymes (GAPDH, ATPase, pgk1 and RAB), constitute a class
of extracellular vehicles (EVs) defined as membrane-bound
nanovesicles of endocytic origin, with a diameter of 40–150 nm
(4–6). The molecular contents of exosomes can
reflect the nature and state of their cells of origin, and these
contents alter the function of recipient cells (7). Since Johnstone et al (8) discovered and named exosomes in 1987,
the complex process of exosome formation has been elaborated in
detail. First, the membrane domain is endocytosed to form early
endosomes, and then, the process of budding forms intraluminal
vesicles, which further become multivesicular bodies (MVBs) by
encapsulating proteins, nucleic acids and peptide bands. Some MVBs
are degraded in lysosomes, while other MVBs fuses with the cell
membrane and release internal vesicles to form exosomes (8). The final steps in exosome biogenesis
also involve Rab enzymes, which regulate the transport of MVBs and
promote the fusion of MVBs with the plasma membrane, thereby
releasing exosomes (8,9).
The number of studies investigating the role of
exosomes in tumour growth and cancer metastasis has grown
exponentially (8,9). From tumour growth to cell metastasis,
the intricate exosomal communication networks between tumour and
non-tumour cells direct all steps of biological changes in tumours
(10). Tumour cells develop
exosome-based mechanisms that promote favourable microenvironments
to support tumour growth by enhancing cell metastasis and avoiding
apoptosis (10). In addition, cancer
exosomes have the ability to induce neovascularization, which
ensures the acquisition of nutrients and oxygen and the removal of
waste, and contributes to sustained tumour cell proliferation
(7,11). The invasion and dissemination of
tumours is highly enhanced by cancer exosomes, which carry
information that contributes to extracellular matrix (ECM)
remodelling, cancer cell migration and invasion (12,13).
Furthermore, it has been demonstrated that exosomal communication
contributes to tumour immune escape and metastatic niche
preparation (14–18).
The present review discusses the interactions
between exosomes and the malignant biology of PC. The potential
clinical application of exosomes are also discussed.
Biological characteristics of exosomes
Exosomes constitute a subpopulation of small
extracellular vesicles that arises from the membranes of MVBs, and
are released from the cell into the extracellular environment with
the plasma membrane (18). Almost
all live cells, including stroma cells, reticulocytes, epithelial
cells and tumour cells, can release exosomes, and such exosomes
have been extracted from blood plasma, serum, urine, bile, saliva
and breast milk (4,6,19–32).
Exosomes are small, membrane-enclosed vesicles (40–150 nm) that can
deliver cargo (proteins, lipids and nucleic acids) from the cells
of origin to recipient cells (13).
A type of small vesicle released from marrow mesenchymal stem cell
(MSC)-derived exosomes has been demonstrated to transfer functional
RNAs to recipient cells, illustrating their promise as an
alternative for cell-based therapy (14). Notably, it has been reported that
exosomes can carry microRNAs (miRNAs), which are involved in cancer
cell proliferation, differentiation and apoptosis (15,16). In
addition, as tumour suppressors or oncogenes, miRNAs regulate gene
expression post-transcriptionally (14). Previous studies have demonstrated
that exosomes from pancreatic cells play an important role in niche
initiation prior to liver metastasis (15,16). A
unknown pre-metastatic circuit has been described, through which
pancreatic adenocarcinoma (PDAC)-derived exosomes induce the
formation of pre-metastatic niches that promote the development of
metastatic disease (17).
Exosome-mediated metastasis of oncogenic miRNAs from pancreas
cancer cells may change the biological characteristics of
non-cancer cells; however, metastasis of tumour-suppressing miRNAs
may inhibit the proliferation of pancreatic cells (18). According to proteomic analyses, some
proteins associated with cytosolic signalling proteins, cell
surface receptors, antigen presentation, metabolic enzymes, the
major histocompatibility complex (MHC), HSPs (such as HSP70, HSP90,
HSP60 and HSC70) and tetraspanins (CD9, CD63, CD81 and CD82) are
selectively enriched in specific exosomes (33,34).
Some of the aforementioned proteins participate in the normal
physiological activities of exosomes, while other proteins mediate
interactions between exosomes and recipient cells. For example, a
family of tetraspanins or integrins on the exosomal membrane can
selectively act on target cells or target organs (35). Another class of proteins is
associated with the specificity of the cell of origin, such as
melanoma exosomes, which express the tumour-associated protein
melanoma antigen recognized by T cell 1, and tumour exosomes of
epithelial cell origin, which express epithelial cell adhesion
molecule (EpCAM) (36,37).
Exosomes are secreted by different types of cells
and play important roles in cellular communication (4,21).
However, only the following three mechanisms by which microvesicles
are released into the extracellular space are known: Exocytic
exosome release from intracellular MVBs, single-membrane vesicle
release from the plasma membrane and apoptotic body release from
cells undergoing apoptosis (33).
Exosomes have pleiotropic effects that influence the physiology of
neighbouring cells. Of these effects, the best studied in
vitro effects include the roles of exosomes in several stages
of the immune response (interactions with immune cells). These
roles range from exosomes acting as a vehicle for antigen
presentation to antigen-independent roles that can inhibit
(immunosuppressive properties) or promote (immune-activating
properties) immune responses. In addition, exosomes play a role in
intercellular communication by acting as conveyors of proteins and
lipids that affect downstream signalling events in recipient cells
(35). Exosomes can also deliver
genetic material that affects the physiology of recipient cells
(33).
Some nucleic acids and lipids also exhibit highly
selective enrichment (5,8,23–25).
Nucleic acids include miRNAs, mRNAs, transfer RNAs, ribosomal RNAs
and non-coding RNAs (23–25). Among these, miRNAs, which are a type
of non-coding RNA, 19–25 nucleotides in length, can
post-transcriptionally inhibit the expression or translation of
target genes (38,39). Furthermore, miRNAs can disrupt the
stability of mRNA and inhibit its translation, regulate the
expression of target genes in different types of cells, and
participate in important biological processes, such as cell
proliferation, differentiation, apoptosis and metabolism (40). In addition, the lipid molecules in
exosomes exhibit great research potential in PC (41). Most lipid molecules in exosomes,
including sphingomyelin, cholesterol and phosphatidylserine, are
located on the membrane (6).
Previous studies have demonstrated that sphingomyelin and
cholesterol can improve the stability of exosomal phospholipid
bilayers (42,43). In addition, phosphatidylserine can
promote the fusion of exosomes with target cell membranes and
participate in signal transduction as a signalling molecule
(41). Exosomal lipids can induce
apoptosis in human PC SOJ-6 cells by inhibiting the Notch1 pathway.
In addition, exosomal lipids can also induce drug resistance in
human PC cells through the C-X-C motif chemokine receptor 4
(CXCR4)/stromal cell-derived factor 1α (SDF1α) signalling pathway
(42,43).
Exosomes are membrane vesicles that are released by
cells upon the fusion of MVBs with the plasma membrane. Their
molecular composition reflects their origin in endosomes as
intraluminal vesicles. In addition to a common set of membrane and
cytosolic molecules, exosomes harbour unique subsets of proteins
linked to cell type-associated functions (40). Exosome secretion participates in the
eradication of obsolete proteins; however, a number of studies,
particularly those investigating the immune system, have
demonstrated that exosomes constitute a potential mode of
intercellular communication (42,43). The
release of exosomes by tumour cells and their involvement in the
propagation of unconventional pathogens, such as prions, are
indicative of their participation in pathological situations
(39).
Exosomes as potential diagnostic markers of
PC
PC is a major threat to human health, with very few
effective therapies and a poor prognosis (1–3). As the
fourth leading primary cause of mortality among cancers, PC has an
incredibly low survival rate (3).
Despite improvements in PC therapies, the mortality of the disease
has remained relatively the same over recent decades, largely due
to the lack of adequate screening methods and biomarkers for early
diagnosis (3).
Progress in the treatment of PDAC remains elusive
despite the substantial time and resources invested in attempts to
improve its dismal prognosis. PC is a malignant disease that
develops rapidly and has a poor prognosis (2). Currently, surgery is the only radical
treatment. The American Cancer Society estimates that ~56,770
people will be diagnosed with, and ~45,750 will die of PC in 2019.
The most recent SEER database reported a 9% 5-year survival rate
from 2008–2014 (3). The early
diagnosis of PC is difficult due to the lack of specific symptoms
(1). In the current stage of
clinical treatment, imaging examinations are extensively used for
qualitative and positional diagnosis of PC. The serum marker,
CA19-9 is also used; however, it has a low specificity for PC
(2). Only 40% of patients with early
PC have elevated serum CA19-9 levels, and several patients are
diagnosed with advanced disease (44,45).
Thus, the search for novel early diagnostic markers that can be
used to differentially diagnose PC and other benign lesions has
become the focus of research concerning PC diagnosis and
treatment.
Glypican 1 (GPC1) is crucial for
metastatic potential of PC cells
GPC1 is a lipid raft-heparan sulfate proteoglycan
located on the cell surface that is involved in several important
cellular signalling pathways such as cellular division,
differentiation and morphogenesis (46). It has been demonstrated that
downregulation of GPC1 expression in the PC cell line, PANC-1, can
slow the proliferation rate of PC cells, and decrease angiogenesis
and metastasis in PC (47). Thus,
both cancer cell- and host-derived GPC1 are crucial for the full
mitogenic, angiogenic and metastatic potential of PC cells
(47). Using flow cytometry to
detect and isolate GPC1 from serum exosomes from patients with PC
and mouse models of PC, Melo et al (28) demonstrated that GPC1 is enriched in
exosomes from PC. In addition, GPC1 in these exosomes has high
specificity and sensitivity, and exhibits potential as a
serological marker for the initial diagnosis and prognosis
prediction of patients with early PC (28,48).
