Introduction
Bone cancer pain (BCP) is one of the most common and
intractable symptoms affecting patients with primary or metastatic
bone cancer, and is characterized by allodynia or hyperalgesia
(1). Despite understanding the
effects of BCP on the health-associated quality of life, BCP
remains inadequately controlled in numerous patients (2), due to limited breakthroughs made in
understanding the underlying mechanisms and developing therapeutics
for BCP (3).
Piezo2, which is a member of the evolutionarily
conserved mechanosensitive Piezo channel family, is a rapidly
adapting mechanically-activated nonselective cation channel that is
most prominently expressed in sensory tissues, especially in the
dorsal root ganglion (DRG) neurons (4). Most rapidly adapting
mechanically-activated currents in DRG neuronal cultures are absent
in Piezo2-conditional knockout mice (5). Mice lacking Piezo2 not only exhibit
impaired nociceptive responses to mechanical stimuli in the sensory
neurons, but also do not react to capsaicin-induced inflammation or
spared nerve injury (6). In
addition, individuals with loss-of-function mutations in Piezo2
fail to develop sensitization and reactions to mechanical stimuli
following skin inflammation (7).
These studies in mice and human patients indicate the essential
role of Piezo2 in mechanical allodynia.
Numerous studies have demonstrated that cyclic
adenosine monophosphate (cAMP) signaling serves an important role
in regulating pain sensitivity through the exchange protein
directly activated by cAMP (Epac), which has two isoforms, namely
Epac1 and Epac2 (8–10). Intraplantar injection of the Epac
agonist 8-pCPT induces long-lasting mechanical allodynia (11); however, nerve damage-induced
mechanical allodynia is markedly decreased in Epac1−/−
mice (12). Eijkelkamp et al
(12) have reported that DRG Epac1
potentiation of Piezo2-mediated mechanotransduction contributes to
mechanical allodynia. Together, these data suggest that the
Epac1-Piezo2 signaling pathway may contribute to the establishment
of mechanical allodynia in multiple forms of pain (12). However, to the best of our knowledge,
there are currently no reports on the role of Piezo2 in BCP.
The present study used a mouse model of
osteosarcoma-associated BCP to determine whether the DRG
Epac1-Piezo2 signaling pathway may be responsible for the
mechanical allodynia of BCP and to further examine whether
N-methyl-D-aspartic acid (NMDA) receptor subunit 2B (NR2B), which
is as an important regulatory factor, is involved in this
mechanism.
Materials and methods
Animals
Male C3H/HeJ mice, weighing 20–25 g (4–6 weeks old),
were provided by the Vital River Laboratory Animal Technology
Company. The animals were housed in a temperature-controlled room
(21±1°C) with a 12-h dark/light cycle and were provided ad
libitum food and water. Mice were used for behavioral
experiments (n=8 mice/group), western blotting and reverse
transcription-quantitative (RT-q) PCR assays (n=3 mice/group). All
protocols were approved by the Animal Care and Use Committee of
Nanjing University (Nanjing, China) and followed the relevant
ethical guidelines for the use of laboratory animals (13).
Cell culture and implantation
Osteosarcoma NCTC 2472 cells (cat. no. 2087787;
American Type Culture Collection) were incubated in NCTC 135 medium
(Sigma-Aldrich; Merck KGaA) supplemented with 10% horse serum
(Gibco; Thermo Fisher Scientific, Inc.) at 37°C in 5%
CO2 and 95% air.
The mouse model of BCP was established according to
previous studies (14,15). Mice were intraperitoneally injected
with 50 mg/kg pentobarbital sodium (1% in normal saline).
Gonarthrotomy was performed to expose the condyles of the distal
femur. A 30-gauge needle was used to perforate the cortex, and
1×105−1×106 NCTC 2472 cells in 20 µl
α-minimal essential medium (α-MEM; Gibco; Thermo Fisher Scientific,
Inc.) was injected into the intramedullary space of the femur with
a microsyringe. The femurs of the mice in the sham group were
injected with 20 µl α-MEM alone. The injection hole was sealed with
bone wax, and the wound was sutured closed.
Drug preparation and injection
The Epac activator 8-pCPT was obtained from the
Biology Life Science Institute, and the NMDA receptor agonist NMDA
and the NR2B-selective antagonist ifenprodil were purchased from
Sigma-Aldrich; Merck KGaA. The Epac1 antisense (AS) or mismatch
(MM) oligodeoxynucleotides (ODNs) were obtained from Thermo Fisher
Scientific, Inc. The following Epac1-ODNs were used (12): ASODN, 5′-AACTCTCCACCCTCTCCCA-3′; and
MMODN, 5′-ACATTCCACCCTCCTCCAC-3′. All reagents were dissolved in
normal saline.
Mice received an intraplantar injection of 2.5 µl
8-pCPT (12.5 nmol) (12) or
intrathecal administration of Epac1-ODN (10 µg/5 µl) (12), NMDA (1 nmol/5 µl) (16) and ifenprodil (5 µg/5 µl) (17).
Mechanical allodynia tests
Mechanical allodynia was evaluated as previously
described (14,18). Mice were individually placed into a
transparent plastic chamber with a metal mesh bottom and allowed to
habituate for 30 min prior to testing. Paw withdrawal mechanical
threshold (PWMT) of the right hind paw was measured using von Frey
filaments (0.16, 0.40, 0.60, 1.00, 1.40 and 2.00 g). The filaments
were used to vertically push against the plantar surface of the
right hind paw. A positive response was brisk withdrawal or licking
of the stimulated hind paw. Each mouse was tested five times per
stimulus force. The lowest filament that evoked ≥3 positive
reactions was set as the PWMT value.
Western blotting
Mice were anesthetized with 50 mg/kg sodium
pentobarbital (1% in normal saline) and sacrificed by cervical
dislocation. The right lumbar DRG L2-L5 segments were rapidly
removed and stored in liquid nitrogen. The tissue samples were
homogenized with 10 µl/mg RIPA lysis buffer (cat. no. C1053;
Applygen Technologies, Inc.) and centrifuged (4°C) at 25,600 × g
for 10 min. The protein concentration was determined using a BCA
Protein Assay kit (Nanjing KeyGen Biotech Co., Ltd.). The protein
samples (50 µg/sample) were separated by 10% SDS-PAGE (Bio-Rad
Laboratories, Inc.) and transferred to a PVDF membrane (EMD
Millipore). The membrane was blocked with a 5% non-fat milk
solution at room temperature for 1 h and subsequently incubated
with the following primary antibodies at 4°C overnight: Rabbit
anti-Epac1 (1:1,000; cat. no. ab124162; Abcam), anti-phosphorylated
(p-)NR2B (1:1,000; cat. no. ab65783; Abcam) and mouse anti-β-actin
(1:2,000; cat. no. TA-09; OriGene Technologies, Inc.). The next
day, the blots were incubated at room temperature with goat
anti-rabbit (1:2,000; cat. no. ZB-2301) or goat anti-mouse
(1:2,000; cat. no. ZB-2305) (both from OriGene Technologies, Inc.)
secondary antibodies conjugated with horseradish peroxidase.
