Introduction
Incidences and deaths caused by lung cancer (LC)
across the world have been on the rise (11.6 and 18.4%) (1,2). As
such, the disease has become an urgent public health concern. In
China, the incidence of LC in 2015 was 733/100,000 individuals. Its
mortality rate in the same year was 610/100,000 individuals, thus
causing the disease to rank first among causes of cancer-associated
deaths. Patients with LC often face physical, emotional, and
financial distress, which not only affects their mental health but
may also impose financial and social burdens to families (3). Currently, chemotherapy is the common
clinical treatment method used to treat LC. However, this method
also damages normal cells, thus leading to other adverse reaction,
such as nausea, vomiting, anorexia and diarrhea (4). As such, patients with LC undergoing
chemotherapy have been using tradition Chinese medicine as an
adjuvant therapy.
The Yiqi Gubiao pill is the most used drug in
treatment of lung-related diseases, such as chronic obstructive
pulmonary disease (COPD), in Xinjiang Uygur Autonomous Region
Hospital (5). The pill is composed
of 13 traditional Chinese medicines namely, Dangshen [DS,
Codonoposis pilosula (Franch)], Baizhu (BZ, Atractylodes
macrocephala), Fuling [FL, Poriacocos (Schw) Wolf],
Chenpi (CP, Citrus reticulata Blanco), Banxia [BX,
Pinelliaternata (Thunb) Breit], Yiyiren [YYR,
Coixlacryma-jobi L.var.mayuen (Roman) Stapf], Fuxiaomai
(FXM, Triticum aestivum L.), Zisu (ZS, Perilla frutescens
(L.) Britt), Kuandonghua (KDH, Tussilago farfara L.),
Huangqin (HQ, Scutellaria baicalensis Ceorgi), Yibeimu (YBM,
Fritillariapallidiflora Schrenk), Pibaye [Eriobotrya
japonica (Thunb) Lindl] and Fangfeng [FF, Saponshnikovia
divaricate (Turcz) Schischk]. Previous studies have reported
that Yiqi Gubiao pills function in COPD treatment via the JAK/STAT
pathway to prolong the stable period of COPD as well as reduce the
number of acute episodes (5,6).
Yiqi Gubiao pills are multi-component traditional
Chinese medicine (TCM) and thus have multi-target properties. As
such, it is challenging to decipher its underlying mechanism(s) in
treating LC. Network pharmacology integrates multi-disciplinary
technologies, such as systems biology, multi-directional
pharmacology, network analysis and computational biology, to help
in investigate the mechanism of TCMs (7,8). The
relationships between drugs and diseases are revealed at the system
level by constructing a multi-level structure network of
‘Disease-Phenotype-Gene-Drug’.
Herein, network pharmacology was employed to analyze
the complex network relationships among the multi-components and
multi-target properties of Yiqi Gubiao and LC. Biological
experiments were set up and used to identify and verify the key
genes involved in Yiqi Gubiao adjuvant treatment of LC. This
revealed the mechanism of action of Yiqi Gubiao pills, thus
providing a theoretical basis for its clinical application
(Fig. 1).
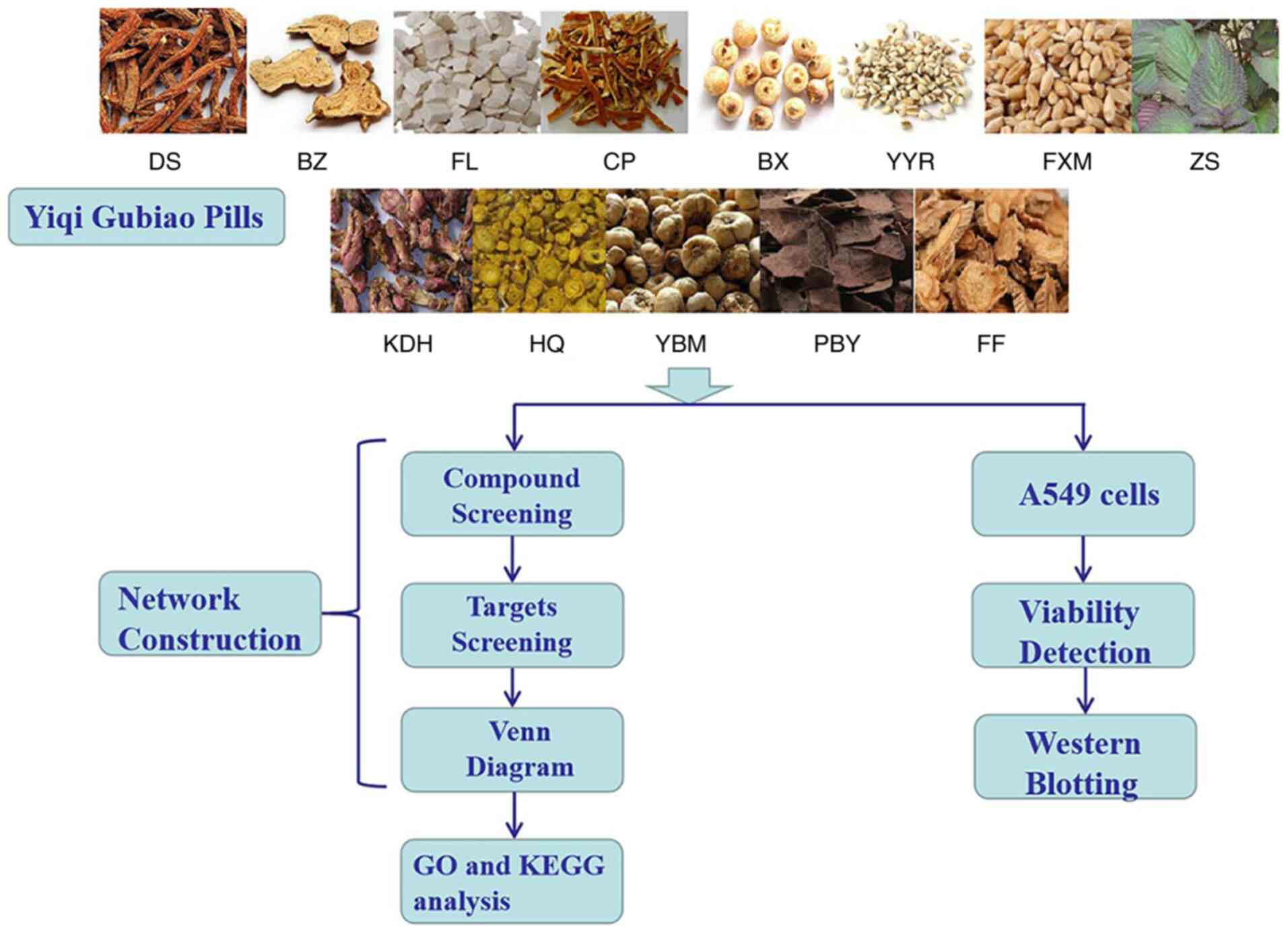 | Figure 1.Complete flow diagram of the study.
