Introduction
Esophageal cancer (EC) and gastric cancer (GC) are
leading causes of cancer-associated mortality. Despite recent
advances in surgical treatment, chemotherapy, radiotherapy and
molecular-targeted treatment, the five-year survival rate of
patients with GC or EC remains poor (1). Further research is therefore required
to elucidate the molecular mechanisms underlying the tumorigenesis
and progression of GC and EC and to identify novel therapeutic
targets. Also, applicable biomarkers for predicting clinical
outcome are imperatively required.
Studies identifying potential biomarkers of
tumorigenesis have routinely focused on protein-coding genes.
However, it has been estimated that 98% of the human genome is
non-protein coding. Ground-breaking discoveries in the field of
non-coding RNAs have helped to improve the characterization of
microRNAs and long non-coding RNAs (lncRNAs) (2). lncRNAs are transcribed RNA molecules
that are >200 nucleotides in length and located in nuclear or
cytosolic fractions, yet do not encode proteins (3,4). The
abnormal expression and roles of lncRNAs in cancer have been
reported in numerous studies. lncRNAs may play important roles in
various biological processes, including immune responses,
differentiation, angiogenesis, apoptosis, cell proliferation and
autophagy. Therefore, they may contribute to the prognosis and
treatment of cancer as novel therapeutic targets (5).
Metastasis-associated lung adenocarcinoma transcript
1 (MALAT1) is a lncRNA that has been revealed to be dysregulated
and have prognostic and therapeutic significance in various
cancers, including lung cancer, pancreatic cancer, hepatocellular
carcinoma, nasopharyngeal carcinoma, osteosarcoma, colorectal
cancer, breast cancer, GC and EC (3). A number of studies have investigated
the pathogenetic pathways of MALAT1, and the association between
MALAT1 expression and the prognosis of gastric and esophageal
malignancies. The present review aims to summarize all current
knowledge on the association between MALAT1 expression and these
malignancies, and to describe the pathogenetic pathways and the
possible prognostic role of MALAT1 in esophagogastric cancer.
MALAT1 and esophageal cancer
MALAT1 pathways in esophageal squamous
cell carcinoma (ESCC)
In patients with ESCC, MALAT-1 is expressed at
higher levels in cancer tissues compared with paired adjacent
normal tissues, and in ESCC cell lines, MALAT-1 knockdown reduces
proliferation, increases apoptosis, inhibits migration, invasion
and colony formation, and leads to cell cycle arrest at the G2/M
phase (6). MALAT1 depletion has been
shown to induce G2/M phase arrest via phosphorylation of the
ataxia-telangiectasia mutated/checkpoint kinase 2 pathway and
increase the percentage of apoptotic cells (7). lncRNAs are able interact with miRNAs
(miRs), modulate the expression of other lncRNAs, and serve as
competing endogenous RNAs (ceRNAs) to miRNAs. Moreover, the
expression of lncRNAs can be inhibited by miRNAs through an
argonaute 2-mediated pathway. miR-101 and miR-217 have been
demonstrated to act as tumor suppressor genes by targeting MALAT1
during ESCC development (8).
Furthermore, miR-101 and miR-217 have been found to be
downregulated in EC. There is evidence to suggest that the
posttranscriptional silencing of MALAT1 by miR-101 and miR-217
occurs in ESCC cells, which may significantly suppress the
proliferation of the cells via G2/M cell cycle arrest. This may be
due to suppression of the MALAT1-mediated upregulation of p21 and
p27 expression and inhibition of B-MYB expression. In addition, the
overexpression of miR-101 or miR-217, or transfection with MALAT1
small interfering RNA (siRNA) inhibits the migration and invasion
of ESCC cells, which may be due to the dysregulation of genes
downstream of MALAT1, such as MIA SH3 domain ER export factor 2,
hepatocyte nuclear factor 4 γ, roundabout guidance receptor 1,
chaperonin containing TCP1 subunit 4 and collagen triple helix
repeat containing 1 (8).
miR-101-3p has been demonstrated to bind directly to
MALAT1. This miRNA blocks the MALAT-1-induced activation of the
PI3K/AKT signaling pathway, resulting in repression of the
proliferation, migration and invasion of ESCC cells (2). Furthermore, with the introduction of
miRNA-based cancer therapy, Li et al (9) designed a poly(glycidyl methacrylate)
(PGEA)-based star-like polycation with flanking folic acid (FA)
ligands (S-PGEA-FA), rich in hydrophilic hydroxyl and secondary
amine groups, as an effective nanovector with the ability to
deliver miR-101 and miR-217 and thereby silence MALAT1 in ESCC
cells. The authors found that miR-101 and miR-217 acted like silent
information regulators-M to inhibit ESCC cells via the
downregulation of MALAT1. S-PGEA-FA was shown to transfer miR-101
and miR-217 into ESCC cells and thereby induce cell cycle arrest,
and inhibit cell proliferation, migration and invasion, indicating
that it has potential as a promising agent in ESCC therapy
(9).
Enhancer of zeste homolog 2 (EZH2) has been shown to
be associated with carcinogenesis and cancer progression, and to
serve a crucial role in the apoptosis, invasion and migration of
cancer cells. MALAT1 is able to promote the malignant development
of ESCC by targeting EZH2. Chen et al (10) found that MALAT1 affects the
epithelial-to-mesenchymal transition (EMT) and metastasis of EC
cells via the EZH2-Notch1 signaling pathway. Another study
indicated that MALAT1 may serve as a ceRNA controlling the
expression of ZEB1 and ZEB2 via the sponging of miR-200a, and
thereby promote the invasion and migration of EC cells through the
induction of EMT (11). Furthermore,
it has been shown that the silencing of MALAT1 downregulates the
expression of OCT4 and NANOG genes, which inhibits EC cell
proliferation, migration and tumor sphere formation, while
increasing cell apoptosis. The expression of β-catenin, Lin28 and
EZH2 genes is also decreased when MALAT1 is downregulated, while
the overexpression of EZH2 completely reverses the MALAT1
siRNA-mediated repression of β-catenin and Lin28 in EC cells
(12).
MALAT1 has been indicated to be associated with the
stemness of ESCC cells, as the knockdown of MALAT1 decreases cancer
stem cell-like traits. The expression levels of the stemness
markers sex-determining region Y-box 2 (SOX2) and NANOG in ESCC
cells are reduced by MALAT1 downregulation, as is the migration
ability of the cells. MALAT1 positively regulates the stemness of
ESCC cells. It binds directly to yes-associated protein (YAP), a
transcriptional factor that plays a critical role in the expansion
of cancer stem cells, and induces YAP protein expression and
transcriptional activity (13).
