Introduction
Pancreatic cancer is one of the most lethal
diseases, with a 5-year survival rate of ~5% (1). Due to the absence of obvious symptoms
during the early stages, patients diagnosed with pancreatic cancer
often have late-stage disease, where the tumour may have
metastasized to other organs, and have missed the opportunity to be
treated with surgical resection. Unfortunately, pancreatic cancer
is relatively resistant to chemotherapy and radiotherapy compared
with other types of cancer, such as breast or lung cancer. Thus, it
is crucial to understand the mechanisms of radioresistance
(2,3).
MicroRNA (miRNA/miR) is a type of small non-coding
RNA of ~23 nucleotides in length (4). They exert their regulatory roles by
binding to the 3′ untranslated region (UTR) of mRNAs and inhibiting
their transcription. Due to the extensiveness of their targets, a
single miRNA can regulate multiple molecules and serve important
roles in a series of physiological and pathological processes,
including cancer (5).
Radioresistance is often thought to be regulated by
cell stemness. In the process of searching for a candidate miRNA
that can modulate radioresistance in pancreatic cancer, it was
found that multiple stem cell-related genes were targeted by
miR-153, including jagged canonical Notch ligand 1 (JAG1) and
Kruppel-like factors (6,7). Of note, miR-153 has previously been
reported to regulate radiosensitivity in human glioma (8,9), and to
influence the efficacy of other therapies (10). However, the role of miR-153 in the
radioresistance of pancreatic cancer remains unknown. In the
present study, it was found that miR-153 could sensitize pancreatic
cancer to radiotherapy by directly targeting JAG1.
Materials and methods
Cell culture
The human pancreatic cancer cell line SW1990
[American Type Culture Collection (ATCC); cat. no. CRL-2172] was
cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.) and the MIA PaCa-2 cell line (ATCC; cat. no. CRM-CRL-1420)
was cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS.
Animal studies
Experimental procedures involving animals were
approved by the Taizhou People's Hospital Institutional Animal Care
and Use Committee (Taizhou, China). Briefly, 5×106
miR-153-overexpressing or control SW1990 cells were subcutaneously
injected into 10, 6–8 week-old (~20 g weight), male BALB/c nude
mice, with 5 mice in each group. The mice were supplied by Shanghai
SLAC Laboratory Animal Co., Ltd and housed at ~22°C, ~50% humidity
with a 12/12 light/dark cycle and free access to the food and
water. When the tumours reached 500 mm3, they were
irradiated by a single dose of 4 Gy for ~2 min. The tumour volume
was measured every 3 days and, 15 days later, the mice were
anaesthetized with sodium pentobarbital (40 mg/kg) by
intraperitoneal injection, and then sacrificed by cervical
dislocation. The largest tumour diameter was 15 mm, and the largest
tumour volume was 1,365 mm3.
Lentivirus infection
The miR-153 overexpression and inhibition lentivirus
and their controls were purchased from OBiO Technology (Shanghai)
Corp., Ltd. Lentivirus was then added to the cell culture media at
a cell:virus ratio of 1:10 with 8 µg/ml polybrene (Merck KGaA).
Following incubation for 24 h, cells were changed with fresh
culture media. The stably transfected cells were selected using
puromycin (final concentration, 1 µg/ml) and fluorescence-activated
cell sorting.
JAG1 treatment
After plating 2×105 SW1990 cells/well in
6-well plates, the JAG1 recombinant protein (50 ng/ml; cat. no.
1277-JG; R&D Systems, Inc.) was added. The medium was changed
every 2 days with fresh medium containing recombinant protein until
the cells were used for analysis.
Dual-luciferase reporter assay
The pmirGLO reporter plasmid containing the
wild-type and mutant 3′UTR segment of the miR-153 binding site in
JAG1, and the control, were constructed by OBiO Technology
(Shanghai) Corp., Ltd. The reporter plasmid was co-transfected with
miR-153 mimic (5′-UUGCAUAGUCACAAAAGUGAUC-3′) and mimic-negative
control (NC) (5′-UUUGUACUACACAAAAGUACUG-3′) (Guangzhou RiboBio Co.,
Ltd.) into SW1990 cells using Lipofectamine 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
instructions. Six hours following transfection, the culture medium
was replaced with fresh culture media, and 72 h following
transfection, SW1990 cells were collected and analysed using
Dual-Luciferase® Reporter Assay (Promega Corporation),
according to the manufacturer's instructions and the results were
normalized to Renilla luciferase activity.
Cell viability assay
Cell viability was analysed using Cell Counting
Kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Inc.),
according to the manufacturer's instructions. Briefly,
1×105 SW1990 cells/well were seeded into 6-well plates.
Following culture for 6 h, the cells were treated with 4 Gy
radiotherapy. Untreated cells were used as control. After 48 h, the
cells were incubated with 10 µl CCK-8 per 100 µl culture media for
2 h at 37°C. The absorbance was measured at 450 nm using a
Varioskan Flash microplate reader (Thermo Fisher Scientific,
Inc.).
Cell apoptosis analysis
Cell apoptosis was analysed by flow cytometry.
