Introduction
Prostate cancer (PCa) is the fifth leading cause of
death in men, with 359,500 deaths worldwide in 2018 (1). The disease phenotypes varied from
indolent to aggressive. The local stage is potentially curable with
local therapy and shows a 5-year survival rate of nearly 100%,
compared with 29.8% for metastatic cases (1). One challenge for clinicians is to
determine the optimal sequencing therapies for patients who present
intermediate, high-risk localized, locally advanced or metastatic
prostate cancer (mPCa) to minimize overtreatment and improve
outcomes. Thus, early and precise detection of cancer is important
for decreasing patient mortality. In addition, the current
therapeutic landscape offers the patient an individualized
treatment approach. Nevertheless, the treatment of mPCa is becoming
increasingly complex (2). The risk
of overdiagnosis and overtreatment remains and has a negative
impact on the quality of life of men with PCa (3). One of the greatest challenges in the
current management of PCa is adequate assessment of the response to
treatment. Prostate-specific antigen (PSA) as a tumor marker for
prostate cancer has limitations as a surrogate for survival end
points because of insufficient sensitivity and specificity
(4). Additionally, PSA determination
is not an adequate marker for the evaluation of treatment response.
However, personalizing PCa treatment with a biomarker, such as
circulating tumor cells (CTCs), offers the possibility to create
risk-adapted strategies to optimize patient care. CTCs represent a
minimally invasive source of spreading tumor cells and provide
important clinical information for the individual patient's
treatment in terms of monitoring metastasis, evaluating the
efficacy of treatment, and/or facilitating the early detection of
treatment resistance (5–7). In recent reports, detection of
androgen-receptor splice variant 7 (AR-V7) in pooled CTCs of men
with progressive metastatic castration-resistant prostate cancer
(mCRPC) was associated with resistance to the androgen receptor
inhibitors abiraterone and enzalutamide. This finding shows that
CTCs can provide insights into drivers of tumor growth in patients
and into the pharmacodynamics effects of targeted therapies
(4,8).
However, it remains difficult to isolate and
characterize CTCs because of their rarity (1–10 CTCs per ml blood)
and heterogeneous phenotype (9).
Additionally, CTCs are present in a large background of
hematopoietic cells. In 2004, the CellSearch® system was
the first US Food and Drug Administration (FDA)-authorized system
for the enumeration of CTCs in 7.5 ml of blood. Clinical studies
demonstrated that CTCs captured with the CellSearch system were
clearly associated with poor patient outcomes (10,11). At
present, several platforms have been developed to detect CTCs. CTC
detection can be achieved based on physical and biological
properties (12,13).
In a previous study, we evaluated the
CellCollector® (GILUPI CellCollector), an in vivo
approach initially ex vivo (in vivo was not allowed
at this time) in blood samples from PCa patients. Our results
showed that the CellCollector could be applied for the sensitive
isolation and molecular characterization of CTCs ex vivo
(14). To date, other study has
evaluated the CellCollector in single-center trails in patients
with breast, lung, high-risk PCa and neuroendocrine tumors in small
cohorts (15–17).
In the present study, we validated the
CellCollector, which allowed in vivo isolation of CTCs
directly from the cubital vein in a cohort of prostate cancer
patients in different clinical stages and control groups. This
included monitoring prostate cancer patients during treatment for a
clinical response correlated to CTC counts and comparison of
CellCollector results to those from the CellSearch System.
Materials and methods
Study population and clinical
information
The patients provided written informed consent and
were enrolled at University Clinic and Outpatient Clinic for
Urology, Medical Faculty of Martin Luther University
Halle-Wittenberg from February 2011 to March 2012. The medical
faculty ethics committee of Martin Luther University
Halle-Wittenberg approved the study protocol. Furthermore, we
obtained a permit from the Federal Institute for Drug and Medical
Devices (Germany, BfArM).
The study population consisted of 14 metastasized
