Allogeneic hematopoietic stem cell transplantation
(allo-HSCT) is an effective treatment for hematological tumors
(1), which has recently been
demonstrated to improve therapeutic effects in patients with
autoimmune diseases (2). Due to the
lengthy procedure of immune reconstruction, particularly after the
use of high-intensity chemotherapy to suppress hematopoiesis and
the application of T-cell depletion, the occurrence of
post-transplant infection has become a prominent complication
following allo-HSCT (3). Viral
infection is the leading cause of infectious mortality in 30% of
patients following transplantation (4). For decades, opportunistic
cytomegalovirus (CMV) infection has been the most common
complication following allo-HSCT, resulting in mortality (5). Recipients may experience primary human
(H)CMV infection, re-infection, re-ignition and co-infection
following transplantation (6). CMV
immunoglobulin G (IgG) is a marker of HCMV infection, the positive
rate of which reaches 50.0–92.2% in healthy adults worldwide, with
rates increasing with age (7–10).
Following initial HCMV infection, healthy individuals may exhibit
no obvious symptoms in their lifetime, and HCMV can also exist in a
latent state (11,12). However, infection in immunosuppressed
individuals may be more likely to occur due to lack of CMV-specific
cytotoxic and helper T cells (13).
Active HCMV infection is one of the most common complications
following allo-HSCT, which may be fatal for patients receiving
transplantation (13). In addition
to HCMV-associated diseases that exhibit a high mortality, such as
asymptomatic viremia, DNAemia, antigenemia, esophagitis,
gastroenteritis, hepatitis, retinitis, pneumonia and encephalitis,
HCMV infection is also associated with graft vs. host disease
(GVHD), and the increased incidence of other pathogenic infections
such as Epstein-Barr virus, varicella-zoster virus and child
adenovirus (13).
HCMV is a double-stranded DNA β-herpes virus
(235,000 base pairs), also known as herpes virus 5, that contains
>200 potential open reading frames (14,15).
HCMV synthesizes a series of proteins after entering the host cell,
which are divided into immediate early protein (IE), delayed early
protein and late protein, according to the time at which they are
produced (16). These proteins are
synthesized within 2, 24 and after 24 h, respectively (17).
HCMV is latent in the peripheral monocytes and
endothelial cells of several organs. Distinct organs, tissues and
cell transplants can transmit HCMV. The latency of primary HCMV
infection relies on its multiple and complex immune evasion
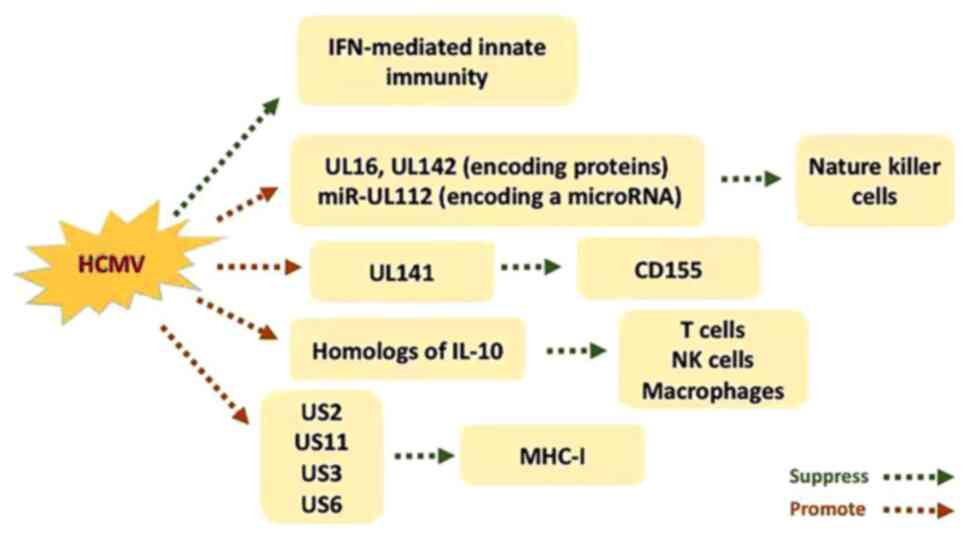
mechanisms to evade the host immune response (18,19).
Interferon (IFN)-mediated innate immunity is one of the first lines
of the host defense mechanism (12,20).
Specific genes encoded by HCMV are associated with the
downregulation of IFN-mediated innate immunity (11). In addition, HCMV infection
upregulates the function of ligands targeting the natural killer
cell activating receptor, natural killer group 2, member D
(21). However, the presentation of
these ligands on the surface of cells is suppressed by certain
HCMV-induced genes, including UL16 and UL142, which encode
proteins, and microRNA (miRNA/miR)-UL112, which encodes a miRNA
(12,22). Furthermore, HCMV influences the
expression of CD155 by upregulating UL141, exposing the receptor on
the cell surface to avoid recognition (23). Interleukin (IL)-10 serves as an
inhibitor, suppressing the secretion of several cytokines from
helper T cells, including IFN-γ and IL-2 (24,25).
This in turn attenuates the production of inflammatory cytokines
from monocytes and macrophages, decreasing the expression of major
histocompatibility complex (MHC)-II molecules and subsequent
antigen presentation (26). IL-10
also encodes proteins that act as host inflammatory cytokines,
resulting in a decrease of local cytokine effectiveness (27). Cheung et al (20) suggested that HCMV is associated with
the production of IL-10 homologs, which serves an immunosuppressive
role during the incubation period of infection. In addition, US2
and US11 have been demonstrated to inhibit the degradation of
target MHC-I molecules within the cytoplasm, resulting in
destruction by proteasomes. US3 interferes with molecular chaperone
related antigen peptide loading by containing MHC-I within the
endoplasmic reticulum. Furthermore, US6 suppresses the transporter
associated with MHC-I antigen processing. The expression of these
genes allow infected cells to escape immune clearance (Fig. 1) (28,29).
However, latent infection is established when the virus spreads to
and is persistently present in various cells, including myeloid
cells (such as monocytes and CD34 cells), endothelial cells,
epithelial cells (including retinal cells), smooth muscle cells,
fibroblasts, leukocytes and dendritic cells (30,31).
Endothelial and hematopoietic cell infection may lead to the spread
of the virus within various systems of the host (32). In addition, the infection of
ubiquitous cell types, such as fibroblasts and smooth muscle cells,
provides a platform for effective virus proliferation (33). CMV-specific CD4 and CD8 T cells
appear successively in the peripheral blood. CD4 T cells secrete
helper T cell-type cytokines, such as IFN-γ and tumor necrosis
factor (TNF)-α. CD8 T cells can lyse CMV-peptides to present target
cells (34,35). However, during latent infection,
these specific T cells fail to eliminate HCMV (12).
Following myeloablative conditioning, recipient
immune cells and malignant or defective cells are eliminated,
meaning that allo-HSCT recipients must go through a period of
pancytopenia for days to weeks depending on the source of stem
cells (5). The adaptive immune
system is subsequently restored slowly over a period of several
months to 1–2 years (36). In the
early stages following allo-HSCT, transferred immunity is only
maintained for a limited period, after which a gradual decrease is
observed (37). In addition to
hematological disease itself and the drugs administered during
myeloablative conditioning, immunosuppressive agents are used to
prevent GVHD, which can further delay immune reconstruction,
increasing patient susceptibility to several opportunistic
infections (12,38). After allo-HSCT, the immune system is
gradually restored following neutrophil engraftment; however, the
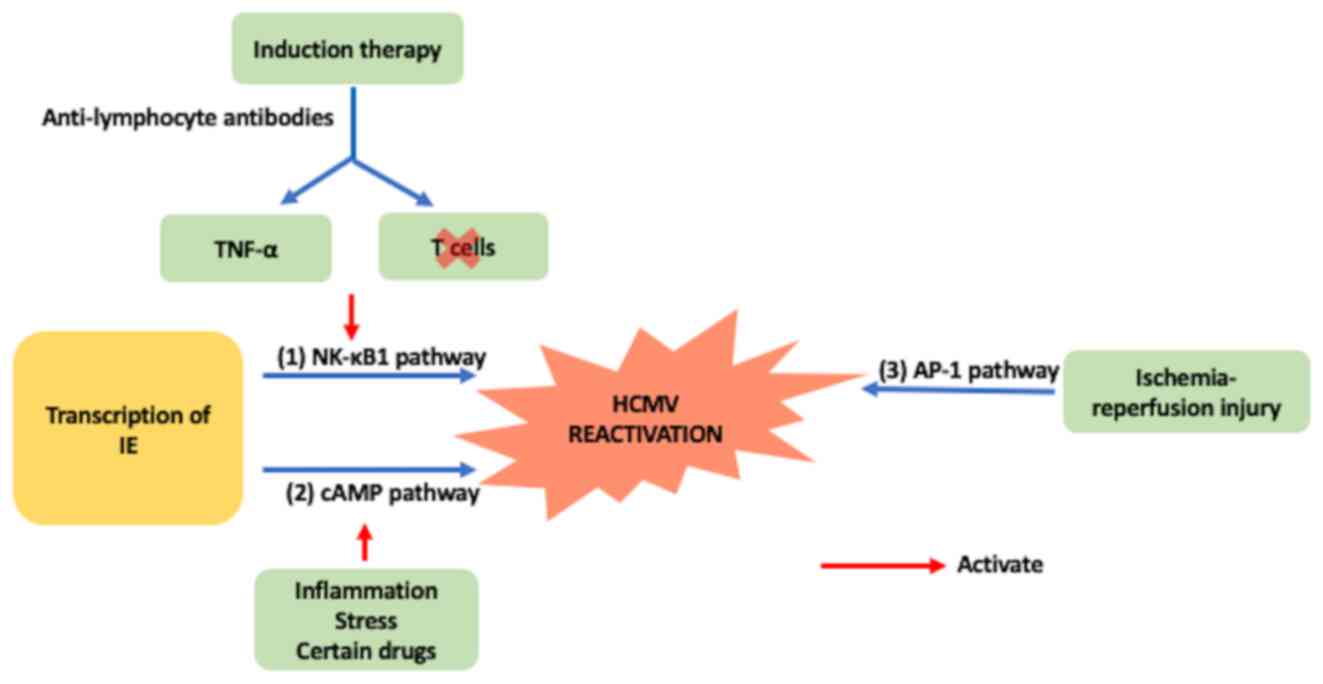
recovery of lymphocyte function takes an extended period (39). At this point, CMV ends its latent
period (3). It has been demonstrated
that the activation of multiple pathways can reactivate latent HCMV
(40). However, whether a cross-over
mechanism exists between each pathway is yet to be elucidated.
Anti-lymphocyte antibodies used for induction therapy can induce
TNF-α secretion and activate the NK-κB1 pathway, stimulating the
transcription of the HCMV IE gene, leading to the resurgence of
latent HCMV (41). Simultaneously,
antibody treatment can clear T cells, resulting in the lack of
T-cell immunity against CMV and decreased immunological
surveillance for HCMV (42). In the
event of rejection, latent HCMV can be activated through the NK-κB1
pathway (38). Inflammation
andstresscan activate the expression of IE through the cAMP pathway
(43). Ischemia-reperfusion injury
activates activator protein-1 (AP-1) through the AP-1 pathway
(38). Rejection following
transplantation typically occurs prior to HCMV activation (44). Activation of the NK-κB1 pathway
results in the transcription of HCMV genes that induce viral
infection (45). Subsequent
anti-rejection therapy, such as hormonal shock therapy or the
application of antithymocyte globulin (ATG) drugs, inhibit or
destroy immune function against HCMV (Fig. 2) (44).
