Introduction
Breast cancer is an intractable type of cancer that
poses a major threat to the physical and mental wellbeing of women
(1), and its incidence rate is
increasing annually worldwide. Advanced diagnostic methods and
comprehensive treatment contribute to effectively improving the
prognosis of patients with early-stage breast cancer (2). Moreover, a significant proportion of
patients are treated with docetaxel chemotherapy. Docetaxel, a
cytotoxic drug that belongs to the taxane family of anticancer
agents, is employed for the treatment of various cancers, including
breast cancer, and acts via interfering in tubulin synthesis
(3). Patients with advanced breast
cancer receiving long- term chemotherapy may develop resistance to
the initial anticancer drugs, which results in treatment failure,
tumor recurrence and metastasis, and increased mortality risk
(4,5). Therefore, an improved understanding of
the regulatory mechanisms underlying breast cancer progression and
the identification of novel effective therapeutic strategies are
urgently required to improve the prognosis.
MicroRNAs (miRNAs/miRs) are a class of RNAs in
eukaryotes that are 18–25 nt in length, lack protein-coding
ability, and have been attracting increasing interest in the field
of cancer research. miRNAs are involved in the post-transcriptional
regulation of numerous human genes via negative regulation of
target gene expression, promotion of target mRNA cleavage and
suppression of translation of proteins that may serve as direct
regulators in biological processes (6). Previous studies have reported a strong
association between miRNAs and numerous biological processes, such
as inflammation, stress response, cell cycle regulation, cell
proliferation, differentiation, invasion and drug resistance
(7–11). miR-26a has been reported to act as a
cancer promoter in some carcinomas, and to suppress tumor
occurrence and development in other types of cancer (12). A recent study revealed that miR-26a
expression is downregulated in breast cancer and is considered to
act as a tumor suppressor (13).
However, the underlying mechanisms through which miRNAs regulate
the cancer development process require further investigation.
Several studies have reported that the abnormal
expression of family with sequence similarity 98 member A (FAM98A)
is associated with multiple types of cancer, including ovarian,
endometrial, colorectal and lung cancer (14–17).
Akter et al (14) revealed
that FAM98A, which is arginine-methylated by protein arginine
methyltransferase 1 (PRMT1), was highly expressed in ovarian cancer
cell lines, and found that the knockdown of FAM98A reduced ovarian
cancer cell migration, invasion and colony formation. Another study
conducted by Akter et al demonstrated that FAM98A and
FAM98B, a new complex binding to DDX1 and C14orf166, are required
for PRMT1 expression. FAM98A and PRMT1, which are highly expressed
in colorectal cancer tissues, act as tumor promoters and facilitate
cancer occurrence and progression (15). A recent study revealed that FAM98A
promoted cancer cell survival and progression of endometrial
carcinoma, whereas its overexpression was associated with poor
prognosis (16). Moreover, another
study identified an association between the presence of FAM98A and
advanced lung cancer. FAM98A was shown to act as an oncogene by
activating the P38/activating transcription factor 2 signaling
pathway to promote lung cancer cell proliferation and colony
formation (17).
The present study was undertaken to investigate the
role of miR-26a in breast cancer by examining its expression and
detecting its effect on cell proliferation, colony formation,
migration and invasion. Furthermore, the association between
miR-26a and FAM98A was elucidated using bioinformatics analysis,
luciferase reporter assay and western blotting.
Materials and methods
Patients and specimens
A total of 13 pairs of breast cancer and matched
non-cancerous breast tissues were obtained from female patients,
aged 30–73 years, undergoing surgery for breast cancer at The First
Affiliated Hospital of University of Science and Technology of
China (Hefei, China) between September 2019 and August 2020.
Corresponding non-cancerous breast tissues at a distance of >5
cm from the edge of the tumor were collected. These tissue
specimens were freshly frozen in liquid nitrogen and stored at
−80°C. Written informed consent was obtained from the patients, and
the study was approved by the Ethics Committee of The First
Affiliated Hospital of University of Science and Technology of
China (approval no. 2019-ky086). The clinicopathological
characteristics of the patients are summarized in Table I.
 | Table I.Clinicopathological characteristics
of patients with breast cancer (n=13). |
Table I.