DS, Danshen; BZ, Baizhu; FL, Fuling; CP, Chenpi; BX, Banxia; YYR,
Yiyiren; FXM, Fuxiaomai; ZS, Zisu; KDH, Kuandonghua; HQ, Huangqin;
YBM, Yibeimu; PBY, Pibaye; FF, Fangfeng. |
Materials and methods
Screening the active ingredients of
the Yiqi Gubiao pill
A search for the ingredients of the Yiqi Gubiao pill
was conducted using the Chinese Medicine System Pharmacology
Analysis Platform (http://lsp.nwu.edu.cn/tcmsp.php) and the SymMap
database (http://www.symmap.org) (9). An oral bioavailability (OB)≥30% and
drug-likeness (DL) ≥0.18 were set as the cut-off values for
choosing the active ingredients.
Collection of targets
A search of the validated targets of the active
ingredients of the Yiqi Gubiao pill was performed using the Herbal
Ingredients' Targets Database (HIT, http://lifecenter.sgst.cn/hit/) (10). The PubChem database (https://pubchem.ncbi.nlm.nih.gov/) was used to
query the SMILES number of the active compounds. The SMILES numbers
were then imported into the Similarity Ensemble Approach database
(SEA, http://sea.bkslab.org) (11) to predict the targets of the active
ingredients. Targets obtained in the SEA and HIT databases were
used for subsequent analysis. The Online Mendelian Inheritance in
Man (OMIM, http://omim.org) (12), Pubmed-Gene (https://www.ncbi.nlm.nih.gov/pubmed/gene) (13) and Genecards (https://www.genecards/org) bioinformatics tools were
employed to identify LC-related targets. All targets were then
calibrated to correspond to the official gene name using the
UniProt database (https://www.uniprot.org/) (14).
Protein-protein interaction (PPI)
network construction and analysis
The online website, Funrich (http://funrich.org/index.html) was used to overlap the
active ingredients and disease targets to obtain the common
targets. These common targets were considered as the potential key
targets of the Yiqi Gubiao pills in LC treatment. Gene targets of
the Yiqi Gubiao pill and LC co-acting targets were then imported
into the online STRING platform (https://string-db.org) to obtain their protein
interaction data. The score was set at >0.7 to filter the
information. In addition, the Cytoscape 3.7.2 platform (15) was used to build the protein-protein
interaction (PPI) network, while the plug-in cytohubba was employed
to identify the top five genes based on degree values (degree
>128). These genes were regarded as the hub genes. Finally, the
information of the target hub genes was identified using the
DisGeNET database (version 5; http://www.disgenent.org/web/DisGeNet/menu).
Gene Ontology (GO) and Kyoto
Encyclopedia of Genes and Genomes (KEGG) enrichment analysis
The GO tool has several functions such as molecular
function (MF), biological process (BP) and cellular component (CC),
which are applied to the biological mechanism of high-throughput
genome or transcriptome data identification (16). On the other hand, the KEGG database
is a functional and biological platform for identifying candidate
genes. Herein, the ‘clusterProfiler’ package in the R software
(version 3.5.1) (17) was used for
the GO function and KEGG pathway analysis of targets in the PPI
network.
Source of drugs and reagents
The Yiqi Gubiao pills were purchased from the
Xinjiang Uygur Autonomous Region Hospital (Xinjiang, batch no.
20121212). They were decocted twice with ddH2O for 30
min and their filtrates mixed and condensed to 2 g crude drug/ml.
The Cell Counting Kit-8 (CCK-8) was purchased from Invitrogen
(Thermo Fisher Scientific, Inc.) while AKT1, ALB, GAPDH, IL6 and
MAPK3 antibodies were purchased from Cell Signaling Technology,
Inc.
Cells lines and cell cultures
The human non-small cell (NSC)LC cell line A549 was
used in the present study. It was purchased from the Cell Bank of
Type Culture Collection of Chinese Academy of Sciences. The cells
were cultured in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal calf serum (FCS) and 100 U/ml
penicillin-streptomycin (all Hyclone; Cyvita), and then maintained
in a cell culture incubator set at 37°C and 5% CO2.
Cell viability assay
The A549 cells were seeded into a 96-well plate at a
density of 5×103 cells/well and incubated in a cell
culture incubator set at 37°C, 5% CO2 and saturated
humidity for 24-h prior to treatment. Cells were then randomly
divided into five groups: Control group and Yiqi Gubiao pill groups
(50, 25, 12.5 and 6.25 µg/ml). The supernatant of the medium was
aspirated and discarded, followed by addition of 100 µl 10% CCK-8
phenol red-free DMEM medium added to the cells. The cultures were
left to stand for 2 h and their absorbance measured at a wavelength
of 450 nm using as a microplate reader (BioTek Instruments). Cell
viability (% of control) was calculated using the formula:
(Absorbance of drug treatment group/absorbance of control)
×100%.
Reverse-transcription quantitative
(RT-q)PCR
Total RNA was isolated from the A549 cells treated
with varying concentrations of Yiqi Gubiao pills (50, 25, 12.5 and
6.25 µg/ml) using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). The RNA was then reverse transcribed to
cDNA and used as a template in the RT-qPCR reaction to amplify the
hub genes using the PrimeScript RT Reagent kit with gDNA Eraser
(cat no. RR037A; Takara Biotechnology Co., Ltd.), according to the
manufacturer's. qPCR was subsequently performed using the SYBR
Premix Taq™ kit (cat. no. DRR820A; Takara Biotechnology Co., Ltd.),
on a Agilent Mx3005P (Agilent Technologies, Inc.), according to the
manufacturer's instructions. The following thermocycling conditions
were used: 1 cycle at 95°C for 10 min, and 40 cycles at 95°C for 30
sec, 95°C for 5 sec and 60°C for 30 sec. The primer sequences used
to amplify the hub genes are listed in Table I. GADPH was used as an internal
control. Reactions were conducted in triplicate for each sample and
the mean value determined for subsequent analysis. The relative
expression of mRNA was calculated using the 2−ΔΔCq
method (18).
 | Table I.Primer sequences used for the
quantitative PCR. |
Table I.
Primer sequences used for the
quantitative PCR.
| Name | Sequence,
5′→3′ |
|---|
| AKT1 | F:
CCTCCACGACATCGCACTG |
|
| R:
TCACAAAGAGCCCTCCATTATCA |
| ALB | F:
GGGGTGTGTTTCGTCGAGAT |
|
| R:
AGGCAATCAACACCAAGGCT |
| IL-6 | F:
GAACTCCTTCTCCACAAGCGCCTT |
|
| R:
CAAAAGACCAGTGATGATTTTCACCAGG |
| MAPK3 | F:
TCCGCCATGAGAATGTTATAGGC |
|
| R:
GGTGGTGTTGATAAGCAGATTGG |
| TP53 | F:
GGAGCCGCAGTCAGATCCTAG |
|
| R:
CAAGGGGGACAGAACGTTG |
| GAPDH | F:
GAAGGTCGGAGTCAACGGATTT |
|
| R:
CCTGGAAGATGGTGATGGGATT |
Western blot analysis
A549 cells were seeded in 6-well plates at a density
of 5×103 cells/well for 24-h before treatment. The cells
were then treated with varying concentrations of Yiqi Gubiao (50,
25, 12.5 and 6.25 µg/ml). They were then lysed in RIPA buffer
(Thermo Fisher Scientific, Inc.) containing 50 mM Tris-HCl (pH
7.5), 150 mM NaCl, 1 mM EDTA, 1 mM MgCl2, 0.5% Triton X-100,
phosphatase inhibitor mix (1 mM NaF, 1 mM Na3VO4, and 1 mM
β-glycerol phosphate) and centrifuged at 5,600 × g for 5 min at
4°C, which contained the proteins. Protein quantification was
measured using the BCA kit (cat. no. p0006; Beyotime Biotechnology,
Inc.), and a microplate reader. The protein lysates (50 µg) were
mixed with 3× sample buffer solution and heated for 5 min. Protein
samples were loaded on a 12% SDS-gel and resolved using SDS-PAGE
for 3 h. Resolved proteins were transferred onto nitrocellulose
membranes and blocked with 0.01% Tween-20 containing 5% skimmed
milk powder for 4 h at room temperature. The membranes were
incubated with primary antibodies against AKT1 (cat. no. AF4718),
ALB (cat no. DF6396), TP53 (cat. no. DF7238), IL6 (cat. no.
