Introduction
Lung cancer is the leading cause of
cancer-associated death among males and females, accounting for an
estimated 600,000 associated deaths in China in 2015 (1). Immune checkpoint blockade has emerged
as a promising strategy in several malignancies, including both
non-small cell lung cancer (NSCLC) and small cell lung cancer
(SCLC) (2–6). The CheckMate-032 study investigated the
efficacy of nivolumab, an anti-programmed cell death-1 (PD-1)
antibody, alone or in combination with ipilimumab, an
anti-cytotoxic T lymphocyte antigen-4 antibody in recurrent
patients with SCLC, who progressed after one or more prior regimens
(6). In the aforementioned study,
the objective response rates (ORRs) were 10, 23 and 19% for
patients treated with 3 mg/kg nivolumab, 1 mg/kg nivolumab + 3
mg/kg ipilimumab and 3 mg/kg nivolumab + 1 mg/kg ipilimumab,
respectively (6). Among the patients
with metastatic NSCLC who progressed following platinum-based
chemotherapy, nivolumab was associated with significantly longer
median overall survival time compared with docetaxel [OS;
non-squamous NSCLC, 12.2 vs. 9.4 months; hazard ratio (HR), 0.73;
95% CI, 0.59–0.89; P=0.002; squamous NSCLC, 9.2 vs. 6.0 months; HR,
0.59; 95% CI, 0.44–0.79; P<0.001] (7,8). Other
immune checkpoint inhibitors, including pembrolizumab, an anti-PD-1
antibody [programmed death-ligand 1 (PD-L1)-positive NSCLC
population, 10.4 vs. 8.5 months; HR, 0.71; 95% CI, 0.58–0.88;
P=0.0008], and atezolizumab, an anti-PD-L1 antibody (13.8 vs. 9.6
months; HR, 0.73; 95% CI, 0.62–0.87; P=0.0003), also exhibited
improved OS compared with chemotherapy (9,10).
However, the response rate of immune checkpoint inhibitors was
relatively low in unselected patients (5). Therefore, multiple biomarkers have been
investigated for selecting patients who can benefit from
immunotherapy.
Tumor mutation burden (TMB) is an emerging biomarker
to independently predict response to immunotherapy (11,12). For
example, the retrospective analysis of the Checkmate-032 study
suggested that the efficacy of nivolumab combined with ipilimumab
was improved in patients with high TMB compared with in those with
low TMB (ORR, 46.2 vs. 22.2%) (11).
Currently, the evaluation of TMB is based on expensive, large
next-generation sequencing (NGS) gene panels or whole-exome
sequencing. Several studies have demonstrated that single gene
mutations, such as driver mutations in polymerase ε catalytic
subunit A (POLE)/polymerase δ catalytic subunit gene 1 (POLD1)
genes and pathogenetic mutations in mismatch repair genes, are
associated with high TMB, which may provide an economical, simple
and complementary method for predicting TMB and response to
immunotherapy (13–15). However, the aforementioned studies
mainly focused on colorectal and endometrial carcinoma. The
molecular characteristics that may contribute to high TMB in lung
cancer have not been fully documented. Therefore, in the present
study, a retrospective, cohort study was conducted to
comprehensively investigate the clinicopathological and molecular
features of patients with lung cancer with extremely high mutation
burden (hypermutation).
In addition to TMB, other molecular biomarkers have
been identified to affect patient response to immunotherapy. High
levels of microsatellite instability (MSI), deficient mismatch
repair and PD-L1 expression have been approved by the Food and Drug
Administration (FDA) as predictive biomarkers of immunotherapy
across multiple types of cancer, such as NSCLC, triple-negative
breast cancer and gastric or gastroesophageal junction
adenocarcinoma (16–19). Additionally, alterations in DNA
damage response (DDR) genes may be associated with high TMB and
improved clinical benefits from immunotherapy in patients with
NSCLC (20). Tumors with
co-occurring TP53/KRAS gene mutations exhibited remarkable clinical
benefit from immunotherapy with PD-1 inhibitors (21). However, some driver mutations in
NSCLC tend to predict a poorer response to immunotherapy, such as
EGFR sensitive mutations and ALK fusions (22). Somatic mutations in PTEN, β-2
microglobulin (B2M), serine-threonine kinase 11 (STK11), Kelch-like
ECH-associated protein 1 (KEAP1), murine double minute 2 (MDM2) and
11q13 amplification have also been negatively associated with
immunotherapy response (23–27). The current study aimed to explore the
prevalence of these immunotherapy-associated biomarkers in a
hypermutant lung cancer cohort.
Materials and methods
Patient samples
A total of 1,000 patients with lung cancer who
underwent genetic testing using the NGS technology at
Geneplus-Beijing (Beijing, China) between November 2017 and
September 2019 were retrospectively enrolled in the present study,
irrespective of the type of prior treatment and treatment response.
The clinical characteristics of the patients are summarized in
Table I. Fresh tissues or tissues
fixed using 10% formalin at room temperature for 24 h and embedded
in paraffin [formalin-fixed paraffin-embedded (FFPE) tissues],
including those prepared from pleural effusions, and 10 ml matched
peripheral blood were obtained from each patient to perform a
matched tumor-normal NGS testing. All procedures were conducted in
accordance with the Declaration of Helsinki and written informed
consent was provided by all participants.
 | Table I.Comparison of clinicopathological
characteristics between patients with hypermutant (n=67) and
non-hypermutant (n=933) lung cancer. |
Table I.
Comparison of clinicopathological
characteristics between patients with hypermutant (n=67) and
non-hypermutant (n=933) lung cancer.
|
Characteristics | Hypermutant
cohort | Non-hypermutant
cohort | P-value |
|---|
| Median age at
diagnosis (range), years | 62 (43–79) | 61 (18–94) | 0.0198 |
| Sex, n (%) |
|
| <0.0001 |
|
Male | 58 (86.6) | 552 (59.2) |
|
|
Female | 9
(13.4) | 381 (40.8) |
|
| Histological
subtype, n (%) |
|
| 0.0215 |
|
Adenocarcinoma | 42 (62.7) | 742 (79.5) |
|
|
Squamous | 15 (22.4) | 122 (13.1) |
|
|
SCLC | 4
(6.0) | 24
(2.6) |
|
|
Other | 2
(3.0) | 15
(1.6) |
|
|
N/A | 4
(6.0) | 30
(3.2) |
|
| Clinical stage, n
(%) |
|
| 0.5087 |
|
I/II | 4
(6.0) | 105 (11.3) |
|
|
III | 6
(9.0) | 75
(8.0) |
|
| IV | 26 (38.8) | 399 (42.8) |
|
|
N/A | 31 (46.3) | 354 (37.9) |
|
| Smoking history, n
(%) |
|
| <0.0001 |
| Never
smokers | 10 (14.9) | 498 (53.4) |
|
|
Smokers | 57 (85.1) | 435 (46.6) |
|
| Previous treatment,
n (%) |
|
| 0.6062 |
| No | 48 (71.6) | 695 (74.5) |
|
|
Yes | 19 (28.4) | 238 (25.5) |
|
| Family history, n
(%) |
|
| 0.5237 |
|
Yes | 13 (19.4) | 151 (16.2) |
|
| No | 39 (58.2) | 560 (60.0) |
|
|
N/A | 15 (22.4) | 222 (23.8) |
|
| Sample type, n
(%) |
|
| 0.1404 |
| FFPE
tissue | 61 (91.0) | 787 (84.4) |
|
| Fresh
tissue | 6
(9.0) | 146 (15.6) |
|
Sequencing and analysis
Comprehensive genomic profiling was performed using
a custom-designed NGS panel containing 1,021 cancer-associated
genes. The captured genomic regions included the most common driver
genes of solid tumors, including lung cancer. Sample processing,
DNA extraction, library preparation, target capture, sequencing and
bioinformatic analysis were conducted as previously described
(28,29). Briefly, the genomic DNA from white
blood cells isolated from peripheral blood, fresh tissues and FFPE
samples was extracted using the CWE9600 Blood DNA kit (CoWin
Biosciences) and the Maxwell® 16 FFPE Plus LEV DNA
Purification kit (Promega Corporation) according to the
manufacturer's protocol. Illumina TruSeq DNA Library Preparation
kits (Illumina, Inc.) were utilized to prepare the sequencing
libraries. The libraries were then hybridized to custom-designed
biotinylated oligonucleotide probes (Roche NimbleGen, Inc.)
