Introduction
Polycythemia vera (PV), essential thrombocythemia
and primary myelofibrosis (1) are
Philadelphia chromosome-negative myeloproliferative neoplasms
(MPNs) that originate from hematopoietic stem cells (HSCs). In
2005, an activating point mutation in Janus kinase 2 (JAK2V617F)
was identified in the majority of patients with MPNs (2–5), which
provided critical insights into MPN pathogenesis and promoted the
development of Janus kinase 2 (JAK2) inhibitors. According to
clinical studies, JAK2 inhibitors effectively decrease spleen size
and relieve MPN-associated symptoms; however, their
disease-modifying activity is limited (6,7).
5-Lipoxygenase (5-LO) is an important dioxygenase,
since it is the key enzyme that catalyzes the transformation of
arachidonic acid into inflammatory leukotrienes (LTs) (8). Accumulating evidence has suggested that
the 5-LO signaling pathway is directly involved in cancer
development by promoting cell proliferation, angiogenesis,
migration and invasion, and inhibiting apoptosis (9–13). As
demonstrated in a study by Chen et al (14), 5-LO is upregulated in a mouse model
of JAK2V617F-induced PV, and inhibition of 5-LO by zileuton, a
selective 5-LO inhibitor, attenuates PV development by blocking
JAK2V617F-expressing HSCs in mice. Therefore, it may be
hypothesized that zileuton could potentially eliminate persistent
malignant HSCs in patients with PV. However, to the best of our
knowledge, no previous reports have described the role of 5-LO in
patients with JAK2V617F-positive PV.
Based on the aforementioned evidence, the
combination of zileuton with a JAK2 inhibitor may be a promising
treatment strategy for patients with PV. The present study first
analyzed 5-LO expression in CD34+ cells from the bone
marrow of patients with JAK2V617F-positive PV using western
blotting and reverse transcription-quantitative PCR (RT-qPCR).
Subsequently, the effects of zileuton combined with ruxolitinib on
colony formation, apoptosis and the cell cycle of CD34+
cells from patients with PV were analyzed in vitro.
Materials and methods
Patient specimens and cell
preparation
Bone marrow and peripheral blood were donated by 18
patients who were newly diagnosed with PV and 10 healthy adult
volunteers at the Affiliated Zhuzhou Hospital Xiangya Medical
College CSU (Zhuzhou, China) between August 2017 and April 2019.
All patients met the World Health Organization diagnostic criteria
for PV (1). Patient characteristics
are shown in Table I. The healthy
volunteers were eligible if they were 18–69 years of age and in
healthy condition without active infections, and serious liver,
kidney, heart and other diseases. Bone marrow and peripheral blood
from 10 healthy volunteers were used as normal controls. The
volunteers included 6 women and 4 men. The mean age was 41.5 years,
and the age ranged between 23 and 69 years. All participants
provided written informed consent according to the protocol
approved by the Medical Ethics Committees of the Affiliated Zhuzhou
Hospital Xiangya Medical College CSU (Zhuzhou, China) and in
accordance with the principles outlined in the Declaration of
Helsinki. Mononuclear cells were separated from bone marrow samples
at 440 × g for 30 min at room temperature using Ficoll-Hypaque
density gradient centrifugation (GE Healthcare). An EasySep™
CD34-positive selection kit (Stemcell Technologies, Inc.) was used
to enrich the CD34+ cell population according to the
manufacturer's protocol. CD34+ cells with a purity ≥85%
were used in each experiment.
 | Table I.Patient characteristics and
experiments performed using patient samples. |
Table I.
Patient characteristics and
experiments performed using patient samples.
|
|
|
|
| Experiments |
|---|
|
|
|
|
|
|
|---|
| Case | Age, years | Sex | JAK2V617F allele
burden, % | Leukotriene B4
ELISA | Reverse
transcription-quantitative PCR | Western
blotting | Hematopoietic
progenitor cell assays | FACS for apoptosis
assay | FACS for cell cycle
analysis |
|---|
| PV1 | 68 | Female | 49 | Y | N | N | Y | Y | Y |
| PV2 | 70 | Female | 26 | Y | Y | N | N | N | Y |
| PV3 | 43 | Male | 43 | Y | Y | N | N | Y | Y |
| PV4 | 46 | Male | 75 | N | Y | N | Y | N | N |
| PV5 | 67 | Male | 90 | Y | Y | N | Y | N | N |
| PV6 | 54 | Female | 80 | Y | N | N | Y | Y | Y |
| PV7 | 69 | Male | 28 | Y | Y | Y | N | Y | N |
| PV8 | 55 | Female | 70 | Y | N | Y | Y | N | N |
| PV9 | 72 | Female | 45 | Y | Y | Y | Y | Y | Y |
| PV10 | 67 | Male | 83 | N | Y | Y | Y | Y | N |
| PV11 | 66 | Female | 58 | Y | N | Y | N | N | Y |
| PV12 | 41 | Female | 47 | Y | Y | Y | Y | Y | Y |
| PV13 | 61 | Male | 61 | Y | Y | Y | Y | N | N |
| PV14 | 48 | Female | 40 | Y | N | Y | N | Y | Y |
| PV15 | 67 | Male | 46 | N | Y | N | Y | Y | Y |
| PV16 | 68 | Male | 57 | Y | N | N | Y | N | N |
| PV17 | 56 | Female | 79 | N | Y | N | N | N | N |
| PV18 | 66 | Male | 84 | Y | Y | N | Y | Y | Y |
Detection of leukotriene B4
(LTB4)
Plasma samples from patients with PV and healthy
volunteers were collected to detect LTB4 levels using a leukotriene
B4 Express ELISA kit (cat. no. 10009292; Cayman Chemical Company).
Briefly, the standard or plasma sample, LTB4 AchE tracer and
anti-LTB4 antibody were sequentially added to each well of a
96-well plate. The plates were then incubated for 60–90 min at room
temperature before measuring the absorbance at 405 nm using an
ELISA microplate reader (Thermo Fisher Scientific, Inc.).
RT-qPCR
Total RNA was extracted from the purified
CD34+ cells using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). cDNA synthesis was
performed using a PrimeScript™ RT reagent Kit with gDNA Eraser
(Perfect Real Time) (cat. no. RR047A; Takara Bio, Inc.) according
to the manufacturer's protocols. Reactions were incubated at 37°C
for 15 min followed by heat inactivation for 5 sec at 85°C for
reverse transcription. The PCR amplification was performed using TB
Green™ Premix Ex Taq™ II (Tli RNaseH Plus) (cat. no. RR820A; Takara
Bio, Inc.). The primer sequences of the human 5-LO gene and the
GAPDH gene are listed in Table II.
The thermocycling conditions were as follows: 95°C for 30 sec,
followed by 40 cycles of 95°C for 5 sec and 60°C for 31 sec.
Amplification was performed using an ABI 7300 system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The 2−ΔΔCq
formula (15) was used to analyze
the relative mRNA expression levels of 5-LO, which were normalized
to the expression levels of GAPDH.
 | Table II.Primer sequences used for reverse
transcription-quantitative PCR. |
Table II.