(PCa-m) and 21 localized (PCa-l) PCa patients. A control group of
16 men with benign prostate hypertrophy (BPH) and a second control
group of 20 women were also included (Table I). The patients were required to have
histologically proven prostate cancer in the localized group and
documented metastases, as confirmed by computed tomography (CT), in
the metastasized group. All patients had PSA levels determined at
every time point of CTC isolation.
 | Table I.Study population demographics. |
Table I.
Study population demographics.
|
| Prostate
cancer | Control group |
|---|
|
|
|
|
|---|
|
Characteristics | Metastatic | Localized | BPH | Women |
|---|
| Patients, n | 14 | 21 | 16 | 20 |
| Median age (range),
years | 52 (53–79) | 59 (56–72) | 67 (58–83) | 25.5 (19–38) |
| Ethnicity | Caucasian | Caucasian | Caucasian | Caucasian |
| Gleason score at
diagnosis |
|
|
|
|
| ≤7, n
(%) | 2 (14.30) | 18
(85.7) |
|
|
| >7,
n (%) | 12 (85.70) | 3
(14.3) |
|
|
| Median PSA at
baseline, ng/ml (range) | 23.6
(3.5–1120) | 7.91
(1.8–39.5) | 1.9
(0.41–15.9) |
|
| Median PSA, ng/ml
(range) | 21.7
(0.04–1120) | 0.04
(0.04–3.3) | 0.4 (0.04–3.1) |
|
| Primary
therapy |
|
|
|
|
| TURP, n
(%) |
|
| 16 (100) |
|
| Surgery
(RP), n (%) | 2
(13.3) | 21 (100) |
|
|
|
Radiation, n (%) | 9
(60.0) | 5
(18.5) |
|
|
| Systemic
therapy |
|
|
|
|
|
Androgen treatment, n (%) | 10 (71.4) | 2
(7.1) |
|
|
|
Chemotherapy, n (%) | 13 (92.8) |
|
|
|
| Site of metastatic
disease |
|
|
|
|
| Bone, n
(%) | 12 (86.7) |
|
|
|
| Lymph
node, n (%) | 6
(42.9) |
|
|
|
| Other
soft tissue, n (%) | 3
(21.4) |
|
|
|
In vivo CTC isolation
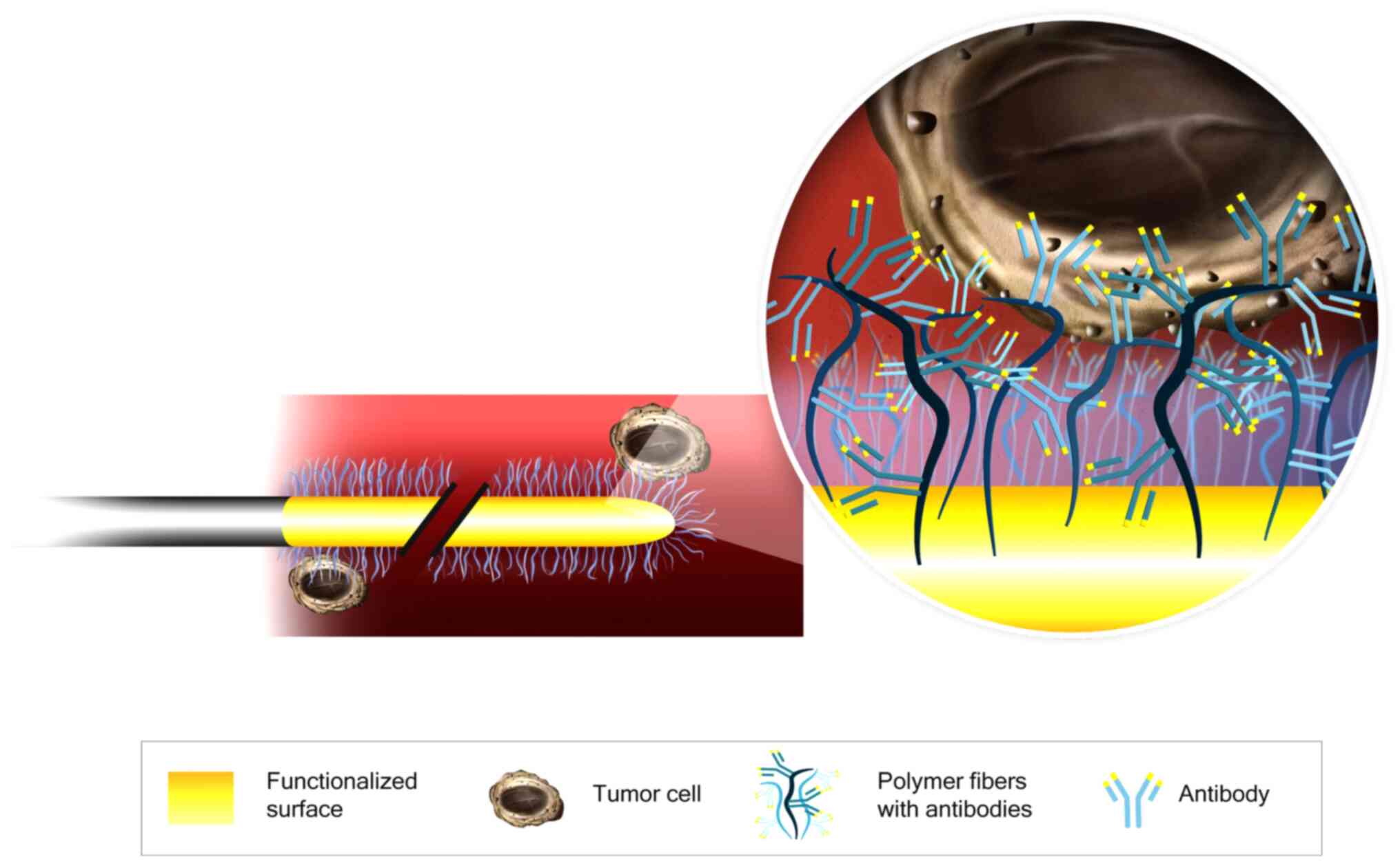
The CellCollector consists of a 160-mm sterile steel
wire with a 20-mm functionalized tip containing epithelial-cell
adhesion molecule (EpCAM) antibodies on its surface. The antibodies
are covalently bonded to a hydrogel that is linked to a gold layer
(Fig. 1). The wire was inserted into
the cubital vein through a 20G cannula and remained in place for 30
min. Then, the CellCollector was washed three times with
phosphate-buffered saline (PBS), and captured cells were fixed with
100% acetone for 10 min at room temperature and blocked with 3%
bovine serum albumin/PBS for 30 min. The captured cells were
identified by immunofluorescence staining, and the CellCollector
was examined for fixed cells using a Nikon microscope (TE2000-E) at
20× magnification. The images were digitally processed with ImageJ
software by altering the contrast and brightness in accordance with
Nature Publishing Guidelines.
CellSearch system
Blood samples were collected into 7.5 ml CellSave
tubes. These samples remained stable for 96 h at room temperature
and were sent overnight to the University Medical Center
Hamburg-Eppendorf. CTCs were isolated using EpCAM-functionalized
immunomagnetic beads with a semiautomated workflow that included
enrichment, fluorescent labeling/characterization and automated
fluorescence imaging of the rare cell population (9,18,19).
CTC enumeration and morphology
CTC enumeration and identification for both
isolation technologies were based on identical criteria. Isolated
cells and/or clusters of immunostained cells of interest were
examined by a blinded experienced researcher. EpCAM-positive cells
were defined as CTCs with the following cytology-based FDA
definition: i) size ≥4 µm, ii) visible cytoplasm, iii) high
nuclear/cytoplasm ratio, iv) positive fluorescent staining of CK 8,
18, and 19 with negative staining of CD45, and v) 50% of the
nucleus contained within the CK border (20).
Statistical evaluation
Since limited data regarding the CellCollector were
available at the time of the study design, no formal sample size
calculations were performed. Therefore, our analyses were
exploratory in nature. We compared the CTC counts between the
control group and the PCa-l and PCa-m groups using Kruskal-Wallis
test followed by Dunn's multiple comparison test. The overall
survival rates were calculated using CTCs value at baseline and
follow up visits. The log-rank test was used for comparing the
Kaplan-Meier survival curves. For all analyses, P<0.05 was
considered statistically significant. The accuracy of the CTC
counts and PSA level were evaluated by receiver operating
characteristic (ROC) analysis. Analyses were performed using
GraphPad Prism version 6.
