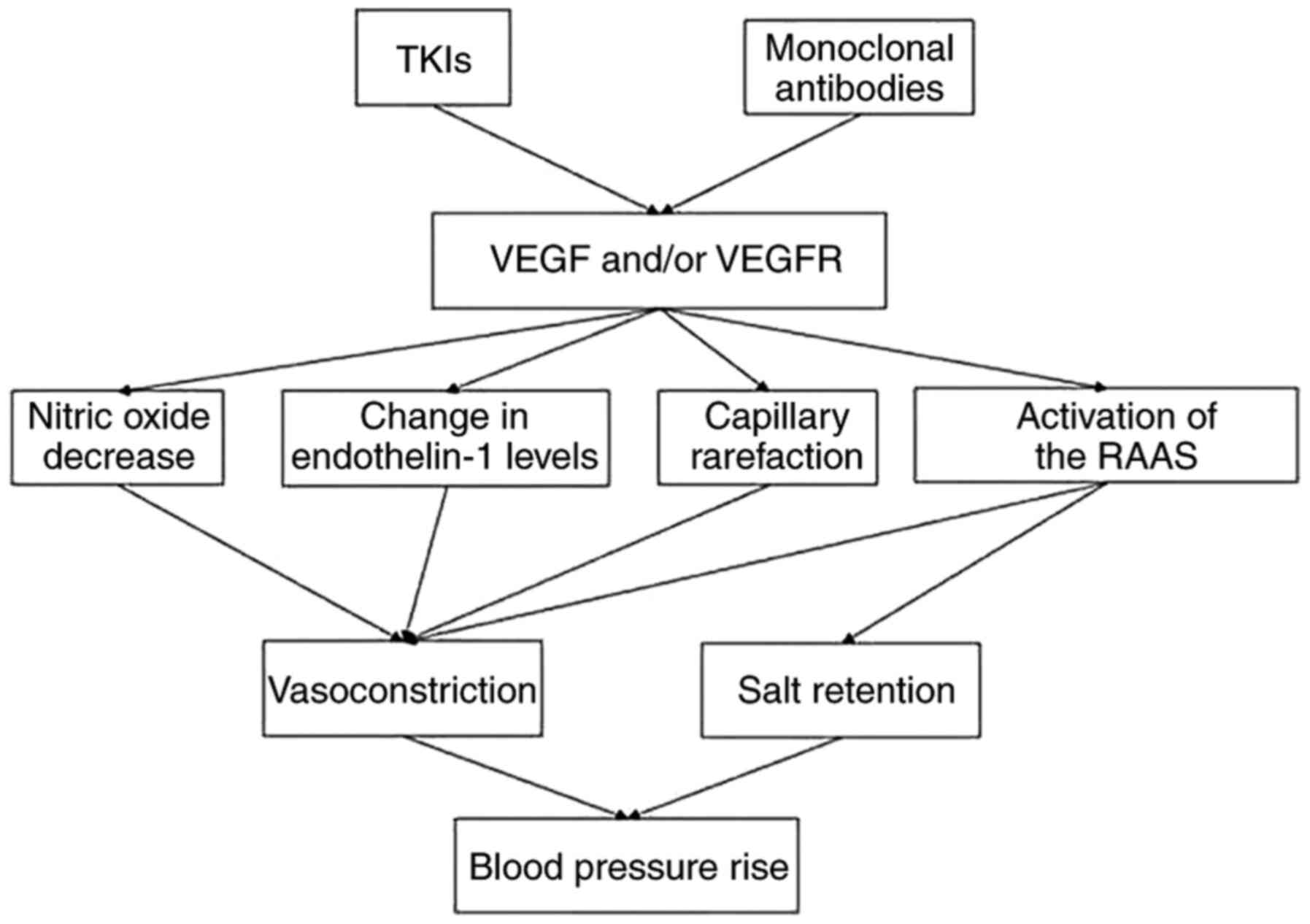
However, a previous study has reported that
antiangiogenic therapy increased arterial blood pressure (BP),
raised the risk of new-onset hypertension, or worsened existing
hypertension (5). The mechanism
underlying the antiangiogenic drug-induced hypertension remains
controversial. The current hypotheses include decreased nitric
oxide (NO) (6), increased
endothelin-1 (7), capillary
rarefaction (8) and activation of
the renin-angiotensin-aldosterone system (RAAS) (9) (Fig. 1).
According to the National Cancer Institute's common terminology
criteria for adverse events (NCI CTCAE), version 5.0 (10), hypertension for adults can be classed
into five categories depending on its severity (Table I). Subsequently, the presence of
hypertension can lead to a reduction or interruption of
antiangiogenic therapy (11).
Considering the complex relationship between
antihypertensive drugs and cancer, the cancer type, the
pre-existing comorbidities and the presence of contraindications
should be considered when selecting antihypertensive drugs for
patients with cancer (12).
Currently, angiotensin-converting enzyme inhibitors (ACEIs) are the
preferred first-line option in treatment of hypertension induced by
anti-VEGF chemotherapy, given its improved outcome in several types
of cancer (13). On the other hand,
several retrospective studies (14,15) have
found that the appearance of hypertension during antiangiogenic
therapy might be associated with improved survival. Thus, it
remains unclear whether hypertension should be considered as an
adverse reaction or as a positive prognostic marker in patients
with various types of cancer. The present review aimed to explore
the relationship between hypertension and antiangiogenic therapy in
different types of tumors.
In total, nine studies have reported the association
between hypertension and antiangiogenic drugs, including sunitinib,
bevacizumab, sorafenib, axitinib and pazopanib, in renal cell
carcinoma (RCC). Of these nice studies, seven were prospective
studies (16–22) and two were retrospective studies
(23,24), involving a total of 6,083 patients
(Table II).