targeting 1,021 genes, including 14 genes with therapeutic value,
recommended by the National Comprehensive Cancer Network guidelines
(30–34) or approved by the FDA, and 220, 98 and
689 genes with therapeutic, diagnostic or prognostic value based on
well-powered studies, multiple small studies, and small studies or
case reports, respectively (Table
SI). The prepared libraries were sequenced on the NextSeq CN
500 system (Illumina, Inc.), according to the manufacturer's
instructions. The calling of somatic single nucleotide variants
(SNVs) and small insertions and deletions (InDels) was executed
using the MuTect (v1.1.4) and GATK (v3.4–46-gbc02625) softwares
(both Broad Institute). Copy number variations and structural
variations were identified using Contra (v2.0.8) (35) and NoahCare structural variations
detection (in house), respectively. TMB was calculated as the
number of somatic non-synonymous SNVs and InDels per Mb in the
coding region, with a variant allele fraction of ≥0.03. The
threshold of hypermutation was determined using the following
formula, previously used by Zehir et al (36) from Memorial Sloan Kettering Cancer
Center: Median (TMB) + 2 × IQR (TMB), where IQR represents the
interquartile range. The MSI status was inferred using the
MSIsensor (v0.5) software (37) and
MSI-high was defined on an empirically defined cut-off of MSI score
>8%. SNVs and InDels, copy number variants, structural variants
and TMB and MSI are provided in Tables
SII, SIII, SIV and SV,
respectively.
Differentially mutated genes (DMGs)
and pathway enrichment analysis
The DMGs between cohorts were identified using
two-tailed Fisher's exact test. P≤0.05 was considered to indicate a
statistically significant difference. Kyoto Encyclopedia of Genes
and Genomes (KEGG) analysis was performed using the Web-based Gene
Set Analysis Toolkit (WebGestalt; http://www.webgestalt.org/) to explore whether the
DMGs that were more frequently mutated in the hypermutant cohort
were enriched in certain signaling pathways. The significance of
mutation enrichment was determined by applying a hypergeometric
test and was adjusted for multiple testing using the
Benjamini-Hochberg false discovery rate.
Statistical analysis
The somatic mutation profiles in The Cancer Genome
Atlas (TCGA) database from 1,031 NSCLC samples, including 562
patients with adenocarcinoma and 469 patients with squamous cell
cancer, were downloaded from CBioPortal (https://www.cbioportal.org). The difference in age at
diagnosis between the cohorts were evaluated using a two-tailed
unpaired Mann-Whitney U test. χ2 test was utilized to
assess the differences in other demographic characteristics.
Missing data regarding histological subtype, clinical stage and
family history were not included in the statistical analyses. The
correlation between sex and smoking history was examined using
Spearman's rank correlation analysis. Cox multivariate regression
analysis was used to further verify the association between
clinical characteristics and TMB. P≤0.05 was considered to indicate
a statistically significant difference. Statistical analyses were
performed using GraphPad Prism 8.0.2 (GraphPad Software, Inc.) or
SPSS v19 software (IBM Corp.).
Results
Study design and patient
demographics
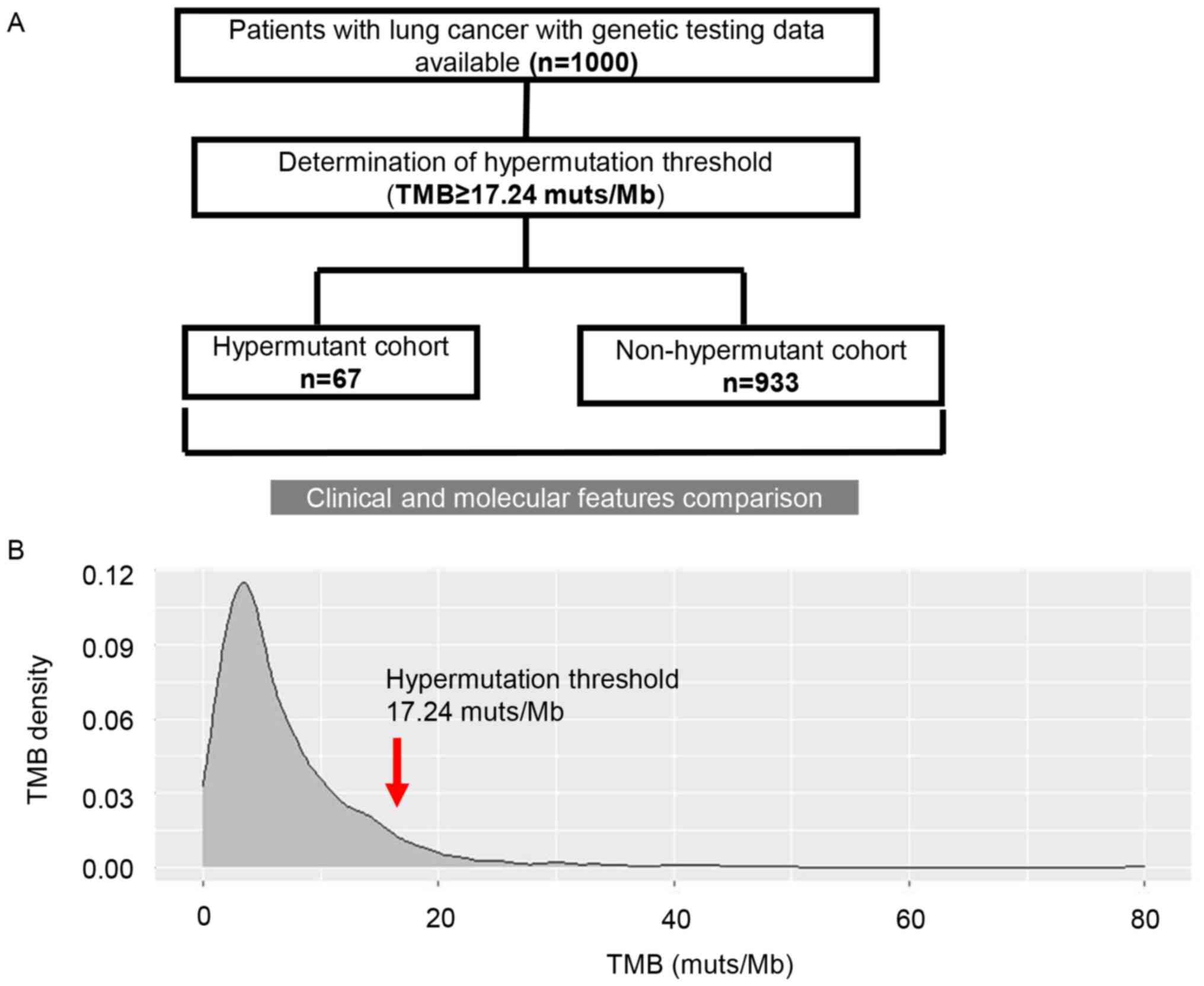
The flow chart of the methodology of the present
study is presented in Fig. 1A. To
determine the threshold of hypermutation in patients with lung
cancer, the TMB status of 1,000 selected patients with lung cancer
was screened. The distribution of TMB is presented in Fig. 1B. Among these patients, the median
TMB was 5 muts/Mb (range, 0–80 muts/Mb). TMB of 17.24 muts/Mb was
defined as the threshold according to the criterion for
hypermutation set in the current study. Consequently, 67 patients
were considered to be hypermutant, and the remaining patients were
non-hypermutant (n=933). The median TMB for the hypermutant and
non-hypermutant cohorts was 22 (range, 17.28–80 muts/Mb) and 4.8
muts/Mb (range, 0–17 muts/Mb), respectively.