DF6087), MAPK3 (cat. no. BF0528) and GADPH (cat. no. AF0911) (all
for 1:1,000 and purchased from Affinity Co., Ltd.), for 24 h at
4°C. The membranes were washed with TBST buffer (10 mM Tris-HCl (pH
7.5), 150 mM NaCl, 0.1% Tween-20) and subsequently incubated with
anti-rabbit IgG secondary antibodies with TBST solution (1:3,000;
cat. no. S0001; Affinity Co., Ltd.) for 1 h at room temperature.
The membranes were re-washed with TBST and the protein bands were
visualized using enhanced chemiluminescence reagent (SuperSignal
Western Pico Chemiluminescent Substrate; Pierce; Thermo Fisher
Scientific, Inc.) and quantified using the FluorChem FC2 Imaging
System (ProteinSimple). Densitomery analysis was performed using
Image-Pro Plus 6.0 software (National Institutes of Health).
Statistical analysis
GraphPad Prism 8.0 software (GraphPad Software,
Inc.) was used for statistical analysis and graphing of the data.
All experiments were performed in triplicate and data are presented
as the mean ± standard error of the mean. Unpaired Student's
t-tests were used to compare the difference between two groups,
while one-way ANOVA was used to compare differences among ≥3
groups, followed by Tukey's post hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Collection of active ingredients in
Yiqi Gubiao pills
Yiqi Gubiao pills had 102 active ingredients,
including 7 components of BZ, 8 components of BX, 4 components of
CP, 12 components of DS, 11 components of FF, 7 components of FL, 2
compounds of FXM, 19 components of HQ, 8 components of KDH, 8
components of PBY, 6 compounds of YBM, 4 components of YYR and 4
components of ZS (Table II).
 | Table II.Active ingredients of Yiqi Gubiao
pills. |
Table II.
Active ingredients of Yiqi Gubiao
pills.
| Molecule name | CAS | OB | DL | Herb |
|---|
| Cavidine | 32728-75-9 | 35.64 | 0.81 | BX |
| Coniferin | 109664-02-0 | 31.11 | 0.32 | BX |
| Cycloartenol | 469-38-5 | 38.69 | 0.78 | BX |
| Gondoic acid | 5561-99-9 | 30.70 | 0.20 | BX |
| Stigmasterol | 83-48-7 | 43.83 | 0.76 | BX, DS, HQ,
YYR |
| Baicalein | 491-67-8 | 33.52 | 0.21 | BX, HQ |
| Baicalin | 31564-28-0 | 40.12 | 0.75 | BX, HQ |
|
Didehydrotuberostemonine | 106861-40-9 | 51.90 | 0.74 | BZ |
| Formononetin | 485-72-3 | 69.67 | 0.21 | BZ |
| Malkangunin | 52691-06-2 | 57.71 | 0.63 | BZ |
| Stemotinine | 85644-15-1 | 38.69 | 0.46 | BZ |
| Suchilactone | 50816-74-5 | 57.51 | 0.56 | BZ |
| α-Amyrin | 638-95-9 | 39.51 | 0.76 | BZ |
| Naringenin | 153-18-4 | 59.29 | 0.21 | CP |
| Nobiletin | 10236-47-2 | 61.67 | 0.78 | CP |
| Pectolinarin | 28978-02-1 | 47.62 | 0.65 | CP |
| Aurantiamide
acetate | 56121-42-7 | 58.38 | 0.52 | DS |
| Daturilin | 111950-78-8 | 50.37 | 0.77 | DS |
| Frutinone A | 38210-27-4 | 65.9 | 0.34 | DS |
| Glycitein | 40957-83-3 | 50.48 | 0.24 | DS |
| Luteolin | 491-70-3 | 36.16 | 0.25 | DS, ZS |
| Perlolyrine | 29700-20-7 | 65.95 | 0.27 | DS |
| Sitosterol | 83-46-5 | 36.91 | 0.75 | DS |
| Spinasterol | 481-18-5 | 42.98 | 0.76 | DS |
| Spinoside A | 524-40-3 | 39.97 | 0.4 | DS |
| Taraxasteryl
palmitate | 29803-90-5 | 33.84 | 0.31 | DS |
| Taraxerol | 22076-46-6 | 38.40 | 0.77 | DS |
| Ammidin | 482-44-0 | 34.55 | 0.22 | FF |
| Anomalin | 81740-07-0 | 59.65 | 0.66 | FF |
| Decursin | 5928-25-6 | 39.27 | 0.38 | FF |
| Mandenol | 544-35-4 | 42.00 | 0.19 | FF, YYR |
| Marmesin | 13849-08-6 | 50.28 | 0.18 | FF |
| Nodakenetin | 495-32-9 | 68.62 | 0.18 | FF |
| Phellopterin | 2543-94-4 | 40.19 | 0.28 | FF |
| Prangenidin | 642-05-7 | 36.31 | 0.22 | FF |