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Product length,
bp | Primer | Sequence
(5′-3′) |
|---|
| 5-lipoxygenase | 168 | Forward |
GCTGAATGACGACTGGTA |
|
|
| Reverse |
CGGTGTTGCTTGAGAATG |
| GAPDH | 263 | Forward |
ATGCTGGCGCTGAGTACGTC |
|
|
| Reverse |
GGTCATGAGTCCTTCCACGATA |
Western blotting
RIPA lysis solution (cat. no. P0013B; Beyotime
Institute of Biotechnology) was used to extract total proteins from
purified CD34+ cells. BCA assays (cat. no. 23227;
Pierce; Thermo Fisher Scientific, Inc.) were used to determine the
protein concentration. An equal amount of total proteins (30
µg/lane) was separated by 10% SDS-PAGE and then transferred onto
PVDF membrane (cat. no. IPVH00010; EMD Millipore). The membranes
were blocked using TBS with 0.05% Tween-20 (TBST) containing 5%
non-fat milk at room temperature for 2 h, and then incubated with
primary antibodies against 5-LO (dilution, 1:500; cat. no.
sc-136195; Santa Cruz Biotechnology, Inc.) and β-actin (dilution,
1:1,000; cat. no. ab8226; Abcam) overnight at 4°C. After washing
with TBST, the membranes were incubated with secondary antibodies
(dilution, 1:2,000; cat. no. 7076; Cell Signaling Technology, Inc.)
conjugated to horseradish peroxidase at room temperature for 1 h.
Finally, the protein blots were visualized using a Hypersensitive
ECL chemiluminescence kit (cat. no. P10018FS; Beyotime Institute of
Biotechnology). The results were semi-quantified using ImageJ
software (v1.52; National Institutes of Health).
Colony formation assay (CFA)
The colony-forming ability of the cells was
estimated by inoculating 500 CD34+ cells in MethoCult
H4435 (Stemcell Technologies, Inc.). Various concentrations of
zileuton (50, 100, 250 and 500 µM; Cayman Chemical Company) and/or
50 nM ruxolitinib (Cayman Chemical Company) were added for 14 days
at 37°C. After 14 days, the presence of colonies (>40 cells) was
scored under a light microscope (Zeiss LSM 800 with Airyscan;
magnification, ×25; Zeiss AG).
Flow cytometry
CD34+ cells (1×106 cells/well)
from patients with PV and healthy volunteers were seeded into a
6-well plate and treated with 100 µM zileuton, 50 nM ruxolitinib or
the combination of 100 µM zileuton and 50 nM ruxolitinib for 48 h
at 37°C. After 2 days, 5×105 cells were collected and
applied to the flow cytometry detection. The apoptosis assay was
performed using an Annexin V-FITC Apoptosis Detection Kit (cat. no.
556547; BD Biosciences). In brief, cells were stained with Annexin
V-FITC and PI for 15 min at room temperature, and detected using a
flow cytometer. Stained cells were analyzed using a FACSCalibur
instrument (BD Biosciences) using a 488 nm excitation wavelength
and emission was detected at 530 nm (for FITC) and 575 nm (for PI).
The data were analyzed using the BD FACSuite™ version 1.01 (BD
Biosciences). The phases of the cell cycle and DNA synthesis
activity of CD34+ cells were determined using a FITC
BrdU Flow kit (cat. no. 559619; BD Biosciences) according to the
manufacturer's protocol. Briefly, cells were incubated with BrdU, a
nucleoside analogue of thymidine, and then stained with anti-human
CD34-allophycocyanin (dilution, 1:5; cat. no. 560940; BD
Biosciences). After fixing in BD Cytofix/Cytoperm Buffer for 30 min
on ice and permeabilizing in BD Cytoperm Permeabilization Buffer
Plus for 10 min on ice, cells were treated with DNase to expose
BrdU epitopes and then incubated with a FITC-conjugated anti-BrdU
antibody (provided in kit; BD Biosciences) for 20 min at room
temperature. DNA was counterstained with 7-aminoactinomycin D
(7AAD; provided in kit; BD Biosciences) for 15 min at room
temperature. Stained cells were then analyzed using a FACSCalibur
instrument using a 488 nm excitation wavelength and emission was
detected at 530 nm (for FITC-conjugated anti-BrdU) and 610 nm (for
7AAD). The data were analyzed using BD FACSuite™ version 1.01.
Statistical analysis
All experiments were repeated three times. Data are
presented as medians and interquartile ranges. Differences in 5-LO
expression and LTB4 levels between groups were compared using a
two-tailed Mann-Whitney test, while data derived from the same
samples after different treatments were analyzed using the Friedman
test, and the Nemenyi post hoc test was subsequently used for
pairwise comparisons between groups. GraphPad Prism 7 software
(GraphPad Software, Inc.) and SPSS 26.0 software (IBM Corp.) were
utilized for statistical analyses. P<0.05 was considered to
indicate a statistically significant difference.
Results
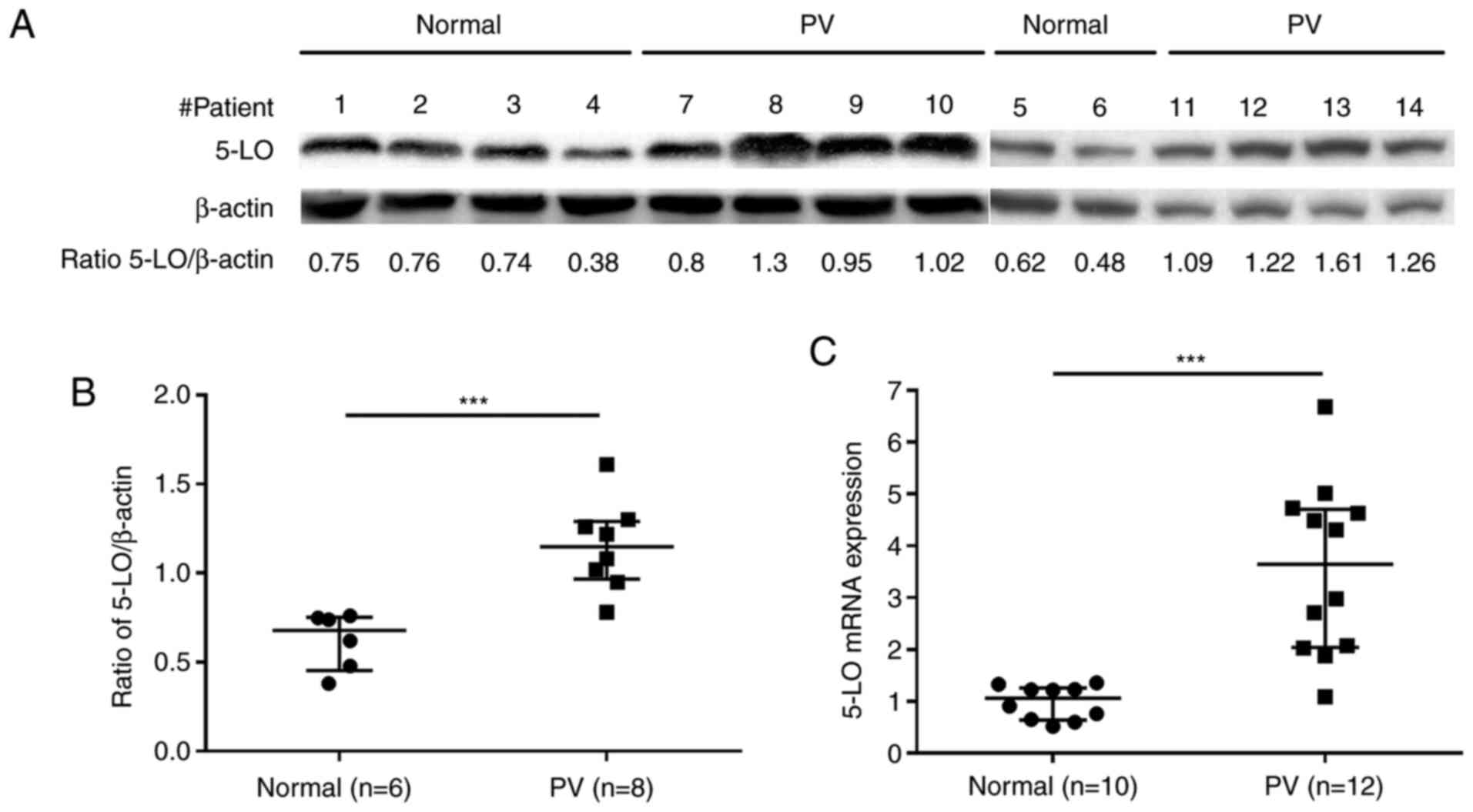
5-LO expression is increased in
CD34+ cells from patients with PV
To determine the potential effects of 5-LO inhibitor
zileuton in the treatment of human PV, the present study first
assessed the basal protein and mRNA expression levels of 5-LO in
CD34+ cells from the bone marrow of patients with PV and
healthy volunteers. Western blot analysis revealed that
CD34+ cells from patients with PV exhibited higher
protein expression levels of 5-LO than those from healthy
volunteers (Fig. 1A and B; Table SI). Consistent with the results
observed for protein expression, 5-LO mRNA expression was also
increased in CD34+ cells from patients with PV compared
with in those from healthy volunteers, as demonstrated by the
results of RT-qPCR (Fig. 1C;
Table SII).