Immunoblots were developed using an enhanced chemiluminescence
detection system [Hangzhou MultiSciences (Lianke) Biotech Co.,
Ltd.) and quantified using Quantity One V4.62 software (Bio-Rad
Laboratories, Inc.). β-actin was used as the loading control.
RT-qPCR
Mouse right lumbar DRG (L2-L5) segments were
isolated and frozen in liquid nitrogen. Total RNA was isolated and
purified using TRIzol® reagent (Beijing CWbio). The RNA
concentration was measured using an ultraviolet-visible
spectrometer (Shanghai Meipuda Instrument Co., Ltd.). Reverse
transcription was performed using an RT-PCR kit (Takara
Biotechnology Co., Ltd), and qPCR was performed using a
SYBR® PrimeScript qRT-PCR kit (Takara Biotechnology Co.,
Ltd.) according to the manufacturer's protocol. The following
primer pairs were used (12): Piezo2
forward, 5′-ATTGGCTGGAGGAGAAA-3′ and reverse,
5′-GAAGGTGGAAGAGTGGGAGT-3′; β-actin forward,
5′-AAGAAGGTGGTGAAGCAGG-3′ and reverse, 5′-GAAGGTGGAAGAGTGGGAGT-3′
(Sangon Biotech Co., Ltd.). The thermocycling conditions were as
follows: 95°C for 10 min, followed by 40 cycles of 95°C for 10 sec,
60°C for 30 sec and 72°C for 30 sec. The relative levels of mRNA
were calculated using the 2−ΔΔCq method and optimized
with a standard curve to confirm specificity (19). The change in the fluorescence of the
amplification product was used to determine the amount of original
template.
Statistical analysis
Data are presented as the mean ± SD. Repeated
measures analysis of variance (ANOVA) was performed to determine
the overall differences at each time point for PWMT. One-way ANOVA
was used to detect differences in the expression levels of proteins
or mRNAs among all experimental groups. Post hoc analysis was
performed using the LSD test for multiple comparisons in order to
determine the sources of difference. P<0.05 was considered to
indicate a statistically significant difference.
Results
Bone cancer-induced mechanical
allodynia and DRG Epac1, NR2B and Piezo2 expression levels over
time
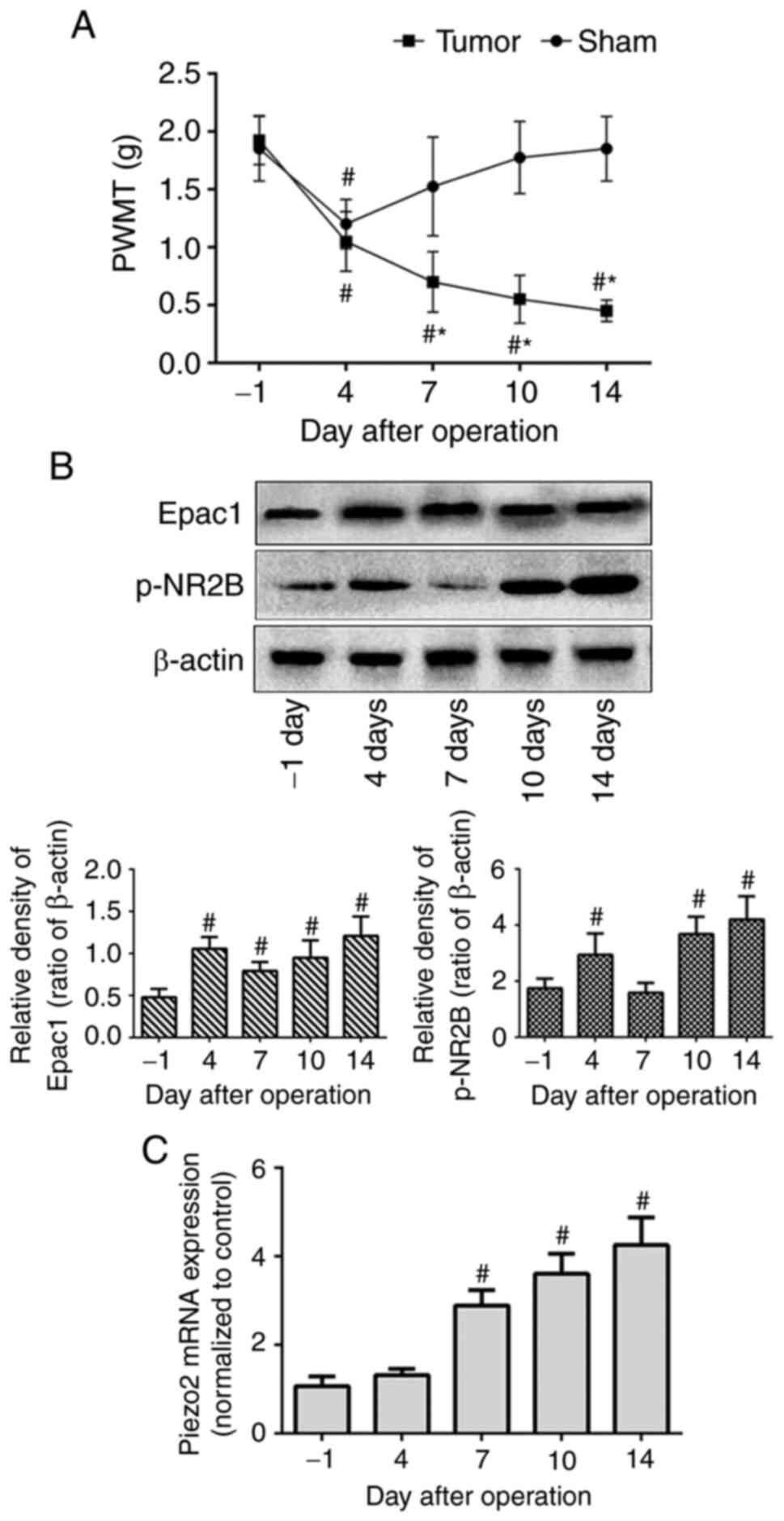
To assess mechanical allodynia in the BCP mouse
model, PWMT of the right hind limb was measured on the day before
modeling (day-1) as the baseline value, and at days 4, 7, 10 and 14
after modeling. No significant differences were observed in the
baseline PWMT (day-1) between the tumor-bearing and sham-operated
mice. In the sham group, the PWMT displayed a significant decrease
on day 4 and recovered to the pre-modeling level on day 7. Although
the tumor-bearing mice also exhibited a marked decrease in PWMT on
day 4, it subsequently continued to decrease over time until day 14
(Fig. 1A). These results
demonstrated the successful establishment of mechanical allodynia
in the BCP mouse model.
