Introduction
Bladder cancer has been categorized as the ninth
most commonly diagnosed cancer worldwide in 2016 and ranked as the
fourth in the USA in 2018 (1).
Unlike other malignancies, the morbidity and mortality of bladder
cancer remained stable from 1975 to 2014 despite oncology advances
in novel medicine treatment or diagnosis over these years, even
with small increases in morbidity and mortality rates during a
specific time period (2). The 5-year
survival rate of patients with bladder cancer was 73% between 1994
and 1998 and 76% between 2009 and 2013 worldwide (3). These data indicate that current
treatment approaches targeting bladder cancer have not
significantly improved when it comes to prognosis. Hence, novel
therapeutic procedures are urgently needed.
Striatin-4 (STRN4), also known as Zinedin, is a
scaffolding protein of the mammalian STRN family of proteins that
do not exert any enzymatic functions but serve as regulatory
cascade subunits for protein phosphatase 2A (PP2A) (4). The STRN family consists of three highly
homologous proteins: STRN, S/G2 Nuclear autoantigen
(SG2NA) and STRN4. These three proteins manifest in a conservative
manner due to their presence both in fungi and higher eukaryotes
(5). Numerous studies have reported
that the STRN proteins are expressed in various organs such as the
central nervous system (6), lung,
kidney and testis (7). However, the
exact expression pattern differs, which indicates that the STRN
family of proteins possess abundant but differential functions at
the organ level or cellular level (8). For example, STRN is found to be
expressed both in the central and peripheral nervous system, but
mainly in the striatum and motor neurons. In addition, STRN is
involved in the control of motor neurons and dendrite growth. SG2NA
is highly expressed in the cerebellum and cortex, but it is most
highly expressed during the S and G2 phases, which
indicates its extensive involvement in cell cycle control (4,9). Similar
to its homologous proteins, STRN4 contains four conserved
functional domains that allow STRN4 to interact with numerous
signaling pathways, such as the estrogen receptor signaling
(STRN4-ERα) and RhoA pathways (10).
These complex structural domains define its extensive involvement
in biological and pathological processes. Previous reports have
demonstrated that STRN4 participates in cell cycle regulation,
Golgi assembly, apoptosis, neuron development and tumorigenesis,
especially concerning its role in vascular formation (8,11,12).
Abnormal STRN4 expression can promote cell cycle
progression and assist cancer cells in gaining invasive properties
by activating downstream transcription factors, including
misshapen-like kinase 1, RhoA, TRAF2 and NCK-interacting protein
kinase (belonging to germinal center kinases) and platelet-derived
growth factor receptor-α (PDGFRA) (13). Reznickova et al (13) have revealed that STRN4 functions as a
fusion protein during the activation process of PDGFRA and
accelerates the development of chronic eosinophilic leukemia
(11,13). In another study, STRN4 was reported
to promote cell proliferation and inhibit apoptosis in the prostate
under the regulation of leukemia/lymphoma-related factor (14). However, to the best of our knowledge,
there are no studies investigating the expression pattern of STRN4
in bladder cancer and the association between and STRN4 expression
and prognosis.
The present study investigated the prognostic role
of STRN4 in bladder cancer and aimed to determine the association
between STRN4 and recurrence.
Materials and methods
Fresh samples and tumor specimens of
bladder cancer
Up to 28 fresh excised bladder transitional cancer
tissues along with 28 normal bladder tissues (3–3.5 cm away from
the tumor) were collected after surgical removal at the China-Japan
Union Hospital of Jilin University (Changchun, China) between
December 2015 and December 2017. None of these patients had
received anticancer therapy (including chemotherapy and targeted
medicine) before surgery. The samples were immediately stored in
liquid nitrogen (−196°C) for further experiments. A total of 112
bladder transitional cancer specimens with different
Tumor-Node-Metastasis (TNM) stages (15) were obtained from the Department of
Tissue Bank, China-Japan Union Hospital of Jilin University.
Informed consent was obtained from all patients and the study was
approved by The Ethics Committee of China-Japan Union Hospital of
Jilin University (Changchun, China).