| Wogonin | 632-85-9 | 30.68 | 0.23 | FF, HQ |
| β
Sitosterol/sitosterol | 83-46-5 | 36.91 | 0.75 | FF, BZ, BX, CP,
FXM, HQ, KDH, YYR, ZS, PBY |
| Ellipticine | 519-23-3 | 30.82 | 0.28 | FL |
| Hederagenin | 465-99-6 | 36.91 | 0.75 | FL |
| Pachymic acid | 29070-92-6 | 33.63 | 0.81 | FL |
| Poricoic acid
A | 137551-38-3 | 30.61 | 0.76 | FL |
| Poricoic acid
B | 137551-39-4 | 30.52 | 0.75 | FL |
| Poricoic acid
C | 151200-89-4 | 38.15 | 0.75 | FL |
| Trametenolic
acid | 24160-36-9 | 38.71 | 0.80 | FL |
| Acacetin | 480-44-4 | 34.97 | 0.24 | HQ |
| Carthamidin | 479-54-9 | 41.15 | 0.24 | HQ |
| Coptisine | 3486-66-6 | 30.67 | 0.86 | HQ |
|
Dihydrooroxylin | 18956-18-8 | 66.06 | 0.23 | HQ |
| Diop | 25103-50-8 | 43.59 | 0.39 | HQ |
| Epiberberine | 1816598 | 43.09 | 0.78 | HQ |
| Glucobrassicin | 4356-52-9 | 53.64 | 0.48 | HQ |
| Glyceryl
linolenate | 18465-99-1 | 38.13 | 0.31 | HQ |
|
Moslosooflavone | 3570-62-5 | 44.09 | 0.25 | HQ |
| Neobaicalein | 55084-08-7 | 104.34 | 0.44 | HQ |
| Norwogonin | 4443-09-8 | 39.40 | 0.21 | HQ |
| Panicolin | 41060-16-6 | 76.26 | 0.29 | HQ |
| Rivularin | 70028-59-0 | 37.94 | 0.37 | HQ |
| Salvigenin | 19103-54-9 | 49.07 | 0.33 | HQ |
| Diosgenin | 512-04-9 | 80.87 | 0.81 | KDH |
| Femara | 112809-51-5 | 66.09 | 0.20 | KDH |
| Kaempferol | 520-18-3 | 41.88 | 0.24 | KDH, PBY |
| Quercetin | 117-39-5 | 46.43 | 0.28 | KDH, PBY, BX |
| Senkirkine | 2318-18-5 | 56.16 | 0.41 | KDH |
| Tussilagin | 104012-37-5 | 61.12 | 0.38 | KDH |
| Cinchonain 1a | 85081-24-9 | 30.12 | 0.93 | PBY |
| Ellagic acid | 476-66-4 | 43.06 | 0.43 | PBY |
| Eugenol | 97-53-0 | 56.24 | 0.32 | PBY |
| Isorhamnetin | 480-19-3 | 49.60 | 0.31 | PBY |
| Mairin | 472-15-1 | 55.38 | 0.78 | PBY |
| Cyclopamine | 4449-51-8 | 55.42 | 0.82 | YBM |
| Peimisine | 19773-24-1 | 57.40 | 0.81 | YBM |
| Pelargonidin | 134-04-3 | 37.99 | 0.21 | YBM |
| Siraitic acid
A | 183374-15-4 | 41.52 | 0.85 | YBM |
| Verticinone | 1357-77-3 | 60.07 | 0.67 | YBM |
| Yibeinoside C | 157536-48-6 | 37.72 | 0.83 | YBM |
| Sitosterol α 1 | 474-40-8 | 43.28 | 0.78 | YYR |
| (+)-Catechin | 154-23-4 | 54.83 | 0.24 | ZS |
| Beta-carotene | 7235-40-7 | 37.18 | 0.58 | ZS |
| Cyanin | 523-42-2 | 47.42 | 0.76 | ZS |
Collection of targets
The targets of the active ingredients were predicted
based on the principle of structural similarity. They included 174
targets of BZ, 311 targets of BX, 140 targets of BX, 246 targets of
DS, 122 targets of FF, 159 targets of FL, 37 targets of FXM, 203
targets of HQ, 243 targets of KQH, 261 targets of PPY, 54 targets
of YBM, 109 targets of YYR and 173 targets of ZS components (Data
SI). Further to this, 7,446 targets associated with LC were
detected in the OMIM database, 22,400 targets were detected in the
Pubmed-Gene Database, and 3,007 targets in the Genecards Database.
Intersection of the targets obtained from the three databases
results in 2,413 targets.
PPI network construction and
analysis
The predicted targets of Yiqi Gubiao pills were
compared with those related to LC. Finally, 229 anti-LC potential
targets of the Yiqi Gubiao pills were selected. These included
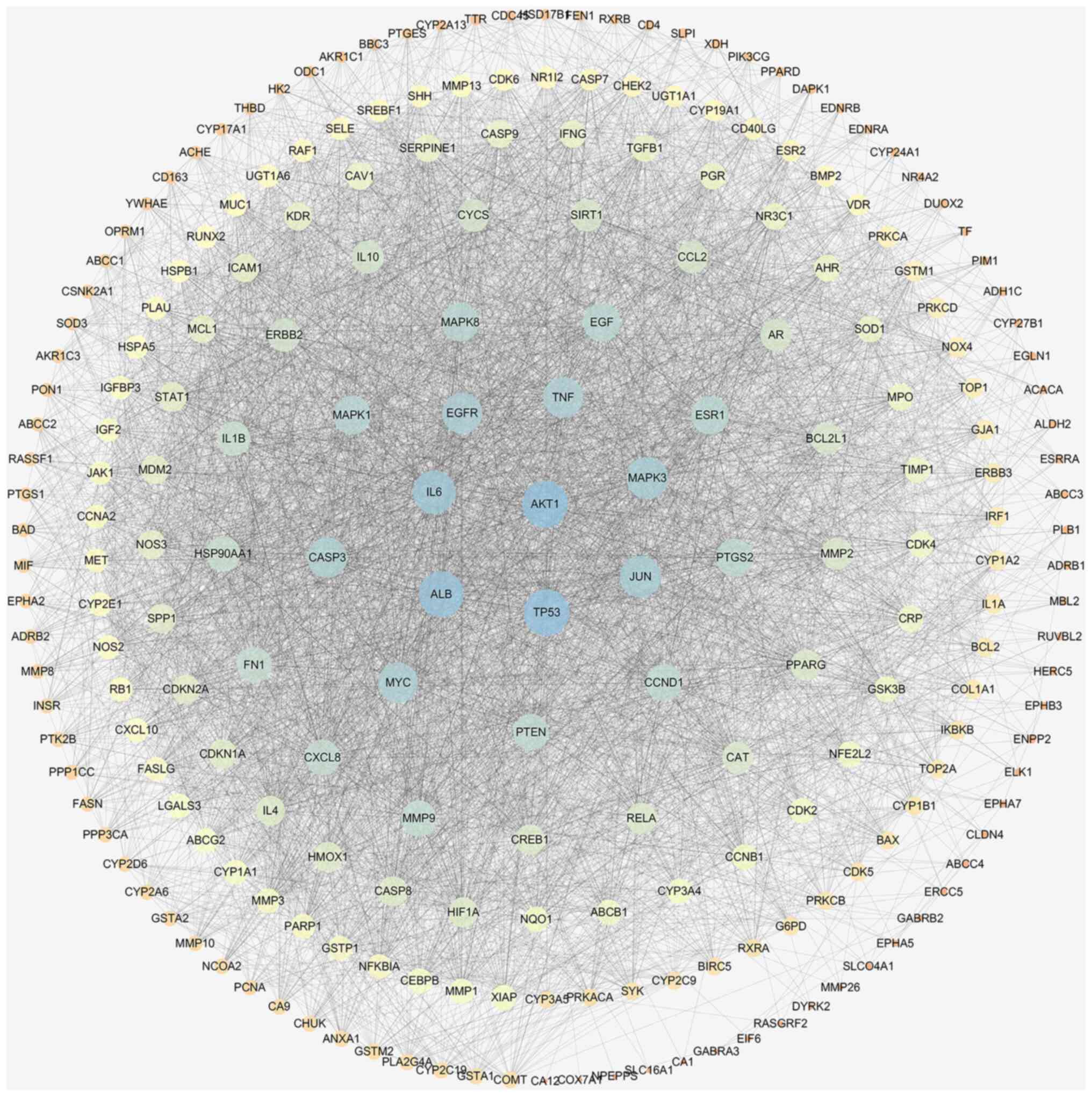
AKT1, TP53, IL-6, ALB and MAPK3, among others. A PPI network
composed of the 229 targets contained 227 nodes and 4,810
interaction edges (Fig. 2). The top
five hub genes where protein kinase B (AKT1) (degree=149), TP53
(degree=148), ALB (degree=144), IL6 (degree=134), and MAPK3
(degree=128) (Table III).
 | Table III.Protein classes of key targets. |
Table III.