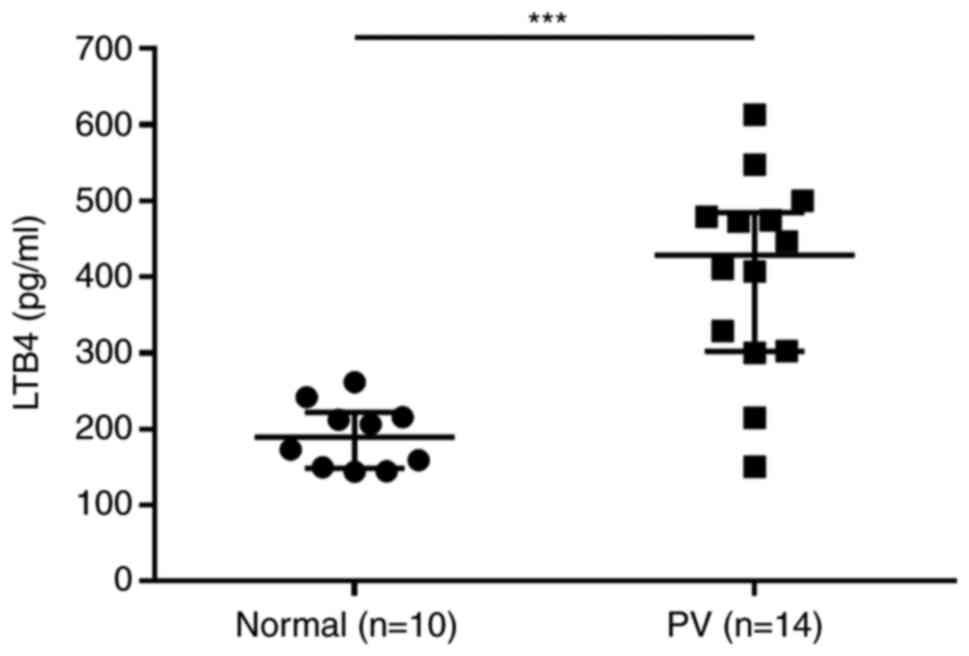
LTB4 levels are elevated in patients
with PV
Plasma levels of LTB4, a metabolite of the 5-LO
signaling pathway, were measured in 14 patients with PV and 10
healthy volunteers using ELISA. Higher LTB4 levels were observed in
patients with PV compared with those in healthy volunteers
(Fig. 2; Table SIII).
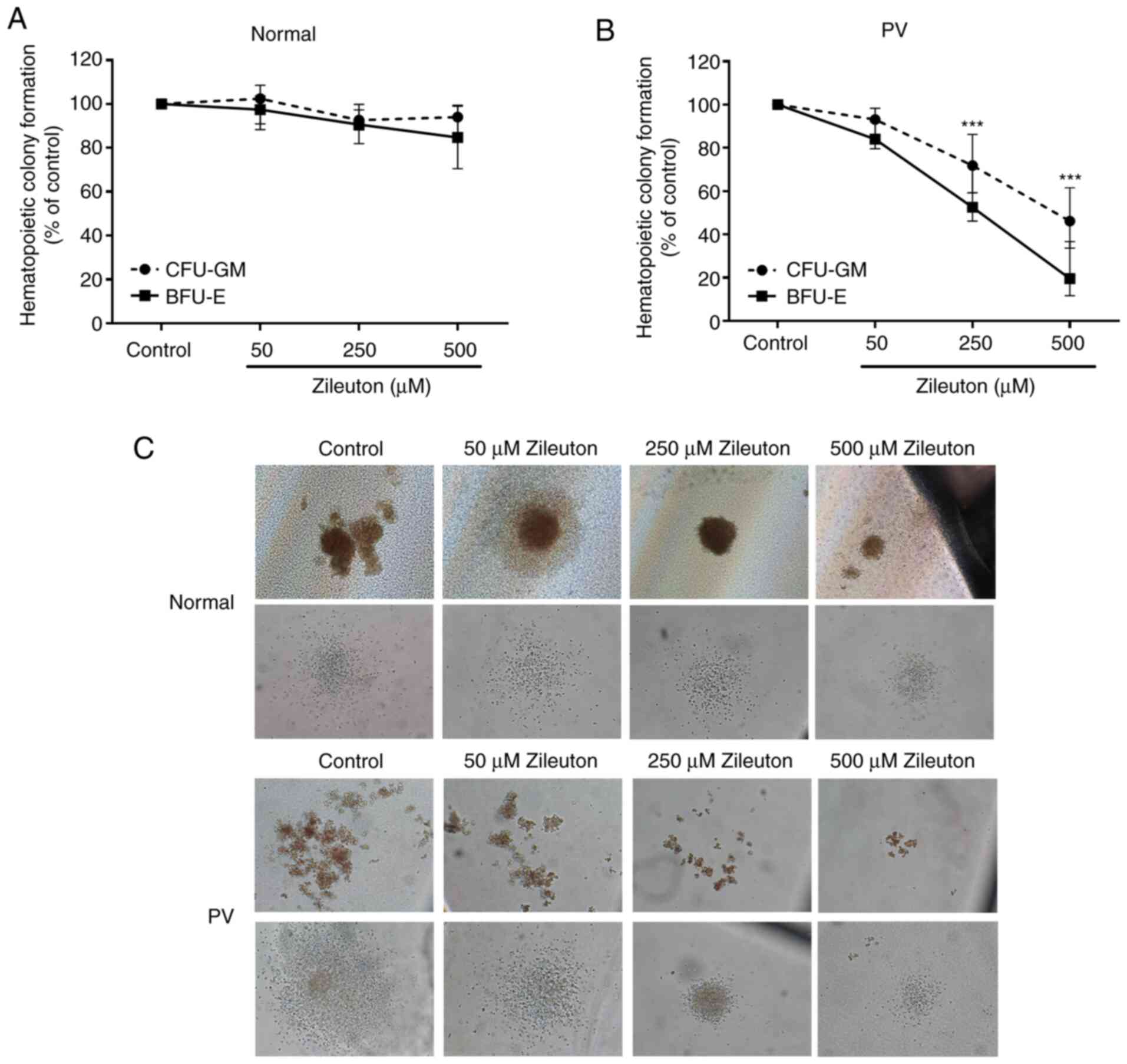
Zileuton suppresses the colony
formation of CD34+ cells from patients with PV in a
dose-dependent manner
A CFA was performed to assess the effect of zileuton
treatment on the colony formation of primary CD34+ cells
in vitro. As shown in Fig. 3,
zileuton treatment inhibited granulocytes and monocytes (CFU-GM)-
and burst-forming unit-erythroid (BFU-E)-derived colony formation
by PV CD34+ cells in a dose-dependent manner, with an
IC50 of 460.4 µM for CFU-GM and 233.5 µM for BFU-E
(Fig. 3B and C; Table SIV). By contrast, zileuton treatment
did not markedly alter the colony formation of the CD34+
cells from healthy volunteers (Fig. 3A
and C; Table SIV).