RNA harvest and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from excised bladder cancer
and normal tissues using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) and reverse transcribed into cDNA
with SMART® MMLV Reverse Transcriptase (cat. no. 639524;
Takara Bio, Inc.). The mixture was heated at 70°C for 3 min, cooled
down and subsequently centrifuged at 17,344 × g for 1 min at 4°C,
incubated at 50°C for 60 min, and a final extension at 70°C for 15
min. RT-qPCR was performed to detect STRN4 expression using the
2−∆∆Cq method (16) with
the PowerUp™ SYBR™ Green Master Mix (cat. no. A25742; Applied
Biosystems; Thermo Fisher Scientific, Inc.) according to the
manufacturer's standard protocol. Primers were designed using the
GPP Web Portal (https://portals.broadinstitute.org/gpp/public) and
screened using the Blast function from both Primer Premier 6
(version 6.00; Premier Biosoft) and National Center for
Biotechnology Information. STRN4 primers were purchased from
Shanghai GenePharma Co., Ltd. The primer sequences were as follows:
STRN4 Forward, 5′-GATCTCACCGTCACCAACGA-3′ and reverse,
5′-GGAACGAATGCCGTCGTAGT-3′. GAPDH was chosen as an internal
control. qPCR was performed on the QuantStudio™ 5 Real-Time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Initial denaturation at 95°C for 30 sec followed by 40 cycles of
three-step PCR amplification comprising denaturation for 5 sec at
95°C, annealing for 34 sec at 60°C, and extension for 10 sec at
72°C.
Immunohistochemistry (IHC)
A total of 112 previously acquired formalin-fixed,
paraffin-embedded bladder transitional cancer specimens along with
28 fresh samples and 28 matched adjacent non-cancerous tissues
(ANCT) were cut into 4-µm thick slices and analyzed as previously
described (17). Two pathologists
who were blinded to the experimental data were invited to score
each specimen's immunostaining degree using a light microscopy
(Olympus Corporation; magnification, ×40). The images were captured
using CellSens (version 2.3; Olympus Corporation). The
immunostaining intensities were set as follows: 0 (No stain=blank),
1 (weak stain=light yellow), 2 (moderate stain=light brown) and 3
(strong stain=brown). In addition, the positive immunostaining
proportions were categorized into four tiers: 0 (<10%), 1
(11–25%), 2 (26–50%) and 3 (>51%) (18). The intensity and proportion values
were multiplied as the composite index. The marginal index
(threshold) was set at 3. Specimens with composite index scores ≤3
were considered to have a low expression of STRN4. The STRN4
primary antibody (cat. no. ab230858; Abcam) titer was set at 1:100.
Mouse IgG1 κ Isotype Control P3.6.2.8.1 (cat. no. 14-4714-82;
eBioscience; Thermo Fisher Scientific, Inc.) was diluted at 1:100.
IHC was performed under standard procedures according to the Abcam
manufacturer's protocol.
Statistical analysis
Data are presented as the mean ± SD (unless
otherwise shown) with each experiment performed in triplicate.
Statistical analysis between two groups was evaluated using
two-tailed unpaired Student's t-tests and Fisher's exact tests