As an adjuvant treatment of gastric cancer,
antiangiogenic drugs significantly prolong the survival of patients
with advanced or metastatic gastric cancer (GC) in addition to
gastroesophageal junction carcinoma (GEJ), and hypertension is a
common adverse reaction that cannot be ignored. Five studies have
reported the association between hypertension and antiangiogenic
drugs, including apatinib and ramucirumab, of which, four were
prospective studies (26–29) and one was a retrospective study
(15). In total, 1,700 patients were
included (Table III).
Two double-blind, randomized, placebo-controlled,
phase III trials for ramucirumab (27,28) and
the phase II and III studies for apatinib (26,29)
showed that ramucirumab and apatinib increased the risk of ≥G3
hypertension in patients with GC or GEJ carcinoma. The incidence of
hypertension in the TKI treatment group (36.80%) was higher
compared with the group treated with monoclonal antibodies
(22.78%). However, the incidence of severe hypertension in the
monoclonal antibody treatment group (11.37%) was higher compared
with the TKI-treated group (6.32%). Of note, the incidence of
severe hypertension was higher in the dose of 425 mg twice daily
compared with the dose of 850 mg once-daily regimen (26), but this comparison lacked statistical
significance.
A retrospective cohort study of 269 patients
demonstrated that the presence of hypertension within the first
four weeks of antiangiogenic therapy was associated with prolonged
median overall survival (15),
suggesting that hypertension is an early prognostic marker.
However, further studies are required to support this
conclusion.
Bevacizumab is the most widely used antiangiogenic
drug for lung cancer treatment. Twelve studies have reported the
association between hypertension and approved antiangiogenic drugs,
including fruquintinib, crediranib anlotinib and bevacizumab. There
are seven prospective studies (30–36), two
retrospective studies (14,37) and three meta-analyses (38–40). A
total of 1,1291 patients were included (Table IV).
Bevacizumab increased the risk of severe
hypertension in the high-dose group (15 mg/kg) (32,39) and
amongst female patients (41). There
was no significant difference between different races (30), pathological types (38) and age (42). Discontinuation of medication due to
hypertension was extremely rare (30,39).
Based on these data, it was found in the present study that the
incidence of hypertension in the TKI treatment group (59.72%) was
higher compared with the group treated with the monoclonal
antibodies (28.61%). The incidence of hypertension was the highest
in the anlotinib group (67.3%). A total of 13.6% patients developed
severe hypertension during therapy. Notably, 23% of patients
developed severe hypertension when receiving bevacizumab plus
erlotinib (34).
Antiangiogenic therapy improved the overall survival
of patients with colorectal cancer, but its benefit is offset
partially by adverse events, such as hypertension. Thirteen studies
have reported significant associations between hypertension and
antiangiogenic drugs, including bevacizumab, ramucirumab and
fruquintinib. There are six prospective studies (43–48),
three retrospective studies (14,49,50),
three meta-analyses (51–53) and one cohort study (54), including a total of 22,639 patients
(Table V).
Other studies have reported that bevacizumab did not
significantly increase the risk of severe hypertension in patients
receiving the drug (60,61) and patients who were aged ≥70
(57). However, the former
conclusion may have selection bias, because amongst the patients
who have previously received bevacizumab treatment, only patients
who have not developed severe hypertension receive bevacizumab
treatment again.
Antiangiogenic drugs have an important role in the
treatment of hepatocellular carcinoma (62). Five studies have reported the
association between hypertension and antiangiogenic drugs,
including cabozantinib, regorafenib, sorafenib and ramucirumab, in
hepatocellular carcinoma. There are four prospective studies
(63–66) and one retrospective study (67). A total of 2,272 patients were
included (Table VI).
Four phase III, randomized, double-blind,
placebo-controlled trials of antiangiogenic drugs, including
cabozantinib, regorafenib and ramucirumab, reported an increasing
risk of severe hypertension in the drug-treated group, with an
incidence of 13–16% (63–66). Based on the given data, it was found
in the present study that the incidence of severe hypertension in
the TKI-treated group (14.51%) was moderately greater compared with
the monoclonal antibodies-treated group (13.66%).
One retrospective study of 38 patients suggested
that hypertension within two weeks of therapy initiation may be a
positive predictor of the anticancer efficacy of sorafenib in
patients with hepatocellular carcinoma (67).
Three multicenter, phase III, double-blind,
placebo-controlled trials showed that sorafenib did not
significantly increase the risk of severe hypertension in patients
with advanced hepatocellular carcinoma (68–70).
This may be a unique manifestation of sorafenib in hepatocellular
carcinoma.
Antiangiogenic agents have been used extensively for
the treatment of breast cancer, but high rates of treatment-induced
hypertension have been reported (71). Six studies have reported the
association between hypertension and antiangiogenic drugs
including, bevacizumab and axitinib, in breast cancer. There are
four prospective studies (72–75), one
retrospective study (76) and one
meta-analysis (77), with a total of
7,414 patients included (Table
VII).
A meta-analysis of five clinical trials reported
that bevacizumab increased the risk of severe hypertension
(77). Severe hypertension was more
frequent in the high-dose group (73) and in some specific genotypes
(76). In specific, Schneider et
al (76) demonstrated that those
with VEGF-1498TT and VEGF-634CC genotypes were largely protected
from severe hypertension. There was no clear correlation between
severe hypertension and baseline blood pressure (78). Based on the given data, it was found
in the present study that the incidence of severe hypertension in
the TKI-treated group (17.5%) was higher compared with the
monoclonal antibodies-treated group (6.6%).