T cell-driven cellular immunity is known to control
CMV replication, and the lack or delay of CMV-specific CD4- and
CD8-T lymphocyte recovery can lead to CMV recurrence and
CMV-associated diseases (46–48). CMV
reactivation is usually associated with a high frequency of GVHD,
which may partially lead to enhanced T-cell reconstitution in
patients with HCMV infection (12).
A previous study demonstrated that the presence of CMV-specific
cytotoxic T lymphocytes (CTL) in CMV seropositive recipients is
associated with faster T-cell reconstitution, which may induce
donor allogeneic reactivity (49).
The successful elimination of residual host hematopoietic function
is therefore reflected by the complete donor chimerism (50). In addition, suppression of cytokine
signaling genes (SOCS) can also explain the close association
between CMV reactivation, GVHD and donor chimerism (51). SOCS is associated with the regulation
of T-cell homeostasis and the negative feedback mechanism induced
by cytokine signaling, involving IFN-γ or interleukins (51). Previous studies on SOCS gene
expression have demonstrated that SOCS1 expression is significantly
lower in patients with GVHD compared with post-transplant patients
without GVHD (37,51,52).
Furthermore, SOCS1 expression is significantly lower in patients
with chronic GVHD than those with acute GVHD (53). In addition, our previous study
revealed that SOCS1 expression is significantly higher in patients
with CMV reactivation than those with non-CMV reactivation
(53). Conversely, SOCS3 expression
is decreased in all HSCT recipients (53,54).
These data explain the molecular association between HCMV
reactivation and allo-HSCT.
CMV serological status, that is, CMV–IgG (+) and
(−), is one of the main risk factors associated with the incidence
and mortality of patients with CMV disease following stem cell
transplantation (55). According to
previous studies, recipients with a negative CMV serostatus
receiving CMV seropositive donor grafts (D+/R-) have the highest
risk of mortality following transplantation (56,57).
However, other studies have demonstrated that although the risk of
infection in patients that are D+/R+ is lower, the survival time of
grafts and recipients is shorter than that of D+/R-individuals
(58–60). However, the association between CMV
serostatus and CMV-positive recipients (R+) remains
controversial.
Currently, the main sources of graft stem cells for
transplantation are bone marrow, peripheral blood stem cells and
umbilical cord blood (61).
Trenschel et al (62)
demonstrated that the incidence of persistent CMV antigenemia and
CMV-related interstitial pneumonia following peripheral blood stem
cell transplantation significantly decreases compared with bone
marrow transplantation, which may be due to the varying immune
reconstitution times following different graft transplantations. In
addition, Uppuluri et al (63) reported that the incidence rate of
HCMV reactivation in pediatric patients receiving allo-HSCT from
matched-related donors, unrelated peripheral blood stem cells,
unrelated umbilical cords and mismatched or haploidentical grafts
were 3.0, 33.3, 17.4 and 36.5%, respectively. Furthermore, Boeckh
et al (64) suggested that
patients receiving autologous stem cell transplantation have a
lower CMV disease morbidity than patients receiving allogeneic stem
cell transplantation.
Patients are routinely administered thymoglobulin,
cyclosporine, alemtuzumab and glucocorticoid shock therapy as
treatment following allo-HSCT (74).
However, these drugs have been reported to increase the risk of
HCMV reactivation (73,75). In addition, the increased use of
immunosuppressive agents is an important factor that affects HCMV
reactivation (76). Kobashigawa
et al (77) revealed that the
combination of tacrolimus and mycophenolate mofetil achieve a more
effective response with less side effects. Furthermore, previous
studies have suggested that the application of everolimus and
calcineurin inhibitors without steroid treatment can markedly
improve the incidence rate of CMV antigenemia (78–80).
Collectively, it has been demonstrated that immunosuppression
regimens are closely associated with CMV infection.
GVHD is caused by a series of ‘cytokine storms’
stimulated by T cells in allogeneic donor grafts following
transplantation, which greatly enhances its immune response to
recipient antigens (81). Target
cells are subsequently used to initiate cytotoxic attacks, of which
the skin, liver and intestine are the primary targets (82). Miller et al (83) demonstrated that CMV-specific
cytotoxic T cells may serve an important role in CMV infection
control. The incidence of GVHD and the treatment of
immunosuppression limits the proliferation of CMV-specific
cytotoxic T cells, thus increasing the chance of CMV infection
(84). Nutrient absorption and the
physical fitness of patients is weakened, which further increases
the risk of CMV infection (85,86).
Univariate analysis has revealed that the rate of CMV infection in
patients with acute grade 0-I GVHD following transplantation is
51.9%, and the rate of patients with acute grade II–IV GVHD is
92.3% (87). In addition,
multivariate analysis has demonstrated that patients with acute
grade II–IV GVHD exhibit a higher CMV infection rate following
transplantation (87).
Other risk factors for reactivation in allo-HSCT
recipients include advanced age, co-infection with human herpes
virus 6 or human herpes virus and HLA incompatibility (88).
The prediction of CMV-related diseases is important.
Due to primary hematological diseases, certain drugs (such as ATG)
and immunosuppressive therapies applied after allo-HSCT or GVHD
compromise the immune system of patients (89). In addition, the speed of immune
system recovery in different recipients is another contributing
factor (90). Given that the
resistance of patients to CMV following allo-HSCT mainly depends on
CMV-specific cytotoxic T-cells, CMV-specific cell-mediated immunity
serves an important role in reducing the risk of CMV-related
diseases (90). Yong et al
(91) revealed that the
quantification of CMV-specific T cells may predict the risk of
CMV-related disease. Furthermore, as CMV-specific T cells can be
measured by the production of IFN-γ, IFN-γ ELISpot assays serve an
important role in predicting the immunity of CMV-specific T cells
(92–94). In addition, Camargo et al
(41) suggested that determining the
phenotype of CMV-specific T cells, the non-protective signature
[NPS; IL-2− IFN-γ+ TNF-α− and
macrophage inflammatory protein (MIP-1β+)] and the
protective signature (PS; IL-2+ IFN-γ+
TNF-α+ and MIP-1β+), alone or in combination
may be used to determine the risk of CMV infection more
efficiently. In addition, patients with high NPS and low PS exhibit
an increased risk of CMV infection (41). Low levels of NKG2C copies within the
donor and the DNA load of torque teno virus may also be a predictor
of CMV infection (95,96). Previous studies have demonstrated
that suppression of SOCS, which is associated with IFN-γ or
interleukin negative feedback, and with measuring the function of T
cells (97), can explain the
association between CMV reactivation, GVHD and donor chimerism
(52–54). SOCS1 is expressed at low levels in
patients with GVHD than those without GVHD, and in patients with
chronic GVHD than those with acute GVHD (54). SOCS1 expression is also higher in
patients with reactivated CMV (53).
In a previous study where patients simultaneously exhibited CMV
reactivation and GVHD, SOCS1 expression decreased compared with
patients only exhibiting CMV reactivation (54). However, SOCS3 expression was
downregulated in all patients following transplantation (51).
For patients with a high risk of CMV-related
disease, several techniques used for immune monitoring, such as
measuring CMV-specific T-cell function, are effective for the
improvement of transplantation outcome. However, further studies
are required to confirm these results.
CMV-mediated disease is diagnosed when patients test
positive for CMV serum antigens or produce a positive viral culture
following infected tissue analysis, whilst demonstrating
corresponding clinical symptoms (98). CMV pp65 antigenemia assays and the
amplification of CMV DNA are currently the most used laboratory
techniques for the detection of CMV infection (99–101).
CMV pp65 antigenemia assays detect CMV pp65 antigens. Furthermore,
PCR is performed to detect CMV DNA viral load (102). Bhatia et al (99) demonstrated that the sensitivity and
specificity of the pp65 antigenemia assay were sufficient to use
for the early diagnosis of CMV infection. The pp65 antigen is
present in neutrophils and has a semi-quantitative association with
CMV virus replication. Since the pp65 antigenemia value usually
significantly increases during the first week of CMV treatment, the
assay results over this period must be taken seriously (103). This method of detection is simple,
easy to implement and requires inexpensive equipment. However, in
the absence of standard values, the results may be affected by
subjective factors (103). In
addition, the requirements for counting neutrophils are high
(104). Despite its low
specificity, quantitative DNA-detection techniques have an
observable sensitivity and can demonstrate patient prognosis by
measuring viral load (99). However,
since the results of PCR are affected by the type of specimen used,
only plasma or whole blood should be selected for serial viral load
testing (105,106). In addition, to differentially
diagnose patients with CMV-mediated pneumonia and pulmonary
shedding, the quantification of CMV DNA load in bronchoalveolar
lavage may be necessary (107,108).
Furthermore, the pp67 assay may determine advanced L-mRNA and
reflect active HCMV infection, which makes it an effective method
for monitoring CMV infection (109).
CMV prophylaxis is mainly aimed at patients with
high-risk CMV infection following allo-HSCT (110). Patients with a high risk of CMV
include those that are anti-CMV positive following transplantation,
those receiving transplants from unrelated donors, those with donor
HLA incompatibility and those receiving T lymphatic transplantation
(111). Preventive measures include
donor selection, blood product handling and the application of
antiviral drugs (112).
If both donors and recipients are CMV–IgG negative,
recipients are less likely to develop CMV infection following
allo-HSCT (13). Thus, for CMV–IgG
negative recipients, priority should be given to CMV–IgG negative
donors (13). The risk of CMV
infection and CMV disease in patients with this combination of
donor and recipient serotypes is significantly lower compared with
patients demonstrating other serotype combinations (113). The main route of CMV infection is
blood transfusion (114). However,
Boeckh and Ljungman (13)
recommended that if the donor and recipient match at HLA-A, HLA-B
or HLA-DR sites, but are seropositive, the matching donor is
preferred. In addition, age and blood type should also be taken
into consideration when selecting suitable donors (43).
A previous study revealed that blood products
obtained after leukocyte depletion effectively decrease the
incidence of CMV infection (115).
Vamvakas (113) reported that CMV
seronegative blood components should be selected over white blood
cell reduced blood components to effectively prevent CMV infection.
The removal of leukocytes from blood products primarily occurs
through filtration, decreasing CMV infection via transfusion.
Traditionally, this process is used to screen CMV seronegative
blood products and prevent CMV infection (115). However, this screening technique is
difficult as it requires increased manpower and material resources
(115). In addition, due to the
high incidence of CMV infection in certain territories, it may be
difficult to obtain CMV seronegative blood products (115).
The role of IVIG in preventing CMV infection is
controversial. Previous studies have demonstrated that IVIG serves
no preventive function in CMV diseases and may also cause other
serious complications, such as interstitial pneumonia (58,116–118).
In addition, Malagola et al (119) affirmed the clinical therapeutic
effect, safety and tolerance of anti-CMV specific immunoglobulins,
such as Megalotect. Furthermore, HCMV immunoglobulin has been
approved for use in high-risk lung transplant recipients by the
Food and Drug Association of the United States (120). Notably, although decreasing
immunosuppression to the greatest extent possible is crucial,
caution must be used when considering IVIG.
The antiviral drugs currently administered to
prevent CMV infection include ganciclovir, valganciclovir,
foscarnet and cidofovir (Table I).
However, the use of antiviral drugs as preventive treatment remains
controversial. A recent retrospective study evaluated the
effectiveness of antiviral drug administration for the prevention
of CMV, the results of which revealed that the regimen was only
partially effective (121). An
additional prospective study compared the use of valganciclovir
with a placebo. The results demonstrated that whilst valganciclovir
prophylaxis was effective in decreasing CMV reactivation, it did
not decrease CMV infection or mortality, indicating that its affect
was not superior when compared with preemptive treatment (122). Thus, due to the disadvantages and
adverse drug reactions associated with antiviral drugs, including
bone marrow suppression, the majority of HSCT recipients receive
preemptive treatment rather than prophylaxis (123).