To examine the expression levels of Epac1, NR2B and
Piezo2 in the DRG of the BCP model mice, western blotting and
RT-qPCR assays were performed. Compared with those on day-1, the
protein expression levels of Epac1 and p-NR2B in the tumor-bearing
mice were increased on day 4 post-modeling and reached the highest
levels on day 14 (Fig. 1B). The mRNA
expression levels of Piezo2 were significantly increased on day 7
compared with those on day-1 and were gradually upregulated over
time until day 14 (Fig. 1C). Thus,
the increasing tendency of protein or mRNA expression, which
coincided with the PWMT changes, suggested that DRG Epac1, NR2B and
Piezo2 may be involved in the pathogenesis and development of
mechanical allodynia in BCP.
Effects of intrathecal administration
of Epac1-ASODN on bone cancer-induced mechanical allodynia and
protein or mRNA expression
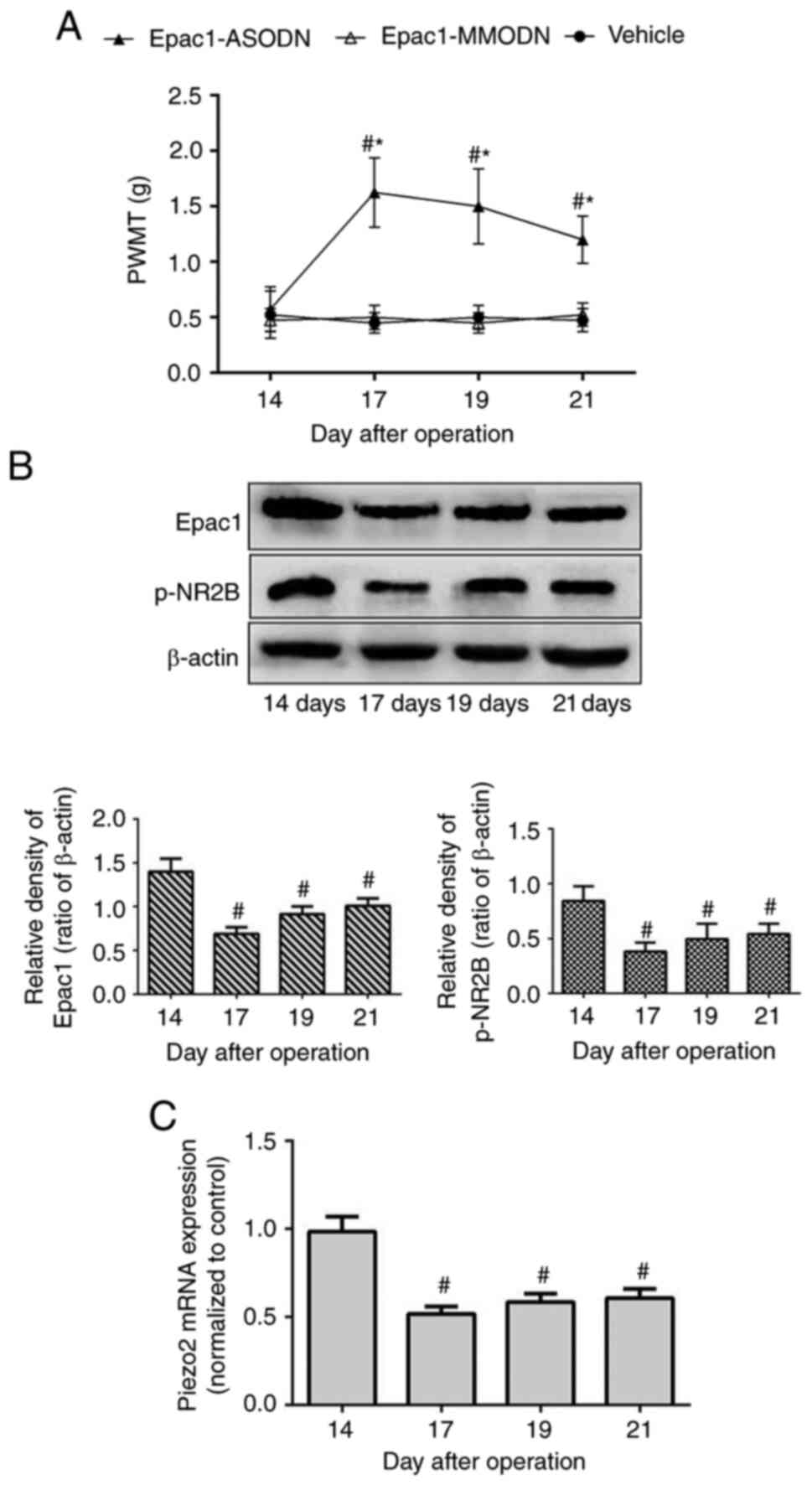
Based on the aforementioned results, the present
study further assessed the effects of intrathecal injections of
Epac1-ODN. Tumor-bearing mice were randomly divided into three
groups on day 14 post-modeling and received Epac1-ASODN,
Epac1-MMODN or saline once daily between days 14 and 16. PWMT was
measured on day 14 prior to administration and days 17, 19 and 21
post-modeling.
As demonstrated in Fig.