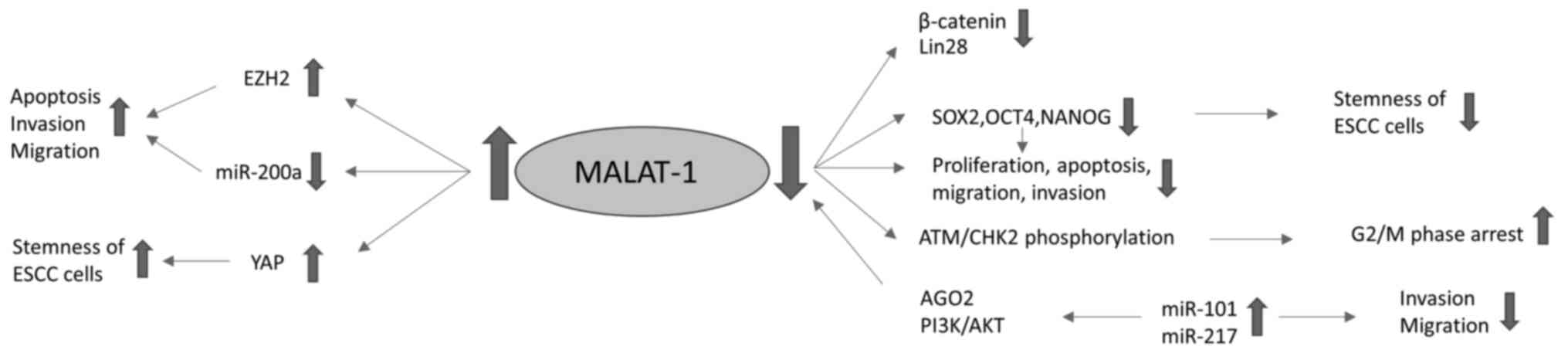
MALAT1 pathways in ESCC are summarized in Table I and presented in Fig. 1.
 | Table I.Summary of MALAT1 pathways in
ESCC. |
Table I.
Summary of MALAT1 pathways in
ESCC.
| First author,
year | Samples | Sample size | MALAT1 pathways in
ESCC | (Refs.) |
|---|
| Yao, 2019 | Human ESCC cell
lines, normal human esophageal epithelial cells, cancer stem
cells | N/A | MALAT1 binds to
YAP, enhances YAP protein expression and regulates the stemness of
ESCC cells | (13) |
| Chen, 2018 | Human ESCC cell
lines, normal human esophageal epithelial cells | N/A | MALAT1 affects EMT
and metastasis through the EZH2/Notch1 signaling pathway | (10) |
| Zhang, 2017 | Human ESCC cell
lines | N/A | MALAT1 regulates
ZEB1 and ZEB2 by sponging miR-200a and induces EMT | (11) |
| Li, 2017 | Human ESCC cell
lines | N/A | miR-101 and miR-217
act as tumor suppressor genes by targeting MALAT1 when delivered
via ligand-functionalized hydroxyl-rich nanovectors | (9) |
| Li, 2017 | Mouse ESCC
xenografts and cells | N/A | MALAT1 regulates
Cks1 expression | (17) |
| Wang, 2016 | Human ESCC tissues,
human ESCC cell lines, mouse xenografts | 106 patients (+
adjacent healthy tissues) | MALAT1 targets
β-catenin via EZH2 | (12) |
| Yao, 2016 | Human ESCC tissues,
human ESCC cell lines | 137 patients (+
adjacent healthy tissues) | MALAT1 contributes
to G2/M phase arrest, cell apoptosis, cell migration and
invasion | (6) |
| Hu, 2015 | Human ESCC tissues,
human ESCC cell lines | 54 patients (+
adjacent healthy tissues) | MALAT1 contributes
to G2/M phase arrest and ATM/CHK2 pathway phosphorylation | (7) |
| Wang, 2015 | Human ESCC tissues,
human ESCC lines, mouse xenografts | 42 patients (+
adjacent healthy tissues) | miR-101 and miR-217
silence MALAT1, inhibit the migration and invasion of ESCC cells
and induce G2/M phase arrest | (8) |
Impact of MALAT1 expression in
patients with ESCC
In a large Chinese study of single nucleotide
polymorphisms of MALAT1, the authors proposed that polymorphism
rs3200401 C>T is associated with a higher risk of ESCC and
polymorphism rs619586 A>G might be protective against ESCC
(14).
The upregulation of MALAT1 in ESCC tissues can
influence tumor progression and may predict postoperative survival;
therefore, the expression level of MALAT1 can be used as an index
to predict the prognosis of patients with ESCC. It has been shown
that the expression levels of MALAT1 in ESCC tissues are higher
than those in adjacent non-carcinoma tissues, and the overall
survival (OS) and disease-free survival (DFS) of patients with high
MALAT1 expression levels are shorter than those with low expression
levels (OS, 32 vs. 57 months; DFS, 27 vs. 54 months, respectively).
Moreover, high MALAT1 expression is associated with a high TNM
stage (stage III vs. I and II) (15). High MALAT1 expression has also been
shown to be significantly associated with lymphatic invasion, large
primary tumor size, distant metastasis and poor tumor
differentiation, but not with age, sex or tumor location (7,16). In
the only published meta-analysis regarding the role of MALAT1 in
ESCC prognosis, a significant association was detected between the
expression of MALAT1 and the OS of patients with ESCC in China.
More specifically, a high MALAT1 expression level in ESCC tissues
was found to be an important independent predictive factor of poor
OS and DFS (3).
MALAT1 has been indicated to be associated with the
chemo- and radiosensitivity of ESCC cells, with the downregulation
of MALAT1 enhancing the chemo- and radiosensitivity of ESCC cells
(2,13). Cyclin-dependent kinase subunit 1
(Cks1) is frequently overexpressed in ESCC, and its overexpression
is associated with the increased resistance of ESCCs to
radiotherapy. Li et al (17)
found that the expression levels of MALAT1 and Cks1 were reduced in
ESCC mouse xenografts and cells when irradiated. Furthermore, the
irradiation-induced reduction in cell viability, increase in
apoptosis and downregulation of Cks1 were inhibited by the
overexpression of MALAT1. The authors concluded that MALAT1 is as
positive regulator of the radioresistance of ESCC, which may be of
great importance for enhancing the radiotherapeutic effect in
ESCC.
The main clinical impacts of MALAT1 dysregulation on
patients with ESCC are summarized in Table II.
 | Table II.Clinical impact of MALAT1
dysregulation on patients with ESCC. |
Table II.