Clinicopathological characteristics
of patients with breast cancer (n=13).
|
Characteristics | No. of
patients |
|---|
| Age (years) |
|
|
≤50 | 7 |
|
>50 | 6 |
| Tumor size
(cm) |
|
| ≤2 | 5 |
|
>2 | 8 |
| Lymph node
status |
|
|
Negative | 5 |
|
Positive | 8 |
| Pathological
stage |
|
|
I–II | 8 |
|
III–IV | 5 |
| Estrogen receptor
status |
|
|
Negative | 6 |
|
Positive | 7 |
| Progesterone
receptor status |
|
|
Negative | 8 |
|
Positive | 5 |
| Human epidermal
growth factor receptor-2 status |
|
|
Negative | 8 |
|
Positive | 5 |
| Ki-67 |
|
|
≤15% | 0 |
|
>15% | 13 |
Cell lines and cell culture
The human breast cancer cell lines SK-BR-3, BT474,
MDA-MB-231, MDA-MB-468 and MCF-7, and the non-tumorigenic
epithelial cell line MCF-10A were obtained from the American Type
Culture Collection. Cells were grown in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) and 100 U/ml penicillin and
streptomycin (Gibco; Thermo Fisher Scientific, Inc.) in a
humidified incubator at 37°C with 5% CO2.
Cell transfection
Briefly, 1 day prior to transfection, MCF-7 and
MDA-MB-231 cells (1×105 cells/well) were seeded into
6-well plates with complete growth medium and no antibiotics. The
cells were then transfected with miR-26a mimic and negative control
(NC) (Shanghai GenePharma Co., Ltd.) for 24 h at room temperature
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
concentration used for miR-26a mimic was 100 nM. The sequences of
the miR-26a mimics and NC used were as follows: miR-26a:
5′-UUCAAGUAAUCCAGGAUAGGCU-3′/5′-CCUAUCCUGGAUUACUUGAAUU-3′ and NC:
5′-UUCUCCGAACGUGUCACGUTT-3′/5′-ACGUGACACGUUCGGAGAATT-3′. RT-qPCR
was conducted to verify whether the transfection of miR-26a mimic
significantly increased miR-26a expression in MCF-7 and MDA-MB-231
cells. Subsequent experiments were carried out 24–72 h after
transfection.
Total RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR) analysis
Total RNA was extracted from the tissue samples and
cell lines using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) and was quantified using an ultraviolet
spectrophotometer (NanoDrop 2000; Thermo Fisher Scientific, Inc.).
cDNA was synthesized using a Reverse Transcription kit (Shanghai
GenePharma Co., Ltd.) according to the manufacturer's protocol. All
PCR reagents and primers were designed and synthesized by Shanghai
GenePharma Co., Ltd. cDNAs were used as templates in a 20-µl PCR
reaction using a LightCycler480II system (Roche Diagnostics). The
reaction mixture was as follows: cDNA (2 µl), miRNA/U6 snRNA
specific primer set (0.4 µl), 5 U/µl Taq DNA polymerase (0.2 µl),
2X Real-time PCR Master Mix (containing SYBR Green) (10 µl) and
sterilized H2O (7.4 µl). U6 was used as an internal
reference. The primers used were as follows: miR-26a forward,
5′-CTCCTCGCTTCAAGTAATCCAG-3′, and reverse,
5′-TATGCTTGTTCTCGTCTCTGTGTC-3′; and U6 forward,
5′-CAGCACATATACTAAAATTGGAACG-3′ and reverse,
5′-ACGAATTTGCGTGTCATCC-3′. The thermocycling conditions were as
follows: Initial denaturation at 95°C for 3 min, 40 cycles at 95°C
for 12 sec and 62°C for 40 sec. The results were quantified using
the 2−ΔΔCq method (18).