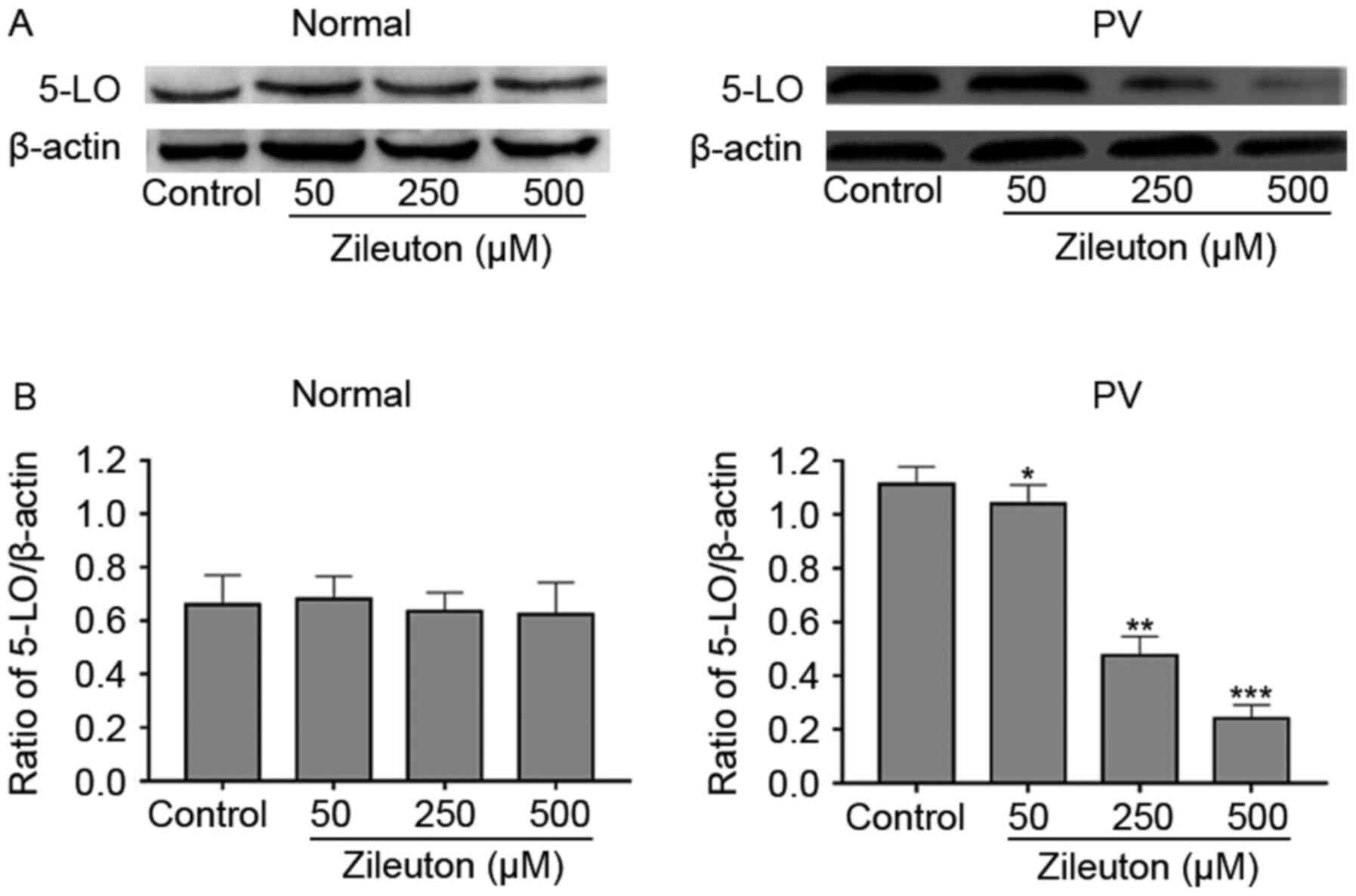
Reduction of colony formation by
zileuton treatment in PV CD34+ cells occurs through a
reduction in 5-LO expression
To explore whether the effect of zileuton on colony
formation of hematopoietic cells was associated with the levels of
5-LO, 5-LO protein expression in CD34+ cells from
patients with PV and healthy volunteers was measured with and
without treatment with increasing concentrations of zileuton using
western blotting. As shown in the Fig.
4, zileuton dose-dependently decreased 5-LO protein expression
in CD34+ cells from a patient with PV, which exhibited
high 5-LO expression, but had no significant effects on 5-LO
expression in CD34+ cells from a healthy volunteer.
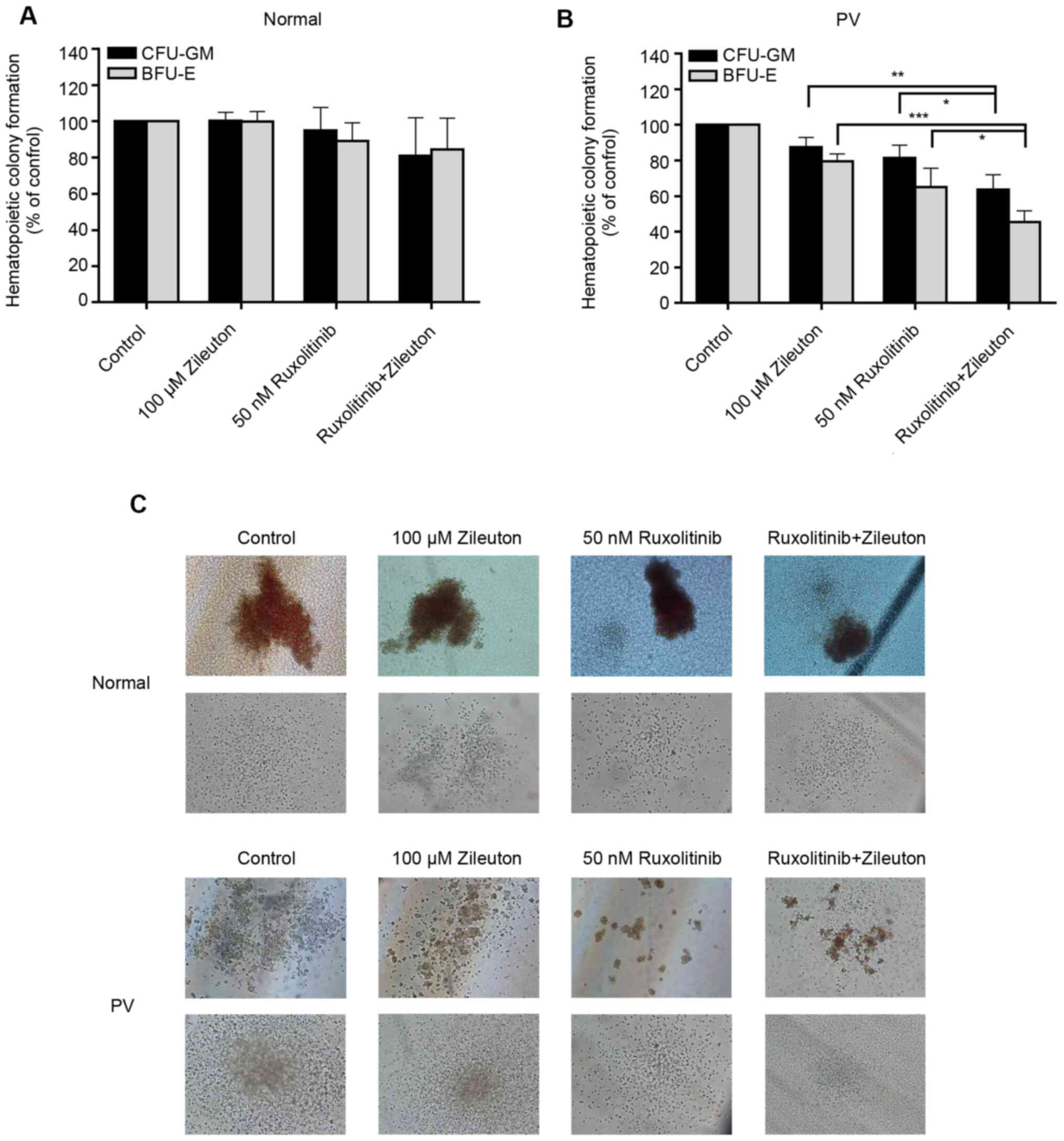
Combination treatment with zileuton
and ruxolitinib synergistically inhibits the colony formation of
hematopoietic cells from patients with PV
The present study investigated the effect of
combination treatment with zileuton and ruxolitinib on the
formation of colonies of primary CD34+ cells from 10
patients with PV and 6 healthy volunteers in vitro. The
doses selected for the present study were 100 µM zileuton and 50 nM
ruxolitinib. In the CD34+ cells from healthy volunteers,
treatment with 100 µM zileuton, 50 nM ruxolitinib or 100 µM
zileuton combined with 50 nM ruxolitinib did not alter colony
formation (Fig. 5A and C; Table SV). By contrast, the yields of
CFU-GM and BFU-E in CD34+ cells from patients with PV
treated with 50 nM ruxolitinib were decreased by 19 and 35%,
respectively, and the yields of CFU-GM and BFU-E in
CD34+ cells from patients with PV treated with 100 µM
zileuton were decreased by 12 and 20%, respectively (Fig. 5B and C; Table SV). However, the combination of
ruxolitinib with zileuton had a greater effect on the colony
formation of CD34+ cells from patients with PV, with a
36% a reduction in CFU-GM and a 55% reduction in BFU-E (Fig. 5B and C; Table SV).