The development of safe and effective vaccines for
CMV has been the focus of recent medical research. As such, there
are currently several vaccines under development (124–127).
Adjuvant recombinant protein vaccines, which comprise envelope
glycoprotein and DNA plasmid, peptide-based vaccines, vectored
vaccines and peptide vaccines are currently used against CMV
(125). Among those proposed, a
specific bivalent DNA vaccine, named ASP0113, is the most studied.
However, phase two clinical trials have indicated that whilst the
vaccine demonstrates certain antiviral effects, its immunogenicity
is not statistically significant (128). Despite these results, phase three
clinical trials are currently underway (126). In addition, another vaccine derived
from soluble recombinant glycoprotein B (gB) with the adjuvant MF59
and CMV monoclonal is being developed (127). In conclusion, the application of
antiviral vaccines requires additional research and
development.
Preemptive treatment refers to immediate antiviral
administration when CMV antigenemia or viremia first occurs
following transplantation. Recently, the application of preemptive
treatment has significantly decreased the incidence and mortality
of CMV-related diseases following allo-HSCT (129). In addition, the length of treatment
required for infection has been shortened, and the incidence of
adverse reactions has improved (130). Thus, the success of preemptive
treatment primarily depends on the sensitive detection of CMV
antigenemia (13). If treatment is
performed before detecting the virus, some patients may be treated
unnecessarily. Any adverse reactions because of drug administration
may therefore increase the probability of infection by other
bacterial or fungal agents (13). In
addition, receiving treatment too late may also affect the
antiviral response of patients (131). With use of the CMV pp65 antigen
test or PCR, preemptive treatment can be undertaken at a targeted
viral load (89). The target viral
load varies according to the risk of developing CMV-related
diseases and current immunosuppression (132). Drugs currently used for preemptive
treatment include ganciclovir, valganciclovir, foscarnet and
cidofovir (Table I). Under normal
circumstances, preemptive treatment should be maintained until the
relevant symptoms are resolved and the CMV serum test is negative
(89). If the patients' initial
viral load or pp65 antigenemia assay is positive, treatment is
maintained until the PCR/pp65 antigenemia assay turns negative.
Subsequently, patients should receive maintenance treatment for a
varying period (89). The length of
maintenance treatment varies from 0–6 weeks depending on factors
such as the patients' sensitivity to treatment, drug side effects
and the risk of relapse (133).
Most transplant centers worldwide use ganciclovir as
the drug of choice for early treatment (134). As an inhibitor of DNA polymerase,
ganciclovir inhibits the replication of viral DNA in vivo to
prevent viral infection (112,135).
Winston et al (136)
revealed that when administering ganciclovir prior to or following
allo-HSCT, the incidence rate and severity of CMV infection
decreases, despite the suppression of bone narrow function. Similar
results have been demonstrated in previous studies (64,137,138).
The myelosuppressive effect of ganciclovir may be improved by
administering granulocyte-colony stimulating factor (G-CSF) alone
or in combination with anti-CMV immunoglobulins (139,140).
However, ganciclovir is inefficient in treating interstitial
pneumonia following transplantation (141).
As the antiviral immunity of patients differs before
and after 100 days of transplantation, the corresponding preemptive
treatment regimens also differ (142). According to guidelines (142), preemptive treatment within 100 days
after transplantation is suitable for patients who have a high risk
of CMV infection following autologous stem cell transplantation,
and for patients receiving allogeneic stem cell transplantation who
have tested positive for CMV antigenemia or viremia for the first
time after transplantation (143).
The preferred treatment for these patients is inducive intravenous
5 mg/kg−1/d−1 ganciclovir administered twice
a day for 7–14 days, with maintenance therapy once a day until two
consecutive tests are negative. In addition, preemptive treatment
after 100 days of transplantation is suitable for various patients
who achieve two consecutive CMV viremia results or PCR positive
tests, including those receiving allogeneic hematopoietic stem cell
transplants, GVHD patients receiving steroid therapy or patients
receiving CMV antiviral therapy within 100 days of transplantation
(131). Due to the myelosuppressive
effect of ganciclovir, previous studies have suggested decreasing
its dose to a degree that does not change the antiviral effect
(89,144). According to a recent study, the
dosage should be adjusted based on viral load and that low-dose
ganciclovir administered at the beginning of preemptive treatment
may be safe and feasible (145). It
may also greatly improve the side effects of treatment (89).
Valganciclovir is a prodrug of ganciclovir and
demonstrates a good oral bioavailability. A previous study has
demonstrated that the blood exposure level of ganciclovir after
oral valciclovir administration is higher than intravenous
ganciclovir (146). Oral
administration is also more convenient and avoids related
infections caused by intravenous administration. In addition, due
to its myelotoxicity and similar side effects to ganciclovir, close
monitoring of patients during treatment is required (147).
Furthermore, a randomized controlled trial revealed
that foscarnet demonstrated similar effects to ganciclovir but
without granulocytopenia, making it suitable for patients
exhibiting bone marrow suppression (148). The main adverse reaction following
foscarnet administration is electrolyte disturbance; however, this
can be easily corrected by intravenous fluid replacement (115).
An additional drug used in preemptive treatment is
cidofovir. The pharmacokinetic characteristics of cidofovir require
its administration once a week (149). Although its main side effect is
renal toxicity, this can be reduced by receiving hydration and
probenecid (115). Cidofovir is
often administrated when ganciclovir or foscarnet treatment has
been ineffective or if the patient demonstrates intolerance
(149).
Letermovir (LET) is a novel antiviral drug that
suppresses the CMV-terminase complex instead of CMV
deoxyribonucleic acid polymerase (150). It can significantly decrease the
incidence of CMV infection with few side effects and demonstrates
no cross-resistance with other CMV antivirals (150). LET is available both orally and
intravenously at 480 and 240 mg dosages, and was approved for use
in CMV infection prophylaxis in CMV-seropositive recipients of
allogeneic HSCT over the age of 18 in 2017 (151). Previous studies have demonstrated
that LET resistance is primarily a result of mutations in the CMV
UL56 gene (152,153).
Recently, clinical trials assessing the
effectiveness and safety of novel drugs against CMV have been
performed or are currently underway (Table II).
Brincidofovir is an orally administered drug that
is a bioavailable lipid conjugate of cidofovir (154). Its antiviral effect has been
confirmed both in vivo and in vitro (155–157).
It has demonstrated a broad spectrum of effects on several viruses,
including herpes virus, polyoma virus, adenovirus, papilloma virus
and smallpox virus (158,159). The long half-life of brincidofovir
and the absence of nephrotoxicity also makes it a desirable
candidate for anti-CMV treatment (160–164).
However, Marty et al (165)
indicated that brincidofovir may be associated with
gastrointestinal reactions following administration (165). Based on the existing research
currently available, a complete evaluation cannot be made for the
clinical application of brincidofovir.
Refractory CMV infection occurs when CMV
antigenemia or DNAemia remains positive, or the CMV DNA copy number
increases or remains unchanged after 14 days of regular antiviral
treatment. When suspected resistance occurs, blood samples should
be obtained from patients and sent for the phenotypic testing of
resistance genes (1). In addition,
certain antiviral drugs, including foscarnet sodium, should be
replaced in the case of ganciclovir resistance. Ganciclovir
administered in combination with phosphonate sodium is a method.
The dose of ganciclovir may be occasionally increased to 15
mg/kg−1/d−1, with G-CSF administered as a
supportive treatment (13). Avery
et al (172) demonstrated
that the administration of oral maribavir may be beneficial for the
treatment of refractory or resistant CMV infection. In addition,
several case reports assessing the antimalarial drug, artesunate,
and the novel anti-rheumatic drug, leflunomide, revealed that each
agent successfully treated refractory CMV infections that were
resistant to multiple antiviral drugs (173,174).
The restoration of the CMV-specific CTL response in
patients receiving transplantation is indispensable (175). Reusser et al (49) assessed the transfer of CMV-specific
CD8+ T cells for the first time in 1991 (49). Since then, many studies have done the
same. Walter et al (176)
selected 14 patients with CMV-specific CTL deficiency following
allo-HSCT and applied CTL clones as treatment. The results
confirmed the safety and efficiency of this immunotherapeutic
technique (176). In a phase two
clinical trial performed in 2013, Blyth et al (177) revealed that the adoptive transfer
of CMV-specific CTL was exceedingly beneficial for the antiviral
response exhibited by patients, the inhibition of virus replication
and the spread of infection. Furthermore, Peggs et al
(178) treated 16 patients with CMV
infection following allo-HSCT with CMV-CTL. The results revealed
that 50% of patients achieved negative CMV DNA without antiviral
treatment (178). In a phase I/IIa
trial, Neuenhahn et al (179) reported that the adoptive transfer
of stem cells from a donor or third-party donor was associated with
the reconstitution of CMV-specific T-cells in transplant
recipients. In addition, the first application of virus specific
T-cell transfer in Turkey exerted a degree of control over CMV
replication (176). However,
antiviral drugs may be administered in combination due to their
lack of effect on CMV specific IgG (180).
The application of CMV-CTL can speed up the immune
reconstruction of patients following allo-HSCT (181,182),
effectively suppressing CMV replication whilst decreasing the use
of antiviral drugs and their accompanying adverse reactions
(183). It may therefore be an
ideal replacement for antiviral drugs in the future (184,185).
The occurrence of GVHD (both acute and chronic) is a significant
concern in initial trials that utilize unmanipulated donor products
(183). According to previous
studies, these concerns can be eliminated with the development of
technologies that select and expand specific T cells (179,186).
Recently, the rates of GVHD following cell therapy have not
exceeded those expected for patients post-HSCT (179,186).
Almost all patients who develop GVHD following treatment do so as
the result of other high-risk factors, including history of chronic
or acute GVHD, subtherapeutic immunosuppression or receiving prior
T cell-replete grafts (187–189)
(Table III). However, there are
still several challenges for the clinical application of this
method (190). Although a study has
suggested that G-CSF can be used for stimulation (191), it is unknown how to practically
prepare T cells for this treatment (192). In addition, there is no uniform
standard for T-cell subsets that are optimal for anti-CMV treatment
(186). Thus, this method needs to
be improved through subsequent research.
Mesenchymal stem cells (MSCs) are one of the most
common adult stem cells, originating from non-hematopoietic stem
cells isolated from bone marrow (193). MSCs participate in the formation of
the bone marrow hematopoietic microenvironment and provide
significant support for the proliferation and differentiation of
hematopoietic stem cells (194).
MSCs can also support hematopoietic reconstitution by cell-cell
contact and the secretion of cytokines to promote the shift from
Th2 to Th1 phenotypes, increasing the expression of T regulatory
cells to regulate the immune system (195). MSCs have been used in the treatment
of GVHD and have wide application prospects (196). However, research on MSCs has
primarily focused on its effect on allogeneic T cells (197). Thus, whether virus-specific T cells
have the same effect is yet to be fully elucidated. In addition,
little is known about how MSCs affect CTLs and the conversion of
memory and effector T-cell subgroups in CMV-CTL. In our previous
study, it was demonstrated that MSCs inhibit the proliferation of
allogeneic CD8+ T cells and CMV-specific T cells in
vitro (198). However, there is
insufficient evidence on whether its molecular mechanism of action
and T-cell immune function are affected.
Although CMV infection can cause high mortality
following transplantation, Elmaagacli et al (199) revealed that patients with acute
myeloid leukemia (AML) demonstrating donor and recipient CMV
seropositivity or early or late post-transplantation CMV
antigenemia have a decreased risk of relapse. This may be due to
the apoptosis of AML cells following HCMV reactivation (200–203).
However, current conclusions and related mechanisms require further
research.