SW1990 cells were treated as described in the ‘Cell viability
assay’ section. At 48 h after radiotherapy, cells were collected
and washed twice with cold PBS. Cell apoptosis was evaluated using
the Annexin V-FITC/PI apoptosis detection kit (BD Biosciences),
according to the manufacturer's instructions. Data were collected
by flow cytometry (BD Accuri C6) BD Bioscience) and analysed by
FlowJo v.10.5.2 (BD Bioscience).
Colony formation assay
SW1990 cells (1×104) were seeded into
6-well plates. After 6 h of culture, cells were exposed to 0, 2, 4
and 6 Gy radiotherapy. The cell culture medium was replaced with
fresh culture media every 3–4 days. After 12–14 days, the colonies
were fixed using 4% paraformaldehyde at room temperature for 30 min
and stained with crystal violet solution (Beyotime Institute of
Biotechnology). Cell plates were scanned and colonies were counted
using ImageJ v.1.8.0 (National Institutes of Health).
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Cells were washed twice with PBS and lysed in
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.).
Briefly, total RNA was separated by chloroform and precipitated by
isopropanol. Following washing with 75% ethanol, RNA was dried and
resuspended in RNase-free ddH2O. RNA from SW1990 cells
was reverse-transcribed with PrimeScript™ RT reagent kit (Takara
Bio, Inc.) according to the manufacturer's protocol. qPCR was
performed using SYBR® Premix Ex Taq™ (Takara Bio, Inc.),
according to the manufacturer's instructions with the following
thermocycling conditions: 95°C for 5 sec, 60°C for 34 sec for 40
cycles. The relative expression levels were determined using the
−2∆∆Cq method (11).
Primers for GAPDH were purchased from Sangon Biotech Co., Ltd.
(cat. no. B661104). The other primers used in this study are as
follows: miR153, forward, 5′-CATGCTAGCTCTCTCTCCCTCCCTCTTTCCC-3′ and
reverse, 5′-GCGGATCCCCGTTAGCAATACAAACCAACCC-3′; RT primer,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGATCAC-3′; U6,
forward, 5′-CAAGGATGACACGCAAA-3′ and reverse,
5′-TCAACTGGTGTCGTGG-3′; RT primer,
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGAAAAATAT-3′; JAG1, forward,
5′-ATTACCAGGATAACTGTGCGAA-3′ and reverse,
5′-CAAATGTGCTCCGTAGTAAGAC-3′.
Western blotting
Cells were subjected to lysis with RIPA buffer
(Thermo Fisher Scientific, Inc.) supplemented with the complete
protease inhibitor cocktail (Roche Diagnostics) for 10 min on ice.
The lysates were then centrifuged at 15,000 × g for 30 min at 4°C
to remove cell debris. Protein concentrations were determined using
a BCA Protein Assay kit (Beyotime Institute of Biotechnology). A
total of 40 µg cellular proteins were then separated by 10%
SDS-PAGE and transferred to a nitrocellulose membrane. Membranes
were blocked with 5% skimmed milk in TBS at room temperature for 1
h and incubated with primary antibody, anti-GAPDH (Cell Signalling
Technology, Inc.; cat. no. 2118; 1:1,000) or anti-JAG1 (Cell
Signalling Technology, Inc.; cat. no. 70109; 1:1,000), according to
the manufacturer's instructions. Following primary antibody
incubation, the secondary antibody IRDye 800CW Goat anti-Rabbit IgG
(1:5,000; cat. no. 925-32211; LI-COR Biosciences) were used to
incubate the membrane at room temperature for 1 h, and the
membranes were then subjected to imaging using the Odyssey Imaging
Systems (LI-COR Biosciences).
Bioinformatics
Survival analysis of pancreatic cancer patients with
different miR-153 expression was performed on the website
http://www.oncolnc.org/, using the data from The
Cancer Genome Atlas (TCGA) Program (https://www.cancer.gov/tcga). In addition, expression
data for miR-153 and JAG1 were download from the oncolnc website
and analysed using GraphPad Prism 7 (GraphPad Software, Inc.).
Statistical analysis
All experiments were repeated at least three times.