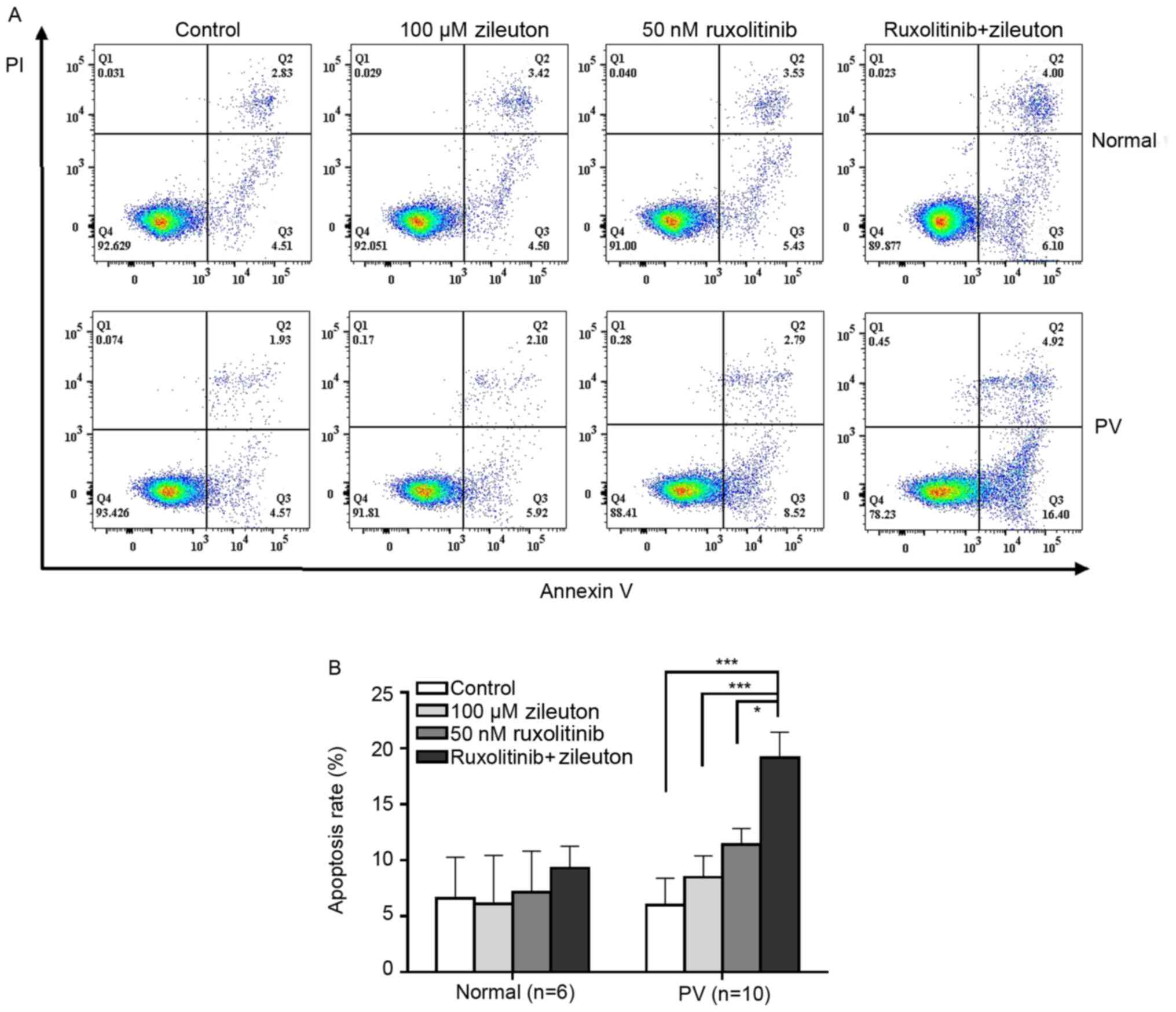
Combination treatment with zileuton
and ruxolitinib induces apoptosis in CD34+ cells from
patients with PV
The apoptosis rate of CD34+ cells from
patients with PV and healthy volunteers after treatment with
zileuton and/or ruxolitinib was detected using flow cytometry. The
apoptosis rate of CD34+ cells from patients with PV was
slightly increased following zileuton or ruxolitinib treatment
(Fig. 6; Table SVI). However, combination treatment
with zileuton and ruxolitinib induced apoptosis in a greater number
of CD34+ cells from patients with PV than treatment with
either individual drug. By contrast, neither ruxolitinib nor
zileuton alone or in combination induced apoptosis of
CD34+ cells from healthy volunteers (Fig. 6; Table
SVI).
