Introduction
Gynecologic cancer is any cancer of the female
reproductive organs, including ovarian, uterine, vaginal, cervical
and vulvar cancers. Generally, all women are at risk of developing
gynecological cancers, and risk increases with age (1). Each gynecologic cancer is unique, with
different signs and symptoms, different risk factors and different
therapeutic strategies (2,3). Gynecological cancers are associated
with high mortality rates worldwide as it is difficult to detect
the cancers in early stage (1).
Gynecological cancers are among the major threats to modern life,
particularly to female health (1–3). An
essential part of the gynecologic assessment is examination of the
breasts. Breast cancer is recognized more often by gynecologists
than any other physician (4). Women
with breast cancer are at risk of developing a second primary
gynecologic cancer, particularly uterine and ovarian cancers
(4).
Breast cancer is the most common invasive cancer in
women that develops from breast tissues. It is the second most
common cancer among women and the leading cause of
cancer-associated mortality, accounting for approximately 500,000
mortalities per year worldwide (5).
It is a multifactorial disease affected by several risk factors,
including age, sex, genetics, ethnicity, environmental factors,
high levels of certain hormones, including estrogen and
progesterone, lifestyle and diet (5–8). Ovarian
cancer is a cancerous growth that begins in the ovaries (9). It is caused by several factors,
including age, genetic mutations, metabolic abnormalities,
endometriosis and hormone replacement therapy (9–12).
Cervical cancer is a cancer arising from the cervix (the entrance
to the womb from the vagina), which predominantly affects sexually
active women aged 30–45 years. Infection with the human papilloma
virus (HPV) is considered a major risk factor for cervical cancer
(13,14). Other risk factors for this disease
include smoking, a weak immune system, birth control pills, early
age at first intercourse, multiple sex partners and low
socioeconomic status (13–15). Endometrial cancer is a common
gynecological cancer that begins in the uterus (16). Several factors may increase the risk
of endometrial cancer, including age, obesity, hormone therapy,
genetic mutations, hypertension, diabetes mellitus and favorable
prognosis (16–18). Other types of gynecological cancers
include vaginal cancer, which forms in the tissues of the vagina,
and vulvar cancer, which forms in the external genital organ of
women. However, these types of gynecological cancers are rare, and
thus infrequently studies (2).
Similar to cervical cancer, both vaginal and vulvar cancers are
associated with HPV infection (2,19).
Long non-coding RNA and cancer
The human genome has been estimated to contain
23,000 long non-coding RNA (lncRNA) genes, which are more abundant
than 20,000 protein-coding genes (20). lncRNA genes are an important
population of non-coding RNAs, without protein-coding capacity
(20,21). lncRNAs transcripts are >200
nucleotides (nt) in length, and represent a stringent
cell-type/tissue specificity (22).
lncRNAs play critical roles in normal development of organisms, as
well as the tumorigenesis process (22,23). The
crucial roles of lncRNAs include dosage compensation, imprinting,
chromatin rearrangement, histone modification, modification of
alternative splicing genes, as well as gene expression (23). According to their functions and
expression patterns in tumor cells/tissues, lncRNAs can be
classified as tumor suppressor genes or oncogenes (24,25).
lncRNAs play different roles in the regulation of cancer-related
pathways, such as the Wnt, Hedgehog, Notch and PI3K/AKT/mTOR
pathways, and regulate the plasticity of cancer stem cells
(26). lncRNAs interact with
microRNAs (miRNAs/miRs), mRNAs, proteins and genomic DNA to exert
their physiological and pathological functions (26,27).
Deregulation of several lncRNAs has been detected in different
types of cancer, such as, breast, ovarian, cervical and prostate
cancer, which suggests the use of lncRNAs as markers for cancer
detection and prognosis, as well therapeutic targets for cancer
treatment (22,28).
MALAT1: Structure and function
Metastasis-associated lung adenocarcinoma transcript
1 (MALAT1) is a large and infrequently spliced lncRNA, also known
as nuclear enriched abundant transcript 2, HCN, LINC00047,
NCRN00047 and PRO2853 (29). MALAT1
is abundant in several human cell types, with the highest
expression levels in pancreas and lung cells (29,30).
MALAT1 is a single-exon gene, located within human chromosome 11q13
and mouse chromosome 19qA. The primary structure of MALAT1 contains
~8 kb in humans and ~7 kb in mice (31,32). Its
3′-end lacks a poly (A) tail structure and can be processed by
RNase P and RNase Z cleavage into a long 6.7-kb transcript, which
yields an additional 3′-short tRNA-like ncRNA and a 5′-long
MALAT1-associated small cytoplasmic RNA (31–33).
Following transcription, the longer form of MALAT1 is retained in
the nucleus and specifically localizes to nuclear speckles. These
structures of MALAT1 are enriched in pre-mRNA splicing factors, and
serve as storage and assembly/modification sites. MALAT1 may
interact with the serine/arginine-rich family of splicing factors,
which affects the distribution of splicing factors in nuclear
speckle domains, and regulates tissue- or cell-type specific
alternative splicing in a phosphorylation-dependent manner
(31,34). MALAT1 acts as an activator of gene
expression by mediating the interaction with the demethylated form
of chromobox homolog 4, also known as polycomb 2, a component of
the Polycomb Repressive Complex 1 (31,34,35).
MALAT1 has also been reported to regulate several
pathological processes, ranging from diabetes complications to
cancer (36). MALAT1 was initially
identified as a factor associated with high metastatic potential
and poor prognosis in stage I non-small cell lung cancer (NSCLC)
(36,37). MALAT1 is overexpressed in different
types of tumors, including lung, liver, gastric, pancreatic, renal,
colon, bladder, breast, bone cancers and gynecological cancers
(38). MALAT1 regulates the
expression of metastasis-associated genes and cell motility via
transcriptional and/or post-transcriptional regulation (38,39). It
has been demonstrated that various upstream regulators may bind to
the promoter to activate the transcription of MALAT1, resulting in
upregulated MALAT1 expression in different types of cancer
(40). Sp1 is associated with lung
cancer, whereas JMJD1A is associated with neuroblastoma, the sex
determining region Y-box (Sox) 17 is associated with esophageal
cancer, and TDP-43 is associated with NSCLC (40,41).