using GraphPad Prism 7 (GraphPad Software, Inc.). The association
between STRN4 expression and clinicopathological parameters of
bladder transitional carcinoma was analyzed using the χ2
test. The survival data of up to 165 patients were collected from
the epidemiology and statistics database (jointly built with First
hospital of Jilin University) from the School of Public Health,
Jilin University. Survival curves were established using the
Kaplan-Meier method and compared using log-rank tests with SPSS
26.0 (IBM Corp.) and multivariate analysis was conducted using a
Cox regression model. P<0.05 was considered to indicate a
statistically significant difference.
Results
STRN4 is overexpressed in bladder
transitional carcinoma
In order to determine STRN4 expression in bladder
transitional cancer, IHC was performed on 28 pairs of fresh bladder
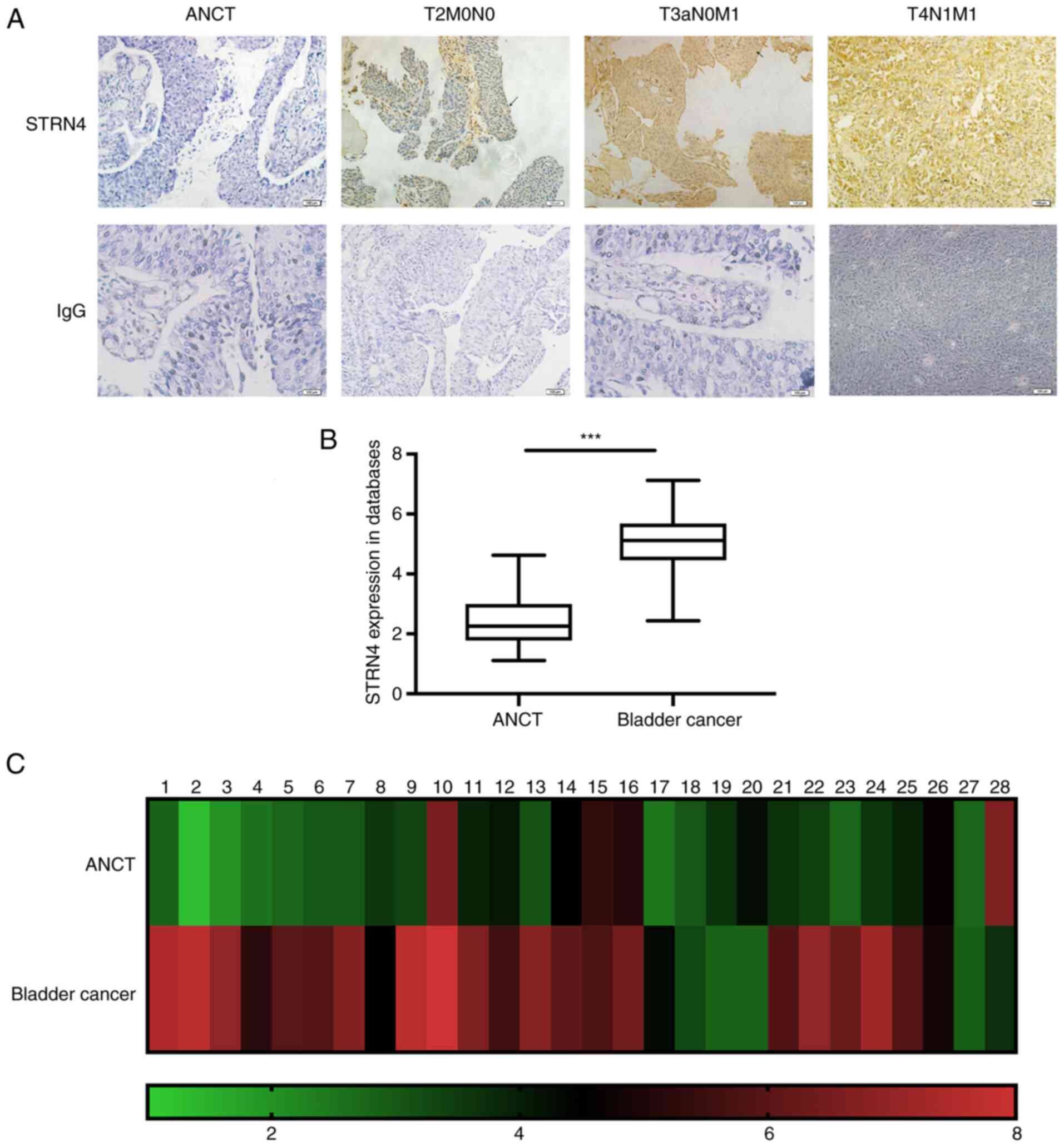
tumor samples. As shown in Fig. 1A,
the location and expression of STRN4 varied in different TNM
stages. In relatively early TNM stages, STRN4 was mainly located at
the cell membrane. Upon progression of TNM stage, STRN4 was located
primarily in the cytoplasm and with increased expression. T2N0M0
indicates stage II in TNM stages. T3aN0M1 and T4N1M1 represents
stage IV in bladder cancer.
Moreover, the present study analyzed STRN4 mRNA
expression of patients with bladder carcinoma from the epidemiology
and statistics database from the School of Public Health, Jilin
University (19). STRN4 was
significantly overexpressed in cancer samples (43 cases) compared
with ANCT (23 cases) (P<0.001; Fig.
1B). Additionally, STRN4 mRNA expression in 28 paired samples
was examined and presented as a heat map (Fig. 1C). RT-qPCR verified that STRN4 mRNA
expression was significantly increased compared with the ANCT group
by an average of 1.81-fold (maximum, 6.05-fold; P<0.001; data
not shown). In summary, these results suggested that STRN4 is
overexpressed in human bladder transitional cell carcinoma.
Aberrant STRN4 expression is
associated with clinicopathological features
In order to investigate the association between
STRN4 expression and specific clinical parameters, certain features
such as age, sex, tumor size and muscle infiltration were chosen to
measure the effects of STRN4 expression. As shown in Table I, STRN4 expression was significantly
associated with tumor size (P=0.005564), muscle infiltration
(P=0.000357), pathological tumor grade (P=0.001500) and
microvascular invasion (P=0.034741). However, STRN4 expression was
not statistically associated with age (P=0.542109), sex
(P=0.240841) and lymph node involvement (P=0.232024). High STRN4
expression in patients with high grade bladder carcinoma was 84.2%
(59/70) compared with 57.1% (24/42) in patients with low grade
bladder carcinoma. STRN4 was detected in 77.5% (69/89) of cases
with deep muscle infiltration (T2-T4) compared with superficial
tumor invasion (Ta-T1), which was 39.1% (9/23). In patients with
microvascular invasion, the detection rate of STRN4 was 81.5%
(53/65) compared with 63.8% (30/47) in the low expression group.