Exosomal microRNAs are important tools
to diagnose PC
Recent studies have also focused on elucidating
miRNAs that can be used as specific markers of PC by comparing
miRNA expression between patients with pancreatic cancer and
healthy controls (28,48). Next-generation sequencing and reverse
transcription-quantitative (RT-q)PCR analyses of exosomal microRNAs
from PC are important tools used to identify biomarkers for the
diagnosis of PC. For example, miR-10b, miR-550, miR-196a, miR-1246
and miR-451a have all been experimentally confirmed to be enriched
in PC exosomes and can be used as markers for the early diagnosis
of PC (49–52). Regarding miRNA-based RT-PCR assays, a
recent study designed Bulge-Loop miRNA RT-qPCR primer sets (one RT
primer and a pair of quantitative PCR primers for each set) for
four types of miRNAs, namely, miR-21, miR-17-5p, miR-155 and
miR-196a. MiRNAs in serum from 49 patients, including 22 patients
with PC, six patients with benign pancreatic tumours, seven
patients with ampullary carcinomas, six patients with chronic
pancreatitis patients and eight healthy volunteers, were used. The
clinicopathological data were collected and the patients with PC
were classified according to the presence and absence of
metastasis, tumour differentiation and advanced stage. Patients
with PC had higher expression levels of serum exosomal miR-17-5p
and miR-21 compared with the control group, suggesting that the
miRNAs in serum exosomes represent serum markers for PC diagnosis
(53). Notably, the expression
profiles of exosomal miR-21 and miR-17-5p were significantly
enhanced in patients with PC compared to multiple controls, whereby
this difference may be used to distinguish patients with PC from
patients with non-malignant chronic pancreatitis (54). Another study focused on investigating
whether exosomal miRNAs can be used to localize PC. Exosomes were
collected from conditioned media of PC cell lines and plasma
samples from patients with localized PC (stage I–IIA, n=15) and
healthy subjects (n=15). The cells and exosomal miRNAs from the
pancreatic cancer cell lines were profiled via next-generation
sequencing, and the plasma exosome miRNA expression was detected
via RT-qPCR analysis. This experiment confirmed that miR-196a and
miR-1246 are highly enriched in PC exosomes. Consistently, the
plasma exosome miR-196a and miR-1246 levels were significantly
higher in patients with PC compared with the controls. The control
group included patients with other pancreatic diseases.
Furthermore, when combined with cancer subtypes in the analysis,
plasma exosome miR-196a was a better indicator of PDAC, whereas
plasma exosome miR-1246 was significantly elevated in the patients
with intraductal papillary mucinous neoplasms. Conversely, miR-196a
and miR-1246 levels did not differ between patients with pancreatic
neuroendocrine tumours and the healthy subjects (51).
Madhavan et al (27) simultaneously assessed both serum
exosomal proteins and miRNA markers derived from the supernatant of
a PC cell line and a gene microarray of patients with PC,
respectively. The PC initiating cell (PaCIC) markers, CD44v6,
Tspan8, EpCAM, MET and CD104 were detected via flow cytometric
analysis. The serum exosomes and exosome-depleted serum were
assessed for miR-1246, miR-3976, miR-4306 and miR-4644 via RT-qPCR
analysis. As a result, the concomitant evaluation of PaCIC and
miRNA serum-exosome markers exhibited significantly improved
sensitivity [1.00; 0.95–1], with a specificity for PC of 0.80 (CI,
0.67–0.90) and 0.93 (CI, 0.81–0.98) after excluding the
non-malignant tumours, compared with all the other groups. Thus,
assessing the expression levels of initial tumour cell markers and
miRNAs in serum exosomes from patients with PC can significantly
improve the sensitivity of the serological detection of PC, and
differentiate patients with PC from healthy subjects, patients with
non-malignant chronic pancreatitis and patients with benign
pancreatic lesions, with a specificity of ~93% (27).
Macrophage migration inhibitory factor
is highly expressed in pancreatic cancer-derived exosomes
Based on a study by Madhavan et al (27), a novel experiment recently
investigated whether exosomal miRNAs in saliva can be used as
biomarkers of pancreatobiliary tract cancer. Saliva was collected
from 12 patients with pancreatobiliary tract cancer; the exosomal
miRNAs in the saliva were extracted via RT-qPCR, and the results
demonstrated that the expression levels of miR-1246 and miR-4644
were significantly higher in the cancer group compared with the
controls. These results suggest that miR-1246 and miR-4644 in
salivary exosomes may be candidate biomarkers of pancreaticobiliary
tract cancer. Macrophage migration inhibitory factor (MIF) is
highly expressed in pancreatic cancer-derived exosomes, and its
inhibition prevents the formation of premetastatic niches and the
progression of PC (55). Compared
with patients whose pancreatic tumours did not progress, patients
with stage I PC who subsequently developed liver metastasis
exhibited a significantly increased expression of MIF, indicating
that exosomal MIF plays an important role in liver metastasis, and
may also be an indicator for predicting liver metastasis in the
future (56).
Taken together, these studies suggest that
components of exosomes are of great significance for PC diagnosis.
However, due to the small sample sizes and lack of
generalizability, whether exosomal miRNA detection can be used as
an early diagnostic marker of PC remains to be further investigated
(Table I).
 | Table I.Specific types and functions of
different miRNAs and their associations with pancreatic cancer. |
Table I.
Specific types and functions of
different miRNAs and their associations with pancreatic cancer.
| Author/Year | miRNA/Protein | Physiological
function | (Refs.) |
|---|
| Joshi et al
(49), 2015 | miR-10b | Early diagnostic
markers of PC | (49) |
| Que et al
(53), 2013 | miR-17-5p | Early diagnostic
markers of PC | (53) |
| Charrier et
al (60), 2014 | miR-21 | 1. Promote
transformation of pancreatic epithelial cells into stromal
cells | (53,60,102) |
|
|
| 2. Promote
metastasis of hypoxic tumour cells |
|
|
|
| 3. Early diagnostic
markers of PC |
|
| Chen et al
(85), 2017 | miR-23b-3p | 1. Promote
pancreatic cancer cell proliferation and migration, and upregulate
CXCL1/CXCL2 2. Associated with CA19-9 levels | (85) |
| Wu et al
(64), 2019 | miR-126-3p | Downregulate ADAM9
and inhibit proliferation, invasion and metastasis of PC cells | (64) |
| Richards et
al (62), 2017 | miR-146a | Increased secretion
due to Snail upregulation, and induce proliferation and
chemoresistance formation of PC cells | (62,63) |
| Pang et al
(61), 2015 | miR-155 | 1. Promote the
proliferation of pancreatic interstitial cells | (53,61,104–106) |
|
|
| 2. Early diagnostic
markers of PC |
|
|
|
| 3. Involved in the
formation of chemoresistance |
|
| Matsushita et
al (71), 2016 | miR-196a | Sensitive index for
early diagnosis of PDAC | (71,93) |
| Zhou et al
(97), 2014 | miR-203 | Downregulate the
expression of TLR4, as well as downstream cytokines in DCs, and
promote formation of immune suppression | (97) |
| Ding et al
(96), 2015 | miR-212-3p | Inhibit the
expression of MHC II and induce the formation of immunological
tolerance in DCs | (96) |
| Wang et al
(82), 2018 | miR-301a-3p | Activate PTEN/PI3Kγ
signalling pathway and promote metastasis of PC cells | (82) |
| Li et al
(83), 2018 | miR-338 | Regulate the
expression of MACC1, and promote metastasis and invasion of PC
cells | (83) |
| Takikawa et
al (84), 2017 | miR-451a | 1. Promote
pancreatic cancer cell proliferation and migration, and upregulate
CXCL1/CXCL2 | (52,84) |
|
|
| 2. Early diagnostic
markers of PC |
|
| Taller et al
(50), 2015 | miR-550 | Early diagnostic
markers of PC | (50) |
| Taller et al
(50), 2015 | miR-1246 | Sensitive index for
early diagnosis of PDAC | (50) |
| Madhavan et
al (27), 2015 | miR-3976, miR-4306,
miR-4644 | Early diagnostic
markers of PC | (27) |
Exosomes regulate proliferation in PC
Generally, tumour patients have more exosomes in
their blood compared with healthy individuals. These exosomes are
rich with proteins, lipids and nucleic acids, which play a pivotal
role in interactive cell-to-cell information transfer (57). Kahlert et al (58) demonstrated that KRAS and p53 DNA are
mutated in both PC cell lines and serum-derived exosomes, which is
a very common type of mutation in PC (59). KRAS mutations indicate the
development of early intraductal tumours, while p53 mutations
indicate the transition of tumours from a low to high grade
(58,59).
Exosomes released from PC cells also have a
stimulatory effect on pancreatic stellate cells (PSCs), which
remain quiescent in healthy individuals. PSCs constitute a
characteristic type of pancreatic stromal cells, similar to the
population of stellate cells in the liver or other organs and can
be converted into activated myofibroblasts upon appropriate
stimulation. Activated PSCs can release exosomes containing miR-21
(59). These exosomes can promote
the transformation of pancreatic epithelial cells into stromal
cells, enhance their proliferative ability and promote the
proliferation of stromal cells (60). In addition, it has been demonstrated
that PC cells promote mesenchymal proliferation by releasing
exosomes rich with miR-155 (61).
Recently, studies investigating cancer-associated
fibroblasts (CAFs) have gradually increased (62–65).
CAFs, which develop from bone marrow derived MSCs, are cellular
components of the desmoplastic stroma that are characteristic of
tumours and inextricably associated with the proliferation of PC
cells. CAFs treated with gemcitabine exhibit significantly
increased exosome release, which consequently increases the
proliferation and survival of PC cells (61). Mechanistically, correlative studies
have demonstrated increased expression of Snail (Snai1) and the
Snail target, mRNA-146a, in these exosomes. Furthermore, inhibiting
the release of CAF exosomes can decrease the proliferation and
survival of PC cells (62). Notably,
the same MSCs inhibit the progression of PC. It has been
demonstrated that MSCs downregulate metalloproteinase-9 by
overexpressing exosomes carrying miR-126-3p, thus inhibiting the
proliferation, invasion and metastasis of PC cells (63). This study aimed to elucidate how
non-tumour-derived exosomes may affect the proliferation, invasion
and apoptosis of PC cell lines, and highlighted the potential of
miR-126-3p as a novel biomarker for the treatment of PC (64). A more advanced study suggested that a
zinc protein, ZIP4, slows PC progression by decreasing the
secretion of HSP70 and HSP90. A subset of exosomes mediate the
transport of these HSPs (65).