Biomarker analysis of the Eastern Cooperative
Oncology Group clinical trial E2100 demonstrated that patients with
severe hypertension had a superior median overall survival, and
that the VEGF-2578 AA genotype was associated with improved outcome
(76). Another study of apatinib
showed that the predictive effect of hypertension was not related
to the grade of hypertension (75).
The present brief review examined the association
between hypertension and antiangiogenic therapy in different types
of cancer. There are several key findings reported in the present
review. First, the use of antiangiogenic drugs was associated with
an increased risk of hypertension in most types of solid cancer.
Based on the analyzed data, the incidence of hypertension (33.39%)
was the highest in lung cancer. In addition, the incidence of
severe hypertension was the highest in hepatocellular carcinoma
(13.48%) and the lowest in breast cancer (7.1%). Second, there was
no significant difference in the incidence of hypertension between
monoclonal antibodies and small molecule TKI treatments. Of note,
the use of several novel TKIs has been associated with a higher
incidence of severe hypertension, such as axitinib in renal cell
cancer (18%) (19), fruquintinib in
colorectal cancer (29.8%) (48),
apatinib in breast cancer (17.5%) (75), and combination of bevacizumab with
erlotinib in lung cancer (23%) (34). However, this effect was not observed
in the combined antiangiogenic immunotherapy arm (79). In addition, hypertension as an
adverse event was more common in patients receiving high doses
(41), however, the effect of
frequency of administration on the occurrence of hypertension
remains unclear. Third, hypertension was more likely to occur in
patients younger than 75 years old (43,56,57),
those who have not previously used bevacizumab (60,61), and
female patients (41). Fourth, the
effect of baseline blood pressure levels on the development of
hypertension is controversial. Pivot et al (78) reported that there was no clear
correlation between baseline hypertension and its development
during study treatment. By contrast, Yang et al (80) found that a history of hypertension
was an independent risk factor for predicting hypertension during
the treatment period. Fifth, discontinuation or death caused by
hypertension was rare. Nevertheless, hypertension was a risk factor
for acute and chronic cardiovascular diseases and ischemic stroke,
with the grade of hypertension associated with mortality (77,81).
Finally, early development of significant hypertension may be a
biomarker associated with greater efficacy of antiangiogenic
therapy and improved survival (14,49,50).
Large doses of antiangiogenic agents are generally
associated with greater inhibitory effects on VEGF. We speculate
that higher sensitivity to angiogenesis inhibitors may be an
explanation, due to different levels of VEGF expression. Patients
with RCC have increased levels of VEGF and VEGFR expression
(82), which is accompanied by
higher rates of hypertension development, compared with
hepatocellular carcinoma patients treated with sorafenib (83). Frey et al (84) have shown that bevacizumab-induced
hypertension is related to genetic variation in WNK lysine
deficient protein kinase 1, kallikrein B1 and G protein-coupled
receptor kinase 4. The performance of sunitinib in patients with
non-small cell lung cancer (85),
bevacizumab in Chinese patients with locally progressed GC
(86), as well as sorafenib
(87) and sunitinib (88) in patients with breast cancer,
confirms our hypothesis: These drugs did not increase the risk of
serious hypertension, but at the same time, they did not improve
survival.
As an adverse event, hypertension caused by
antiangiogenic drugs should be monitored regularly by physicians.
There is a role for home or ambulatory blood pressure monitoring,
which can increase the sensitivity of diagnosing hypertension
(89). Nevertheless, blood pressure
monitoring in the clinic is recommended for the first cycle of
therapy (90). When blood pressure
remains <140/90 mmHg, lifestyle intervention is recommended,
which includes lower salt intake, reduced alcohol consumption,
normalization of the body mass index, no cigarette smoking and
increased physical activity (91).
Antihypertensive therapy should be initiated when blood pressure is
>140/90 mmHg or 20 mmHg greater than the baseline blood pressure
(90). The association between
antihypertensive drugs and cancer is a matter of large debate in
the last several years. At present, renin inhibitors, including
ACEIs and angiotensin receptor blockers, are the first-line agents
preferred for antiangiogenetic therapy, since they can improve
remodeling by reducing left ventricular afterload and by direct
inhibition of angiotensin II type 1 receptor-mediated hypertrophy
and fibrosis (13). After the end of
antiangiogenic drug treatment, blood pressure should be regularly
monitored, and antihypertensive treatment should be discontinued if
it normalizes.
On the other hand, the presence of hypertension has
been reported as a positive prognostic biomarker of improved
survival in patients receiving antiangiogenic therapy. However, the
underlying mechanisms, the timing and value of blood pressure that
best predicts survival needs to be elucidated. Previous studies
have shown that significant hypertension [≥G2 (22,23) or
≥G3 (76)] and early occurrence of
hypertension [in the first two (67), four (15) or six weeks of treatment initiation
(21)] may be associated with
improved survival. However, it is unclear whether patients who do
not develop significant hypertension in the early stage need
alterations in the medication regimen (15).
In conclusion, the use of antiangiogenic drugs is
associated with an increased risk of hypertension in most types of
solid cancer. Early development of significant hypertension may be
a potential biomarker of improved survival. Prospective studies are
needed to support these findings.
The authors would like to thank Dr Sharen Lee
(Cardiovascular Analytics Group, Laboratory of Cardiovascular
Physiology, Hong Kong) for his contribution to the revision of the
manuscript and data interpretation.
No funding was received.
Not applicable.
GT, PS and LZ designed and arranged the manuscript.