With the continuous advancement of transplantation
technology, an increased number of patients with hematological
tumors are undergoing HSCT. Correspondingly, the number of CMV
infections following transplantation is also increasing. Antiviral
treatment still occupies the mainstream position in the prevention
and treatment of CMV, and drugs currently used for prophylaxis and
preemptive treatment include ganciclovir, valganciclovir, foscarnet
and cidofovir. Although the application of post-transplantation CMV
infection antiviral prophylaxis and preemptive therapy has
significantly decreased the risk of post-transplant CMV infection
and disease, adverse reactions are commonplace. Thus, other methods
that decrease the incidence of CMV infection and disease following
transplantation are urgently required. Notable advancements have
been established in recent years, including the elucidation of
novel drugs, the adoptive transfer of CMV-specific CTLs and the
application of MSCs. Although the effectiveness of these novel
methods has not yet been determined, it is believed that with the
progress of research, the prophylaxis and treatment of CMV
infection following transplantation will further improved.
Not applicable.
The present review was supported by the Natural
Science Foundation of Jiangsu Province (grant no. BK20150639), the
Key Medical of Jiangsu Province (grant no. ZDXKB2016020), the Six
Talent Peaks Project in Jiangsu Province (grant no. WSW-033) and
the Innovative and entrepreneurial doctors of Jiangsu province.
Not applicable.
XZ, NJ and BC were responsible for confirming the
topic, and collecting and analyzing the data. XZ designed and
drafted the initial manuscript, and edited the figure legends and
tables. All authors have read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Ljungman P, de la Camara R, Robin C,
Crocchiolo R, Einsele H, Hill JA, Hubacek P, Navarro D, Cordonnier
C and Ward KN; 2017 European Conference on Infections in Leukaemia
group, : Guidelines for the management of cytomegalovirus infection
in patients with haematological malignancies and after stem cell
transplantation from the 2017 European Conference on Infections in
Leukaemia (ECIL 7). Lancet Infect Dis. 19:e260–e272. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gyurkocza B, Rezvani A and Storb RF:
Allogeneic hematopoietic cell transplantation: The state of the
art. Expert Rev Hematol. 3:285–299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gratwohl A, Baldomero H, Aljurf M,
Pasquini MC, Bouzas LF, Yoshimi A, Szer J, Lipton J, Schwendener A,
Gratwohl M, et al: Hematopoietic stem cell transplantation: A
global perspective. JAMA. 303:1617–1624. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Borchers S, Luther S, Lips U, Hahn N,
Kontsendorn J, Stadler M, Buchholz S, Diedrich H, Eder M, Koehl U,
et al: Tetramer monitoring to assess risk factors for recurrent
cytomegalovirus reactivation and reconstitution of antiviral
immunity post allogeneic hematopoietic stem cell transplantation.
Transpl Infect Dis. 13:222–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moss P and Rickinson A: Cellular
immunotherapy for viral infection after HSC transplantation. Nat
Rev Immunol. 5:9–20. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haidar G and Singh N: Viral infections in
solid organ transplant recipients: Novel updates and a review of
the classics. Curr Opin Infect Dis. 30:579–588. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Enders G, Daiminger A, Lindemann L, Knotek
F, Bäder U, Exler S and Enders M: Cytomegalovirus (CMV)
seroprevalence in pregnant women, bone marrow donors and
adolescents in Germany, 1996–2010. Med Microbiol Immunol.
201:303–309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ouédraogo AS, Yaméogo JT, Poda GEA,
Kientega Y and Ouédraogo Traore R: Prevalence of anti-CMV
antibodies in blood donors in Ouagadougou (Burkina Faso). Med Sante
Trop. 22:107–109. 2012.PubMed/NCBI
|
|
9
|
Revello MG, Vauloup-Fellous C,
Grangeot-Keros L, van Helden J, Dickstein Y, Lipkin I, Mühlbacher A
and Lazzarotto T: Clinical evaluation of new automated
cytomegalovirus IgM and IgG assays for the Elecsys(®)
analyser platform. Eur J Clin Microbiol Infect Dis. 31:3331–3339.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Strand KM, Odland ML, Iversen AC, Nordbø
SA, Vik T and Austgulen R: Cytomegalovirus antibody status at 17–18
weeks of gestation and pre-eclampsia: A case-control study of
pregnant women in Norway. BJOG. 119:1316–1323. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Amsler L, Verweij M and DeFilippis VR: The
tiers and dimensions of evasion of the type I interferon response
by human cytomegalovirus. J Mol Biol. 425:4857–4871. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2013. View Article : Google Scholar
|
|
13
|
Boeckh M and Ljungman P: How we treat
cytomegalovirus in hematopoietic cell transplant recipients. Blood.
113:5711–5719. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bankier AT, Beck S, Bohni R, Brown CM,
Cerny R, Chee MS, Hutchison CA III, Kouzarides T, Martignetti JA,
Preddie E, et al: The DNA sequence of the human cytomegalovirus
genome. DNA Seq. 2:1–12. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murphy E and Shenk T: Human
cytomegalovirus genome. Curr Top Microbiol Immunol. 325:1–19.
2008.PubMed/NCBI
|
|
16
|
Scherer M, Schilling EM and Stamminger T:
The human CMV IE1 protein: An offender of PML NUCLEAR BOdies. Adv
Anat Embryol Cell Biol. 223:77–94. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fox HL, Dembowski JA and DeLuca NA: A
herpesviral immediate early protein promotes transcription
elongation of viral transcripts. mBio. 8:e00745–17. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gardner TJ and Tortorella D: Virion
glycoprotein-mediated immune evasion by human cytomegalovirus: A
sticky virus makes a slick getaway. Microbiol Mol Biol Rev.
80:663–677. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sezgin E, An P and Winkler CA: Host
genetics of cytomegalovirus pathogenesis. Front Genet. 10:6162019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheung AK, Gottlieb DJ, Plachter B,
Pepperl-Klindworth S, Avdic S, Cunningham AL, Abendroth A and
Slobedman B: The role of the human cytomegalovirus UL111A gene in
down-regulating CD4+ T-cell recognition of latently
infected cells: Implications for virus elimination during latency.
Blood. 114:4128–4137. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Slavuljica I, Busche A, Babić M, Mitrović
M, Gašparović I, Cekinović D, Markova Car E, Pernjak Pugel E,
Ciković A, Lisnić VJ, et al: Recombinant mouse cytomegalovirus
expressing a ligand for the NKG2D receptor is attenuated and has
improved vaccine properties. J Clin Invest. 120:4532–4545. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Griffiths PD: Burden of disease associated
with human cytomegalovirus and prospects for elimination by
universal immunisation. Lancet Infect Dis. 12:790–798. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wilkinson GW, Tomasec P, Stanton RJ,
Armstrong M, Prod'homme V, Aicheler R, McSharry BP, Rickards CR,
Cochrane D, Llewellyn-Lacey S, et al: Modulation of natural killer
cells by human cytomegalovirus. J Clin Virol. 41:206–212. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fang D and Zhu J: Molecular switches for
regulating the differentiation of inflammatory and IL-10-producing
anti-inflammatory T-helper cells. Cell Mol Life Sci. 77:289–303.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou L, Chong MM and Littman DR:
Plasticity of CD4+ T cell lineage differentiation.
Immunity. 30:646–655. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Opal SM and DePalo VA: Anti-inflammatory
cytokines. Chest. 117:1162–1172. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McSharry BP, Avdic S and Slobedman B:
Human cytomegalovirus encoded homologs of cytokines, chemokines and
their receptors: Roles in immunomodulation. Viruses. 4:2448–2470.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barel MT, Ressing M, Pizzato N, van
Leeuwen D, Le Bouteiller P, Lenfant F and Wiertz EJ: Human
cytomegalovirus-encoded US2 differentially affects surface
expression of MHC class I locus products and targets
membrane-bound, but not soluble HLA-G1 for degradation. J Immunol.
171:6757–6765. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khan N, Bruton R, Taylor GS, Cobbold M,
Jones TR, Rickinson AB and Moss PA: Identification of
cytomegalovirus-specific cytotoxic T lymphocytes in vitro is
greatly enhanced by the use of recombinant virus lacking the US2 to
US11 region or modified vaccinia virus Ankara expressing individual
viral genes. J Virol. 79:2869–2879. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ciáurriz M, Zabalza A, Beloki L, Mansilla
C, Pérez-Valderrama E, Lachén M, Bandrés E, Olavarría E and Ramírez
N: The immune response to cytomegalovirus in allogeneic
hematopoietic stem cell transplant recipients. Cell Mol Life Sci.
72:4049–4062. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Minton K: Viral immunity: How CMV bypasses
immune memory. Nat Rev Immunol. 10:2882010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Reddehase MJ and Lemmermann NAW: Cellular
reservoirs of latent cytomegaloviruses. Med Microbiol Immunol.
208:391–403. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sinzger C, Digel M and Jahn G:
Cytomegalovirus cell tropism. Curr Top Microbiol Immunol.
325:63–83. 2008.PubMed/NCBI
|
|
34
|
Higdon LE, Trofe-Clark J, Liu S, Margulies
KB, Sahoo MK, Blumberg E, Pinsky BA and Maltzman JS:
Cytomegalovirus-responsive CD8+ T cells expand after
solid organ transplantation in the absence of CMV disease. Am J
Transplant. 17:2045–2054. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Albiero E, Amati E, Baumeister E, Einsele
H, Grigoleit GU and Rodeghiero F: Heterogeneity of Specific
CD4+ and CD8+ T cells stimulated by CMV pp65
and IE1 antigens. J Immunother. 39:329–342. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Styczynski J: Who is the patient at risk
of CMV recurrence: A review of the current scientific evidence with
a focus on hematopoietic cell transplantation. Infect Dis Ther.
7:1–16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cho SY, Lee DG and Kim HJ: Cytomegalovirus
infections after hematopoietic stem cell transplantation: Current
status and future immunotherapy. Int J Mol Sci. 20:26662019.
View Article : Google Scholar
|
|
38
|
Kim SJ, Varghese TK, Zhang Z, Zhao LC,
Thomas G, Hummel M and Abecassis M: Renal ischemia/reperfusion
injury activates the enhancer domain of the human cytomegalovirus
major immediate early promoter. Am J Transplant. 5:1606–1613. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kurata K, Yakushijin K, Mizuno I, Gomyo H,
Okamura A, Ichikawa H, Sakai R, Mizutani Y, Kakiuchi S, Miyata Y,
et al: Early lymphocyte recovery predicts clinical outcome after
HSCT with mycophenolate mofetil prophylaxis in the Japanese
population. Int J Hematol. 108:58–65. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Theobald SJ, Khailaie S, Meyer-Hermann M,
Volk V, Olbrich H, Danisch S, Gerasch L, Schneider A, Sinzger C,
Schaudien D, et al: Signatures of T and B cell development,
functional responses and PD-1 upregulation after HCMV latent
infections and reactivations in Nod.Rag.Gamma mice humanized with
cord blood CD34+ cells. Front Immunol. 9:27342018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Camargo JF, Wieder ED, Kimble E, Benjamin
CL, Kolonias DS, Kwon D, Chen XS and Komanduri KV: Deep functional
immunophenotyping predicts risk of cytomegalovirus reactivation
after hematopoietic cell transplantation. Blood. 133:867–877. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
van Gent R, Metselaar HJ and Kwekkeboom J:
Immunomodulation by hyperimmunoglobulins after solid organ
transplantation: Beyond prevention of viral infection. Transplant
Rev (Orlando). 31:78–86. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Prösch S, Wendt CE, Reinke P, Priemer C,
Oppert M, Krüger DH, Volk HD and Döcke WD: A novel link between
stress and human cytomegalovirus (HCMV) infection: Sympathetic
hyperactivity stimulates HCMV activation. Virology. 272:357–365.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Atabani SF, Smith C, Atkinson C, Aldridge
RW, Rodriguez-Perálvarez M, Rolando N, Harber M, Jones G, O'Riordan
A, Burroughs AK, et al: Cytomegalovirus replication kinetics in
solid organ transplant recipients managed by preemptive therapy. Am
J Transplant. 12:2457–2464. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yong MK, Lewin SR and Manuel O: Immune
monitoring for CMV in transplantation. Curr Infect Dis Rep.