Results
Study population and clinical
information
The baseline characteristics and clinical parameters
of the different study groups are summarized in Table I. In total, 71 study subjects were
enrolled in our trial, and 92.8% of the 14 PCa-m patients received
chemotherapy. Ten prostate cancer patients (71.4%) were treated
with androgen deprivation therapy (ADT). The primary therapy for
all 21 PCa-l patients was radical prostatectomy (RP). Five patients
(18.5%) received postoperative radiation. All patients with benign
prostatic hyperplasia in our control group were treated with
transurethral resection of the prostate. The second control group
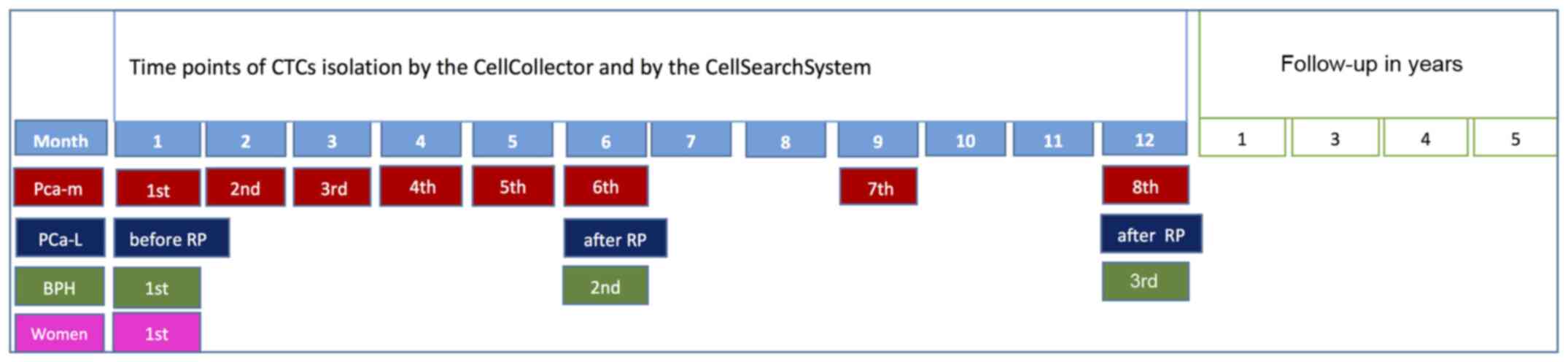
consisted of healthy women. A schedule of the in vivo
application and the comparison method is presented in Fig. 2.
In vivo CTC isolation
Overall, 188 CellCollector applications were
included in the analysis (Fig. 3).
The CellCollector was well tolerated, and no adverse events (AEs)
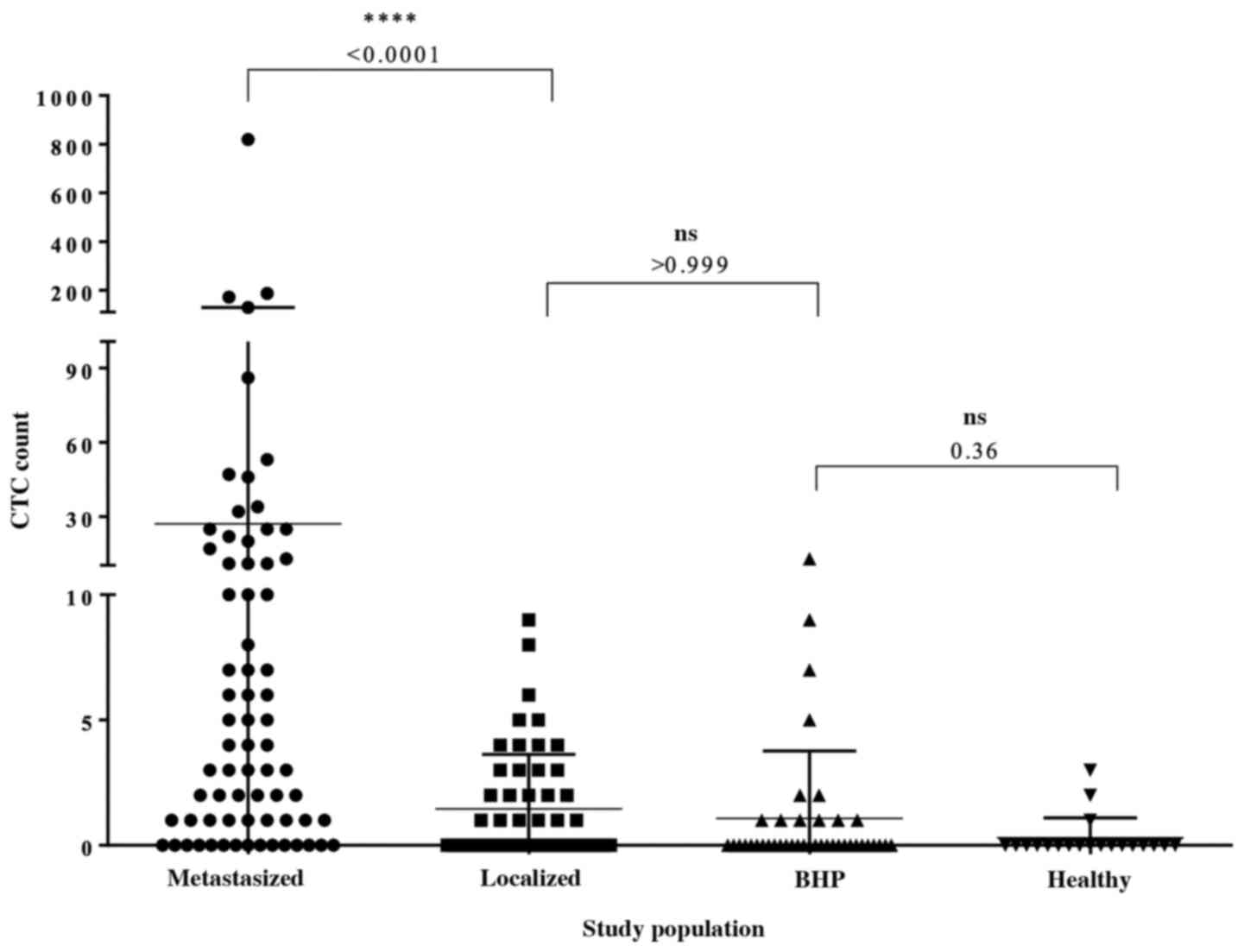
or serious adverse events (SAEs) were reported. In the metastatic
group, 78.9% (n=57) of the 71 applications were positive for ≥1
CTCs. Among the metastatic patients with detectable CTCs, the
median CTC count was 4 (range, 0–820), and the mean CTC count was
27. In the localized group, 45.3% (n=24) of the 53 CellCollector
applications were positive for CTCs. Most of the identified CTCs
were single cells, and cell clusters were rarely present. Among the
CTC-positive localized PCa patients, the CTC median count was 0
(range, 0–9.0) and the mean CTC count was 1.45, and the CTC count
was significantly different (P<0.0001) between the cancer groups
(Fig. 3). A total of 70.7% (n=29) of
the BPH patients and 85% (n=17) of the women in our control group
were negative for CTCs. The median CTC count of the BPH patients
was 0 (range, 0–13); a median CTC value of 0 (range, 0–3) was also
detected in the female controls. With the exception of the female
control group, one wire per group from the PCa-m, PCa-l and BPH
groups could not be evaluated.