Serine/arginine-rich splicing factor 1 (SRSF1) is associated with
breast cancer, while yes-associated protein and SRSF1 are
associated with liver cancer (40,41).
Previous studies have identified the common genetic variants
(single nucleotide polymorphisms) of MALAT1 associated with the
risk of different types of cancer (42–44).
Thus, MALAT1 plays an important factor in carcinogenesis. The
present review discusses the expression patterns of MALAT1 in
breast cancer and gynecological cancers, in the perspectives of
carcinogenesis and therapeutics.
Pathogenic role of MALAT1 in gynecological
cancers
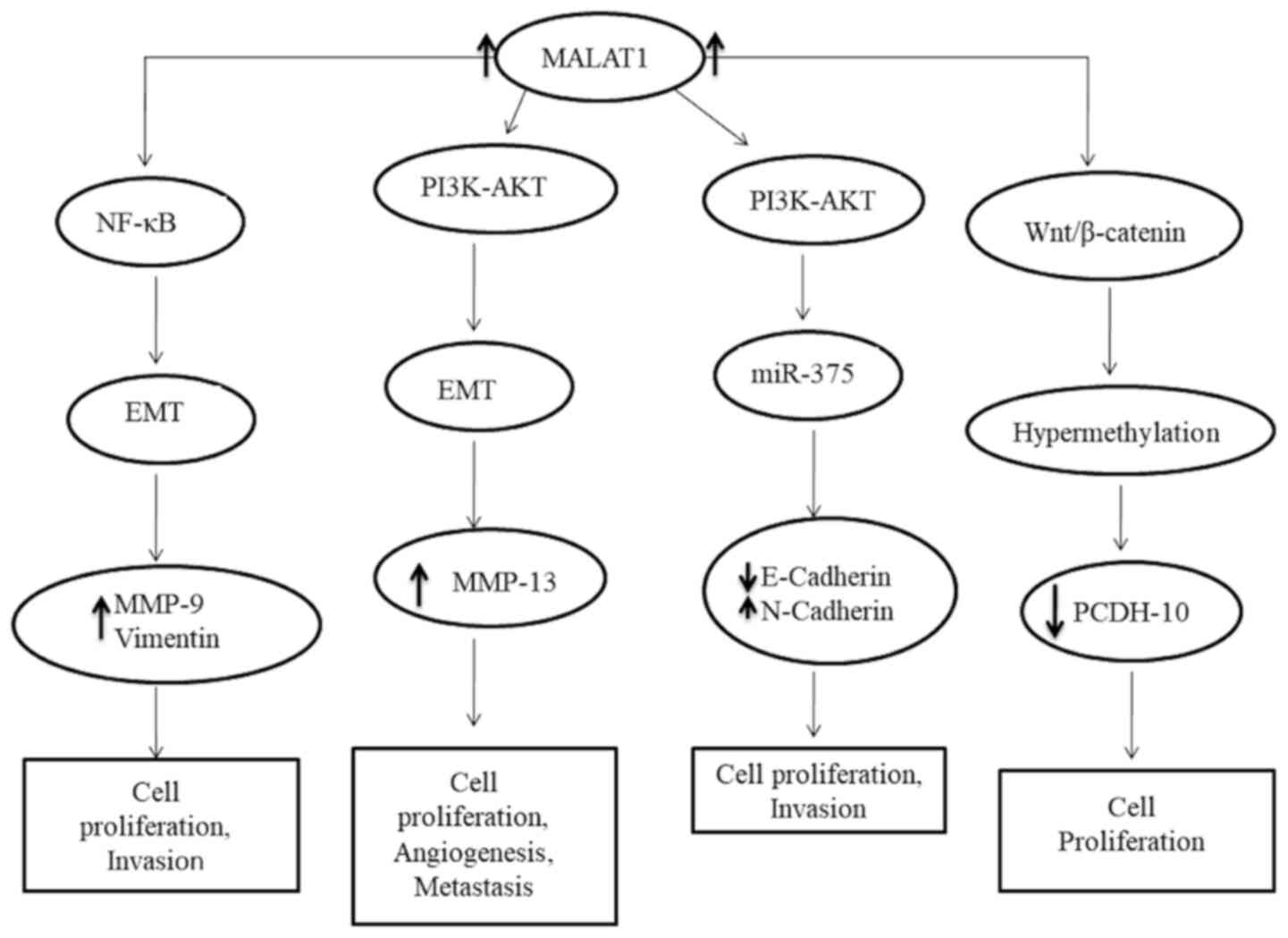
Fig. 1 and Table I present the pathogenic role of
MALAT1 in gynecological cancers. Breast cancer exosomes promote
cancer cell proliferation by modulating exosomal MALAT1 regulation.
MALAT1 is considered a proinflammatory factor, which regulates the
lipopolysaccharide (LPS)-induced inflammatory response by
interacting with nuclear factor kappa-light-chain-enhancer of
activated B cells (NF-κB) of breast cancer cells (39,45).
NF-κB also plays an important role in regulating the
epithelial-to-mesenchymal transition (EMT) process, and promotes
matrix metalloproteinase (MMP)-9 and vimentin expression by binding
to the promoter of vimentin (39,45).
Previous studies have demonstrated that MALAT1 expression is
upregulated in breast cancer cells and tissues (46–49).
Gomes et al (50) reported
that an antisense transcript of MALAT1, named TALAM1, mediates
MALAT1 response in human breast cancer. They reported that MALAT1
locus is spanned by TALAM1, and downregulation of TALAM1 induces
breast cancer cell aggressiveness and malignancy. In addition, Wu
et al (51) observed the
highest level of MALAT1 expression in metastatic triple-negative
breast cancer and trastuzumab-resistant human epidermal growth
factor receptor 2 (HER2) overexpressing (HER2+) cells. They
demonstrated that upregulated MALAT1 expression via activation of
the PI3K/AKT pathway induced EMT-like phenotypes and cell
invasiveness in HER2+ cells (51).