20:42018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Blyth E, Withers B, Clancy L and Gottlieb
D: CMV-specific immune reconstitution following allogeneic stem
cell transplantation. Virulence. 7:967–980. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Król L, Stuchlý J, Hubáček P, Keslová P,
Sedláček P, Starý J, Hrušák O and Kalina T: Signature profiles of
CMV-specific T-cells in patients with CMV reactivation after
hematopoietic SCT. Bone Marrow Transplant. 46:1089–1098. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lilleri D, Gerna G, Zelini P, Chiesa A,
Rognoni V, Mastronuzzi A, Giorgiani G, Zecca M and Locatelli F:
Monitoring of human cytomegalovirus and virus-specific T-cell
response in young patients receiving allogeneic hematopoietic stem
cell transplantation. PLoS One. 7:e416482012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Reusser P, Riddell SR, Meyers JD and
Greenberg PD: Cytotoxic T-lymphocyte response to cytomegalovirus
after human allogeneic bone marrow transplantation: Pattern of
recovery and correlation with cytomegalovirus infection and
disease. Blood. 78:1373–1380. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ogonek J, Varanasi P, Luther S, Schweier
P, Kühnau W, Göhring G, Dammann E, Stadler M, Ganser A, Borchers S,
et al: Possible impact of cytomegalovirus-specific CD8+
T cells on immune reconstitution and conversion to complete donor
chimerism after allogeneic stem cell transplantation. Biol Blood
Marrow Transplant. 23:1046–1053. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hill GR, Kuns RD, Raffelt NC, Don AL,
Olver SD, Markey KA, Wilson YA, Tocker J, Alexander WS, Clouston
AD, et al: SOCS3 regulates graft-versus-host disease. Blood.
116:287–296. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Alexander WS: Suppressors of cytokine
signalling (SOCS) in the immune system. Nat Rev Immunol. 2:410–416.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lee TH, Lee JY, Park S, Shin SH, Yahng SA,
Yoon JH, Lee SE, Cho BS, Kim YJ, Lee S, et al: Expression of SOCS1
and SOCS3 genes in human graft-versus-host disease after allogeneic
hematopoietic stem cell transplantation. Blood Res. 48:16–23. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Shin SH, Lee JY, Lee TH, Park SH, Yahng
SA, Yoon JH, Lee SE, Cho BS, Lee DG, Kim YJ, et al: SOCS1 and SOCS3
are expressed in mononuclear cells in human cytomegalovirus viremia
after allogeneic hematopoietic stem cell transplantation. Blood
Res. 50:40–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Meyers JD, Flournoy N and Thomas ED: Risk
factors for cytomegalovirus infection after human marrow
transplantation. J Infect Dis. 153:478–488. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kuo HT, Ye X, Sampaio MS, Reddy P and
Bunnapradist S: Cytomegalovirus serostatus pairing and deceased
donor kidney transplant outcomes in adult recipients with antiviral
prophylaxis. Transplantation. 90:1091–1098. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Nichols WG, Corey L, Gooley T, Davis C and
Boeckh M: High risk of death due to bacterial and fungal infection
among cytomegalovirus (CMV)-seronegative recipients of stem cell
transplants from seropositive donors: Evidence for indirect effects
of primary CMV infection. J Infect Dis. 185:273–282. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Schnitzler MA, Woodward RS, Brennan DC,
Spitznagel EL, Dunagan WC and Bailey TC: Impact of cytomegalovirus
serology on graft survival in living related kidney
transplantation: Implications for donor selection. Surgery.
121:563–568. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Gerstenkorn C, Balupuri S, Mohamed MA,
Manas DM, Ali S, Kirby J and Talbot D: The impact of
cytomegalovirus serology for 7-year graft survival in cadaveric
kidney transplantation-the Newcastle experience. Transpl Int. 13
(Suppl 1):S372–S374. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Harvala H, Stewart C, Muller K, Burns S,
Marson L, MacGilchrist A and Johannessen I: High risk of
cytomegalovirus infection following solid organ transplantation
despite prophylactic therapy. J Med Virol. 85:893–898. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Amouzegar A, Dey BR and Spitzer TR:
Peripheral blood or bone marrow stem cells? Practical
considerations in hematopoietic stem cell transplantation. Transfus
Med Rev. 33:43–50. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Trenschel R, Ross S, Hüsing J, Ottinger H,
Elmaagacli A, Roggendorf M, Schaefer UW and Runde V: Reduced risk
of persisting cytomegalovirus pp65 antigenemia and cytomegalovirus
interstitial pneumonia following allogeneic PBSCT. Bone Marrow
Transplant. 25:665–672. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Uppuluri R, Subburaj D, Jayaraman D,
Swaminathan VV, Mullanfiroze K, Vaidhyanathan L and Raj R:
Cytomegalovirus reactivation posthematopoietic stem cell
transplantation (HSCT) and type of graft: A step toward
rationalizing CMV testing and positively impacting the economics of
HSCT in developing countries. Pediatr Blood Cancer. 64:2017.doi:
10.1002/pbc.26639. View Article : Google Scholar
|
|
64
|
Boeckh M, Gooley TA, Myerson D, Cunningham
T, Schoch G and Bowden RA: Cytomegalovirus pp65 antigenemia-guided
early treatment with ganciclovir versus ganciclovir at engraftment
after allogeneic marrow transplantation: A randomized double-blind
study. Blood. 88:4063–4071. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Gatault P, Al-Hajj S, Noble J, Chevallier
E, Piollet M, Forconi C, Gaudy-Graffin C, Thibault G,
Miquelestorena-Standley E, Halimi JM, et al: CMV-infected kidney
grafts drive the expansion of blood-borne CMV-specific T cells
restricted by shared class I HLA molecules via presentation on
donor cells. Am J Transplant. 18:1904–1913. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Sylwester AW, Mitchell BL, Edgar JB,
Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA,
Kern F, et al: Broadly targeted human cytomegalovirus-specific
CD4+ and CD8+ T cells dominate the memory
compartments of exposed subjects. J Exp Med. 202:673–685. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Essa S, Pacsa A, Said T, Nampoory MR,
Raghupathy R, Johny KV, Al-Nakib W and Al-Mosawy M: Is combined
pretransplantation seropositivity of kidney transplant recipients
for cytomegalovirus antigens (pp150 and pp28) a predictor for
protection against infection? Med Princ Pract. 17:66–70. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wills MR, Okecha G, Weekes MP, Gandhi MK,
Sissons PJ and Carmichael AJ: Identification of naive or
antigen-experienced human CD8+ T cells by expression of
costimulation and chemokine receptors: Analysis of the human
cytomegalovirus-specific CD8+ T cell response. J
Immunol. 168:5455–5464. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Maecker HT and Maino VC: Analyzing T-cell
responses to cytomegalovirus by cytokine flow cytometry. Hum
Immunol. 65:493–499. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Baumann NS, Welten SPM, Torti N, Pallmer
K, Borsa M, Barnstorf I, Oduro JD, Cicin-Sain L and Oxenius A:
Early primed KLRG1-CMV-specific T cells determine the size of the
inflationary T cell pool. PLoS Pathog. 15:e10077852019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Scheinberg P, Melenhorst JJ, Brenchley JM,
Hill BJ, Hensel NF, Chattopadhyay PK, Roederer M, Picker LJ, Price
DA, Barrett AJ and Douek DC: The transfer of adaptive immunity to
CMV during hematopoietic stem cell transplantation is dependent on
the specificity and phenotype of CMV-specific T cells in the donor.
Blood. 114:5071–5080. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Liu J, Chang YJ, Yan CH, Xu LP, Jiang ZF,
Zhang XH, Liu KY and Huang XJ: Poor CMV-specific CD8+ T central
memory subset recovery at early stage post-HSCT associates with
refractory and recurrent CMV reactivation. J Infect. 73:261–270.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
LaMattina JC, Mezrich JD, Hofmann RM,
Foley DP, D'Alessandro AM, Sollinger HW and Pirsch JD: Alemtuzumab
as compared to alternative contemporary induction regimens. Transpl
Int. 25:518–526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wang QY, Dong YJ, Liang ZY, Yin Y, Liu W,
Xu WL, Sun YH, Han N, Li Y and Ren HY: Clinical analysis for
patients with AML treated after Allo-HSCT. Zhongguo Shi Yan Xue Ye
Xue Za Zhi. 28:1105–1114. 2020.(In Chinese). PubMed/NCBI
|
|
75
|
Kanter J, Pallardó L, Gavela E, Escudero
V, Beltrán S, Morales A, Avila A and Crespo JF: Cytomegalovirus
infection renal transplant recipients: Risk factors and outcome.
Transplant Proc. 41:2156–2158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hosseini-Moghaddam SM, Rotstein C and
Husain S: Effects of the intensity of immunosuppressive therapy on
outcome of treatment for CMV disease in organ transplant
recipients. Am J Transplant. 11:4072011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kobashigawa JA, Miller LW, Russell SD,
Ewald GA, Zucker MJ, Goldberg LR, Eisen HJ, Salm K, Tolzman D, Gao
J, et al: Tacrolimus with mycophenolate mofetil (MMF) or sirolimus
vs. cyclosporine with MMF in cardiac transplant patients: 1-year
report. Am J Transplant. 6:1377–1386. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Viganò M, Dengler T, Mattei MF, Poncelet
A, Vanhaecke J, Vermes E, Kleinloog R, Li Y, Gezahegen Y and
Delgado JF; RAD A2411 Study Investigators, : Lower incidence of
cytomegalovirus infection with everolimus versus mycophenolate
mofetil in de novo cardiac transplant recipients: A randomized,
multicenter study. Transpl Infect Dis. 12:23–30. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Axelrod D, Leventhal JR, Gallon LG, Parker
MA and Kaufman DB: Reduction of CMV disease with steroid-free
immunosuppresssion in simultaneous pancreas-kidney transplant
recipients. Am J Transplant. 5:1423–1429. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ekberg H, Bernasconi C, Nöldeke J, Yussim
A, Mjörnstedt L, Erken U, Ketteler M and Navrátil P: Cyclosporine,
tacrolimus and sirolimus retain their distinct toxicity profiles
despite low doses in the Symphony study. Nephrol Dial Transplant.
25:2004–2010. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Muffly L, Sheehan K, Armstrong R, Jensen
K, Tate K, Rezvani AR, Miklos D, Arai S, Shizuru J, Johnston L, et
al: Infusion of donor-derived CD8+ memory T cells for
relapse following allogeneic hematopoietic cell transplantation.
Blood Adv. 2:681–690. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Teijaro JR: Cytokine storms in infectious
diseases. Semin Immunopathol. 39:501–503. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Miller W, Flynn P, McCullough J, Balfour
HH Jr, Goldman A, Haake R, McGlave P, Ramsay N and Kersey J:
Cytomegalovirus infection after bone marrow transplantation: An
association with acute graft-v-host disease. Blood. 67:1162–1167.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Stern A and Papanicolaou GA: CMV
prevention and treatment in transplantation: What's New in 2019.
Curr Infect Dis Rep. 21:452019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Murata M, Ikegame K, Morishita Y, Ogawa H,
Kaida K, Nakamae H, Ikeda T, Nishida T, Inoue M, Eto T, et al:
Low-dose thymoglobulin as second-line treatment for
steroid-resistant acute GvHD: An analysis of the JSHCT. Bone Marrow
Transplant. 52:252–257. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lin HC, Han SM, Hwang WL, Chou CW, Chang
KH, Shi ZY and Jerry Teng CL: Cytomegalovirus infection and
treatment in allogeneic hematopoietic stem cell transplantation: A
retrospective study from a single institution in an endemic area.