Effectivity of CTC isolation
technologies
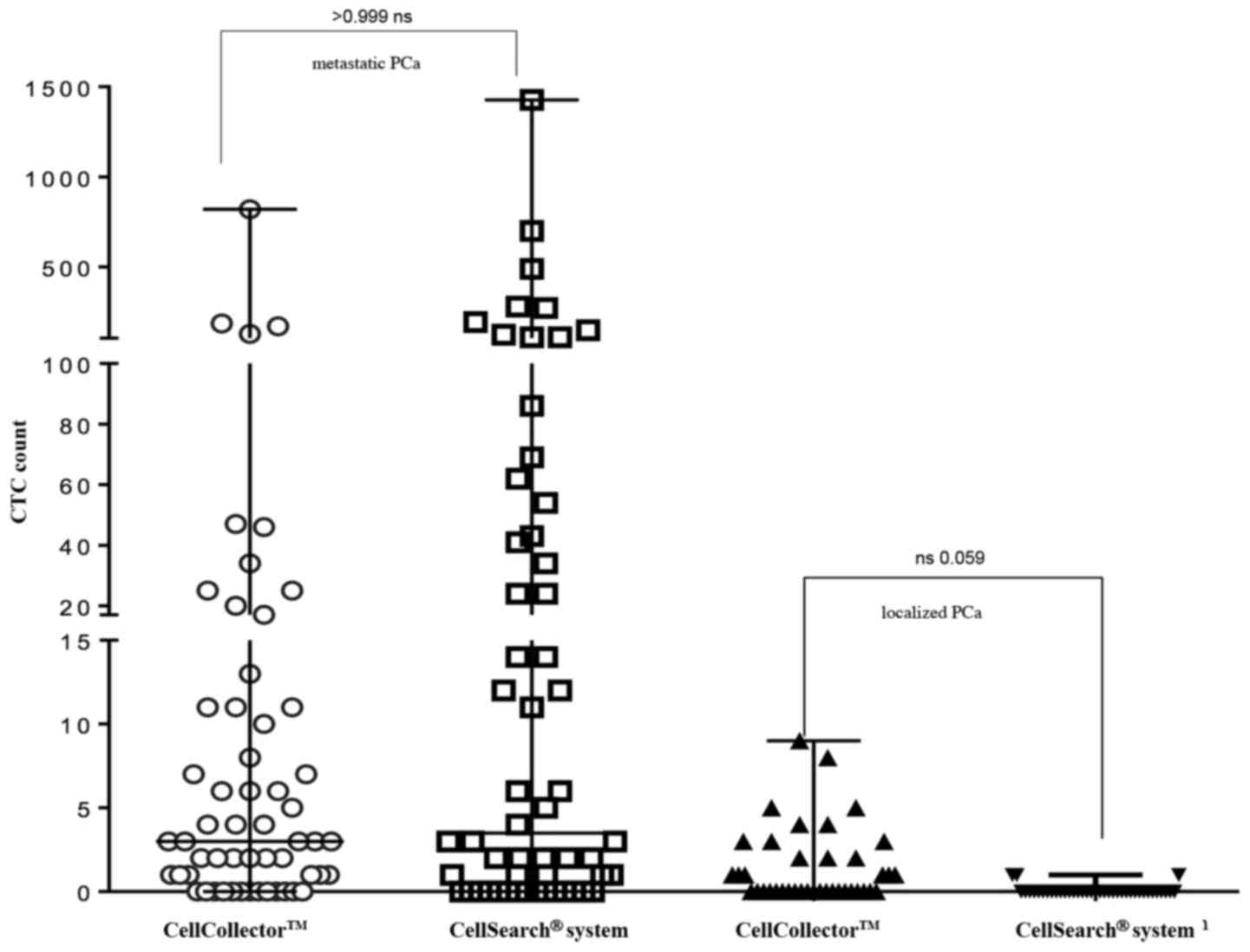
For direct comparison of the CTC isolation
technology, 95 analyzable blood samples (7.5 ml CellSave) were
taken prior to the CellCollector applications and detected by the
CellSearch system. As shown in Fig.
4, the CellCollector captured in vivo CTCs in 18 of 39
PCa-l patients (46.2%) with a median (range) of 0 (0–9); in PCa-m
patients, the CellCollector detected CTCs in 47 of 60 patients
(78.4%) with a median (range) of 3 (0–820) CTCs.
The CellSearch system isolated CTCs in 4 of 39 of
the PCa-l patients (10.3%) with a median (range) of 0 (0–1) and
showed positive results for CTCs in 40 of 60 of the PCa-m patients
(67%) with a median (range) of 3.5 (0–1,428) (Fig. 4). Both systems demonstrated no
correlation between CTC counts.