Huang et al (52) reported an
interaction between MALAT1 and miR-145, and observed that
upregulated MALAT1 expression significantly enhanced the
proliferation, migration and tube formation of MCF-7 cells, whereas
miR-145 expression inversely changed in breast cancer tissues. Kim
et al (53) demonstrated
contradictory effects of MALAT1 in breast cancer cell proliferation
and invasion. They reported that MALAT1 acts as a
metastasis-suppressing lncRNA rather than a metastasis promoter in
breast cancer. Furthermore, overexpression of MALAT1 was
demonstrated to suppress breast cancer cell migration and invasion
by binding and inactivating the pro-metastatic transcription
factor, TEAD (53). Studies by Kwok
et al (54) and Eastlack
et al (55) have reported
that MALAT1 can act as a tumor suppressor in breast cancer. As
these findings contradict the role of MALAT1 as an important
therapeutic target for breast cancer, it was suggested that MALAT1
should be thoroughly investigated to determine its dual roles
across different types of cancer (56). Peng et al (42) demonstrated the association of MALAT1
rs619586 A>G polymorphism with a reduced risk of breast cancer
in the Chinese Han population.
 | Table I.Molecular mechanisms of MALAT1 in
gynecological cancers. |
Table I.
Molecular mechanisms of MALAT1 in
gynecological cancers.
| Cancer type | Mechanism | Effects | (Refs.) |
|---|
| Breast cancer | Downregulates
TALAM1 expression | Induces cell
aggressiveness and malignancy | (50) |
|
| Activates the
PI3K/AKT pathway | Induces EMT-like
phenotype and cell invasiveness | (51) |
|
| Downregulates
microRNA-145 expression | Enhances
proliferation, migration and tube formation | (52) |
| Ovarian cancer | Activates the
JAK2/STAT3 signaling pathway | Promotes
proliferation and inhibits cell apoptosis | (59) |
|
| Activates the
PI3K/AKT and Wnt/β-catenin pathways | Induces EMT-like
phenotype | (62) |
|
| Influences HUVECs
and stimulates angiogenesis related genes | Promotes
angiogenesis | (65) |
|
| Upregulates MMP13
expression and downregulates MMP19 and ADAMTS1 expression | Induces cell
migration and invasion | (66) |
| Cervical
cancer | Activates the
PI3K/AKT pathway | Induces EMT-like
phenotype | (71) |
|
| Activates
AKT/mTOR | Increases cell
viability, cell migration and invasion, and EMT | (70) |
| Endometrial | Activates the
Wnt/β-catenin pathway | Induces cell
migration and invasion | (72) |
| cancer | MALAT1 rs664589
C>G polymorphism | Associated with
cancer risk | (73) |
Several studies have suggested that MALAT1 is highly
expressed in ovarian cancer tissues and cell lines. Overexpression
of MALAT1 promotes tumor initiation in ovarian cancer by inducing
EMT (57,58). For example, Sun et al
(59) demonstrated that
overexpression of MALAT1 promotes proliferation and suppresses the
apoptosis of ovarian cancer cells by activating the JAK2/STAT3
signaling pathway. EMT maintains the mesenchymal cell phenotype and
plays an essential role in cancer cell migration and invasion
through E-cadherin, N-cadherin, vimentin and EMT-inducing
transcription factors, such as Twist, Snail, Slug and Zeb (60,61).
MALAT1 may influence EMT in ovarian cancer by activating the
PI3K/AKT pathway (62). Jin et
al (62) reported that MALAT1
regulates ovarian cancer cell proliferation, migration and
apoptosis via the Wnt/β-catenin signaling pathway. Wnt is a cell
growth factor that promotes Frizzled receptor expression and
activates the scaffolding protein, Dishevelled, to induce
intracellular signal transduction and glycogen synthase-kinase 3 β
phosphorylation. β-catenin (a subunit of the cadherin protein
complex) protein binds to E-cadherin to increase the adhesion
between cells (63). β-catenin
accumulates in the nucleus, which activates the transcription of
the downstream cyclin D1 and other target genes to increase the
cell proliferation, tumor formation and cell migratory and invasive
abilities (63,64). Qiu et al (65) reported that MALAT1 can be transferred
from ovarian cancer cells to recipient human umbilical vein
endothelial cells (HUVECs) via exosomes. They indicated that the
metastatic ovarian cancer cells promote angiogenesis by
transferring exosomal MALAT1 to recipient HUVECs, which in turn,
triggers HUVECs to promote angiogenesis by stimulating
angiogenesis-related genes, including vascular endothelial growth
factor (VEGF)-A, VEGF-D, epithelial neutrophil-activating
peptide-78, placental growth factor, interleukin-8, angiogenin,
basic fibroblast growth factor and leptin. Zhou et al
(66) demonstrated that the efficacy
of MALAT1 in promoting ovarian cancer progression can also be
mediated by upregulating MMP13 expression and downregulating MMP19
and ADAMTS1 expression.
MALAT1 expression is upregulated in cervical cancer
tissues compared with normal cervix tissues (67). Jiang et al (68) demonstrated that MALAT1 expression is
upregulated in HPV-positive cervical squamous cells, which suggests
that high MALAT1 expression is associated with HPV in cervical
cancer. Upregulated MALAT1 expression promotes the proliferation
and invasion, and decreases the apoptosis of cervical cancer cells
(67). MALAT1 can act as an oncogene
by sponging miRNA in HR-HPV positive cervical cancer cells
(69). MALAT1 is involved in
regulating the EMT process in cervical cancer (70). Wang et al (71) reported that overexpressing MALAT1
significantly increases BRWD1 mRNA expression by activating the
PI3K/AKT pathway in HeLa and C-33A cells. Han et al
(70) demonstrated that
overexpression of periostin in cervical cells is positively
associated with MALAT1 expression and negatively associated with
miR-202-3p expression. Furthermore, it was suggested that the
MALAT1/miR-202-3p/periostin axis increases cell viability, cell
migration and invasion, and EMT in HeLa or SiHa cells by activating
the AKT/mTOR signaling pathway (70).