Western blot analysis
Total protein was extracted from MCF-7 and
MDA-MB-231 cells transfected with miR-26a mimic and miR-NC,
respectively using RIPA lysis buffer (Beyotime Institute of
Biotechnology Co., Ltd.) and the concentration of total protein was
determined with the BCA protein assay kit (Pierce; Thermo Fisher
Scientific, Inc.). Protein samples (25 µg) were separated via 10%
SDS-PAGE and blotted onto PVDF membranes (EMD Millipore). The
membranes were blocked with 5% non-fat milk for 1 h at room
temperature and incubated with primary antibodies against FAM98A
(dilution 1:500; ab204083), sonic hedgehog (Hh) signaling molecule
(SHH; dilution 1:1,000; ab53281), smoothened, frizzled class
receptor (SMO; dilution 1:1,000; ab124964), GLI family zinc finger
1 (GLI1; dilution 1:1,000; ab134906) and GAPDH (dilution 1:2,000;
ab8245) (all from Abcam) overnight at 4°C. The membranes were
subsequently incubated with horseradish peroxidase (HRP)-conjugated
secondary antibody (dilution 1:2,000; cat. nos. 58802 and 93702;
Cell Signaling Technology, Inc.) at room temperature for 1 h. The
western blots were visualized with enhanced chemiluminescence
reagents (EMD Millipore).
Tumor sphere formation assay
At 24 h post-transfection, MCF-7 and MDA-MB-231
cells were seeded in 6-well plates at a density of 2,000 cells/well
with ultra-low attachment surface polystyrene (Corning, Inc.).
Cells were cultured in DMEM-F12 (BioWhittaker), supplemented with
B27 (1:50, Invitrogen; Thermo Fisher Scientific, Inc.), 20 ng/m
basic fibroblast growth factor and 20 ng/ml epidermal growth factor
(BD Biosciences). After incubation for 4 and 9 days in a 5%
CO2 incubator, the number of tumor spheres was counted
and images were captured using a light microscope (Olympus
Corporation; magnification, ×100).
Cell proliferation assay
A Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc.) assay was conducted for cell viability and drug
toxicity analysis according to the manufacturer's protocol., MCF-7
and MDA-MB-231 cells were seeded into 96-well microplates at a
density of 3,000 cells per well with or without different
concentrations (0.1, 0.5, 1.0 or 1.5 µM) of docetaxel 24 h after
transfection. After the cells had been incubated at 37°C for 24,
48, 72 and 96 h, 10 µl CCK-8 solution was added into each well and
incubated at 37°C for 2 h in an incubator. Cell proliferation was
detected on an INFINITE 200 Promultimode reader (Tecan Group, Ltd.)
with the optical density measured at 450 nm.
Colony formation assay
At 24 h post-transfection, cells in the logarithmic
growth phase were collected and seeded in 6-well plates (500 cells
per well). After incubation for 2 weeks at 37°C in a 5%
CO2 incubator, the medium was discarded. The cells were
fixed in 4% paraformaldehyde for 15 min at room temperature after
being rinsed twice with PBS, and were then stained with 0.1%
crystal violet solution for 30 min at room temperature. The number
of cells per clone (>50 cells) was counted under a light
microscope (Olympus Corporation; magnification, ×40).
Cell migration and invasion assay
MCF-7 and MDA-MB-231 cells were seeded in a 6-well
plate at a density of 4×105 cells/well and were cultured
overnight. After transfection, when the cell confluence reached
~90%, a 200-µl pipette was used to scratch the wells. Then, the
cells were rinsed twice with PBS and cultured with serum-free
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.). At 0 and
48 h after scratching, the wound healing was imaged using an
inverted fluorescence microscope (Olympus Corporation;
magnification, ×10).