The immunostaining scores of 112 samples are listed in graphical
form (Fig. 2). The number of
specimens with different immunostaining scores are listed below: 3
(score 0), 4 (score 1), 7 (score 2), 15 (score 3), 38 (score 4), 30
(score 6), 15 (score 9). High STRN4 expression (score >3) was
detected in 74.1% (83/112) of cases compared with cases with low
expression (score ≤3), which was 25.9% (29/112).
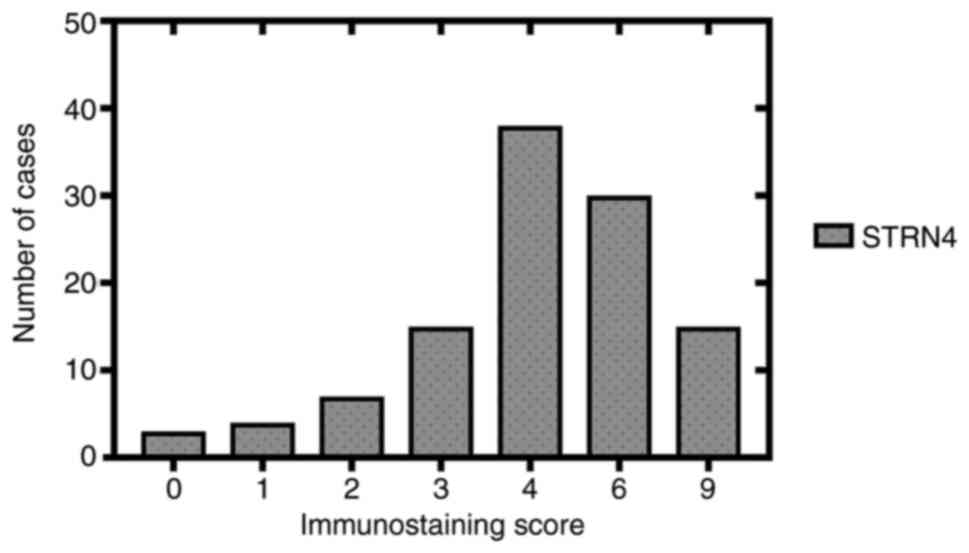 | Figure 2.Immunostaining scores of STRN4
expression from 112 clinical bladder transitional cancer specimens
in graphical form. Horizontal coordinates represent immunostaining
scores (0, 1, 2, 3, 4, 6 and 9), which consists of different
immunostaining intensities (0, 1, 2 and 3) multiplied by the
immunostaining proportion (0, 1, 2 and 3). STRN4, striatin-4. |
 | Table I.Correlation between STRN4 expression
and clinicopathological parameters in patients with bladder
transitional carcinoma. |
Table I.
Correlation between STRN4 expression
and clinicopathological parameters in patients with bladder
transitional carcinoma.
|
|
| STRN4 expression |
|
|---|
|
|
|
|
|
|---|
| Clinical pathological
variable | Number of
patients | Low, n (%) | High, n (%) | P-value |
|---|
| Total | 112 | 29 (25.9) | 83 (74.1) |
|
| Age, years |
|
|
| 0.542109 |
|
<65 | 35 | 12 (34.3) | 23 (65.7) |
|
| ≥65 | 77 | 22 (28.6) | 55 (71.4) |
|
| Sex |
|
|
| 0.240841 |
| Male | 81 | 36 (44.4) | 45 (55.6) |
|
|
Female | 31 | 10 (32.3) | 21 (67.7) |
|
| Tumor size, cm |
|
|
| 0.005564 |
|
<3 | 82 | 31 (37.8) | 51 (62.2) |
|
| ≥3 | 30 | 6 (20.0) | 24 (80.0) |
|
| Muscle
infiltration |
|
|
| 0.000357 |
|
Ta-I | 23 | 14 (60.9) | 9 (39.1) |
|
|
T2-T4 | 89 | 20 (22.5) | 69 (77.5) |
|
| Lymph node
involvement |
|
|
| 0.232024 |