Clinical impact of MALAT1
dysregulation on patients with ESCC.
| First author,
year | Samples | Sample size | Clinical
impact | (Refs.) |
|---|
| Yao, 2019 | Human ESCC cell
lines, normal human esophageal epithelial cells, cancer stem
cells | N/A | Knockdown of MALAT1
enhances the chemo- and radiosensitivity of ESCC cells | (13) |
| Wang, 2019 | Meta-analysis | 3 studies | High MALAT1
expression is a significant independent predictor of poor overall
and disease-free survival | (3) |
| Qu, 2019 | Human plasma | 245 ESCC patients
and 490 controls | MALAT1 polymorphism
rs3200401 C>T increases the risk of ESCC and polymorphism
rs619586 A>G is protective against ESCC | (14) |
| Kang, 2018 | Human ESCC
tissues | 100 patients (+
adjacent healthy tissues) | MALAT1 expression
determines prognosis and is associated with high TNM stage | (15) |
| Li, 2017 | Mouse ESCC
xenografts and cells | N/A | MALAT1 affects the
efficacy of radiotherapy | (17) |
| Huang, 2016 | Human ESCC tissues,
human EC and esophageal epithelial cell lines | 132 patients, 50
controls | High MALAT1
expression is associated with lymphatic invasion, large primary
tumor size, distant metastasis and poor tumor differentiation | (16) |
| Hu, 2015 | Human ESCC tissues,
human ESCC cell lines | 54 patients (+
adjacent healthy tissues) | High MALAT1
expression is associated with lymphatic invasion and large primary
tumor size | (7) |
MALAT1 and esophageal
adenocarcinoma
At present, to the best of our knowledge, no
published literature has investigated in depth the pathways and
roles of MALAT1 dysregulation in esophageal adenocarcinomas.
However, new possibilities for the diagnosis, prognosis and
treatment of patients with esophageal adenocarcinoma may emerge in
future studies.
MALAT1 and gastric cancer
MALAT1 pathways in GC
As research into lncRNAs has increased, new and
additional insights into the pathways of MALAT1 in GC have been
gained. EZH2 is overexpressed in various cancer subtypes and
contributes to tumorigenesis and progression through the
transcriptional silencing of tumor suppressor genes. In GC, the
binding of MALAT1 to EZH2 inhibits the expression of the tumor
suppressor gene protocadherin 10 and promotes the migration and
invasion of GC cells (18). MALAT1
may also promote the proliferation of GC cells via the regulation
of splicing factor 2/alternative splicing factor (SF2/ASF) in GC
cell lines, MALAT1 induces the upregulation of SF2/ASF in the
nucleolus, while the downregulation of MALAT1 or SF2/ASF induces
cell cycle arrest at the G0/G1 phase and suppresses cell
proliferation. Although the nuclear distribution and expression of
SF2/ASF are disturbed when MALAT1 is depleted, the overexpression
of SF2/ASF does not attenuate the MALAT1 depletion-induced
reduction in cell proliferation (19). MALAT1 knockdown also promotes
apoptosis, and suppresses the growth, migration and invasion of GC
cells (2).
As aforementioned, MALAT1 functions as a ceRNA,
indirectly regulating miRNAs and functionally liberating various
RNA transcripts. The expression levels of miR-122 and MALAT1 are
negatively associated in gastric cells, while those of MALAT1 and
insulin-like growth factor 1 receptor (IGF-1R), an miR-122 target,
are positively associated. Furthermore, the overexpression of
miR-122 inhibits MALAT1 expression, while the inhibition of miR-122
upregulates MALAT1 overexpression. This suggests that the
miR-122/IGF-1R axis may modulate the expression of MALAT1 in GC
cells (20). Zhang et al
(21) demonstrated that MALAT1
directly targets miR-202 and the downregulation of MALAT1
significantly decreases the expression of Gli2 via the negative
regulation of miR-202. They also revealed that the knockdown of
MALAT1 in GC cells inhibits cell proliferation, reduces the number
of cells in the S-phase, and induces apoptosis via the negative
regulation of miR-202. Li et al (22) identified a negative association
between MALAT1 and miR-1297, and revealed that MALAT1 promotes high
mobility group protein B2 (HMGB2) through the negative regulation
of miR-1297. Their results indicated that the MALAT1/miR-1297/HMGB2
axis is important for the regulation of GC tumorigenesis and
progression, and may serve as a therapeutic target for GC. In a
study conducted by Li et al (23), transfected cells with overexpressed
or silenced MALAT1 were prepared, and their proliferation and
apoptosis, as well as the tumor volume and weight when injected
into mice were evaluated. The overexpression of MALAT1 promoted
proliferation and inhibited apoptosis via the upregulation of
miR-22-3p and downregulation of ErbB3, while the knockdown of
MALAT1 inhibited GC tumor growth in mice. Zhang et al
(24) demonstrated that the sponging
of miR-22-3p by MALAT1 modulated the oxaliplatin resistance of GC
xenografts. The knockdown of MALAT1 or overexpression of miR-22-3p
inhibited the survival, proliferation and drug resistance of GCs
subjected to oxaliplatin treatment, and those effects were
attenuated by the inhibition of miR-22-3p.
The interleukin-21 receptor (IL-21R) contributes to
the progression of GC, and its knockdown suppresses the invasion
and proliferation of GC cells. The expression of IL-21R is
negatively associated with that of miR-125a-3p, a tumor suppressive
miRNA, in GC, and MALAT1 is able to sponge miR-125a-3p. It appears
that the upregulation of MALAT1 dysregulates the MALAT1/miR-125a-3p
axis, which leads to the activation of IL-21R and its oncogenic
role in GC (25).
The levels of MALAT1 are elevated in the serum of
patients with gastric adenocarcinoma and in gastric adenocarcinoma
cell lines, and the knockdown of MALAT1 inhibits the proliferation
of gastric adenocarcinoma cells and promotes their apoptosis.
MALAT1 directly targets and decreases the expression of
miR-181a-5p, which upregulates the expression of AKT3 and promotes
tumor growth (26).
Activation of the PI3K/AKT pathway increases the
migration, proliferation and invasion of GC cells. MALAT1 increases
the phosphorylation levels of PI3K and AKT, which may promote the
progression of GC (27). The
cisplatin resistance of GC has also been attributed to the
regulatory effect of MALAT1 on the PI3K/AKT pathway (4).
The expression of miR-183 is downregulated in GC
cells, and the knockdown of miR-183 has been shown to increase the
viability and reduce the apoptosis of GC cells by modulating the
activation of cell autophagy. The underlying mechanisms have been
suggested to involve the MALAT1/miR-183/sirtuin 1 axis and
PI3K/AKT/mTOR pathway, and miR-183 has been proposed as a
therapeutic target for GC due to its dysregulating effect on those
pathways (28). Elevated levels of
MALAT1 are associated with increased levels of
microtubule-associated protein 1 light chain 3β (LC3B), an
autophagy marker, and antigen Ki67, a proliferation marker. miR-204
represses the autophagy of GC cells through the downregulation of
LC3B and transient receptor potential melastatin 3 expression,
while MALAT1 inhibits the expression of miR-204 and thereby
activates autophagy (5). Studies of
human gastric tumors have been conducted to elucidate the
functional role of MALAT1 in autophagy-associated chemoresistance.