Protein classes of key targets.
| Gene name | UniProt ID | Target | Protein class | Degree |
|---|
| AKT1 | P31749 | AKT
serine/threonine kinase 1 | Calcium-binding
protein; kinase; transfer/carrier protein transderase | 149 |
| TP53 | P04637 | Tumor protein
p53 | Transcription
factor | 148 |
| MAPK3 | P27361 | Mitogen-activated
protein kinase 3 | Kinase;
transferase | 128 |
| IL6 | P05231 | Interleukin 6 | None | 134 |
| ALB | P02768 | Albumin | Transporter | 144 |
GO and KEGG enrichment analysis
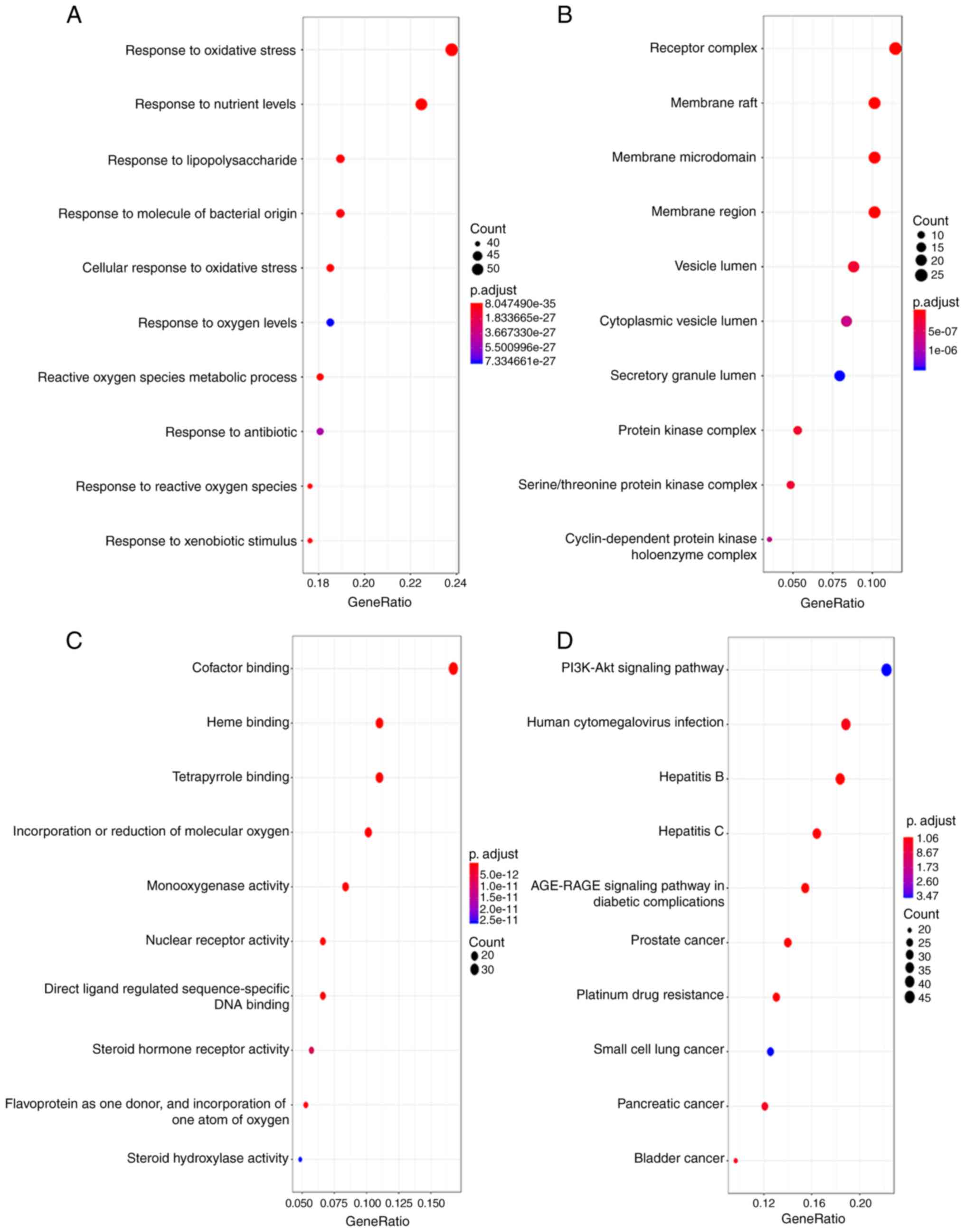
GO enrichment and KEGG pathway analysis was
performed using the 227 genes in the PPI network. An adjusted
P<0.05 was set as the screening value. In CC enrichment
analysis, the genes were mainly enriched in the signaling pathways
such as ‘membrane raft’ and ‘membrane microdomain’ signaling
pathways (Fig. 3B). In BP, the genes
were involved in response ‘oxidative stresses’, ‘nutrient levels’
and ‘lipopolysaccharides’. In MF, the genes were mainly involved in
binding activities, such as ‘heme binding’ (Fig. 3C). KEGG pathway analysis further
revealed that the ‘PI3K-Akt’ and ‘AGE-RAGE’ signaling pathways were
the most common pathways related to LC treatment using Yiqi Gubiao
pills (Fig. 3D).
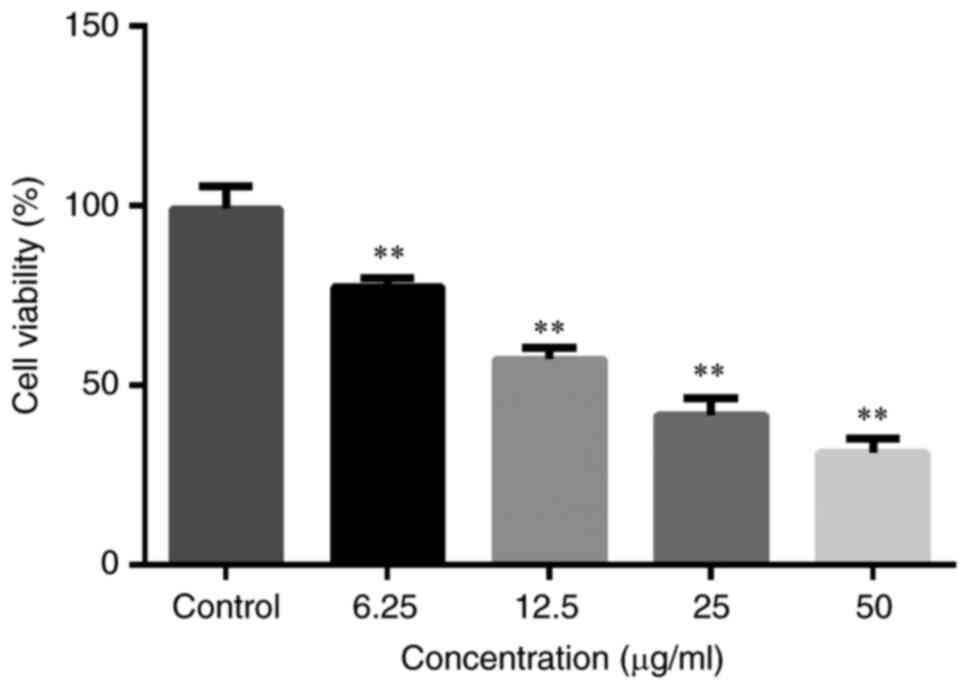
Cell viability
Viability of cells in the Yiqi Gubiao pill groups
(50, 25, 12.5 and 6.25 µg/ml) decreased significantly in a
concentration-dependent manner compared with those of the control
group (all P<0.01; Fig. 4).