Data are presented as the mean ± SD. Statistical analysis was
performed using a one-way ANOVA followed by Tukey's honest
significant difference test on GraphPad Prism 7 (GraphPad Software,
Inc.). When comparing two groups, unpaired Student's t test was
performed. Pearson's r correlation was used for correlation
analysis of miR-153 and JAG1 in pancreatic ductal adenocarcinoma
tissues. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-153 is downregulated in pancreatic
cancer and associated with poor prognosis
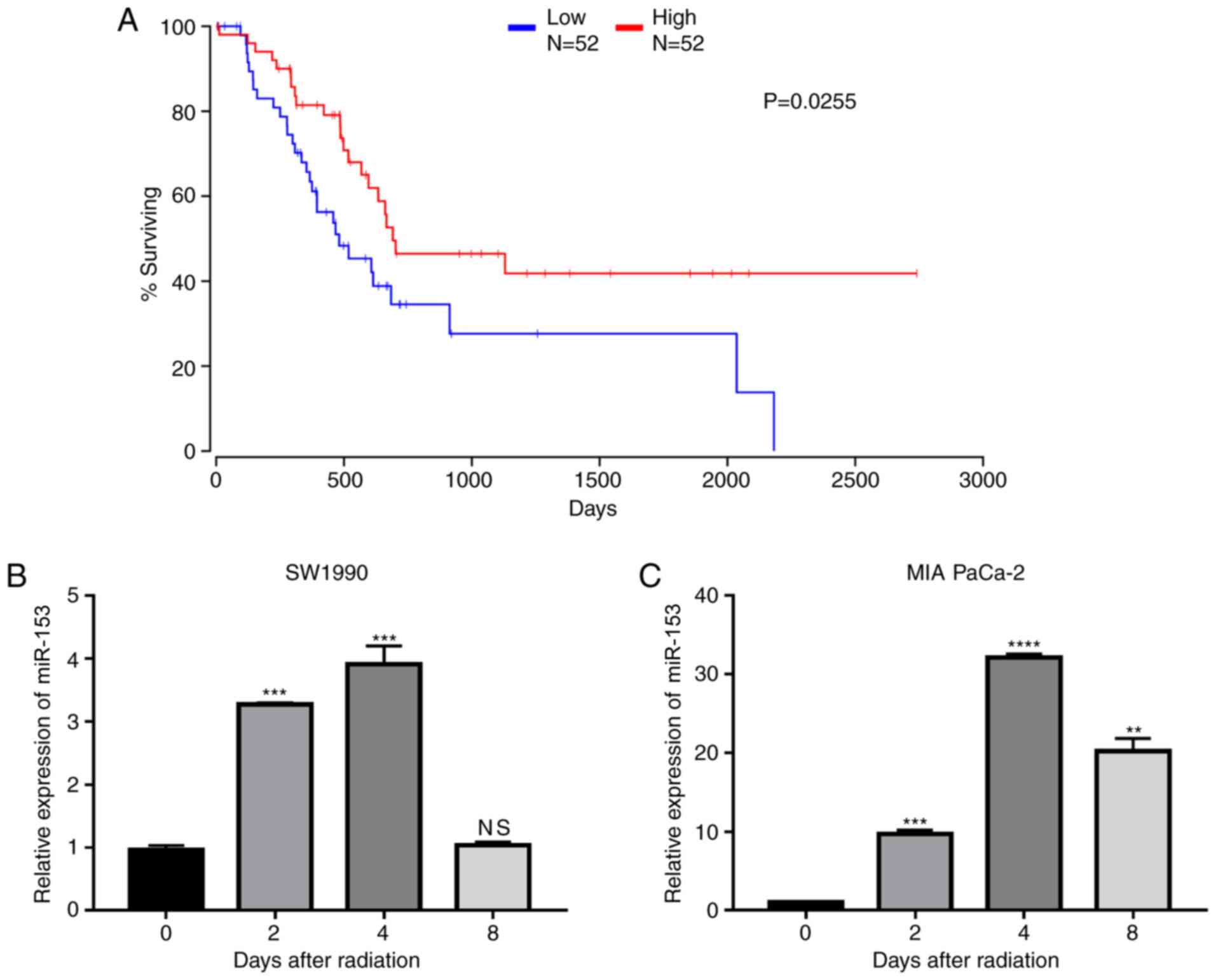
To explore the role of miR-153 in pancreatic cancer,
data from TCGA were analysed and it was found that miR-153
expression levels were positively associated with patient survival
(Fig. 1A), indicating that miR-153
may serve as a target in the treatment of pancreatic cancer. The
expression changes of miR-153 following radiation in two pancreatic
cancer cell lines were further investigated. The results
demonstrated that miR-153 expression levels were significantly
upregulated in both cell lines following radiation (Fig. 1B and C).
miR-153 sensitizes SW1990 cells to
radiotherapy