| No | 100 | 46 (46.0) | 54 (54.0) |
|
|
Yes | 12 | 5 (41.7) | 7 (58.3) |
|
| Pathological tumor
grade |
|
|
| 0.001500 |
|
Low | 42 | 18 (42.9) | 24 (57.1) |
|
|
High | 70 | 11 (15.8) | 59 (84.2) |
|
| Microvascular
invasion |
|
|
| 0.034741 |
| No | 47 | 17 (36.2) | 30 (63.8) |
|
|
Yes | 65 | 12 (18.5) | 53 (81.5) |
|
High STRN4 expression in bladder
transitional cancer is associated with adverse survival
outcomes
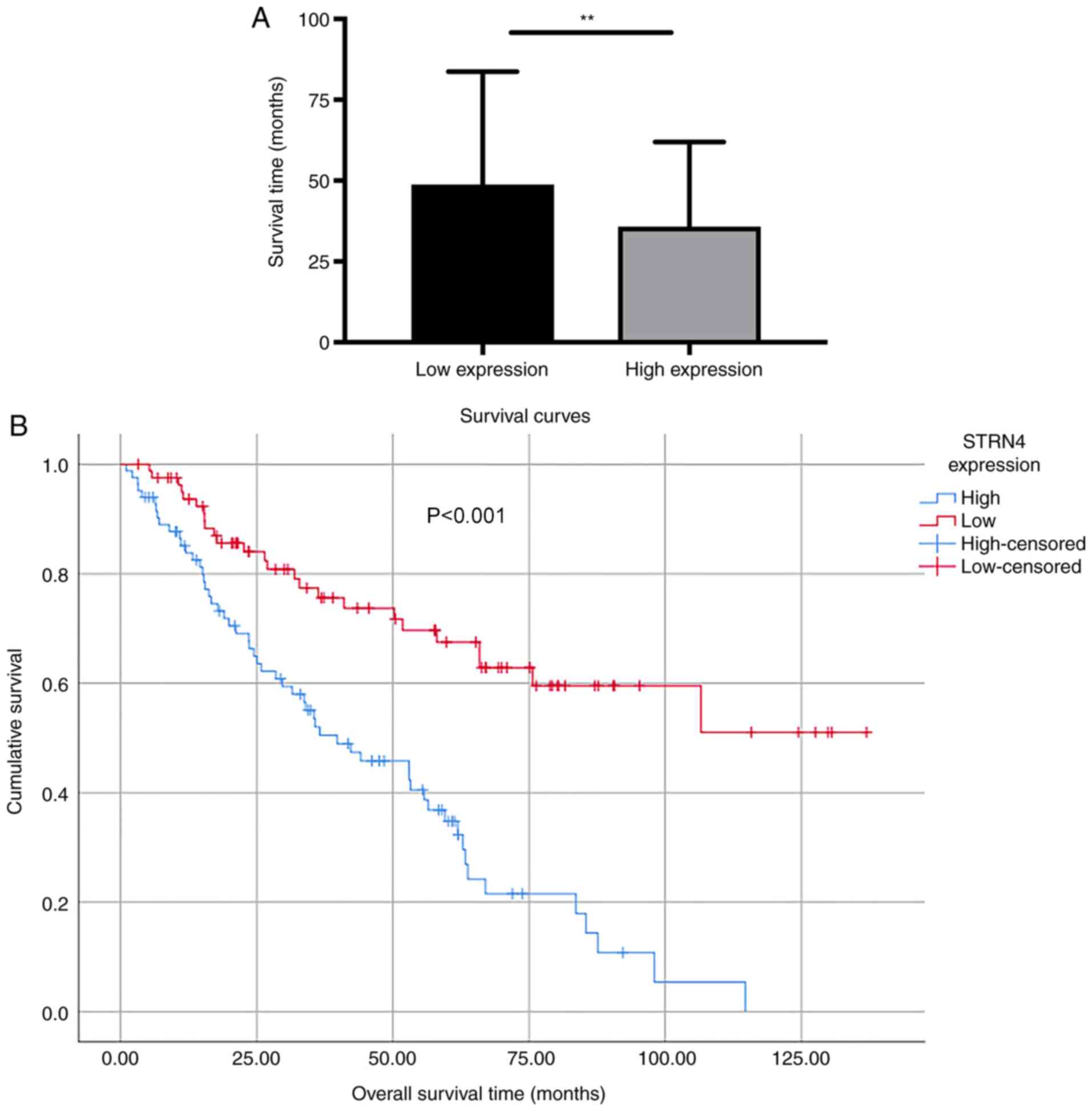
As shown in Fig. 3A,
the mean survival time of patients with high STRN4 expression was
35.82±2.874 months (n=83), which was significantly lower compared
with the low expression group (48.77±3.86 months; n=82; P=0.0077).
Kaplan-Meier survival curves demonstrated that patients with high
STRN4 expression ended up having a significantly shorter overall
survival time compared with patients with lower STRN4 expression
(P<0.001; Fig. 3B). Multivariate
analysis was conducted to determine whether STRN4 expression could
serve as a risk factor. STRN4 expression, along with other possible
prognostic factors, were analyzed using Cox regression analysis. As
shown in Table II, low pathological
tumor grade could serve as a protective factor
(coefficient=−0.951). The risk level of patients with low
pathological tumor grades was 0.386 [hazard ratio (HR)=0.386] when
compared with the high tumor grade group. The risk of patients with
no relapse was 0.242 [hazard ratio (HR)=0.242] compared with
patients with recurrence. STRN4 expression could serve as an
independent factor for evaluating prognosis in patients with
bladder transitional carcinoma (P=0.0018).
 | Table II.Multivariate analysis of the
association between clinical indexes and survival time of patients
with bladder cancer. |
Table II.
