Introduction
Lung cancer is a leading cause of tumor-related
mortality worldwide (1). Every year,
1.8 million people are diagnosed with lung cancer, and 1.6 million
people die as a result of the disease, as well as 5-year survival
rates vary from 4–17% depending on stage and regional differences
(2). Non-small cell lung cancer
(NSCLC) accounts for ~85% of lung cancer cases, and most deaths
from lung cancer can be attributed to NSCLC (3). After radical surgery, >60% of
patients with early-stage NSCLC can experience in situ
recurrence of the tumor or distal metastasis (4). Recently, adjuvant chemotherapy based on
platinum drugs has been widely applied to the clinical treatment of
NSCLC after surgery (5). Platinum
drugs can destroy the structure of DNA by forming platinum-DNA
complexes, thereby contributing to the apoptosis of tumor cells
(6). However, the long-term use of
platinum drugs leads to drug resistance, which is one of the major
obstacles of cancer treatment (7).
Thus, an improved understanding of the mechanisms of platinum drug
resistance, as well as the development of methods that can overcome
resistance, is necessary to improve the prognosis of patients with
cancer.
As the major target of platinum drugs is DNA, the
sensitivity/resistance to these drugs may be affected by the
ability of cells to recognize and repair DNA damage (8). Nucleotide excision repair (NER) is the
major pathway for the removal of platinum-DNA adducts (9). ERCC excision repair 1, endonuclease
non-catalytic subunit (ERCC1), a key component of NER, is involved
in interstrand cross-linking repair, double-strand break repair,
homologous recombination and telomere maintenance (10). Increasing evidence has indicated that
the differential expression of ERCC1 may be a cause of cell
resistance to platinum drugs. Selvakumaran et al (11) found that downregulation of
ERCC1 altered the DNA repairing ability of
cisplatin-resistant ovarian cancer cells and increased their
sensitivity to cisplatin. In a previous meta-analysis, patients
with lung cancer that had low/negative ERCC1 expression had
a higher response to platinum drugs and longer median survival time
compared with those with high/positive ERCC1 expression
(12).
In addition, poly(ADP-ribose) polymerase 1 (PARP1),
is a sensor for DNA strand break that responds to platinum-induced
DNA damage and participates in DNA repair (13). PARP inhibitors can improve the
sensitivity of tumor cells to chemotherapy drugs or directly kill
tumor cells through a homozygous lethal mechanism (14,15). A
randomized clinical study demonstrated that the PARP inhibitor
olaparib significantly increased the sensitivity to platinum drugs
and prolonged the median progression-free survival time of breast
cancer (16). However, the effects
of PARP inhibitors combined with ERCC1 expression on the
sensitivity of platinum drugs remain unclear in NSCLC.
Hence, the present study aimed to investigate
whether the expression of ERCC1 enhanced the sensitivity of
platinum drugs in combination with PARP inhibitors, thereby
improving the prognosis of NSCLC. These results may provide novel
insight for the improvement of platinum drug sensitivity and
treatment of NSCLC.
Materials and methods
Cell culture
NSCLC cell lines, including the NCI-H1299
(adenocarcinoma) and SK-MES-1 (squamous carcinoma) cell lines were
purchased from the Cell Resource Center, Shanghai Institute of
Biotechnology, Chinese Academy of Sciences. NCI-H1299 and SK-MES-1
cells were cultured in RPMI-1640 medium (Thermo Fisher Scientific,
Inc.) with 10% fetal bovine serum (FBS; Thermo Fisher Scientific,
Inc.) and minimum essential medium (Thermo Fisher Scientific, Inc.)
with 10% FBS, respectively. Both cell lines were incubated in an
incubator with 5% CO2 at 37°C.
Cell transfection
Small interfering (si)RNA-negative control (NC,
non-targeting; forward, 5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′), siRNA-ERCC1-1 (GCCCTTATTCCGATCTACA),
siRNA-ERCC1-2 (CGACGTAATTCCCGACTAT), and siRNA-ERCC1-3
(CCGTGAAGTCAGTCAACAA) were designed and synthesized by Guangzhou
RiboBio Co., Ltd. GV230 and GV230-ERCC1+ were purchased from
Shanghai Genechem Co., Ltd. Cell transfection was performed as
previously described (17).
NCI-H1299 or SK-MES-1 cells were cultured in serum-free medium,
then seeded into 6-well plates (5×105 cells/well). Next, 3.5 µg
GV230, 3.5 µg GV230-ERCC1+, 50 nM siRNA-NC, 50 nM siRNA-ERCC1-1, 50
nM siRNA-ERCC1-2 or 50 nM siRNA-ERCC1-3 were transfected into the
cells at 24±2°C for 20 min using Lipofectamine 2000®
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. After transfection at 37°C for 6 h,
the medium was replaced with 10% serum-containing medium and
cultured at 37°C for another 48 h. Total RNA and total protein of
the cells from different cells were extracted. The transfection
efficiency was evaluated by determining the expression of ERCC1
using reverse transcription-quantitative (RT-q) PCR and western
blotting.
RT-qPCR
Total RNA was extracted from the transfected cells
(5×105 cells/well) using TRIzol® reagent (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Total
RNA was reverse transcribed into cDNA using the PrimeScript™ II 1st
Strand cDNA Synthesis kit (Takara Bio, Inc.). The temperature
protocol used for reverse transcription was 37°C for 60 min and
85°C for 5 sec. Subsequently, qPCR was performed using SYBR Premix
EX Taq (2X; Thermo Fisher Scientific Inc.), and the primer sequence
of ERCC1 was as follows: Forward, 5′-TTGTCCAGGTGGATGTGAAA-3′
and reverse, 5′-GCTGGTTTCTGCTCATAGGC-3′. The qPCR thermocycling
conditions were as follows: 95°C for 3 min; 95°C for 10 sec;
followed by 40 cycles at 60°C for 30 sec and 60°C for 30 sec. The
mRNA expression of ERCC1 was quantified using the 2-ΔΔCq
method (18) and normalized to the
reference gene GAPDH forward, 5′-AGACAGCCGCATCTTCTTGT-3′ and
reverse, 5′-CTTGCCGTGGGTAGAGTCAT-3′.
Western blotting
Total protein was isolated from transfected cells
(5×105 cells/well using radioimmunoprecipitation assay protein
lysis buffer (Beyotime Institute of Biotechnology). Protein
concentrations were measured using a Bicinchoninic Acid Protein
Assay kit (Wuhan Boster Biological Technology, Ltd.) following the
manufacturer's protocol. Protein samples (20 µg) were separated by
10% SDS-PAGE and transferred to PVDF membranes. After blocking with
5% skimmed milk for 2 h at 37°C, the membranes were incubated with
anti-ERCC1 antibody (1:2,000; cat. no. 14586-1-AP; ProteinTech
Group, Inc.) and anti-β-actin antibody (1:10,000; cat. no.