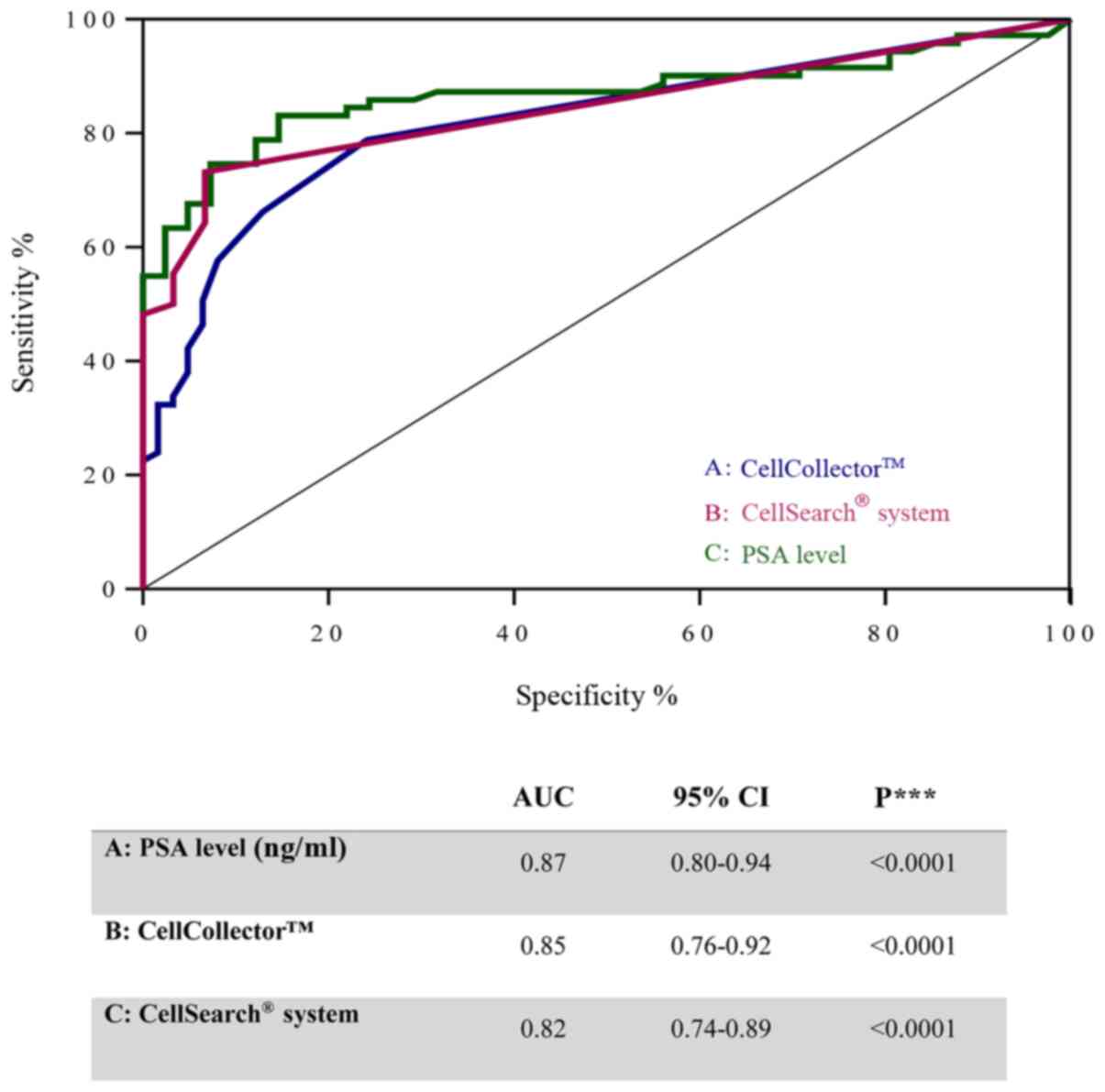
To investigate the diagnostic accuracy of the
CellCollector, we compared the PSA level and CTCs detected using
the CellSearch system in PCa-m patients, BPH patients and healthy
donors. Notably, the ROC curves for these three parameters showed
similar areas under the curve (AUCs): 0.87 (95% CI, 0.8–0.94) for
PSA, 0.82 (95% CI, 0.74–0.89) for the CellCollector and 0.84 (95%
CI, 0.76–0.92) for the CellSearch system (Fig. 5). These values indicated a low
likelihood of false-negative and false-positive results.
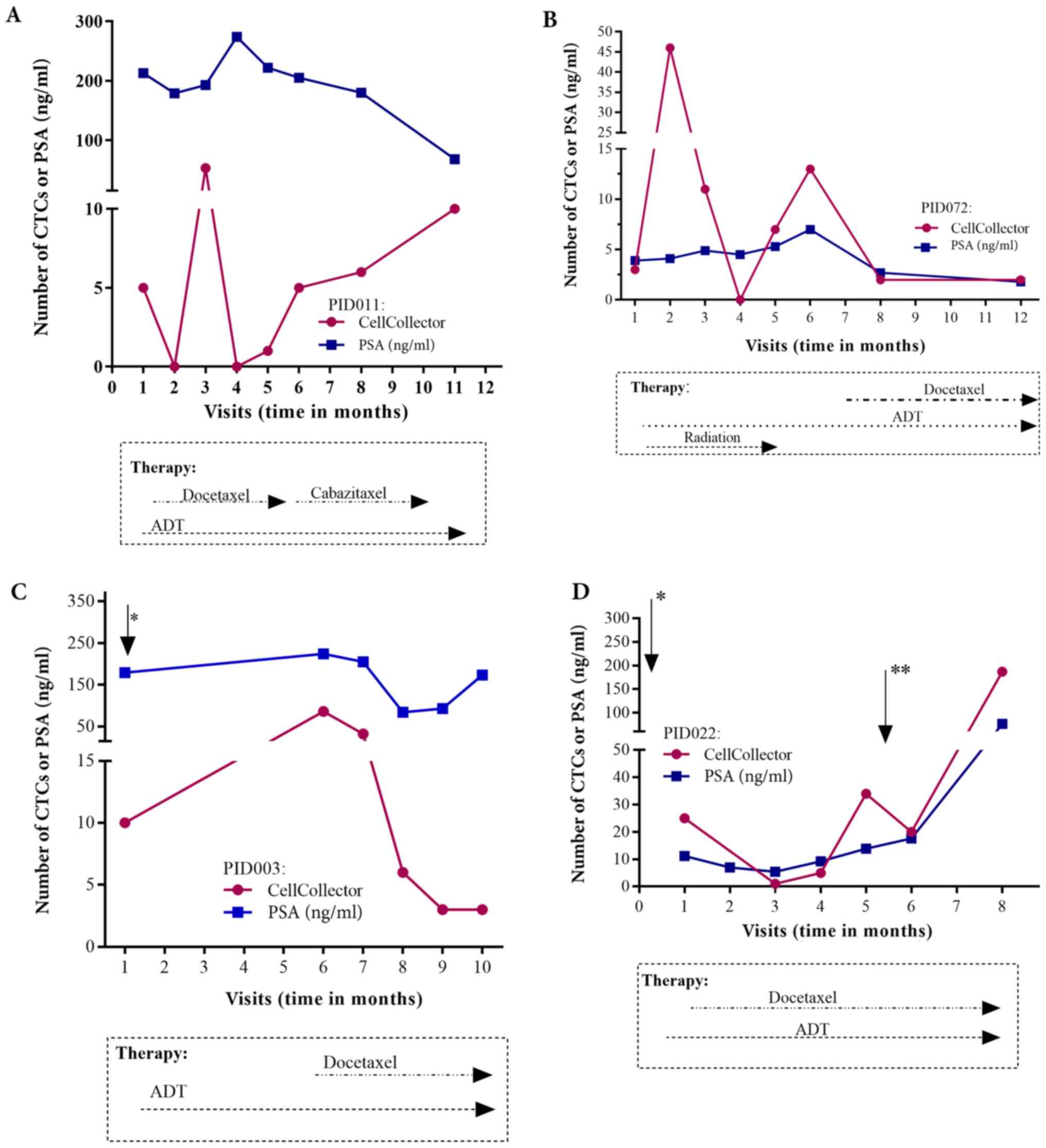
Individual patient CTC/PSA
profiles
Regarding the individual CTC/PSA profiles for
therapy monitoring in the PCa-m group, we included only
metastasized patients with more than three visits. The CTC/PSA
profiles over time are illustrated for 4 patients in Fig. 6A-D. The onset of hormonal therapy in
patient P072 led to a short decline in the PSA level and CTC count.