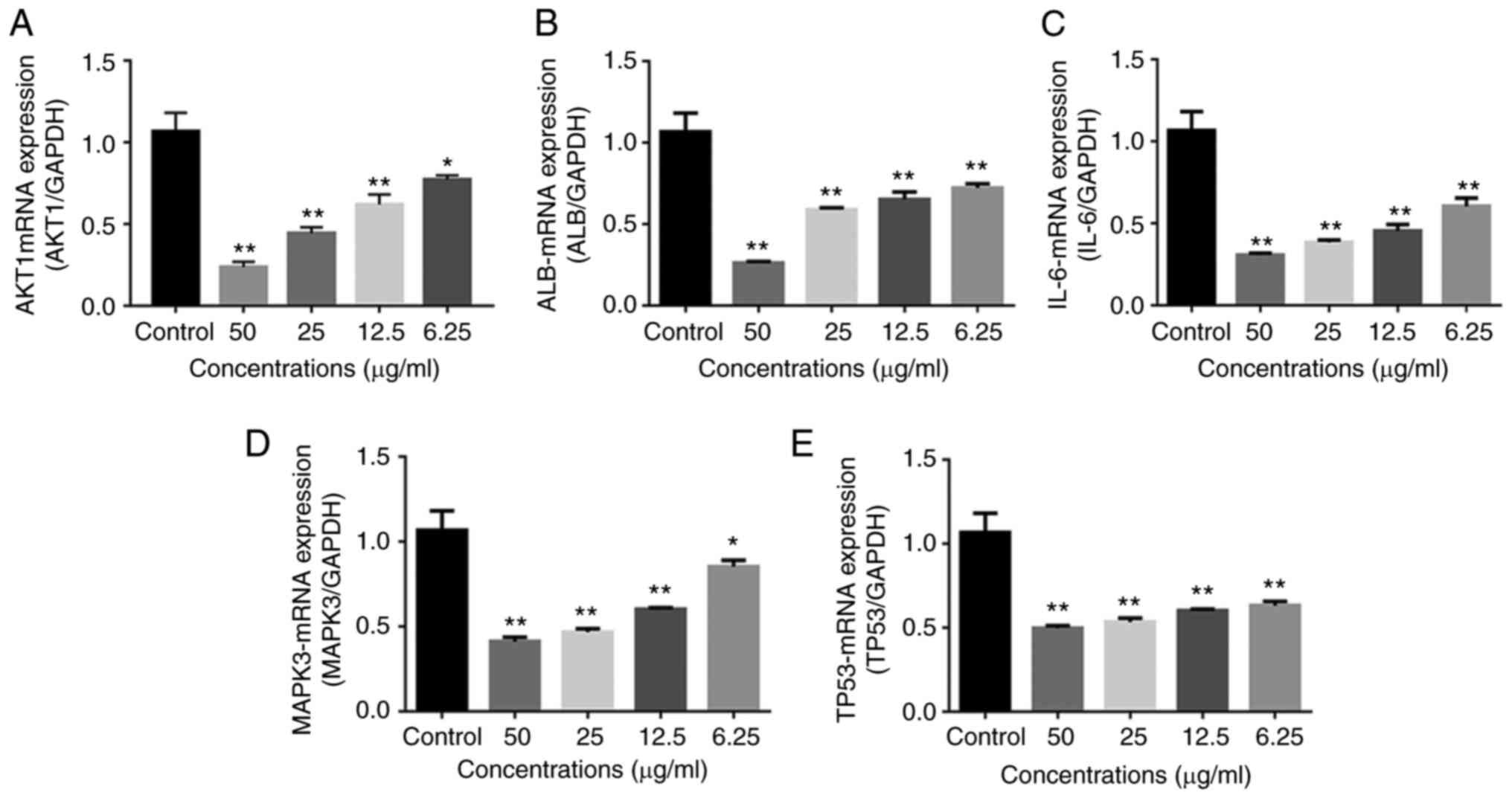
mRNA suppression and protein
expression of the hub genes by Yiqi Gubiao pills
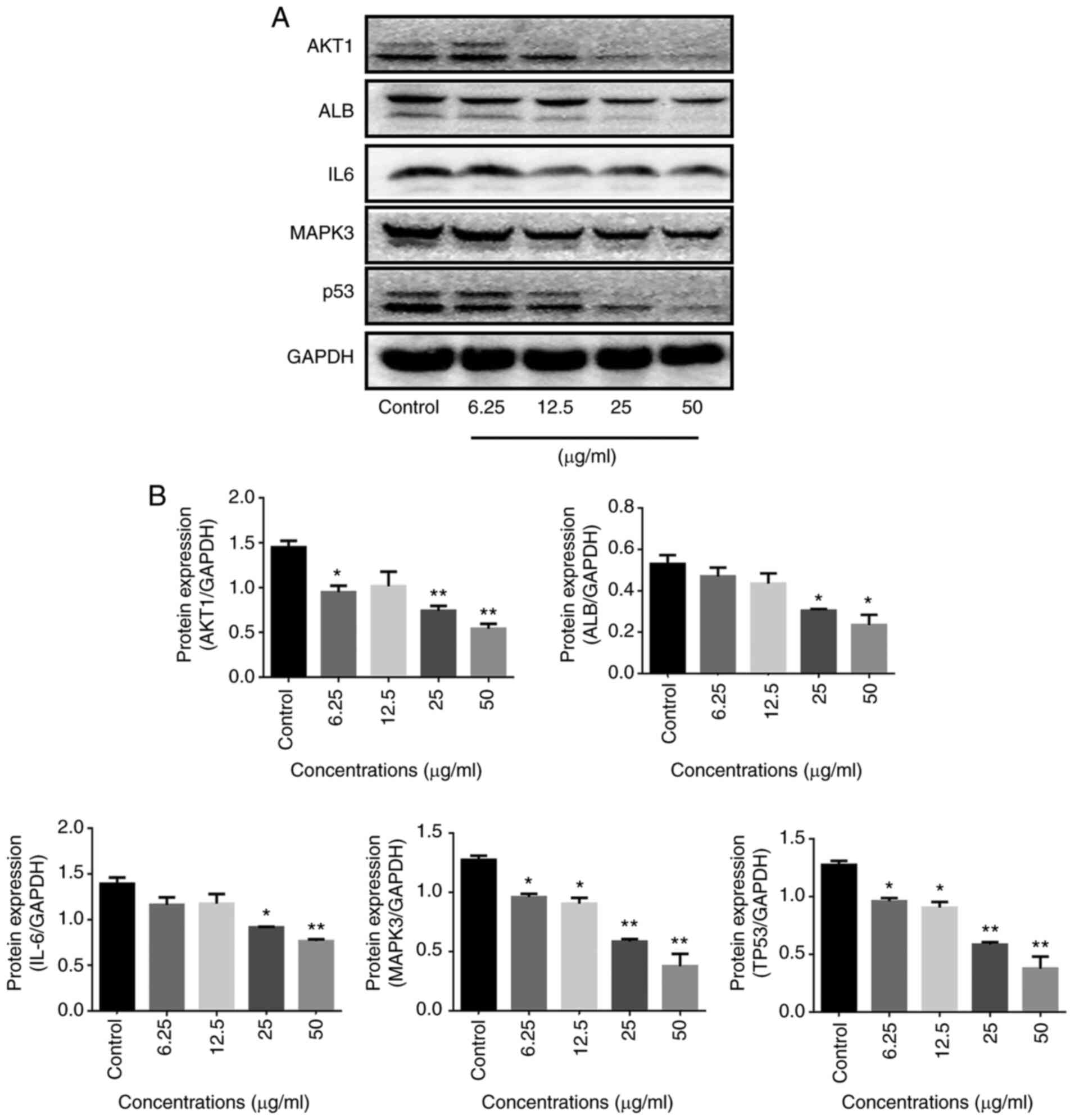
The mRNA levels and protein expression levels of
AKT1, ALB, TP53, IL6 and MAPK3 were detected using RT-qPCR and
western blotting, respectively, to further validate the effects of
Yiqi Gubiao pills on the predicted hub genes. The mRNA expression
of AKT1, ALB, TP53, IL6 and MAPK3 was significantly suppressed by
Yiqi Gubiao pills in a concentration-dependent manner (all
P<0.05 or P<0.01; Fig. 5).