66009-1-Ig; ProteinTech Group, Inc.) overnight at 4°C. After
washing 3 times with PBST (0.05% Tween-20 in PBS), the membranes
were incubated with goat anti-rabbit mouse IgG (1:10,000; cat. no.
115-035-003; Jackson ImmunoResearch Laboratories, Inc.) at 37°C for
2 h. After 3 washes, protein bands were visualized using the ECL
assay kit (Beyotime Institute of Biotechnology) and analyzed using
Image-Pro Plus software v.6.0, (Media Cybernetics Inc.).
Cell viability assay
Cell viability of NCI-H1299 and SK-MES-1 cells was
determined using the Cell Counting Kit-8 (CCK-8; Beyotime Institute
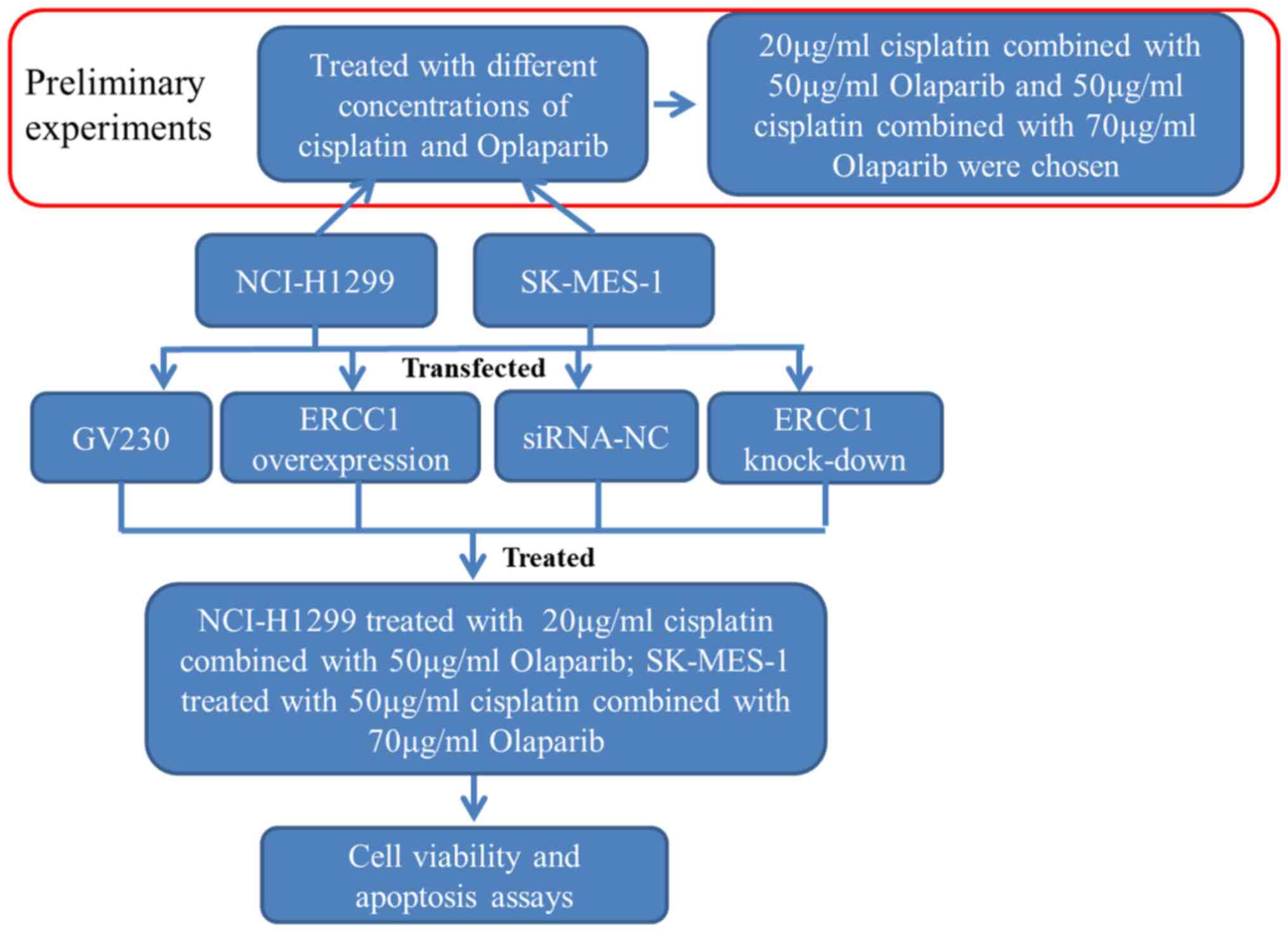
of Biotechnology). The schematic workflow of the cellular
experimentation is presented in Fig.
1. Briefly, different concentrations of cisplatin
(Sigma-Aldrich; Merck KGaA) and PARP inhibitor olaparib (Selleck
Chemicals) were prepared in dimethyl sulfoxide (Beijing Solarbio
Science & Technology Co., Ltd.). The control,
ERCC1-overexpressing and ERCC1-knockdown cells were
seeded into 6-well plates (5×105 cells/well), then treated with
cisplatin alone or in combination with olaparib at 37KC, as shown
in Fig. 1. Following treatment for
24 h, 10 µl of CCK-8 reagent (Beyotime Institute of Biotechnology)
was added to the cells and incubated at 37°C for 2 h. Absorbance
was detected at 450 nm using a microplate reader.
Cell apoptosis assay
Effects of ERCC1 expression on apoptosis in
NCI-H1299 and SK-MES-1 cells were determined using an Annexin
V-FITC cell apoptosis assay kit (Beyotime Institute of
Biotechnology) according to the manufacturer's instructions. The
NCI-H1299 or SK-MES-1 cells with ERCC1 overexpression or
interference (1×104 cells/well) were treated with cisplatin and
olaparib at 37°C for 24 h as detailed in Fig. 1. Subsequently, the cells were
harvested and resuspended in pre-cooled phosphate-buffered saline.
After centrifugation at 1,000 × g for 5 min at room temperature,
195 µl binding buffer and 5 µl Annexin V-FITC (20 µg/ml) were added
to the cells. After incubation at room temperature for 30 min in
the dark, the cells were stained with 5 µl of propidium iodide (PI;
50 µg/ml) and incubated at room temperature in the dark for 10 min.