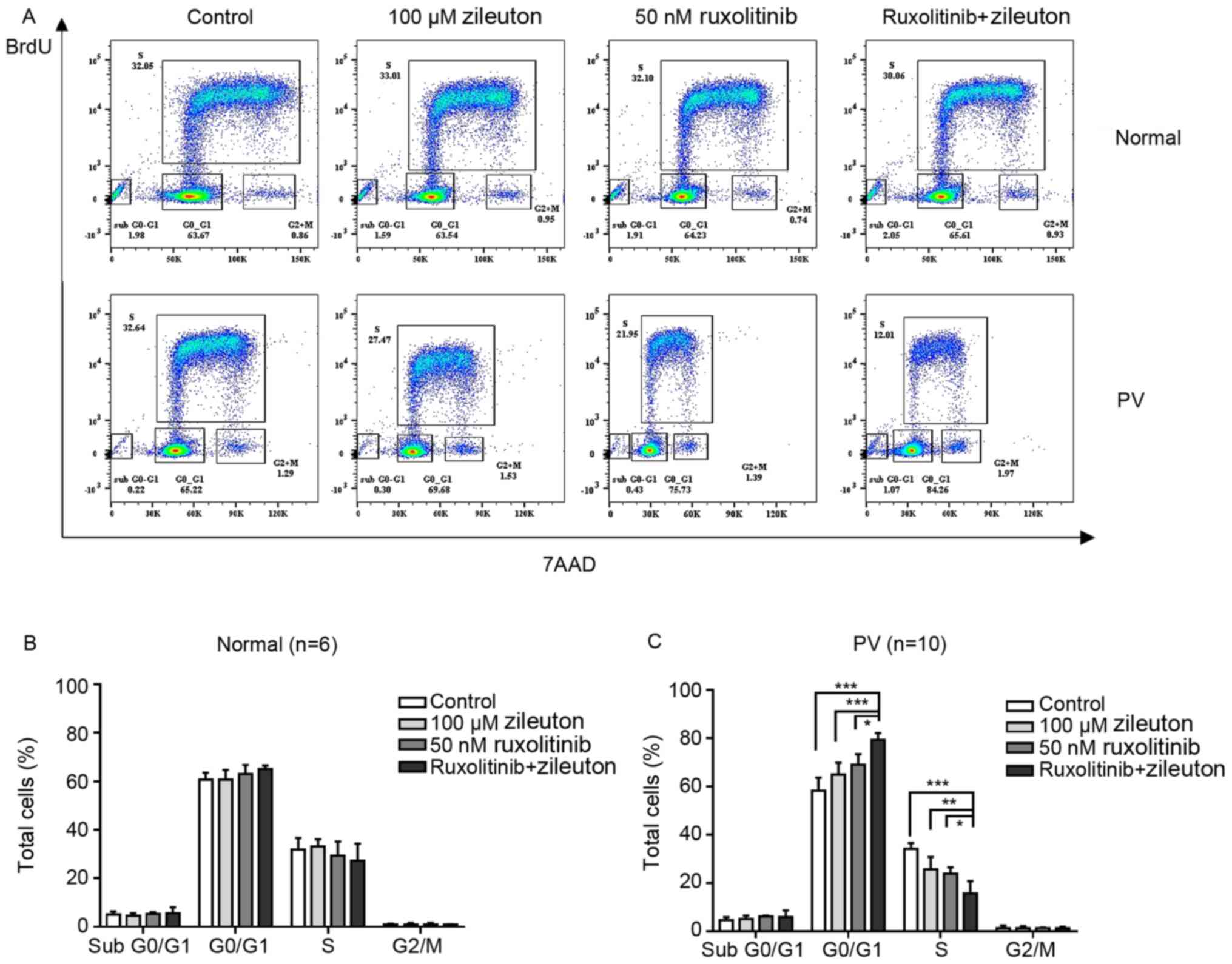
Treatment with zileuton and
ruxolitinib arrests CD34+ cells from patients with PV at
the G0/G1 phase of the cell cycle
Cell cycle arrest is an important effect of numerous
anticancer agents. The present study investigated the effects of
zileuton and/or ruxolitinib on the cell cycle of CD34+
cells from patients with PV in vitro. As shown in Fig. 7A and C, treatment with zileuton and
ruxolitinib alone slightly increased the number of CD34+
cells from patients with PV that were arrested in the
G0/G1 phase of the cell cycle, with a
concomitant decrease in the percentage of cells in S phase
(Table SVII). However, combination
treatment with zileuton and ruxolitinib caused more
CD34+ cells from patients with PV to be arrested in the
G0/G1 phase and a significant decrease in the
proportion of cells in S phase compared with either monotherapy. By
contrast, the percentage of CD34+ cells from healthy
volunteers in each phase of the cell cycle did not markedly change,
regardless of whether they were treated with zileuton, ruxolitinib
or the combination treatment (Fig. 7A
and B; Table SVII).
Discussion
PV is the most common Philadelphia
chromosome-negative MPN (16).
Thrombosis and hemorrhage, as well as myelofibrotic and/or leukemic
transformation (17,18), are potential complications occurring
in patients with PV. The JAK2V617F mutation in the pseudokinase
domain leads to constitutive phosphorylation of JAK2 and
overactivation of downstream signaling pathways, ultimately
resulting in uncontrolled myeloid cell proliferation (19,20).
These findings motivated the clinical development of JAK kinase
inhibitors for patients with MPNs. Ruxolitinib was the first
selective JAK1/2 inhibitor that was demonstrated to be effective in
patients with PV and myelofibrosis (21–23), and
it provides substantial benefits, including a marked decrease in
splenomegaly and disease symptoms (24). However, treatment with ruxolitinib
rarely induces molecular or pathological remission in patients with
MPNs (25–27). Furthermore, the dose-dependent
hematological toxicity of ruxolitinib represents a major concern
for a number of patients (28).
Researchers have expressed an interest in identifying novel agents
with different mechanisms of action other than targeting the
JAK-STAT signaling pathway.
5-LO expression is upregulated in numerous different
types of cancer, including pancreatic, breast, prostate, esophageal
and colon cancer (29–33). The products of 5-LO, such as
5-hydroxyeicosatetraenoic acid (5-HETE) and LTs, are able to
promote cell proliferation, suppress apoptosis, promote
angiogenesis and enhance tumor cell invasion (34–36).
According to previous studies, epidermal growth factors and
neurotensin are involved in the 5-LO-mediated tumor progression in
individuals with prostate cancer (37,38). A
study of patients with colorectal cancer revealed that 5-HETE
stimulates angiogenesis by inducing the expression of VEGF
(39,40). Furthermore, increased activities of
5-LO and matrix metalloproteinases are associated with
extracellular matrix stiffness (41)
and enhance the invasiveness of cancer cells (42,43). The
enzyme 5-LO is involved in the development of not only solid
malignancies but also certain forms of leukemia (44,45).
Recently, the 5-LO gene was shown to be a critical regulator for
mouse leukemic stem cells (LSCs) in BCR-ABL-induced chronic myeloid
leukemia (CML), and the combination of zileuton and imatinib
extends the survival time of mice with CML (46). This finding motivated an initial
clinical trial combining zileuton with imatinib as a treatment for
CML (clinicaltrials.com; NCT02047149,
NCT01130688) with no available efficacy data at present. Zileuton
has been revealed to selectively deplete JAK2V617F-expressing HSCs,
thereby preventing PV development in mice (14). The molecular mechanism of 5-LO in PV
is associated with the β-catenin signaling pathway, which is
required for the maintenance of both HSCs (47) and LSCs in individuals with CML
(48–50). Based on these results, drugs
targeting the 5-LO signaling pathway can eradicate human
JAK2V617F+ malignant HSCs and thus are potentially
curative treatments for MPNs. However, a previous study reported
low expression levels of 5-LO and LTB4 receptor 1 in
CD34+ cells from patients with BCR-ABL-positive CML
compared with healthy donors (51).
Another study reported that 5-LO expression was undetectable in
more primitive CML LSCs (52).
Therefore, 5-LO has distinct and important regulatory and
functional roles in human and murine CML. To the best of our
knowledge, no previous reports have described the role of 5-LO in
human PV.
In the present study, 5-LO mRNA and protein
expression was increased in CD34+ cells from patients
with PV compared with in CD34+ cells from healthy
volunteers. Higher LTB4 levels were detected in patients with PV
compared with healthy volunteers. Based on these results, the 5-LO
signaling pathway was upregulated in patients with
JAK2V617F-positive PV, consistent with previous findings from PV
mice (14), indicating that 5-LO may
be involved in the pathogenesis of human JAK2V617F-positive PV.
Zileuton treatment decreased the colony formation of
CD34+ cells from patients with PV in a dose-dependent
manner by reducing 5-LO expression in PV CD34+ cells.