These studies substantiate that the effects of
exosomes on PC cell proliferation depend on the cells from which
the exosomes are derived. Not all molecules carried by exosomes
play a role in promoting proliferation in PC. Conversely, some
signalling molecules delay the progression of PC. Undoubtedly,
additional exosomal signalling pathways remain to be
investigated.
Exosomes guide the premetastatic stage
of PC
The liver is the most common metastatic site in PC.
The liver premetastatic niche comprises Kupffer cells, hepatic
stellate cells (HSCs), bone marrow-derived cells, ECM and soluble
factors, such as cytokines and chemokines (66). Primary tumour cells secrete large
amounts of cytokines and growth factors that promote the
mobilization and replenishment of bone marrow-derived cells to
future metastatic sites, and promote the formation of the tumour
microenvironment. By injecting PDAC-derived exosomes into mice,
Costa-Silva et al (56)
demonstrated that exosomes play a crucial role in the liver
pre-metastasis of PDAC, ultimately leading to an increased
metastatic burden in the liver. When ingested by Kupffer cells,
these exosomes cause TGF-β secretion and the upregulation of
fibronectin, which is an ECM component produced by activated HSCs
(56). In addition, this fibrotic
microenvironment enhances the recruitment of bone marrow-derived
macrophages. These bone marrow-derived cells modulate the tumour
microenvironment through ECM remodelling, immune suppression and
inflammation. Furthermore, the experiment demonstrated that
macrophage MIF is highly expressed in PDAC-derived exosomes. By
blocking this signalling molecule, all successive steps of
pre-metastasis niches in the liver were blocked to prevent exosome
induced PDAC transfer. In addition, it was experimentally
demonstrated that MIF is elevated in plasma exosomes isolated from
a mouse model of PC with pancreatic intraepithelial neoplasia or
PDAC injury (67). MIF expression in
patients with stage I PC who subsequently developed liver
metastases was significantly higher than that in patients whose
pancreatic tumours did not progress (56).
Furthermore, Nielsen et al (68) demonstrated that metastasis-associated
macrophages are exclusively derived from the bone marrow and can
activate HSCs to transform into myofibroblasts, leading to the
formation of a fibrotic microenvironment in the liver that supports
the growth of metastatic PDAC. Simultaneously, another type of
macrophages in the liver, embryo-derived tissue-resident
macrophages (Kupffer cells), can promote the activation and
fibrosis of HSCs, which is an important process in the formation of
the premetastatic niche (69).
Tumour-derived exosomes also regulate the formation
of premetastatic niches through the binding of integrins on their
own membrane structure to specific target cells. For example,
targeting integrins α6β4 and αvβ5 can decrease exosome uptake and
decreased lung and liver metastasis, respectively. Notably, most
PDAC-derived exosomes accumulate in the liver. Although exosomes
were administered via retroorbital injection, they were unable to
reach the lungs (70). The liver is
the most common metastatic organ in PC, not only because of the
anatomy associated with the entry of pancreatic blood into the
liver through the portal vein but also because PDAC-derived
exosomes are recruited into the liver (71). While melanoma cell-derived exosomes
are taken up by liver macrophages and lung endothelial cells, the
molecular mechanism by which pancreatic cancer-derived exosomes
enter the liver remains unclear.
Mechanisms by which exosomes promote PC
metastasis
Exosomes promote metastasis and invasion in PC.
Exosomes are released into bodily fluids by tumour cells and
participate in the formation of the tumour microenvironment. Due to
its high metastatic potential and invasiveness, PC has very poor
therapeutic outcomes (66). Several
studies have demonstrated the important role of exosomes in
promoting metastasis and invasion in PC (66,72–74). On
one hand, exosomes promote this process by modulating the tumour
microenvironment since the metastasis of tumour cells is closely
associated with the tumour microenvironment, and hypoxia and
inflammatory cell infiltration (particularly macrophage
infiltration) are two important factors (75,76).
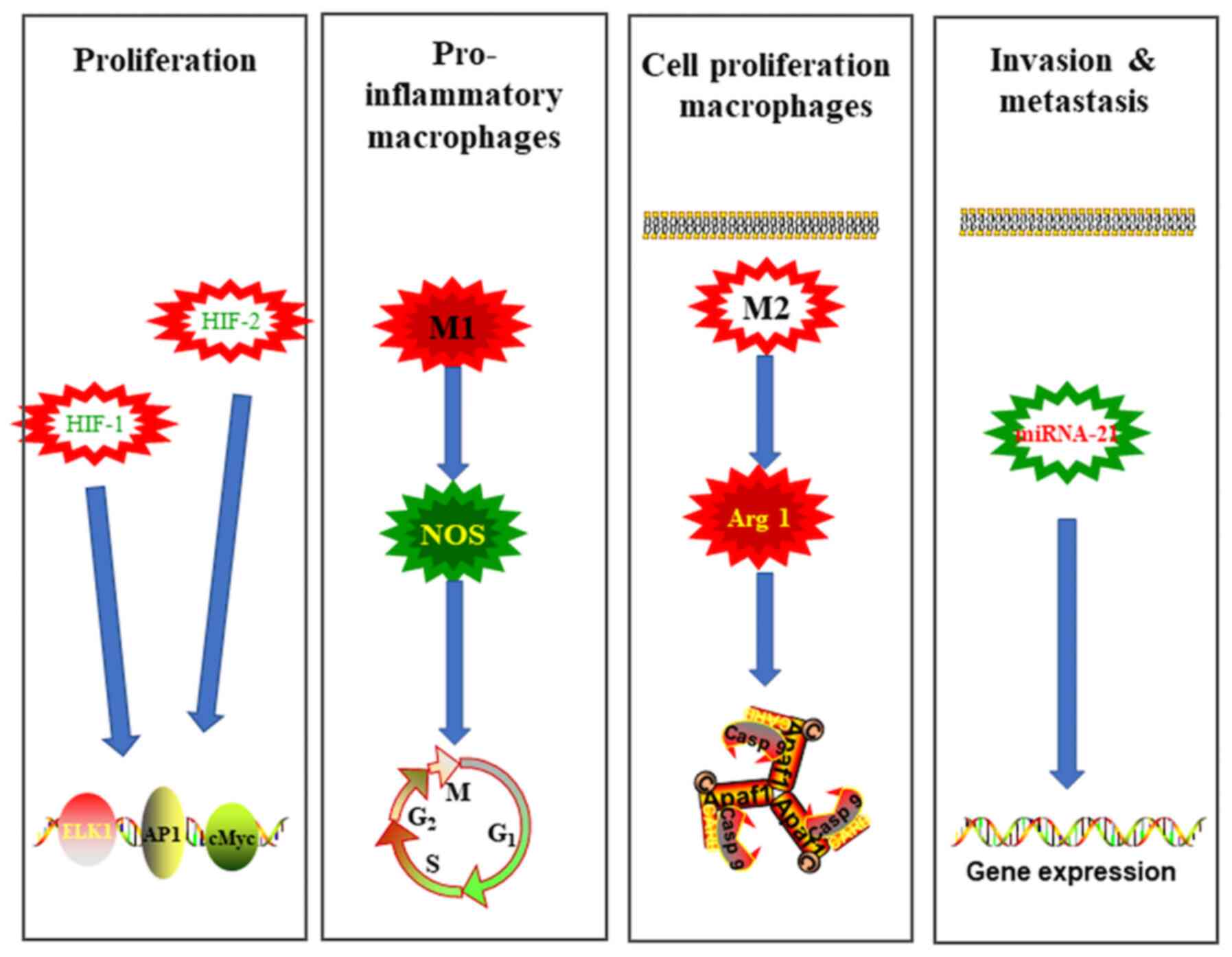
Hypoxia may contribute to tumour progression by modulating
cell-to-cell communication by modifying exosome release. PC cells
can produce miRNA-21-enriched exosomes to promote the metastasis of
hypoxic tumour cells (77). In
addition, the stabilization and activation of hypoxia-inducible
factors (HIFs), particularly HIF-1α and HIF-2α, which activate
proto-oncogenes that promote tumour growth, angiogenesis and cell
metastasis, are major mechanisms by which PC cells respond to
hypoxia (78). Macrophages are the
most abundant infiltrating immune-related stromal cells near tumour
cells. Depending on the microenvironment, macrophages can be
polarized into the classically activated type (M1) or alternatively
activated type (M2) (79). M1
macrophages are characterized by the expression of inducible nitric
oxide synthase and are pro-inflammatory, while M2 macrophages
express higher levels of anti-inflammatory cytokines and a more
active arginase-1, which favours the proliferation of tumour cells
(80). A hypoxic microenvironment
can activate the phosphatase and tensin homologue/phosphoinositol
3-kinase (PI3K) gamma signalling pathway via the secretion of
miRNA-301a-3p-enriched exosomes (81). Subsequently, M2 macrophage
polarization is stimulated in a manner that induces HIF1α or HIF2a,
thereby promoting metastasis of tumour cells (82).
On the other hand, exosomes can also directly affect
the metastatic ability and invasiveness through certain signalling
pathways, such as circ-PDE8A (83).
Recently, a circular RNA (circ-PDE8A) was extracted from liver
metastatic PDAC cells via microarray analysis, and it was
demonstrated that high circ-PDE8A expression is associated with
lymphatic invasion, the TNM stage and poor survival in patients
with PDAC (84). Further studies
revealed that circ-PDE8A promotes the invasive proliferation of
PDAC cells by upregulating MET. For example, circ-PDE8A regulates
metastasis-associated colon cancer-1 (MACC1) as a ceRNA of miR-338,
and stimulates invasive proliferation through the MACC/MET/ERK or
AKT pathways (83). In addition,
PSCs can secrete exosomes carrying miR-451a, which promotes PC cell
proliferation and migration, and upregulates the expression of the
chemokine ligands, CXCL1 and CXCL2 (84). Overexpression of miR-23b-3p also
promotes this process, and miR-23b-3p expression is associated with
the serum carcinoembryonic antigen 199 levels (Fig. 1) (85).