Finally, flow cytometry (FACSCalibur; Becton-Dickinson and Company)
was used to observe cell apoptosis, and the apoptosis rate (early
plus late apoptosis) was calculated using the CellQuest software
v.4.0 (Becton-Dickinson and Company).
Statistical analysis
Each experiment was performed in triplicate. All
data are expressed as the mean ± standard deviation. GraphPad Prism
5.0 (GraphPad Software, Inc.) was used for statistical analysis.
For multiple comparisons, one-way analysis of variance followed by
Bonferroni correction was performed. Student's t-test with unpaired
test was applied for comparisons between two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Selecting optimal concentrations of
cisplatin and olaparib for cellular treatment
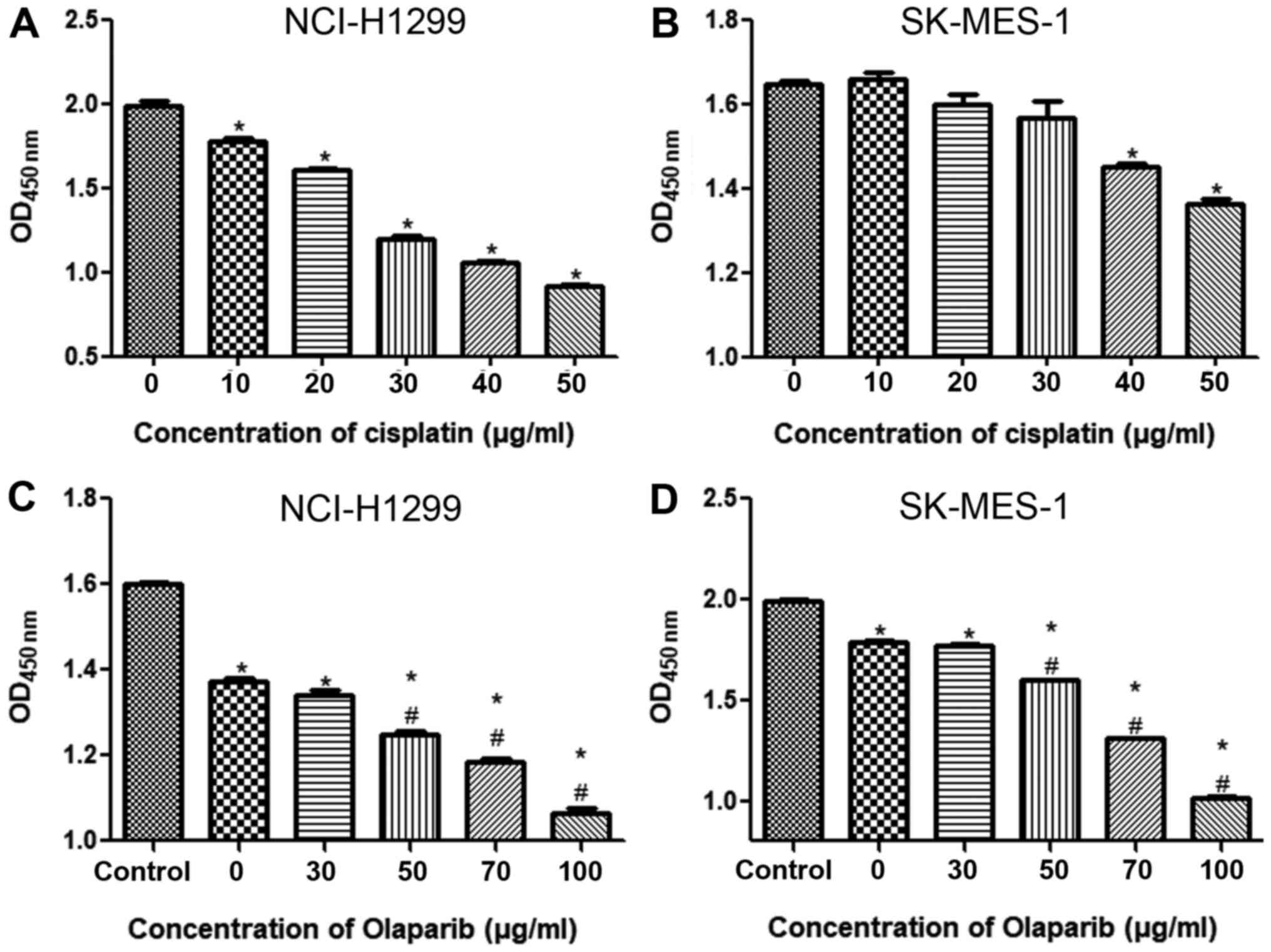
To select the optimum concentration of cisplatin,
different concentrations were used to treat NSCLC cell lines for 24
h. In the NCI-H1299 cell line, when the cisplatin concentration was
10 µg/ml, the cell viability was significantly inhibited compared
with the control group (P<0.05) and gradually decreased with an
increase in cisplatin concentration (Fig. 2A). In the SK-MES-1 cell line, when
the cisplatin concentration was 30 µg/ml, the cell viability began
to be significantly inhibited compared with the control group
(P<0.05), and when the concentration of cisplatin was 50 µg/ml,
cell viability was further suppressed compared with the control
group (P<0.05; Fig. 2B). Thus, in
subsequent experiments, 20 and 50 µg/ml cisplatin were applied to
the NCI-H1299 and SK-MES-1 cell lines, respectively.
To confirm the concentrations required for the PARP
inhibitor olaparib, the NSCLC cell lines were treated with
cisplatin combined with different concentrations of olaparib. When
the NCI-H1299 and SK-MES-1 cell lines were treated with 20 and 50
µg/ml cisplatin, respectively, cell viability was significantly
decreased compared with that of the control group (P<0.05;
Figs. 2C and D). However, in the
NCI-H1299 cell line, the cell viability was significantly lower
after 50 µg/ml olaparib combined with cisplatin administration
compared with after cisplatin treatment alone (P<0.05; Fig. 2C). Similarly, in the SK-MES-1 cell
line, when the concentration of olaparib was 70 µg/ml, the cell
viability was further significantly reduced compared with that of
the cells treated without olaparib (P<0.05; Fig. 2D). These results indicated that in
subsequent experiments, the NCI-H1299 and SK-MES-1 cells should be
treated with 20 µg/ml cisplatin combined with 50 µg/ml olaparib and
50 µg/ml cisplatin combined with 70 µg/ml olaparib,
respectively.