The Transwell assay was conducted using Transwell
inserts in 24-well plates (pore size, 8 µm; Corning, Inc.). The
upper surface of the membrane was pre-coated with Matrigel (BD
Biosciences) at 37°C for 30 min. A total of 1×105 cells
suspended in RPMI-1640 medium without FBS were seeded into the
upper chamber, and the lower chamber was filled with cell medium
supplemented with 10% FBS. After incubation for 48 h, the cells
invading in the lower chamber were fixed in 4% paraformaldehyde for
15 min at room temperature and stained with 0.1% crystal violet
solution for 30 min at room temperature. Then, invading cells were
counted and imaged using an inverted fluorescence microscope
(Olympus Corporation; magnification, ×100).
Luciferase reporter assay
The target binding sites of miR-26a in FAM98A 3′-UTR
were predicted using TargetScanHuman 7.2 (http://www.targetscan.org). The FAM98A 3′-UTR
containing miR-26a sequences binding sites was amplified and cloned
into the pGL3 Basic vector (Promega Corporation). Then, pGL3-FAM98A
3′-UTR-wild-type (WT) and pGL3-FAM98A 3′-UTR-mutant (MT) (100 ng)
were co-transfected with miR-NC or miR-26a mimic (100 nM) into
MCF-7 and MDA-MB-231 cells using Lipofectamine® 2000
according to the manufacturer's protocol. After transfection for 48
h, a Dual-Luciferase Reporter Assay system (Promega Corporation)