The clinicopathological characteristics of patients
are summarized in Table I. The
proportion of males (86.6 vs. 59.2%) and smokers (85.1 vs. 46.6%)
was higher in the hypermutant cohort compared with in the
non-hypermutant cohort (both P<0.0001). In addition, a strong
correlation between smoking and sex (male smokers vs. female
smokers, 79.5 vs. 9.1%; Spearman rank correlation, 0.659; 95% CI,
0.551–0.748) was revealed in the present study (data not shown).
Additionally, compared with in the non-hypermutant cohort, the
proportion of patients with squamous cell carcinoma and SCLC was
higher in the hypermutant cohort (22.4 vs. 13.1% and 6.0 vs. 2.6%,
respectively; P=0.0215). In addition, the age at diagnosis of
hypermutant patients was significantly increased compared with that
of non-hypermutant patients (P=0.0198). Cox multivariate regression
analyses further confirmed that TMB was associated with sex,
smoking history and histological subtype, but not with age at
diagnosis (Table SVI).
Mutation profiles of hypermutant and
non-hypermutant lung cancer
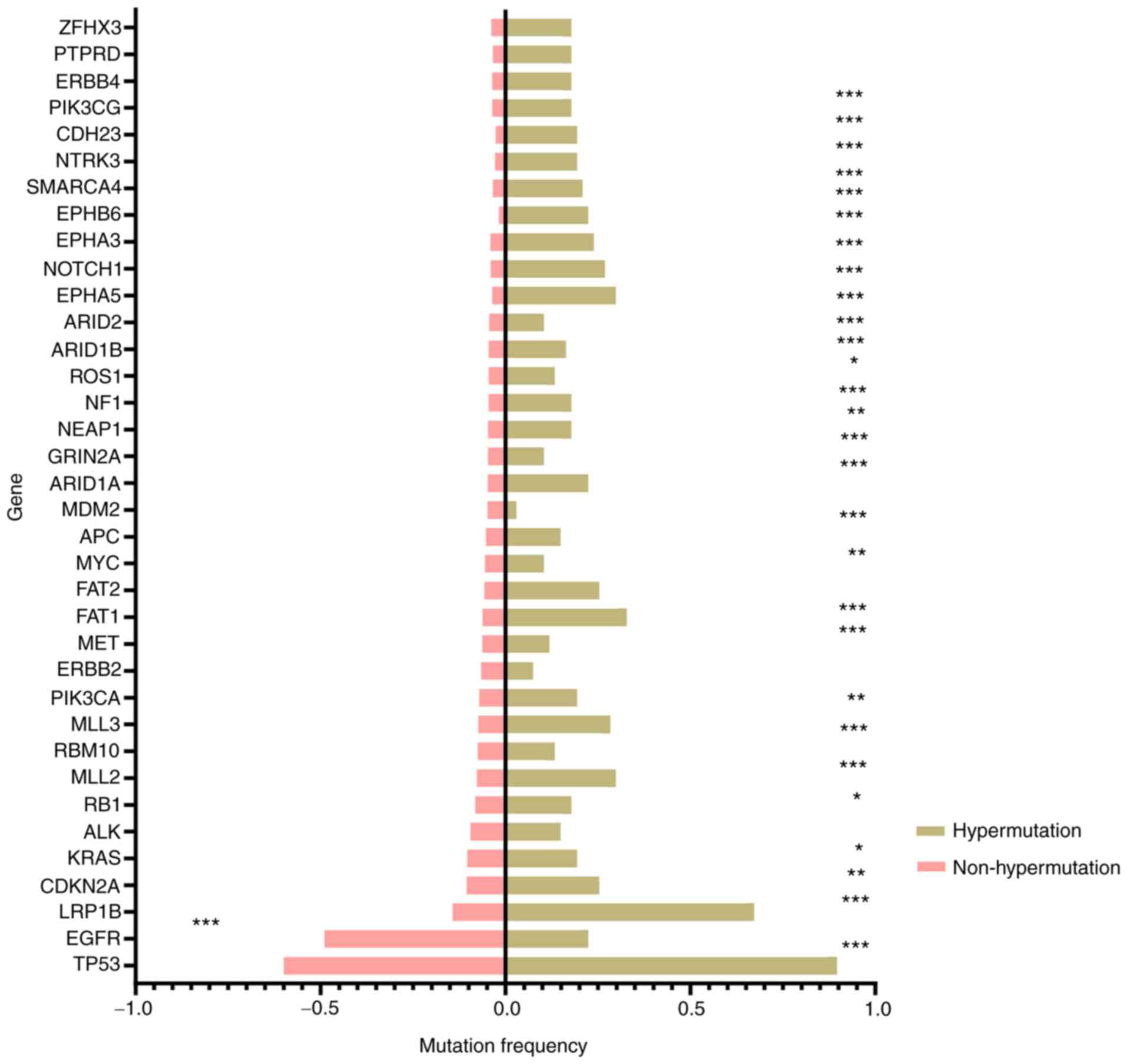
The somatic mutation profiles of hypermutant and
non-hypermutant samples are presented in Fig. 2 (top 25 genes for each group). As
shown in Fig. 2, the occurrence of
mutations in multiple genes was higher in the hypermutant compared
with in the non-hypermutant cohort. TP53 was the most frequently
mutated gene in both groups, with a mutation frequency of 89.6 and
59.9% in the hypermutant and non-hypermutant group, respectively
(P<0.001). Low-density lipoprotein receptor-related protein 1B
(LRP1B) exerted the most significant difference on mutation rate
between the hypermutant and non-hypermutant cohorts (67.2 vs.
14.3%; P<0.001). In addition, 51.1% of cases with LRP1B
mutations in the hypermutant cohort harbored >2 mutations, which
was significantly higher compared with in the non-hypermutant
cohort (21.8%; P=0.0002) (data not shown). Only EGFR mutations were
more frequently observed in the non-hypermutant cohort compared
with in the hypermutant cohort (48.9 vs. 22.4%; P<0.001).