Cell transfection efficiency analyses
by RT-qPCR and western blotting
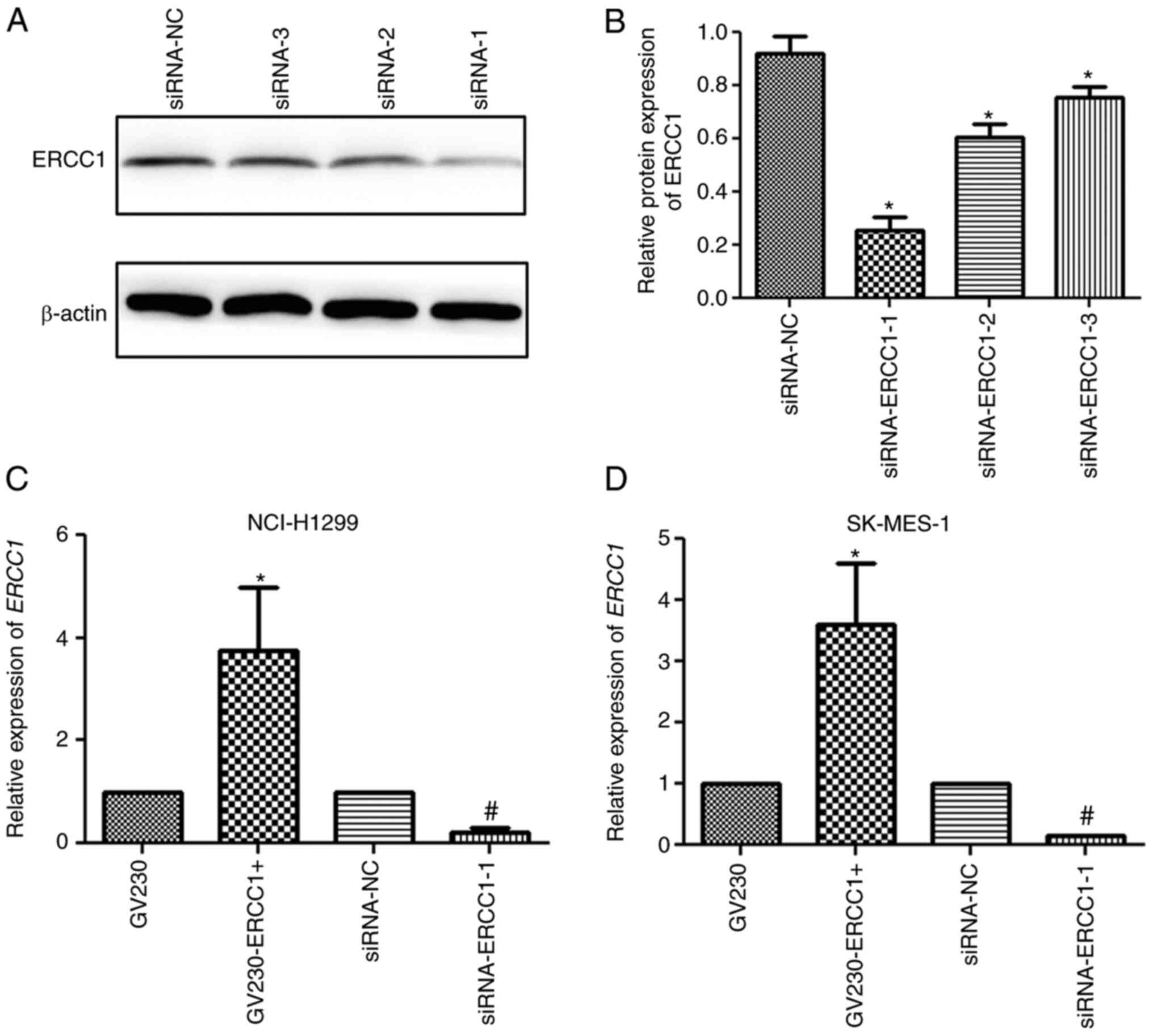
To evaluate the transfection efficiency, the
expression level of ERCC1 in NSCLC cell lines was determined by
RT-qPCR and western blotting. In the NCI-H1299 cell line, western
blot analysis demonstrated that the expression of ERCC1
significantly decreased following transfection with
siRNA-ERCC1-1/2/3 compared with the siRNA-NC group (P<0.05) and
that the siRNA-ERCC1-1 group had the best transfection efficiency
as the ERCC1 mRNA expression was the lowest in the
siRNA-ERCC1-1 group compared with the siRNA-NC, si-RNA-ERCC1-2 and
si-RNA-ERCC1-3 groups (Fig. 3A and
B). In addition, RT-qPCR was performed to determine the mRNA
expression levels of ERCC1 in the GV230, GV230-ERCC1+,
siRNA-NC, and siRNA-ERCC1-1 groups. The expression level of
ERCC1 in the GV230-ERCC1+ group was significantly higher
compared with that in the GV230 group, while the expression level
was significantly downregulated in the siRNA-ERCC1-1 group compared
with that in the siRNA-NC group (both P<0.05; Fig. 3C). The trend of ERCC1 mRNA expression
in the SK-MES-1 cell line detected by RT-qPCR was similar to that
in the NCI-H1299 cell line (Fig.
3D). These results suggested that the NCI-H1299 or SK-MES-1
cells with ERCC1 overexpression and ERCC1 knockdown
were successfully generated.