Furthermore, zileuton and ruxolitinib exerted their anticancer
effects by suppressing the colony formation of hematopoietic cells,
inducing apoptosis and blocking the cell cycle of CD34+
cells from patients with PV. The combination of the two drugs was
more effective than either agent alone. Similar effects were not
observed in CD34+ cells from healthy volunteers after
treatment with zileuton or ruxolitinib, either alone or in
combination. Therefore, zileuton enhanced the antitumor activity of
ruxolitinib against hematopoietic progenitor cells from patients
with PV, suggesting that zileuton and ruxolitinib may represent an
effective therapeutic combination. Notably, activities of both 100
µM zileuton and 50 nM ruxolitinib were relatively specific for
hematopoietic progenitor cells from patients with PV, while sparing
CD34+ cells from healthy volunteers. The latter finding
suggests that the combination treatment may exhibit improved safety
and low hematological toxicity.
In conclusion, zileuton exerted a synergistic effect
with ruxolitinib on CD34+ cells from patients with PV by
suppressing cell proliferation, inducing apoptosis and arresting
the cell cycle, which provides conceptual validation for further
clinical applications of combination therapy with ruxolitinib and
zileuton for patients with PV. However, because CD34+
cells collected from some patients were not amplified enough to
complete all downstream experiments, only a portion of the samples
were analyzed in some experiments. This was a limitation of the
present study. Future studies should focus on investigating the
molecular mechanisms by which 5-LO inhibitors block colony
formation, and induce apoptosis and cell cycle arrest, in patients
with PV.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the Clinical Medical
Technology Innovation Guidance Project of Hunan Province (grant no.
2018SK52803).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GH and YC proposed and designed the current study.
JL and YL selected patients and collected samples/clinical data. YC
and HZ conducted the experiments. YC, HZ and KT were responsible
for acquiring, analyzing and interpreting the data. YC and HZ
drafted the initial manuscript. YC, HZ, JL and GH reviewed and
edited the manuscript. GH, YC and HZ assessed the authenticity of
all the raw data and ensured its legitimacy. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The experimental protocol was established according
to the ethical guidelines of the Helsinki Declaration and was
approved by the Medical Ethics Committees of The Affiliated Zhuzhou
Hospital Xiangya Medical College CSU (Zhuzhou, China). Written
informed consent was obtained from each individual.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Arber DA, Orazi A, Hasserjian R, Thiele J,
Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M and Vardiman JW:
The 2016 revision to the World Health Organization classification
of myeloid neoplasms and acute leukemia. Blood. 127:2391–2405.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baxter EJ, Scott LM, Campbell PJ, East C,
Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N,
et al: Acquired mutation of the tyrosine kinase JAK2 in human
myeloproliferative disorders. Lancet. 365:1054–1061. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
James C, Ugo V, Le Couédic JP, Staerk J,
Delhommeau F, Lacout C, Garçon L, Raslova H, Berger R,
Bennaceur-Griscelli A, et al: A unique clonal JAK2 mutation leading
to constitutive signalling causes polycythaemia vera. Nature.
434:1144–1148. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jones AV, Kreil S, Zoi K, Waghorn K,
Curtis C, Zhang L, Score J, Seear R, Chase AJ, Grand FH, et al:
Widespread occurrence of the JAK2 V617F mutation in chronic
myeloproliferative disorders. Blood. 106:2162–2168. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kralovics R, Passamonti F, Buser AS, Teo
SS, Tiedt R, Passweg JR, Tichelli A, Cazzola M and Skoda RC: A
gain-of-function mutation of JAK2 in myeloproliferative disorders.
N Engl J Med. 352:1779–1790. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deininger M, Radich J, Burn TC, Huber R
and Verstovsek S: The effect of long-term ruxolitinib treatment on
JAK2p.V617F allele burden in patients with myelofibrosis. Blood.
126:1551–1554. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cervantes F and Pereira A: Does
ruxolitinib prolong the survival of patients with myelofibrosis?
Blood. 129:832–837. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Needleman P, Turk J, Jakschik BA, Morrison
AR and Lefkowith JB: Arachidonic acid metabolism. Ann Rev Biochem.
55:69–102. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peters-Golden M and Henderson WR Jr:
Leukotrienes. N Engl J Med. 357:1841–1854. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pidgeon GP, Lysaght J, Krishnamoorthy S,
Reynolds JV, O'Byrne K, Nie D and Honn KV: Lipoxygenase metabolism:
Roles in tumor progression and survival. Cancer Metastasis Rev.
26:503–524. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang D and Dubois RN: Eicosanoids and
cancer. Nat Rev Cancer. 10:181–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bishayee K and Khuda-Bukhsh AR:
5-lipoxygenase antagonist therapy: A new approach towards targeted
cancer chemotherapy. Acta Biochim Biophys Sin (Shanghai).
45:709–719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moore GY and Pidgeon GP: Cross-talk
between cancer cells and the tumour microenvironment: The role of
the 5-lipoxygenase pathway. Int J Mol Sci. 18:2362017. View Article : Google Scholar
|
|
14
|
Chen Y, Shan Y, Lu M, DeSouza N, Guo Z,
Hoffman R, Liang A and Li S: Alox5 blockade eradicates
JAK2V617F-induced polycythemia Vera in mice. Cancer Res.
77:164–174. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mehta J, Wang H, Iqbal SU and Mesa R:
Epidemiology of myeloproliferative neoplasms in the United States.
Leuk Lymphoma. 55:595–600. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hoffman R, Prchal JT, Samuelson S, Ciurea
SO and Rondelli D: Philadelphia chromosome-negative
myeloproliferative disorders: Biology and treatment. Biol Blood
Marrow Transplant. 13 (Suppl 1):S64–S72. 2007. View Article : Google Scholar
|
|
18
|
Mascarenhas J: A concise update on risk
factors, therapy, and outcome of leukemic transformation of
myeloproliferative neoplasms. Clin Lymphoma Myeloma Leuk. 16
(Suppl):S124–S129. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Silvennoinen O and Hubbard SR: Molecular
insights into regulation of JAK2 in myeloproliferative neoplasms.
Blood. 125:3388–3392. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Levine RL, Wadleigh M, Cools J, Ebert BL,
Wernig G, Huntly BJ, Boggon TJ, Wlodarska I, Clark JJ, Moore S, et
al: Activating mutation in the tyrosine kinase JAK2 in polycythemia
Vera, essential thrombocythemia, and myeloid metaplasia with
myelofibrosis. Cancer Cell. 7:387–397. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Verstovsek S, Mesa RA, Gotlib J, Levy RS,
Gupta V, DiPersio JF, Catalano JV, Deininger M, Miller C, Silver
RT, et al: A double-blind, placebo-controlled trial of ruxolitinib
for myelofibrosis. N Engl J Med. 366:799–807. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hasselbalch HC and Bjørn ME: Ruxolitinib
versus standard therapy for the treatment of polycythemia Vera. N
Engl J Med. 372:16702015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pardanani A, Harrison C, Cortes JE,
Cervantes F, Mesa RA, Milligan D, Masszi T, Mishchenko E, Jourdan
E, Vannucchi AM, et al: Safety and efficacy of fedratinib in
patients with primary or secondary myelofibrosis: A randomized
clinical trial. JAMA Oncol. 1:643–651. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kiladjian J, Verstovsek S, Griesshammer M,
Masszi T, Durrant S, Passamonti F, Harrison CN, Pane F, Zachee P,
Kirito K, et al: Results from the 208-week (4-year) Follow-up of
RESPONSE Trial, a Phase 3 study comparing ruxolitinib (Rux) with
best available therapy (BAT) for the treatment of polycythemia Vera
(PV). Blood. 130 (Suppl 1):S3222017.
|
|
25
|
Verstovsek S, Gotlib J, Mesa RA, Vannucchi
AM, Kiladjian JJ, Cervantes F, Harrison CN, Paquette R, Sun W, Naim
A, et al: Long-term survival in patients treated with ruxolitinib
for myelofibrosis: COMFORT-I and -II pooled analyses. J Hematol
Oncol. 10:1562017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Harrison CN, Vannucchi AM, Kiladjian JJ,
Al-Ali HK, Gisslinger H, Knoops L, Cervantes F, Jones MM, Sun K,
McQuitty M, et al: Long-term findings from COMFORT-II, a phase 3
study of ruxolitinib vs. best available therapy for myelofibrosis.
