Hepatocyte nuclear receptor factor 4 α (HNF4α;
NR2A1) is a highly conserved orphan member of the nuclear receptor
superfamily. It was first cloned by Frances Sladek in James
Darnell's laboratory for nuclear factor expression in the liver and
was demonstrated to act as a central regulator of gene expression
in certain types of cells that play critical roles in metabolic
homeostasis, including hepatocytes, enterocytes and pancreatic β
cells (1). In humans, HNF4α gene is
widely expressed in the liver, kidney, pancreas, stomach, small
intestine and colon (2), is located
on chromosome 20 and comprises at least 12 exons. Two promoters of
HNF4α, named P1 and P2, have been identified to drive the
expression of at least six different splicing variants, HNF4α1-α3
and HNF4α7-α9 (2–4). Four variants have been well
characterized: HNF4α1 and HNF4α2 from the P1 promoter and HNF4α7
and HNF4α8 from the P2 promoter. HNF4α3 (P1-driven) and HNF4α9
(P2-driven), which both have a different F domain, are less well
characterized, although some recent studies reported that these
isomers are expressed in human pancreas and may play a role in the
development of diabetes (4).
HNF4α is classified as an orphan nuclear receptor
for which ligand has not been found yet, and its regulatory role
remains unclear (5). Previously,
research on HNF4α ligands have reported conflicting evidence. For
example, long-chain fatty acids have been shown to bind as acyl-CoA
thioesters to the ligand binding pocket of HNF4α and to act as
transactivators or antagonists, depending on their chain length and
saturation (6–9). However, these fatty acids are bound
firmly and not exchangeable, and seem to bind irreversibly to the
receptor, suggesting that they might act more as structural
co-factors than classical regulatory ligands (10,11).
Conversely, HNF4α expressed in mammals was shown to be bound to the
essential fatty acid linoleic acid (LA; C18:2), which is considered
as a potential endogenous ligand of HNF4α (12). Although this binding is reversible,
it does not appear to have any significant effects on the
transactivation function of HNF4α (5,12).
Recently, several synthetic HNF4α antagonists were reported to bind
to the ligand binding domain (LBD) of HNF4α with high affinity and
to regulate the expression of known HNF4α target genes. In
particular, these antagonists were found to be selectively
cytotoxic to cancer cells in vitro and in vivo
(1,13). For example, one small molecule,
1-(2′-chloro-5′-nitrobenzenesulfonyl)-2-methylbenzimidazole
(BIM5078), which has been discovered by a novel high-throughput
screening for insulin promoter modulators (14) exhibited a dose-response inhibition of
the expression of known HNF4α target genes (1). BIM5078 was demonstrated to be
structurally similar to FK614, which is a PPARγ agonist formerly
described as a therapeutic agent for type II diabetes (15). BIM5078 may directly bind and interact
with the LBD of HNF4α repress target genes and may be cytotoxic to
hepatocellular carcinoma cells (1).
HNF4α is a highly conserved member of the nuclear
receptor superfamily of ligand-dependent transcription factors,
acts as a homodimer and plays a critical role in early liver
morphogenesis, fatal liver development, liver differentiation and
metabolism by regulating the transcription of genes involved in
each of these biological processes (5,16–18).
Numerous studies have also reported the central role of HNF4α in
regulating a number of genes, such as cytochrome P450 genes
(CYP2C8, CYP2C9, CYP2C19, CYP7A1, CYP3A4 and CYP8B1) that essential
for the xenobiotic and drug metabolism, to protect individuals from
toxic effects and provide key building blocks and nutrients to
promote the growth or maintain the survival of the organism
(5,19–21). Due
to its multiple functions, HNF4α is described as a master regulator
in multiple signal channels. For example, HNF4α-deficient embryonic
livers showed decreased expression of most hepatic factors,
including apolipoprotein B, liver fatty acid-binding protein and
microsomal triglyceride transfer protein as well as retinol-binding
protein, which indicated that HNF4α is a hepatocyte differentiation
factor critical for maintaining normal liver structure and normal
liver development (12,22–25).