MD and RW wrote the article. DZ, ZZ and JZ found and analyzed the
references in Medline, and participated in writing the article. All
authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vasudev NS and Reynolds AR:
Anti-angiogenic therapy for cancer: Current progress, unresolved
questions and future directions. Angiogenesis. 17:471–494. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brinda BJ, Viganego F, Vo T, Dolan D and
Fradley MG: Anti-VEGF-induced hypertension: A review of
pathophysiology and treatment options. Curr Treat Options
Cardiovasc Med. 18:332016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eskens FA and Verweij J: The clinical
toxicity profile of vascular endothelial growth factor (VEGF) and
vascular endothelial growth factor receptor (VEGFR) targeting
angiogenesis inhibitors; A review. Eur J Cancer. 42:3127–3139.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abi Aad S, Pierce M, Barmaimon G, Farhat
FS, Benjo A and Mouhayar E: Hypertension induced by
chemotherapeutic and immunosuppresive agents: A new challenge. Crit
Rev Oncol Hematol. 93:28–35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Neagoe PE, Lemieux C and Sirois MG:
Vascular endothelial growth factor (VEGF)-A165-induced prostacyclin
synthesis requires the activation of VEGF receptor-1 and −2
heterodimer. J Biol Chem. 280:9904–9912. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neves KB, Rios FJ, Jones R, Evans TRJ,
Montezano AC and Touyz RM: Microparticles from vascular endothelial
growth factor pathway inhibitor-treated cancer patients mediate
endothelial cell injury. Cardiovasc Res. 115:978–988. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hasinoff BB and Patel D: Mechanisms of
myocyte cytotoxicity induced by the multikinase inhibitor
sorafenib. Cardiovasc Toxicol. 10:1–8. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Semeniuk-Wojtaś A, Lubas A, Stec R,
Szczylik C and Niemczyk S: Influence of tyrosine kinase inhibitors
on hypertension and nephrotoxicity in metastatic renal cell cancer
patients. Int J Mol Sci. 17:20732016. View Article : Google Scholar
|
|
10
|
U.S. Department of Health and Human
Services, . Common Terminology Criteria For Adverse Events (CTCAE).
Version 5.0, 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5×7.pdfNovember
27–2017
|
|
11
|
Bæk Møller N, Budolfsen C, Grimm D, Krüger
M, Infanger M, Wehland M and Magnusson N: Drug-induced hypertension
caused by multikinase inhibitors (sorafenib, sunitinib, lenvatinib
and axitinib) in renal cell carcinoma treatment. Int J Mol Sci.
20:47122019. View Article : Google Scholar
|
|
12
|
Tadic M, Cuspidi C, Belyavskiy E and
Grassi G: Intriguing relationship between antihypertensive therapy
and cancer. Pharmacol Res. 141:501–511. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pinter M, Kwanten WJ and Jain RK:
Renin-angiotensin system inhibitors to mitigate cancer
treatment-related adverse events. Clin Cancer Res. 24:3803–3812.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakaya A, Kurata T, Yokoi T, Iwamoto S,
Torii Y, Katashiba Y, Ogata M, Hamada M, Kon M and Nomura S:
Retrospective analysis of bevacizumab-induced hypertension and
clinical outcome in patients with colorectal cancer and lung
cancer. Cancer Med. 5:1381–1387. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu X, Qin S, Wang Z, Xu J, Xiong J, Bai
Y, Wang Z, Yang Y, Sun G, Wang L, et al: Early presence of
anti-angiogenesis-related adverse events as a potential biomarker
of antitumor efficacy in metastatic gastric cancer patients treated
with apatinib: A cohort study. J Hematol Oncol. 10:1532017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ravaud A, Motzer RJ, Pandha HS, George DJ,
Pantuck AJ, Patel A, Chang YH, Escudier B, Donskov F, Magheli A, et
al: Adjuvant sunitinib in high-risk renal-cell carcinoma after
nephrectomy. N Engl J Med. 375:2246–2254. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Escudier B, Pluzanska A, Koralewski P,
Ravaud A, Bracarda S, Szczylik C, Chevreau C, Filipek M, Melichar
B, Bajetta E, et al: Bevacizumab plus interferon alfa-2a for
treatment of metastatic renal cell carcinoma: A randomised,
double-blind phase III trial. Lancet. 370:2103–2111. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Escudier B, Eisen T, Stadler WM, Szczylik
C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA,
et al: Sorafenib in advanced clear-cell renal-cell carcinoma. N
Engl J Med. 356:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rini BI, Melichar B, Ueda T, Grünwald V,
Fishman MN, Arranz JA, Bair AH, Pithavala YK, Andrews GI, Pavlov D,
et al: Axitinib with or without dose titration for first-line
metastatic renal-cell carcinoma: A randomised double-blind phase 2
trial. Lancet Oncol. 14:1233–1242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sternberg CN, Davis ID, Mardiak J,
Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA,
Kavina A, et al: Pazopanib in locally advanced or metastatic renal
cell carcinoma: Results of a randomized phase III trial. J Clin
Oncol. 28:1061–1068. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Akaza H, Naito S, Ueno N, Aoki K, Houzawa
H, Pitman Lowenthal S and Lee SY: Real-world use of sunitinib in
Japanese patients with advanced renal cell carcinoma: Efficacy,
safety and biomarker analyses in 1689 consecutive patients. Jpn J
Clin Oncol. 45:576–583. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rini BI, Halabi S, Rosenberg JE, Stadler
WM, Vaena DA, Archer L, Atkins JN, Picus J, Czaykowski P, Dutcher J
and Small EJ: Phase III trial of bevacizumab plus interferon alfa
versus interferon alfa monotherapy in patients with metastatic
renal cell carcinoma: Final results of CALGB 90206. J Clin Oncol.