Increased levels of MALAT1 have been detected in
endometrial cancer. Zhao et al (72) identified MALAT1 as a direct
transcriptional target of Wnt/β-catenin in endometrial cancer
cells. The protocadherin 10 (PCDH10) is a novel Wnt pathway
regulatory element that acts as a tumor suppressor. In endometrial
cancer, PCDH10 expression is downregulated by promoter
hypermethylation, which induces aberrant activation of the Wnt/
β-catenin signaling pathway via the direct binding site of TCF4 in
MALAT1 promoter region (72). Chen
et al (73) demonstrated the
association of MALAT1 rs664589 C>G polymorphism with the risk of
endometrial cancer in Southern Chinese women. It was demonstrated
that individuals with CGG haplotypes have a higher risk of
developing endometrial cancer compared with the wild-type GCG
haplotype carriers (73).
Collectively, these findings suggest the potential role of MALAT1
in inducing the development of different gynecological cancers.
Targeting MALAT1 in gynecological cancer
therapeutics
The treatment of gynecologic cancer depends on
several factors, including the type, stage and grade, as well as
the general health of the patient. The main treatment options for
gynecologic cancer include surgery, chemotherapy, radiation therapy
and hormone therapy, either alone or in a combination with each
other (2,58). Despite advancements in modern
multimodality chemotherapeutic strategies, there is a high chance
of local relapse and tumor metastasis (2,58). Thus,
targeted therapies are used to target both metastatic progressions
and decrease the risk of recurrence in the treatment of gynecologic
cancer (2). Targeting MALAT1 is an
important strategy to combat gynecological cancers. The important
strategies to target MALAT1 are summarized in Table II.
 | Table II.Major strategies in cancer
therapeutics by targeting MALAT1. |
Table II.
Major strategies in cancer
therapeutics by targeting MALAT1.
| Strategies |
|---|
| Inactivation of
MALAT1 by small RNA technologies |
| Development of
MALAT1-specific chemotherapeutic drugs |
| Inactivation of
MALAT1 by applying inhibitors of MALAT1 |
| Degradation of
MALAT1 by using microRNA response elements |
| Epigenetic
modification of MALAT1 gene to silence its expression |
Several studies have reported that high MALAT1
expression is associated with increased stage, recurrence and
decreased survival in gynecological cancers (56,66,67).
Thus, suppressing MALAT1 expression may be a novel target for the
treatment of gynecological cancers. MALAT1 knockdown via small
interfering RNA (siRNA) impairs the exosome-mediated pro-angiogenic
activity of HUVECs through certain key angiogenesis-related genes,
and significantly decreases cancer cell proliferation, migration
and invasion (74,75).
Li et al (39)
performed RNA reverse transcription-associated trap sequencing and
demonstrated that MALAT1 bound to the promoter regulatory element
of the translation elongation factor 1-α 1 gene (EEF1α1). Knockdown
of MALAT1 significantly downregulated EEF1α1 expression, which in
turn decreased cell proliferation and invasion by arresting cells
at the G0/G1 phase in breast cancer cells
(39). Liu et al (75) reported that miR-1 functions as a
tumor suppressor by targeting K-RAS and MALAT1. They found that
downregulated hsa-miR-1 expression in breast cancer tissues and
restoration of miR-1 in breast cancer cells inhibits tumor growth
and cell migration and invasion, and increases apoptosis (75). Knockdown of endogenous MALAT1 using
MALAT1 short hairpin RNA (shRNA) significantly increases miR-145
expression and can inhibit proliferation, migration and tube
formation by decreasing VEGF expression in breast cancer cells
(52). Arun et al (76) reported that genetic loss or systemic
MALAT1 knockdown using antisense oligonucleotides (ASOs) in the
MMTV (mouse mammary tumor virus)-PyMT mouse mammary carcinoma model
decreased tumor growth and metastatic capacity (76). In addition, MALAT1 knockdown in 4T1
×enograft mice significantly decreased the inflammatory responses
by decreasing tumor necrosis factor-α, and weakened tumor
metastasis of lung induced by LPS (39).
Gordon et al (77) reported that MALAT1 promotes ovarian
cancer progression by regulating RBFOX2-mediated alternative
splicing. It was demonstrated that suppression of MALAT1 decreased
the proliferation, invasion, anchorage independent growth, and
increased anoikis in multiple anoikis-resistant ovarian cancer cell
lines by decreasing RBFOX2 expression and EMT-related genes
(77). Guo et al (63) demonstrated that downregulating MALAT1
expression inhibits cell proliferation, invasion and migration,
arrests cell cycle progression in the S phase and induces cell
apoptosis in ovarian cancer cell lines by inhibiting activation of
the Wnt/β-catenin signaling pathway. Bai et al (78) reported that MALAT1 knockdown
significantly attenuates cisplatin resistance and induces apoptosis
in cisplatin-resistant ovarian cancer cells by inhibiting the
Notch1 signaling pathway. Several studies have performed
lentivirus-mediated artificial miRNA interference to determine the
effect of MALAT1 in ovarian cancer cells (66,79). It
has been reported that miR-200c is negatively associated with
MALAT1 expression (80).
Furthermore, MALAT1 knockdown suppresses the viability, and the
invasive and migratory abilities of ovarian cancer cells (80). MALAT1 knockdown may also suppress
tumor growth via miR-506-dependent regulation (81). MALAT1 knockdown decreases MMP13
protein expression, while increasing MMP19 and ADAMTS1 expression,
resulting in G0/G1 cell cycle arrest and
apoptosis in ovarian cancer cell lines (66).
Silencing MALAT1 expression via shRNA decreases
cervical cancer cell viability, induces cell apoptosis, represses
the cell invasive capacity, increases G1 phase cells and
decreases S phase or G2/M ratio (82). Overexpression of several miRNAs, such
as, miR-1, miR-145, miR-506 and miR-200c or the use of MALAT1 siRNA
decreases the cell invasive and migratory abilities, downregulates
mesenchymal markers, β-catenin and Vimentin, and upregulates
E-cadherin expression (52,75,81,83). Xia
et al (84) used metformin
for type 2 diabetes, to assess its effects on the migratory and
invasive abilities of human cervical cancer cells, and it was
demonstrated that metformin markedly inhibits the proliferation and
angiogenesis of human cervical cancer cells and cervical cancer
cell xenografts in nude mice (84).