Multivariate analysis of the
association between clinical indexes and survival time of patients
with bladder cancer.
|
|
|
|
|
|
|
| 95.0% CI for
HR |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Variable | Coefficient | Standard error | χ2 | df | HR | P-value | Lower | Upper |
|---|
| Age, ≥65 vs. <65
years | 0.022 | 0.010 | 4.633 | 1 | 0.0314 | 1.022 | 1.002 | 1.043 |
| Sex, male vs.
female | −0.193 | 0.294 | 0.432 | 1 | 0.5112 | 0.824 | 0.463 | 1.467 |
| Muscle
infiltration, T2-T4 vs. Ta-T1 | 1.140 | 0.304 | 14.092 | 1 | 0.0002 | 3.126 | 1.724 | 5.668 |
| Expression group,
high vs. low | 0.832 | 0.267 | 9.690 | 1 | 0.0018 | 2.297 | 1.361 | 3.879 |
| Pathological tumor
grade high vs. low | −0.951 | 0.410 | 5.386 | 1 | 0.0203 | 0.386 | 0.173 | 0.863 |
| Recurrence status,
yes vs. no | −1.420 | 0.647 | 4.814 | 1 | 0.0282 | 0.242 | 0.068 | 0.859 |
Discussion
Bladder transitional cancer is known for its
frequent recurrence after transurethral resection. The probability
of 5-year recurrence for patients (mainly from UK, France, Spain
and Netherlands in 2016) with European Organization for Research
and Treatment of Cancer grades 1–4 is ~46%. Higher grade is
accompanied by higher recurrence possibilities (20–22).
Hence, identifying novel indicators of recurrence is particularly
important for patients with bladder tumors.
Through proteomic analysis, researchers have noted
that STRN4 is included in the polyprotein complex known as
STRN-interacting phosphatase and kinase (STRIPAK) as one of its
core components (8,12,23).
STRIPAK consists of several crucial proteins such as PP2A, germinal
center kinase III, cerebral cavernous malformation 3 and monopolar
spindle one-binder family 3 (24–26).
Accumulating evidence has indicated that STRN4 participates in the
development of various cancer types and cardiac dysfunction via
STRIPAK and STRIPAK-like complexes (27,28).
Other reports have stated that the dysregulation of STRN4
expression exhibits potential for diagnosis and prognostic
prediction (29–31). However, the prognostic role of STRN4
in bladder cancer is unclear and needs to be further
determined.
The present study first examined STRN4 expression in
postoperative samples. The results demonstrated that STRN4 was
distinctly overexpressed in bladder transitional cancer samples
when compared with ANCT. Meanwhile, STRN4 mRNA expression was
analyzed using qPCR and compared with database results. The present
study concluded that STRN4 mRNA expression was significantly higher
compared with the ANCT group and the overexpression pattern was
consistent with database results. These results indicated that
STRN4 expression was upregulated in bladder transitional
cancer.
To further elucidate the clinical relevance of
STRN4, the association between STRN4 and several
clinicopathological indexes were analyzed. STRN4 was significantly
associated with tumor size, muscle infiltration, pathological grade
and microvascular invasion. Muscle infiltration, pathological grade
and microvascular invasion were listed as some of the most crucial
factors for evaluating patient survival time and prognosis. The
association between these variables and patient outcome is not
strictly confined to bladder cancer, but also in other malignancies
(32–34). Hence, STRN4 may serve a role as an
independent prognostic factor. Furthermore, since the main
metastasis approach for bladder transitional carcinoma is still
muscle intrusion, these results suggested that STRN4 is important
in tumor metastasis and recurrence, and may serve as an effective
indicator for progression.
To determine the prognostic value of STRN4, survival
analysis was performed to determine the effects of STRN4 on patient
OS time. It was demonstrated that patients with high STRN4
expression had a lower OS time compared with the low expression
group. Multivariate analysis indicated that STRN4 was an
independent positive prognostic factor for OS. Taken together, the
present results demonstrated that STRN4 may be a potential
indicator for the prognosis of patients with bladder cancer and
could be a new therapeutic target. To conclude, the present study
indicated that STRN4 is an independent prognostic factor and
potential therapeutic target for patients with bladder transitional
carcinoma.