28:2137–2143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ravaud A and Sire M: Arterial hypertension
and clinical benefit of sunitinib, sorafenib and bevacizumab in
first and second-line treatment of metastatic renal cell cancer.
Ann Oncol. 20:966–967. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Donskov F, Michaelson MD, Puzanov I, Davis
MP, Bjarnason GA, Motzer RJ, Goldstein D, Lin X, Cohen DP,
Wiltshire R and Rini BI: Sunitinib-associated hypertension and
neutropenia as efficacy biomarkers in metastatic renal cell
carcinoma patients. Br J Cancer. 113:1571–1580. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goldstein D, Rosenberg JE, Figlin RA,
Townsend RR, McCann L, Carpenter C and Pandite L: Is change in
blood pressure a biomarker of pazopanib and sunitinib efficacy in
advanced/metastatic renal cell carcinoma? Eur J Cancer. 53:96–104.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li J, Qin S, Xu J, Guo W, Xiong J, Bai Y,
Sun G, Yang Y, Wang L, Xu N, et al: Apatinib for
chemotherapy-refractory advanced metastatic gastric cancer: Results
from a randomized, placebo-controlled, parallel-arm, phase II
trial. J Clin Oncol. 31:3219–3225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fuchs CS, Tomasek J, Yong CJ, Dumitru F,
Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry
DR, et al: Ramucirumab monotherapy for previously treated advanced
gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An
international, randomised, multicentre, placebo-controlled, phase 3
trial. Lancet. 383:31–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wilke H, Muro K, Van Cutsem E, Oh SC,
Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et
al: Ramucirumab plus paclitaxel versus placebo plus paclitaxel in
patients with previously treated advanced gastric or
gastro-oesophageal junction adenocarcinoma (RAINBOW): A
double-blind, randomised phase 3 trial. Lancet Oncol. 15:1224–1235.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y,
Liu W, Tong J, Liu Y, Xu R, et al: Randomized, double-blind,
placebo-controlled phase III trial of apatinib in patients with
chemotherapy-refractory advanced or metastatic adenocarcinoma of
the stomach or gastroesophageal junction. J Clin Oncol.
34:1448–1454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou C, Wu YL, Chen G, Liu X, Zhu Y, Lu S,
Feng J, He J, Han B, Wang J, et al: BEYOND: A randomized,
double-blind, placebo-controlled, multicenter, phase III study of
first-line carboplatin/paclitaxel plus bevacizumab or placebo in
Chinese patients with advanced or recurrent nonsquamous
non-small-cell lung cancer. J Clin Oncol. 33:2197–2204. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Reck M, von Pawel J, Zatloukal P, Ramlau
R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N and
Manegold C: Phase III trial of cisplatin plus gemcitabine with
either placebo or bevacizumab as first-line therapy for nonsquamous
non-small-cell lung cancer: AVAil. J Clin Oncol. 27:1227–1234.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lu S, Chang J, Liu X, Shi J, Lu Y, Li W,
Yang JJ, Zhou J, Wang J, An T, et al: Randomized, double-blind,
placebo-controlled, multicenter phase II study of fruquintinib
after two prior chemotherapy regimens in chinese patients with
advanced nonsquamous nonsmall-cell lung cancer. J Clin Oncol.
36:1207–1217. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Saito H, Fukuhara T, Furuya N, Watanabe K,
Sugawara S, Iwasawa S, Tsunezuka Y, Yamaguchi O, Okada M, Yoshimori
K, et al: Erlotinib plus bevacizumab versus erlotinib alone in
patients with EGFR-positive advanced non-squamous non-small-cell
lung cancer (NEJ026): Interim analysis of an open-label,
randomised, multicentre, phase 3 trial. Lancet Oncol. 20:625–635.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou M, Chen X, Zhang H, Xia L, Tong X,
Zou L, Hao R, Pan J, Zhao X, Chen D, et al: China national medical
products administration approval summary: Anlotinib for the
treatment of advanced non-small cell lung cancer after two lines of
chemotherapy. Cancer Commun (Lond). 39:362019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Goodwin R, Ding K, Seymour L, LeMaitre A,
Arnold A, Shepherd FA, Dediu M, Ciuleanu T, Fenton D, Zukin M, et
al: Treatment-emergent hypertension and outcomes in patients with
advanced non-small-cell lung cancer receiving chemotherapy with or
without the vascular endothelial growth factor receptor inhibitor
cediranib: NCIC clinical trials group study BR24. Ann Oncol.
21:2220–2226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Koyama N: Adverse cardiovascular events
predict survival benefit in non-small lung cancer patients treated
with bevacizumab. Cancer Biomark. 14:259–265. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lin H, Li L, Luo S, Zhou S, Shen R, Yang
H, Chen H and Xie X: Efficacy and safety of angiogenesis inhibitors
in small-cell lung cancer. Oncology. 8:1141–1155. 2017.