It has been reported that miR-200 family members are
enriched in endometrial cancer, while MALAT1 is expressed at low
levels. Li et al (85) used a
xenograft tumor model to demonstrate that targeting the
miR-200c/MALAT1 axis inhibits endometrial cancer cell proliferation
and EMT-associated protein expression in vivo. Thus, MALAT1
may act as a key target in therapeutic research on gynecological
cancers.
Future research
Gynecological cancers are among the most common
causes of mortality in women worldwide as it is difficult to detect
the cancer in early stage (2).
Dysregulation of MALAT1 has been reported in gynecological cancers,
and thus may be used as a potential therapeutic target (86–88).
Several studies have reported on the interference approaches of
MALAT1 knockdown by RNA (78,81).
However, MALAT1 is less accessible than mRNAs to siRNAs as it is in
the nucleus. Direct targeting of MALAT1 through RNA interference is
not possible as this technology is not yet feasible in routine
clinical practice. ASOs are regarded as a valuable approach to
antagonize MALAT1. Systemic knockdown of MALAT1 using ASOs may
provide an exciting prospective avenue for investigating the use of
MALAT1 ASOs in a therapeutic setting to decrease tumor progression.
ASOs are considered more advantageous over siRNAs due to their
higher specificity and fewer off-target effects, as well as their
independency on the RNA-induced silencing complex machinery. The
limitations of ASOs include off-target toxicity effects, low
affinity for the target, high vulnerability to degradation and poor
delivery to the target tissues. Chemical modifications may overcome
some of these limitations of ASOs by enhancing affinity,
specificity and improving effective delivery to target tissues,
with lower toxicity (28,38,88,89). In
addition, targeting MALAT1 with specific drugs or stimulating its
functions in clinically feasible ways may lead to the suppression
of cancer development (28,38,88–90).
Conclusions
Gynecological cancers are a major threat to modern
life in women. The conventional treatments of gynecological cancers
remain unsatisfactory due to the adverse side effects. Early
diagnosis is essential for the effective treatment of gynecological
cancers. As MALAT1 expression is upregulated in different types of
human cancer, including gynecological cancers, MALAT1 may act as a
potential diagnostic marker, and may be recognized as an effective
therapeutic target for gynecological cancers. However, more
clinical studies are required to determine the prognostic potential
of MALAT1 in gynecological cancers. A better understanding of the
role of MALAT1 in tumor progression and tumor metastasis may help
discover novel anti-metastatic targets for the effective treatment
of gynecological cancers.
Acknowledgements
Not applicable.
Funding
The present review was supported by the Provincial
Science Foundation of Hubei, China (grant no. 2020cfb702).
Availability of data and materials
Not applicable.
Authors' contributions
FHQ drafted the initial manuscript, MT and HYL
conceived the present review and revised the manuscript for
important intellectual content. MT and HYL confirmed the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jeong HM, Kwon MJ and Shin YK:
Overexpression of cancer-associated genes via epigenetic
derepression mechanisms in gynecologic cancer. Front Oncol.
4:122014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou XM, Zhang H and Han X: Role of
epithelial to mesenchymal transition proteins in gynecological
cancers: Pathological and therapeutic perspectives. Tumour Biol.
35:9523–9530. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu DT: EMMPRIN in gynecologic cancers:
Pathologic and therapeutic aspects. Tumour Biol. 36:4883–4888.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gusberg SB: The gynecologist and breast
cancer. Isr J Med Sci. 17:843–846. 1981.PubMed/NCBI
|
|
5
|
Imani S, Hosseinifard H, Cheng J, Wei C
and Fu J: Prognostic value of EMT-inducing transcription factors
(EMT-TFs) in metastatic breast cancer: A systematic review and
meta-analysis. Sci Rep. 6:285872016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu S, Wang H, Zhang L, Tang C, Jones L,
Ye H, Ban L, Wang A, Liu Z, Lou F, et al: Rapid detection of
genetic mutations in individual breast cancer patients by
next-generation DNA sequencing. Hum Genomics. 9:22015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brody JG, Rudel RA, Michels KB, Moysich
KB, Bernstein L, Attfield KR and Gray S: Environmental pollutants,
diet, physical activity, body size, and breast cancer: Where do we
stand in research to identify opportunities for prevention? Cancer.
109 (12 Suppl):S2627–S2634. 2007. View Article : Google Scholar
|
|
8
|
Rezaeian M, Sharifirad G, Mostafavi F,
Moodi M and Abbasi MH: The effects of breast cancer educational
intervention on knowledge and health beliefs of women 40 years and
older, Isfahan, Iran. J Educ Health Promot. 3:432014.PubMed/NCBI
|
|
9
|
Tania M, Khan MA and Song Y: Association
of lipid metabolism with ovarian cancer. Curr Oncol. 17:6–11. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goff BA: Ovarian cancer: Screening and
early detection. Obstet Gynecol Clin North Am. 39:183–194. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Piek JM, van Diest PJ and Verheijen RH:
Ovarian carcinogenesis: An alternative hypothesis. Adv Exp Med
Biol. 622:79–87. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vargas-Hernández VM, Moreno-Eutimio MA,
Acosta-Altamirano G and Vargas-Aguilar VM: Management of recurrent
epithelial ovarian cancer. Gland Surg. 3:198–202. 2014.PubMed/NCBI
|
|
13
|
Canavan TP and Doshi NR: Cervical cancer.
Am Fam Physician. 61:1369–1376. 2000.PubMed/NCBI
|
|
14
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Muñoz N, Castellsagué X, de González AB
and Gissmann L: Chapter 1: HPV in the etiology of human cancer.