Approximately one month before visit 8, chemotherapy with docetaxel
was started. This patient presented radiologic and PSA progression
and a rising CTC count (Fig. 6B). As
observed in patient P022, the CTC number and the PSA level were
clearly associated with additional therapies, such as palliative
transurethral resection of the prostate (TURP) and removal of brain
metastases (Fig. 6D). However, the
PSA and CTC profiles could be related to the need for additional
therapy in only 4 of the 10 patients with metastatic disease
(Fig. 6A-D).
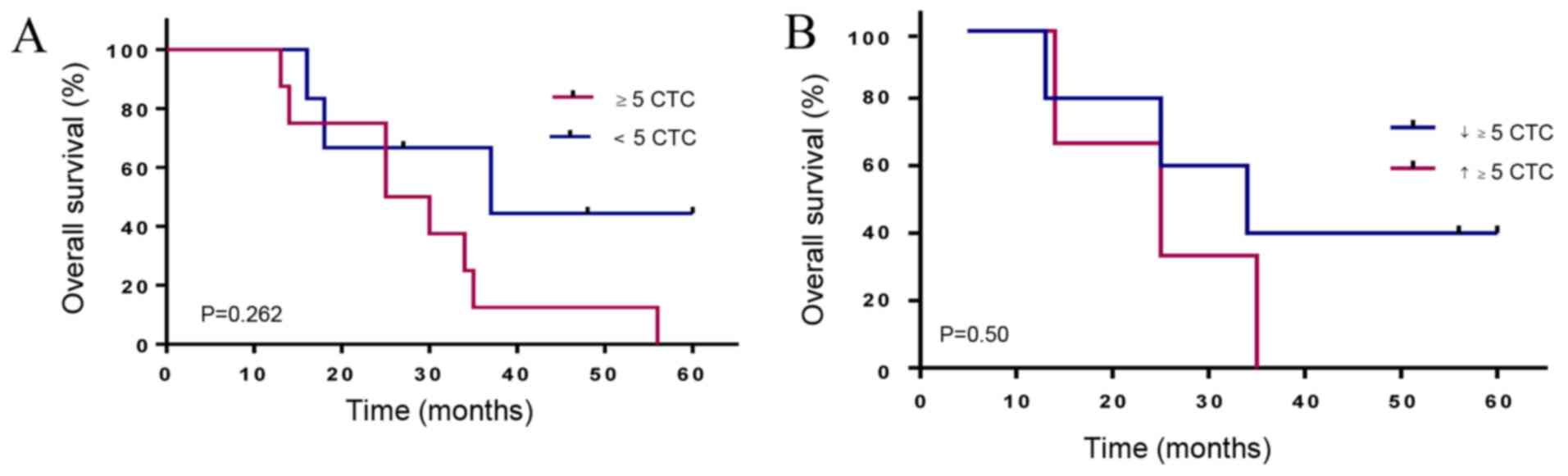
Correlation of in vivo CTC count with
clinical outcome and CTC kinetics
The median follow-up time in the cancer group was 37
months. The Kaplan-Meier curves, according to the CTC count for
metastatic prostate patients, showed that a CTC count of 5 or more
has a lower median OS of 27.5 months compared to 37 months for
patients with less than 5 CTCs (HR 2.6, 95% CI, 0.78–8.3) (Fig. 7A). Interestingly, PCa-m patients with
more than 5 CTCs at all time points and increasing CTC counts
showed the shortest median OS of 25 months compared to 34 months
(HR 1.9, 95% CI 0.4–11.6) for patients with declining CTC counts
(Fig. 7B).
In a match analysis of localized PCa patients before
and after RP, 6 of 14 patients (43%) showed at least one CTC. The
6- and 12-month visits after surgery included 8 (58%) and 5 (36%)
patients who were CTC-positive. In the follow-up of PCa-l patients
after RP, we observed no significant variation in the CTC number
and no prognosis of OS. Furthermore, the presence of CTCs did not
correlate with the PSA level, Gleason score or tumor, node,
metastasis (TNM) classification in any PCa group.
Discussion
The development of sensitive and specific assays for
the detection, isolation and characterization of CTCs in the blood
of cancer patients is still in progress. At this time, only one
assay (CellSearch system) has achieved the level of validity for
approval by the FDA (11).
Our objective was to prove the clinical feasibility
of the in vivo isolation of CTCs in PCa patients. The
CellCollector was inserted into the vein of the patient six times
per year per patients with metastatic prostate cancer. The wire was
well tolerated in patients and the control group.
We demonstrated a significant difference between
in vivo-captured CTCs in localized (median=0 CTCs) and
metastasized PCa (median, 4 CTCs) patients (Fig. 3). According to our data, CTC counts
relative to the criterion threshold of five-displayed individual
association with overall survival in metastasized PCa patients
(Fig. 7A). This threshold status of
more than five CTCs was validated in mPCa patients by the
CellSearch system (11).
Metastasized prostate cancer patients who showed rising CTC counts
at all-time points demonstrated an OS period of 25 months compared
to 34 months for those who showed declining CTCs (Fig. 7B). Our data demonstrated that a
rinsing CTC count may be involved in a cancer progression resulting
in active tumor spreading.
In the current investigation, we further revealed
that the area under the curve (AUC) value did not differ
significantly between the in vivo captured CTC and PSA
levels and ex vivo captured CTCs (Fig. 5). This indicates a similar diagnostic
performance of the CTC count and PSA level. In contrast, Goldkorn
et al (21) demonstrated in
the SWOGS0421 trial that the ROC curves for the day-0 CTC count had
considerably higher AUCs than those for the day-0 PSA level. One
possible reason could be that CTCs, in contrast to PSA, are not
directly affected by hormonal treatment.
Furthermore, we were able to measure the CTC/PSA
profile in metastatic prostate cancer patients during therapeutic
layering. Importantly, the individual profiles showed promise for
therapeutic decision-making in this patient group (Fig. 6). This offers the opportunity for
disease monitoring and for making a tailored treatment decision in
individual metastatic prostate cancer patients. In the area of
personalized medicine, such profiling allows to protect patients
from unnecessary side effects of ineffective treatment. Our results
confirm the potential of CTCs as pharmacodynamics and potential
intermediate endpoint biomarkers for overall survival (7,11).