2A, mice in the vehicle (treated with saline) and Epac1-MMODN
groups exhibited a stable PWMT from day 14 throughout the
experiment. In contrast, Epac1-ASODN-treated mice exhibited an
increase in PWMT on day 17, and this effect was maintained until
day 21. In addition, Epac1-ASODN significantly downregulated the
protein expression levels of DRG Epac1 and p-NR2B, as well as the
Piezo2 mRNA expression levels, in a time-dependent manner (Fig. 2B and C). These results suggested that
the DRG Epac1-Piezo2 signaling pathway may be required for the
mechanical allodynia of BCP, and NR2B may be involved in the
underlying mechanism.
Effects of administration the Epac1 or
NR2B agonists on mechanical allodynia and protein or mRNA
expression
To elucidate the association between NR2B and the
Epac1-Piezo2 signaling pathway, the present study first tested
whether selective activation of Epac1 or NR2B increased mechanical
allodynia. The Epac agonist 8-pCPT was injected into the right hind
paw of normal mice as previously described (12). Compared with that in the vehicle
group treated with saline, 8-pCPT significantly decreased the PWMT
in the ipsilateral hind paw of normal mice ~5 min after
administration, and the lowest PWMT values were observed at 30 min.
Similarly, intrathecal injection of NMDA decreased the PWMT in the
right hind paw ~5 min after injection, with the lowest values
observed at 15 min post-injection (Fig.
3A). In addition to increasing the expression levels of the
tested proteins (Fig. 3B), 8-pCPT
and NMDA also increased the ipsilateral DRG Piezo2 mRNA expression
levels in normal mice (Fig. 3D).
These results demonstrated that not only Epac1, but also NR2B was
involved in the regulation of the Piezo2-mediated mechanical
allodynia.
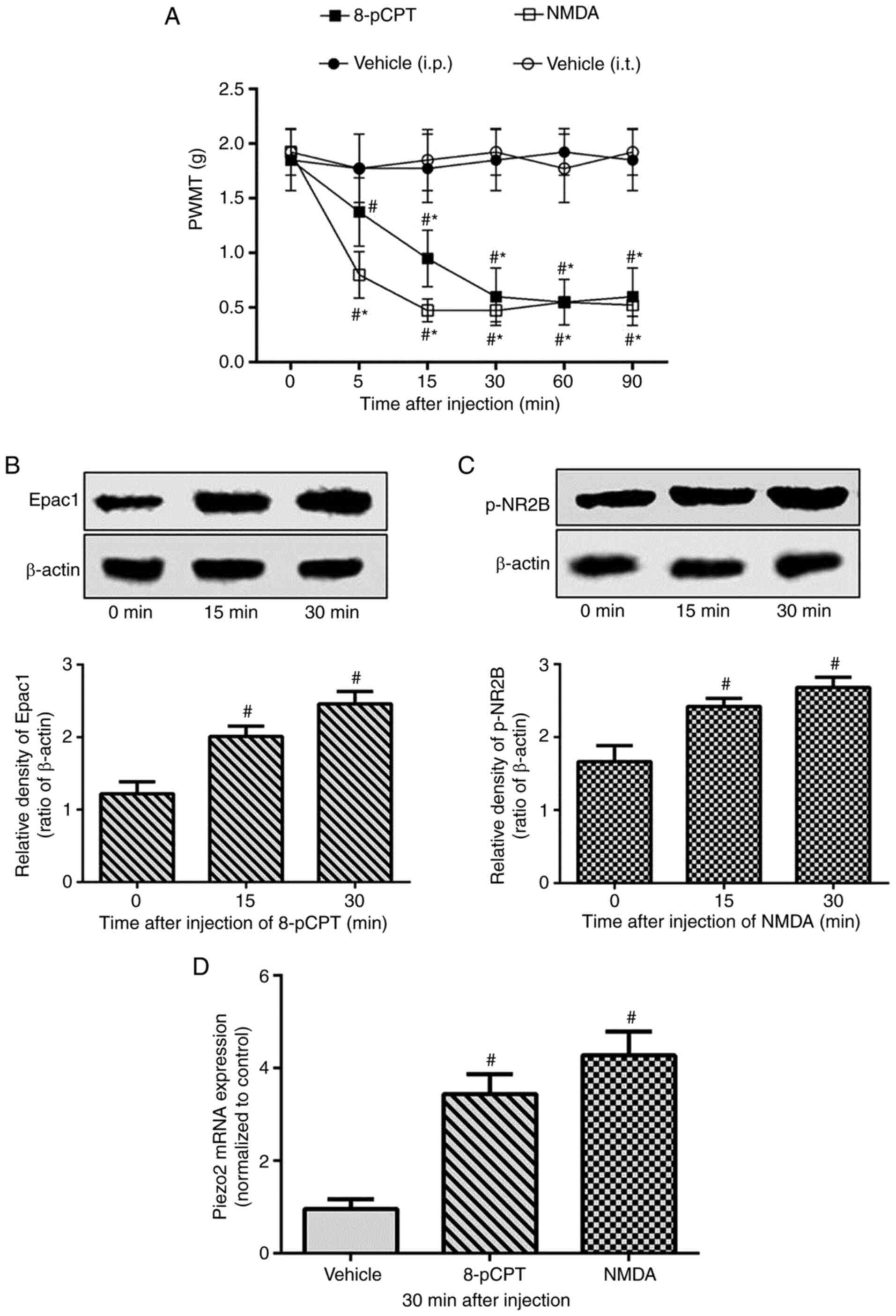 | Figure 3.Exacerbation of mechanical allodynia
and upregulation of DRG Piezo2 expression by selective activation
of Epac1 or NR2B in normal mice. Normal mice were randomly divided
into three groups (n=8/group) and received intrathecal (i.t.)
saline (5 µl) and NMDA (1 nmol/5 µl), or intraplantar saline (5 µl)
and 8-pCPT (12.5 nmol/5 µl), respectively. (A) PWMT was measured at
0 (before injection), 5, 15, 30, 60 and 90 min after intrathecal or
intraplantar injection. (B-D) The expression level of the right
lumbar DRG L2-L5 segments of Epac1in 8-pCPT group, p-NR2B in NMDA
group and Piezo2 mRNA in both groups were detected at the time of
0, 15 or 30 min after injection. Data represents means ± SD.