The potential role of miR-153 in the radioresistance
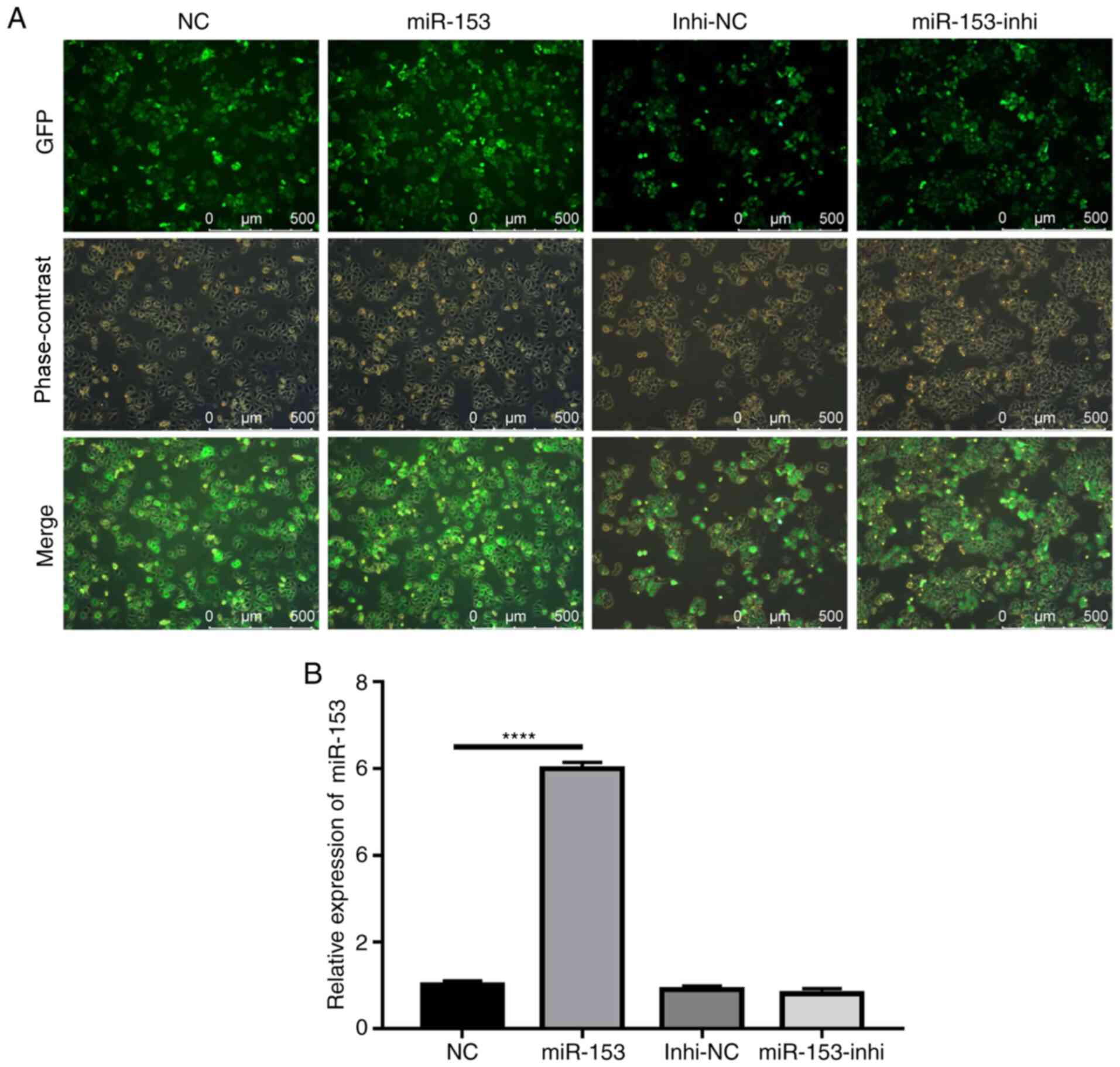
of pancreatic cancer was subsequently explored. Using lentiviruses
expressing miR-153 mimic or miR-153 inhibitor, SW1990 cell lines
that stably overexpressed miR-153 mimic or miR-153 inhibitor were
successfully established. The efficiency of stable infection was
detected by green fluorescent protein expression (Fig. 2A). The miR-153 expression levels in
the stably infected cell lines were confirmed by RT-qPCR (Fig. 2B). The results indicated that miR-153
was significantly overexpressed in the mimic-transduced cells, and
miR-153 inhibition did not cause any significant changes to miR-153
expression, as compared with the control.
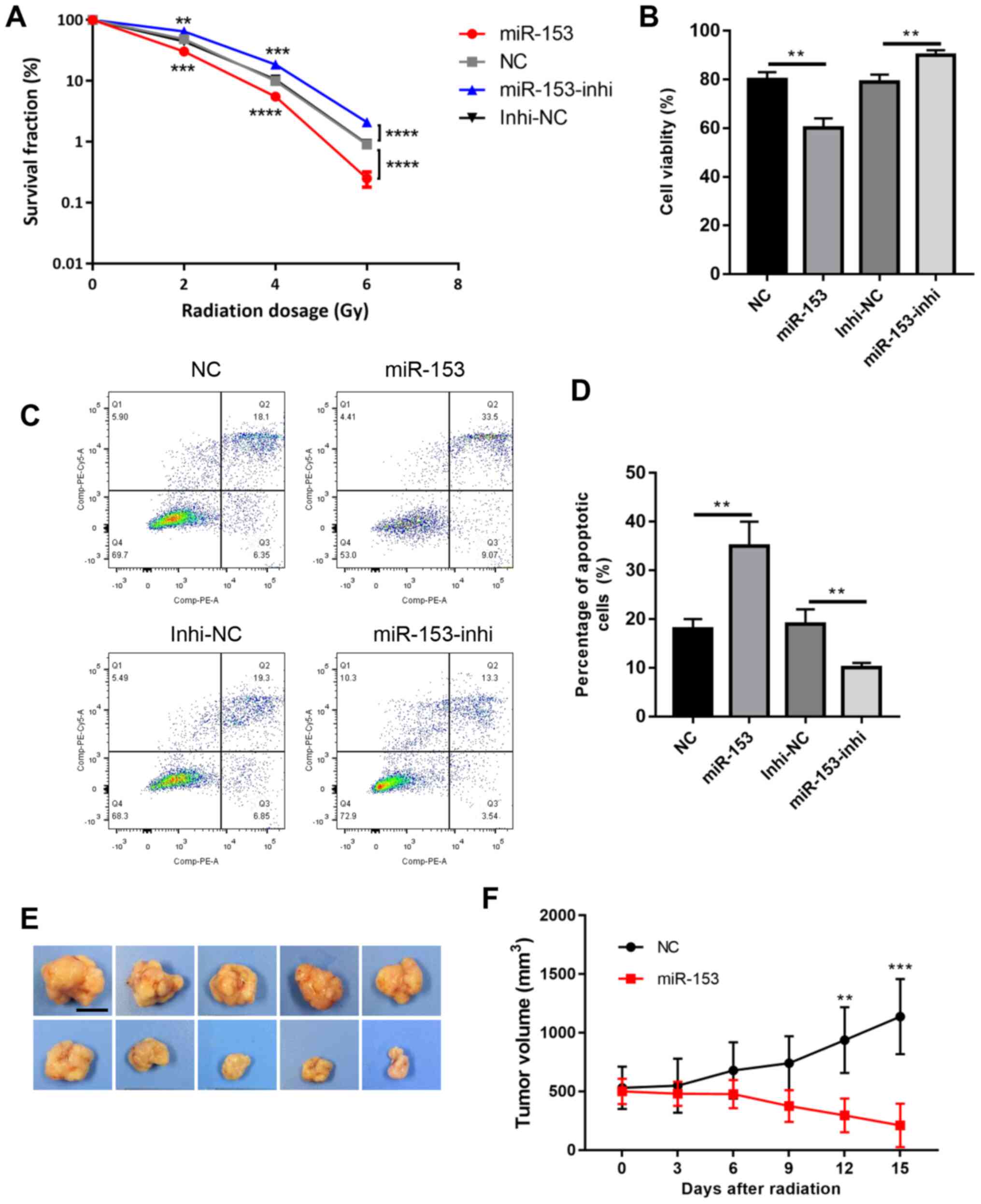
These cells were then subjected to radiotherapy and
the colony formation survival assay, which is considered the
classic method of evaluating radioresistance. The results revealed
that miR-153 overexpression significantly reduced colony formation
following radiation, while miR-153 inhibition increased cell
survival in a dose-dependent manner (Fig. 3A). Cell viability was then analysed
using a CCK-8 assay, and it was found that cell viability was