Exosomes promote immune tolerance in
PC
Macrophages can be polarized into two key
phenotypes, M1 and M2 macrophages. M1 macrophages express high
levels of MHC I and MHC II antigens, and secrete complement factors
that promote complement-mediated phagocytosis (60,80). M1
macrophages also produce high levels of pro-inflammatory factors,
such as interleukin (IL)-1, IL-6, IL-23 and TNF (80). Conversely, M2 macrophages are
characterized by lower pro-inflammatory cytokine production,
leading to suppression of inflammatory responses, suppression of T
cell proliferation and attenuation of adaptive immune responses
(27). Previous studies have
demonstrated that the invasion of PC is mostly supported by M2
macrophages, which exhibit decreased phagocytosis of tumour cells,
and their number is also positively associated with the degree of
peripheral lymph node metastasis and early distant metastasis
(80,86). Furthermore, Di Caro et al
(87) demonstrated that most
tumour-associated macrophages at the tumour-stroma interface in
patients with PDAC were of the M2 type, and that the prognostic
relevance of postsurgical adjuvant chemotherapy for PDAC was
associated with a decrease in the density of CD206(+) and IL-10(+)
macrophages at the tumour-stroma interface. In addition, exosomes
extracted from the saliva of PDAC mice had inhibitory effects on
immune surveillance and decreased the tumour-killing ability of NK
cells (88). Exosomes inhibit the
cytotoxicity of NK cells against tumour cells, which may be
associated with the expression levels of TGF-β1, MICA/MICB and
myeloid blast markers (CD34, CD33 and CD117) (88). In particular, TGF-β, which
downregulates the NK cell-activating receptor NKG2D, inhibits NK
cell activity and cytotoxicity.
Fridlender et al (89) demonstrated that the switch of
neutrophils to a protumour phenotype depends on TGF-β exposure, and
that the recruitment of these cells to the tumour microenvironment
is partially driven by macrophages. Neutrophils have also been
demonstrated to be abundant at the invasive front of liver
metastases in PC (90). Similar to
the transformation mechanism of macrophages, neutrophils appear to
adopt an alternative tumour-promoting phenotype to promote cell
proliferation, angiogenesis, tumour invasion and suppression of the
adaptive immune response (91).
However, more cancer clinical data and additional evidence are
required to determine the molecular mechanism by which exosomes
regulate this type of cell.
Exosomes regulate adaptive innate
immune responses in PC
Tumour-derived exosomes are widely present in the
tumour microenvironment and plasma from tumour patients. These
exosomes carry and deliver various stimulatory and inhibitory
molecules to human immune cells, giving tumour cells the
opportunity to achieve immunosuppression and immune escape.
Increasing evidence suggests that T cell infiltration in the tumour
microenvironment is closely associated with patient outcomes.
Tumours that can escape recognition by cytotoxic T cells generally
have a poor prognosis (91). In
patients with PC, it has been demonstrated that a high ratio of
tumour-infiltrating regulatory T cells (T-regs), defined as
FoxP3(+) CD4(+) T cells, is significantly associated with shortened
survival, whereas high levels of tumour-infiltrating CD(+) T and
CD8(+) T cells are significantly associated with prolonged survival
(92). Among these cells, T-regs
have been demonstrated to support tumour growth and expansion by
suppressing host immune responses and accelerating angiogenesis and
tissue remodelling (93). Their role
in the immune response from premalignant lesions to the established
stage of PDAC suggests that a high prevalence of T-regs can serve
as a marker for evaluating a poor prognosis (93). Increasing evidence suggests that
tumour-derived exosomes have immunomodulatory properties, which are
able to induce T-reg polarization, promote T-reg expansion,
upregulate T-reg suppressive function and enhance T-reg resistance
to apoptosis (94). The critical
role of TGF-β in FOXP3 expression in T-regs was further
demonstrated by Wada et al (95), who isolated exosomes from malignant
effusions from patients with cancer to help maintain cultured
T-regs. Exosomes can release TGF-β following treatment with Kupffer
cells in premetastatic niches, although it is not possible to
determine whether PDAC-derived exosomes contain TGF-β (54). These experiments demonstrate that
exosomes can induce TGF-β production in immune cells, which may
play an important role in maintaining tumour immune tolerance.
In addition, it is well known that dendritic cells
(DCs) play an important role in activating immune responses.
Previous studies have investigated how exosomal miRNAs derived from
PC suppress mRNA expression in DCs and induce immune tolerance
(96). Compared with immature DCs,
exosome-stimulated DCs exhibited upregulation of 9 PC-related
miRNAs and the inhibition of 208 mRNAs. This result validates the
experimental prediction that regulatory factor X-associated protein
(RFXAP), which is an important transcription factor of MHC II, is
inhibited by miR-212-3p transferred from PC-secreted exosomes,
resulting in decreased MHC II expression. In addition, miR-212-3p
was negatively associated with RFXAP in PC tissues (97). Based on this study, it appears that
PC-derived exosomes inhibit RFXAP expression via miR-212-3p,
thereby decreasing MHC II expression and inducing immune tolerance
in dendritic cells (96). Another
study aimed to investigate the effects of exosomes on Toll-like
receptors (TLRs) in DCs. The effect of miR-203 on TLR4 and
downstream cytokines was studied as an entry point. First, it was
established that miR-203 is expressed in PANC-1 cells and exosomes,
and that its levels are upregulated in exosome-treated DCs. The
results demonstrated that TLR4 expression was decreased in DCs
treated with exosomes and miR-203, while TLR4 was increased in
exosome-treated DCs treated with miR-203 inhibitors. The expression
levels of tumour necrosis factor-α and IL-12 also decreased
following treatment with exosomes and miR-203, and increased in
exosome-treated DCs treated with miR-203 inhibitors. In conclusion,
PC-derived exosomes downregulate TLR4 and downstream cytokines in
DCs via miR-203 (97).
Exosome-induced chemoresistance in
PC
Recently, gemcitabine-based chemotherapy regimens
have remained the mainstay of treatment for advanced or metastatic
PC. However, with the activation of oncogenic miRNAs,
anti-apoptotic enzymes and signalling pathways associated with
cellular chemoresistance, PC cells have gradually developed
resistance to chemotherapy (98). In
addition, stromal tissues in PC are characterized by low blood
perfusion and hypoxia; thus, the dense stroma can affect the
release of chemotherapeutic agents through physical barriers, high
interstitial pressure, compression of blood vessels and dense
stromal cells. Exosomes are important vehicles for intercellular
communication between genes and signalling molecules (98). Previous studies have demonstrated the
important role of exosomes in the chemoresistance of other types of
cancer cells, such as lung, breast, prostate and gastric cancer
cells (99–103). In addition, several experiments
have indicated that exosomes can improve the resistance of PC cells
to chemotherapeutic drugs through various molecular mechanisms
(99).
Exosomes derived from PC can deliver multiple drug
resistance-associated miRNAs and proteins to target cells to
decrease chemotherapy efficacy. CAFs occupy most of the tumour
volume in PDAC (98). Previous
studies have demonstrated that CAFs are intrinsically resistant to
gemcitabine and that CAFs exposed to gemcitabine significantly
increase exosome release (62).
These exosomes upregulate the expression of the
chemoresistance-inducing factor Snail and the Snail target,
mRNA-146a, in recipient epithelial cells and promote proliferation
and chemoresistance in PC cells (98). Further treatment of
gemcitabine-exposed CAFs with the exosome release inhibitor,
GW4869, significantly decreases the survival rate of the exosomal
recipient epithelial cells, indicating the important role of CAF
exosomes in chemoresistance (62).
Given that chemoresistance can spread to all PDAC tissues in a
patient's body, it is assumed that a series of specific miRNAs are
involved in this process, and that changes in their expression
levels or related cellular communication factors can affect the
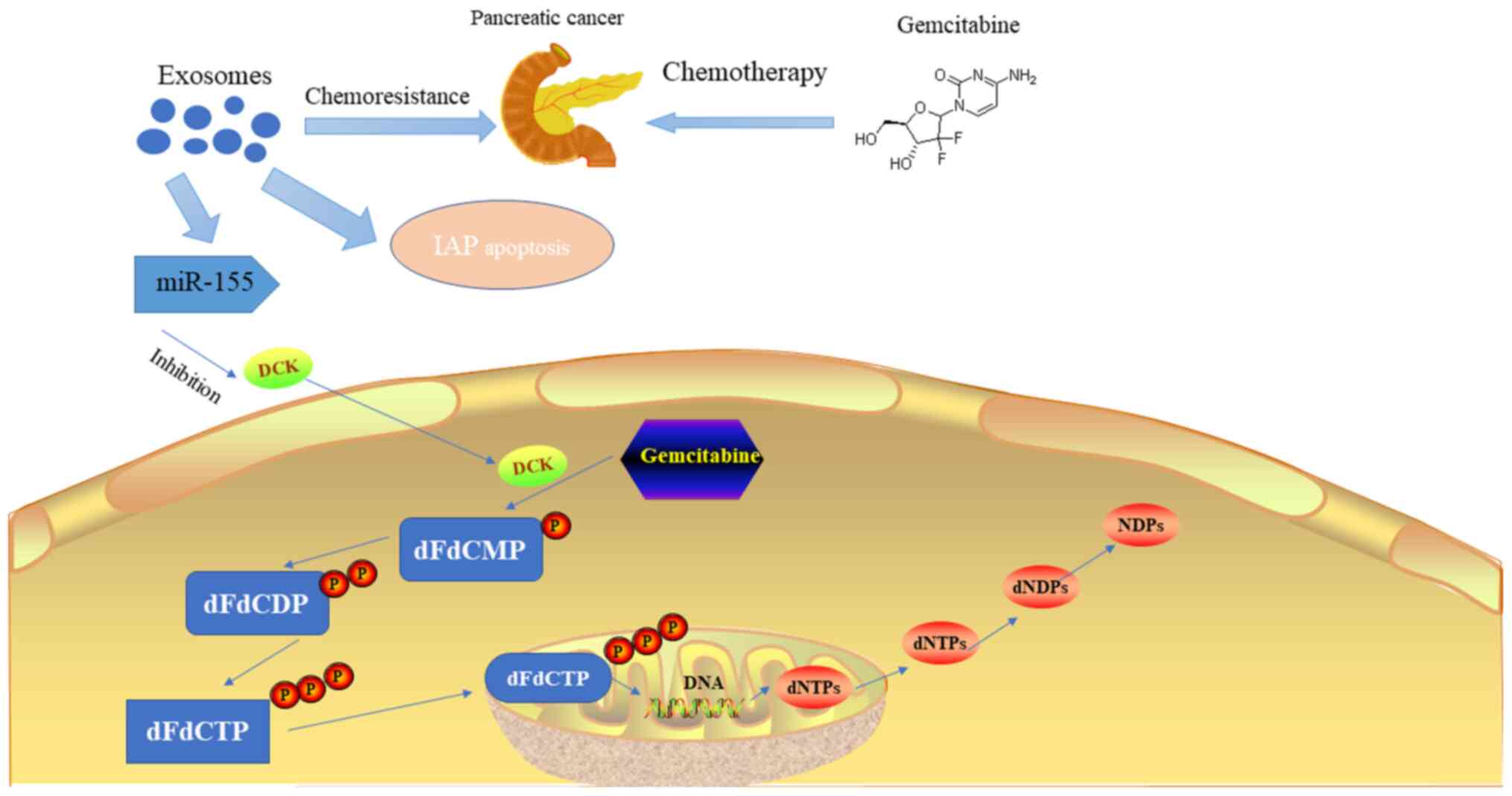
development of chemoresistance (98). miRNA-155 is a typical miRNA that has
multiple effects mediated by its downstream genes and is involved
in several physiological and pathological processes, such as
inflammation, immunity and tumour development. A recent study
suggested the existence of an acquired chemoresistance mechanism in
PC that is mediated by miRNA-155. Conditioned medium from PC cells
pre-treated with gemcitabine provided significant chemoprotection
against subsequent gemcitabine toxicity. A gene expression analysis
demonstrated that superoxide dismutase 2 (SOD2) and catalase (CAT)
are upregulated, while deoxycytidine kinase (DCK) is downregulated
in PC cells following this pretreatment (62). Previous studies have suggested that
exosomes may increase the level of the ROS detoxification gene
expression products, SOD2 and CAT, by lateral transfer of their
transcripts, resulting in chemoresistance in pre-treated PC cells
(104,105). However, exosomes secreted by
pre-treated PC cells can also interfere with the metabolic process
of gemcitabine by inhibiting the activity of DCK via miRNA-155.