#P<0.05 vs. 0 min; *P<0.05 vs. vehicle group at
each time point. DRG, dorsal root ganglion; PWMT, paw withdrawal
mechanical threshold. |
Effects of intrathecal pretreatment on
the Epac1 or NR2B agonist-induced mechanical allodynia and protein
or mRNA expression
To further clarify the association between Epac1 and
NR2B in the regulation of Piezo2, normal mice were intrathecally
pretreated with ifenprodil or Epac1-ASODN prior to the injection of
8-pCPT or NMDA. Compared with the control group (pretreated with
saline 5 min before 8-pCPT), intrathecal pretreatment with
ifenprodil prevented the decrease in the PWMT (Fig. 4A) and the increase in ipsilateral DRG
Piezo2 mRNA expression levels (Fig.
4B) induced by 8-pCPT, but had no effects on Epac1 expression
levels (Fig. 4C). In contrast,
intrathecal injection of NMDA induced a decrease in the PWMT
(Fig. 4A) and elevated the levels of
Piezo2 and p-NR2B expression in normal mice (Fig. 4B and D), which was not affected by
pretreatment with Epac1-ASODN. These results suggested that NR2B,
which is a critical downstream regulator of Epac1, may be involved
in the regulation of mechanical allodynia by the Epac1-Piezo2
signaling pathway.
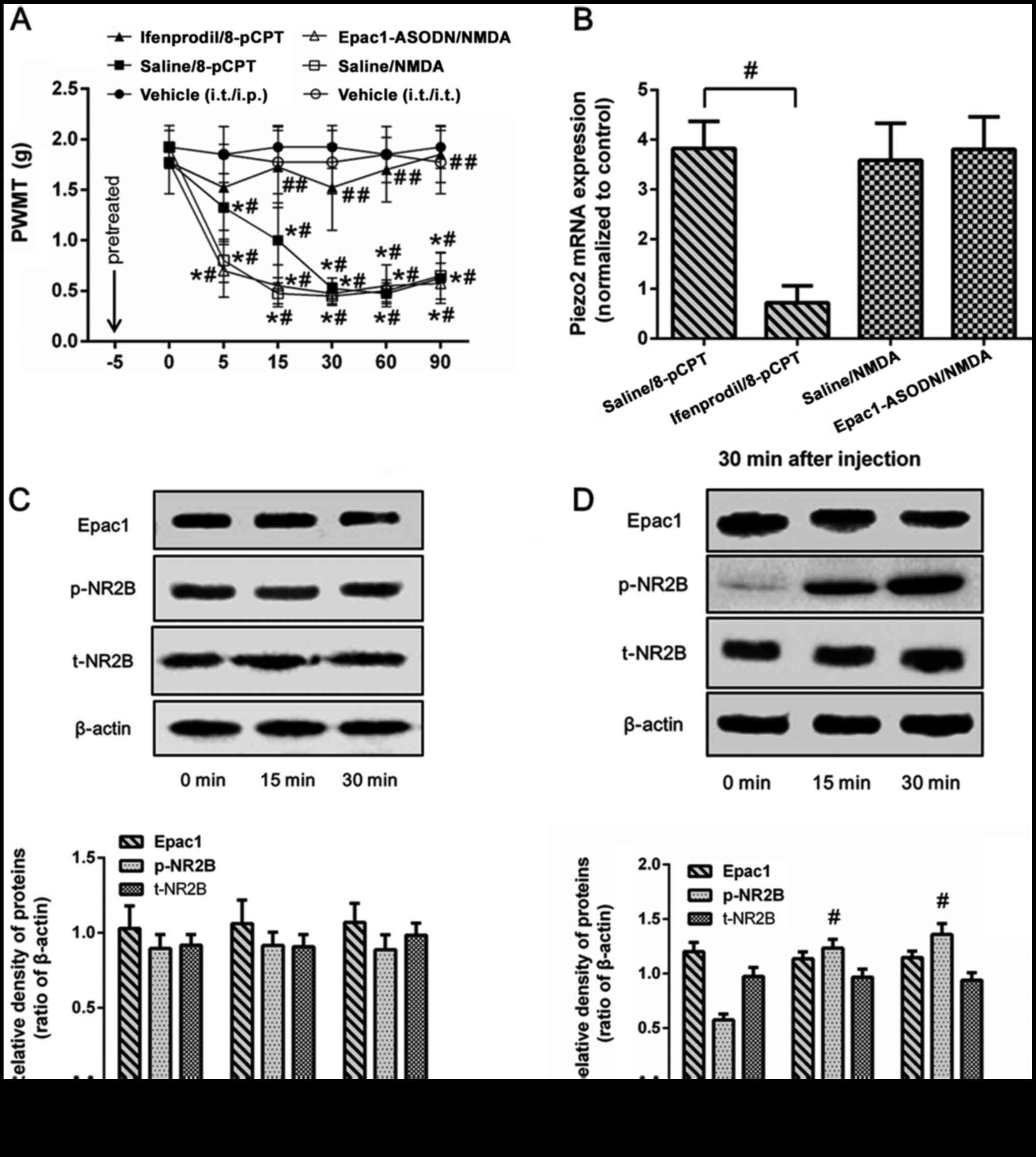 | Figure 4.Different effects of antagonist
pretreatment on Epac1 or NR2B agonist-induced mechanical allodynia
and DRG Piezo2 expression in normal mice. Normal mice were randomly
divided in five groups (n=8/group): Pretreated with ifenprodil or
saline and 5 min later intraplantar injection of 8-pCPT
(ifenprodil/8-pCPT group and saline/8-pCTP group); pretreated with
Epac1-ASODN or saline and then intrathecal administration of NMDA
(Epac1-ASODN/NMDA group and saline/NMDA group); intrathecal only or
intrathecal and intraplantar injection of saline (vehicle group).
(A) PWMT was measured at 0, 5, 15, 30, 60 and 90 min after injected
with agonist. (B-D) Expression levels of Epac1, p-NR2B and Piezo2
in the ipsilateral lumbar DRG L2-L5 segments were detected at the
time of 0, 15 or 30 min after injection. Data represents means ±
SD. #P<0.05 vs. 0 min; *P<0.05 vs. vehicle group
at each time point. ##P<0.05 vs. saline group. ASODN,
antisense oligodeoxynucleotides; DRG, dorsal root ganglion; PWMT,
paw withdrawal mechanical threshold; p-, phosphorylated. |
Discussion
Mechanical allodynia, which is the painful
perception of mechanical stimuli, is one of the typical symptoms in
clinical BCP (20,21). The DRG serves an essential
integratory role in the modulation of the peripheral and central
sensory processing of pain, and is an excellent target for pain
research and therapy (22). The
results of the present study demonstrated that the mechanical
allodynia induced by bone cancer in a mouse model was associated
with increased expression levels of DRG Epac1, NR2B and Piezo2. The
selected Epac1-ASODN markedly increased the PWMT and decreased the
expression levels of Epac1, NR2B and Piezo2 in the mouse model of
BCP. Pretreatment with the NR2B antagonist prevented the
aggravation in mechanical allodynia and DRG Piezo2 expression
levels induced by the Epac1 agonist. However, the NR2B
agonist-induced increase in the Piezo2 expression levels was not
reversed by pretreatment with Epac1-ASODN. Overall, the results of
the present study may enrich the theoretical knowledge of the
mechanical allodynia of BCP and provide a potential analgesic
strategy for clinical treatment.