Leukemia. 31:7752016. View Article : Google Scholar
|
|
27
|
Verstovsek S, Mesa RA, Gotlib J, Gupta V,
DiPersio JF, Catalano JV, Deininger MW, Miller CB, Silver RT,
Talpaz M, et al: Long-term treatment with ruxolitinib for patients
with myelofibrosis: 5-year update from the randomized,
double-blind, placebo-controlled, phase 3 COMFORT-I trial. J
Hematol Oncol. 10:552017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Harrison C, Kiladjian JJ, Al-Ali HK,
Gisslinger H, Waltzman R, Stalbovskaya V, McQuitty M, Hunter DS,
Levy R, Knoops L, et al: JAK inhibition with ruxolitinib versus
best available therapy for myelofibrosis. N Engl J Med.
366:787–798. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Avis I, Hong SH, Martínez A, Moody T, Choi
YH, Trepel J, Das R, Jett M and Mulshine JL: Five-lipoxygenase
inhibitors can mediate apoptosis in human breast cancer cell lines
through complex eicosanoid interactions. FASEB J. 15:2007–2009.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Melstrom LG, Bentrem DJ, Salabat MR,
Kennedy TJ, Ding XZ, Strouch M, Rao SM, Witt RC, Ternent CA,
Talamonti MS, et al: Overexpression of 5-lipoxygenase in colon
polyps and cancer and the effect of 5-LOX inhibitors in vitro and
in a murine model. Clin Cancer Res. 14:6525–6530. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hennig R, Ding XZ, Tong WG, Schneider MB,
Standop J, Friess H, Büchler MW, Pour PM and Adrian TE:
5-Lipoxygenase and leukotriene B(4) receptor are expressed in human
pancreatic cancers but not in pancreatic ducts in normal tissue. Am
J Pathol. 161:421–428. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hoque A, Lippman SM, Wu TT, Xu Y, Liang
ZD, Swisher S, Zhang H, Cao L, Ajani JA and Xu XC: Increased
5-lipoxygenase expression and induction of apoptosis by its
inhibitors in esophageal cancer: A potential target for prevention.
Carcinogenesis. 26:785–791. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Matsuyama M, Yoshimura R, Mitsuhashi M,
Hase T, Tsuchida K, Takemoto Y, Kawahito Y, Sano H and Nakatani T:
Expression of lipoxygenase in human prostate cancer and growth
reduction by its inhibitors. Int J Oncol. 24:821–827.
2004.PubMed/NCBI
|
|
34
|
Hyde CA and Missailidis S: Inhibition of
arachidonic acid metabolism and its implication on cell
proliferation and tumour-angiogenesis. Int Immunopharmacol.
9:701–715. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ding XZ, Iversen P, Cluck MW, Knezetic JA
and Adrian TE: Lipoxygenase inhibitors abolish proliferation of
human pancreatic cancer cells. Biochem Biophys Res Commun.
261:218–223. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wen Z, Liu H, Li M, Li B, Gao W, Shao Q,
Fan B, Zhao F, Wang Q, Xie Q, et al: Increased metabolites of
5-lipoxygenase from hypoxic ovarian cancer cells promote
tumor-associated macrophage infiltration. Oncogene. 34:1241–1252.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hassan S and Carraway RE: Involvement of
arachidonic acid metabolism and EGF receptor in neurotensin-induced
prostate cancer PC3 cell growth. Regul Pept. 133:105–114. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Karlage KL, Mogalian E, Jensen A and
Myrdal PB: Inhalation of an ethanol-based zileuton formulation
provides a reduction of pulmonary adenomas in the A/J mouse model.
AAPS PharmSciTech. 11:168–173. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Romano M, Catalano A, Nutini M, D'Urbano
E, Crescenzi C, Claria J, Libner R, Davi G and Procopio A:
5-Lipoxygenase regulates malignant mesothelial cell survival:
Involvement of vascular endothelial growth factor. FASEB J.
15:2326–2336. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ye YN, Wu WK, Shin VY, Bruce IC, Wong BC
and Cho CH: Dual inhibition of 5-LOX and COX-2 suppresses colon
cancer formation promoted by cigarette smoke. Carcinogenesis.
26:827–834. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Levental KR, Yu H, Kass L, Lakins JN,
Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et
al: Matrix crosslinking forces tumor progression by enhancing
integrin signaling. Cell. 139:891–906. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Erler JT, Bennewith KL, Cox TR, Lang G,
Bird D, Koong A, Le QT and Giaccia AJ: Hypoxia-induced lysyl
oxidase is a critical mediator of bone marrow cell recruitment to
form the premetastatic niche. Cancer Cell. 15:35–44. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kirschmann DA, Seftor EA, Fong SF, Nieva
DR, Sullivan CM, Edwards EM, Sommer P, Csiszar K and Hendrix MJ: A
molecular role for lysyl oxidase in breast cancer invasion. Cancer
Res. 62:4478–4483. 2002.PubMed/NCBI
|
|
44
|
Graham SM, Vass JK, Holyoake TL and Graham
GJ: Transcriptional analysis of quiescent and proliferating CD34+
human hemopoietic cells from normal and chronic myeloid leukemia
sources. Stem Cells. 25:3111–3120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Roos J, Oancea C, Heinssmann M, Khan D,
Held H, Kahnt AS, Capelo R, La Buscato E, Proschak E, Puccetti E,
et al: 5-Lipoxygenase is a candidate target for therapeutic
management of stem cell-like cells in acute myeloid leukemia.
Cancer Res. 74:5244–5255. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen Y, Hu Y, Zhang H, Peng C and Li S:
Loss of the Alox5 gene impairs leukemia stem cells and prevents
chronic myeloid leukemia. Nat Genet. 41:783–792. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ruiz-Herguido C, Guiu J, D'Altri T,
Inglés-Esteve J, Dzierzak E, Espinosa L and Bigas A: Hematopoietic
stem cell development requires transient Wnt/β-catenin activity. J
Exp Med. 209:1457–1468. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hu Y, Chen Y, Douglas L and Li S:
Beta-catenin is essential for survival of leukemic stem cells
insensitive to kinase inhibition in mice with BCR-ABL-induced
chronic myeloid leukemia. Leukemia. 23:109–116. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhao C, Blum J, Chen A, Kwon HY, Jung SH,
Cook JM, Lagoo A and Reya T: Loss of beta-catenin impairs the
renewal of normal and CML stem cells in vivo. Cancer Cell.
12:528–541. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Heidel FH, Bullinger L, Feng Z, Wang Z,
Neff TA, Stein L, Kalaitzidis D, Lane SW and Armstrong SA: Genetic
and pharmacologic inhibition of beta-catenin targets
imatinib-resistant leukemia stem cells in CML. Cell Stem Cell.
10:412–424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lucas CM, Harris RJ, Giannoudis A,
Mcdonald E and Clark RE: Low leukotriene B4 receptor 1 leads to
ALOX5 downregulation at diagnosis of chronic myeloid leukemia.
Haematologica. 99:1710–1715. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Dolinska M, Piccini A, Wong WM, Gelali E,
Johansson AS, Klang J, Xiao P, Yektaei-Karin E, Strömberg UO,
Mustjoki S, et al: Leukotriene signaling via ALOX5 and cysteinyl
leukotriene receptor 1 is dispensable for in vitro growth of
CD34+ CD38− stem and progenitor cells in
chronic myeloid leukemia. Biochem Biophys Res Commun. 490:378–384.
2017. View Article : Google Scholar : PubMed/NCBI
|