Chemoresistant GC cells exhibit elevated levels of MALAT1 and
increased autophagy. The chemo-induced autophagy of GC cells is
promoted by MALAT1 and the knockdown of MALAT1 sensitizes GC cells
to chemotherapeutics. A proposed mechanism is that MALAT1 acts as a
ceRNA for miR-23b-3p and attenuates the inhibitory effect of
miR-23b-3p on the autophagy regulator ATG12, leading to elevated
ATG12 levels, chemo-induced autophagy and chemoresistance in GC
cells (29). Also, MALAT1 has been
shown to potentiate autophagy-associated cisplatin resistance via
suppression of the miR-30b/ATG5 axis and the facilitation of
autophagy, indicating that MALAT1 may be targeted to overcome
chemoresistance in GC. MALAT1 directly binds to miR-30b and
inhibits its expression, and the upregulation of miR-30b attenuates
MALAT1-induced cisplatin resistance via the suppression of
autophagy (30). Another study
confirmed that GC cells presented increased autophagy and high
expression levels of MALAT1, and demonstrated that propofol induced
apoptosis and reduced autophagic activity through the
downregulation of MALAT1. The knockdown of MALAT1 inhibited
chemo-induced autophagy, and MALAT1 overexpression promoted
autophagy by directly binding with miR-30e and thereby regulating
ATG5 expression. Furthermore, in a GC xenograft model, mice treated
with both propofol and cisplatin exhibited a significant reduction
in tumor size and weight, and the knockdown of MALAT1 enhanced
these effects (31).
Up-frameshift suppressor 1 homolog (UPF1) has been
shown to be a modulator of MALAT1, and the UPF1/MALAT1 pathway may
be a potential therapeutic target for GC. The overexpression of
UPF1 inhibits proliferation, cell cycle, migration and invasion,
and promotes cell apoptosis in GC cells, and the overexpression of
MALAT1 attenuates the UPF1-mediated inhibition of GC progression.
The overexpression of UPF1 is indicated to downregulate MALAT1 by
increasing the efficiency of nonsense-mediated mRNA decay (32).
MALAT1 has been demonstrated to promote
tumorigenesis and metastasis in GC by facilitating vasculogenic
mimicry and angiogenesis via the vascular endothelial
(VE)-cadherin/β-catenin complex and ERK/matrix metalloproteinase
(MMP) and focal adhesion kinase (FAK)/paxillin signaling pathways.
The mechanism of MALAT1 involves the modulation of VE-cadherin,
β-catenin, MMP2, MMP9 and membrane type 1-MMP expression, and
phosphorylated (p)-ERK, p-FAK and p-paxillin levels, which are
classical markers of vasculogenic mimicry and angiogenesis and
components of several associated signaling pathways (33).
In a study of GC, MALAT1 silencing promoted a
reduction in GC cell invasion and migration, and those effects were
reversed by transfection with epidermal growth factor-like
domain-containing protein 7 (EGFL7). Furthermore, in a GC
xenotransplant mouse model, the downregulation of MALAT1 inhibited
tumor metastasis. MALAT1 was demonstrated to regulate EGFL7
expression by altering the H3 histone acetylation level in the
EGFL7 promoter (34).
MALAT1 promotes invasion and migration in GC via
EMT, with reduced expression of snail and N-cadherin, and increased
expression of E-cadherin. Transfection with MALAT1 siRNA
significantly decreases the expression of Wnt/β-catenin-associated
genes (35). Also, the suppression
of MALAT-1 expression decreases the expression of the
EMT-associated marker vimentin and increases the expression of
E-cadherin (36). Yang et al
(37) investigated the anticancer
effects of resveratrol, a traditional Chinese medicine, and found
that they proceeded via the inhibition of MALAT1 expression. EMT
was suppressed by resveratrol, and the knockdown of MALAT1
expression suppressed the viability, migration, invasion and EMT of
EC cells (37).
The MALAT1/SOX2 axis has been reported to induce the
stemness of GC cells. The knockdown of MALAT1 attenuates the
stemness of non-adherent GC cell spheroids and, conversely, the
overexpression of MALAT1 increases the stemness of adherent GC
cells. The expression of MALAT1 and SOX2 is positively correlated.
MALAT1 directly binds to SOX2 mRNA and increases its expression.
The promoting effect of MALAT1 overexpression on the stemness of GC
cells is attenuated by the knockdown of SOX2 (38).
MALAT1 pathways in GC are summarized in Table III and presented in Fig. 2
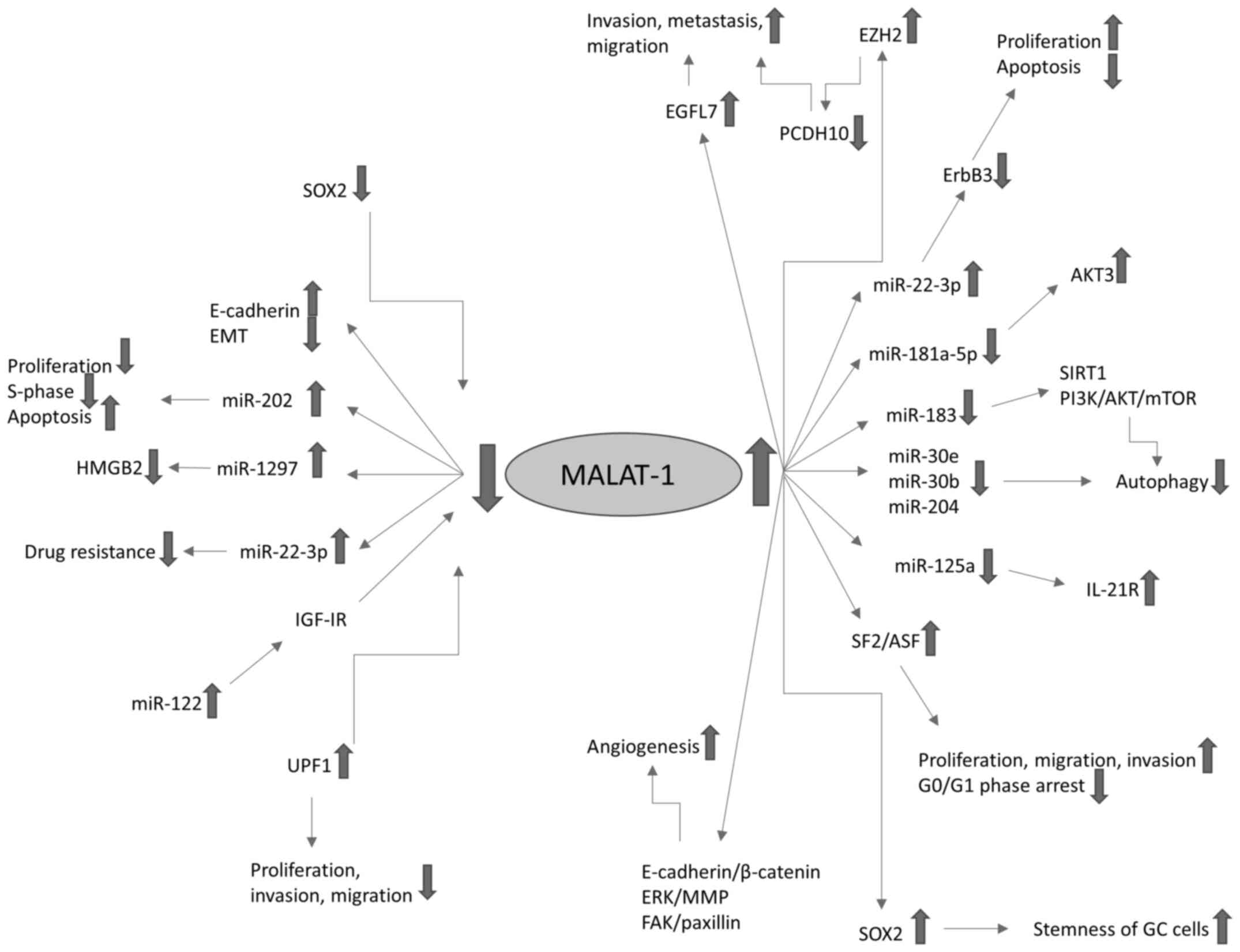 | Figure 2.MALAT1 pathways in GC. Fine arrows
indicate regulatory effects, upward pointing thick arrows represent
overexpression and downward pointing thick arrows indicate
downregulation. MALAT-1, metastasis-associated lung adenocarcinoma
transcript 1; GC, gastric cancer; miR, microRNA; EMT,
epithelial-to-mesenchymal transition; SOX2, sex-determining region
Y-box 2; HMGB2, high mobility group protein B2; IGF-1R,
insulin-like growth factor 1 receptor; UPF1, up-frameshift
suppressor 1 homolog; MMP, matrix metalloproteinase; FAK, focal
adhesion kinase; EZH2, enhancer of zeste homolog 2; EGFL7,
epidermal growth factor-like domain-containing protein 7; PCDH10,
protocadherin-10; SIRT1, sirtuin 1; IL-21R, interleukin-21
receptor; SF2/ASF, splicing factor 2/alternative splicing
factor. |
 | Table III.Summary of MALAT1 pathways in GC. |
Table III.