Similarly, HNF4α was demonstrated to promote the
differentiation of intestinal epithelial cells (26) and embryonic development of the colon
in mice (27). HNF4α also serves an
important role in hepatic progenitor cells differentiation by
governing the expression of the transcription factors, including
forkhead box protein A2, (T-Box transcription factor 3),
hematopoietically-expressed homeobox protein, GATA4 and GATA6 that
control the hepatocyte cell development and regeneration (28). Other studies reported that ectopic
expression of HNF4α significantly inhibits the proliferation of
HEK293 (29) and pancreatic
INS-1β-cells (30). Furthermore,
HNF4α may act as a mesenchymal-to-epithelial transition
(MET)-inducing factor in hepatocytes (31–33), and
accumulated evidence indicates that HNF4α is involved in
inflammatory networks (34,35).
The present review will summarize the expression
patterns, alterations and regulatory effects of HNF4α in human
cancers, and introduce the novel functions of this ancient
receptor.
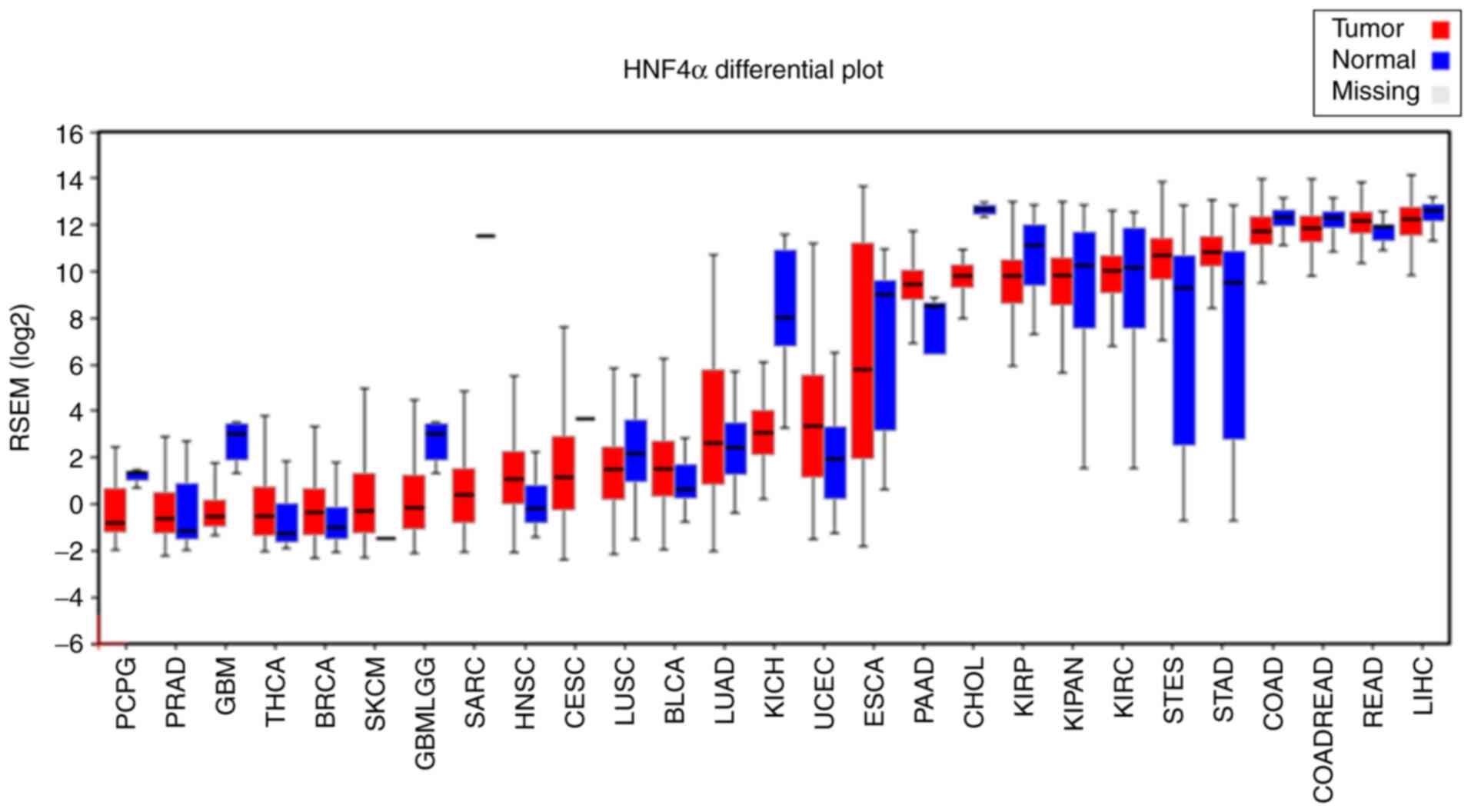
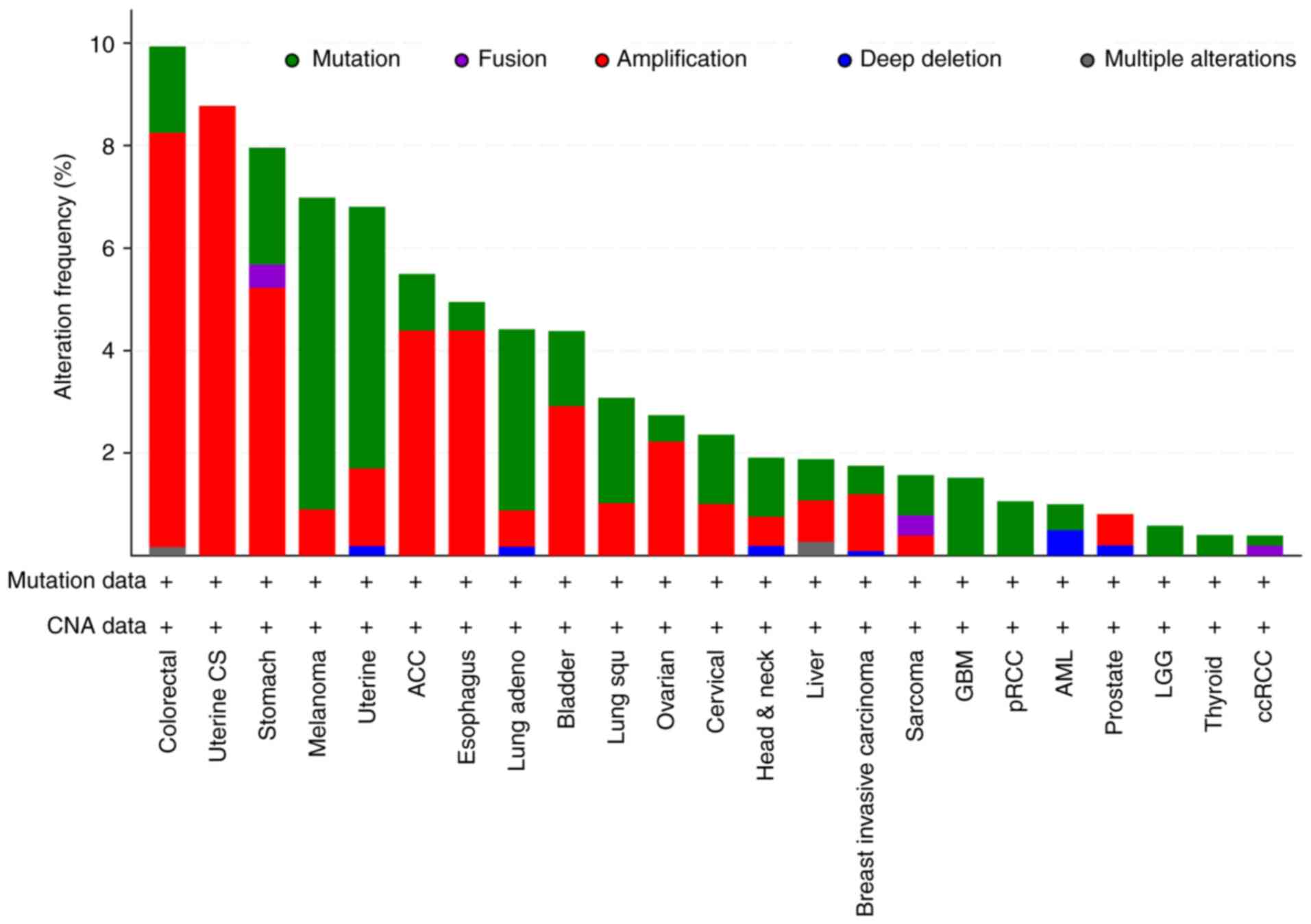
The frequency of HNF4α gene alterations, including
mutation, deletion, fusion and amplification, was determined across
multiple types of cancer using the cBioPortal for Cancer Genomics
database (http://www.cbioportal.org), which
contains 147 common cancer studies that included the
clinicopathological characteristics of almost 23,000 patients. All
searches were carried out according to the online instructions of
cBioPortal website. By pan-cancers analysis, we found that
amplifications and mutations were the most common alterations of
HNF4α in human cancers, particularly in colorectal and uterine
cancers (Fig. 2). Notably, HNF4α
alterations were mostly observed in one of the CRC studies (TCGA,
Pan-Can) (42), in which HNF4α was
altered in 59 cases of 594 patients (9.93%), where 48 cases (81.4%)
of these alterations were amplifications. As a core transcription
factor, HNF4α was demonstrated to be associated with the
tumorigenesis and development of CRC (43). However, the expression and functional
role of HNF4α in CRC was controversial (44). It was reported that HNF4α expression
is downregulated in CRC specimens and is positively correlated with
pT typing, lymph node metastasis, distant metastasis and clinical
stage in patients with CRC (45).
Furthermore, HNF4α plays an inhibitory role in the progression of
colon cancer by interacting with Wnt/β-catenin/transcription factor
4 (TCF4) pathway and influencing apoptosis and cell cycle
progression (45). In addition,
P2-driven HNF4α has been shown to promote inflammation and
carcinogenesis in colon (40,44).
Numerous proteins, including RAD50, PARP1 (double strand break
repair protein, poly(ADP-ribose) polymerase 1) and DNA-PKCS
(DNA-dependent protein kinase) have been demonstrated to interact
with HNF4α in order to improve the response of CRC cells to DNA
damage (46). These different
functional roles and controversial reports may be due at least in
part to the different transcriptional or translational changes in
HNF4α gene.
The protein expression level, sub-nuclear
distribution and post-translational modifications (PTMs) of HNF4α
are also critical determinants of its transactivation potency.
Yokoyama et al (47) analysed
the PTMs in HNF4α proteins by mass-spectrometry (MS) and identified
eight PTMs, including phosphorylation sites (S142, T166, S167 and
S436), ubiquitylation sites (K234 and K307) and an ubiquitination
and acetylation site at K458. Zhou et al (48) reported that the stability of HNF4α
was regulated by SUMOylation in a human embryonic stem cell-based
model, and that it serves a critical role during hepatocellular
differentiation. Daigo et al (49) validated and identified several
phosphorylation sites (Ser134, Ser133,
Ser158 and Thr420 + Ser427) of
HNF4α by MS/MS neutral loss ion spectra analysis, suggesting a
contribution of phosphorylation status alterations to the
multi-functional roles of HNF4α. Due to the development and
application of high-throughput genomic and proteomic technologies,
the expression and modification of HNF4α have become the focus of
attention in the recent decades. However, the post-transcriptional
role of HNF4α in the carcinogenesis process remains unclear.