Effects of ERCC1 combined with
olaparib on the cell viability of cisplatin-treated cells
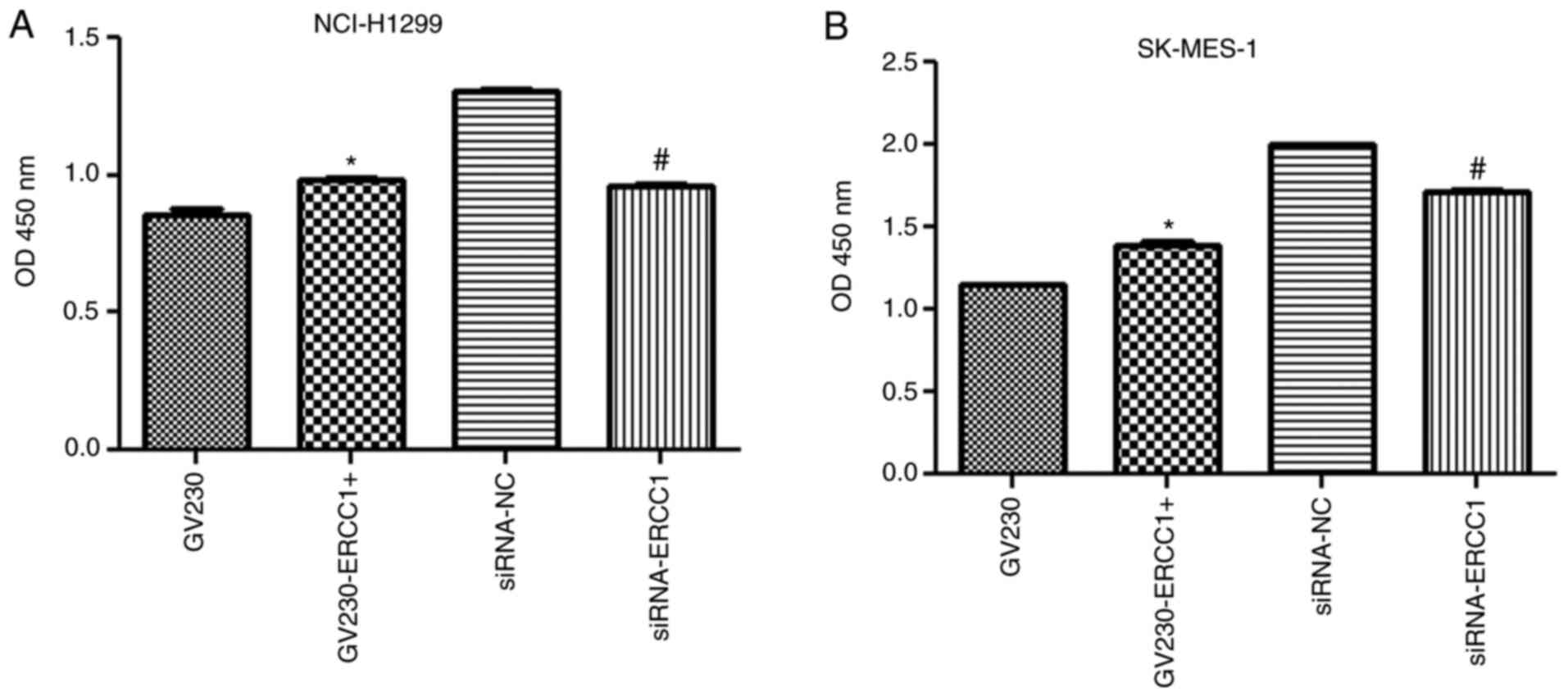
In the NCI-H1299 cell line, after the cells were
treated with 20 µg/ml cisplatin combined with 50 µg/ml olaparib for
24 h, cell viability was significantly increased in the
GV230-ERCC1+ group compared with the GV230 group, but significantly
decreased in the siRNA-ERCC1 group compared with the siRNA-NC group
(both P<0.05; Fig. 4A).
Additionally, the change in cell viability in the SK-MES-1 cell
line was in accordance with that in the NCI-H1299 cell line
(Fig. 4B). Indeed, after the
SK-MES-1 cells were treated with 50 µg/ml cisplatin in combination
with 70 µg/ml olaparib, the cell viability was enhanced in
ERCC1-overexpressing cells, whereas it was inhibited in
ERCC1-knockdown cells (P<0.05; Fig. 4B). These results suggested that
ERCC1 combined with olaparib may enhance the sensitivity of
NCI-H1299 and SK-MES-1 cells to cisplatin by affecting cell
viability.
Effects of ERCC1 combined with
olaparib on the cell apoptosis of cisplatin-treated cells
Effects of ERCC1 on the apoptosis of cells
treated with cisplatin and olaparib were determined using flow
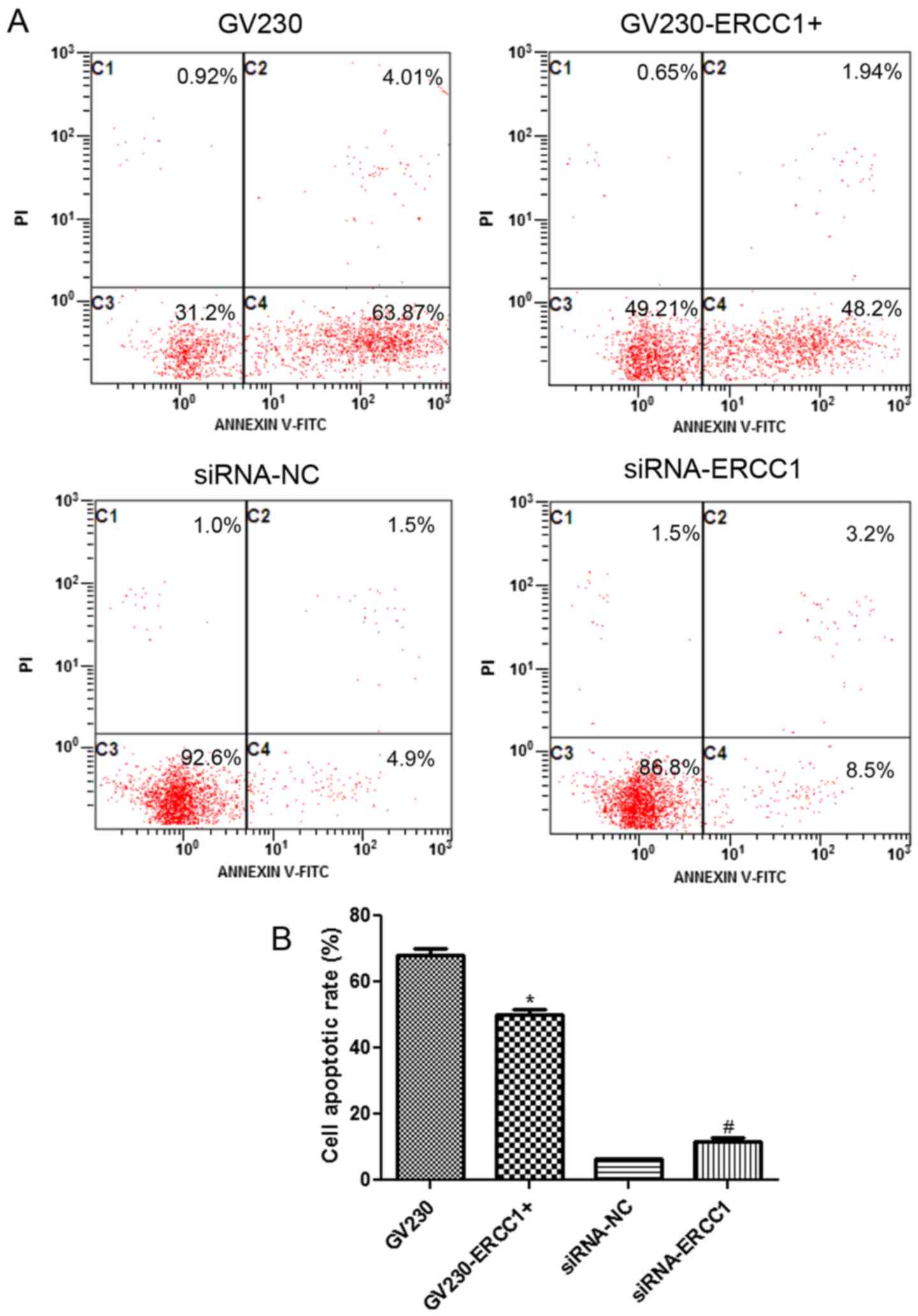
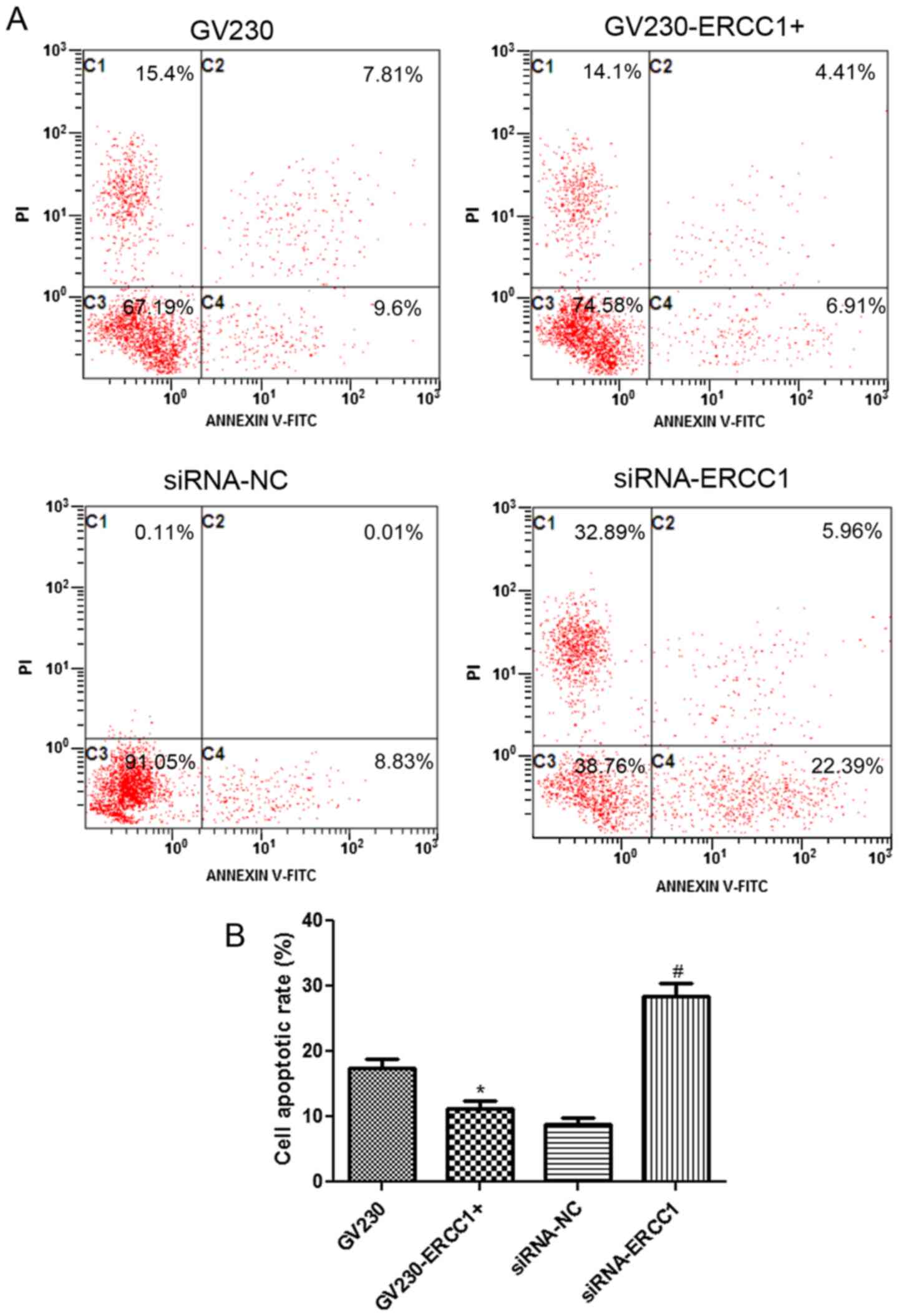
cytometry. The flow cytometry dotplots evaluating the apoptosis of
the NCI-H1299 and SK-MES-1 cell lines are shown in Figs. 5A and 6A, respectively. In the NCI-H1299 cell
line, the cell apoptosis rates in the GV230, GV230-ERCC1+,
siRNA-NC, and siRNA-ERCC1 groups were 67.88±2.1%, 50.14±1.53%,
6.3±0.29% and 11.7±0.98%, respectively (Fig. 5B). These results demonstrated that
after NCI-H1299 cells were treated with 20 µg/ml cisplatin combined
with 50 µg/ml olaparib for 24 h, the cell apoptosis rate
significantly decreased in ERCC1-overexpressing cells
compared with the GV230 group, and markedly increased in