was used to detect luciferase activity. Renilla luciferase
activity was used for normalization.
Statistical analysis
Data management and analysis were performed using
SPSS 23.0 (IBM, Corp.). P<0.05 was considered to indicate a
statistically significant difference. Two-tailed unpaired Student's
t-test was used to analyze the association between two independent
groups. Statistically significant differences among multiple groups
were determined using one-way ANOVA followed by Tukey's post hoc
test. To assess differences in cell proliferation curves,
repeated-measures ANOVAs was used. All cell biological assays were
evaluated in triplicate.
Results
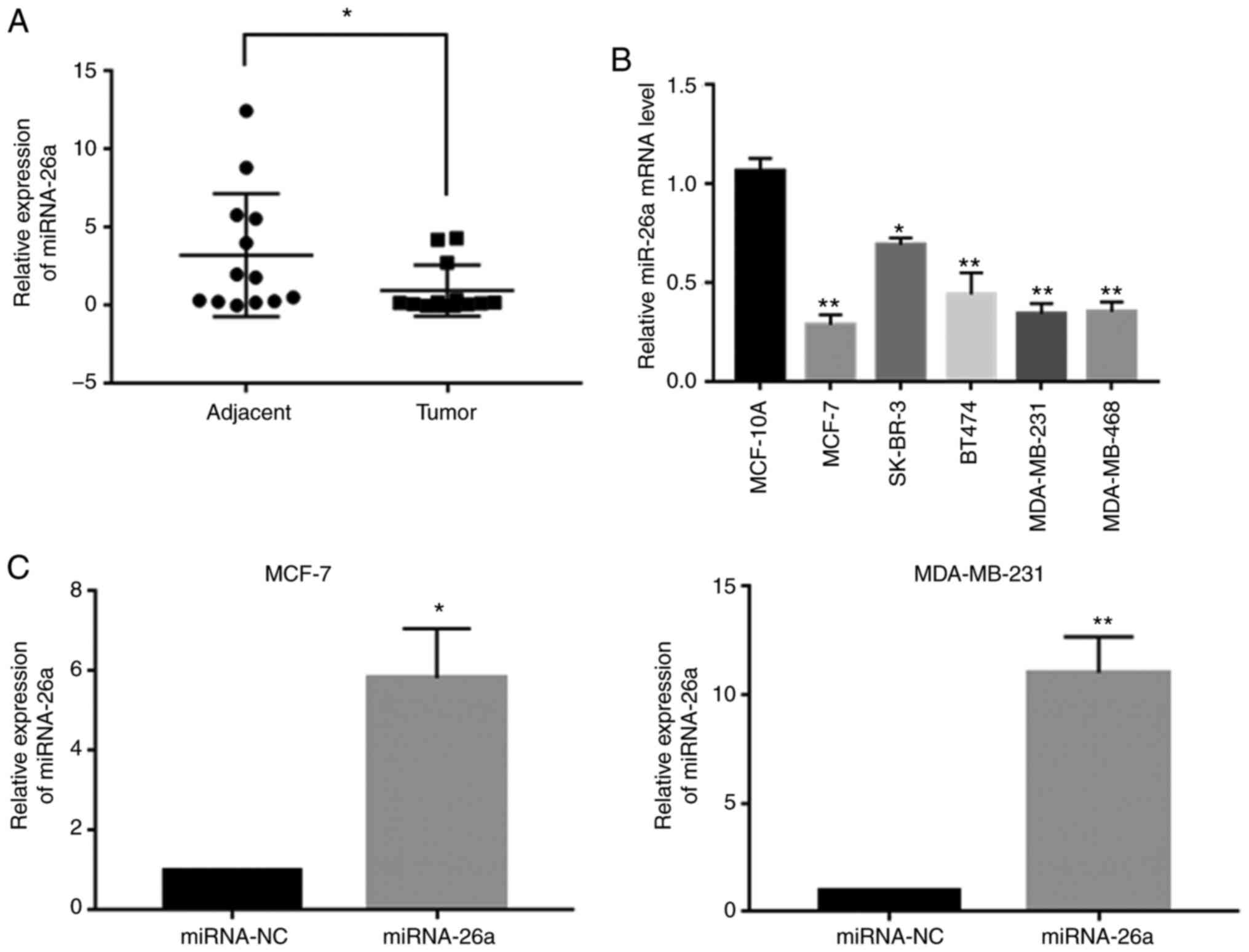
miR-26a is downregulated in human
breast cancer tissues and cell lines
RT-qPCR analysis was conducted to determine the
differential expression of miR-26a in 13 pairs of clinical tissue
specimens. The results indicated that the relative mRNA expression
level of miR-26a was decreased in cancer tissues compared with that
in the adjacent non-cancerous tissues (Fig. 1A). Moreover, further experiments
demonstrated that miR-26a expression was upregulated in MCF-10A
cells and was downregulated in all breast carcinoma cell lines
(MDA-MB-468, MDA-MB-231, BT474, SK-BR-3 and MCF-7), particularly in
MCF-7 and MDA-MB-231 cells (Fig.