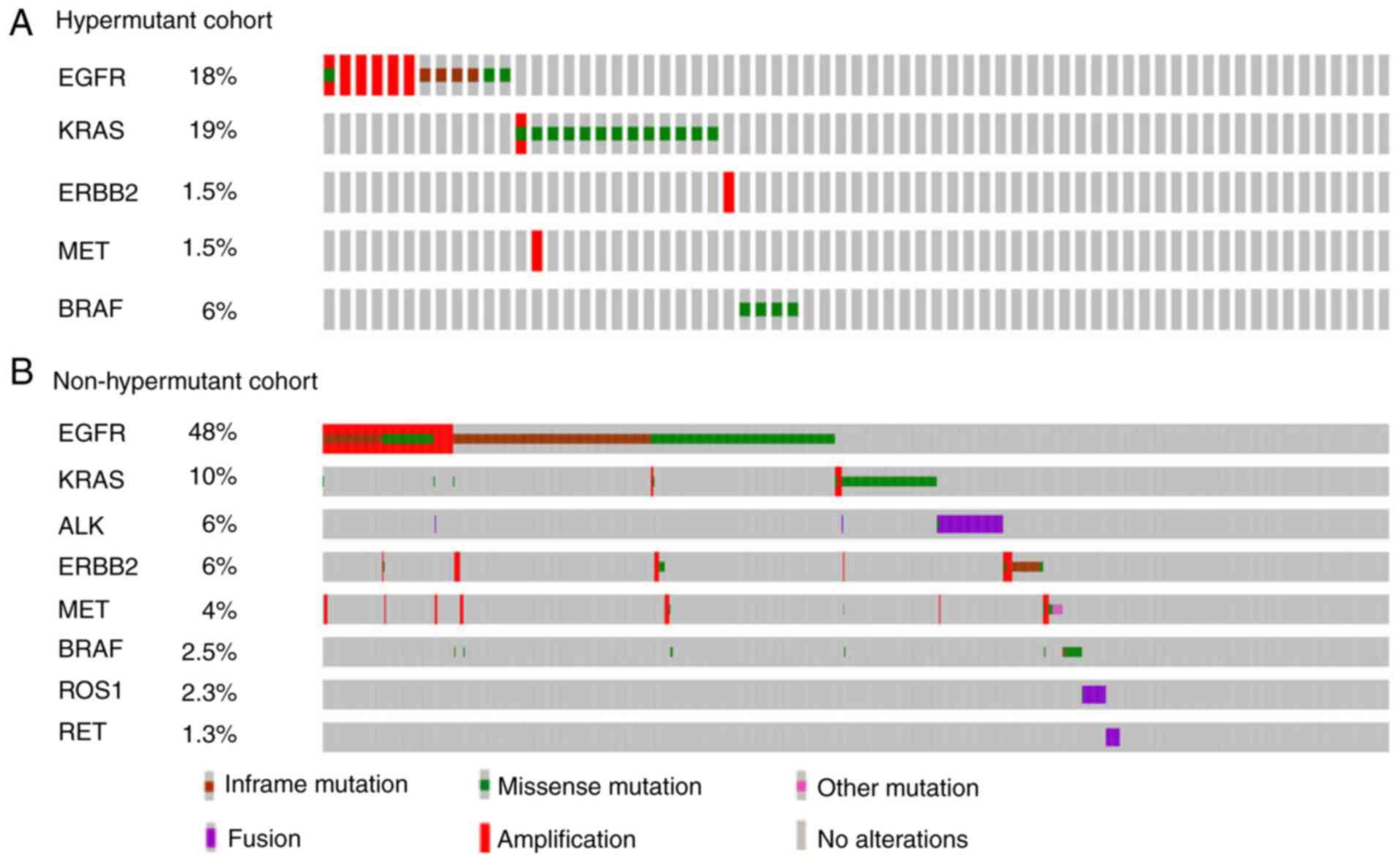
Subsequently, the mutational profiles of common
driver genes in NSCLC were compared (Fig. 3). In the hypermutant cohort (Fig. 3A), the most frequently mutated genes
were EGFR and KRAS, with a mutation rate of 18 and 19%,
respectively. EGFR sensitive mutations (exon 19 deletions,
Leu858Arg and other missense mutations that are sensitive to first-
and second-generation EGFR tyrosine kinase inhibitors) and
amplification were identified in only 7.5 and 9% of hypermutant
patients, respectively. BRAF non-V600E mutations were found in 4
cases in the hypermutant cohort. In the non-hypermutant cohort
(Fig. 3B), EGFR mutations were
detected in 48% of cases. Furthermore, EGFR sensitive mutations
were identified in 43.2% of non-hypermutant patients, and among
them, 45 cases harbored EGFR secondary resistance mutations. EGFR
amplification was found in 12.2% of non-hypermutant patients, with
76.3% of cases accompanied with EGFR sensitive mutations. Compared
with in the hypermutant cohort, fewer patients in the
non-hypermutant cohort harbored KRAS mutations (10%). ALK, ROS1 and
RET rearrangements were only observed in the non-hypermutant cohort
with a frequency of 6, 2.3 and 1.3%, respectively. Finally, BRAF
mutations were detected in 23 cases, including 11 V600E and 12
non-V600E mutations, representing 2.5% of all patients in the
non-hypermutant cohort.
Molecular features associated with
immunotherapy efficacy
The genetic factors associated with immunotherapy
efficacy in the hypermutant cohort were analyzed. The results
revealed that MSI-high was observed in 6 cases in the hypermutant
cohort (9.0%) and only 1 patient in the non-hypermutant cohort
(0.1%) (data not shown). Subsequently, the prevalence of DDR
variants (20,38,39)
(Table SVII) between the
hypermutant and non-hypermutant cohorts was compared. A total of
105 mutations were identified in DDR genes in 70.1% of patients in
the hypermutant cohort, while 321 mutations in DDR genes were
detected in 27.0% of patients in the non-hypermutant cohort (data
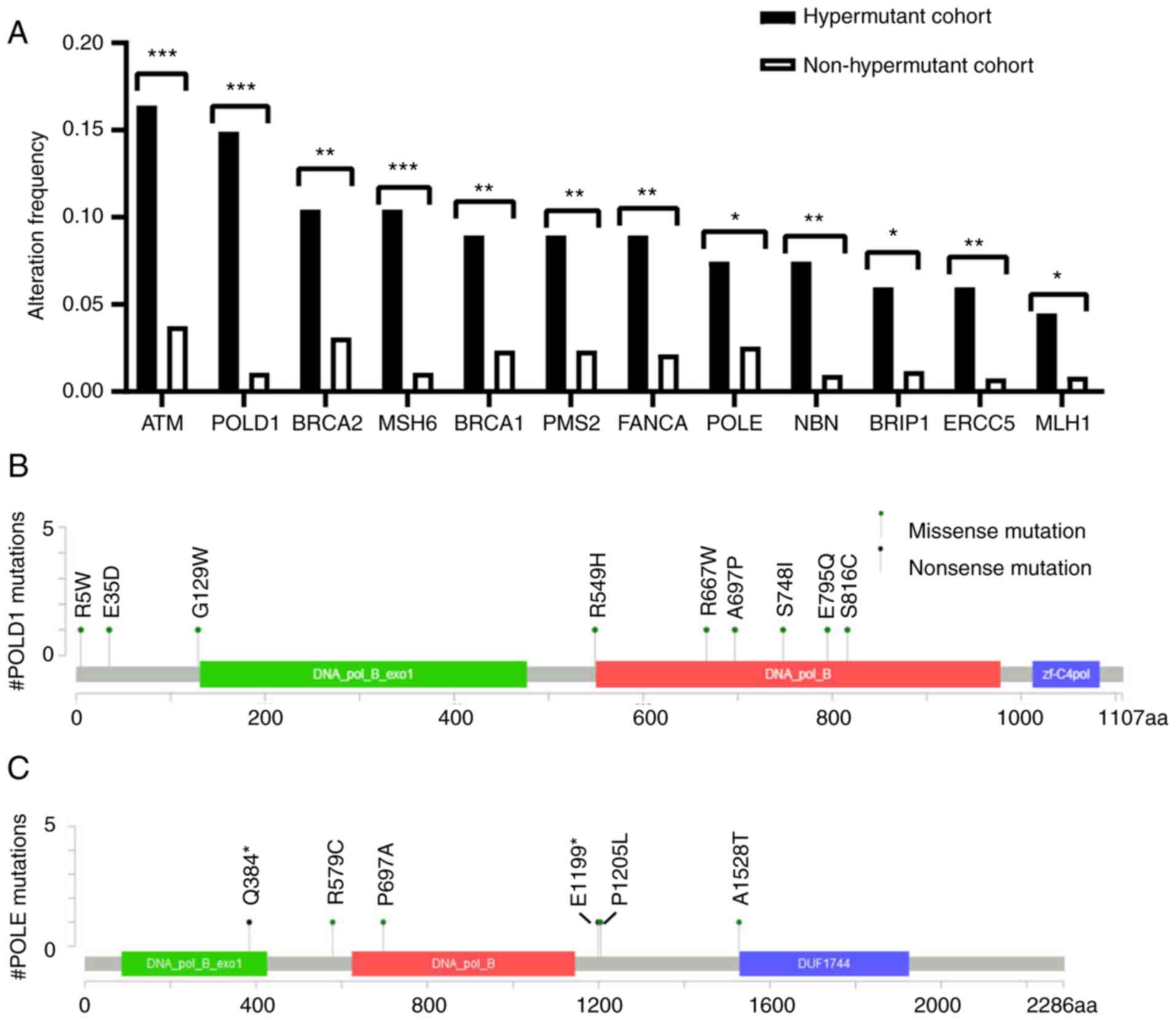
not shown). Mutations in multiple DDR genes were enriched in the
hypermutant cohort, including mutations in ATM, POLE/POLD1 and
BRCA1/2 genes (Fig. 4A). As shown in
Fig. 4B and C, 16 mutations
(including one copy number variant) were detected in POLE/POLD1
genes. According to the criteria for driver mutations proposed by
Campbell et al (40), no
known driver mutations were detected in POLE/POLD1 genes. In
addition, several mutations in POLD1 (E795Q and S816C) and POLE
(A1528T and P1205L) genes were considered as non-driver mutations,
according to the POLE/POLD1 variants and associated mutation burden
referred by Campbell et al (40). The function of other variants remains
to be characterized. TP53 and KRAS co-alterations were identified
in 16.4% of patients in the hypermutant cohort, and significantly
fewer in the non-hypermutant cohort (5.8%; P=0.002; data not
shown). Furthermore, several genetic alterations were negatively
associated with response to immunotherapy, including three PTEN
loss-of-function (LOF) mutations, two B2M LOF mutations, five EGFR
sensitive mutations, three STK11 LOF mutations, one KEAP1 LOF
mutation, two cases with 11q13 amplification (CCND1, FGF3, FGF4 and
FGF19) and one case with MDM2 amplification (data not shown).