significantly decreased in the miR-153 overexpression group
following radiation, while miR-153 inhibition had the opposite
effect (Fig. 3B). In addition, cell
apoptosis was evaluated following radiation, and the results showed
that miR-153 overexpression increased cell apoptosis, while miR-153
inhibition had the opposite effect (Fig.
3C and D). Furthermore, the effect of miR-153 in
radioresistance was investigated in vivo. Radiation had
little effect in the control group and the tumours continued to
grow following radiation treatment. By contrast, miR-153
overexpression significantly inhibited radioresistance and the
tumours shrank following radiation treatment (Fig. 3E and F). In combination, the present
results indicated that miR-153 sensitized SW1990 cells to
radiotherapy.
miR-153 affects radioresistance by
directly targeting JAG1
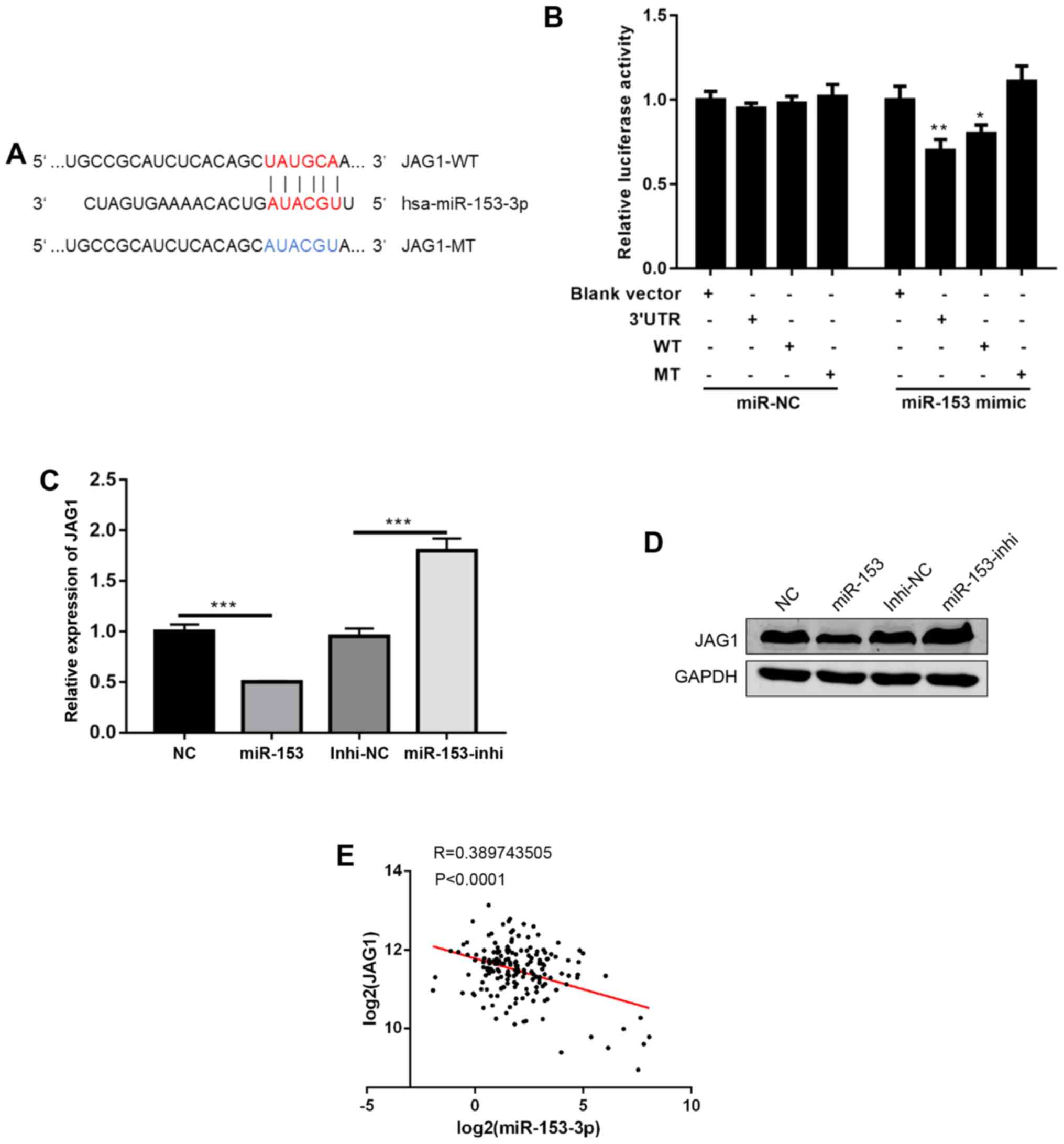
To further investigate the mechanisms of miR-153, a
commonly used miRNA target prediction website, TargetScan
(http://www.targetscan.org/vert_72/),
was used. It was found that miR-153 could bind to the 3′UTR of
JAG1, a ligand in NOTCH signalling (Fig.