The present study is not without limitations. First,
the analysis between STRN4 expression and the clinical parameters
only consisted of 112 samples. Larger sample sizes are required to
validate the results presented here. In addition, STRN4 mRNA
expression analysis was performed using 43 cancer samples and only
23 ANCT samples. For prospective studies, equivalent samples are
required to exclude interference from confounding and mismatching
factors. Further studies are also required to determine the
molecular mechanisms underlying the abnormal expression of STRN4 in
the tumorigenesis of bladder transitional carcinoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Doctor
Training Fund Subdivision of China-Japan Union Hospital of Jilin
University (grant no. 4700404B003C).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
XG, XL planned and designed the experiments and
collected and analyzed the data. YZ validated the results,
performed the experiments and drafted the manuscript. FJ, PS
analyzed and interpreted the data. XL and YZ confirmed the
authenticity of all raw data. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was supervised and approved by the
Ethics Committee of China-Japan Union Hospital of Jilin University
(Changchun, China; approval no. 2015-wjw014), and performed in
compliance with the Declaration of Helsinki. Written informed
consent was provided by all patients prior to the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Antoni S, Ferlay J, Soerjomataram I, Znaor
A, Jemal A and Bray F: Bladder cancer incidence and mortality: A
global overview and recent trends. Eur Urol. 71:96–108. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Malmström PU: Why has the survival of
patients with bladder cancer not improved? BJU Int. 101:267–269.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Benoist M, Gaillard S and Castets F: The
striatin family: A new signaling platform in dendritic spines. J
Physiol Paris. 99:146–153. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanabe O, Hirata D, Usui H, Nishito Y,
Miyakawa T, Igarashi K and Takeda M: Fission yeast homologues of
the B' subunit of protein phosphatase 2A: Multiple roles in mitotic
cell division and functional interaction with calcineurin. Genes
Cells. 6:455–473. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Castets F, Rakitina T, Gaillard S, Moqrich
A, Mattei MG and Monneron A: Zinedin, SG2NA, and striatin are
calmodulin-binding, WD repeat proteins principally expressed in the
brain. J Biol Chem. 275:19970–19977. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Soni S, Jain BP, Gupta R, Dharavath S, Kar
K, Komath SS and Goswami SK: Biophysical characterization of SG2NA
variants and their interaction with DJ-1 and Calmodulin in vitro.
Cell Biochem Biophys. 76:451–461. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hwang J and Pallas DC: STRIPAK complexes:
Structure, biological function, and involvement in human diseases.
Int J Biochem Cell Biol. 47:118–148. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Castets F, Bartoli M, Barnier JV, Baillat
G, Salin P, Moqrich A, Bourgeois JP, Denizot F, Rougon G, Calothy G
and Monneron A: A novel calmodulin-binding protein, belonging to
the WD-repeat family, is localized in dendrites of a subset of CNS
neurons. J Cell Biol. 134:1051–1062. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pojoga LH, Coutinho P, Rivera A, Yao TM,
Maldonado ER, Youte R, Adler GK, Williams J, Turchin A, Williams GH
and Romero JR: Activation of the mineralocorticoid receptor
increases striatin levels. Am J Hypertens. 25:243–249. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin L, Lo LH, Lyu Q and Lai KO:
Determination of dendritic spine morphology by the striatin
scaffold protein STRN4 through interaction with the phosphatase
PP2A. J Biol Chem. 292:9451–9464. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wong M, Hyodo T, Asano E, Funasaka K,
Miyahara R, Hirooka Y, Goto H, Hamaguchi M and Senga T: Silencing
of STRN4 suppresses the malignant characteristics of cancer cells.
Cancer Sci. 105:1526–1532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reznickova E, Gucky T, Kovacova V, Ajani
H, Jorda R and Krystof V: Activity of 2,6,9-trisubstituted purines
as potent PDGFRα kinase inhibitors with antileukaemic activity. Eur
J Med Chem. 182:1116632019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang F, Zheng Q, Chang L, Li X, Wang X
and Gu X: Pro-oncogene pokemon promotes prostate cancer progression
by inducing STRN4 expression. J Cancer. 10:1833–1845. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cornejo KM, Rice-Stitt T and Wu CL:
Updates in staging and reporting of genitourinary malignancies.
Arch Pathol Lab Med. 144:305–319. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang X, Jiang F, Song H, Li X, Xian J and
Gu X: MicroRNA-200a-3p suppresses tumor proliferation and induces
apoptosis by targeting SPAG9 in renal cell carcinoma. Biochem
Biophys Res Commun. 470:620–626. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li X, Jiang F, Wang X and Gu X: SPAG9
regulates HEF1 expression and drives EMT in bladder transitional
cell carcinoma via rac1 signaling pathway. Am J Cancer Res.