Identification of DMGs and enrichment
analysis
To identify genes with significantly higher
alteration frequency in the hypermutant group compared with in the
non-hypermutant cohort, Fisher's exact test was performed. The DMGs
enriched in the hypermutant cohort are summarized in Table SVIII. Webgestalt was used for the
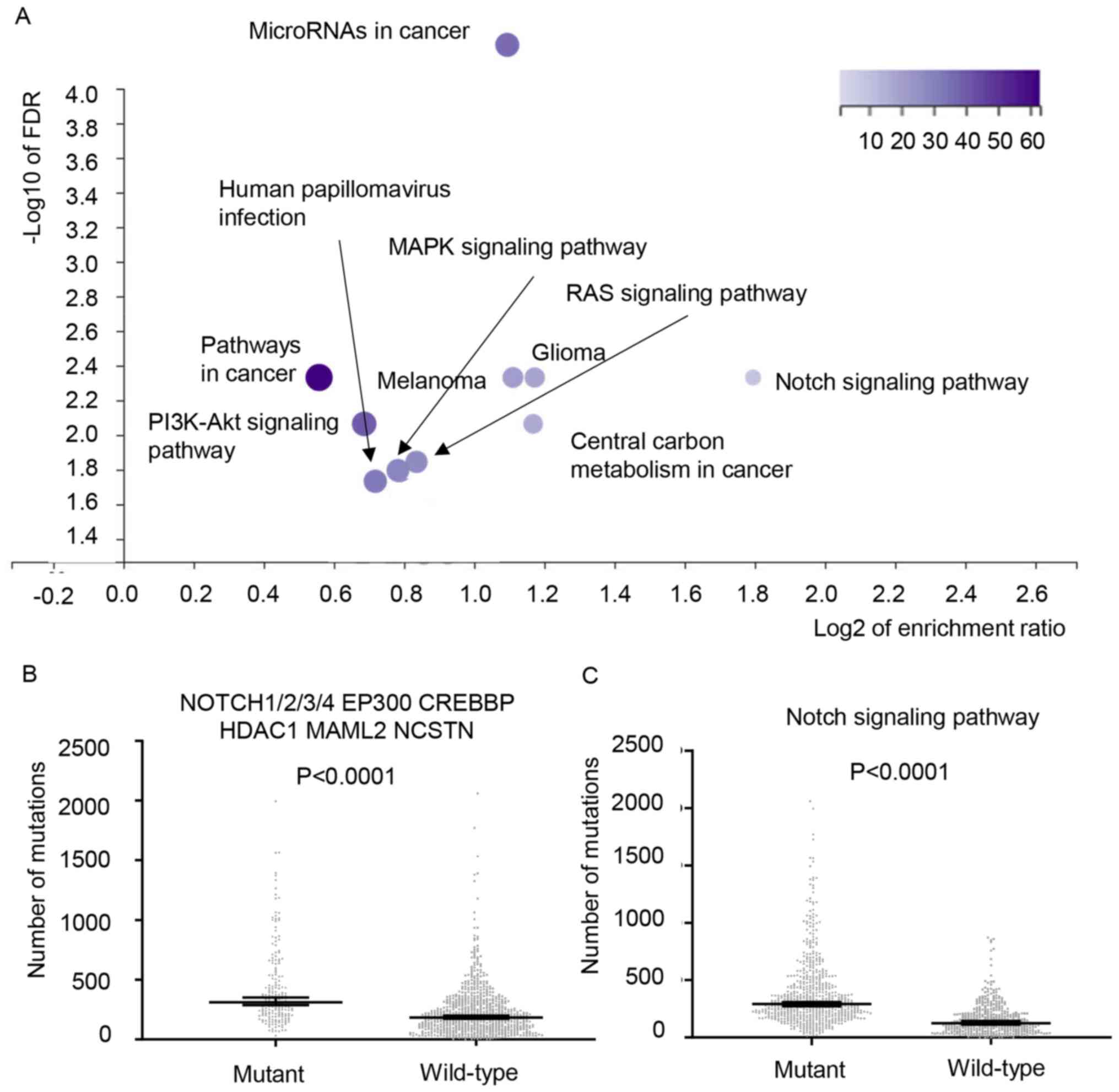
enrichment analysis. The top 10 pathways are shown in Fig. 5A, including the Notch signaling
pathway, MAPK signaling pathway and RAS signaling pathway.
Mutations in the Notch signaling pathway were enriched in the
hypermutant cohort, with an enrichment ratio of 3.49. This
signaling pathway included NOTCH1/2/3/4, CREB-binding protein
(CREBBP), E1A binding protein P300 (EP300), mastermind-like
transcriptional coactivator 2 (MAML2), nicastrin (NCSTN) and
histone deacetylase 1 (HDAC1); these genes were completely mutated
in 64.2% of cases in the hypermutant cohort (data not shown). To
validate the enrichment of mutations in the Notch signaling pathway
in the hypermutant cohort, lung adenocarcinoma and squamous cell
carcinoma data from TCGA database were analyzed (n=1,031). Patients
with mutations in these nine genes exhibited more non-synonymous
mutations in the coding regions compared with those without these
mutations (median, 310 vs. 183.5; P<0.0001; Fig. 5B). Similarly, when the validated
genes were expanded to all the genes involved in the Notch
signaling pathway (41) (ARRDC1,
CNTN6, CREBBP, EP300, HES1, HES2, HES3, HES4, HES5, HEY1, HEY2,
HEYL, KAT2B, KDM5A, NOTCH1, NOTCH2, NOTCH3, NOTCH4, NOV, NRARP,
PSEN2, LFNG, ITCH, NCSTN, SPEN, JAG1, APH1A, FBXW7, FHL1, THBS2,
HDAC2, MFAP2, CUL1, RFNG, NCOR1, NCOR2, MFAP5, HDAC1, NUMB, JAG2,
MAML3, MFNG, CIR1, CNTN1, MAML1, MAML2, NUMBL, PSEN1, PSENEN, RBPJ,
RBPJL, RBX1, SAP30, SKP1, SNW1, CTBP1, CTBP2, ADAM10, APH1B,
ADAM17, DLK1, DLL1, DLL3, DLL4, DNER, DTX1, DTX2, DTX3, DTX3L,
DTX4, EGFL7), a significant difference was also observed (median,
292 vs. 125; P<0.0001; Fig.
5B).
Discussion
It has been reported that hypermutation is
frequently found in melanoma, lung and bladder cancer (42–44). The
present retrospective cohort study comprehensively explored the
clinicopathological and molecular features of patients with
hypermutant lung cancer. The present study may provide important
insights into hypermutant lung cancer among the Chinese population.
To the best of our knowledge, the present study was the first to
comprehensively investigate the clinical and genetic
characteristics of hypermutant lung cancer in a Chinese
population.
First, the clinical characteristics between cohorts
were compared, and the results revealed that hypermutation was more
frequently observed in certain groups of patients, including males,
smokers and specific histological subtypes. A previous study
investigated the association between smoking and mutational burden.