Epac1 is one of the primary downstream sensors of
cAMP, which is an intracellular signaling messenger that alters the
pain threshold (23,24). In vitro cultures of sensory
neurons, the activation of Epac1 enhances the Piezo2 currents,
whereas specific activation of other cAMP sensors PKA or Epac2 does
not affect Piezo2 sensitivity (12).
In addition, mechanical allodynia induced by an intraplantar
injection of an Epac agonist is attenuated by Piezo2 knockdown
(12). These results suggest that
Piezo2 serves a crucial role in the development of mechanical
allodynia, which involves Epac1. The present results demonstrated
that an intrathecal injection of Epac1-ASODN effectively alleviated
mechanical allodynia and decreased the DRG Piezo2 expression levels
in a mouse model of BCP, which may be due to the inhibition of the
Epac1-dependent Piezo2 pathway.
Previous studies using cryo-electron microscopy have
revealed that Piezo2 is a large membrane protein with a complicated
structure that contains >30 potential phosphorylation sites
(25,26). The potentiation of Piezo2 currents is
associated with the increases in cytosolic Ca2+ after
Epac1 activation (12). These
studies indicate that phosphorylation may be the mechanism by which
Epac1 regulates Piezo2 activity. However, based on the structure
and function, Epac1 is unlikely to directly activate Piezo2
(24). Therefore, the underlying
mechanism may involve protein kinases or other factors that induce
the increase in cytosolic Ca2+.
Previous studies have demonstrated that NMDA
receptors (especially NR2B) are essential in the nociceptive
processing during BCP (14,27). The activation of NMDA receptors leads
to excessive Ca2+ influx or marked increases in
cytosolic Ca2+, which triggers a complex cascade of
events, including the activation of Ca2+-dependent
protein kinases, and further activates a number of downstream
effectors (28,29). Previous studies have reported that
Epac may exert positive regulatory effects on NMDA receptor
activation, which causes an increase in the intracellular
Ca2+ levels (30,31). Thus, it was hypothesized that NR2B
may be involved in the regulation of Piezo2. In the present study,
with the exacerbation or remission of the mechanical allodynia of
BCP, NR2B and Piezo2 expression levels exhibited a similar pattern.
In addition, pretreatment with the NRB antagonist reversed the
increase in Piezo2-mediated mechanical allodynia induced by the
Epac1 agonist. However, pretreatment with the Epac1 antagonist
followed by the NR2B agonist was not sufficient. These results
suggested that NR2B may mediate the Epac1-Piezo2 pathway,
contributing to the development of the mechanical allodynia of
BCP.
Certain details of the results of the present study
require further illustration. Firstly, the present results
suggested that disturbing the Epac1-Piezo2 signaling pathway
alleviated the mechanical allodynia of BCP, which was similar to
the results of previous studies (7,32).
Secondly, behavioral changes are the gold standard for judging the
occurrence and development of pain. The proficiency in the
establishment of cancer-induced femur pain model and the
corresponding imaging and histological changes have been fully
illustrated in a previous study (33). Thus, inoculation of osteosarcoma
cells into the femur induced progressive mechanical allodynia in
this study, indicating the successful establishment of a
cancer-induced pain model in mice, similarly to previous studies
(14,34,35).
Thirdly, the up-down method descripted by Chaplan et al
(18) is a classical method of
assessing mechanical allodynia. The studies were not only conducted
by sequentially increasing and decreasing the stimulus strength,
but repeated five times. The lowest von Frey filaments which had
three or more positive responses were regarded as PWMT. Hence, the
method modified is more practical. In addition, the mechanism of
bone cancer pain is complex and many factors are involved at
different stages. Therefore, other mechanism involved in mediating
Piezo2 and influencing the expression of Epac1 and NR2B on day 7
cannot be excluded. It is a very interesting phenomenon and we will
continue to study in depth. Moreover, the biological activity, the
affinity for intended target sequences, and the safety profile of
antisense oligodeoxynucleotides (AS-ODNs) cannot be neglected.
Future study will be required for validation and translation.
Besides, as summarized in Fig. 5,
Piezo2 is activated by complicated signal transduction mechanisms
and produces mechanically activated cationic current (7). This current further induces strong
depolarization in somatosensory neurons and ultimately increases
the transmission of peripheral nociceptive signals to the center,
which is postulated to lead to the production and maintenance of
mechanical hyperalgesia in bone cancer pain. Finally, since BCP
possesses the characteristics of inflammatory, neuropathic and
tumorigenic components, it is more complicated compared with other
forms of pain (36,37); therefore, based on previous studies
(12,16,38,39), the
present study applied agonists by intraplantar or intrathecal
injection to induce mechanical allodynia to partly simulate the
mechanical allodynia of BCP. However, further in-depth studies are
required to further explore the detailed underlying mechanisms.