ERCC1-knockdown cells compared with si-NC group (P<0.05;
Fig. 5B). In addition, when the
SK-MES-1 cells were treated with 50 µg/ml cisplatin in combination
with 70 µg/ml olaparib, the trend of cell apoptosis rate in the
SK-MES-1 cell line was similar to that in the NCI-H1299 cell line
(Fig. 6B). In summary, after the
treatment of NSCLC cells with cisplatin and olaparib, cell
apoptosis was inhibited in ERCC1-overexpressing cells, but
enhanced ERCC1-knockdown cells.
Discussion
NSCLC is one of the most common malignant tumors and
is harmful to human health and life (19). Platinum-based chemotherapy is usually
used to assist in the treatment of NSCLC (20). However, clinical treatment is not
always satisfactory due to the progression of drug resistance
(21). Hence, there is an urgent
need to develop new ways to combat drug resistance and improve the
sensitivity of these drugs. The expression of ERCC1
regulates DNA damage induced by platinum drugs and is a candidate
biomarker for predicting the sensitivity of platinum drugs
(22,23). Additionally, olaparib can enhance the
sensitivity of platinum drugs by inhibiting PARP-related pathways
(24).
In the present study, 50 µg/ml olaparib combined
with 20 µg/ml cisplatin significantly inhibited the viability of
NCI-H1299 cells, while 70 µg/ml olaparib combined with 50 µg/ml
cisplatin further inhibited the viability of SK-MES-1 cells. The
findings of the present study indicated that olaparib can enhance
the sensitivity of cisplatin in NSCLC. Ledermann and
Pujade-Lauraine (25) demonstrated
that olaparib increased the sensitivity of platinum-based drugs and
prolonged the progression-free survival time of relapsed ovarian
cancer, which is consistent the results of the present study. The
present study further demonstrated that olaparib combined with
cisplatin can inhibit the viability of NSCLC cell lines.