The results demonstrated that the total number of point mutations
in the coding regions was higher in smokers (median, 209; range,
104–1,363) compared with non-smokers (median, 18; range, 10–22)
(43). Other studies also obtained
similar conclusions (45,46). Additionally, the present findings
were consistent with the aforementioned studies. In addition, the
strong correlation between smoking and sex observed in the present
study may be associated with smoking habits in the Chinese
population. In China, the smoking prevalence was 47.2% (range,
46.9–47.5%) for men and 2.7% (range, 2.6–2.8%) for women in 2013
(47). Therefore, the present study
hypothesized that the significantly higher rate of males in the
hypermutant cohort may result from the higher number of smokers in
this cohort. The current findings were consistent with a previous
study demonstrating higher TMB in squamous carcinoma and SCLC
compared with in adenocarcinoma (48).
Subsequently, the mutation spectra of both cohorts
were analyzed. LRP1B was more frequently mutated in the hypermutant
cohort. LRP1B encodes a member of the low-density lipoprotein
receptor family. These receptors serve a wide variety of roles in
normal cell function and development by interacting with multiple
ligands (49). Several studies have
demonstrated an association between LRP1B mutations and a high
level of TMB in patients with NSCLC and melanoma; these studies
suggested that in patients with LRP1B mutations, immune response
and cell cycle regulation circuits were among the top enriched
pathways (50,51). Although the mechanism underlying the
association between TMB and LRP1B is not entirely clear, the
current study supported the aforementioned findings. The
distribution of KRAS and EGFR mutations identified in the present
study was also consistent with previous studies, suggesting the
positive effect of KRAS mutations and negative effect of EGFR
mutations on immunotherapy response (21,52).
ALK, ROS1 and RET rearrangements were only found in the
non-hypermutant cohort, which may suggest a negative association
with hypermutation, thereby affecting the response to immunotherapy
(53).
The genetic factors affecting immunotherapy response
were also analyzed. MSI-high was reported in 0.2% of patients with
non-squamous NSCLC (54). In the
present study, the prevalence of MSI-high was extremely high in the
hypermutant cohort (9%), indicating a potential important role of
MSI in hypermutation in lung cancer. The enrichment of DDR gene
mutations in the hypermutant cohort suggested that mutations in DDR
genes may serve as biomarkers for predicting TMB and patient
response to immunotherapy. A previous study has confirmed elevated
TMB and improved efficacy of immunotherapy in patients with
pathogenic DDR alterations (20).
Co-mutations of KRAS and TP53 were more frequently identified in
the hypermutation cohort, supporting the improved clinical outcomes
of patients with KRAS and TP53 co-mutations during the period of
immunotherapy (21). In addition,
several negative biomarkers, such as PTEN mutations and MDM2/4
amplification, in the hypermutant cohort were identified,
suggesting hyper-progressive disease or disease resistant to
immunotherapy, which should raise concern.
DMGs enriched in the hypermutant cohort were
identified in the present study, and an enrichment analysis was
performed. The results suggested that mutations in the Notch
signaling pathway were associated with high TMB, which was
confirmed using molecular profiles of lung cancer in TCGA database.
The Notch signaling pathway activates cell proliferation and
antagonizes apoptosis, as well as cross-talks with several
transcriptional factors to promote epithelial-mesenchymal
transition in lung cancer, thus leading to enhanced motility,
invasion and metastasis of cancer cells (55). Recently, NOTCH1 has been reported to
contribute to an immune-suppressive tumor microenvironment in
melanoma (56). Targeting NOTCH1 may
therefore affect cell proliferation and survival, and provide an
immune-responsive tumor microenvironment, thus improving the
efficacy of immunotherapy (56).
Another study uncovered a marked association between mutations in
NOTCH1/2/3 and improved outcomes in EGFR- and ALK-wild-type
patients with NSCLC treated with immune checkpoint inhibitors
(57). In addition, deleterious
NOTCH mutations exhibited an improved effect compared with
non-deleterious mutations (57).
However, the underlying mechanism remains unknown. Previous studies
found that tumors with Notch family gene (NOTCH1/2/3/4) mutations
exerted higher TMBs in multiple types of cancer, including
hepatocellular carcinoma, esophageal carcinoma, breast cancer,
SCLC, head and neck cancer and cutaneous carcinoma (58,59),
which may explain the predictive value of NOTCH mutations to
immunotherapy response. In the present study, the strong
association between NOTCH gene mutations and high TMB indicated a
potential strategy for immunotherapy in patients with lung cancer
with mutations in the Notch signaling pathway. However, the
mutation types in genes involved in the Notch signaling pathway and
the specific genes exhibiting strong predictive value to
immunotherapy response should be further investigated.
The FDA has approved FoundationOne®CDx as
the first companion diagnostic to identify patients with solid
tumors that are TMB-high (≥10 muts/Mb) and suitable for treatment
with pembrolizumab (60). A previous
study conducted by Foundation Medicine described the distribution
of TMB across a diverse cohort of 100,000 cases of cancer, with TMB
>20 muts/Mb designated as high TMB (61). The percentages of cases with TMB
>20 muts/Mb were 17, 12.3, 11.3 and 9% for NSCLC (not otherwise
specified) (n=2,636), lung adenocarcinoma (n=11,855), lung squamous
cell carcinoma (n=2,102) and lung small cell undifferentiated
carcinoma (n=913), respectively (61). However, in the present study,
hypermutant patients (TMB ≥17.24 muts/Mb) constituted 6.7% of the
whole population. TMB cut-offs may differ depending on sample type,
tumor type, patient subgroup, therapy investigated and assay used.
More specifically, the capture region, number of genes, sequencing
depth and TMB calculation method of a panel may affect the TMB
threshold. Despite the difference between TMB cut-offs and
sequencing details, the findings of the present study may be
valuable thanks to the scientific method for TMB cut-off
determination and controlled study design.
However, there were a few limitations due to the
retrospective nature of the present study. Information on whether
the patients received immunotherapy after genetic testing and
corresponding treatment response was not available. The efficacy of
immune checkpoint inhibitors in the hypermutant cohort was of great
value and could be further researched. In addition, ~78% of
patients in the current study were diagnosed with adenocarcinoma.
It may be interesting to explore TMB-associated molecular
characteristics in adenocarcinoma only in a Chinese population.
In conclusion, the present cohort study suggested
that hypermutant lung cancer exhibited distinctive genetic
profiles, including high occurrence of MSI-high, high frequency of
mutations in DDR genes and genes involved in the Notch signaling
pathway, which may be associated with high levels of TMB. In
addition, patients with hypermutant lung cancer may be more likely
to have a history of tobacco use and exhibit the histological
subtypes of squamous carcinoma and SCLC. These characteristics may
be used as complementary indicators for screening patients
sensitive to immunotherapy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Government-funded
clinical excellence training programme.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ, YW and MZ contributed to conception and design
of the study. MZ contributed to the provision of study materials or
patients. HZ and MY confirm the authenticity of all the raw data.
QJ, HC, RC, XL, JX and MY contributed to acquisition of data. HL,
JW, GZ, ET and LZ performed data analysis and interpretation. All
authors wrote the manuscript and read and approved the final
manuscript.
Ethics approval and consent to
participate
All procedures were in accordance with the 1964
Declaration of Helsinki and its later amendments. The study was
performed under a protocol approved by the Institutional Review
Board of Geneplus-Beijing. Written informed consent was provided by
all participants included in the study.
Patient consent for publication
Not applicable.
Competing interests
MY and RC are employees of Geneplus-Beijing. All
other authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
SCLC
|
small cell lung cancer
|
|
ORR
|
objective response rate
|
|
TMB
|
tumor mutation burden
|
|
NGS
|
next-generation sequencing
|
|
MSI
|
microsatellite instability
|
|
DDR
|
DNA damage response
|
|
FFPE
|
formalin-fixed, paraffin-embedded
|
|
SNV
|
single nucleotide variant
|
|
InDel
|
insertions and deletions
|
|
DMG
|
differentially mutated gene
|
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chung HC, Piha-Paul SA, Lopez-Martin J,
Schellens JHM, Kao S, Miller WH Jr, Delord JP, Gao B, Planchard D,
Gottfried M, et al: Pembrolizumab after two or more lines of prior
therapy in patients with advanced small-cell lung cancer (SCLC):
Results from the KEYNOTE-028 and KEYNOTE-158 studies. Cancer Res.