Increasing evidence indicated that disruption of
HNF4α expression is widely involved in the initiation and
development of numerous types of human cancer, including gastric,
hepatocellular and colorectal carcinomas (36). Many studies have focused on
clarifying the regulatory role and underlying mechanism of HNF4α in
cancer. However, there have been conflicting reports about the role
of HNF4α in promoting and inhibiting cancer in humans.
HNF4α is the main regulator of liver specific gene
expression and has strong tumor suppressive activity. The tumor
suppressive effect of HNF4α was determined after discovering that
HNF4α expression was lost or significantly decreased in several
human cancers, and that restoration of HNF4α expression could
inhibit cancer cell proliferation in different types of cancer,
including mouse liver cells (50–53),
intestinal cancer (54), lung
endothelial cells and embryonic cancer (55), islet tumor cells (30) and embryonic kidney cells (29). However, the underlying mechanism of
HNF4α-mediated tumor inhibition is not fully understood. The
mechanisms by which HNF4α can inhibit cancer in humans are
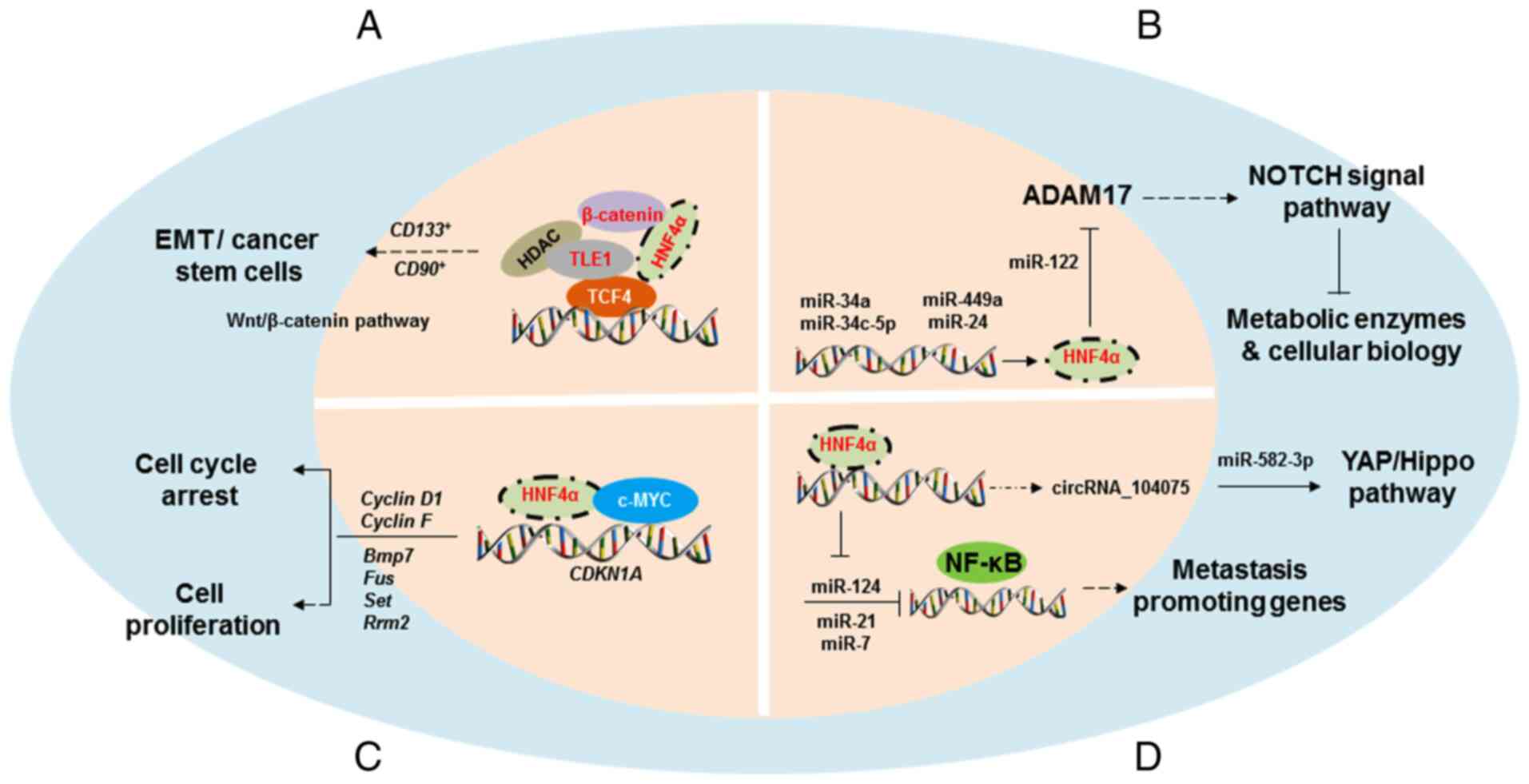
therefore summarized in the present review (Fig. 3 and Table
I).
A recent study from our group demonstrated that
HNF4α expression is decreased in prostate cancer cells, and that
ectopic overexpression of HNF4α could significantly inhibit
prostate cancer cell proliferation, induce cell-cycle arrest at
G2/M phase and trigger the cellular senescence via activation of
p21 signal pathway in a p53-independent manner and direct
transactivation of cyclin-dependent kinase inhibitor 1, suggesting
that HNF4α might have a tumor suppressor role in prostate cancer
cells (56). Hwang and Sladek
(57) reported that HNF4α competes
with the oncoprotein c-Myc for targeting the p21 promoter in order
to activate its expression, which could significantly inhibit HCC
and colorectal carcinoma cell proliferation. These findings
confirmed the critical role of p21 protein in HNF4α-mediated tumor
growth inhibition. The loss of HNF4α expression may therefore be
considered as a key event in the development and progression of
cancer; however, its underlying mechanism remains to be further
investigated. Previous studies on the HNF4α gene knockout mouse
model reported the negative correlation between deletion/deletion
expression of HNF4α and activation of c-MYC network, which involves
many pro-growth genes, such as bone morphogenetic protein 7
(58), FUS RNA binding protein, SET
nuclear proto-oncogene, ribonucleotide reductase regulatory subunit
M2 and Myc (59,60). Cyclin D1 was also demonstrated to
directly bind to HNF4α and cause a decrease in downstream genes
expression (61). Previous studies
in renal cell carcinoma reported that decreased HNF4α expression is
positively correlated with e-cadherin expression, suggesting a poor
prognosis in patients (62–64). Furthermore, HNF4α expression is
blocked by mutated IDH, which could promote biliary cancer
progression (65). Previous studies
in hepatocellular carcinoma (HCC) demonstrated that HNF4α exhibits
a decreased expression pattern, which could inhibit hepatocellular
carcinoma growth by downregulating miR-122 expression and
inhibition of the ADAM metallopeptidase domain 17 and NOTCH signal
pathway (52,66,67).