|
|
39
|
Sun L, Ma JT, Zhang SL, Zou HW and Han CB:
Efficacy and safety of chemotherapy or tyrosine kinase inhibitors
combined with bevacizumab versus chemotherapy or tyrosine kinase
inhibitors alone in the treatment of non-small cell lung cancer: A
systematic review and meta-analysis. Med Oncol. 32:4732015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Soria JC, Mauguen A, Reck M, Sandler AB,
Saijo N, Johnson DH, Burcoveanu D, Fukuoka M, Besse B and Pignon
JP; meta-analysis of bevacizumab in advanced NSCLC collaborative
group, : Systematic review and meta-analysis of randomised, phase
II/III trials adding bevacizumab to platinum-based chemotherapy as
first-line treatment in patients with advanced non-small-cell lung
cancer. Ann Oncol. 24:20–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Brahmer JR, Dahlberg SE, Gray RJ, Schiller
JH, Perry MC, Sandler A and Johnson DH: Sex differences in outcome
with bevacizumab therapy: Analysis of patients with advanced-stage
non-small cell lung cancer treated with or without bevacizumab in
combination with paclitaxel and carboplatin in the Eastern
cooperative oncology group trial 4599. J Thorac Oncol. 6:103–108.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Leighl NB, Zatloukal P, Mezger J, Ramlau
R, Moore N, Reck M and Manegold C: Efficacy and safety of
bevacizumab-based therapy in elderly patients with advanced or
recurrent nonsquamous non-small cell lung cancer in the phase III
BO17704 study (AVAiL). J Thorac Oncol. 5:1970–1976. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Price TJ, Zannino D, Wilson K, Simes RJ,
Cassidy J, Van Hazel GA, Robinson BA, Broad A, Ganju V, Ackland SP
and Tebbutt NC: Bevacizumab is equally effective and no more toxic
in elderly patients with advanced colorectal cancer: A subgroup
analysis from the AGITG MAX trial: An international randomised
controlled trial of capecitabine, bevacizumab and mitomycin C. Ann
Oncol. 23:1531–1536. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Aparicio T, Bouché O, Taieb J, Maillard E,
Kirscher S, Etienne PL, Faroux R, Khemissa Akouz F, El Hajbi F,
Locher C, et al: Bevacizumab+chemotherapy versus chemotherapy alone
in elderly patients with untreated metastatic colorectal cancer: A
randomized phase II trial-PRODIGE 20 study results. Ann Oncol.
29:133–138. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Allegra CJ, Yothers G, O'Connell MJ,
Sharif S, Colangelo LH, Lopa SH, Petrelli NJ, Goldberg RM, Atkins
JN, Seay TE, et al: Initial safety report of NSABP C-08: A
randomized phase III study of modified FOLFOX6 with or without
bevacizumab for the adjuvant treatment of patients with stage II or
III colon cancer. J Clin Oncol. 27:3385–3390. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tabernero J, Yoshino T, Cohn AL,
Obermannova R, Bodoky G, Garcia-Carbonero R, Ciuleanu TE, Portnoy
DC, Van Cutsem E, Grothey A, et al: Ramucirumab versus placebo in
combination with second-line FOLFIRI in patients with metastatic
colorectal carcinoma that progressed during or after first-line
therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine
(RAISE): A randomised, double-blind, multicentre, phase 3 study.
Lancet Oncol. 16:499–508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Grothey A, Cutsem EV, Sobrero A, Siena S,
Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et al:
Regorafenib monotherapy for previously treated metastatic
colorectal cancer (CORRECT): An international, multicentre,
randomised, placebo-controlled, phase 3 trial. Lancet. 381:303–312.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xu RH, Li J, Bai Y, Xu J, Liu T, Shen L,
Wang L, Pan H, Cao J, Zhang D, et al: Safety and efficacy of
fruquintinib in patients with previously treated metastatic
colorectal cancer: A phase Ib study and a randomized double-blind
phase II study. J Hematol Oncol. 10:222017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Scartozzi M, Galizia E, Chiorrini S,
Giampieri R, Berardi R, Pierantoni C and Cascinu S: Arterial
hypertension correlates with clinical outcome in colorectal cancer
patients treated with first-line bevacizumab. Ann Oncol.
20:227–230. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tahover E, Uziely B, Salah A, Temper M,
Peretz T and Hubert A: Hypertension as a predictive biomarker in
bevacizumab treatment for colorectal cancer patients. Med Oncol.
30:3272013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
da Silva WC, de Araujo VE, Lima EMEA, Dos
Santos JBR, Silva MRRD, Almeida PHRF, de Assis Acurcio F, Godman B,
Kurdi A, Cherchiglia ML and Andrade EIG: Comparative effectiveness
and safety of monoclonal antibodies (bevacizumab, cetuximab, and
panitumumab) in combination with chemotherapy for metastatic
colorectal cancer: A systematic review and meta-analysis. BioDrugs.
32:585–606. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hurwitz HI, Tebbutt NC, Kabbinavar F,
Giantonio BJ, Guan ZZ, Mitchell L, Waterkamp D and Tabernero J:
Efficacy and safety of bevacizumab in metastatic colorectal cancer:
Pooled analysis from seven randomized controlled trials.
Oncologist. 18:1004–1012. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Galfrascoli E, Piva S, Cinquini M, Rossi
A, La Verde N, Bramati A, Moretti A, Manazza A, Damia G, Torri V,
et al: Risk/benefit profile of bevacizumab in metastatic colon
cancer: A systematic review and meta-analysis. Dig Liver Dis.
43:286–294. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tahover E, Hubert A, Temper M, Salah A,
Peretz T, Hamburger T and Uziely B: An observational cohort study
of bevacizumab and chemotherapy in metastatic colorectal cancer
patients: Safety and efficacy with analysis by age group. Target
Oncol. 10:55–63. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tabernero J, Hozak RR, Yoshino T, Cohn AL,
Obermannova R, Bodoky G, Garcia-Carbonero R, Ciuleanu TE, Portnoy
DC, Prausová J, et al: Analysis of angiogenesis biomarkers for
ramucirumab efficacy in patients with metastatic colorectal cancer
from RAISE, a global, randomized, double-blind, phase III study.