Furthermore, chronic exposure to gemcitabine can increase miR-155
expression (105). The increase in
miR-155 expression continues to promote the secretion of exosomes
and the formation of chemoresistance capacity, thereby forming a
positive cycle through which exosomal miR-155 regulates
chemoresistance (106).
Inhibitor of apoptosis protein (IAP) can promote
apoptosis in tumour cells; however, its expression significantly
decreases in different types of tumour cells (54). In a study on PC tissues and cell
lines, Asuncion Valenzuela et al (107) demonstrated that IAP expression is
significantly upregulated by nuclear factor-κB. Exosomes derived
from PC contain the associated mRNA of IAP, and following
chemotherapy, the protein or mRNA IAP levels in the cytoplasm of PC
cells remain unchanged or moderately upregulated. Thus, exosomes
may also enhance the resistance of PC cells to chemotherapeutic
agents by inhibiting IAP expression. Another study reported that
exosomal lipids can induce chemoresistance in human pancreatic
tumour cells (MiaPaCa-2) through CXCR4-stromal cell-derived
factor-1α signalling (43). A recent
study identified a candidate chemoresistance transfer factor,
Ephrin type-A receptor 2 (EphA2). Exosomes from chemoresistant
PANC-1 cells increased the resistance of MIA PaCa-2 and BxPC-3
cells to gemcitabine, and exosomes can be isolated from
chemoresistant PANC-1 cells overexpressing EphA2. However,
treatment of MIA PaCa-2 and BxPC-3 cells with soluble EphA2 did not
promote chemoresistance, indicating that membrane-borne EphA2 is
important for the EphA2 chemoresistance effect (108). Collectively, exosomes can promote
chemoresistance in PC cells by regulating miRNAs, proteins, lipids
and signalling pathways. However, the regulatory molecular
mechanism of exosomes requires verification and further
investigation in additional experiments (Fig. 2).
Exosomes and novel PC therapies
Exosomes have the potential to be targeted
therapeutic carriers affecting processes, such as cell signalling
and material transport, because of their low immunogenicity,
nontoxicity and highly stable biological characteristics in blood.
Compared with other carriers, exosomes not only retain a more
stable lipid bilayer structure to protect their cargoes but also
have a higher targeted delivery capacity, and their smaller
diameter further ensures that they can selectively enter tumour
tissues to achieve better therapeutic effects (43). A previous study has increasingly
focused on how exosomes as natural endogenous carriers can be used
to deliver certain interfering signals or transport cancer drugs
(109).
Mutations in the KRAS gene are widely present in
several malignancies, and G12D and G12V mutations at codon 12 in
the second exon are the most common in PDAC, accounting for 70–95%
of cases (43). The combination of
GTP enzymes and KRAS mutant genes is a key driver of PC. Normally,
KRAS is inactivated immediately after activation (109). However, following KRAS gene
mutation, the KRAS protein maintains a continuous activation state
and no longer depends on stimulation by an upstream signal; thus,
the KRAS protein is in a state of continuous binding with GTP,
leading to abnormal activity in downstream signalling pathways,
such as PI3K-AKT-mTOR, thereby promoting tumour cell proliferation,
transformation, adhesion and survival (109). Buscail et al (110) targeted, silenced and delivered KRAS
G12D via the exosome-mediated delivery of small interfering (si)RNA
to PC cells. As a result, the proliferation and metastasis of the
cancer cells significantly decreased. Recent studies have also
investigated a novel approach to directly and specifically target
oncogenic KRAS in tumours by using engineered exosomes known as
iExosomes (110). SiRNA targeting
mutant KRAS was introduced into fibroblast-derived exosomes to
generate iExosomes (104). As this
exosome carries CD47, it decreases the clearance of iExosomes in
circulation by monocytes and macrophages. Subsequently, the
iExosome is delivered to cancer cells, thereby inhibiting KRAS
GTPase activity and the activation of the downstream MEK-ERK or
PI3K-AKT-mTOR signalling pathways, ultimately inhibiting tumour
growth and metastasis (111).
Another strategy for exosome-targeted therapy is
inhibiting the uptake of exosomes by recipient cells. Heparan
sulfate proteoglycans (HSPGs) serve as internalization receptors
for tumour cell-derived EVs with exosome-like characteristics
(111). Internalized exosomes
colocalize with syndecan and glypican types of cell surface HSPGs,
and the uptake of exosomes is specifically inhibited by free
heparan sulfate (HS) chains, thus suggesting that tumour
cell-derived exosomes use HSPGs for internalization and functional
activity (111). In addition,
syntenin genes can bind the cytoplasmic tail of syndecans, which
are internalized into sorting endosomes along with their intact HS
chains (112). In target cells,
syntenin genes are also involved in maintaining the pool of HSPG at
the cell membrane by stimulating the recycling of the intact form
of HSPG through direct interaction with phosphatidylinositol 4,5
bisphosphate. Mouse fibroblasts isolated from syntenin knockout
mice exhibited low amounts of HSPGs that were associated with the
decreased uptake of exosomes, further confirming that the presence
of HSPGs is essential for the efficient internalization and
function of exosomes (112). In
addition, the syntenin gene is a potential target for future cancer
therapy (112).
Due to the nontoxicity and low immunogenicity of
exosomes in serum, recent experiments have used exosomes as
targeted carriers of chemotherapeutic agents to avoid causing
systemic chemotherapy toxicity in off-target tissues and achieve
better therapeutic effects (113).
MSCs have been proposed for the delivery of anticancer agents
because of their ability to home in on the tumour microenvironment.
MSCs can acquire strong antitumour activity after priming with
paclitaxel (PTX) through their capacity to take up and then release
the drug (114). Pascucci et
al (113) loaded exosomes
secreted by MSCs with PTX to inhibit the proliferation of PANC-1
cells, verifying the possibility of using exosomes to package and
deliver active drugs. Subsequently, Kim et al (114) added PTX-loaded exosomes to
drug-resistant cells and obtained good anticancer effects in a
mouse model. These experiments suggest the feasibility of this
novel exosome system for further development to carry other
chemotherapeutic agents in the future.
Conclusions and future perspectives
The molecular composition of exosomes reflects the
specialized functions of their cells of origin. Through their
ability to bind target cells, exosomes are likely to modulate
selected cellular activities, such as vascular homeostasis and
antigen presentation. The presence of exosomes in blood and tissues
in vivo suggests their participation in physiological and/or
pathological processes, such as PC. Their lipid composition and
presence of proteins that protect against complement, such as CD55
and CD59, may contribute to their stability in the extracellular
environment. The following advantages of an exosomal-acellular mode
of communication should aid the development of diagnostic and
therapeutic strategies: Exosomes are non-living, contain sorted
sets of molecules involved in different cellular processes, have
the capacity to transmit antigenic information and can be easily
recovered from fluids. Based on these properties, diagnostic
protocols, and clinical assays for anti-tumoural immunotherapy are
under development. However, the advantages must be weighed against
the potential consequences that exosomes pose to human health as
exosomes may be used by tumour cells to invade normal tissue and
pathogens, such as prions and HIV, to maximize their spread between
cells.
The present review discusses the interactions
between exosomes and the malignant processes of PC. The potential
clinical applications of exosomes are also discussed. First, the
biological characteristics of exosomes are introduced.
Subsequently, exosomes as diagnostic markers of PC are discussed.
In addition, exosomes regulate the proliferation, metastasis and
invasion of PC cells. Several mechanistic questions remain, thus
prospective studies are required to confirm current findings.
Furthermore, exosomes that promote immune tolerance and induce
chemoresistance are discussed in the present review, along with
novel therapies for PC. The establishment of new bioanalytical
technologies and novel experimental animal models may help
researchers uncover the secrets of exosomes. In the present review,
the molecular mechanisms and functions of several exosomes were
introduced in detail. The roles of these proteins in malignancy
were also discussed, with the ultimate aim of determining the exact
role of exosomes in human cancer cells.
Recently, increasing evidence suggests that exosomes
are associated with cancer and have the capacity to accelerate PC
cell proliferation, migration and invasion (113,114).
Prospective studies are required to provide detailed information
regarding the molecular mechanism by which exosomes regulate the
proliferation of tumour cells, and help establish exosomes as novel
targets for the treatment of PC. It is speculated that the
application of exosomes for the treatment of PC can be highly
effective, suggesting that all human malignant tumours and
associated challenges can be overcome in the future. Recent studies
have demonstrated that some technical methods, such as engineering
and scientific modification of exosomes may treat PC (113–115).