Previous studies demonstrated that non-coding RNAs
(ncRNAs), including micro (mi)RNAs, long ncRNAs and circular
(circ)RNA, serve important roles in the regulation of
HNF4α-mediated transcriptional level (68–70)
(Fig. 3B). Takagi et al
(71) reported that HNF4α expression
is decreased in HepG2 cells and is negatively regulated by
miR-24-mediated mRNA degradation and miR-34a-mediated
transcriptional suppression, affecting therefore the expression of
metabolic enzymes and cellular biology (68,72).
miR-34a, miR-34c-5p and miR-449a are reported to share the same
target elements located at two distinct locations within the 3′-UTR
of HNF4α, which overexpression could significantly repress HNF4α
protein level by blocking mRNA translation (68,73). Koh
et al (74) demonstrated that
high expression level of miRNAs from let-7 family could regulate
self-renewal and differentiation pathways by suppressing the
downstream target HNF4α. It was reported that HNF4α expression is
decreased in HCC via nuclear factor kappa B (NF-κB) mediated miR-21
upregulation (70). In addition,
decreased expression of HNF4α could promote HCC metastasis by
regulating the expression and translocation of RelA and affecting
NF-κB activation (70). Zhan et
al (75) demonstrated that HNF4α
could bind to the promotor region of circRNA_104075 to stimulate
its expression, and that circRNA_104075 can act as a ceRNA able to
upregulate YAP-Hippo pathway by absorbing miR-582-3p. ncRNAs are
the most abundant regulatory factors that possess great
post-transcriptional regulatory potential. However, the underlying
mechanism by which miRNAs act and regulate HNF4α expression remains
to be further investigated.
HNF4α has been reported to suppress hepatocyte
epithelial-mesenchymal transition (EMT) and cancer stem cell
generation via inhibition of β-catenin signalling pathway (45,53). EMT
is a critical developmental process during cancer invasion and
metastasis. Wnt-β-catenin signalling pathway plays a crucial role
in triggering EMT progression in both embryonic development and
tumorigenesis (33,76,77).
Previously, HNF4α was reported to be a potential EMT regulator in
HCC cells (77), since its ectopic
expression induces MET and blocks HCC progression (25). Previous studies demonstrated that the
repression of mesenchymal program of HNF4α is subsequent to
inhibition of Snail (31) and
competition with β-catenin for binding to TCF4 in HCC cells
(77,78). Other studies also indicated that
HNF4α expression is lost or significantly decreased in cirrhotic
tissues and decreased in HCC tissues compared with healthy tissues
(50,53,79).
Restoration of HNF4α expression by an adenovirus-mediated gene
delivery system could attenuate hepatocyte EMT during
hepatocarcinogenesis through inhibition of Wnt/β-catenin signalling
pathway (77,80,81),
significantly reducing the proportion of cells with stem cell gene
expression and CD133+ and CD90+ cells, which
are considered as tumor stem cells in the start and development of
HCC (82). These findings
highlighted the central role of HNF4α in the Wnt-β-catenin/snail
signalling pathway involved in the EMT/MET progression, which could
be a critical inhibitory mechanism for tumorigenesis.
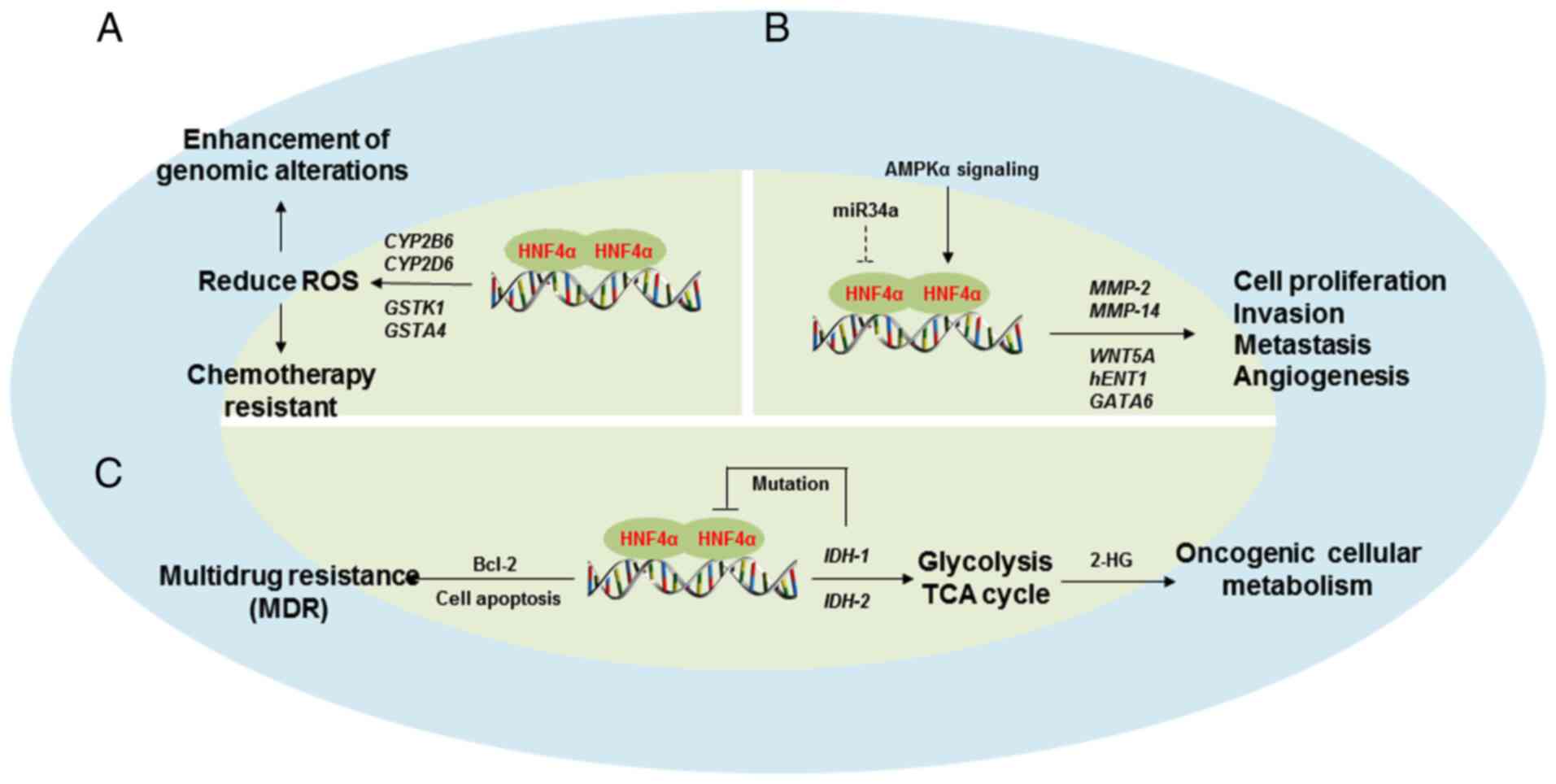
Considering the differences in the biologic
properties of experimental systems and tumor samples, increased
conflicting reports about the role of HNF4α in HCC progression and
in several other types of cancer (51,83–87;
Table II) have been observed. The
known oncogenic roles of HNF4α in human cancers are summarized in
Fig. 4.
Recent studies have reported that HNF4α is
significantly upregulated in gastric cancer (GC), head and neck
squamous cell carcinoma and pancreatic adenocarcinoma tissues
compared with normal tissues (94,95). In
particular, HNF4α acts by sustaining an oncogenic metabolism in GC
via the direct binding to the promoter region of isocitrate
dehydrogenase-1 (IDH-1), which is a key enzyme for TCA cycle
required for GC development (95).