Ann Oncol. 602–609. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Obermannová R, Van Cutsem E, Yoshino T,
Bodoky G, Prausová J, Garcia-Carbonero R, Ciuleanu T, Garcia
Alfonso P, Portnoy D, Cohn A, et al: Subgroup analysis in RAISE: A
randomized, double-blind phase III study of irinotecan, folinic
acid, and 5-fluorouracil (FOLFIRI) plus ramucirumab or placebo in
patients with metastatic colorectal carcinoma progression. Ann
Oncol. 27:2082–2090. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Cunningham D, Lang I, Marcuello E, Lorusso
V, Ocvirk J, Shin DB, Jonker D, Osborne S, Andre N, Waterkamp D, et
al: Bevacizumab plus capecitabine versus capecitabine alone in
elderly patients with previously untreated metastatic colorectal
cancer (AVEX): An open-label, randomised phase 3 trial. Lancet
Oncol. 14:1077–1085. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Shah SR, Gressett Ussery SM, Dowell JE,
Marley E, Liticker J, Arriaga Y and Verma U: Shorter bevacizumab
infusions do not increase the incidence of proteinuria and
hypertension. Ann Oncol. 24:960–965. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, Xu
J, Bai Y, Chi Y, Wang L, et al: Regorafenib plus best supportive
care versus placebo plus best supportive care in Asian patients
with previously treated metastatic colorectal cancer (CONCUR): A
randomised, double-blind, placebo-controlled, phase 3 trial. Lancet
Oncol. 16:619–629. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bennouna J, Sastre J, Arnold D, Österlund
P, Greil R, Van Cutsem E, von Moos R, Viéitez JM, Bouché O, Borg C,
et al: Continuation of bevacizumab after first progression in
metastatic colorectal cancer (ML18147): A randomised phase 3 trial.
Lancet Oncol. 14:29–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hegewisch-Becker S, Graeven U,
Lerchenmüller CA, Killing B, Depenbusch R, Steffens CC, Al-Batran
SE, Lange T, Dietrich G, Stoehlmacher J, et al: Maintenance
strategies after first-line oxaliplatin plus fluoropyrimidine plus
bevacizumab for patients with metastatic colorectal cancer (AIO
0207): A randomised, non-inferiority, open-label, phase 3 trial.
Lancet Oncol. 16:1355–1369. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Morse MA, Sun W, Kim R, He AR, Abada PB,
Mynderse M and Finn RS: The role of angiogenesis in hepatocellular
carcinoma. Clin Cancer Res. 25:912–920. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Abou-Alfa GK, Meyer T, Cheng AL,
El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park
JW, et al: Cabozantinib in patients with advanced and progressing
hepatocellular carcinoma. N Engl J Med. 379:54–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bruix J, Qin S, Merle P, Granito A, Huang
YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al:
Regorafenib for patients with hepatocellular carcinoma who
progressed on sorafenib treatment (RESORCE): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet. 389:56–66.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhu AX, Park JO, Ryoo BY, Yen CJ, Poon R,
Pastorelli D, Blanc JF, Chung HC, Baron AD, Pfiffer TE, et al:
Ramucirumab versus placebo as second-line treatment in patients
with advanced hepatocellular carcinoma following first-line therapy
with sorafenib (REACH): A randomised, double-blind, multicentre,
phase 3 trial. Lancet Oncol. 16:859–870. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhu AX, Kang YK, Yen CJ, Finn RS, Galle
PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, et al:
Ramucirumab after sorafenib in patients with advanced
hepatocellular carcinoma and increased α-fetoprotein concentrations
(REACH-2): A randomised, double-blind, placebo-controlled, phase 3
trial. Lancet Oncol. 20:282–296. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Akutsu N, Sasaki S, Takagi H, Motoya M,
Shitani M, Igarashi M, Hirayama D, Wakasugi H, Yamamoto H, Kaneto
H, et al: Development of hypertension within 2 weeks of initiation
of sorafenib for advanced hepatocellular carcinoma is a predictor
of efficacy. Int J Clin Oncol. 20:105–110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Meyer T, Fox R, Ma YT, Ross PJ, James MW,
Sturgess R, Stubbs C, Stocken DD, Wall L, Watkinson A, et al:
Sorafenib in combination with transarterial chemoembolisation in
patients with unresectable hepatocellular carcinoma (TACE 2): A
randomised placebo-controlled, double-blind, phase 3 trial. Lancet
Gastroenterol Hepatol. 2:565–575. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Aalders KC, Tryfonidis K, Senkus E and
Cardoso F: Anti- angiogenic treatment in breast cancer: Facts,
successes, failures and future perspectives. Cancer Treat Rev.
53:98–110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Bear HD, Tang G, Rastogi P, Geyer CE Jr,
Robidoux A, Atkins JN, Baez-Diaz L, Brufsky AM, Mehta RS,
Fehrenbacher L, et al: Bevacizumab added to neoadjuvant
chemotherapy for breast cancer. N Engl J Med. 366:310–320. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Miles DW, Chan A, Dirix LY, Cortes J,
Pivot X, Tomczak P, Delozier T, Sohn JH, Provencher L, Puglisi F,
et al: Phase III study of bevacizumab plus docetaxel compared with
placebo plus docetaxel for the first-line treatment of human
epidermal growth factor receptor 2-negative metastatic breast
cancer. J Clin Oncol. 28:3239–3247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Robert NJ, Diéras V, Glaspy J, Brufsky AM,
Bondarenko I, Lipatov ON, Perez EA, Yardley DA, Chan SY, Zhou X, et
al: RIBBON-1: Randomized, double-blind, placebo-controlled, phase
III trial of chemotherapy with or without bevacizumab for
first-line treatment of human epidermal growth factor receptor
2-negative, locally recurrent or metastatic breast cancer. J Clin
Oncol. 29:1252–1260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Fan M, Zhang J, Wang Z, Wang B, Zhang Q,
Zheng C, Li T, Ni C, Wu Z, Shao Z and Hu X: Phosphorylated VEGFR2
and hypertension: Potential biomarkers to indicate VEGF-dependency
of advanced breast cancer in anti-angiogenic therapy. Breast Cancer
Res Treat. 143:141–151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Schneider BP, Wang M, Radovich M, Sledge
GW, Badve S, Thor A, Flockhart DA, Hancock B, Davidson N, Gralow J,
et al: Association of vascular endothelial growth factor and
vascular endothelial growth factor receptor-2 genetic polymorphisms
with outcome in a trial of paclitaxel compared with paclitaxel plus
bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol.