Vaccine. 24 (Suppl 3):S3/1–10. 2006. View Article : Google Scholar
|
|
16
|
Pessoa JN, Freitas AC, Guimaraes RA, Lima
J, Dos Reis HL and Filho AC: Endometrial assessment: When is it
necessary? J Clin Med Res. 6:21–25. 2014.PubMed/NCBI
|
|
17
|
Colombo N, Preti E, Landoni F, Carinelli
S, Colombo A, Marini C and Sessa C; ESMO Guidelines Working Group,
: Endometrial cancer: ESMO clinical practice guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 24 (Suppl
6):vi33–vi38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lozano R, Naghavi M, Foreman K, Lim S,
Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et
al: Global and regional mortality from 235 causes of death for 20
age groups in 1990 and 2010: A systematic analysis for the global
burden of disease study 2010. Lancet. 380:2095–2128. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dittmer C, Katalinic A, Mundhenke C, Thill
M and Fischer D: Epidemiology of vulvar and vaginal cancer in
Germany. Arch Gynecol Obstet. 284:169–174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kopp F and Mendell JT: Functional
classification and experimental dissection of long noncoding RNAs.
Cell. 172:393–407. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bassett AR, Akhtar A, Barlow DP, Bird AP,
Brockdorff N, Duboule D, Ephrussi A, Ferguson-Smith AC, Gingeras
TR, Haerty W, et al: Considerations when investigating lncRNA
function in vivo. Elife. 3:e030582014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Soudyab M, Iranpour M and Ghafouri-Fard S:
The role of long non-coding RNAs in breast cancer. Arch Iran Med.
19:508–517. 2016.PubMed/NCBI
|
|
23
|
Geisler S and Coller J: RNA in unexpected
places: Long non-coding RNA functions in diverse cellular contexts.
Nat Rev Mol Cell Biol. 14:699–712. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grote P and Herrmann BG: Long noncoding
RNAs in organogenesis: Making the difference. Trends Genet.
31:329–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nagano T and Fraser P: No-nonsense
functions for long noncoding RNAs. Cell. 145:178–181. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu Y, Luo M, Brooks M, Clouthier SG and
Wicha MS: Biological and clinical significance of cancer stem cell
plasticity. Clin Transl Med. 3:322014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ørom UA and Shiekhattar R: Long noncoding
RNAs usher in a new era in the biology of enhancers. Cell.
154:1190–1193. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wahlestedt C: Targeting long non-coding
RNA to therapeutically upregulate gene expression. Nat Rev Drug
Discov. 12:433–446. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang X, Hamblin MH and Yin KJ: The long
noncoding RNA Malat1: Its physiological and pathophysiological
functions. RNA Biol. 14:1705–1714. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hutchinson JN, Ensminger AW, Clemson CM,
Lynch CR, Lawrence JB and Chess A: A screen for nuclear transcripts
identifies two linked noncoding RNAs associated with SC35 splicing
domains. BMC Genomics. 8:392007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang B, Arun G, Mao YS, Lazar Z, Hung G,
Bhattacharjee G, Xiao X, Booth CJ, Wu J, Zhang C and Spector DL:
The lncRNA Malat1 is dispensable for mouse development but its
transcription plays a cis-regulatory role in the adult. Cell Rep.
2:111–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bernard D, Prasanth KV, Tripathi V,
Colasse S, Nakamura T, Xuan Z, Zhang MQ, Sedel F, Jourdren L,
Coulpier F, et al: A long nuclear-retained non-coding RNA regulates
synaptogenesis by modulating gene expression. EMBO J. 29:3082–3093.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wilusz JE, Freier SM and Spector DL: 3′
end processing of a long nuclear-retained noncoding RNA yields a
tRNA-like cytoplasmic RNA. Cell. 135:919–932. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tano K, Mizuno R, Okada T, Rakwal R,
Shibato J, Masuo Y, Ijiri K and Akimitsu N: MALAT-1 enhances cell
motility of lung adenocarcinoma cells by influencing the expression
of motility-related genes. FEBS Lett. 584:4575–4580. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang L, Lin C, Liu W, Zhang J, Ohgi KA,
Grinstein JD, Dorrestein PC and Rosenfeld MG: ncRNA- and Pc2
methylation-dependent gene relocation between nuclear structures
mediates gene activation programs. Cell. 147:773–788. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ji P, Diederichs S, Wang W, Böing S,
Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, et
al: MALAT-1, a novel noncoding RNA, and thymosin beta4 predict
metastasis and survival in early-stage non-small cell lung cancer.
Oncogene. 22:8031–8041. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gutschner T, Hämmerle M, Eissmann M, Hsu
J, Kim Y, Hung G, Revenko A, Arun G, Stentrup M, Gross M, et al:
The noncoding RNA MALAT1 is a critical regulator of the metastasis
phenotype of lung cancer cells. Cancer Res. 73:1180–1189. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shi X, Sun M, Wu Y, Yao Y, Liu H, Wu G,
Yuan D and Song Y: Post-transcriptional regulation of long
noncoding RNAs in cancer. Tumour Biol. 36:503–513. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li Z, Xu L, Liu Y, Fu S, Tu J, Hu Y and
Xiong Q: LncRNA MALAT1 promotes relapse of breast cancer patients
with postoperative fever. Am J Transl Res. 10:3186–3197.
2018.PubMed/NCBI
|
|
40
|
Zhao M, Wang S, Li Q, Ji Q, Guo P and Liu
X: MALAT1: A long non-coding RNA highly associated with human
cancers. Oncol Lett. 16:19–26. 2018.PubMed/NCBI
|
|
41
|
Tee AE, Ling D, Nelson C, Atmadibrata B,
Dinger ME, Xu N, Mizukami T, Liu PY, Liu B, Cheung B, et al: The
histone demethylase JMJD1A induces cell migration and invasion by
up-regulating the expression of the long noncoding RNA MALAT1.
Oncotarget. 5:1793–1804. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Peng R, Luo C, Guo Q, Cao J, Yang Q, Dong
K, Wang S, Wang K and Song C: Association analyses of genetic
variants in long non-coding RNA MALAT1 with breast cancer
susceptibility and mRNA expression of MALAT1 in Chinese Han
population. Gene. 642:241–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen Q, Zhu C and Jin Y: The oncogenic and
tumor suppressive functions of the long noncoding RNA MALAT1: An
emerging controversy. Front Genet. 11:932020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ni W, Wang X, Sun Y and Gao X:
Meta-analysis of the association between MALAT1 rs619586 A>G
polymorphism and cancer risk. J Int Med Res.