1B). The results indicated that the expression level of miR-26a
may be associated with breast carcinogenesis and metastasis.
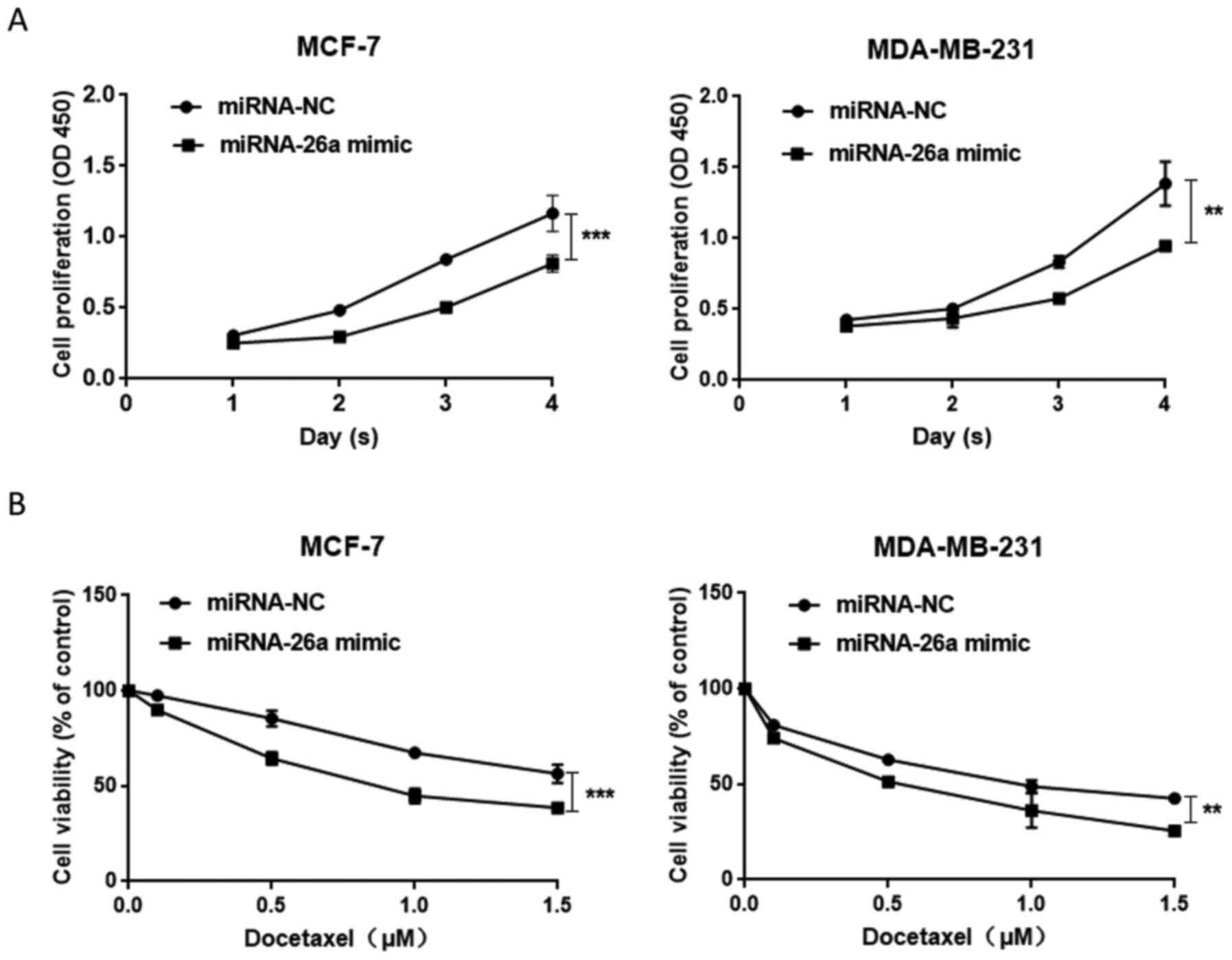
Overexpression of miR-26a inhibits
cell proliferation and colony formation in breast cancer and
enhances the sensitivity of cancer cells to docetaxel
In the present study, it was observed that miR-26a
expression was significantly decreased in the MCF-7 and MDA-MB-231
cell lines (Fig. 1B). Therefore,
these two cell lines were used to examine the role of miR-26a in
breast cancer cells. Subsequently, miR-26a mimic and miR-NC were
transfected into the breast cancer cells to determine whether
miR-26a affected cell proliferation and colony formation. The
transfection of miR-26a mimic significantly increased miR-26a
expression compared with that observed in the miR-NC group
(Fig. 1C). Proliferation assay
results suggested that the proliferation of cells transfected with
miR-26a mimic was decreased (Fig.
2A) and the overexpression of miR-26a sensitized carcinoma
cells to docetaxel (Fig. 2B).
Furthermore, sphere formation assay was performed to evaluate
weather miR-26a modified the ability of the cell lines to grow in
suspension as tumor spheres. However, there was no significant
difference compared with the control group (data not shown).