Turk J Haematol. 34:159–166. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Xue H, Hu Y, Feng S, Liu Z and Gao F: Risk
factor analysis for cytomegalovirus infection after peripheral
blood allogeneic hematopoietic stem cell transplantation. J China
Med Univ. 48:417–420. 2019.
|
|
88
|
Fuji S, Einsele H and Kapp M:
Cytomegalovirus disease in hematopoietic stem cell transplant
patients: Current and future therapeutic options. Curr Opin Infect
Dis. 30:372–376. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Park SY, Lee SO, Choi SH, Kim YS, Woo JH,
Baek S, Sung H, Kim MN, Kim DY, Lee JH, et al: Efficacy and safety
of low-dose ganciclovir preemptive therapy in allogeneic
haematopoietic stem cell transplant recipients compared with
conventional-dose ganciclovir: A prospective observational study. J
Antimicrob Chemother. 67:1486–1492. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhu MX, Wan WL, Li HS, Wang J, Wang YF, Hu
K and Ke XY: Early immune reconstitution after hematopoietic stem
cell transplantation. Beijing Da Xue Xue Bao Yi Xue Ban.
48:515–522. 2016.(In Chinese). PubMed/NCBI
|
|
91
|
Yong MK, Cameron PU, Slavin M, Morrissey
CO, Bergin K, Spencer A, Ritchie D, Cheng AC, Samri A, Carcelain G,
et al: Identifying cytomegalovirus complications using the
quantiferon-CMV assay after allogeneic hematopoietic stem cell
transplantation. J Infect Dis. 215:1684–1694. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wagner-Drouet E, Teschner D, Wolschke C,
Janson D, Schäfer-Eckart K, Gärtner J, Mielke S, Schreder M, Kobbe
G, Kondakci M, et al: Standardized monitoring of
cytomegalovirus-specific immunity can improve risk stratification
of recurrent cytomegalovirus reactivation after hematopoietic stem
cell transplantation. Haematologica. 106:363–374. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
El Haddad L, Ariza-Heredia E, Shah DP,
Jiang Y, Blanchard T, Ghantoji SS, El Chaer F, El-Haddad D, Prayag
A, Nesher L, et al: The ability of a cytomegalovirus ELISPOT assay
to predict outcome of low-level CMV reactivation in hematopoietic
cell transplant recipients. J Infect Dis. 219:898–907. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Krawczyk A, Ackermann J, Goitowski B,
Trenschel R, Ditschkowski M, Timm J, Ottinger H, Beelen DW, Grüner
N and Fiedler M: Assessing the risk of CMV reactivation and
reconstitution of antiviral immune response post bone marrow
transplantation by the QuantiFERON-CMV-assay and real time PCR. J
Clin Virol. 99-100:61–66. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Cao K, Marin D, Sekine T, Rondon G, Zhao
W, Smith NT, Daher M, Wang Q, Li L, Saliba RM, et al: Donor NKG2C
copy number: An independent predictor for CMV reactivation after
double cord blood transplantation. Front Immunol. 9:24442018.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Albert E, Solano C, Giménez E, Focosi D,
Pérez A, Macera L, Piñana JL, Boluda JCH, Maggi F and Navarro D:
The kinetics of torque teno virus plasma DNA load shortly after
engraftment predicts the risk of high-level CMV DNAemia in
allogeneic hematopoietic stem cell transplant recipients. Bone
Marrow Transplant. 53:180–187. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Takahashi R, Nakatsukasa H, Shiozawa S and
Yoshimura A: SOCS1 is a key molecule that prevents regulatory T
cell plasticity under inflammatory conditions. J Immunol.
199:149–158. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Lodding IP, Schultz HH, Jensen JU, Kirkby
N, Perch M, Andersen C, Lundgren JD and Iversen M: Cytomegalovirus
viral load in bronchoalveolar lavage to diagnose lung transplant
associated CMV pneumonia. Transplantation. 102:326–332. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Bhatia J, Shah BV, Mehta AP, Deshmukh M,
Sirsat RA and Rodrigues C: Comparing serology, antigenemia assay
and polymerase chain reaction for the diagnosis of cytomegalovirus
infection in renal transplant patients. J Assoc Physicians India.
52:297–300. 2004.PubMed/NCBI
|
|
100
|
Griscelli F, Barrois M, Chauvin S, Lastere
S, Bellet D and Bourhis JH: Quantification of human cytomegalovirus
DNA in bone marrow transplant recipients by real-time PCR. J Clin
Microbiol. 39:4362–4369. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Drew WL: Laboratory diagnosis of
cytomegalovirus infection and disease in immunocompromised
patients. Curr Opin Infect Dis. 20:408–411. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Pollack M, Heugel J, Xie H, Leisenring W,
Storek J, Young JA, Kukreja M, Gress R, Tomblyn M and Boeckh M: An
international comparison of current strategies to prevent
herpesvirus and fungal infections in hematopoietic cell transplant
recipients. Biol Blood Marrow Transplant. 17:664–673. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Malouli D, Hansen SG, Nakayasu ES,
Marshall EE, Hughes CM, Ventura AB, Gilbride RM, Lewis MS, Xu G,
Kreklywich C, et al: Cytomegalovirus pp65 limits dissemination but
is dispensable for persistence. J Clin Invest. 124:1928–1944. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Lee H, Park KH, Ryu JH, Choi AR, Yu JH,
Lim J, Han K, Kim SI, Yang CW, Chung BH and Oh EJ: Cytomegalovirus
(CMV) immune monitoring with ELISPOT and QuantiFERON-CMV assay in
seropositive kidney transplant recipients. PLoS One.
12:e01894882017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Lisboa LF, Asberg A, Kumar D, Pang X,
Hartmann A, Preiksaitis JK, Pescovitz MD, Rollag H, Jardine AG and
Humar A: The clinical utility of whole blood versus plasma
cytomegalovirus viral load assays for monitoring therapeutic
response. Transplantation. 91:231–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Gerna G, Lilleri D, Caldera D, Furione M,
Zenone Bragotti L and Alessandrino EP: Validation of a DNAemia
cutoff for preemptive therapy of cytomegalovirus infection in adult
hematopoietic stem cell transplant recipients. Bone Marrow
Transplant. 41:873–879. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Boeckh M, Stevens-Ayers T, Travi G, Huang
M-L, Cheng GS, Xie H, Leisenring W, Erard V, Seo S, Kimball L, et
al: Cytomegalovirus (CMV) DNA quantitation in bronchoalveolar
lavage fluid from hematopoietic stem cell transplant recipients
with CMV pneumonia. J Infect Dis. 215:1514–1522. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Beam E, Germer JJ, Lahr B, Yao JDC, Limper
AH, Binnicker MJ and Razonable RR: Cytomegalovirus (CMV) DNA
quantification in bronchoalveolar lavage fluid of immunocompromised
patients with CMV pneumonia. Clin Transplant. 32:2018.doi:
10.1111/ctr.13149. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Hebart H, Rudolph T, Loeffler J,
Middeldorp J, Ljubicic T, Jahn G and Einsele H: Evaluation of the
NucliSens CMV pp67 assay for detection and monitoring of human
cytomegalovirus infection after allogeneic stem cell
transplantation. Bone Marrow Transplant. 30:181–187. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Rea F, Potena L, Yonan N, Wagner F and
Calabrese F: Cytomegalovirus hyper immunoglobulin for CMV
prophylaxis in thoracic transplantation. Transplantation. 100
(Suppl 3):S19–S26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Kotton CN: CMV: Prevention, diagnosis and
therapy. Am J Transplant. 13 (Suppl 3):S24–S40. 2013. View Article : Google Scholar
|
|
112
|
Griffiths PD: An explanation for
posttransplant late-onset disease associated with CMV prophylaxis.
Rev Med Virol. 29:e20802019. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Vamvakas EC: Is white blood cell reduction
equivalent to antibody screening in preventing transmission of
cytomegalovirus by transfusion? A review of the literature and
meta-analysis. Transfus Med Rev. 19:181–199. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Bowden RA, Sayers M, Flournoy N, Newton B,
Banaji M, Thomas ED and Meyers JD: Cytomegalovirus immune globulin
and seronegative blood products to prevent primary cytomegalovirus
infection after marrow transplantation. N Engl J Med.
314:1006–1010. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Ljungman P, Larsson K, Kumlien G, Aschan
J, Barkholt L, Gustafsson-Jernberg A, Lewensohn-Fuchs I and Ringdén
O: Leukocyte depleted, unscreened blood products give a low risk
for CMV infection and disease in CMV seronegative allogeneic stem
cell transplant recipients with seronegative stem cell donors.
Scand J Infect Dis. 34:347–350. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Boeckh M, Bowden RA, Storer B, Chao NJ,
Spielberger R, Tierney DK, Gallez-Hawkins G, Cunningham T, Blume
KG, Levitt D and Zaia JA: Randomized, placebo-controlled,
double-blind study of a cytomegalovirus-specific monoclonal
antibody (MSL-109) for prevention of cytomegalovirus infection
after allogeneic hematopoietic stem cell transplantation. Biol
Blood Marrow Transplant. 7:343–351. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Raanani P, Gafter-Gvili A, Paul M,
Ben-Bassat I, Leibovici L and Shpilberg O: Immunoglobulin
prophylaxis in hematopoietic stem cell transplantation: Systematic
review and meta-analysis. J Clin Oncol. 27:770–781. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Alexander BT, Hladnik LM, Augustin KM,
Casabar E, McKinnon PS, Reichley RM, Ritchie DJ, Westervelt P and
Dubberke ER: Use of cytomegalovirus intravenous immune globulin for
the adjunctive treatment of cytomegalovirus in hematopoietic stem
cell transplant recipients. Pharmacotherapy. 30:554–561. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Malagola M, Greco R, Santarone S, Natale
A, Iori AP, Quatrocchi L, Barbieri W, Bruzzese A, Leotta S, Carotti
A, et al: CMV management with specific immunoglobulins: A
multicentric retrospective analysis on 92 allotransplanted
patients. Mediterr J Hematol Infect Dis. 11:e20190482019.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Grossi P, Mohacsi P, Szabolcs Z and Potena
L: Cytomegalovirus immunoglobulin after thoracic transplantation:
An overview. Transplantation. 100 (Suppl 3):S1–S4. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Hammerstrom AE, Lombardi LR, Pingali SR,
Rondon G, Chen J, Milton DR, Chemaly RF, Champlin RE, Gulbis A and
Ciurea SO: Prevention of cytomegalovirus reactivation in
haploidentical stem cell transplantation. Biol Blood Marrow
Transplant. 24:353–358. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Boeckh M, Nichols WG, Chemaly RF,
Papanicolaou GA, Wingard JR, Xie H, Syrjala KL, Flowers ME,
Stevens-Ayers T, Jerome KR and Leisenring W: Valganciclovir for the
prevention of complications of late cytomegalovirus infection after
allogeneic hematopoietic cell transplantation: A randomized trial.
Ann Intern Med. 162:1–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Green ML, Leisenring W, Xie H, Mast TC,
Cui Y, Sandmaier BM, Sorror ML, Goyal S, Özkök S, Yi J, et al:
Cytomegalovirus viral load and mortality after haemopoietic stem
cell transplantation in the era of pre-emptive therapy: A
retrospective cohort study. Lancet Haematol. 3:e119–e127. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Wloch MK, Smith LR, Boutsaboualoy S, Reyes
L, Han C, Kehler J, Smith HD, Selk L, Nakamura R, Brown JM, et al:
Safety and immunogenicity of a bivalent cytomegalovirus DNA vaccine
in healthy adult subjects. J Infect Dis. 197:1634–1642. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Schleiss MR: Cytomegalovirus vaccines
under clinical development. J Virus Erad. 2:198–207. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Kharfan-Dabaja MA, Boeckh M, Wilck MB,
Langston AA, Chu AH, Wloch MK, Guterwill DF, Smith LR, Rolland AP
and Kenney RT: A novel therapeutic cytomegalovirus DNA vaccine in
allogeneic haemopoietic stem-cell transplantation: A randomised,
double-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis.