4A) (12). Using a dual
luciferase reporter assay, the direct binding of miR-153 to the
3′UTR of JAG1 was confirmed (Fig.
4B). Next, JAG1 mRNA and protein expression levels were
detected following miR-153 overexpression or inhibition, and it was
found that miR-153 overexpression decreased JAG1 expression levels,
while miR-153 inhibition increased the JAG1 expression levels
(Fig. 4C and D). Of note, data from
human pancreatic cancer tissues were analysed, and it was found
that the expression of miR-153 was negatively correlated with JAG1
expression (Fig. 4E). In
combination, the present results suggested that JAG1 is a direct
target of miR-153.
Next, the present study investigated whether miR-153
exerted its function through JAG1. Since JAG1 expression was
decreased by miR-153, a recombinant JAG1 protein was used to
determine if it could rescue the phenotypic effects of miR-153
overexpression in pancreatic cancer cells. In the miR-153
overexpression group, JAG1 treatment significantly increased the
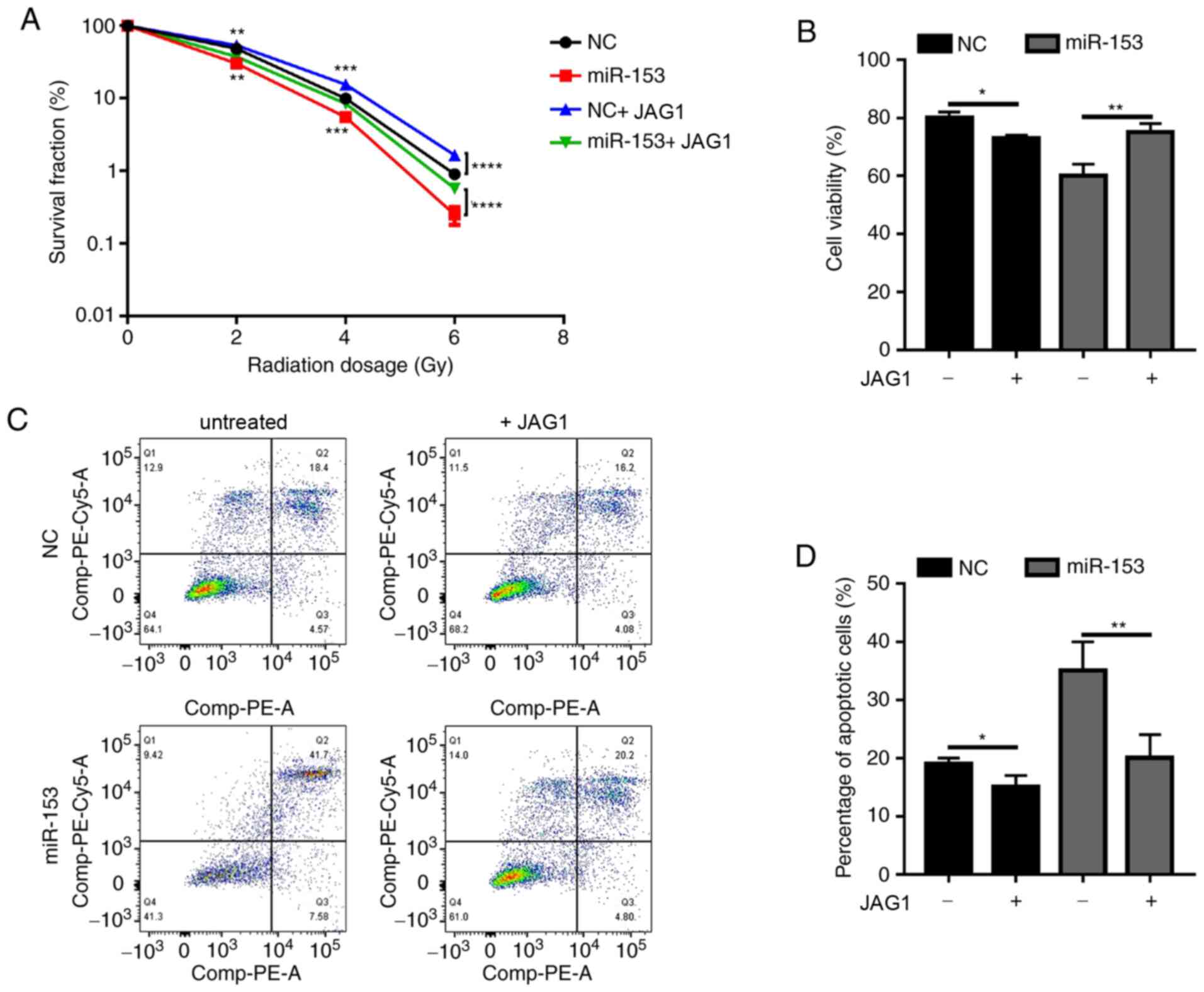
colony formation ability of irradiated cancer cells (Fig. 5A). Of note, when JAG1 was added in
the NC group, it was also able to promote radioresistance (Fig. 5A). The results of the cell viability
assay (Fig. 5B) and apoptosis
analysis (Fig. 5C and D) were
similar. Addition of exogenous JAG1 protein in the cultures
significantly increased cell viability and decreased the percentage
of apoptotic cells. The present results demonstrated that miR-153
sensitized pancreatic cancer cells to radiotherapy by inhibiting
JAG1.