Unfortunately, this option is not possible in all our PCa-m
patients, partially due to the heterogeneous phenotypes of CTCs in
PCa patients. These phenotypes reflect the epithelial-mesenchymal
transition (EMT) process, which plays a critical role in cancer
metastasis. Indeed, CTCs undergo phenotypic changes from epithelial
to more mesenchymal transitional states during the metastatic
transition (22–24). An enhancement of the in vivo
CTC isolation in metastasized prostate cancer patients should be
considered to use an EMT stable marker such as Prostate-Specific
Membrane Antigen (PSMA) (25) for
functionalizing the CellCollector.
Interestingly, in the metastasized group, the
detection rate of 79.2% was slightly reduced compared to the 100%
rate of ex vivo CTC isolation in our previous study
(14). An explanation (as described
above) could be the heterogeneous phenotypes of CTCs in patients
with progressive cancer status. Furthermore, we detected more
leucocytes and cells positive for EpCAM, CD45 and pan-CK (data not
shown) in comparison to the ex vivo application of the wire
(14). It remains challenging to
examine and analyze CTCs, as this phenomenon indicates activation
of the immune system and may signal a therapeutic response
(9). The results of our trial are in
general agreement with previous results of other CTC trials in
PCa-m patients (11,26–28).
The role of CTCs in PCa-l has been proposed as an
option for postoperative risk stratification. We isolated CTCs in
45.3% of the wire applications in PCa-l patients. The monitoring of
the CTC count (>1 CTC) before and after radical prostatectomy
demonstrated no correlation with clinical and pathological
parameters. Meyer et al (29)
analyzed 152 localized patients preoperatively for CTCs and
reported an 11% (n=17) CTC-positive rate. They showed no difference
in biochemical recurrence in patients with or without CTCs.
Todenhofer et al (30) used
an EpCAM-independent isolation system and detected CTCs in 50% of
PCa-l patients. They also revealed no correlation between CTC
detection and PSA level, tumor stage or Gleason score (30). Taken together, our results did not
verify a significant association of CTC positivity in patients
undergoing RP for preoperative localized PCa. Our control groups
also demonstrated a median CTC of 0, which was similar to the
median CTC of the local PCa group. We demonstrated in Fig. 5 a good diagnostic accuracy of the
CellCollector for CTC capturing comparable to the CellSearch System
and the PSA level. The in vivo CTC isolation system allows
to detect the low number of cells. From the other hand, it create
the risk of the false-positive results. The localized prostate
cancer group consist of 85.7% patients with a low Gleason score ≤7.
It is very unlikely that such tumors release high invasive CTCs.
False-positive results in control groups (BPH and healthy women)
can have different causes. One reason may be the used
characterization of CTCs, which does not distinguish between
possible different CTCs phenotypes. CTCs can infiltrate normal
tissue and form metastases or, which is more likely, be attacked by
immune cells and killed. It is also possible to misinterpret cells,
like larger CD45 negative leucocytes or low number of epithelial
cells, which potentially enter in blood by the peripheral
intravenous cannulation. Castle et al (31) discussed also such subtype of cells,
which remained to be characterized. In our previous in vitro
study, we demonstrated that 54% of the BPH patients' blood samples
are positive for CTCs (14). In the
present trail, only 20.3% of the BPH patients were positive for
CTCs. This might suggest a better diagnostic accuracy of the in
vivo CTC isolation compared the ex vivo CellCollector
application (14). Our findings
suggest that a cutoff value of in vivo captured CTCs must be
developed. The role of CTCs as prognostic markers in localized PCa
seems to be more relevant in locally advanced prostate cancer
patients, such as risk stratification for additional treatment.
The limitations of our investigation are of course
the small number of patients in the cancer groups. The unknown
blood volume of the reported CTC counts must also be noted. Thus,
future studies are needed to discover the clinical potential of the
wire.
In conclusion, our present findings indicate that
in vivo capture of CTCs by the CellCollector overcomes the
blood volume limitations of other diagnostic approaches and thereby
increases the diagnostic sensitivity of CTC analysis. The
CTC/PSA-Profile reveals the possibility of personalized therapy
monitoring which can help to prevent patients for side effects of
invalidly treatment. The CellCollector was well tolerated, and no
side effects were reported. Thus, future studies are needed to
explore this method in the clinic.
Acknowledgements
The authors would like to thank the Department of
Tumor Biology, University Medical Center Hamburg-Eppendorf, Hamburg
for providing the CellSearch analysis.
Funding
Financial funding for the implementation of this
study was received from the GILUPI GmbH. The sponsor played no role
in the study.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GT designed the study, performed the experiments,
analyzed the data and wrote the manuscript. GT and PF confirm the
authenticity of all the raw data. CB and KF performed the
experiments and were involved in drafting the paper. JB performed
the analysis and interpretation of data, and was involved in
drafting the paper and revised it critically for important
intellectual content. RH and EW designed the study. FK and SS
performed the analysis and interpretation of data, and gave final
approval of the version to be published. The conception and design
of the study, as well as drafting and approving the article, is
attributed to PF. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Medical
Faculty Ethics Committee of MLU Halle-Wittenberg. Furthermore, we
obtained a permit from the Federal Institute for Drug and Medical
Devices (Germany, BfArM). The patients provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that GILUPI GmbH supported the
present study. We received funding from GILUPI GmbH; they provided
the CellCollector and the patients received travel expenses. The
sponsor played no role in the study. GILUPI GmbH provided the
schematic image of the wire (Fig.
1).
Authors' information
Raschid Hoda was employed until February 2013 at the
Medical Faculty of Martin Luther University Halle-Wittenberg,
University Clinic and Outpatient Clinic for Urology, 06120 Halle
(Saale), Germany.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nuhn P, De Bono JS, Fizazi K, Freedland
SJ, Grilli M, Kantoff PW, Sonpavde G, Sternberg CN,
Yegnasubramanian S and Antonarakis ES: Update on systemic prostate
cancer therapies: Management of metastatic castration-resistant
prostate cancer in the era of precision oncology. Eur Urol.