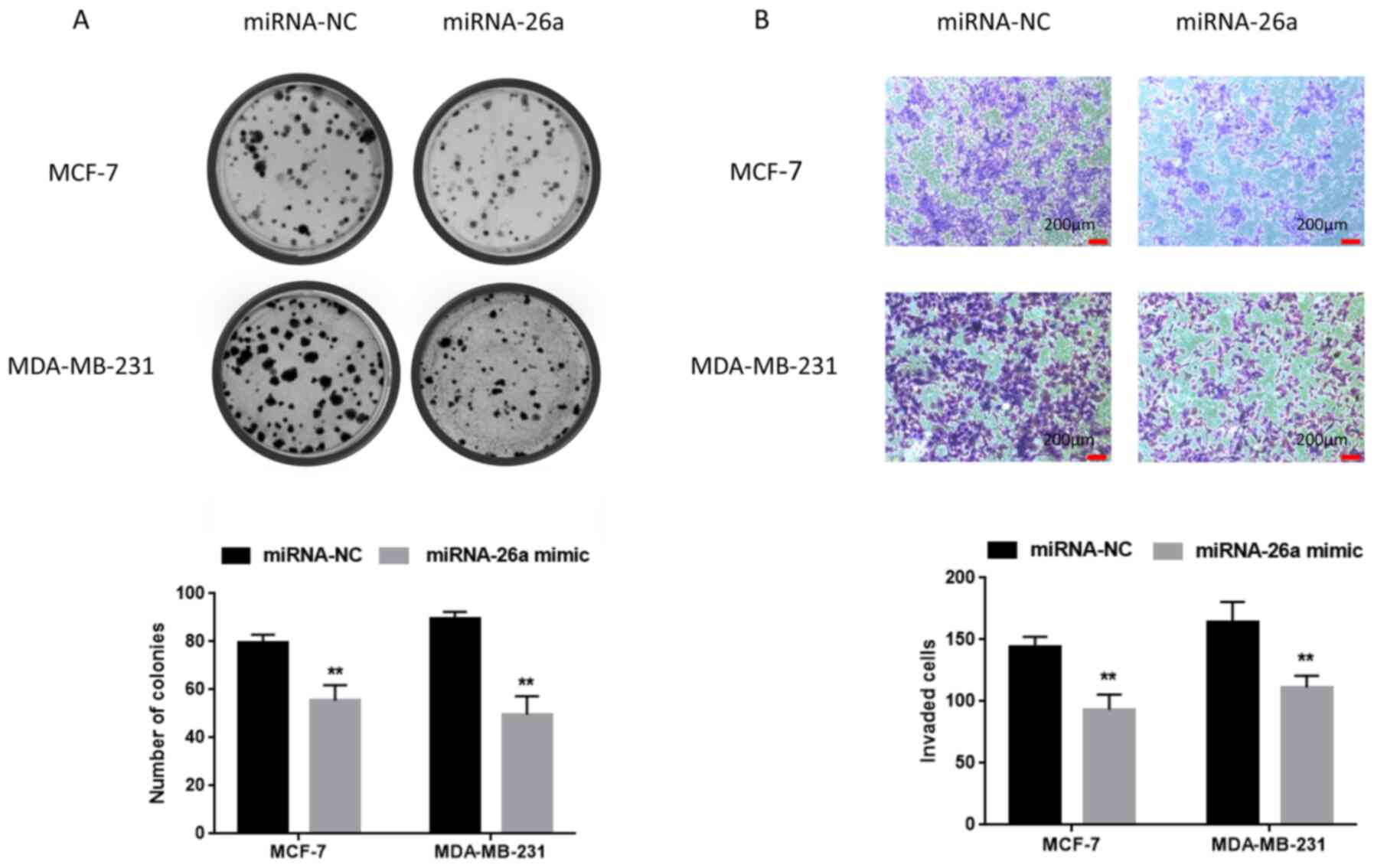
Moreover, the effect of miR-26a on the colony formation ability of
cancer cells was examined, and it was found that the number of the
colonies in the miR-26a mimic group was lower compared with that in
the miR-NC group (Fig. 3A).