However, mutated IDH-1 and IDH-2 can inhibit HNF4α to block
hepatocyte differentiation and promote the development of
premalignant biliary lesions and progression to metastatic HCC
(65). Chang et al (96) reported that HNF4α is upregulated by
AMPK signalling and acts as an upstream regulator of the WNT signal
pathway through its target gene Wnt family member 5A in GC. In
addition, the overexpression of HNF4α in GC tissues is
significantly associated with tumor stage and lymph node metastasis
in patients with GC, which may cause drug resistance to multiple
chemotherapeutics due to regulation of cell apoptosis and Bcl-2
expression (97). Nakajima et
al (98) demonstrated that HNF4α
is a direct target gene of kruppel like factor 5/GATA binding
protein (GATA)4/GATA6 that can interact with GATA6 and contribute
to the development of mucinous-type lung adenocarcinomas and GC
(99). A previous study from our
laboratory demonstrated that HNF4α can be significantly upregulated
in prostate cancer cells-derived prostatospheroids, suggesting that
HNF4α may also work in the regulation of prostate cancer stem cells
(100). In summary, HNF4α had
demonstrated an upregulation pattern and an oncogenic role in many
types of cancer, suggesting that HNF4α may be considered as a
therapeutic target in numerous human carcinomas.
As an important regulator of tumorigenesis and tumor
development, HNF4α is expressed at different levels in different
types of tumor and serves various roles that are tissue-specific.
This suggests the ubiquitinal expression patterns of HNF4α and the
changes in HNF4α expression, as well as the controversial
mechanisms that may be involved in cancer progression, which
provide further clues to the better understanding of HNF4α role in
cancer. Over the years, numerous studies have provided significant
advances in the role of HNF4α in human cancers; however, the
underlying mechanisms involved remain unclear and require urgent
further investigation. Considering the different roles of HNF4α
isoforms in the transcriptional control of cell proliferation, EMT,
stemness and other cellular processes in different types of cancer,
further research should focus on the potential therapeutic
approaches of targeting HNF4α.
Not applicable.
This work was partially supported by the Shenzhen
Science and Technology Program (Basic Research Project; grant no.
JCYJ20180228163919346), the National Natural Science Foundation of
China (grant no. 81802566) and Longhua Science and Technology
Innovation Fund (grant nos. 2020013 and 2020003).
The data that support the findings of this study are
available from The Cancer Genome Atlas (http://cancergenome.nih.gov/).
ZW and YZ drafted the manuscript. ZW obtained
funding, drafted and revised the manuscript. QD, JZ and HL helped
to revise the manuscript for important intellectual content. ZW and
HL confirm the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Kiselyuk A, Lee SH, Farber-Katz S, Zhang
M, Athavankar S, Cohen T, Pinkerton AB, Ye M, Bushway P, Richardson
AD, et al: HNF4α antagonists discovered by a high-throughput screen
for modulators of the human insulin promoter. Chem Biol.
19:806–818. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Duncan SA, Manova K, Chen WS, Hoodless P,
Weinstein DC, Bachvarova RF and Darnell JE Jr: Expression of
transcription factor HNF-4 in the extraembryonic endoderm, gut, and
nephrogenic tissue of the developing mouse embryo: HNF-4 is a
marker for primary endoderm in the implanting blastocyst. Proc Natl
Acad Sci USA. 91:7598–7602. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang J, Karakucuk V, Levitsky LL and
Rhoads DB: Expression of HNF4alpha variants in pancreatic islets
and Ins-1 beta cells. Diabetes Metab Res Rev. 24:533–543. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Harries LW, Locke JM, Shields B, Hanley
NA, Hanley KP, Steele A, Njølstad PR, Ellard S and Hattersley AT:
The diabetic phenotype in HNF4A mutation carriers is moderated by
the expression of HNF4A isoforms from the P1 promoter during fetal
development. Diabetes. 57:1745–1752. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hwang-Verslues WW and Sladek FM:
HNF4alpha-role in drug metabolism and potential drug target? Curr
Opin Pharmacol. 10:698–705. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hertz R, Magenheim J, Berman I and
Bar-Tana J: Fatty acyl-CoA thioesters are ligands of hepatic
nuclear factor-4alpha. Nature. 392:512–516. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bogan AA, Dallas-Yang Q, Ruse MD Jr, Maeda
Y, Jiang G, Nepomuceno L, Scanlan TS, Cohen FE and Sladek FM:
Analysis of protein dimerization and ligand binding of orphan
receptor HNF4alpha. J Mol Biol. 302:831–851. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dhe-Paganon S, Duda K, Iwamoto M, Chi YI
and Shoelson SE: Crystal structure of the HNF4 alpha ligand binding
domain in complex with endogenous fatty acid ligand. J Biol Chem.
277:37973–37976. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wisely GB, Miller AB, Davis RG, Thornquest
AD Jr, Johnson R, Spitzer T, Sefler A, Shearer B, Moore JT, Miller
AB, et al: Hepatocyte nuclear factor 4 is a transcription factor
that constitutively binds fatty acids. Structure. 10:1225–1234.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Benoit G, Malewicz M and Perlmann T:
Digging deep into the pockets of orphan nuclear receptors: Insights
from structural studies. Trends Cell Biol. 14:369–376. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sladek F: Desperately seeking…something.
Mol Cell. 10:219–221. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan X, Ta TC, Lin M, Evans JR, Dong Y,
Bolotin E, Sherman MA, Forman BM and Sladek FM: Identification of
an endogenous ligand bound to a native orphan nuclear receptor.
PLoS One. 4:e56092009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee SH, Piran R, Keinan E, Pinkerton A and
Levine F: Induction of beta-cell replication by a synthetic
HNF4alpha antagonist. Stem Cells. 31:2396–2407. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kiselyuk A, Farber-Katz S, Cohen T, Lee
SH, Geron I, Azimi B, Heynen-Genel S, Singer O, Price J, Mercola M,
et al: Phenothiazine neuroleptics signal to the human insulin
promoter as revealed by a novel high-throughput screen. J Biomol
Screen. 15:663–670. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fujimura T, Sakuma H, Konishi S, Oe T,
Hosogai N, Kimura C, Aramori I and Mutoh S: FK614, a novel
peroxisome proliferator-activated receptor gamma modulator, induces
differential transactivation through a unique ligand-specific
interaction with transcriptional coactivators. J Pharmacol Sci.
99:342–352. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Inoue Y, Yu AM, Yim SH, Ma X, Krausz KW,
Inoue J, Xiang CC, Brownstein MJ, Eggertsen G, Björkhem I and
Gonzalez FJ: Regulation of bile acid biosynthesis by hepatocyte
nuclear factor 4alpha. J Lipid Res. 47:215–227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chiang JY: Hepatocyte nuclear factor
4alpha regulation of bile acid and drug metabolism. Expert Opin
Drug Metab Toxicol. 5:137–147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nitta S, Kusakari Y, Yamada Y, Kubo T, Neo
S, Igarashi H and Hisasue M: Conversion of mesenchymal stem cells
into a canine hepatocyte-like cells by Foxa1 and Hnf4a. Regen Ther.
14:165–176. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen S, Lencinas A, Nunez M, Selmin OI and
Runyan RB: HNF4a transcription is a target of trichloroethylene
toxicity in the embryonic mouse heart. Environ Sci Process Impacts.
22:824–832. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jover R, Moya M and Gomez-Lechon MJ:
Transcriptional regulation of cytochrome p450 genes by the nuclear
receptor hepatocyte nuclear factor 4-alpha. Curr Drug Metab.