Summary of MALAT1 pathways in GC.
| First author,
year | Samples | Sample size | MALAT1 pathways in
GC | (Refs.) |
|---|
| Dai, 2020 | Human GC cell
lines | 343 GC samples, 30
normal controls | MALAT1 regulates
the PI3K/AKT pathway | (4) |
| Zhang, 2020 | Human GC tissues,
human normal gastric epithelial cells, human GC cells, human
oxaliplatin-treated GC cells, mouse tumor xenografts | 24 patients (+
adjacent healthy tissues), 24 GC oxaliplatin-treated patients | MALAT1 sponges
miR-22-3p | (24) |
| Li, 2020 | Human GC tissues,
human GC cell lines, mouse tumor xenotransplants | 37 patients (+
adjacent healthy tissues), 24 mice | Silencing MALAT1
inhibits proliferation and promotes apoptosis by regulating
miR-22-3p-mediated ErbB3 | (23) |
| Zhang, 2020 | Human GC cell
lines, mouse GC xenografts | 36 mice | MALAT1/miR-30e/ATG5
axis suppresses autophagy | (31) |
| Shao, 2020 | Human GC tissues,
human GC cell lines, normal gastric epithelial cell line | 57 patients (+
adjacent healthy tissues) | MALAT1 activates
autophagy and promotes cell proliferation by downregulating
miR-204 | (5) |
| Li, 2019 | Human GC tissues,
human GC and gastric mucosal cell lines | 20 patients (+
adjacent healthy tissues) | miR-183 affects the
development of GC by regulating autophagy via MALAT1/miR-183/SIRT1
and PI3K/AKT/mTOR | (28) |
| Zhu, 2019 | Human GC tissues,
human GC cell lines, plasma | 64 patients (+
adjacent healthy tissues) | MALAT1
overexpression promotes proliferation, migration and invasion of GC
by activating the PI3K/AKT pathway | (27) |
| Yan, 2019 | Human GC tissues,
human GC cell lines | 89 patients (+
adjacent healthy tissues) | IL-21R functions as
an oncogenic factor and is regulated by the MALAT1/miR-125a-3p
axis | (25) |
| Lu, 2019 | Serum, gastric
adenocarcinoma cell line, normal gastric epithelial cell line | 70 patients and 70
healthy controls | MALAT1 promotes GC
through the MALAT1/miR-181a-5p/AKT3 axis | (26) |
| Xiao, 2019 | Human GC tissues,
human GC cell lines, cancer stem cells | 30 patients (+
adjacent healthy tissues) | MALAT1/SOX2 axis
promotes the stemness of GC cells | (38) |
| Xi, 2019 | Human GC cell
lines | N/A | MALAT1 regulates
the miR-30b/autophagy-related gene 5 axis | (30) |
| Yang, 2019 | Human GC cell
lines, human gastric epithelium cell line | N/A | Resveratrol
suppresses GC via inhibition of MALAT1-mediated EMT | (37) |
| Zhang, 2017 | Human GC tissues,
human GC cell lines and human gastric mucosal cell line | 60 patients (+
adjacent healthy tissues) | MALAT1 regulates
the expression of Gli2 via miR-202 | (21) |
| Li, 2017 | Human GC tissues,
human GC cell lines and normal gastric epithelial cell line | 78 patients (+
adjacent healthy tissues) |
MALAT1/miR-1297/HMGB2 axis acts as
critical regulator pathway in GC tumorigenesis | (22) |
| Li, 2017 | Human GC tissues,
human GC cell lines | 38 patients (+
adjacent healthy tissues) | UPF1 expression is
downregulated in GC and negatively correlated with MALAT1
expression. UPF1-mediated inhibition of GC progression is reversed
by overexpression of MALAT1 | (32) |
| Li, 2017 | Human GC tissues,
human GC cell lines, human gastric epithelial cells, mouse tumor
xenografts | 150 patients, 15
healthy controls | MALAT1 promotes
tumorigenicity and metastasis via the VE-cadherin/β-catenin complex
and ERK/MMP and FAK/paxillin signaling pathways | (33) |
| Lee, 2017 | Human GC tissues,
human GC cell lines | 50 patients (+
adjacent healthy tissues) | MALAT1 promotes GC
via EMT | (35) |
| Chen, 2017 | Human GC tissues,
human GC and gastric epithelial cell lines | 20 patients and 20
healthy controls | MALAT-1 suppression
results in reduced EMT | (36) |
| Hu, 2017 | Human GC cell
lines, mouse tumor xenotransplants | 8 mice | MALAT1 acts as a
competing endogenous RNA for miR-23b-3p and attenuates the
inhibitory effect of miR-23b-3p on ATG12 | (29) |
| Qi, 2016 | Human GC cell
lines | N/A | MALAT1 binds EZH2,
suppresses PCDH10 and promotes GC cell migration and invasion | (18) |
| Xia, 2016 | Human GC tissues,
human GC cell lines, plasma | Tissues from 39
patients (+ adjacent healthy tissues), plasma from 72 patients and
36 healthy controls | miR-122/IGF-1R axis
regulates the expression of MALAT1 | (20) |
| Deng, 2016 | Human GC tissues,
human GC cell lines, mouse tumor xenotransplants | 25 patients (+
adjacent healthy tissues), 25 mice | MALAT1 regulates
EGFL7 expression by altering the level of H3 histone
acetylation | (34) |
| Wang, 2014 | Human GC and
gastric epithelium cell lines | N/A | MALAT1 regulates
SF2/ASF and G0/G1 cell cycle arrest | (19) |
Impact of MALAT1 expression on
patients with GC
In patients with GC, it has been observed that
MALAT1 is upregulated. In particular, MALAT1 is overexpressed in
the serum of patients with gastric adenocarcinoma and GC tissues,
as well as in GC cell lines (23,26).