In the present study, to further explore the
synergistic effects of ERCC1 expression combined with
olaparib on the sensitivity of cisplatin, NSCLC cell lines with
ERCC1 overexpression and knockdown were successfully
constructed. In the NCI-H1299 or SK-MES-1 cells with ERCC1
knockdown, olaparib combined with cisplatin inhibited cell
viability and promoted cell apoptosis. In the present study, the
trends of cell viability and apoptosis in the cells with ERCC1
overexpression were opposite to those in cells with ERCC1
knockdown. The low expression of some genes related to DNA damage
repair, including ERCC1, cyclin dependent kinase 1
(CDK1), serine/threonine protein kinase CHK1 (CHK1),
CHK2 DNA damage checkpoint kinase (CHK2), BRCA1 DNA repair
associated (BRCA1), sperm hammerhead 2 (SH2), ATM
serine/threonine kinase (ATM), RAD51 recombinase
(RAD51), MRE11 homolog, double strand break repair nuclease
(MRE11), phosphatase and tensin homolog (PTEN), X-ray
repair cross complementing 1 (XRCC1), damage specific DNA
binding protein 1 (DDB1), and XPA binding protein 2
(XAB2), can improve the sensitivity of PARP inhibitor
therapy (26–28). A study by Xie et al (29) demonstrated that patients with NSCLC
with low expression of both ERCC1 and PARP1 had the
best prognosis in platinum-based chemotherapy compared to patients
with NSCLC. Based on the findings of the aforementioned and present
studies, it can be hypothesized that ERCC1 expression may
have an effect on the sensitivity of olaparib to cisplatin and
there may be a synergistic effect between PARP inhibitors and
ERCC1 knockdown in NSCLC. Additionally, cisplatin-induced
DNA damage triggers G2/M cell cycle arrest by activating checkpoint
signaling (30). Hence, it can be
hypothesized that olaparib combined with ERCC1 knockdown may
mediate the proliferation and apoptosis of tumor cells by
regulating DNA damage repair. However, this needs to be
investigated in future studies.
In addition, another study indicated that the
predictive effect of ERCC1 expression on cisplatin treatment
may be associated with the histological types of lung cancer
(31). In the present study,
olaparib combined with ERCC1 overexpression or ERCC1
knockdown enhanced the sensitivity of cisplatin in adenocarcinoma
cells (NCI-H1299) and squamous carcinoma cells (SK-MES-1), thereby
regulating cell viability and apoptosis. Pierceall et al
(31) indicated that ERCC1
expression in patients with squamous cell carcinoma displayed
significant predictive value, while in patients with adenocarcinoma
it was not significant. This was somewhat different from the
findings of the present study, possibly due to the joint action of
ERCC1 and other genes related to DNA damage repair. BRCA1
expression is also considered a predictive biomarker for the
sensitivity of platinum drugs and is associated with the expression
of PARP in NSCLC (32,33).
Further studies need to be performed to explore the combined
effects of different genes related to DNA damage repair on the
sensitivity/resistance of platinum drugs.
However, there are some limitations to our study.
The relationship between Olaparib combined with ERCC1 and DNA
damage repair in NSCLC requires further investigation, and all
underlying mechanisms need to be further explored. Additionally,
the combination effects of Olaparib and ERCC1 knockdown
should be further verified in vivo; moreover, further
studies associated with preclinical or clinical models are also
required.
In conclusion, in the present study, olaparib
combined with ERCC1 knockdown enhanced the sensitivity of
cisplatin, which inhibited cell viability and promoted cell
apoptosis in NSCLC cell lines. The findings of the present study
provide evidence for drugs that block ERCC1 function during
the treatment of NSCLC and may assist in the future development of
new therapeutic strategies with olaparib combined with ERCC1
for the treatment of NSCLC.
Acknowledgements
Not applicable.
Funding
This study was supported by a Shaoxing City Science
and Technology Bureau Grant (grant no. 2015B70043).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HH conceived the study. KX performed the
experiments. KX, XN and SL collected and analyzed the data. KX and
HH drafted the manuscript and HH and GZ revised it for important
intellectual content. HH supervised the study. HH and KX confirm
the authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
NER
|
nucleotide excision repair
|
|
ERCC1
|
ERCC excision repair 1 endonuclease
non-catalytic subunit
|
|
PARP1
|
poly(ADP-ribose) polymerase 1
|
|
FBS
|
fetal bovine serum
|
|
NC
|
negative control
|
|
CCK-8
|
Cell Counting Kit-8.
|
References
|
1
|
Bade BC and Dela Cruz CS: Lung Cancer
2020: Epidemiology, Etiology, and Prevention. Clin Chest Med.
41:1–24. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ardizzoni A, Boni L, Tiseo M, Fossella FV,
Schiller JH, Paesmans M, Radosavljevic D, Paccagnella A, Zatloukal
P, Mazzanti P, et al CISCA (CISplatin versus CArboplatin)
Meta-analysis Group, : Cisplatin- versus carboplatin-based
chemotherapy in first-line treatment of advanced non-small-cell
lung cancer: An individual patient data meta-analysis. J Natl
Cancer Inst. 99:847–857. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yin JY, Huang Q, Zhao YC, Zhou HH and Liu
ZQ: Meta-analysis on pharmacogenetics of platinum-based
chemotherapy in non small cell lung cancer (NSCLC) patients. PLoS
One. 7:e381502012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jassem J, Skokowski J, Dziadziuszko R,
Jassem E, Szymanowska A, Rzyman W and Roszkiewicz A: Results of
surgical treatment of non-small cell lung cancer: Validation of the
new postoperative pathologic TNM classification. J Thorac
Cardiovasc Surg. 119:1141–1146. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Choi J, Oh JY, Lee YS, Min KH, Shim JJ,
Choi SI, Park DW, Park CK, Kang EJ, Yong HS, et al: Clinical
efficacy of adjuvant chemotherapy in stage IB (<4 cm) non-small
cell lung cancer patients with high-risk factors. Korean J Intern
Med. Aug 21–2020.(Epub ahead of print). doi: 10.3904/kjim.2020.011.
View Article : Google Scholar
|
|
6
|
Martin LP, Hamilton TC and Schilder RJ:
Platinum resistance: he role of DNA repair pathways. Clin Cancer
Res. 14:1291–1295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Damia G and Broggini M: Platinum
resistance in ovarian cancer: Role of DNA repair. Cancers (Basel).