10:508–519. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tavares-Sanchez OL, Rodriguez C,
Gortares-Moroyoqui P and Estrada MI: Hepatocyte nuclear
factor-4alpha, a multifunctional nuclear receptor associated with
cardiovascular disease and cholesterol catabolism. Int J Environ
Health Res. 25:126–139. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kyrmizi I, Hatzis P, Katrakili N, Tronche
F, Gonzalez FJ and Talianidis I: Plasticity and expanding
complexity of the hepatic transcription factor network during liver
development. Genes Dev. 20:2293–2305. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li J, Ning G and Duncan SA: Mammalian
hepatocyte differentiation requires the transcription factor
HNF-4alpha. Genes Dev. 14:464–474. 2000.PubMed/NCBI
|
|
24
|
Hayhurst GP, Lee YH, Lambert G, Ward JM
and Gonzalez FJ: Hepatocyte nuclear factor 4alpha (nuclear receptor
2A1) is essential for maintenance of hepatic gene expression and
lipid homeostasis. Mol Cell Biol. 21:1393–1403. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Parviz F, Matullo C, Garrison WD, Savatski
L, Adamson JW, Ning G, Kaestner KH, Rossi JM, Zaret KS and Duncan
SA: Hepatocyte nuclear factor 4alpha controls the development of a
hepatic epithelium and liver morphogenesis. Nat Genet. 34:292–296.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lussier CR, Babeu JP, Auclair BA,
Perreault N and Boudreau F: Hepatocyte nuclear factor-4alpha
promotes differentiation of intestinal epithelial cells in a
coculture system. Am J Physiol Gastrointest Liver Physiol.
294:G418–G428. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Garrison WD, Battle MA, Yang C, Kaestner
KH, Sladek FM and Duncan SA: Hepatocyte nuclear factor 4alpha is
essential for embryonic development of the mouse colon.
Gastroenterology. 130:1207–1220. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
DeLaForest A, Nagaoka M, Si-Tayeb K, Noto
FK, Konopka G, Battle MA and Duncan SA: HNF4A is essential for
specification of hepatic progenitors from human pluripotent stem
cells. Development. 138:4143–4153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grigo K, Wirsing A, Lucas B, Klein-Hitpass
L and Ryffel GU: HNF4 alpha orchestrates a set of 14 genes to
down-regulate cell proliferation in kidney cells. Biol Chem.
389:179–187. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Erdmann S, Senkel S, Arndt T, Lucas B,
Lausen J, Klein-Hitpass L, Ryffel GU and Thomas H: Tissue-specific
transcription factor HNF4alpha inhibits cell proliferation and
induces apoptosis in the pancreatic INS-1 beta-cell line. Biol
Chem. 388:91–106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Santangelo L, Marchetti A, Cicchini C,
Conigliaro A, Conti B, Mancone C, Bonzo JA, Gonzalez FJ, Alonzi T,
Amicone L and Tripodi M: The stable repression of mesenchymal
program is required for hepatocyte identity: A novel role for
hepatocyte nuclear factor 4α. Hepatology. 53:2063–2074. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ahn SH, Shah YM, Inoue J, Morimura K, Kim
I, Yim S, Lambert G, Kurotani R, Nagashima K, Gonzalez FJ and Inoue
Y: Hepatocyte nuclear factor 4alpha in the intestinal epithelial
cells protects against inflammatory bowel disease. Inflamm Bowel
Dis. 14:908–920. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Darsigny M, Babeu JP, Dupuis AA, Furth EE,
Seidman EG, Lévy E, Verdu EF, Gendron FP and Boudreau F: Loss of
hepatocyte-nuclear-factor-4alpha affects colonic ion transport and
causes chronic inflammation resembling inflammatory bowel disease
in mice. PLoS One. 4:e76092009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tanaka T, Jiang S, Hotta H, Takano K,
Iwanari H, Sumi K, Daigo K, Ohashi R, Sugai M, Ikegame C, et al:
Dysregulated expression of P1 and P2 promoter-driven hepatocyte
nuclear factor-4alpha in the pathogenesis of human cancer. J
Pathol. 208:662–672. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Oshima T, Kawasaki T, Ohashi R, Hasegawa
G, Jiang S, Umezu H, Aoyagi Y, Iwanari H, Tanaka T, Hamakubo T, et
al: Downregulated P1 promoter-driven hepatocyte nuclear
factor-4alpha expression in human colorectal carcinoma is a new
prognostic factor against liver metastasis. Pathol Int. 57:82–90.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Takano K, Hasegawa G, Jiang S, Kurosaki I,
Hatakeyama K, Iwanari H, Tanaka T, Hamakubo T, Kodama T and Naito
M: Immunohistochemical staining for P1 and P2 promoter-driven
hepatocyte nuclear factor-4alpha may complement mucin phenotype of
differentiated-type early gastric carcinoma. Pathol Int.
59:462–470. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lambert E, Babeu JP, Simoneau J, Raisch J,
Lavergne L, Lévesque D, Jolibois É, Avino M, Scott MS, Boudreau F
and Boisvert FM: Human hepatocyte nuclear factor 4-alpha encodes
isoforms with distinct transcriptional functions. Mol Cell
Proteomics. 19:808–827. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vuong LM, Chellappa K, Dhahbi JM, Deans
JR, Fang B, Bolotin E, Titova NV, Hoverter NP, Spindler SR,
Waterman ML and Sladek FM: Differential effects of hepatocyte
nuclear factor 4alpha isoforms on tumor growth and T-cell factor
4/AP-1 interactions in human colorectal cancer cells. Mol Cell
Biol. 35:3471–3490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Babeu JP, Jones C, Geha S, Carrier JC and
Boudreau F: P1 promoter-driven HNF4alpha isoforms are specifically
repressed by beta-catenin signaling in colorectal cancer cells. J
Cell Sci. 131:jcs2147342018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hoadley KA, Yau C, Hinoue T, Wolf DM,
Lazar AJ, Drill E, Shen R, Taylor AM, Cherniack AD, Thorsson V, et
al: Cell-of-origin patterns dominate the molecular classification
of 10,000 tumors from 33 types of cancer. Cell. 173:291–304.e6.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhou B and Guo R: Genomic and regulatory
characteristics of significant transcription factors in colorectal
cancer metastasis. Sci Rep. 8:178362018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chellappa K, Deol P, Evans JR, Vuong LM,
Chen G, Briançon N, Bolotin E, Lytle C, Nair MG and Sladek FM:
Opposing roles of nuclear receptor HNF4α isoforms in colitis and
colitis-associated colon cancer. Elife. 5:e109032016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yao HS, Wang J, Zhang XP, Wang LZ, Wang Y,
Li XX, Jin KZ, Hu ZQ and Wang WJ: Hepatocyte nuclear factor 4α
suppresses the aggravation of colon carcinoma. Mol Carcinog.
55:458–472. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Babeu JP, Wilson SD, Lambert E, Levesque
D, Boisvert FM and Boudreau F: Quantitative proteomics identifies
DNA repair as a novel biological function for hepatocyte nuclear
factor 4α in colorectal cancer cells. Cancers (Basel). 11:6262019.