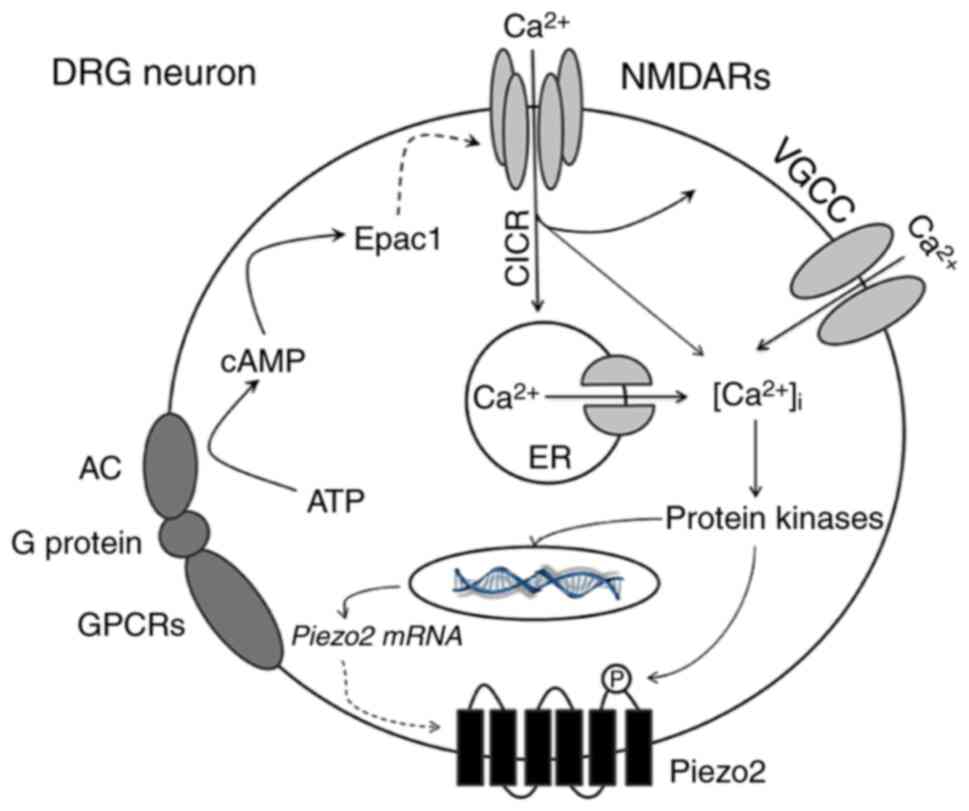 | Figure 5.Schematic representation of
NR2B-mediated Epac1-Piezo2 signaling pathway possibly participating
in the mechanical allodynia of BCP. In the mouse model of BCP,
peripheral nociceptive stimulation leads to the activation of GPCRs
in DRG neurons which triggers the conversion of ATP into cAMP. As
an important second messenger, cAMP directly activates Epac1
protein and then regulates the activity of NR2B-containing NMDA
receptors. Activated NMDA receptors cause a significant increase in
[Ca2+]i through multiple ways, such as the
directly influx of calcium ions, CICR and VGCC. Various
calcium-dependent protein kinases (such as ERK or CaMK) may be
subsequently activated. On the one hand, the synthesis of Piezo2
mRNA or protein is increased; on the other hand, phosphorylation of
Piezo2 enhances its function which mediate large amount of cations
influx. Based on these mechanisms, the excitability of neurons
continues to escalate and ultimately increases the transmission of
peripheral nociceptive signals to the center, which leads to the
production and maintenance of mechanical hyperalgesia in BCP.
GPCRs, G protein-coupled receptors; ATP, adenosine triphosphate;
cAMP, cyclic adenosine monophosphate; DRG, dorsal root ganglion;
CICR, calcium-induced calcium release; VGCC, voltage-gated calcium
channel; ERK, extracellular-signal regulated kinase; CaMK,
calmodulin-dependent kinase. |
In conclusion, the results of the present study
demonstrated that the DRG Epac1-Piezo2 signaling pathway may
contribute towards mechanical allodynia signal processing in the
development of BCP. NR2B, which is a crucial downstream regulator
of Epac1, was implicated in the regulation of mechanical allodynia
mediated by Epac1-Piezo2 pathway. As an Epac1 inhibitor,
Epac1-ASODN alleviated the BCP-induced mechanical allodynia,
suggesting a potential application in BCP. Piezo2 may be a valuable
clinical target, and specific Piezo2 blockers may be efficient
tools for understanding mechanical allodynia and provide an
analgesic strategy for clinical therapy.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81500951 and
81870875), Six Talent Peaks Project of Jiangsu Province (grant no.
YY-077), Young Medical Talents of Jiangsu Province (grant no.
QNRC2016014) and Fundamental Research Funds for the Central
Universities (grant no. 021414380014).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
KN, WZ and ZM conceived and designed the
experiments. YN and YM participated in the behavior test, western
blotting and RT-qPCR. YW carried out the collection of tissues. XG
performed the design of study and coordination. KN and WZ confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
All animal experimental procedures were carried out
in accordance with the guidelines of the National Institutes of
Health Guide for Care and were approved by the Medical College of
Nanjing University Animal Care and Use Committee (approval no.
20210122; Nanjing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mantyh P: Bone cancer pain: Causes,
consequences, and therapeutic opportunities. Pain. 154 (Suppl
1):S54–S62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scarborough BM and Smith CB: Optimal pain
management for patients with cancer in the modern era. CA Cancer J
Clin. 68:182–196. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Colvin L and Fallon M: Challenges in
cancer pain management-bone pain. Eur J Cancer. 44:1083–1090. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coste B, Mathur J, Schmidt M, Earley TJ,
Ranade S, Petrus MJ, Dubin AE and Patapoutian A: Piezo1 and Piezo2
are essential components of distinct mechanically activated cation
channels. Science. 330:55–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ranade SS, Woo SH, Dubin AE, Moshourab RA,
Wetzel C, Petrus M, Mathur J, Bégay V, Coste B, Mainquist J, et al:
Piezo2 is the major transducer of mechanical forces for touch
sensation in mice. Nature. 516:121–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Murthy SE, Loud MC, Daou I, Marshall KL,
Schwaller F, Kühnemund J, Francisco AG, Keenan WT, Dubin AE, Lewin
GR and Patapoutian A: The mechanosensitive ion channel Piezo2
mediates sensitivity to mechanical pain in mice. Sci Transl Med.
10:eaat98972018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Szczot M, Liljencrantz J, Ghitani N, Barik
A, Lam R, Thompson JH, Bharucha-Goebel D, Saade D, Necaise A,
Donkervoort S, et al: PIEZO2 mediates injury-induced tactile pain
in mice and humans. Sci Transl Med. 10:eaat98922018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berkey SC, Herrera JJ, Odem MA, Rahman S,
Cheruvu SS, Cheng X, Walters ET, Dessauer CW and Bavencoffe AG:
EPAC1 and EPAC2 promote nociceptor hyperactivity associated with
chronic pain after spinal cord injury. Neurobiol Pain.