Xu et al (116) developed a
surface modification method for in vitro and in vivo
exosome uptake research through Click Chemistry, which can be used
as the basis for rational design of preclinical exosome therapy. A
number of studies have reported the potential of using exosomes as
nanocarriers to improve exogenous loaded drug therapy as a novel
treatment strategy for PC (16,116–118).
As an important tool for communication and
transport, exosomes mediate cell-to-cell information exchange by
transmitting their carried molecules, such as miRNAs and proteins,
to recipient cells, affecting several physiological functions, such
as the growth and metabolism of recipient cells (16). Recent studies concerning exosomes
have demonstrated their important role in the regulation of PC
proliferation, apoptosis, metastasis and other processes, providing
a new perspective for the understanding of the molecular mechanism
underlying the malignant biological characteristics of PC, and are
expected to provide a new direction for the treatment and early
diagnosis of PC (119–121). Although current research and
experimental results are novel and exciting, there are still many
questions regarding the molecular mechanism of exosomes that remain
to be further investigated.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
HL and SH contributed to the design and drafted the
initial manuscript. HL and SQ revised the manuscript for important
intellectual content. HL, XF, YG and YZ wrote and prepared the
original draft of the manuscript. All authors have read have read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deplanque G and Demartines N: Pancreatic
cancer: Are more chemotherapy and surgery needed? Lancet.
389:985–986. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Théry C: Exosomes: Secreted vesicles and
intercellular communications. F1000 Biol Rep. 3:152011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kalluri R: The biology and function of
exosomes in cancer. J Clin Invest. 126:1208–1215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vader P, Breakefield XO and Wood MJ:
Extracellular vesicles: Emerging targets for cancer therapy. Trends
Mol Med. 20:385–393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Skog J, Wurdinger T, van Rijn S, Meijer
DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky
AM and Breakefield XO: Glioblastoma microvesicles transport RNA and
proteins that promote tumour growth and provide diagnostic
biomarkers. Nat Cell Biol. 10:1470–1476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Johnstone RM, Adam M, Hammond JR, Orr L
and Turbide C: Vesicle formation during reticulocyte maturation.
Association of plasma membrane activities with released vesicles
(exosomes). J Biol Chem. 262:9412–9420. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kowal J, Tkach M and Théry C: Biogenesis
and secretion of exosomes. Curr Opin Cell Biol. 29:116–125. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meehan K and Vella LJ: The contribution of
tumour-derived exosomes to the hallmarks of cancer. Crit Rev Clin
Lab Sci. 53:121–131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fong MY, Zhou W, Liu L, Alontaga AY,
Chandra M, Ashby J, Chow A, O'Connor STF, Li S, Chin AR, et al:
Breast-cancer-secreted miR-122 reprograms glucose metabolism in
premetastatic niche to promote metastasis. Nat Cell Biol.
17:183–194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mu W, Rana S and Zöller M: Host matrix
modulation by tumor exosomes promotes motility and invasiveness.
Neoplasia. 15:875–887. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sung BH, Ketova T, Hoshino D, Zijlstra A
and Weaver AM: Directional cell movement through tissues is
controlled by exosome secretion. Nat Commun. 6:71642015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zitvogel L, Regnault A, Lozier A, Wolfers
J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G and
Amigorena S: Eradication of established murine tumors using a novel
cell-free vaccine: Dendritic cell-derived exosomes. Nat Med.
4:594–600. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abusamra AJ, Zhong Z, Zheng X, Li M, Ichim
TE, Chin JL and Min WP: Tumor exosomes expressing Fas ligand
mediate CD8+ T-cell apoptosis. Blood Cells Mol Dis.
35:169–173. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kamerkar S, LeBleu VS, Sugimoto H, Yang S,
Ruivo CF, Melo SA, Lee JJ and Kalluri R: Exosomes facilitate
therapeutic targeting of oncogenic KRAS in pancreatic cancer.
Nature. 546:498–503. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bobrie A, Krumeich S, Reyal F, Recchi C,
Moita LF, Seabra MC, Ostrowski M and Théry C: Rab27a supports
exosome-dependent and -independent mechanisms that modify the tumor
microenvironment and can promote tumor progression. Cancer Res.
72:4920–4930. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bhatia A and Kumar Y: Cellular and
molecular mechanisms in cancer immune escape: A comprehensive
review. Expert Rev Clin Immunol. 10:41–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mateescu B, Kowal EJ, van Balkom BW,
Bartel S, Bhattacharyya SN, Buzás EI, Buck AH, de Candia P, Chow
FWN, Das S, et al: Obstacles and opportunities in the functional
analysis of extracellular vesicle RNA-an ISEV position paper. J
Extracell Vesicles. 6:12860952017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Colombo M, Raposo G and Théry C:
Biogenesis, secretion, and intercellular interactions of exosomes
and other extracellular vesicles. Annu Rev Cell Dev Biol.
30:255–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang HG and Grizzle WE: Exosomes: A novel
pathway of local and distant intercellular communication that
facilitates the growth and metastasis of neoplastic lesions. Am J
Pathol. 184:28–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Valadi H, Ekström K, Bossios A, Sjöstrand
M, Lee JJ and Lötvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hong CS, Funk S and Whiteside TL:
Isolation of biologically active exosomes from plasma of patients
with cancer. Methods Mol Biol. 1633:257–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie JX, Fan X, Drummond CA, Majumder R,
Xie Y, Chen T, Liu L, Haller ST, Brewster PS, Dworkin LD, et al:
MicroRNA profiling in kidney disease: Plasma versus plasma-derived
exosomes. Gene. 627:1–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou X, Lu Z, Wang T, Huang Z, Zhu W and
Miao Y: Plasma miRNAs in diagnosis and prognosis of pancreatic
cancer: A miRNA expression analysis. Gene. 673:181–193. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Théry C, Ostrowski M and Segura E:
Membrane vesicles as conveyors of immune responses. Nat Rev
Immunol. 9:581–593. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Madhavan B, Yue S, Galli U, Rana S, Gross
W, Müller M, Giese NA, Kalthoff H, Becker T, Büchler MW and Zöller
M: Combined evaluation of a panel of protein and miRNA
serum-exosome biomarkers for pancreatic cancer diagnosis increases
sensitivity and specificity. Int J Cancer. 136:2616–2627. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Melo SA, Luecke LB, Kahlert C, Fernandez
AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari
N, et al: Glypican-1 identifies cancer exosomes and detects early
pancreatic cancer. Nature. 523:177–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Street JM, Koritzinsky EH, Glispie DM,
Star RA and Yuen PST: Urine exosomes: An emerging trove of
biomarkers. Adv Clin Chem. 78:103–122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Katsiougiannis S, Chia D, Kim Y, Singh RP
and Wong DTW: Saliva exosomes from pancreatic tumor-bearing mice
modulate NK cell phenotype and antitumor cytotoxicity. FASEB J.
31:998–1010. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun Y, Liu S, Qiao Z, Shang Z, Xia Z, Niu
X, Qian L, Zhang Y, Fan L, Cao CX and Xiao H: Systematic comparison
of exosomal proteomes from human saliva and serum for the detection
of lung cancer. Anal Chim Acta. 982:84–95. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Qin W, Tsukasaki Y, Dasgupta S,
Mukhopadhyay N, Ikebe M and Sauter ER: Exosomes in human breast
milk promote EMT. Clin Cancer Res. 22:4517–4524. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mathivanan S, Ji H and Simpson RJ:
Exosomes: Extracellular organelles important in intercellular
communication. J Proteomics. 73:1907–1920. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
van Niel G, Porto-Carreiro I, Simoes S and
Raposo G: Exosomes: A common pathway for a specialized function. J
Biochem. 140:13–21. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nazarenko I, Rana S, Baumann A, McAlear J,
Hellwig A, Trendelenburg M, Lochnit G, Preissner KT and Zöller M:
Cell surface tetraspanin Tspan8 contributes to molecular pathways
of exosome-induced endothelial cell activation. Cancer Res.
70:1668–1678. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Raimondo F, Morosi L, Chinello C, Magni F
and Pitto M: Advances in membranous vesicle and exosome proteomics
improving biological understanding and biomarker discovery.
Proteomics. 11:709–720. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Runz S, Keller S, Rupp C, Stoeck A, Issa
Y, Koensgen D, Mustea A, Sehouli J, Kristiansen G and Altevogt P:
Malignant ascites-derived exosomes of ovarian carcinoma patients
contain CD24 and EpCAM. Gynecol Oncol. 107:563–571. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J,
Chen D, Gu J, He X and Huang S: Circular RNA is enriched and stable
in exosomes: A promising biomarker for cancer diagnosis. Cell Res.
25:981–984. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Salido-Guadarrama I, Romero-Cordoba S,
Peralta-Zaragoza O, Hidalgo-Miranda A and Rodríguez-Dorantes M:
MicroRNAs transported by exosomes in body fluids as mediators of
intercellular communication in cancer. OncoTargets Ther.
7:1327–1338. 2014.
|
|
40
|
Medina PP, Nolde M and Slack FJ: OncomiR
addiction in an in vivo model of microRNA-21-induced pre-B-cell
lymphoma. Nature. 467:86–90. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schutters K and Reutelingsperger C:
Phosphatidylserine targeting for diagnosis and treatment of human
diseases. Apoptosis. 15:1072–1082. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Beloribi S, Ristorcelli E, Breuzard G,
Silvy F, Bertrand-Michel J, Beraud E, Verine A and Lombardo D:
Exosomal lipids impact notch signaling and induce death of human
pancreatic tumoral SOJ-6 cells. PLoS One. 7:e474802012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Beloribi-Djefaflia S, Siret C and Lombardo
D: Exosomal lipids induce human pancreatic tumoral MiaPaCa-2 cells
resistance through the CXCR4-SDF-1α signaling axis. Oncoscience.
2:15–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang Y, Yang J, Li H, Wu Y, Zhang H and
Chen W: Tumor markers CA19-9, CA242 and CEA in the diagnosis of
pancreatic cancer: A meta-analysis. Int J Clin Exp Med.