It is known that lifestyle choices such as
vegetarianism, smoking and drinking may affect the expression of
lncRNAs; for example, the expression of the lncRNA SCAL1 may be
induced by smoking (27). This may
impact the diagnostic and prognostic utility of a particular
lncRNA. To the best of our knowledge, the only study that has
investigated the effects of patients' lifestyle choices on the
expression of MALAT1 in GC is a study by Zhu et al (27), which recorded MALAT1 expression
levels in patients with different lifestyles. The study found that
MALAT1 expression levels in the plasma and tumor tissues of
patients GC are not significantly associated with sex, age,
smoking, drinking or vegetarianism. These results indicate that
plasma MALAT1 levels may be used for the accurate diagnosis of GC
in patients with different lifestyles (27,35).
Elevated MALAT1 expression is associated with a
larger tumor size, lymph node metastasis and higher TNM stage
(21,22). Patients with elevated levels of
MALAT1 expression are likely to have a greater depth of tumor
invasion and more advanced TNM stage compared with patients who
have lower levels of MALAT1. MALAT1 expression is also higher in
patients with GC with poorly differentiated histology or signet
ring cell carcinoma than in those with well-to-moderately
differentiated adenocarcinoma (35).
In addition, the upregulation of MALAT1 promotes the metastasis and
peritoneal dissemination of GC, while the downregulation of MALAT1
inhibits tumor metastasis (20,33,34,20).
Therefore, the expression level of MALAT1 is a potential biomarker
for the distant metastasis of GC. Okugawa et al (39) and Wang et al (3) did not observe that patients with high
MALAT1 expression have a poorer prognosis than patients with low
MALAT1 expression, whereas Xia et al (20) and Li et al (23) found that high levels of plasma MALAT1
were independently associated with a worse prognosis for patients
with GC. Li et al (22) also
reported that higher MALAT1 expression predicted a shorter survival
and poor prognosis, and Dai et al (4) proposed that the upregulation of MALAT1
in GC tissues is an independent risk factor for OS among patients
with GC. Moreover, Qi et al (18) found that the high expression of
MALAT1 may be significantly associated with poor OS among patients
with stage III or IV GC, but not those with stage I and II GC.
Finally, Yan et al (25)
suggested that MALAT1 contributes to gastric tumorigenesis and is a
risk factor for survival and recurrence since patients with GC with
high MALAT1 expression exhibit a lower survival rate and higher
probability of recurrence compared with those with low MALAT1
expression. These data suggest that MALAT1 may serve as a
diagnostic and prognostic indicator in GC, and is able to provide
an indication of the severity of the disease.
Chemoresistance and radioresistance are major
obstacles to the successful treatment of GC, and MALAT1 may serve
as a potential therapeutic marker. The overexpression of MALAT1
reduces chemotherapeutical sensitivity, while the knockdown of
MALAT1 improves the chemosensitivity of GC (2,29,32). The
overexpression of MALAT1 reduces doxorubicin sensitivity and
potentiates cisplatin resistance in GC, while the knockdown of
MALAT1 inhibits oxaliplatin resistance (4,30,24,32).
In addition, the knockdown of MALAT1 enhances the radiosensitivity
of GC cells while MALAT1 overexpression attenuates it (38).
The main clinical impacts of MALAT1 dysregulation in
patients with GC are summarized in Table IV.
 | Table IV.Clinical impact of MALAT1
dysregulation on patients with GC. |
Table IV.
Clinical impact of MALAT1
dysregulation on patients with GC.
| First author,
year | Samples | Sample size | Clinical
impact | (Refs.) |
|---|
| Li, 2020 | Human GC tissues,
human GC cell lines, mouse tumor xenotransplants | 37 patients (+
adjacent healthy tissues), 24 mice | Silencing MALAT1
inhibits tumor growth and is associated with the survival of
patients with GC | (23) |
| Zhang, 2020 | Human GC tissues,
human normal gastric epithelial cells, human GC cells, human
oxaliplatin-treated GC cells, mouse tumor xenografts | 24 patients (+
adjacent healthy tissues), 24 GC oxaliplatin-treated patients | MALAT1 modulates
oxaliplatin resistance | (24) |
| Dai, 2020 | Human GC cell
lines | 343 GC samples, 30
normal controls | MALAT1 regulates
cisplatin resistance and is an independent risk factor for OS | (4) |
| Zhang, 2020 | Human GC cell
lines, mouse GC xenografts | 36 mice | Propofol
facilitates cisplatin sensitivity via MALAT1 through suppressing
autophagy in GC | (31) |
| Yan, 2019 | Human GC tissues,
human GC cell lines | 89 patients (+
adjacent healthy tissues) | MALAT1 is a
potential therapeutic and prognostic marker for survival and
recurrence | (25) |
| Lu, 2019 | Serum, gastric
adenocarcinoma cell line and normal gastric epithelial cell
line | 70 patients and 70
healthy controls | MALAT1 is present
at high levels in the serum of patients with GC and in GC cell
lines | (26) |
| Zhu, 2019 | Human GC tissues,
human GC cell lines, plasma | 64 patients (+
adjacent healthy tissues) | Plasma MALAT1
levels can accurately diagnose GC in patients with different
lifestyles and are prognostic indicators | (27) |
| Xiao, 2019 | Human GC tissues,
human GC cell lines, cancer stem cells | 30 patients (+
adjacent healthy tissues) | MALAT1 knockdown
enhances the chemosensitivity and radiosensitivity of GC | (38) |
| Xi, 2019 | Human GC cell
lines | N/A | MALAT1 potentiates
autophagy-related cisplatin resistance | (30) |
| Yang, 2019 | Human GC cell
lines, human gastric epithelium cell line | N/A | Resveratrol (which
has anticancer effects) inhibits MALAT1 expression and exhibits
prognostic significance in gastric cancer cells through MALAT1
expression levels | (37) |
| Zhang, 2017 | Human GC tissues,
human GC cell lines and human gastric mucosal cell line | 60 patients (+
adjacent healthy tissues) | MALAT1
overexpression is associated with larger tumor size, lymph node
metastasis and TNM stage | (21) |
| Li, 2017 | Human GC tissues,
human GC cell lines and normal gastric epithelial cell line | 78 patients (+
adjacent healthy tissues) | MALAT1 expression
is associated with local invasion, lymph node metastasis and TNM
stage | (22) |
| Li, 2017 | Human GC tissues,