26:4672–4678. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Cortes J, Calvo V, Ramírez-Merino N,
O'Shaughnessy J, Brufsky A, Robert N, Vidal M, Muñoz E, Perez J,
Dawood S, et al: Adverse events risk associated with bevacizumab
addition to breast cancer chemotherapy: A meta-analysis. Ann Oncol.
23:1130–1137. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Pivot X, Schneeweiss A, Verma S, Thomssen
C, Passos-Coelho JL, Benedetti G, Ciruelos E, von Moos R, Chang HT,
Duenne AA and Miles DW: Efficacy and safety of bevacizumab in
combination with docetaxel for the first-line treatment of elderly
patients with locally recurrent or metastatic breast cancer:
Results from AVADO. Eur J Cancer. 47:2387–2395. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Rini BI, Powles T, Atkins MB, Escudier B,
McDermott DF, Suarez C, Bracarda S, Stadler WM, Donskov F, Lee JL,
et al: Atezolizumab plus bevacizumab versus sunitinib in patients
with previously untreated metastatic renal cell carcinoma
(IMmotion151): A multicentre, open-label, phase 3, randomised
controlled trial. Lancet. 393:2404–2415. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Yang L, Chen Y, Qin S, Wang L, Hua H, Liu
X, et al: Clinical observation on hypertenison induced by
anti-angiogenic agents for cancer. Chin Clin Oncol. 19:603–607.
2014.
|
|
81
|
Yang WY, Melgarejo JD, Thijs L, Zhang ZY,
Boggia J, Wei FF, Hansen TW, Asayama K, Ohkubo T, Jeppesen J, et
al: Association of office and ambulatory blood pressure with
mortality and cardiovascular outcomes. JAMA. 322:409–420. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Choueiri TK, Vaziri SAJ, Jaeger E, Elson
P, Wood L, Bhalla IP, Small EJ, Weinberg V, Sein N, Simko J, et al:
von Hippel-Lindau gene status and response to vascular endothelial
growth factor targeted therapy for metastatic clear cell renal cell
carcinoma. J Urol. 180:860–866. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Li Y, Li S, Zhu Y, Liang X, Meng H, Chen
J, Zhang D, Guo H and Shi B: Incidence and risk of
sorafenib-induced hypertension: A systematic review and
meta-analysis. J Clin Hypertens (Greenwich). 16:177–185. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Frey MK, Dao F, Olvera N, Konner JA,
Dickler MN and Levine DA: Genetic predisposition to
bevacizumab-induced hypertension. Gynecol Oncol. 147:621–625. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Scagliotti GV, Krzakowski M, Szczesna A,
Strausz J, Makhson A, Reck M, Wierzbicki RF, Albert I, Thomas M,
Miziara JE, et al: Sunitinib plus erlotinib versus placebo plus
erlotinib in patients with previously treated advanced
non-small-cell lung cancer: A phase III trial. J Clin Oncol.
30:2070–2078. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Shen L, Li J, Xu J, Pan H, Dai G, Qin S,
Wang L, Wang J, Yang Z, Shu Y, et al: Bevacizumab plus capecitabine
and cisplatin in Chinese patients with inoperable locally advanced
or metastatic gastric or gastroesophageal junction cancer:
Randomized, double-blind, phase III study (AVATAR study). Gastric
Cancer. 18:168–176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Schwartzberg LS, Tauer KW, Hermann RC,
Makari-Judson G, Isaacs C, Beck JT, Kaklamani V, Stepanski EJ, Rugo
HS, Wang W, et al: Sorafenib or placebo with either gemcitabine or
capecitabine in patients with HER-2-negative advanced breast cancer
that progressed during or after bevacizumab. Clin Cancer Res.
19:2745–2754. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Crown JP, Diéras V, Staroslawska E,
Yardley DA, Bachelot T, Davidson N, Wildiers H, Fasching PA,
Capitain O, Ramos M, et al: Phase III trial of sunitinib in
combination with capecitabine versus capecitabine monotherapy for
the treatment of patients with pretreated metastatic breast cancer.
J Clin Oncol. 31:2870–2878. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Syrigos KN, Karapanagiotou E, Boura P,
Manegold C and Harrington K: Bevacizumab-induced hypertension:
Pathogenesis and management. BioDrugs. 25:159–169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Williams B, Mancia G, Spiering W, Agabiti
Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G,
Dominiczak A, et al: 2018 ESC/ESH guidelines for the management of
arterial hypertension. Eur Heart J. 39:3021–3104. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Maitland ML, Bakris GL, Black HR, Chen HX,
Durand JB, Elliott WJ, Ivy SP, Leier CV, Lindenfeld J, Liu G, et
al: Initial assessment, surveillance, and management of blood
pressure in patients receiving vascular endothelial growth factor
signaling pathway inhibitors. J Natl Cancer Inst. 102:596–604.
2010. View Article : Google Scholar : PubMed/NCBI
|