Discussion
Pancreatic cancer is a deadly disease that is
resistant to most current treatments for cancer, particularly
radiotherapy. Unfortunately, the underlying mechanism of
radioresistance in pancreatic cancer remains unclear. In the
present study, it was found that miR-153 could sensitize pancreatic
cancer cells to radiotherapy by directly targeting and
downregulating JAG1.
In the present study, a new target of miR-153 was
confirmed. In addition, it was found that miR-153 contributed to
radioresistance in pancreatic cancer cells. miR-153 has been
previously reported to regulate radiosensitivity (8,9), as well
as other processes, in various types of cancer. For example,
miR-153 was reported to mediate cell proliferation and apoptosis in
renal cancer (13), inhibit
migration in breast (14,15) and lung cancer (16), act as a prognostic marker for gastric
(17) and cervical cancer (18), mediate immune escape from natural
killer cells in pancreatic cancer (19), and enhance chimeric antigen receptor
T cell immunotherapy in colon cancer (20). In pancreatic cancer, miR-153 mainly
has antitumour properties. miR-153 was reported to act as a
prognostic marker in pancreatic cancer, inhibit cell migration and
invasion (21), and enhance the
therapeutic effect of gemcitabine by targeting snail family
transcriptional repressor 1 (22).
As multiple cancer stem cell-related genes have been predicted to
be targeted by miR-153 (6,7), the effect of miR-153 may be mediated by
suppressing cancer cell stemness. Indeed, miR-153 was previously
found to inhibit cancer cell stemness and hinder tumour growth
(6).
The present study revealed a new role of JAG1 in
pancreatic cancer, which was its potential to increase resistance
to radiotherapy. JAG1 is one of the ligands for NOTCH signalling, a
well-known stem cell signalling pathway. JAG1 also participates in
numerous physiological and pathological processes. Consistent with
the present results, as the ligand for NOTCH signalling, JAG1
upregulation was previously reported to mediate
epithelial-mesenchymal transition and subsequently chemoresistance
of pancreatic cancer (23).
Furthermore, JAG1 was found to be highly expressed in pancreatic
cancer compared with normal pancreatic tissue, and JAG1 silencing
by small interfering RNA or miRNA significantly inhibited cell
growth, migration, and invasion in pancreatic cancer cells
(24,25). A higher JAG1 expression was reported
to be associated with a poorer prognosis in patients with
pancreatic ductal adenocarcinoma (26).
In conclusion, the present study determined the
important role of miR-153 in mediating radioresistance in
pancreatic cancer cells and demonstrated that miR-153 exerted its
function by directly targeting and inhibiting JAG1. These findings
provided two new potential therapeutic targets, miR-153 and JAG1,
for pancreatic cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analysed during the
current study are available in the TCGA Research Network
(https://www.cancer.gov/tcga).
Authors' contributions
ZZ and XS performed experiments, analysed data and
wrote the manuscript. DZ, HX, HK, and BY performed experiments and
analyzed data. LY designed the experiments, supervised the project
and revised the manuscript. ZZ, XS and LY confirm the authenticity
of all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Experimental procedures involving animals were
approved by the Taizhou People's Hospital Institutional Animal Care
and Use Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
miR-153
|
microRNA-153
|
|
WT
|
wild-type
|
|
MT
|
mutant
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Halbrook CJ and Lyssiotis CA: Employing
metabolism to improve the diagnosis and treatment of pancreatic
cancer. Cancer Cell. 31:5–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wolfgang CL, Herman JM, Laheru DA, Klein
AP, Erdek MA, Fishman EK and Hruban RH: Recent progress in
pancreatic cancer. CA Cancer J Clin. 63:318–348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu TX and Rothenberg ME: MicroRNA. J
Allergy Clin Immunol. 141:1202–1207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao G, Zhang Y, Zhao Z, Cai H, Zhao X,
Yang T, Chen W, Yao C, Wang Z, Wang Z, et al: MiR-153 reduces stem
cell-like phenotype and tumor growth of lung adenocarcinoma by
targeting Jagged1. Stem Cell Res Ther. 11:1702020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu R, Shi P, Nie Z, Liang H, Zhou Z, Chen
W, Chen H, Dong C, Yang R, Liu S, et al: Mifepristone suppresses
basal triple-negative breast cancer stem cells by down-regulating
KLF5 expression. Theranostics. 6:533–544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang W, Shen Y, Wei J and Liu F:
MicroRNA-153/Nrf-2/GPx1 pathway regulates radiosensitivity and
stemness of glioma stem cells via reactive oxygen species.