11:112019. View Article : Google Scholar
|
|
8
|
Lazic A, Popović J, Paunesku T, Woloschak
GE and Stevanović M: Insights into platinum-induced peripheral
neuropathy-current perspective. Neural Regen Res. 15:1623–1630.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang D and Lippard SJ: Cellular processing
of platinum anticancer drugs. Nat Rev Drug Discov. 4:307–320. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kirschner K and Melton DW: Multiple roles
of the ERCC1-XPF endonuclease in DNA repair and resistance to
anticancer drugs. Anticancer Res. 30:3223–3232. 2010.PubMed/NCBI
|
|
11
|
Selvakumaran M, Pisarcik DA, Bao R, Yeung
AT and Hamilton TC: Enhanced cisplatin cytotoxicity by disturbing
the nucleotide excision repair pathway in ovarian cancer cell
lines. Cancer Res. 63:1311–1316. 2003.PubMed/NCBI
|
|
12
|
Chen S, Zhang J, Wang R, Luo X and Chen H:
The platinum-based treatments for advanced non-small cell lung
cancer, is low/negative ERCC1 expression better than high/positive
ERCC1 expression? A meta-analysis. Lung Cancer. 70:63–70. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu G and Lippard SJ: Photoaffinity
labeling reveals nuclear proteins that uniquely recognize
cisplatin-DNA interstrand cross-links. Biochemistry. 48:4916–4925.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gandhi L, Rodríguez-Abreu D, Gadgeel S,
Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ,
Powell SF, et al KEYNOTE-189 investigators, : Pembrolizumab plus
chemotherapy in metastatic non-small-cell lung cancer. N Engl J
Med. 378:2078–2092. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Le DT, Uram JN, Wang H, Bartlett BR,
Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et
al: PD-1 blockade in tumors with mismatch-repair deficiency. N Engl
J Med. 372:2509–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Robson M, Im SA, Senkus E, Xu B, Domchek
SM, Masuda N, Delaloge S, Li W, Tung N, Armstrong A, et al:
Olaparib for metastatic breast cancer in patients with a germline
BRCA mutation. N Engl J Med. 377:523–533. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sahoo S, Meijles DN, Al Ghouleh I, Tandon
M, Cifuentes-Pagano E, Sembrat J, Rojas M, Goncharova E and Pagano
PJ: MEF2C-MYOCD and leiomodin1 suppression by miRNA-214 promotes
smooth muscle cell phenotype switching inpulmonary arterial
Hypertension. PLoS One. 11:e01537802016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Patel AJ, Richter A, Drayson MT and
Middleton GW: The role of B lymphocytes in the immuno-biology of
non-small-cell lung cancer. Cancer Immunol Immunother. 69:325–342.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ferrara MG, Di Noia V, D'Argento E, Vita
E, Damiano P, Cannella A, Ribelli M, Pilotto S, Milella M, Tortora
G, et al: Oncogene-addicted non-small-cell lung cancer: Treatment
opportunities and future perspectives. Cancers (Basel). 12:122020.
View Article : Google Scholar
|
|
21
|
Zhou J, Kang Y, Chen L, Wang H, Liu J,
Zeng S and Yu L: The drug-resistance mechanisms of five
platinum-based antitumor agents. Front Pharmacol. 11:3432020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mesquita KA, Alabdullah M, Griffin M, Toss
MS, Fatah TM, Alblihy A, Moseley P, Chan SY, Rakha EA and
Madhusudan S: ERCC1-XPF deficiency is a predictor of olaparib
induced synthetic lethality and platinum sensitivity in epithelial
ovarian cancers. Gynecol Oncol. 153:416–424. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Postel-Vinay S and Soria JC: ERCC1 as
predictor of platinum benefit in non-small-cell lung cancer. J Clin
Oncol. 35:384–386. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oza AM, Cibula D, Benzaquen AO, Poole C,
Mathijssen RH, Sonke GS, Colombo N, Špaček J, Vuylsteke P, Hirte H,
et al: Olaparib combined with chemotherapy for recurrent
platinum-sensitive ovarian cancer: A randomised phase 2 trial.
Lancet Oncol. 16:87–97. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ledermann JA and Pujade-Lauraine E:
Olaparib as maintenance treatment for patients with
platinum-sensitive relapsed ovarian cancer. Ther Adv Med Oncol. May
22–2019.(Epub ahead of print). doi: 10.1177/1758835919849753.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Johnson N, Li YC, Walton ZE, Cheng KA, Li
D, Rodig SJ, Moreau LA, Unitt C, Bronson RT, Thomas HD, et al:
Compromised CDK1 activity sensitizes BRCA-proficient cancers to
PARP inhibition. Nat Med. 17:875–882. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vilar E, Bartnik CM, Stenzel SL, Raskin L,
Ahn J, Moreno V, Mukherjee B, Iniesta MD, Morgan MA, Rennert G, et
al: MRE11 deficiency increases sensitivity to poly(ADP-ribose)
polymerase inhibition in microsatellite unstable colorectal
cancers. Cancer Res. 71:2632–2642. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Weil MK and Chen AP: PARP inhibitor
treatment in ovarian and breast cancer. Curr Probl Cancer. 35:7–50.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xie KJ, He HE, Sun AJ, Liu XB, Sun LP and
Dong XJ: Expression of ERCC1, MSH2 and PARP1 in non-small cell lung
cancer and prognostic value in patients treated with platinum-based
chemotherapy. Asian Pac J Cancer Prev. 15:2591–2596. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roos WP and Kaina B: DNA damage-induced
cell death: From specific DNA lesions to the DNA damage response
and apoptosis. Cancer Lett. 332:237–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pierceall WE, Olaussen KA, Rousseau V,
Brambilla E, Sprott KM, Andre F, Pignon JP, Le Chevalier T, Pirker
R, Jiang C, et al: Cisplatin benefit is predicted by
immunohistochemical analysis of DNA repair proteins in squamous
cell carcinoma but not adenocarcinoma: Theranostic modeling by
NSCLC constituent histological subclasses. Ann Oncol. 23:2245–2252.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo ZP, Hu YC, Xie Y, Jin F, Song ZQ, Liu
XD, Ma T and Zhou PK: MLN4924 suppresses the BRCA1 complex and
synergizes with PARP inhibition in NSCLC cells. Biochem Biophys Res
Commun. 483:223–229. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Paul I, Savage KI, Blayney JK, Lamers E,
Gately K, Kerr K, Sheaff M, Arthur K, Richard DJ, Hamilton PW, et
al: PARP inhibition induces BAX/BAK-independent synthetic lethality
of BRCA1-deficient non-small cell lung cancer. J Pathol.
224:564–574. 2011. View Article : Google Scholar : PubMed/NCBI
|