human GC cell lines | 38 patients (+
adjacent healthy tissues) | MALAT1
overexpression contributes to tumorigenesis and metastasis and
reduces doxorubicin sensitivity | (32) |
| Li, 2017 | Human GC tissues,
human GC cell lines, human gastric epithelial cells, mouse tumor
xenografts | 150 patients, 15
healthy controls | MALAT1 promotes GC
tumorigenicity and metastasis | (33) |
| Lee, 2017 | Human, GC tissues,
human GC cell lines | 50 patients (+
adjacent healthy tissues) | Higher expression
of MALAT-1 is associated with greater depth of tumor invasion | (35) |
| Chen, 2017 | Human GC tissues,
human GC cell lines and human gastric epithelial cell line | 20 patients and 20
healthy controls | MALAT1 expression
is associated with the invasion and metastasis of GC | (36) |
| Hu, 2017 | Human GC cell
lines, mouse tumor xenotransplants | 8 mice | Knockdown of MALAT1
sensitizes GC cells to chemotherapeutics | (29) |
| Xia, 2016 | Human GC tissues,
human GC cell lines, plasma | Tissues from39
patients (+ adjacent healthy tissues), plasma from 72 patients and
36 healthy controls | High MALAT1 level
serves as biomarker for the distant metastasis and prognosis of
GC | (20) |
| Qi, 2016 | Human GC cell
lines | N/A | Highly expressed
MALAT1 is associated with poor OS in stage III and IV and promotes
GC metastasis | (18) |
| Deng, 2016 | Human GC tissues,
human GC cell lines, mouse tumor xenotransplants | 25 patients (+
adjacent healthy tissues), 25 mice | Upregulated MALAT1
promotes the invasion and metastasis of GC | (34) |
| Okugawa, 2014 | Human GC tissues,
human GC cell lines, mouse tumor xenografts | 150 patients (+
adjacent healthy tissues), 14 mice | MALAT1 drives GC
development and promotes peritoneal metastasis | (39) |
Conclusions
The identification of biomarkers and use of
molecular targeting has expanded the field of cancer therapeutics.
The research and identification of lncRNAs and their interactions
with oncogenic pathways offer crucial information to the clinical
aspects of cancer management. Research elucidating the pathways of
MALAT1 in esophagogastric malignancies is suggesting new
possibilities for the diagnosis, prognosis and therapy of GC and
EC. MALAT1 appears to be a promising diagnostic, prognostic and
therapeutic biomarker in esophagogastric malignancies. Ongoing
research into its interaction with different oncogenic pathways may
provide new avenues for EC and GC therapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AS contributed to study conception and design, the
acquisition of data and drafting the manuscript. DM contributed to
drafting the manuscript and the analysis and interpretation of
data. GSK, SS and IK contributed to the analysis and interpretation
of data and drafting the manuscript. MG contributed to the analysis
and interpretation of data, and the drafting and critical revision
of the manuscript. DS contributed to study conception and design,
the acquisition of data, and the drafting and critical revision of
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global Lnds - an update. Cancer Epidemiol Biomarkers Prev.
25:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Farooqi AA, Legaki E, Gazouli M, Rinaldi S
and Berardi R: Withdrawal Notice: MALAT1 as a versatile regulator
of cancer: overview of the updates from predatory role as
competitive endogenous RNA to mechanistic in-sights. Curr Cancer
Drug Targets. Jul 30–2020.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang C, Zhang Q, Hu Y, Zhu J and Yang J:
Emerging role of long non-coding RNA MALAT1 in predicting clinical
outcomes of patients with digestive system malignancies: A
meta-analysis. Oncol Lett. 17:2159–2170. 2019.PubMed/NCBI
|
|
4
|
Dai Q, Zhang T and Li C: LncRNA MALAT1
regulates the cell proliferation and cisplatin resistance in
gastric cancer via PI3K/AKT pathway. Cancer Manag Res.
12:1929–1939. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shao G, Zhao Z, Zhao W, Hu G, Zhang L, Li
W, Xing C and Zhang X: Long non-coding RNA MALAT1 activates
autophagy and promotes cell proliferation by downregulating
microRNA-204 expression in gastric cancer. Oncol Lett. 19:805–812.
2020.PubMed/NCBI
|
|
6
|
Yao W, Bai Y, Li Y, Guo L, Zeng P, Wang Y,
Qi B, Liu S, Qin X, Li Y, et al: Upregulation of MALAT-1 and its
association with survival rate and the effect on cell cycle and
migration in patients with esophageal squamous cell carcinoma.
Tumour Biol. 37:4305–4312. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu L, Wu Y, Tan D, Meng H, Wang K, Bai Y
and Yang K: Up-regulation of long noncoding RNA MALAT1 contributes
to proliferation and metastasis in esophageal squamous cell
carcinoma. J Exp Clin Cancer Res. 34:72015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang X, Li M, Wang Z, Han S, Tang X, Ge Y,
Zhou L, Zhou C, Yuan Q and Yang M: Silencing of long noncoding RNA
MALAT1 by miR-101 and miR-217 inhibits proliferation, migration,
and invasion of esophageal squamous cell carcinoma cells. J Biol
Chem. 290:3925–3935. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li RQ, Ren Y, Liu W, Pan W, Xu FJ and Yang
M: MicroRNA-mediated silence of onco-lncRNA MALAT1 in different
ESCC cells via ligand-functionalized hydroxyl-rich nanovectors.
Nanoscale. 9:2521–2530. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen M, Xia Z, Chen C, Hu W and Yuan Y:
LncRNA MALAT1 promotes epithelial-to-mesenchymal transition of
esophageal cancer through Ezh2-Notch1 signaling pathway. Anticancer
Drugs. 29:767–773. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang QQ, Cui YH, Wang Y, Kou WZ, Cao F,
Cao XJ, Miao ZH and Kang XH: Mechanism of long non-coding
RNA-metastasis associated lung adenocarcinoma transcript 1 induced
invasion and metastasis of esophageal cancer cell EC-109. Zhonghua
Zhong Liu Za Zhi. 39:405–411. 2017.(In Chinese). PubMed/NCBI
|
|
12
|
Wang W, Zhu Y, Li S, Chen X, Jiang G, Shen
Z, Qiao Y, Wang L, Zheng P and Zhang Y: Long noncoding RNA MALAT1
promotes malignant development of esophageal squamous cell
carcinoma by targeting β-catenin via Ezh2. Oncotarget.
7:25668–25682. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yao Q, Yang J, Liu T, Zhang J and Zheng Y:
Long noncoding RNA MALAT1 promotes the stemness of esophageal
squamous cell carcinoma by enhancing YAP transcriptional activity.