48:3000605209419692020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang P, Zhou H, Lu K, Lu Y, Wang Y and
Feng T: Exosome-mediated delivery of MALAT1 induces cell
proliferation in breast cancer. Onco Targets Ther. 11:291–299.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang Y, Zhang Y, Hu K, Qiu J, Hu Y, Zhou M
and Zhang S: Elevated long noncoding RNA MALAT-1 expression is
predictive of poor prognosis in patients with breast cancer: A
meta-analysis. Biosci Rep. 40:BSR202002152020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Stone JK, Kim JH, Vukadin L, Richard A,
Giannini HK, Lim SS, Tan M and Ahn EE: Hypoxia induces cancer
cell-specific chromatin interactions and increases MALAT1
expression in breast cancer cells. J Biol Chem. 294:11213–11224.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zheng L, Zhang Y, Fu Y, Gong H, Guo J, Wu
K, Jia Q and Ding X: Long non-coding RNA MALAT1 regulates BLCAP
mRNA expression through binding to miR-339-5p and promotes poor
prognosis in breast cancer. Biosci Rep. 39:BSR201812842019.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fattahi Dolatabadi N, Dehghani A, Shahand
E, Yazdanshenas M, Tabatabaeian H, Zamani A, Azadeh M and Ghaedi K:
The interaction between MALAT1 target, miR-143-3p, and RALGAPA2 is
affected by functional SNP rs3827693 in breast cancer. Hum Cell.
33:1229–1239. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gomes CP, Nóbrega-Pereira S,
Domingues-Silva B, Rebelo K, Alves-Vale C, Marinho SP, Carvalho T,
Dias S and Bernardes de Jesus B: An antisense transcript mediates
MALAT1 response in human breast cancer. BMC Cancer. 19:7712019.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wu Y, Sarkissyan M, Ogah O, Kim J and
Vadgama JV: Expression of MALAT1 promotes trastuzumab resistance in
HER2 overexpressing breast cancers. Cancers (Basel). 12:19182020.
View Article : Google Scholar
|
|
52
|
Huang XJ, Xia Y, He GF, Zheng LL, Cai YP,
Yin Y and Wu Q: MALAT1 promotes angiogenesis of breast cancer.
Oncol Rep. 40:2683–2689. 2018.PubMed/NCBI
|
|
53
|
Kim J, Piao HL, Kim BJ, Yao F, Han Z, Wang
Y, Xiao Z, Siverly AN, Lawhon SE, Ton BN, et al: Long noncoding RNA
MALAT1 suppresses breast cancer metastasis. Nat Genet.
50:1705–1715. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kwok ZH, Roche V, Chew XH, Fadieieva A and
Tay Y: A non-canonical tumor suppressive role for the long
non-coding RNA MALAT1 in colon and breast cancers. Int J Cancer.
143:668–678. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Eastlack SC, Dong S, Mo YY and Alahari SK:
Expression of long noncoding RNA MALAT1 correlates with increased
levels of Nischarin and inhibits oncogenic cell functions in breast
cancer. PLoS One. 13:e01989452018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Arun G and Spector DL: MALAT1 long
non-coding RNA and breast cancer. RNA Biol. 16:860–863. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu SP, Yang JX, Cao DY and Shen K:
Identification of differentially expressed long non-coding RNAs in
human ovarian cancer cells with different metastatic potentials.
Cancer Biol Med. 10:138–141. 2013.PubMed/NCBI
|
|
58
|
Hosseini ES, Meryet-Figuiere M,
Sabzalipoor H, Kashani HH, Nikzad H and Asemi Z: Dysregulated
expression of long noncoding RNAs in gynecologic cancers. Mol
Cancer. 16:1072017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sun Q, Li Q and Xie F: LncRNA-MALAT1
regulates proliferation and apoptosis of ovarian cancer cells by
targeting miR-503-5p. Onco Targets Ther. 12:6297–6307. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Khan MA, Chen HC, Zhang D and Fu J: Twist:
A molecular target in cancer therapeutics. Tumour Biol.
34:2497–2506. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tania M, Khan MA and Fu J: Epithelial to
mesenchymal transition inducing transcription factors and
metastatic cancer. Tumour Biol. 35:7335–7342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Jin Y, Feng SJ, Qiu S, Shao N and Zheng
JH: LncRNA MALAT1 promotes proliferation and metastasis in
epithelial ovarian cancer via the PI3K-AKT pathway. Eur Rev Med
Pharmacol Sci. 21:3176–3184. 2017.PubMed/NCBI
|
|
63
|
Guo C and Wang X, Chen LP, Li M, Li M, Hu
YH, Ding WH and Wang X: Long non-coding RNA MALAT1 regulates
ovarian cancer cell proliferation, migration and apoptosis through
Wnt/β-catenin signaling pathway. Eur Rev Med Pharmacol Sci.
22:3703–3712. 2018.PubMed/NCBI
|
|
64
|
Wang ZM, Wan XH, Sang GY, Zhao JD, Zhu QY
and Wang DM: miR-15a-5p suppresses endometrial cancer cell growth
via Wnt/β-catenin signaling pathway by inhibiting WNT3A. Eur Rev
Med Pharmacol Sci. 21:4810–4818. 2017.PubMed/NCBI
|
|
65
|
Qiu JJ, Lin XJ, Tang XY, Zheng TT, Lin YY
and Hua KQ: Exosomal metastasis-associated lung adenocarcinoma
transcript 1 promotes angiogenesis and predicts poor prognosis in
epithelial ovarian cancer. Int J Biol Sci. 14:1960–1973. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhou Y, Xu X, Lv H, Wen Q, Li J, Tan L, Li
J and Sheng X: The long noncoding RNA MALAT-1 is highly expressed
in ovarian cancer and induces cell growth and migration. PLoS One.