View Article : Google Scholar
|
|
47
|
Yokoyama A, Katsura S, Ito R, Hashiba W,
Sekine H, Fujiki R and Kato S: Multiple post-translational
modifications in hepatocyte nuclear factor 4alpha. Biochem Biophys
Res Commun. 410:749–753. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhou W, Hannoun Z, Jaffray E, Medine CN,
Black JR, Greenhough S, Zhu L, Ross JA, Forbes S, Wilmut I, et al:
SUMOylation of HNF4α regulates protein stability and hepatocyte
function. J Cell Sci. 125:3630–3635. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Daigo K, Kawamura T, Ohta Y, Ohashi R,
Katayose S, Tanaka T, Aburatani H, Naito M, Kodama T, Ihara S and
Hamakubo T: Proteomic analysis of native hepatocyte nuclear
factor-4α (HNF4α) isoforms, phosphorylation status, and interactive
cofactors. J Biol Chem. 286:674–686. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lazarevich NL, Cheremnova OA, Varga EV,
Ovchinnikov DA, Kudrjavtseva EI, Morozova OV, Fleishman DI,
Engelhardt NV and Duncan SA: Progression of HCC in mice is
associated with a downregulation in the expression of hepatocyte
nuclear factors. Hepatology. 39:1038–1047. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xu L, Hui L, Wang S, Gong J, Jin Y, Wang
Y, Ji Y, Wu X, Han Z and Hu G: Expression profiling suggested a
regulatory role of liver-enriched transcription factors in human
hepatocellular carcinoma. Cancer Res. 61:3176–3181. 2001.PubMed/NCBI
|
|
52
|
Wu N, Zhang YL, Wang HT, Li DW, Dai HJ,
Zhang QQ, Zhang J, Ma Y, Xia Q, Bian JM and Hang HL: Overexpression
of hepatocyte nuclear factor 4α in human mesenchymal stem cells
suppresses hepatocellular carcinoma development through
Wnt/β-catenin signaling pathway downregulation. Cancer Biol Ther.
17:558–565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ning BF, Ding J, Yin C, Zhong W, Wu K,
Zeng X, Yang W, Chen YX, Zhang JP, Zhang X, et al: Hepatocyte
nuclear factor 4 alpha suppresses the development of hepatocellular
carcinoma. Cancer Res. 70:7640–7651. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Saandi T, Baraille F, Derbal-Wolfrom L,
Cattin AL, Benahmed F, Martin E, Cardot P, Duclos B, Ribeiro A,
Freund JN and Duluc I: Regulation of the tumor suppressor homeogene
Cdx2 by HNF4α in intestinal cancer. Oncogene. 32:3782–3788. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chiba H, Itoh T, Satohisa S, Sakai N,
Noguchi H, Osanai M, Kojima T and Sawada N: Activation of
p21CIP1/WAF1 gene expression and inhibition of cell proliferation
by overexpression of hepatocyte nuclear factor-4alpha. Exp Cell
Res. 302:11–21. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang Z, Li Y, Wu D, Yu S, Wang Y and Leung
Chan F: Nuclear receptor HNF4alpha performs a tumor suppressor
function in prostate cancer via its induction of p21-driven
cellular senescence. Oncogene. 39:1572–1589. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hwang-Verslues WW and Sladek FM: Nuclear
receptor hepatocyte nuclear factor 4alpha1 competes with
oncoprotein c-Myc for control of the p21/WAF1 promoter. Mol
Endocrinol. 22:78–90. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lu JW, Hsia Y, Yang WY, Lin YI, Li CC,
Tsai TF, Chang KW, Shieh GS, Tsai SF, Wang HD and Yuh CH:
Identification of the common regulators for hepatocellular
carcinoma induced by hepatitis B virus X antigen in a mouse model.
Carcinogenesis. 33:209–219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Walesky C, Edwards G, Borude P,
Gunewardena S, O'Neil M, Yoo B and Apte U: Hepatocyte nuclear
factor 4 alpha deletion promotes diethylnitrosamine-induced
hepatocellular carcinoma in rodents. Hepatology. 57:2480–2490.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Walesky C and Apte U: Role of hepatocyte
nuclear factor 4α (HNF4α) in cell proliferation and cancer. Gene
Expr. 16:101–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hanse EA, Mashek DG, Becker JR, Solmonson
AD, Mullany LK, Mashek MT, Towle HC, Chau AT and Albrecht JH:
Cyclin D1 inhibits hepatic lipogenesis via repression of
carbohydrate response element binding protein and hepatocyte
nuclear factor 4α. Cell Cycle. 11:2681–2690. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Gao Y, Yan Y, Guo J, Zhang Q, Bi D, Wang
F, Chang Z, Lu L, Yao X and Wei Q: HNF4α downregulation promotes
tumor migration and invasion by regulating Ecadherin in renal cell
carcinoma. Oncol Rep. 42:1066–1074. 2019.PubMed/NCBI
|
|
63
|
Zhou H, Guo L, Yao W, Shi R, Yu G, Xu H
and Ye Z: Silencing of tumor-suppressive NR_023387 in renal cell
carcinoma via promoter hypermethylation and HNF4A deficiency. J
Cell Physiol. 235:2113–2128. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lu J, Chen Z, Zhao H, Dong H, Zhu L, Zhang
Y, Wang J, Zhu H, Cui Q, Qi C, et al: ABAT and ALDH6A1, regulated
by transcription factor HNF4A, suppress tumorigenic capability in
clear cell renal cell carcinoma. J Transl Med. 18:1012020.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Saha SK, Parachoniak CA, Ghanta KS,
Fitamant J, Ross KN, Najem MS, Gurumurthy S, Akbay EA, Sia D,
Cornella H, et al: Mutant IDH inhibits HNF-4α to block hepatocyte
differentiation and promote biliary cancer. Nature. 513:110–114.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yang G, Zhang M, Zhao Y, Pan Y, Kan M, Li
J, He K and Zhang X: HNF-4α inhibits hepatocellular carcinoma cell
proliferation through mir-122-adam17 pathway. PLoS One.
15:e02304502020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lv DD, Zhou LY and Tang H: Hepatocyte
nuclear factor 4α and cancer-related cell signaling pathways: A
promising insight into cancer treatment. Exp Mol Med. 53:8–18.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wang Z and Burke PA: The role of microRNAs
in hepatocyte nuclear factor-4alpha expression and transactivation.
Biochim Biophys Acta. 1829:436–442. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Deng XG, Qiu RL, Wu YH, Li ZX, Xie P,
Zhang J, Zhou JJ, Zeng LX, Tang J, Maharjan A and Deng JM:
Overexpression of miR-122 promotes the hepatic differentiation and
maturation of mouse ESCs through a miR-122/FoxA1/HNF4a-positive
feedback loop. Liver Int. 34:281–295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ning BF, Ding J, Liu J, Yin C, Xu WP, Cong
WM, Zhang Q, Chen F, Han T, Deng X, et al: Hepatocyte nuclear
factor 4α-nuclear factor-κB feedback circuit modulates liver cancer
progression. Hepatology. 60:1607–1619. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Takagi S, Nakajima M, Kida K, Yamaura Y,
Fukami T and Yokoi T: MicroRNAs regulate human hepatocyte nuclear
factor 4alpha, modulating the expression of metabolic enzymes and
cell cycle. J Biol Chem. 285:4415–4422. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Salloum-Asfar S, Arroyo AB, Teruel-Montoya
R, García-Barberá N, Roldán V, Vicente V, Martínez C and
González-Conejero R: MiRNA-based regulation of hemostatic factors
through hepatic nuclear Factor-4 alpha. PLoS One. 11:e01547512016.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ramamoorthy A, Li L, Gaedigk A, Bradford
LD, Benson EA, Flockhart DA and Skaar TC: In silico and in vitro
identification of microRNAs that regulate hepatic nuclear factor 4α
expression. Drug Metab Dispos. 40:726–733. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Koh W, Sheng CT, Tan B, Lee QY, Kuznetsov
V, Kiang LS and Tanavde V: Analysis of deep sequencing microRNA
expression profile from human embryonic stem cells derived
mesenchymal stem cells reveals possible role of let-7 microRNA
family in downstream targeting of hepatic nuclear factor 4 alpha.