Oncotarget. 6:22006–22027. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun D, Mu Y and Piao H: MicroRNA-153-3p
enhances cell radiosensitivity by targeting BCL2 in human glioma.
Biol Res. 51:562018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang L, Lv X, Fu X, Su L, Yang T and Xu P:
MiR-153 inhibits the resistance of lung cancer to gefitinib via
modulating expression of ABCE1. Cancer Biomark. 25:361–369. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grochowski CM, Loomes KM and Spinner NB:
Jagged1 (JAG1): Structure, expression, and disease associations.
Gene. 576:381–384. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou B, Zheng P, Li Z, Li H, Wang X, Shi Z
and Han Q: CircPCNXL2 sponges miR-153 to promote the proliferation
and invasion of renal cancer cells through upregulating ZEB2. Cell
Cycle. 17:2644–2654. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liang H, Ge F, Xu Y, Xiao J, Zhou Z, Liu R
and Chen C: miR-153 inhibits the migration and the tube formation
of endothelial cells by blocking the paracrine of angiopoietin 1 in
breast cancer cells. Angiogenesis. 21:849–860. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang J, Liang S and Duan X: Molecular
mechanism of miR-153 inhibiting migration, invasion and
epithelial-mesenchymal transition of breast cancer by regulating
transforming growth factor beta (TGF-β) signaling pathway. J Cell
Biochem. 120:9539–9546. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shan N, Shen L, Wang J, He D and Duan C:
MiR-153 inhibits migration and invasion of human non-small-cell
lung cancer by targeting ADAM19. Biochem Biophys Res Commun.
456:385–391. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Z, Sun J, Bai Z, Li H, He S, Chen R
and Che X: MicroRNA-153 acts as a prognostic marker in gastric
cancer and its role in cell migration and invasion. Onco Targets
Ther. 8:357–364. 2015.PubMed/NCBI
|
|
18
|
Yang D and Zhang Q: miR-152 may function
as an early diagnostic and prognostic biomarker in patients with
cervical intraepithelial neoplasia and patients with cervical
cancer. Oncol Lett. 17:5693–5698. 2019.PubMed/NCBI
|
|
19
|
Ou ZL, Luo Z, Wei W, Liang S, Gao TL and
Lu YB: Hypoxia-induced shedding of MICA and HIF1A-mediated immune
escape of pancreatic cancer cells from NK cells: Role of
circ_0000977/miR-153 axis. RNA Biol. 16:1592–1603. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang Q, Xia J, Wang L, Wang X, Ma X, Deng
Q, Lu Y, Kumar M, Zhou Z, Li L, et al: miR-153 suppresses IDO1
expression and enhances CAR T cell immunotherapy. J Hematol Oncol.
11:582018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bai Z, Sun J, Wang X, Wang H, Pei H and
Zhang Z: MicroRNA-153 is a prognostic marker and inhibits cell
migration and invasion by targeting SNAI1 in human pancreatic
ductal adenocarcinoma. Oncol Rep. 34:595–602. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu F, Liu B, Qian J, Wu G, Li J and Ma Z:
miR-153 enhances the therapeutic effect of gemcitabine by targeting
Snail in pancreatic cancer. Acta Biochim Biophys Sin (Shanghai).
49:520–529. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Z, Li Y, Kong D, Banerjee S, Ahmad A,
Azmi AS, Ali S, Abbruzzese JL, Gallick GE and Sarkar FH:
Acquisition of epithelial-mesenchymal transition phenotype of
gemcitabine-resistant pancreatic cancer cells is linked with
activation of the notch signaling pathway. Cancer Res.
69:2400–2407. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee J, Lee J and Kim JH: Association of
Jagged1 expression with malignancy and prognosis in human
pancreatic cancer. Cell Oncol (Dordr). 43:821–834. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cao TH, Ling X, Chen C, Tang W, Hu DM and
Yin GJ: Role of miR-214-5p in the migration and invasion of
pancreatic cancer cells. Eur Rev Med Pharmacol Sci. 22:7214–7221.
2018.PubMed/NCBI
|
|
26
|
Huang SF, Yang ZL, Li DQ, Liu ZY, Wang CW,
Miao XY, Zou Q and Yuan Y: Jagged1 and DLL4 expressions in benign
and malignant pancreatic lesions and their clinicopathological
significance. Hepatobiliary Pancreat Dis Int. 15:640–646. 2016.
View Article : Google Scholar : PubMed/NCBI
|