11:e01552502016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yang L, Bai HS, Deng Y and Fan L: High
MALAT1 expression predicts a poor prognosis of cervical cancer and
promotes cancer cell growth and invasion. Eur Rev Med Pharmacol
Sci. 19:3187–3193. 2015.PubMed/NCBI
|
|
68
|
Jiang Y, Li Y, Fang S, Jiang B, Qin C, Xie
P, Zhou G and Li G: The role of MALAT1 correlates with HPV in
cervical cancer. Oncol Lett. 7:2135–2141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lu H, He Y, Lin L, Qi Z, Ma L, Li L and Su
Y: Long non-coding RNA MALAT1 modulates radiosensitivity of HR-HPV+
cervical cancer via sponging miR-145. Tumour Biol. 37:1683–1691.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Han X, Wang Q, Wang Y, Hu B, Dong X, Zhang
H and Wang W: Long non-coding RNA metastasis-associated lung
adenocarcinoma transcript 1/microRNA-202-3p/periostin axis
modulates invasion and epithelial-mesenchymal transition in human
cervical cancer. J Cell Physiol. 234:14170–14180. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang N, Hou MS, Zhan Y, Shen XB and Xue
HY: MALAT1 promotes cisplatin resistance in cervical cancer by
activating the PI3K/AKT pathway. Eur Rev Med Pharmacol Sci.
22:7653–7659. 2018.PubMed/NCBI
|
|
72
|
Zhao Y, Yang Y, Trovik J, Sun K, Zhou L,
Jiang P, Lau TS, Hoivik EA, Salvesen HB, Sun H and Wang H: A novel
wnt regulatory axis in endometrioid endometrial cancer. Cancer Res.
74:5103–5117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chen G, Zhang M, Liang Z, Chen S, Chen F,
Zhu J, Zhao M, He J, Hua W and Duan P: Association of polymorphisms
in MALAT1 with the risk of endometrial cancer in Southern Chinese
women. J Clin Lab Anal. 34:e231462020.PubMed/NCBI
|
|
74
|
Cao X, Zhao R, Chen Q, Zhao Y, Zhang B,
Zhang Y, Yu J, Han G, Cao W, Li J and Chen X: MALAT1 might be a
predictive marker of poor prognosis in patients who underwent
radical resection of middle thoracic esophageal squamous cell
carcinoma. Cancer Biomark. 15:717–723. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Liu R, Li J, Lai Y, Liao Y, Liu R and Qiu
W: Hsa-miR-1 suppresses breast cancer development by
down-regulating K-ras and long non-coding RNA MALAT1. Int J Biol
Macromol. 81:491–497. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Arun G, Diermeier S, Akerman M, Chang KC,
Wilkinson JE, Hearn S, Kim Y, MacLeod AR, Krainer AR, Norton L, et
al: Differentiation of mammary tumors and reduction in metastasis
upon Malat1 lncRNA loss. Genes Dev. 30:34–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Gordon MA, Babbs B, Cochrane DR, Bitler BG
and Richer JK: The long non-coding RNA MALAT1 promotes ovarian
cancer progression by regulating RBFOX2-mediated alternative
splicing. Mol Carcinog. 58:196–205. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Bai L, Wang A, Zhang Y, Xu X and Zhang X:
Knockdown of MALAT1 enhances chemosensitivity of ovarian cancer
cells to cisplatin through inhibiting the Notch1 signaling pathway.
Exp Cell Res. 366:161–171. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Worku T, Bhattarai D, Ayers D, Wang K,
Wang C, Rehman ZU, Talpur HS and Yang L: Long non-coding RNAs: The
new horizon of gene regulation in ovarian cancer. Cell Physiol
Biochem. 44:948–966. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Pa M, Naizaer G, Seyiti A and Kuerbang G:
Long noncoding RNA MALAT1 functions as a sponge of MiR-200c in
ovarian cancer. Oncol Res. Sep 11–2017.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lei R, Xue M, Zhang L and Lin Z: Long
noncoding RNA MALAT1-regulated microRNA 506 modulates ovarian
cancer growth by targeting iASPP. Onco Targets Ther. 10:35–46.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zhu P, Wang FQ and Li QR: Correlation
study between long non-coding RNA MALAT1 and radiotherapy
efficiency on cervical carcinoma and generation of radiotherapy
resistant model of cancer. Eur Rev Med Pharmacol Sci. 22:5140–5148.
2018.PubMed/NCBI
|
|
83
|
Sun R, Qin C, Jiang B, Fang S, Pan X, Peng
L, Liu Z, Li W, Li Y and Li G: Down-regulation of MALAT1 inhibits
cervical cancer cell invasion and metastasis by inhibition of
epithelial-mesenchymal transition. Mol Biosyst. 12:952–962. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Xia C, Liang S, He Z, Zhu X, Chen R and
Chen J: Metformin, a first-line drug for type 2 diabetes mellitus,
disrupts the MALAT1/miR-142-3p sponge to decrease invasion and
migration in cervical cancer cells. Eur J Pharmacol. 830:59–67.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Li Q, Zhang C, Chen R, Xiong H, Qiu F, Liu
S, Zhang M, Wang F, Wang Y, Zhou X, et al: Disrupting
MALAT1/miR-200c sponge decreases invasion and migration in
endometrioid endometrial carcinoma. Cancer Lett. 383:28–40. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Tian X and Xu G: Clinical value of lncRNA
MALAT1 as a prognostic marker in human cancer: Systematic review
and meta-analysis. BMJ Open. 5:e0086532015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Du Z, Fei T, Verhaak RG, Su Z, Zhang Y,
Brown M, Chen Y and Liu XS: Integrative genomic analyses reveal
clinically relevant long noncoding RNAs in human cancer. Nat Struct
Mol Biol. 20:908–913. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Liao H, Chen Q and Xiao J: Reflections on
the role of Malat1 in gynecological cancer. Cancer Manag Res.
12:13489–13500. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Sun Y and Ma L: New insights into long
non-coding RNA MALAT1 in cancer and metastasis. Cancers (Basel).
11:2162019. View Article : Google Scholar
|
|
90
|
Amodio N, Raimondi L, Juli G, Stamato MA,
Caracciolo D, Tagliaferri P and Tassone P: MALAT1: A druggable long
non-coding RNA for targeted anti-cancer approaches. J Hematol
Oncol. 11:632018. View Article : Google Scholar : PubMed/NCBI
|