12:290–299. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Griffiths PD, Stanton A, McCarrell E,
Smith C, Osman M, Harber M, Davenport A, Jones G, Wheeler DC,
O'Beirne J, et al: Cytomegalovirus glycoprotein-B vaccine with MF59
adjuvant in transplant recipients: A phase 2 randomised
placebo-controlled trial. Lancet. 377:1256–1263. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Vincenti F, Budde K, Merville P, Shihab F,
Ram Peddi V, Shah M, Wyburn K, Cassuto-Viguier E, Weidemann A, Lee
M, et al: A randomized, phase 2 study of ASP0113, a DNA-based
vaccine, for the prevention of CMV in CMV-seronegative kidney
transplant recipients receiving a kidney from a CMV-seropositive
donor. Am J Transplant. 18:2945–2954. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Singh N, Winston DJ, Razonable RR, Lyon
GM, Silveira FP, Wagener MM, Stevens-Ayers T, Edmison B, Boeckh M
and Limaye AP: Effect of preemptive therapy vs. antiviral
prophylaxis on cytomegalovirus disease in seronegative liver
transplant recipients with seropositive donors: A randomized
clinical trial. JAMA. 323:1378–1387. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Einsele H, Ehninger G, Hebart H,
Wittkowski KM, Schuler U, Jahn G, Mackes P, Herter M, Klingebiel T,
Löffler J, et al: Polymerase chain reaction monitoring reduces the
incidence of cytomegalovirus disease and the duration and side
effects of antiviral therapy after bone marrow transplantation.
Blood. 86:2815–2820. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Tomblyn M, Chiller T, Einsele H, Gress R,
Sepkowitz K, Storek J, Wingard JR, Young JA, Boeckh MJ; Center for
International Blood and Marrow Researchm, ; et al: Guidelines for
preventing infectious complications among hematopoietic cell
transplantation recipients: A global perspective. Biol Blood Marrow
Transplant. 15:1143–1238. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Natori Y, Alghamdi A, Tazari M, Miller V,
Husain S, Komatsu T, Griffiths P, Ljungman P, Orchanian-Cheff A,
Kumar D, et al: Use of viral load as a surrogate marker in clinical
studies of cytomegalovirus in solid organ transplantation: A
systematic review and meta-analysis. Clin Infect Dis. 66:617–631.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Locatelli F, Bertaina A, Bertaina V and
Merli P: Cytomegalovirus in hematopoietic stem cell transplant
recipients-management of infection. Expert Rev Hematol.
9:1093–1105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Limaye AP, Stapleton RD, Peng L, Gunn SR,
Kimball LE, Hyzy R, Exline MC, Files DC, Morris PE, Frankel SK, et
al: Effect of ganciclovir on IL-6 levels among
cytomegalovirus-seropositive adults with critical Illness: A
randomized clinical trial. JAMA. 318:731–740. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Paya C, Humar A, Dominguez E, Washburn K,
Blumberg E, Alexander B, Freeman R, Heaton N and Pescovitz MD;
Valganciclovir Solid Organ Transplant Study Group, : Efficacy and
safety of valganciclovir vs. oral ganciclovir for prevention of
cytomegalovirus disease in solid organ transplant recipients. Am J
Transplant. 4:611–620. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Winston DJ, Ho WG, Bartoni K, Du Mond C,
Ebeling DF, Buhles WC and Champlin RE: Ganciclovir prophylaxis of
cytomegalovirus infection and disease in allogeneic bone marrow
transplant recipients. Results of a placebo-controlled,
double-blind trial. Ann Intern Med. 118:179–184. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Griffiths P, Whitley R, Snydman DR, Singh
N and Boeckh M; International Herpes Management Forum, :
Contemporary management of cytomegalovirus infection in transplant
recipients: Guidelines from an IHMF workshop, 2007. Herpes.
15:4–12. 2008.PubMed/NCBI
|
|
138
|
Winston DJ, Yeager AM, Chandrasekar PH,
Snydman DR, Petersen FB and Territo MC; Valacyclovir
Cytomegalovirus Study Group, : Randomized comparison of oral
valacyclovir and intravenous ganciclovir for prevention of
cytomegalovirus disease after allogeneic bone marrow
transplantation. Clin Infect Dis. 36:749–758. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Rubin RH, Lynch P, Pasternack MS,
Schoenfeld D and Medearis DN Jr: Combined antibody and ganciclovir
treatment of murine cytomegalovirus-infected normal and
immunosuppressed BALB/c mice. Antimicrob Agents Chemother.
33:1975–1979. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Lukacova V, Goelzer P, Reddy M, Greig G,
Reigner B and Parrott N: A physiologically based pharmacokinetic
model for ganciclovir and its prodrug valganciclovir in adults and
children. AAPS J. 18:1453–1463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Weiner RS, Bortin MM, Gale RP, Gluckman E,
Kay HE, Kolb HJ, Hartz AJ and Rimm AA: Interstitial pneumonitis
after bone marrow transplantation. Assessment of risk factors. Ann
Intern Med. 104:168–175. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Maffini E, Giaccone L, Festuccia M,
Brunello L, Busca A and Bruno B: Treatment of CMV infection after
allogeneic hematopoietic stem cell transplantation. Expert Rev
Hematol. 9:585–596. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Ritchie BM, Barreto JN, Barreto EF, Crow
SA, Dierkhising RA, Jannetto PJ, Tosh PK and Razonable RR:
Relationship of ganciclovir therapeutic drug monitoring with
clinical efficacy and patient safety. Antimicrob Agents Chemother.
63:e01855–18. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Kim ST, Lee MH, Kim SY, Kim SJ, Kim DH,
Jang JH, Kim K, Kim WS and Jung CW: A randomized trial of
preemptive therapy for prevention of cytomegalovirus disease after
allogeneic hematopoietic stem cell transplantation. Int J Hematol.
91:886–891. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
El Chaer F, Shah DP and Chemaly RF: How I
treat resistant cytomegalovirus infection in hematopoietic cell
transplantation recipients. Blood. 128:2624–2636. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Einsele H, Reusser P, Bornhäuser M, Kalhs
P, Ehninger G, Hebart H, Chalandon Y, Kröger N, Hertenstein B and
Rohde F: Oral valganciclovir leads to higher exposure to
ganciclovir than intravenous ganciclovir in patients following
allogeneic stem cell transplantation. Blood. 107:3002–3008. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Barkam C, Kamal H, Dammann E, Diedrich H,
Buchholz S, Eder M, Krauter J, Ganser A and Stadler M: Improving
safety of preemptive therapy with oral valganciclovir for
cytomegalovirus infection after allogeneic hematopoietic stem cell
transplantation. Bone Marrow Res. 2012:8746012012. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Moretti S, Zikos P, Van Lint MT, Tedone E,
Occhini D, Gualandi F, Lamparelli T, Mordini N, Berisso G, Bregante
S, et al: Forscarnet vs. ganciclovir for cytomegalovirus (CMV)
antigenemia after allogeneic hemopoietic stem cell transplantation
(HSCT): A randomised study. Bone Marrow Transplant. 22:175–180.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Meesing A and Razonable RR: New
developments in the management of cytomegalovirus infection after
transplantation. Drugs. 78:1085–1103. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Marty FM, Ljungman P, Chemaly RF, Maertens
J, Dadwal SS, Duarte RF, Haider S, Ullmann AJ, Katayama Y, Brown J,
et al: Letermovir prophylaxis for cytomegalovirus in
hematopoietic-cell transplantation. N Engl J Med. 377:2433–2444.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Bowman LJ, Melaragno JI and Brennan DC:
Letermovir for the management of cytomegalovirus infection. Expert
Opin Investig Drugs. 26:235–241. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Phoompoung P, Ferreira VH, Tikkanen J,
Husain S, Viswabandya A, Kumar D and Humar A: Letermovir as salvage
therapy for Cytomegalovirus infection in transplant recipients.
Transplantation. 104:404–409. 2019. View Article : Google Scholar
|
|
153
|
Popping S, Dalm VASH, Lübke N,
Cristanziano VD, Kaiser R, Boucher CAB and Van Kampen JJA:
Emergence and persistence of letermovir-resistant cytomegalovirus
in a patient with primary immunodeficiency. Open Forum Infectious
Diseases. 6:ofz3752019. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Griffiths P and Lumley S: Cytomegalovirus.
Curr Opin Infect Dis. 27:554–559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Beadle JR, Hartline C, Aldern KA,
Rodriguez N, Harden E, Kern ER and Hostetler KY: Alkoxyalkyl esters
of cidofovir and cyclic cidofovir exhibit multiple-log enhancement
of antiviral activity against cytomegalovirus and herpesvirus
replication in vitro. Antimicrob Agents Chemother. 46:2381–2386.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Bidanset DJ, Beadle JR, Wan WB, Hostetler
KY and Kern ER: Oral activity of ether lipid ester prodrugs of
cidofovir against experimental human cytomegalovirus infection. J
Infect Dis. 190:499–503. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
157
|
Kern ER, Collins DJ, Wan WB, Beadle JR,
Hostetler KY and Quenelle DC: Oral treatment of murine
cytomegalovirus infections with ether lipid esters of cidofovir.
Antimicrob Agents Chemother. 48:3516–3522. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Chemaly RF, Hill JA, Voigt S and Peggs KS:
In vitro comparison of currently available and investigational
antiviral agents against pathogenic human double-stranded DNA
viruses: A systematic literature review. Antiviral Res. 163:50–58.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Gagelmann N, Ljungman P, Styczynski J and
Kröger N: Comparative efficacy and safety of different antiviral
agents for cytomegalovirus prophylaxis in allogeneic hematopoietic
cell transplantation: A systematic review and meta-analysis. Biol
Blood Marrow Transplant. 24:2101–2109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Hostetler KY: Alkoxyalkyl prodrugs of
acyclic nucleoside phosphonates enhance oral antiviral activity and
reduce toxicity: Current state of the art. Antiviral Res.
82:A84–A98. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Painter W, Robertson A, Trost LC, Godkin
S, Lampert B and Painter G: First pharmacokinetic and safety study
in humans of the novel lipid antiviral conjugate CMX001, a
broad-spectrum oral drug active against double-stranded DNA
viruses. Antimicrob Agents Chemother. 56:2726–2734. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Aldern KA, Ciesla SL, Winegarden KL and
Hostetler KY: Increased antiviral activity of
1-O-hexadecyloxypropyl-[2-(14)C]cidofovir in MRC-5 human lung
fibroblasts is explained by unique cellular uptake and metabolism.
Mol Pharmacol. 63:678–681. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Ciesla SL, Trahan J, Wan WB, Beadle JR,
Aldern KA, Painter GR and Hostetler KY: Esterification of cidofovir
with alkoxyalkanols increases oral bioavailability and diminishes
drug accumulation in kidney. Antiviral Res. 59:163–171. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Tippin TK, Morrison ME, Brundage TM and
Momméja-Marin H: Brincidofovir is not a substrate for the human
organic anion transporter 1: A mechanistic explanation for the lack
of nephrotoxicity observed in clinical studies. Ther Drug Monit.