7:1000402019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang LY and Gu Y: Epac and nociceptor
sensitization. Mol Pain. 13:17448069177162342017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li ZH, Cui D, Qiu CJ and Song XJ: Cyclic
nucleotide signaling in sensory neuron hyperexcitability and
chronic pain after nerve injury. Neurobiol Pain. 6:1000282019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hucho TB, Dina OA and Levine JD: Epac
mediates a cAMP-to-PKC signaling in inflammatory pain: An isolectin
B4(+) neuron-specific mechanism. J Neurosci. 25:6119–6126. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eijkelkamp N, Linley JE, Torres JM, Bee L,
Dickenson AH, Gringhuis M, Minett MS, Hong GS, Lee E, Oh U, et al:
A role for Piezo2 in EPAC1-dependent mechanical allodynia. Nat
Commun. 4:16822013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zimmermann M: Ethical guidelines for
investigations of experimental pain in conscious animals. Pain.
16:109–110. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ni K, Zhou Y, Sun YE, Liu Y, Gu XP and Ma
ZL: Intrathecal injection of selected peptide Myr-RC-13 attenuates
bone cancer pain by inhibiting KIF17 and NR2B expression. Pharmacol
Biochem Behav. 122:228–233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schwei MJ, Honore P, Rogers SD,
Salak-Johnson JL, Finke MP, Ramnaraine ML, Clohisy DR and Mantyh
PW: Neurochemical and cellular reorganization of the spinal cord in
a murine model of bone cancer pain. J Neurosci. 19:10886–10897.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen G, Xie RG, Gao YJ, Xu ZZ, Zhao LX,
Bang S, Berta T, Park CK, Lay M, Chen W and Ji RR: β-arrestin-2
regulates NMDA receptor function in spinal lamina II neurons and
duration of persistent pain. Nat Commun. 7:125312016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiaoping G, Xiaofang Z, Yaguo Z, Juan Z,
Junhua W and Zhengliang M: Involvement of the spinal NMDA
receptor/PKCγ signaling pathway in the development of bone cancer
pain. Brain Res. 1335:83–90. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chaplan SR, Bach FW, Pogrel JW, Chung JM
and Yaksh TL: Quantitative assessment of tactile allodynia in the
rat paw. J Neurosci Methods. 53:55–63. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luger NM, Mach DB, Sevcik MA and Mantyh
PW: Bone cancer pain: From model to mechanism to therapy. J Pain
Symptom Manage. 29 (5 Suppl):S32–S46. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goblirsch MJ, Zwolak PP and Clohisy DR:
Biology of bone cancer pain. Clin Cancer Res. 12:6231s–6235s. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Krames ES: The role of the dorsal root
ganglion in the development of neuropathic pain. Pain Med.
15:1669–1685. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hucho T and Levine JD: Signaling pathways
in sensitization: Toward a nociceptor cell biology. Neuron.
55:365–376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bos JL: Epac proteins: Multi-purpose cAMP
targets. Trends Biochem Sci. 31:680–686. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bagriantsev SN, Gracheva EO and Gallagher
PG: Piezo proteins: Regulators of mechanosensation and other
cellular processes. J Biol Chem. 289:31673–31681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang L, Zhou H, Zhang M, Liu W, Deng T,
Zhao Q, Li Y, Lei J, Li X and Xiao B: Structure and mechanogating
of the mammalian tactile channel PIEZO2. Nature. 573:225–229. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gu X, Zhang J, Ma Z, Wang J, Zhou X, Jin
Y, Xia X, Gao Q and Mei F: The role of N-methyl-D-aspartate
receptor subunit NR2B in spinal cord in cancer pain. Eur J Pain.
14:496–502. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lau CG, Takeuchi K, Rodenas-Ruano A,
Takayasu Y, Murphy J, Bennett MVL and Zukin RS: Regulation of NMDA
receptor Ca2+ signalling and synaptic plasticity. Biochem Soc
Trans. 37:1369–1374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu XG and Zhou LJ: Long-term potentiation
at spinal C-fiber synapses: A target for pathological pain. Curr
Pharm Des. 21:895–905. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sánchez-Pérez AM, Llansola M and Felipo V:
Modulation of NMDA receptors by AKT kinase. Neurochem Int.
49:351–358. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gonzalez-Robayna IJ, Falender AE, Ochsner
S, Firestone GL and Richards JS: Follicle-Stimulating hormone (FSH)
stimulates phosphorylation and activation of protein kinase B
(PKB/Akt) and serum and glucocorticoid-lnduced kinase (Sgk):
Evidence for A kinase-independent signaling by FSH in granulosa
cells. Mol Endocrinol. 14:1283–1300. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Singhmar P, Huo X, Eijkelkamp N, Berciano
SR, Baameur F, Mei FC, Zhu Y, Cheng X, Hawke D, Mayor F Jr, et al:
Critical role for Epac1 in inflammatory pain controlled by
GRK2-mediated phosphorylation of Epac1. Proc Natl Acad Sci USA.
113:3036–3041. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ren BX, Gu XP, Zheng YG, Liu CL, Wang D,
Sun YE and Ma ZL: Intrathecal injection of metabotropic glutamate
receptor subtype 3 and 5 agonist/antagonist attenuates bone cancer
pain by inhibition of spinal astrocyte activation in a mouse model.
Anesthesiology. 116:122–132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang Y, Zhang J, Gao Q, Bo J and Ma Z:
Etanercept attenuates thermal and mechanical hyperalgesia induced
by bone cancer. Exp Ther Med. 13:2565–2569. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun Y, Jiang M, Hou B, Lu C, Lei Y, Ma Z
and Gu X: Mas-related gene (Mrg) C activation attenuates bone
cancer pain via modulating Gi and NR2B. PLoS One. 11:e01548512016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mercadante S and Fulfaro F: Management of
painful bone metastases. Curr Opin Oncol. 19:308–314. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Basbaum AI, Bautista DM, Scherrer G and
Julius D: Cellular and molecular mechanisms of pain. Cell.
139:267–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li S, Cao J, Yang X, Suo ZW, Shi L, Liu
YN, Yang HB and Hu XD: NR2B phosphorylation at tyrosine 1472 in
spinal dorsal horn contributed to N-methyl-D-aspartate-induced pain
hypersensitivity in mice. J Neurosci Res. 89:1869–1876. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Alvarez-Vega M, Baamonde A, Gutiérrez M,
Hidalgo A and Menéndez L: Intrathecal N-methyl-D-aspartate (NMDA)
induces paradoxical analgesia in the tail-flick test in rats.
Pharmacol Biochem Behav. 65:621–625. 2000. View Article : Google Scholar : PubMed/NCBI
|