Western blotting further revealed that the extract of the Yiqi
Gubiao pills modulated protein expression of AKT1, ALB, TP53, IL6
and MAPK3. The protein expression levels of the TP53 and MAPK3 were
significantly decreased in the Yiqi Gubiao pill groups compared
with the control group, while protein expression levels of the
AKT1, ALB and IL-6 were not improved at Yiqi Gubiao Pills (12.5
µg/ml) (Fig. 6).
Discussion
Yiqi Gubiao pills are composed of 13 TCMs used to
treat a variety of lung diseases. They are often used as an adjunct
treatment in clinical treatment of LC (5,6).
Preparation of TCM involves a combination of multiple ingredients
of TCM. This in turn causes the action mechanism of TCMs to be
complex because each component corresponds to several targets
(19). Network pharmacology is
performed to search for TCM ingredients, ingredient targets and
disease targets in verified results of clinical trials (8,20), which
may systematically reveal the interaction between the ingredients
and the diseases as well as the mechanisms of action of TCM in
treating diseases (21).
In the present study, 102 active ingredients of Yiqi
Gubiao pills were identified including 7 ingredients of BZ, 8
ingredients of BX, 4 ingredients of CP, 12 ingredients of DS, 11
ingredients of FF, 7 ingredients of FL, 2 ingredients of FXM, 19
ingredients of HQ, 8 ingredients of KDH, 8 ingredients of PPY, 6
ingredients of YBM, 4 ingredients of YYR and 6 ingredients of ZS.
Moreover, 2,658 ingredient targets were obtained after deleting the
duplicate targets and 2,413 disease-related targets were
identified. The disease related targets were further intersected to
obtain 229 common targets. The 229 targets were regarded as
potential targets for the Yiqi Gubiao pills in LC treatment. The
PPI network of the hub genes further revealed that ATK1, TP53, ALB,
IL6 and MAPK3 were the top five hub genes associated with LC
treatment. These results were further verified using western
blotting and RT-qPCR experiments.
Zhang et al (22) reported that PRMT5 activates AKT and
induces the positive regulator PI3K and the negative regulator PTEN
thereby inhibiting LC cell proliferation. In addition, Olivier
et al (23) reported that
TP53 is a key tumor suppressor gene. As such, its mutation may lead
to the loss of function to inhibit tumor growth, thereby promoting
cancer cell proliferation and cell invasion. Similarly, Zheng et
al (24) suggested that
upregulation of long non-coding RNA H19 induces the expression of
microRNA (miR)-675-5p that targets the TP53 gene. This promotes
tumor progression and development in NSCLC. The level of ALB is
usually regarded as the key parameter that reflects an individual's
nutritional status. The ratio of albumin-to-fibrinogen has been
used as a biomarker to judge the clinical prognosis of patients
with NSCLS (25). Studies have
reported that the ERK1 signaling pathway plays a key role in
promoting the proliferation of NSCLC mesenchymal stem cells
(26,27). As such, blocking the ERK signaling
pathway improves the therapeutic response of patients with NSCLC to
EGFR inhibitors (28). In the same
line, the IL6 family of proteins is mainly involved in
inflammation, immune response and development of tumors, thus
leading to NSCLC progression (29).
Yang et al have reported that miR-218 targets the IL6/STAT3
signaling pathway can prevent LC progression.
Targets of the Yiqi Gubiao pills were mainly
enriched in the PI3K-Akt and AGE-RAGE signaling pathways. The
PI3K-Akt signaling pathway is an intracellular signaling pathway
mainly involved in the cell cycle process. It is related to
cellular quiescence, proliferation, cancer and longevity (30). Wu et al (31) reported that the PAX6-zinc finger
E-box-binding homeobox 2 axis promotes tumor metastasis via the
PI3K/AKT signaling pathway. On the other hand, the AGE-RAGE
signaling pathway is an important signal transduction pathway. It
is mainly involved in major mechanisms of vascular oxidative
stresses through activation of the MAPK and NF-κB pathways
(32). Zeng et al postulated
that RHOJ mediates the AGE-RAGE signaling pathway in NSCLC
(33).
Overall, in the present study network pharmacology
was used to decipher the active ingredients and potential targets
of the Yiqi Gubiao pill, thus enabling further related studies. The
results suggested that Yiqi Gubiao pills partially inhibit
progression of LC by regulating the expression of hub genes (AKT1,
TP53, ALB, IL6 and MAPK3) through the PI3K/AKT and AGE-RAGE
signaling pathways. The findings of the study provide a theoretical
basis for the clinical application of Yiqi Gubiao pills in LC
treatment.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Opening Project of
the Key Laboratory of Xinjiang Uygur Autonomous Region (grant no.
2018D04004), and the Special Project for Young Medical Science and
Technology Talents of the Health Commission of Xinjiang Uygur
Autonomous Region (grant no. WJWY-201812).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
ZL made substantial contributions to the conception
and performed the experiments. DX and JJ analyzed the data and
revised the manuscript. FL gave final approval of the version to be
published, made substantial contributions to design, and revised
the manuscript critically for important intellectual content. ZL,
DX, JJ and FL confirmed the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mao Y, Yang D, He J and Krasna MJ:
Epidemiology of lung cancer. Surg Oncol Clin N Am. 25:439–345.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rivera GA and Wakelee H: Lung cancer in
never smokers. Adv Exp Med Biol. 893:43–57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Glinski K, Socha J, Wasilewska-Tesluk E,
Komosinska K and Kepka L: Accelerated hypofractionated radiotherapy
with concurrent full dose chemotherapy for locally advanced
non-small cell lung cancer: A phase I/II study. Radiother Oncol.