BMC Genomics. 11 (Suppl 1):S62010. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhang X, Xu Y, Qian Z, Zheng W, Wu Q, Chen
Y, Zhu G, Liu Y, Bian Z, Xu W, et al: circRNA_104075 stimulates
YAP-dependent tumorigenesis through the regulation of HNF4a and may
serve as a diagnostic marker in hepatocellular carcinoma. Cell
Death Dis. 9:10912018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Moon RT and Miller JR: The APC tumor
suppressor protein in development and cancer. Trends Genet.
13:256–258. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Yang M, Li SN, Anjum KM, Gui LX, Zhu SS,
Liu J, Chen JK, Liu QF, Ye GD, Wang WJ, et al: A double-negative
feedback loop between Wnt-β-catenin signaling and HNF4α regulates
epithelial-mesenchymal transition in hepatocellular carcinoma. J
Cell Sci. 126:5692–5703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Battle MA, Konopka G, Parviz F, Gaggl AL,
Yang C, Sladek FM and Duncan SA: Hepatocyte nuclear factor 4alpha
orchestrates expression of cell adhesion proteins during the
epithelial transformation of the developing liver. Proc Natl Acad
Sci USA. 103:8419–8424. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lazarevich NL, Shavochkina DA, Fleishman
DI, Kustova IF, Morozova OV, Chuchuev ES and Patyutko YI:
Deregulation of hepatocyte nuclear factor 4 (HNF4) as a marker of
epithelial tumors progression. Exp Oncol. 32:167–171.
2010.PubMed/NCBI
|
|
80
|
Yao D, Peng S and Dai C: The role of
hepatocyte nuclear factor 4alpha in metastatic tumor formation of
hepatocellular carcinoma and its close relationship with the
mesenchymal-epithelial transition markers. BMC Cancer. 13:4322013.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Huang Q, Pu M, Zhao G, Dai B, Bian Z, Tang
H, Chen C, Liu W, Qu X, Shen L and Tao K: Tg737 regulates
epithelial-mesenchymal transition and cancer stem cell properties
via a negative feedback circuit between Snail and HNF4α during
liver stem cell malignant transformation. Cancer Lett. 402:52–60.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Yin C, Lin Y, Zhang X, Chen YX, Zeng X,
Yue HY, Hou JL, Deng X, Zhang JP, Han ZG and Xie WF:
Differentiation therapy of hepatocellular carcinoma in mice with
recombinant adenovirus carrying hepatocyte nuclear factor-4alpha
gene. Hepatology. 48:1528–1539. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Stumpf H, Senkel S, Rabes HM and Ryffel
GU: The DNA binding activity of the liver transcription factors
LFB1 (HNF1) and HNF4 varies coordinately in rat hepatocellular
carcinoma. Carcinogenesis. 16:143–145. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Flodby P, Liao DZ, Blanck A, Xanthopoulos
KG and Hallstrom IP: Expression of the liver-enriched transcription
factors C/EBP alpha, C/EBP beta, HNF-1, and HNF-4 in preneoplastic
nodules and hepatocellular carcinoma in rat liver. Mol Carcinog.
12:103–109. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Choi JK, Choi JY, Kim DG, Choi DW, Kim BY,
Lee KH, Yeom YI, Yoo HS, Yoo OJ and Kim S: Integrative analysis of
multiple gene expression profiles applied to liver cancer study.
FEBS Lett. 565:93–100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Cai SH, Lu SX, Liu LL, Zhang CZ and Yun
JP: Increased expression of hepatocyte nuclear factor 4 alpha
transcribed by promoter 2 indicates a poor prognosis in
hepatocellular carcinoma. Therap Adv Gastroenterol. 10:761–771.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhang X, Du L, Qiao Y, Zhang X, Zheng W,
Wu Q, Chen Y, Zhu G, Liu Y, Bian Z, et al: Ferroptosis is governed
by differential regulation of transcription in liver cancer. Redox
Biol. 24:1012112019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Darsigny M, Babeu JP, Seidman EG, Gendron
FP, Levy E, Carrier J, Perreault N and Boudreau F: Hepatocyte
nuclear Factor-4alpha promotes gut neoplasia in mice and protects
against the production of reactive oxygen species. Cancer Res.
70:9423–9433. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Kriegsmann M, Harms A, Longuespée R, Muley
T, Winter H, Kriegsmann K, Kazdal D, Goeppert B, Pathil A and Warth
A: Role of conventional immunomarkers, HNF4-α and SATB2, in the
differential diagnosis of pulmonary and colorectal adenocarcinomas.
Histopathology. 72:997–1006. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Sugai M, Umezu H, Yamamoto T, Jiang S,
Iwanari H, Tanaka T, Hamakubo T, Kodama T and Naito M: Expression
of hepatocyte nuclear factor 4 alpha in primary ovarian mucinous
tumors. Pathol Int. 58:681–686. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Xiang X, Zhao X, Qu H, Li D, Yang D, Pu J,
Mei H, Zhao J, Huang K, Zheng L and Tong Q: Hepatocyte nuclear
factor 4 alpha promotes the invasion, metastasis and angiogenesis
of neuroblastoma cells via targeting matrix metalloproteinase 14.
Cancer Lett. 359:187–197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Sugano M, Nagasaka T, Sasaki E, Murakami
Y, Hosoda W, Hida T, Mitsudomi T and Yatabe Y: HNF4α as a marker
for invasive mucinous adenocarcinoma of the lung. Am J Surg Pathol.
37:211–218. 2012. View Article : Google Scholar
|
|
93
|
Li Z and Chen H: miR-34a inhibits
proliferation, migration and invasion of paediatric neuroblastoma
cells via targeting HNF4α. Artif Cells Nanomed Biotechnol.
47:3072–3078. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Sun Q, Xu W, Ji S, Qin Y, Liu W, Hu Q,
Zhang Z, Liu M, Yu X and Xu X: Role of hepatocyte nuclear factor 4
alpha in cell proliferation and gemcitabine resistance in
pancreatic adenocarcinoma. Cancer Cell Int. 19:492019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Xu C, Ooi WF, Qamra A, Tan J, Chua BY, Ho
SWT, Das K, Adam Isa ZF, Li Z, Yao X, et al: HNF4α pathway mapping
identifies wild-type IDH1 as a targetable metabolic node in gastric
cancer. Gut. 69:231–242. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Chang HR, Nam S, Kook MC, Kim KT, Liu X,
Yao H, Jung HR, Lemos R Jr, Seo HH, Park HS, et al: HNF4α is a
therapeutic target that links AMPK to WNT signalling in early-stage
gastric cancer. Gut. 65:19–32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Ma Y, Wei X and Wu Z: HNF-4α promotes
multidrug resistance of gastric cancer cells through the modulation
of cell apoptosis. Oncol Lett. 14:6477–6484. 2017.PubMed/NCBI
|
|
98
|
Nakajima N, Yoshizawa A, Nakajima T,
Hirata M, Furuhata A, Sumiyoshi S, Rokutan-Kurata M, Sonobe M,
Menju T, Miyamoto E, et al: GATA6-positive lung adenocarcinomas are
associated with invasive mucinous adenocarcinoma morphology,
hepatocyte nuclear factor 4α expression, and KRAS mutations.
Histopathology. 73:38–48. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Chia NY, Deng N, Das K, Huang D, Hu L, Zhu
Y, Lim KH, Lee MH, Wu J, Sam XX, et al: Regulatory crosstalk
between lineage-survival oncogenes KLF5, GATA4 and GATA6
cooperatively promotes gastric cancer development. Gut. 64:707–719.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wang Z, Wu D, Ng CF, Teoh JY, Yu S, Wang Y
and Chan FL: Nuclear receptor profiling in prostatospheroids and
castration-resistant prostate cancer. Endocr Relat Cancer.
25:35–50. 2018. View Article : Google Scholar : PubMed/NCBI
|