38:777–786. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Marty FM, Winston DJ, Chemaly RF, Mullane
KM, Shore TB, Papanicolaou GA, Chittick G, Brundage TM, Wilson C,
Morrison ME, et al: A randomized, double-blind, placebo-controlled
phase 3 trial of oral brincidofovir for cytomegalovirus prophylaxis
in allogeneic hematopoietic cell transplantation. Biol Blood Marrow
Transplant. 25:369–381. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Papanicolaou GA, Silveira FP, Langston AA,
Pereira MR, Avery RK, Uknis M, Wijatyk A, Wu J, Boeckh M, Marty FM
and Villano S: Maribavir for refractory or resistant
cytomegalovirus infections in hematopoietic-cell or solid-organ
transplant recipients: A randomized, dose-ranging, Double-blind,
phase 2 study. Clin Infect Dis. 68:1255–1264. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Frange P and Leruez-Ville M: Maribavir,
brincidofovir and letermovir: Efficacy and safety of new antiviral
drugs for treating cytomegalovirus infections. Med Mal Infect.
48:495–502. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Marty FM and Boeckh M: Maribavir and human
cytomegalovirus-what happened in the clinical trials and why might
the drug have failed? Curr Opin Virol. 1:555–562. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Piret J and Boivin G: Clinical development
of letermovir and maribavir: Overview of human cytomegalovirus drug
resistance. Antiviral Res. 163:91–105. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Bright PD, Gompels M, Donati M and
Johnston S: Successful oral treatment of Ganciclovir resistant
cytomegalovirus with Maribavir in the context of primary
immunodeficiency: First case report and review. J Clin Virol.
87:12–16. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Maertens J, Cordonnier C, Jaksch P, Poiré
X, Uknis M, Wu J, Wijatyk A, Saliba F, Witzke O and Villano S:
Maribavir for preemptive treatment of cytomegalovirus reactivation.
N Engl J Med. 381:1136–1147. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Avery RK, Marty FM, Strasfeld L, Lee I,
Arrieta A, Chou S, Tatarowicz W and Villano S: Oral maribavir for
treatment of refractory or resistant cytomegalovirus infections in
transplant recipients. Transpl Infect Dis. 12:489–496. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Germi R, Mariette C, Alain S, Lupo J,
Thiebaut A, Brion JP, Epaulard O, Saint Raymond C, Malvezzi P and
Morand P: Success and failure of artesunate treatment in five
transplant recipients with disease caused by drug-resistant
cytomegalovirus. Antiviral Res. 101:57–61. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Avery RK, Mossad SB, Poggio E, Lard M,
Budev M, Bolwell B, Waldman WJ, Braun W, Mawhorter SD, Fatica R, et
al: Utility of leflunomide in the treatment of complex
cytomegalovirus syndromes. Transplantation. 90:419–426. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Feuchtinger T, Opherk K, Bethge WA, Topp
MS, Schuster FR, Weissinger EM, Mohty M, Or R, Maschan M, Schumm M,
et al: Adoptive transfer of pp65-specific T cells for the treatment
of chemorefractory cytomegalovirus disease or reactivation after
haploidentical and matched unrelated stem cell transplantation.
Blood. 116:4360–4367. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Walter EA, Greenberg PD, Gilbert MJ, Finch
RJ, Watanabe KS, Thomas ED and Riddell SR: Reconstitution of
cellular immunity against cytomegalovirus in recipients of
allogeneic bone marrow by transfer of T-cell clones from the donor.
N Engl J Med. 333:1038–1044. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Blyth E, Clancy L, Simms R, Ma CK, Burgess
J, Deo S, Byth K, Dubosq MC, Shaw PJ, Micklethwaite KP and Gottlieb
DJ: Donor-derived CMV-specific T cells reduce the requirement for
CMV-directed pharmacotherapy after allogeneic stem cell
transplantation. Blood. 121:3745–3758. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Peggs KS, Verfuerth S, Pizzey A, Khan N,
Guiver M, Moss PA and Mackinnon S: Adoptive cellular therapy for
early cytomegalovirus infection after allogeneic stem-cell
transplantation with virus-specific T-cell lines. Lancet.
362:1375–1377. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Neuenhahn M, Albrecht J, Odendahl M,
Schlott F, Dössinger G, Schiemann M, Lakshmipathi S, Martin K,
Bunjes D, Harsdorf S, et al: Transfer of minimally manipulated
CMV-specific T cells from stem cell or third-party donors to treat
CMV infection after allo-HSCT. Leukemia. 31:2161–2171. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Celilova S, Toret E, Adaklı Aksoy B, Ovalı
E and Bozkurt C: CMV Specific T cells for treatment of CMV
infection after hematopoietic stem cell transplantation in a
pediatric case: First application in Turkey. Turk J Haematol.
37:65–67. 2020.PubMed/NCBI
|
|
181
|
Tzannou I, Papadopoulou A, Naik S, Leung
K, Martinez CA, Ramos CA, Carrum G, Sasa G, Lulla P, Watanabe A, et
al: Off-the-shelf virus-specific T cells to treat BK virus, human
herpesvirus 6, cytomegalovirus, Epstein-Barr virus, and adenovirus
infections after allogeneic hematopoietic stem-cell
transplantation. J Clin Oncol. 35:3547–3557. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Kállay K, Kassa C, Réti M, Karászi É,
Sinkó J, Goda V, Stréhn A, Csordás K, Horváth O, Szederjesi A, et
al: Early experience with CliniMACS prodigy CCS (IFN-gamma) system
in selection of virus-specific T cells from third-party donors for
pediatric patients with severe viral infections after hematopoietic
stem cell transplantation. J Immunother. 41:158–163. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Ogonek J, Verma K, Schultze-Florey C,
Varanasi P, Luther S, Schweier P, Kühnau W, Göhring G, Dammann E,
Stadler M, et al: Characterization of high-avidity
cytomegalovirus-specific T cells with differential tetramer binding
coappearing after allogeneic stem cell transplantation. J Immunol.
199:792–805. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Bao L, Cowan MJ, Dunham K, Horn B, McGuirk
J, Gilman A and Lucas KG: Adoptive immunotherapy with CMV-specific
cytotoxic T lymphocytes for stem cell transplant patients with
refractory CMV infections. J Immunother. 35:293–298. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Koehne G, Hasan A, Doubrovina E, Prockop
S, Tyler E, Wasilewski G and O'Reilly RJ: Immunotherapy with donor
T cells sensitized with overlapping pentadecapeptides for treatment
of persistent cytomegalovirus infection or viremia. Biol Blood
Marrow Transplant. 21:1663–1678. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Pei XY, Zhao XY, Chang YJ, Liu J, Xu LP,
Wang Y, Zhang XH, Han W, Chen YH and Huang XJ:
Cytomegalovirus-specific T-cell transfer for refractory
cytomegalovirus infection after haploidentical stem cell
transplantation: The quantitative and qualitative immune recovery
for cytomegalovirus. J Infect Dis. 216:945–956. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Peggs KS, Thomson K, Samuel E, Dyer G,
Armoogum J, Chakraverty R, Pang K, Mackinnon S and Lowdell MW:
Directly selected cytomegalovirus-reactive donor T cells confer
rapid and safe systemic reconstitution of virus-specific immunity
following stem cell transplantation. Clin Infect Dis. 52:49–57.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Micklethwaite K, Hansen A, Foster A, Snape
E, Antonenas V, Sartor M, Shaw P, Bradstock K and Gottlieb D: Ex
vivo expansion and prophylactic infusion of CMV-pp65
peptide-specific cytotoxic T-lymphocytes following allogeneic
hematopoietic stem cell transplantation. Biol Blood Marrow
Transplant. 13:707–714. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Houghtelin A and Bollard CM:
Virus-specific T cells for the immunocompromised patient. Front
Immunol. 8:12722017. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Einsele H, Roosnek E, Rufer N, Sinzger C,
Riegler S, Löffler J, Grigoleit U, Moris A, Rammensee HG, Kanz L,
et al: Infusion of cytomegalovirus (CMV)-specific T cells for the
treatment of CMV infection not responding to antiviral
chemotherapy. Blood. 99:3916–3922. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Perruccio K, Tosti A, Burchielli E, Topini
F, Ruggeri L, Carotti A, Capanni M, Urbani E, Mancusi A, Aversa F,
et al: Transferring functional immune responses to pathogens after
haploidentical hematopoietic transplantation. Blood. 106:4397–4406.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Gary R, Aigner M, Moi S, Schaffer S,
Gottmann A, Maas S, Zimmermann R, Zingsem J, Strobel J, Mackensen
A, et al: Clinical-grade generation of peptide-stimulated
CMV/EBV-specific T cells from G-CSF mobilized stem cell grafts. J
Transl Med. 16:1242018. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Friedenstein AJ, Deriglasova UF, Kulagina
NN, Panasuk AF, Rudakowa SF, Luriá EA and Ruadkow IA: Precursors
for fibroblasts in different populations of hematopoietic cells as
detected by the in vitro colony assay method. Exp Hematol. 2:83–92.
1974.PubMed/NCBI
|
|
194
|
Méndez-Ferrer S, Michurina TV, Ferraro F,
Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A,
Enikolopov GN and Frenette PS: Mesenchymal and haematopoietic stem
cells form a unique bone marrow niche. Nature. 466:829–834. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Martínez-Peinado P, Pascual-García S,
Roche E and Sempere-Ortells JM: Differences of clonogenic
mesenchymal stem cells on immunomodulation of lymphocyte subsets. J
Immunol Res. 2018:72327172018. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Wolff D, Schleuning M, von Harsdorf S,
Bacher U, Gerbitz A, Stadler M, Ayuk F, Kiani A, Schwerdtfeger R,
Vogelsang GB, et al: Consensus conference on clinical practice in
chronic GVHD: Second-line treatment of chronic Graft-versus-Host
disease. Biol Blood Marrow Transplant. 17:1–17. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Tse WT, Pendleton JD, Beyer WM, Egalka MC
and Guinan EC: Suppression of allogeneic T-cell proliferation by
human marrow stromal cells: Implications in transplantation.
Transplantation. 75:389–397. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Malcherek G, Jin N, Huckelhoven AG, Mani
J, Wang L, Gern U, Diehlmann A, Wuchter P, Schmitt A, Chen B, et
al: Mesenchymal stromal cells inhibit proliferation of
virus-specific CD8+ T cells. Leukemia. 28:2388–2394.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Elmaagacli AH, Steckel NK, Koldehoff M,
Hegerfeldt Y, Trenschel R, Ditschkowski M, Christoph S, Gromke T,
Kordelas L, Ottinger HD, et al: Early human cytomegalovirus
replication after transplantation is associated with a decreased
relapse risk: Evidence for a putative virus-versus-leukemia effect
in acute myeloid leukemia patients. Blood. 118:1402–1412. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Inagaki J, Noguchi M, Kurauchi K, Tanioka
S, Fukano R and Okamura J: Effect of cytomegalovirus reactivation
on relapse after allogeneic hematopoietic stem cell transplantation
in pediatric acute leukemia. Biol Blood Marrow Transplant.
22:300–306. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Litjens NHR, van der Wagen L, Kuball J and
Kwekkeboom J: Potential beneficial effects of cytomegalovirus
infection after transplantation. Front Immunol. 9:3892018.
View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Yoon JH, Lee S, Kim HJ, Jeon YW, Lee SE,
Cho BS, Lee DG, Eom KS, Kim YJ, Min CK, et al: Impact of
cytomegalovirus reactivation on relapse and survival in patients
with acute leukemia who received allogeneic hematopoietic stem cell
transplantation in first remission. Oncotarget. 7:17230–17241.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Jang JE, Kim SJ, Cheong JW, Hyun SY, Kim
YD, Kim YR, Kim JS and Min YH: Early CMV replication and subsequent
chronic GVHD have a significant anti-leukemic effect after
allogeneic HSCT in acute myeloid leukemia. Ann Hematol. 94:275–282.
2015. View Article : Google Scholar : PubMed/NCBI
|