FEBS Open Bio. 9:1392–1402. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qu Y, Shao N, Yang W, Wang J and Cheng Y:
Association of polymorphisms in MALAT1 with the risk of esophageal
squamous cell carcinoma in a Chinese population. Onco Targets Ther.
12:2495–2503. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kang K, Huang YH, Li HP and Guo SM:
Expression of UCA1 and MALAT1 long-chain non-coding RNAs in
esophageal squamous cell carcinoma tissues is predictive of patient
prognosis. Arch Med Sci. 14:752–759. 2018.PubMed/NCBI
|
|
16
|
Huang C, Yu Z, Yang H and Lin Y: Increased
MALAT1 expression predicts poor prognosis in esophageal cancer
patients. Biomed Pharmacother. 83:8–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Z, Zhou Y, Tu B, Bu Y, Liu A and Kong
J: Long noncoding RNA MALAT1 affects the efficacy of radiotherapy
for esophageal squamous cell carcinoma by regulating Cks1
expression. J Oral Pathol Med. 46:583–590. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qi Y, Ooi HS, Wu J, Chen J, Zhang X, Tan
S, Yu Q, Li YY, Kang Y, Li H, et al: MALAT1 long ncRNA promotes
gastric cancer metastasis by suppressing PCDH10. Oncotarget.
7:12693–12703. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Su L, Chen X, Li P, Cai Q, Yu B,
Liu B, Wu W and Zhu Z: MALAT1 promotes cell proliferation in
gastric cancer by recruiting SF2/ASF. Biomed Pharmacother.
68:557–564. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xia H, Chen Q, Chen Y, Ge X, Leng W, Tang
Q, Ren M, Chen L, Yuan D, Zhang Y, et al: The lncRNA MALAT1 is a
novel biomarker for gastric cancer metastasis. Oncotarget.
7:56209–56218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Chen Z, Li MJ, Guo HY and Jing
NC: Long non-coding RNA metastasis-associated lung adenocarcinoma
transcript 1 regulates the expression of Gli2 by miR-202 to
strengthen gastric cancer progression. Biomed Pharmacother.
85:264–271. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li J, Gao J, Tian W, Li Y and Zhang J:
Long non-coding RNA MALAT1 drives gastric cancer progression by
regulating HMGB2 modulating the miR-1297. Cancer Cell Int.
17:442017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li X, Zhao J, Zhang H and Cai J: Silencing
of lncRNA metastasis-associated lung adenocarcinoma transcript 1
inhibits the proliferation and promotes the apoptosis of gastric
cancer cells through regulating microRNA-22-3p-mediated ErbB3. Onco
Targets Ther. 13:559–571. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Z, Li M and Zhang Z: lncRNA MALAT1
modulates oxaliplatin resistance of gastric cancer via sponging
miR-22-3p. Onco Targets Ther. 13:1343–1354. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yan L, Zhang J, Guo D, Ma J, Shui SF and
Han XW: IL-21R functions as an oncogenic factor and is regulated by
the lncRNA MALAT1/miR-125a-3p axis in gastric cancer. Int J Oncol.
54:7–16. 2019.PubMed/NCBI
|
|
26
|
Lu Z, Luo T, Pang T, Du Z, Yin X, Cui H,
Fang G and Xue X: MALAT1 promotes gastric adenocarcinoma through
the MALAT1/miR-181a-5p/AKT3 axis. Open Biol. 9:1900952019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu K, Ren Q and Zhao Y: lncRNA MALAT1
overexpression promotes proliferation, migration and invasion of
gastric cancer by activating the PI3K/AKT pathway. Oncol Lett.
17:5335–5342. 2019.PubMed/NCBI
|
|
28
|
Li H, He C, Wang X, Wang H, Nan G and Fang
L: MicroRNA-183 affects the development of gastric cancer by
regulating autophagy via MALAT1-miR-183-SIRT1 axis and
PI3K/AKT/mTOR signals. Artif Cells Nanomed Biotechnol.
47:3163–3171. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu YR, Yu YC, You S, Li K, Tong X, Chen S,
Chen E, Lin XZ and Chen Y: Long noncoding RNA MALAT1 regulates
autophagy associated chemoresistance via miR-23b-3p sequestration
in gastric cancer. Mol Cancer. 16:1742017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xi Z, Si J and Nan J: LncRNA MALAT1
potentiates autophagy-associated cisplatin resistance by regulating
the microRNA-30b/autophagy-related gene 5 axis in gastric cancer.
Int J Oncol. 54:239–248. 2019.PubMed/NCBI
|
|
31
|
Zhang YF, Li CS, Zhou Y and Lu XH:
Propofol facilitates cisplatin sensitivity via lncRNA
MALAT1/miR-30e/ATG5 axis through suppressing autophagy in gastric
cancer. Life Sci. 244:1172802020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li L, Geng Y, Feng R, Zhu Q, Miao B, Cao J
and Fei S: The human RNA surveillance factor UPF1 modulates gastric
cancer progression by targeting long non-coding RNA MALAT1. Cell
Physiol Biochem. 42:2194–2206. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Y, Wu Z, Yuan J, Sun L, Lin L, Huang N,
Bin J, Liao Y and Liao W: Long non-coding RNA MALAT1 promotes
gastric cancer tumorigenicity and metastasis by regulating
vasculogenic mimicry and angiogenesis. Cancer Lett. 395:31–44.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Deng QJ, Xie LQ and Li H: Overexpressed
MALAT1 promotes invasion and metastasis of gastric cancer cells via
increasing EGFL7 expression. Life Sci. 157:38–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee NK, Lee JH, Ivan C, Ling H, Zhang X,
Park CH, Calin GA and Lee SK: MALAT1 promoted invasiveness of
gastric adenocarcinoma. BMC Cancer. 17:462017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen D, Liu L, Wang K, Yu H, Wang Y, Liu
J, Guo Y and Zhang H: The role of MALAT-1 in the invasion and
metastasis of gastric cancer. Scand J Gastroenterol. 52:790–796.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang Z, Xie Q, Chen Z, Ni H, Xia L, Zhao
Q, Chen Z and Chen P: Resveratrol suppresses the invasion and
migration of human gastric cancer cells via inhibition of
MALAT1-mediated epithelial-to-mesenchymal transition. Exp Ther Med.
17:1569–1578. 2019.PubMed/NCBI
|
|
38
|
Xiao Y, Pan J, Geng Q and Wang G: LncRNA
MALAT1 increases the stemness of gastric cancer cells via enhancing
SOX2 mRNA stability. FEBS Open Bio. 9:1212–1222. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Okugawa Y, Toiyama Y, Hur K, Toden S,
Saigusa S, Tanaka K, Inoue Y, Mohri Y, Kusunoki M, Boland CR, et
al: Metastasis-associated long non-coding RNA drives gastric cancer
development and promotes peritoneal metastasis. Carcinogenesis.
35:2731–2739. 2014. View Article : Google Scholar : PubMed/NCBI
|