79:CT0732019.
|
|
3
|
Horn L, Mansfield AS, Szczęsna A, Havel L,
Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio
M, et al: First-line atezolizumab plus chemotherapy in
extensive-stage small-cell lung cancer. N Engl J Med.
379:2220–2229. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reck M, Rodriguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Antonia SJ, López-Martin JA, Bendell J,
Ott PA, Taylor M, Eder JP, Jäger D, Pietanza MC, Le DT, de Braud F,
et al: Nivolumab alone and nivolumab plus ipilimumab in recurrent
small-cell lung cancer (CheckMate 032): A multicentre, open-label,
phase 1/2 trial. Lancet Oncol. 17:883–895. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rittmeyer A, Barlesi F, Waterkamp D, Park
K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols
MC, et al: Atezolizumab versus docetaxel in patients with
previously treated non-small-cell lung cancer (OAK): A phase 3,
open-label, multicentre randomised controlled trial. Lancet.
389:255–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hellmann MD, Callahan MK, Awad MM, Calvo
E, Ascierto PA, Atmaca A, Rizvi NA, Hirsch FR, Selvaggi G,
Szustakowski JD, et al: Tumor mutational burden and efficacy of
nivolumab monotherapy and in combination with ipilimumab in
small-cell lung cancer. Cancer Cell. 35:3292019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hellmann MD, Ciuleanu TE, Pluzanski A, Lee
JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers
S, Salman P, et al: Nivolumab plus ipilimumab in lung cancer with a
high tumor mutational burden. N Engl J Med. 378:2093–2104. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang F, Zhao Q, Wang YN, Jin Y, He MM, Liu
ZX and Xu RH: Evaluation of POLE and POLD1 mutations as biomarkers
for immunotherapy outcomes across multiple cancer types. JAMA
Oncol. 5:1504–1506. 2019. View Article : Google Scholar
|
|
14
|
Song Z, Cheng G, Xu C, Wang W, Shao Y and
Zhang Y: Clinicopathological characteristics of POLE mutation in
patients with non-small-cell lung cancer. Lung Cancer. 118:57–61.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stadler ZK, Battaglin F, Middha S,
Hechtman JF, Tran C, Cercek A, Yaeger R, Segal NH, Varghese AM,
Reidy-Lagunes DL, et al: Reliable detection of mismatch repair
deficiency in colorectal cancers using mutational load in
next-generation sequencing panels. J Clin Oncol. 34:2141–2147.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marcus L, Lemery SJ, Keegan P and Pazdur
R: FDA approval summary: Pembrolizumab for the treatment of
microsatellite Instability-High solid tumors. Clin Cancer Res.
25:3753–3758. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Narayan P, Wahby S, Gao JJ,
Amiri-Kordestani L, Ibrahim A, Bloomquist E, Tang S, Xu Y, Liu J,
Fu W, et al: FDA approval summary: Atezolizumab plus paclitaxel
protein-bound for the treatment of patients with advanced or
metastatic TNBC whose tumors express PD-L1. Clin Cancer Res.
26:2284–2289. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fashoyin-Aje L, Donoghue M, Chen H, He K,
Veeraraghavan J, Goldberg KB, Keegan P, McKee AE and Pazdur R: FDA
approval summary: Pembrolizumab for recurrent locally advanced or
metastatic gastric or gastroesophageal junction adenocarcinoma
expressing PD-L1. Oncologist. 24:103–109. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sul J, Blumenthal GM, Jiang X, He K,
Keegan P and Pazdur R: FDA approval summary: Pembrolizumab for the
treatment of patients with metastatic non-small cell lung cancer
whose tumors express programmed Death-Ligand 1. Oncologist.
21:643–650. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ricciuti B, Cheng ML, Recondo G, Nishino
M, Umeton R, Sholl LM and Awad MM: DNA damage response gene
alterations are associated with high tumor mutational burden and
clinical benefit from programmed death 1 axis inhibition in
non-small cell lung cancer. J Clin Oncol. 37 (Suppl 15):S90772019.
View Article : Google Scholar
|
|
21
|
Dong ZY, Zhong WZ, Zhang XC, Su J, Xie Z,
Liu SY, Tu HY, Chen HJ, Sun YL, Zhou Q, et al: Potential predictive
value of TP53 and KRAS mutation status for response to PD-1
blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res.
23:3012–3024. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gainor JF, Shaw AT, Sequist LV, Fu X,
Azzoli CG, Piotrowska Z, Huynh TG, Zhao L, Fulton L, Schultz KR, et
al: EGFR Mutations and ALK rearrangements are associated with low
response rates to PD-1 pathway blockade in non-small cell lung
cancer: A retrospective analysis. Clin Cancer Res. 22:4585–4593.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Peng W, Chen JQ, Liu C, Malu S, Creasy C,
Tetzlaff MT, Xu C, McKenzie JA, Zhang C, Liang X, et al: Loss of
PTEN promotes resistance to T cell-mediated immunotherapy. Cancer
Discov. 6:202–216. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sade-Feldman M, Jiao YJ, Chen JH, Rooney
MS, Barzily-Rokni M, Eliane JP, Bjorgaard SL, Hammond MR, Vitzthum
H, Blackmon SM, et al: Resistance to checkpoint blockade therapy
through inactivation of antigen presentation. Nat Commun.
8:11362017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arbour K, Shen R, Plodkowski A, Rizvi H,
Ni A, Long N, Halpenny D, Sanchez-Vega F, Rudin C, Riely G and
Hellmann M: MA19. 09 concurrent mutations in STK11 and KEAP1 is
associated with resistance to PD-(L) 1 blockade in patients with
NSCLC despite high TMB. J Thorac Oncol. 13 (Suppl 10):S4242018.
View Article : Google Scholar
|
|
26
|
Kato S, Goodman A, Walavalkar V,
Barkauskas DA, Sharabi A and Kurzrock R: Hyperprogressors after
immunotherapy: Analysis of genomic alterations associated with
accelerated growth rate. Clin Cancer Res. 23:4242–4250. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang F, Ren C, Zhao Q, Shen L, Dai G, Yuan
X, Chen Y, Yang S, Shi J, Hu X, et al: Association of frequent
amplification of chromosome 11q13 in esophageal squamous cell
cancer with clinical benefit to immune check point blockade. J Clin
Oncol. 37 (Suppl 15):S40362019. View Article : Google Scholar
|
|
28
|
Sun S, Liu Y, Eisfeld AK, Zhen F, Jin S,
Gao W, Yu T, Chen L, Wang W, Chen W, et al: Identification of
germline mismatch repair gene mutations in lung cancer patients
with paired tumor-normal next generation sequencing: A
retrospective study. Front Oncol. 9:5502019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen Y, Chen G, Li J, Huang YY, Li Y, Lin
J, Chen LZ, Lu JP, Wang YQ, Wang CX, et al: Association of tumor
protein p53 and ataxia-telangiectasia mutated comutation with
response to immune checkpoint inhibitors and mortality in patients
with non-small cell lung cancer. JAMA Netw Open. 2:e19118952019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Benson AB, Venook AP, Al-Hawary MM,
Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D,
Engstrom PF, et al: NCCN guidelines insights: Colon cancer, version
2.2018. J Natl Compr Canc Netw. 16:359–369. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ettinger DS, Wood DE, Aggarwal C, Aisner
DL, Akerley W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac
LR, et al: NCCN guidelines insights: Non-small cell lung cancer,
version 1.2020. J Natl Compr Canc Netw. 17:1464–1472. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Haddad RI, Nasr C, Bischoff L, Busaidy NL,
Byrd D, Callender G, Dickson P, Duh QY, Ehya H, Goldner W, et al:
NCCN guidelines insights: Thyroid carcinoma, version 2.2018. J Natl
Compr Canc Netw. 16:1429–1440. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Armstrong DK, Alvarez RD, Bakkum-Gamez JN,
Barroilhet L, Behbakht K, Berchuck A, Berek JS, Chen LM, Cristea M,
DeRosa M, et al: NCCN guidelines insights: Ovarian cancer, version
1.2019. J Natl Compr Canc Netw. 17:896–909. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Goetz MP, Gradishar WJ, Anderson BO,
Abraham J, Aft R, Allison KH, Blair SL, Burstein HJ, Dang C, Elias
AD, et al: NCCN guidelines insights: Breast cancer, version 3.2018.