8:11683–11691. 2015.PubMed/NCBI
|
|
45
|
Frebourg T, Bercoff E, Manchon N, Senant
J, Basuyau JP, Breton P, Janvresse A, Brunelle P and Bourreille J:
The evaluation of CA 19-9 antigen level in the early detection of
pancreatic cancer. A prospective study of 866 patients. Cancer.
62:2287–2290. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Awad W, Adamczyk B, Ornros J, Karlsson NG,
Mani K and Logan DT: Structural aspects of N-glycosylations and the
C-terminal region in human glypican-1. J Biol Chem.
290:22991–23008. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Aikawa T, Whipple CA, Lopez ME, Gunn J,
Young A, Lander AD and Korc M: Glypican-1 modulates the angiogenic
and metastatic potential of human and mouse cancer cells. J Clin
Invest. 118:89–99. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Frampton AE, Prado MM, Lopez-Jimenez E,
Fajardo-Puerta AB, Jawad ZAR, Lawton P, Giovannetti E, Habib NA,
Castellano L, Stebbing J, et al: Glypican-1 is enriched in
circulating-exosomes in pancreatic cancer and correlates with tumor
burden. Oncotarget. 9:19006–19013. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Joshi GK, Deitz-McElyea S, Liyanage T,
Lawrence K, Mali S, Sardar R and Korc M: Label-Free
nanoplasmonic-based short noncoding RNA sensing at attomolar
concentrations allows for quantitative and highly specific assay of
MicroRNA-10b in biological fluids and circulating exosomes. ACS
Nano. 9:11075–11089. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Taller D, Richards K, Slouka Z, Senapati
S, Hill R, Go DB and Chang HC: On-chip surface acoustic wave lysis
and ion-exchange nanomembrane detection of exosomal RNA for
pancreatic cancer study and diagnosis. Lab Chip. 15:1656–1666.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xu YF, Hannafon BN, Zhao YD, Postier RG
and Ding WQ: Plasma exosome miR-196a and miR-1246 are potential
indicators of localized pancreatic cancer. Oncotarget.
8:77028–77040. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Takahasi K, Iinuma H, Wada K, Minezaki S,
Kawamura S, Kainuma M, Ikeda Y, Shibuya M, Miura F and Sano K:
Usefulness of exosome-encapsulated microRNA-451a as a minimally
invasive biomarker for prediction of recurrence and prognosis in
pancreatic ductal adenocarcinoma. J Hepatobiliary Pancreat Sci.
25:155–161. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Que R, Ding G, Chen J and Cao L: Analysis
of serum exosomal microRNAs and clinicopathologic features of
patients with pancreatic adenocarcinoma. World J Surg Oncol.
11:2192013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yan Y, Fu G and Ming L: Role of exosomes
in pancreatic cancer. Oncol Lett. 15:7479–7488. 2018.PubMed/NCBI
|
|
55
|
Machida T, Tomofuji T, Maruyama T, Yoneda
T, Ekuni D, Azuma T, Miyai H, Mizuno H, Kato H, Tsutsumi K, et al:
miR1246 and miR4644 in salivary exosome as potential biomarkers for
pancreatobiliary tract cancer. Oncol Rep. 36:2375–2381. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Costa-Silva B, Aiello NM, Ocean AJ, Singh
S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, et
al: Pancreatic cancer exosomes initiate pre-metastatic niche
formation in the liver. Nat Cell Biol. 17:816–826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tsukamoto M, Iinuma H, Yagi T, Matsuda K
and Hashiguchi Y: Circulating exosomal MicroRNA-21 as a biomarker
in each tumor stage of colorectal cancer. Oncology. 92:360–370.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kahlert C, Melo SA, Protopopov A, Tang J,
Seth S, Koch M, Zhang J, Weitz J, Chin L, Futreal A and Kalluri R:
Identification of double-stranded genomic DNA spanning all
chromosomes with mutated KRAS and p53 DNA in the serum exosomes of
patients with pancreatic cancer. J Biol Chem. 289:3869–3875. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sessa F, Bonato M, Bisoni D, Ranzani GN
and Capella C: Ki-ras and p53 gene mutations in pancreatic ductal
carcinoma: A relationship with tumor phenotype and survival. Eur J
Histochem. 42:Spec No. 67–76. 1998.PubMed/NCBI
|
|
60
|
Charrier A, Chen R, Chen L, Kemper S,
Hattori T, Takigawa M and Brigstock DR: Connective tissue growth
factor (CCN2) and microRNA-21 are components of a positive feedback
loop in pancreatic stellate cells (PSC) during chronic pancreatitis
and are exported in PSC-derived exosomes. J Cell Commun Signal.
8:147–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Pang W, Su J, Wang Y, Feng H, Dai X, Yuan
Y, Chen X and Yao W: Pancreatic cancer-secreted miR-155 implicates
in the conversion from normal fibroblasts to cancer-associated
fibroblasts. Cancer Sci. 106:1362–1369. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Richards KE, Zeleniak AE, Fishel ML, Wu J,
Littlepage LE and Hill R: Cancer-associated fibroblast exosomes
regulate survival and proliferation of pancreatic cancer cells.
Oncogene. 36:1770–1778. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
von Ahrens D, Bhagat TD, Nagrath D, Maitra
A and Verma A: The role of stromal cancer-associated fibroblasts in
pancreatic cancer. J Hematol Oncol. 10:762017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wu DM, Wen X, Han XR, Wang S, Wang YJ,
Shen M, Fan SH, Zhang ZF, Shan Q, Li MQ, et al: Bone marrow
mesenchymal stem cell-derived exosomal MicroRNA-126-3p inhibits
pancreatic cancer development by targeting ADAM9. Mol Ther Nucleic
Acids. 16:229–245. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yang J, Zhang Z, Zhang Y, Ni X, Zhang G,
Cui X, Liu M, Xu C, Zhang Q, Zhu H, et al: ZIP4 promotes muscle
wasting and cachexia in mice with orthotopic pancreatic tumors by
stimulating RAB27B-regulated release of extracellular vesicles from
cancer cells. Gastroenterology. 156:722–734. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yu Z, Zhao S, Ren L, Wang L, Chen Z,
Hoffman RM and Zhou J: Pancreatic cancer-derived exosomes promote
tumor metastasis and liver pre-metastatic niche formation.
Oncotarget. 8:63461–63483. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Sceneay J, Smyth MJ and Möller A: The
pre-metastatic niche: Finding common ground. Cancer Metastasis Rev.
32:449–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Nielsen SR, Quaranta V, Linford A, Emeagi
P, Rainer C, Santos A, Ireland L, Sakai T, Sakai K, Kim YS, et al:
Macrophage-secreted granulin supports pancreatic cancer metastasis
by inducing liver fibrosis. Nat Cell Biol. 18:549–560. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Dey A, Allen J and Hankey-Giblin PA:
Ontogeny and polarization of macrophages in inflammation: Blood
monocytes versus tissue macrophages. Front Immunol. 5:6832015.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Hoshino A, Costa-Silva B, Shen TL,
Rodrigues G, Hashimoto A, Mark MT, Molina H, Kohsaka S, Di
Giannatale A, Ceder S, et al: Tumour exosome integrins determine
organotropic metastasis. Nature. 527:329–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Matsushita H, Yang YM, Pandol SJ and Seki
E: Exosome migration inhibitory factor as a marker and therapeutic
target for pancreatic cancer. Gastroenterology. 150:1033–1035.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Milane L, Singh A, Mattheolabakis G,
Suresh M and Amiji MM: Exosome mediated communication within the
tumor microenvironment. J Control Release. 219:278–294. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Syn N, Wang L, Sethi G, Thiery JP and Goh
BC: Exosome-mediated metastasis: From epithelial-mesenchymal
transition to escape from immunosurveillance. Trends Pharmacol Sci.
37:606–617. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Steinbichler TB, Dudás J, Riechelmann H
and Skvortsova II: The role of exosomes in cancer metastasis. Semin
Cancer Biol. 44:170–181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ackerman D and Simon MC: Hypoxia, lipids,
and cancer: Surviving the harsh tumor microenvironment. Trends Cell
Biol. 24:472–478. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lewis CE and Pollard JW: Distinct role of
macrophages in different tumor microenvironments. Cancer Res.
66:605–612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
King HW, Michael MZ and Gleadle JM:
Hypoxic enhancement of exosome release by breast cancer cells. BMC
Cancer. 12:4212012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Semenza GL: Defining the role of
hypoxia-inducible factor 1 in cancer biology and therapeutics.
Oncogene. 29:625–634. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Löfstedt T, Fredlund E,
Holmquist-Mengelbier L, Pietras A, Ovenberger M, Poellinger L and
Påhlman S: Hypoxia inducible factor-2alpha in cancer. Cell Cycle.
6:919–926. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Singh A, Talekar M, Raikar A and Amiji M:
Macrophage-targeted delivery systems for nucleic acid therapy of
inflammatory diseases. J Control Release. 190:515–530. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wang J, Cao Z, Zhang XM, Nakamura M, Sun
M, Hartman J, Harris RA, Sun Y and Cao Y: Novel mechanism of
macrophage-mediated metastasis revealed in a zebrafish model of
tumor development. Cancer Res. 75:306–315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wang X, Luo G, Zhang K, Cao J, Huang C,
Jiang T, Liu B, Su L and Qiu Z: Hypoxic tumor-derived exosomal
miR-301a mediates M2 macrophage polarization via PTEN/PI3Kγ to
promote pancreatic cancer metastasis. Cancer Res. 78:4586–4598.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Li Z, Yanfang W, Li J, Jiang P, Peng T,
Chen K, Zhao X, Zhang Y, Zhen P, Zhu J and Li X: Tumor-released
exosomal circular RNA PDE8A promotes invasive growth via the
miR-338/MACC1/MET pathway in pancreatic cancer. Cancer Lett.
432:237–250. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Takikawa T, Masamune A, Yoshida N, Hamada
S, Kogure T and Shimosegawa T: Exosomes derived from pancreatic
stellate cells: MicroRNA signature and effects on pancreatic cancer
cells. Pancreas. 46:19–27. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Chen D, Wu X, Xia M, Wu F, Ding J, Jiao Y,
Zhan Q and An F: Upregulated exosomic miR-23b-3p plays regulatory
roles in the progression of pancreatic cancer. Oncol Rep.