148:174–180. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kehoe S, Hook J, Nankivell M, Jayson GC,
Kitchener H, Lopes T, Luesley D, Perren T, Bannoo S, Mascarenhas M,
et al: Primary chemotherapy versus primary surgery for newly
diagnosed advanced ovarian cancer (CHORUS): An open-label,
randomised, controlled, non-inferiority trial. Lancet. 386:249–257.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li FS, Zhang YL, Li Z, Xu D, Liao CY, Ma
H, Gong L, Su J, Sun Q, Xu Q, et al: Randomized, double-blind,
placebo-controlled superiority trial of the Yiqigubiao pill for the
treatment of patients with chronic obstructive pulmonary disease at
a stable stage. Exp Ther Med. 12:2477–2488. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang L, Li F, Sun J, Huo H, Li X and Li
H: Efficacy of Yiqigubiao pill on chronic obstructive pulmonary
disease in rats with the disease induced by lipopolysaccharide and
cigarette-smoke fumigation. J Tradit Chin Med. 40:983–991.
2020.PubMed/NCBI
|
|
7
|
Boezio B, Audouze K, Ducrot P and
Taboureau O: Network-based approaches in pharmacology. Mol Inform.
36:2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kibble M, Saarinen N, Tang J, Wennerberg
K, Mäkelä S and Aittokallio T: Network pharmacology applications to
map the unexplored target space and therapeutic potential of
natural products. Nat Prod Rep. 32:1249–1266. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu Y, Zhang F, Yang K, Fang S, Bu D, Li H,
Sun L, Hu H, Gao K, Wang W, et al: SymMap: An integrative database
of traditional Chinese medicine enhanced by symptom mapping.
Nucleic Acids Res. 47(D1): D1110–D1117. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ye H, Ye L, Kang H, Zhang D, Tao L, Tang
K, Liu X, Zhu R, Liu Q, Chen YZ, et al: HIT: Linking herbal active
ingredients to targets. Nucleic Acids Res. 39((Database Issue)):
D1055–D1059. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Keiser MJ, Roth BL, Armbruster BN,
Ernsberger P, Irwin JJ and Shoichet BK: Relating protein
pharmacology by ligand chemistry. Nat Biotechnol. 25:197–206. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Amberger JS, Bocchini CA, Schiettecatte F,
Scott AF and Hamosh A: OMIM.org: Online mendelian inheritance in
man (OMIM®), an online catalog of human genes and
genetic disorders. Nucleic Acids Res. 43((Database Issue)):
D789–D798. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou J and Rudd KE: EcoGene 3.0. Nucleic
Acids Res. 41((Database Issue)): D613–D624. 2013.PubMed/NCBI
|
|
14
|
The UniProt Consortium: UniProt: The
universal protein knowledgebase. Nucleic Acids Res. 45(D1):
D158–D169. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
The Gene Ontology Consortium: Expansion of
the gene ontology knowledgebase and resources. Nucleic Acids Res.
45(D1): D331–D338. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu G, Wang LG, Han Y and He QY:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kang Y, Zhu X, Xu Y, Tang Q, Huang Z, Zhao
Z, Lu J, Song G, Xu H, Deng C and Wang J: Energy stress-induced
lncRNA HAND2-AS1 represses HIF1α-mediated energy metabolism and
inhibits osteosarcoma progression. Am J Cancer Res. 8:526–537.
2018.PubMed/NCBI
|
|
20
|
Li S and Zhang B: Traditional Chinese
medicine network pharmacology: Theory, methodology and application.
Chin J Nat Med. 11:110–120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang R, Zhu X, Bai H and Ning K: Network
pharmacology databases for traditional Chinese medicine: Review and
assessment. Front Pharmacol. 10:1232019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang S, Ma Y, Hu X, Zheng Y and Chen X:
Targeting PRMT5/Akt signalling axis prevents human lung cancer cell
growth. J Cell Mol Med. 23:1333–1342. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Olivier M, Hollstein M and Hainaut P: TP53
mutations in human cancers: Origins, consequences, and clinical
use. Cold Spring Harb Perspect Biol. 2:a0010082010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng ZH, Wu DM, Fan SH, Zhang ZF, Chen GQ
and Lu J: Upregulation of miR-675-5p induced by lncRNA H19 was
associated with tumor progression and development by targeting
tumor suppressor p53 in non-small cell lung cancer. J Cell Biochem.
120:18724–18735. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Du X, Shao Y, Qin HF, Tai YH and Gao HJ:
ALK-rearrangement in non-small-cell lung cancer (NSCLC). Thorac
Cancer. 9:423–430. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang J, Zhou Y, Ma L, Cao S, Gao W, Xiong
Q, Wang K and Yang L: CIAPIN1 targeted NHE1 and ERK1/2 to suppress
NSCLC Cells' metastasis and predicted good prognosis in NSCLC
patients receiving pulmonectomy. Oxid Med Cell Longev.
2019:19708182019.PubMed/NCBI
|
|
27
|
Moncho-Amor V, Pintado-Berninches L,
Ibañez de Cáceres I, Martín-Villar E, Quintanilla M, Chakravarty P,
Cortes-Sempere M, Fernández-Varas B, Rodriguez-Antolín C, de Castro
J, et al: Role of dusp6 phosphatase as a tumor suppressor in
non-small cell lung cancer. Int J Mol Sci. 20:20362019. View Article : Google Scholar
|
|
28
|
Sun C, Li C, Li X, Zhu Y, Su Z, Wang X, He
Q, Zheng G and Feng B: Scutellarin induces apoptosis and autophagy
in NSCLC cells through ERK1/2 and AKT signaling pathways in vitro
and in vivo. J Cancer. 9:3247–3256. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tang H, Bai Y, Pan G, Wang X, Wei Y, Yang
Z and Zhao J: Interleukin-6 and insulin-like growth factor-1
synergistically promote the progression of NSCLC. Autoimmunity.
51:399–407. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xie Y, Shi X, Sheng K, Han G, Li W, Zhao
Q, Jiang B, Feng J, Li J and Gu Y: PI3K/Akt signaling transduction
pathway, erythropoiesis and glycolysis in hypoxia (Review). Mol Med
Rep. 19:783–791. 2019.PubMed/NCBI
|
|
31
|
Wu DM, Zhang T, Liu YB, Deng SH, Han R,
Liu T, Li J and Xu Y: The PAX6-ZEB2 axis promotes metastasis and
cisplatin resistance in non-small cell lung cancer through PI3K/AKT
signaling. Cell Death Dis. 10:3492019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sparvero LJ, Asafu-Adjei D, Kang R, Tang
D, Amin N, Im J, Rutledge R, Lin B, Amoscato AA, Zeh HJ and Lotze
MT: RAGE (receptor for advanced glycation endproducts), RAGE
ligands, and their role in cancer and inflammation. J Transl Med.
7:172009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zeng T, Chen C, Yang P, Zuo W, Liu X and
Zhang Y: A protective role for RHOJ in nonsmall cell lung cancer
based on integrated bioinformatics analysis. J Comput Biol.
27:1092–1103. 2020. View Article : Google Scholar : PubMed/NCBI
|