J Natl Compr Canc Netw. 17:118–126. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li J, Lupat R, Amarasinghe KC, Thompson
ER, Doyle MA, Ryland GL, Tothill RW, Halgamuge SK, Campbell IG and
Gorringe KL: CONTRA: Copy number analysis for targeted
resequencing. Bioinformatics. 28:1307–1313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zehir A, Benayed R, Shah RH, Syed A,
Middha S, Kim HR, Srinivasan P, Gao J, Chakravarty D, Devlin SM, et
al: Mutational landscape of metastatic cancer revealed from
prospective clinical sequencing of 10,000 patients. Nat Med.
23:703–713. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Niu B, Ye K, Zhang Q, Lu C, Xie M,
McLellan MD, Wendl MC and Ding L: MSIsensor: Microsatellite
instability detection using paired tumor-normal sequence data.
Bioinformatics. 30:1015–1016. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Teo MY, Seier K, Ostrovnaya I, Regazzi AM,
Kania BE, Moran MM, Cipolla CK, Bluth MJ, Chaim J, Al-Ahmadie H, et
al: Alterations in DNA damage response and repair genes as
potential marker of clinical benefit from PD-1/PD-L1 blockade in
advanced urothelial cancers. J Clin Oncol. 36:1685–1694. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mehnert JM, Panda A, Zhong H, Hirshfield
K, Damare S, Lane K, Sokol L, Stein MN, Rodriguez-Rodriquez L,
Kaufman HL, et al: Immune activation and response to pembrolizumab
in POLE-mutant endometrial cancer. J Clin Invest. 126:2334–2340.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Campbell BB, Light N, Fabrizio D, Zatzman
M, Fuligni F, de Borja R, Davidson S, Edwards M, Elvin JA, Hodel
KP, et al: Comprehensive analysis of hypermutation in human cancer.
Cell. 171:1042–1056.e10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sanchez-Vega F, Mina M, Armenia J, Chatila
WK, Luna A, La KC, Dimitriadoy S, Liu DL, Kantheti HS, Saghafinia
S, et al: Oncogenic signaling pathways in the cancer genome atlas.
Cell. 173:321–337.e10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cancer Genome Atlas Network, . Genomic
classification of cutaneous melanoma. Cell. 161:1681–1696. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Govindan R, Ding L, Griffith M,
Subramanian J, Dees ND, Kanchi KL, Maher CA, Fulton R, Fulton L,
Wallis J, et al: Genomic landscape of non-small cell lung cancer in
smokers and never-smokers. Cell. 150:1121–1134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cancer Genome Atlas Research Network, .
Comprehensive molecular characterization of urothelial bladder
carcinoma. Nature. 507:315–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ding L, Getz G, Wheeler DA, Mardis ER,
McLellan MD, Cibulskis K, Sougnez C, Greulich H, Muzny DM, Morgan
MB, et al: Somatic mutations affect key pathways in lung
adenocarcinoma. Nature. 455:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lee W, Jiang Z, Liu J, Haverty PM, Guan Y,
Stinson J, Yue P, Zhang Y, Pant KP, Bhatt D, et al: The mutation
spectrum revealed by paired genome sequences from a lung cancer
patient. Nature. 465:473–477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang M, Luo X, Xu S, Liu W, Ding F, Zhang
X, Wang L, Liu J, Hu J and Wang W: Trends in smoking prevalence and
implication for chronic diseases in China: Serial national
cross-sectional surveys from 2003 to 2013. Lancet Respir Med.
7:35–45. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yarchoan M, Hopkins A and Jaffee EM: Tumor
mutational burden and response rate to PD-1 inhibition. N Engl J
Med. 377:2500–2501. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu CX, Li Y, Obermoeller-McCormick LM,
Schwartz AL and Bu G: The putative tumor suppressor LRP1B, a novel
member of the low density lipoprotein (LDL) receptor family,
exhibits both overlapping and distinct properties with the LDL
receptor-related protein. J Biol Chem. 276:28889–28896. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen H, Chong W, Wu Q, Yao Y, Mao M and
Wang X: Association of LRP1B mutation with tumor mutation burden
and outcomes in melanoma and non-small cell lung cancer patients
treated with immune check-point blockades. Front Immunol.
10:11132019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lan S, Li H, Liu Y, Ma L, Liu X, Liu Y,
Yan S and Cheng Y: Somatic mutation of LRP1B is associated with
tumor mutational burden in patients with lung cancer. Lung Cancer.
132:154–156. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Rizvi H, Sanchez-Vega F, La K, Chatila W,
Jonsson P, Halpenny D, Plodkowski A, Long N, Sauter JL, Rekhtman N,
et al: Molecular determinants of response to anti-programmed cell
death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in
patients with non-small-cell lung cancer profiled with targeted
next-generation sequencing. J Clin Oncol. 36:633–641. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mazieres J, Drilon A, Lusque A, Mhanna L,
Cortot AB, Mezquita L, Thai AA, Mascaux C, Couraud S, Veillon R, et
al: Immune checkpoint inhibitors for patients with advanced lung
cancer and oncogenic driver alterations: Results from the
IMMUNOTARGET registry. Ann Oncol. 30:1321–1328. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mahadevan N, Shivadasani P, Nowak J, Awad
M and Sholl L: MA11. 10 Identification of mismatch repair deficient
lung adenocarcinomas using targeted next-generation sequencing. J
Thorac Oncol. 13 (Suppl 10):S3952018. View Article : Google Scholar
|
|
55
|
Yuan X, Wu H, Han N, Xu H, Chu Q, Yu S,
Chen Y and Wu K: Notch signaling and EMT in non-small cell lung
cancer: Biological significance and therapeutic application. J
Hematol Oncol. 7:872014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Qiu H, Zmina PM, Huang AY, Askew D and
Bedogni B: Inhibiting Notch1 enhances immunotherapy efficacy in
melanoma by preventing Notch1 dependent immune suppressive
properties. Cancer Lett. 434:144–151. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang K, Hong X, Song Z, Xu Y, Li C, Wang
G, Zhang Y, Zhao X, Zhao Z, Zhao J, et al: Identification of
deleterious mutation as novel predictor to efficacious
immunotherapy in NSCLC. Clin Cancer Res. 26:3649–3661. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jiang D, Niu Z, Zhang J, Wang Y, Shang L,
Li B, Guo J, Wang B, Zhao LQ, Wang W, et al: Notch family gene
mutations associate with high tumor mutational burden in diverse
cancers. J ClinOncol. 37 (Suppl 15):e146162019. View Article : Google Scholar
|
|
59
|
Severson EA, Ramkissoon S, Daniel S,
Vergilio JA, Gay LM, Elvin JA, Suh J, Frampton GM, Ali SM, Miller
VA and Ross JS: Association of tumor mutational burden in cutaneous
squamous cell carcinoma with genomic alterations in Notch family
receptors. J Clin Oncol. 35 (Suppl 15):e130312017. View Article : Google Scholar
|
|
60
|
Subbiah V, Solit DB, Chan TA and Kurzrock
R: The FDA approval of pembrolizumab for adult and pediatric
patients with tumor mutational burden (TMB) ≥10: A decision
centered on empowering patients and their physicians. Ann Oncol.
31:1115–1118. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chalmers ZR, Connelly CF, Fabrizio D, Gay
L, Ali SM, Ennis R, Schrock A, Campbell B, Shlien A, Chmielecki J,
et al: Analysis of 100,000 human cancer genomes reveals the
landscape of tumor mutational burden. Genome Med. 9:342017.
View Article : Google Scholar : PubMed/NCBI
|