8:2467–2480. 2018.PubMed/NCBI
|
|
19
|
Du B, Liu S, Cui C, Wang S and Cui W:
Association between glucose transporter 1 rs841853 polymorphism and
type 2 diabetes mellitus risk may be population specific
(1rs8418532). J Diabetes. 5:291–299. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alfred Witjes J, Lebret T, Comperat EM,
Cowan NC, De Santis M, Bruins HM, Hernandez V, Espinos EL, Dunn J,
Rouanne M, et al: Updated 2016 EAU guidelines on muscle-invasive
and metastatic bladder cancer. Eur Urol. 71:462–475. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Babjuk M, Böhle A, Burger M, Capoun O,
Cohen D, Comperat EM, Hernandez V, Kaasinen E, Palou J, Roupret M,
et al: EAU guidelines on non-muscle-invasive urothelial carcinoma
of the bladder: Update 2016. Eur Urol. 71:447–461. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gakis G, Witjes JA, Comperat E, Cowan NC,
De Santis M, Lebret T, Ribal MJ and Sherif AM; European Association
of Urology, : EAU guidelines on primary urethral carcinoma. Eur
Urol. 64:823–830. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goudreault M, D'Ambrosio LM, Kean MJ,
Mullin MJ, Larsen BG, Sanchez A, Chaudhry S, Chen GI, Sicheri F,
Nesvizhskii AI, et al: A PP2A phosphatase high density interaction
network identifies a novel striatin-interacting phosphatase and
kinase complex linked to the cerebral cavernous malformation 3
(CCM3) protein. Mol Cell Proteomics. 8:157–171. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gordon J, Hwang J, Carrier KJ, Jones CA,
Kern QL, Moreno CS, Karas RH and Pallas DC: Protein phosphatase 2a
(PP2A) binds within the oligomerization domain of striatin and
regulates the phosphorylation and activation of the mammalian
Ste20-Like kinase Mst3. BMC Biochemistry. 12:542011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bergametti F, Denier C, Labauge P, Arnoult
M, Boetto S, Clanet M, Coubes P, Echenne B, Ibrahim R, Irthum B, et
al: Mutations within the programmed cell death 10 gene cause
cerebral cavernous malformations. Am J Hum Genet. 76:42–51. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baillat G, Moqrich A, Castets F, Baude A,
Bailly Y, Benmerah A and Monneron A: Molecular cloning and
characterization of Phocein, a protein found from the golgi complex
to dendritic spines. Mol Biol Cell. 12:663–673. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng Y, Liu B, Wang L, Lei H, Pulgar
Prieto KD and Pan D: Homeostatic control of Hpo/MST kinase activity
through autophosphorylation-dependent recruitment of the STRIPAK
PP2A phosphatase complex. Cell Rep. 21:3612–3623. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wagh V, Doss MX, Sabour D, Niemann R,
Meganathan K, Jagtap S, Gaspar JA, Ardestani MA, Papadopoulos S,
Gajewski M, et al: Fam40b is required for lineage commitment of
murine embryonic stem cells. Cell Death Dis. 5:e13202014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shi Z, Jiao S and Zhou Z: STRIPAK
complexes in cell signaling and cancer. Oncogene. 35:4549–4557.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Madsen CD, Hooper S, Tozluoglu M,
Bruckbauer A, Fletcher G, Erler JT, Bates PA, Thompson B and Sahai
E: STRIPAK components determine mode of cancer cell migration and
metastasis. Nat Cell Biol. 17:68–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen M, Zhang H, Shi Z, Li Y, Zhang X, Gao
Z, Zhou L, Ma J, Xu Q, Guan J, et al: The MST4-MOB4 complex
disrupts the MST1-MOB1 complex in the Hippo-YAP pathway and plays a
pro-oncogenic role in pancreatic cancer. J Biol Chem.
293:14455–14469. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wahlin S, Nodin B, Leandersson K, Boman K
and Jirstrom K: Clinical impact of T cells, B cells and the
PD-1/PD-L1 pathway in muscle invasive bladder cancer: A comparative
study of transurethral resection and cystectomy specimens.
Oncoimmunology. 8:e16441082019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lionello M, Bertolin A, Nardello E,
Giacomelli L, Canal F, Rizzotto G, Marioni G and Lucioni M: Could
the infiltration of the thyroarytenoid muscle define the pT2
glottic carcinoma? Head Neck. 41:3639–3646. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Botling J, Lamarca A, Bajic D, Norlen O,
Lonngren V, Kjaer J, Eriksson B, Welin S, Hellman P, Rindi G, et
al: High-grade progression confers poor survival in pancreatic
neuroendocrine tumors. Neuroendocrinology. 110:891–898. 2020.
View Article : Google Scholar : PubMed/NCBI
|