38:2182–2188. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kurahara H, Shinchi H, Mataki Y, Maemura
K, Noma H, Kubo F, Sakoda M, Ueno S, Natsugoe S and Takao S:
Significance of M2-polarized tumor-associated macrophage in
pancreatic cancer. J Surg Res. 167:e211–e219. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Di Caro G, Cortese N, Castino GF, Grizzi
F, Gavazzi F, Ridolfi C, Capretti G, Mineri R, Todoric J, Zerbi A,
et al: Dual prognostic significance of tumour-associated
macrophages in human pancreatic adenocarcinoma treated or untreated
with chemotherapy. Gut. 65:1710–1720. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Szczepanski MJ, Szajnik M, Welsh A,
Whiteside TL and Boyiadzis M: Blast-derived microvesicles in sera
from patients with acute myeloid leukemia suppress natural killer
cell function via membrane-associated transforming growth
factor-beta1. Haematologica. 96:1302–1309. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Fridlender ZG, Sun J, Kim S, Kapoor V,
Cheng G, Ling L, Worthen GS and Albelda SM: Polarization of
tumor-associated neutrophil phenotype by TGF-beta: ‘N1’ versus ‘N2’
TAN. Cancer Cell. 16:183–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Benson DD, Meng X, Fullerton DA, Moore EE,
Lee JH, Ao L, Silliman CC and Barnett CC Jr: Activation state of
stromal inflammatory cells in murine metastatic pancreatic
adenocarcinoma. Am J Physiol Regul Integr Comp Physiol.
302:R1067–R1075. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Galdiero MR, Bonavita E, Barajon I,
Garlanda C, Mantovani A and Jaillon S: Tumor associated macrophages
and neutrophils in cancer. Immunobiology. 218:1402–1410. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki
M, Kosuge T, Kanai Y and Hiraoka N: Immune cell infiltration as an
indicator of the immune microenvironment of pancreatic cancer. Br J
Cancer. 108:914–923. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Hiraoka N, Onozato K, Kosuge T and
Hirohashi S: Prevalence of FOXP3+ regulatory T cells increases
during the progression of pancreatic ductal adenocarcinoma and its
premalignant lesions. Clin Cancer Res. 12:5423–5434. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Szajnik M, Czystowska M, Szczepanski MJ,
Mandapathil M and Whiteside TL: Tumor-derived microvesicles induce,
expand and up-regulate biological activities of human regulatory T
cells (Treg). PLoS One. 5:e114692010. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wada J, Onishi H, Suzuki H, Yamasaki A,
Nagai S, Morisaki T and Katano M: Surface-bound TGF-beta1 on
effusion-derived exosomes participates in maintenance of number and
suppressive function of regulatory T-cells in malignant effusions.
Anticancer Res. 30:3747–3757. 2010.PubMed/NCBI
|
|
96
|
Ding G, Zhou L, Qian Y, Fu M, Chen J, Chen
J, Xiang J, Wu Z, Jiang G and Cao L: Pancreatic cancer-derived
exosomes transfer miRNAs to dendritic cells and inhibit RFXAP
expression via miR-212-3p. Oncotarget. 6:29877–29888. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zhou M, Chen J, Zhou L, Chen W, Ding G and
Cao L: Pancreatic cancer derived exosomes regulate the expression
of TLR4 in dendritic cells via miR-203. Cell Immunol. 292:65–69.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Nagathihalli NS, Castellanos JA, Shi C,
Beesetty Y, Reyzer ML, Caprioli R, Chen X, Walsh AJ, Skala MC,
Moses HL and Merchant NB: Signal transducer and activator of
transcription 3, mediated remodeling of the tumor microenvironment
results in enhanced tumor drug delivery in a mouse model of
pancreatic cancer. Gastroenterology. 149:1932–1943, e1939. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Lobb RJ, van Amerongen R, Wiegmans A, Ham
S, Larsen JE and Möller A: Exosomes derived from mesenchymal
non-small cell lung cancer cells promote chemoresistance. Int J
Cancer. 141:614–620. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Santos JC, da Silva Lima N, Sarian LO,
Matheu A, Ribeiro ML and Derchain SFM: Exosome-mediated breast
cancer chemoresistance via miR-155 transfer. Sci Rep. 8:8292018.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Li J, Yang X, Guan H, Mizokami A, Keller
ET, Xu X, Liu X, Tan J, Hu L, Lu Y and Zhang J: Exosome-derived
microRNAs contribute to prostate cancer chemoresistance. Int J
Oncol. 49:838–846. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zheng P, Chen L, Yuan X, Luo Q, Liu Y, Xie
G, Ma Y and Shen L: Exosomal transfer of tumor-associated
macrophage-derived miR-21 confers cisplatin resistance in gastric
cancer cells. J Exp Clin Cancer Res. 36:532017. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Wang M, Qiu R, Yu S, Xu X, Li G, Gu R, Tan
C, Zhu W and Shen B: Paclitaxelresistant gastric cancer MGC803
cells promote epithelialtomesenchymal transition and
chemoresistance in paclitaxelsensitive cells via exosomal delivery
of miR1555p. Int J Oncol. 54:326–338. 2019.PubMed/NCBI
|
|
104
|
Marques-Rocha JL, Garcia-Lacarte M,
Samblas M, Bressan J, Martínez JA and Milagro FI: Regulatory roles
of miR-155 and let-7b on the expression of inflammation-related
genes in THP-1 cells: Effects of fatty acids. J Physiol Biochem.
74:579–589. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Patel GK, Khan MA, Bhardwaj A, Srivastava
SK, Zubair H, Patton MC, Singh S, Khushman M and Singh AP: Exosomes
confer chemoresistance to pancreatic cancer cells by promoting ROS
detoxification and miR-155-mediated suppression of key
gemcitabine-metabolising enzyme, DCK. Br J Cancer. 116:609–619.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Mikamori M, Yamada D, Eguchi H, Hasegawa
S, Kishimoto T, Tomimaru Y, Asaoka T, Noda T, Wada H, Kawamoto K,
et al: MicroRNA-155 controls exosome synthesis and promotes
gemcitabine resistance in pancreatic ductal adenocarcinoma. Sci
Rep. 7:423392017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Asuncion Valenzuela MM, Castro I, Gonda A,
Diaz Osterman CJ, Jutzy JM, Aspe JR, Khan S, Neidigh JW and Wall
NR: Cell death in response to antimetabolites directed at
ribonucleotide reductase and thymidylate synthase. Onco Targets
Ther. 8:495–507. 2015.PubMed/NCBI
|
|
108
|
Fan J, Wei Q, Koay EJ, Liu Y, Ning B,
Bernard PW, Zhang N, Han H, Katz MH, Zhao Z and Hu Y:
Chemoresistance transmission via exosome-mediated EphA2 transfer in
pancreatic cancer. Theranostics. 8:5986–5994. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
van den Boorn JG, Schlee M, Coch C and
Hartmann G: SiRNA delivery with exosome nanoparticles. Nat
Biotechnol. 29:325–326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Buscail L: Pancreatic cancer: Exosomes for
targeting KRAS in the treatment of pancreatic cancer. Nat Rev
Gastroenterol Hepatol. 14:636–638. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Christianson HC, Svensson KJ, van
Kuppevelt TH, Li JP and Belting M: Cancer cell exosomes depend on
cell-surface heparan sulfate proteoglycans for their
internalization and functional activity. Proc Natl Acad Sci USA.
110:17380–17385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Fares J, Kashyap R and Zimmermann P:
Syntenin: Key player in cancer exosome biogenesis and uptake? Cell
Adh Migr. 11:124–126. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Pascucci L, Cocce V, Bonomi A, Ami D,
Ceccarelli P, Ciusani E, Viganò L, Locatelli A, Sisto F, Doglia AM,
et al: Paclitaxel is incorporated by mesenchymal stromal cells and
released in exosomes that inhibit in vitro tumor growth: A new
approach for drug delivery. J Control Release. 192:262–270. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Kim MS, Haney MJ, Zhao Y, Mahajan V,
Deygen I, Klyachko NL, Inskoe E, Piroyan A, Sokolsky M, Okolie O,
et al: Development of exosome-encapsulated paclitaxel to overcome
MDR in cancer cells. Nanomedicine. 12:655–664. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Oldrini B, Vaquero-Siguero N, Mu Q, Kroon
P, Zhang Y, Galán-Ganga M, Bao Z, Wang Z, Liu H, Sa JK, et al: MGMT
genomic rearrangements contribute to chemotherapy resistance in
gliomas. Nat Commun. 11:38832020. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Xu L, Faruqu FN, Liam-Or R, Abed OA, Li D,
Venner K, Errington RJ, Summers H, Wang JTW and Al-Jamal KT: Design
of experiment (DoE)-driven in vitro and in vivo uptake studies of
exosomes for pancreatic cancer delivery enabled by copper-free
click chemistry-based labelling. J Extracell Vesicles.
9:17794582020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Zhou Y, Zhou G, Tian C, Jiang W, Jin L,
Zhang C and Chen X: Exosome-mediated small RNA delivery for gene
therapy. Wiley Interdiscip Rev RNA. 7:758–771. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Papandreou ME and Tavernarakis N:
Autophagy and the endo/exosomal pathways in health and disease.
Biotechnol J. 12:2017. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Pan JH, Zhou H, Zhao XX, Ding H, Li W, Qin
L and Pan YL: Role of exosomes and exosomal microRNAs in
hepatocellular carcinoma: Potential in diagnosis and antitumour
treatments (Review). Int J Mol Med. 41:1809–1816. 2018.PubMed/NCBI
|
|
120
|
Zhang Y, Liu YT, Tang H, Xie WQ, Yao H, Gu
WT, Zheng YZ, Shang HB, Wang Y, Wei YX, et al: Exosome-transmitted
lncRNA H19 inhibits the growth of pituitary adenoma. J Clin
Endocrinol Metab. 104:6345–6356. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Zhao X, Ren Y, Cui N, Wang X and Cui Y:
Identification of key microRNAs and their targets in exosomes of
pancreatic cancer using bioinformatics analysis. Medicine
(Baltimore). 97:e126322018. View Article : Google Scholar : PubMed/NCBI
|