75:88–99. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Resnick MJ, Koyama T, Fan KH, Albertsen
PC, Goodman M, Hamilton AS, Hoffman RM, Potosky AL, Stanford JL,
Stroup AM, et al: Long-term functional outcomes after treatment for
localized prostate cancer. N Engl J Med. 368:436–445. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scher HI, Lu D, Schreiber NA, Louw J, Graf
RP, Vargas HA, Johnson A, Jendrisak A, Bambury R, Danila D, et al:
Association of AR-V7 on circulating tumor cells as a
treatment-specific biomarker with outcomes and survival in
castration-resistant prostate cancer. JAMA Oncol. 2:1441–1449.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hofman P and Popper HH: Pathologists and
liquid biopsies: To be or not to be? Virchows Arch. 469:601–609.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alix-Panabieres C and Pantel K: Challenges
in circulating tumour cell research. Nat Rev Cancer. 14:623–631.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yap TA, Lorente D, Omlin A, Olmos D and de
Bono JS: Circulating tumor cells: A multifunctional biomarker. Clin
Cancer Res. 20:2553–2568. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Antonarakis ES, Lu C, Wang H, Luber B,
Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL, et
al: AR-V7 and resistance to enzalutamide and abiraterone in
prostate cancer. N Engl J Med. 371:1028–1038. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Andree KC, van Dalum G and Terstappen LW:
Challenges in circulating tumor cell detection by the CellSearch
system. Mol Oncol. 10:395–407. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cristofanilli M, Budd GT, Ellis MJ,
Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ,
Terstappen LW and Hayes DF: Circulating tumor cells, disease
progression, and survival in metastatic breast cancer. N Engl J
Med. 351:781–791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de Bono JS, Scher HI, Montgomery RB,
Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ
and Raghavan D: Circulating tumor cells predict survival benefit
from treatment in metastatic castration-resistant prostate cancer.
Clin Cancer Res. 14:6302–6309. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Habli Z, AlChamaa W, Saab R, Kadara H and
Khraiche ML: Circulating tumor cell detection technologies and
clinical utility: Challenges and opportunities. Cancers (Basel).
12:19302020. View Article : Google Scholar
|
|
13
|
Ferreira MM, Ramani VC and Jeffrey SS:
Circulating tumor cell technologies. Mol Oncol. 10:374–394. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Theil G, Fischer K, Weber E, Medek R, Hoda
R, Lucke K and Fornara P: The use of a new CellCollector to isolate
circulating tumor cells from the blood of patients with different
stages of prostate cancer and clinical outcomes-a proof-of-concept
study. PLoS One. 11:e01583542016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gorges TM, Penkalla N, Schalk T, Joosse
SA, Riethdorf S, Tucholski J, Lücke K, Wikman H, Jackson S, Brychta
N, et al: Enumeration and molecular characterization of tumor cells
in lung cancer patients using a novel in vivo device for capturing
circulating tumor cells. Clin Cancer Res. 22:2197–2206. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saucedo-Zeni N, Mewes S, Niestroj R,
Gasiorowski L, Murawa D, Nowaczyk P, Tomasi T, Weber E, Dworacki G,
Morgenthaler NG, et al: A novel method for the in vivo
isolation of circulating tumor cells from peripheral blood of
cancer patients using a functionalized and structured medical wire.
Int J Oncol. 41:1241–1250. 2012.PubMed/NCBI
|
|
17
|
Mandair D, Vesely C, Ensell L, Lowe H,
Spanswick V, Hartley JA, Caplin ME and Meyer T: A comparison of
cellcollector with cellsearch in patients with neuroendocrine
tumours. Endocr Relat Cancer. 23:L29–L32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Bono JS, Attard G, Adjei A, Pollak MN,
Fong PC, Haluska P, Roberts L, Melvin C, Repollet M, Chianese D, et
al: Potential applications for circulating tumor cells expressing
the insulin-like growth factor-I receptor. Clin Cancer Res.
13:3611–3616. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cristofanilli M: Circulating tumor cells,
disease progression, and survival in metastatic breast cancer.
Semin Oncol. 33 (Suppl 9):S9–S14. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Allard WJ, Matera J, Miller MC, Repollet
M, Connelly MC, Rao C, Tibbe AG, Uhr JW and Terstappen LW: Tumor
cells circulate in the peripheral blood of all major carcinomas but
not in healthy subjects or patients with nonmalignant diseases.
Clin Cancer Res. 10:6897–6904. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goldkorn A, Ely B, Quinn DI, Tangen CM,
Fink LM, Xu T, Twardowski P, Van Veldhuizen PJ, Agarwal N, Carducci
MA, et al: Circulating tumor cell counts are prognostic of overall
survival in SWOG S0421: A phase III trial of docetaxel with or
without atrasentan for metastatic castration-resistant prostate
cancer. J Clin Oncol. 32:1136–1142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Armstrong AJ: Epithelial-mesenchymal
transition in cancer progression. Clin Adv Hematol Oncol.
9:941–943. 2011.PubMed/NCBI
|
|
23
|
Alix-Panabieres C and Pantel K:
Circulating tumor cells: Liquid biopsy of cancer. Clin Chem.
59:110–118. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lambert AW, Pattabiraman DR and Weinberg
RA: Emerging biological principles of metastasis. Cell.
168:670–691. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gleghorn JP, Pratt ED, Denning D, Liu H,
Bander NH, Tagawa ST, Nanus DM, Giannakakou PA and Kirby BJ:
Capture of circulating tumor cells from whole blood of prostate
cancer patients using geometrically enhanced differential
immunocapture (GEDI) and a prostate-specific antibody. Lab Chip.
10:27–29. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Danila DC, Anand A, Sung CC, Heller G,
Leversha MA, Cao L, Lilja H, Molina A, Sawyers CL, Fleisher M and
Scher HI: TMPRSS2-ERG status in circulating tumor cells as a
predictive biomarker of sensitivity in castration-resistant
prostate cancer patients treated with abiraterone acetate. Eur
Urol. 60:897–904. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lorente D, Olmos D, Mateo J, Bianchini D,
Seed G, Fleisher M, Danila DC, Flohr P, Crespo M, Figueiredo I, et
al: Decline in circulating tumor cell count and treatment outcome
in advanced prostate cancer. Eur Urol. 70:985–992. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Scher HI, Heller G, Molina A, Attard G,
Danila DC, Jia X, Peng W, Sandhu SK, Olmos D, Riisnaes R, et al:
Circulating tumor cell biomarker panel as an individual-level
surrogate for survival in metastatic castration-resistant prostate
cancer. J Clin Oncol. 33:1348–1355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Meyer CP, Pantel K, Tennstedt P, Stroelin
P, Schlomm T, Heinzer H, Riethdorf S and Steuber T: Limited
prognostic value of preoperative circulating tumor cells for early
biochemical recurrence in patients with localized prostate cancer.
Urol Oncol. 34:235.e11–6. 2016. View Article : Google Scholar
|
|
30
|
Todenhofer T, Park ES, Duffy S, Deng X,
Jin C, Abdi H, Ma H and Black PC: Microfluidic enrichment of
circulating tumor cells in patients with clinically localized
prostate cancer. Urol Oncol. 34:483.e9–e16. 2016. View Article : Google Scholar
|
|
31
|
Castle J, Morris K, Pritchard S and Kirwan
CC: Challenges in enumeration of CTCs in breast cancer using
techniques independent of cytokeratin expression. PLoS One.
12:e01756472017. View Article : Google Scholar : PubMed/NCBI
|