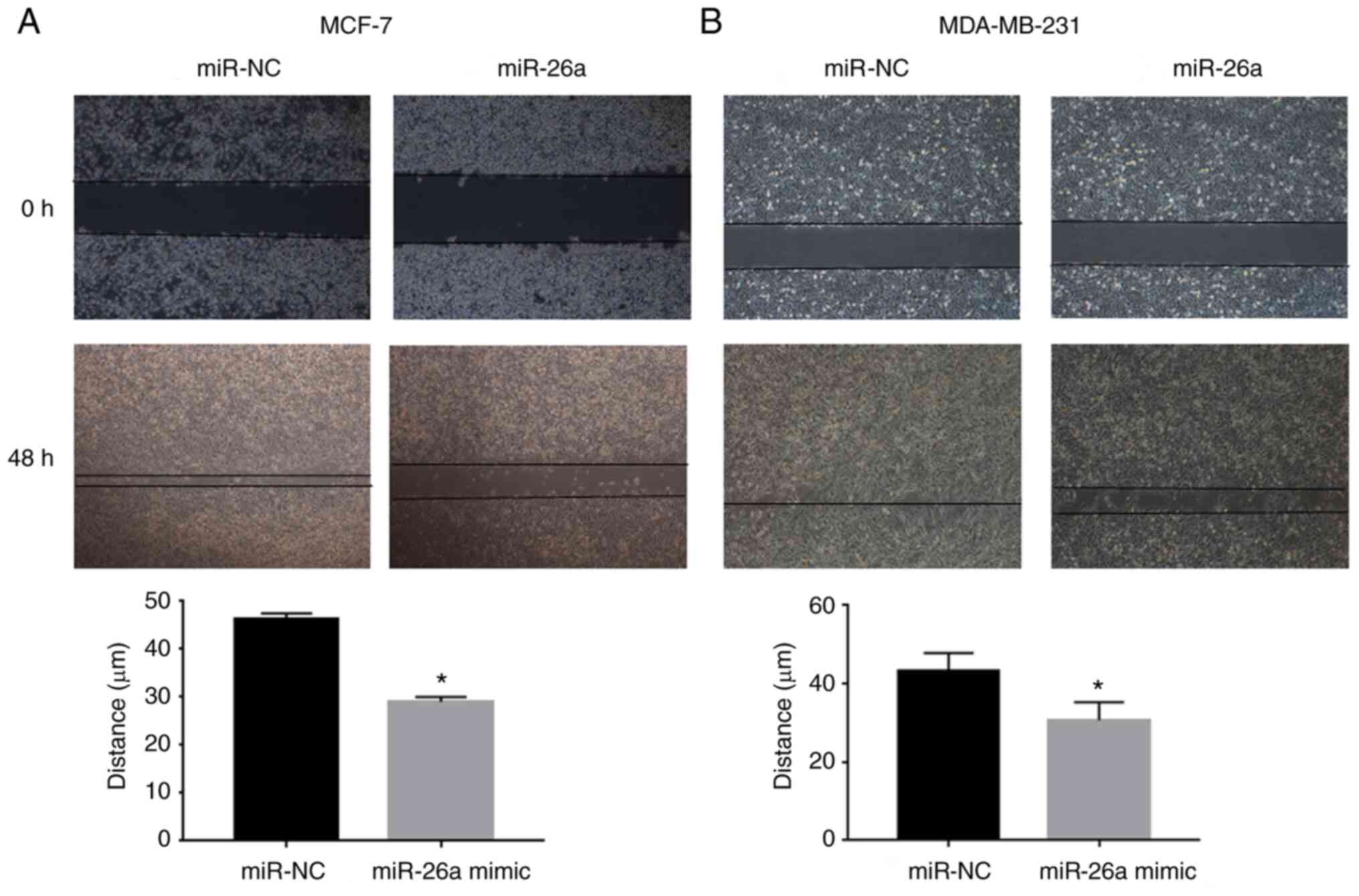
Overexpression of miR-26a suppresses
cell migration and invasion
To determine the effects of miR-26a on cell
migration and invasion, a series of transfections were performed.
Transwell assay was used to assess cell invasion. As shown in
Fig. 3B, the number of invading
cells among breast cancer cells transfected with miR-26a mimic was
lower compared with that of control cells. Moreover, cell migration
was examined, and the results suggested that the migration of
miR-26a mimic-transfected cells was markedly suppressed compared
with that of the NC group (Fig. 4A).
Collectively, these results indicated that miR-26a may attenuate
breast carcinogenesis and progression.
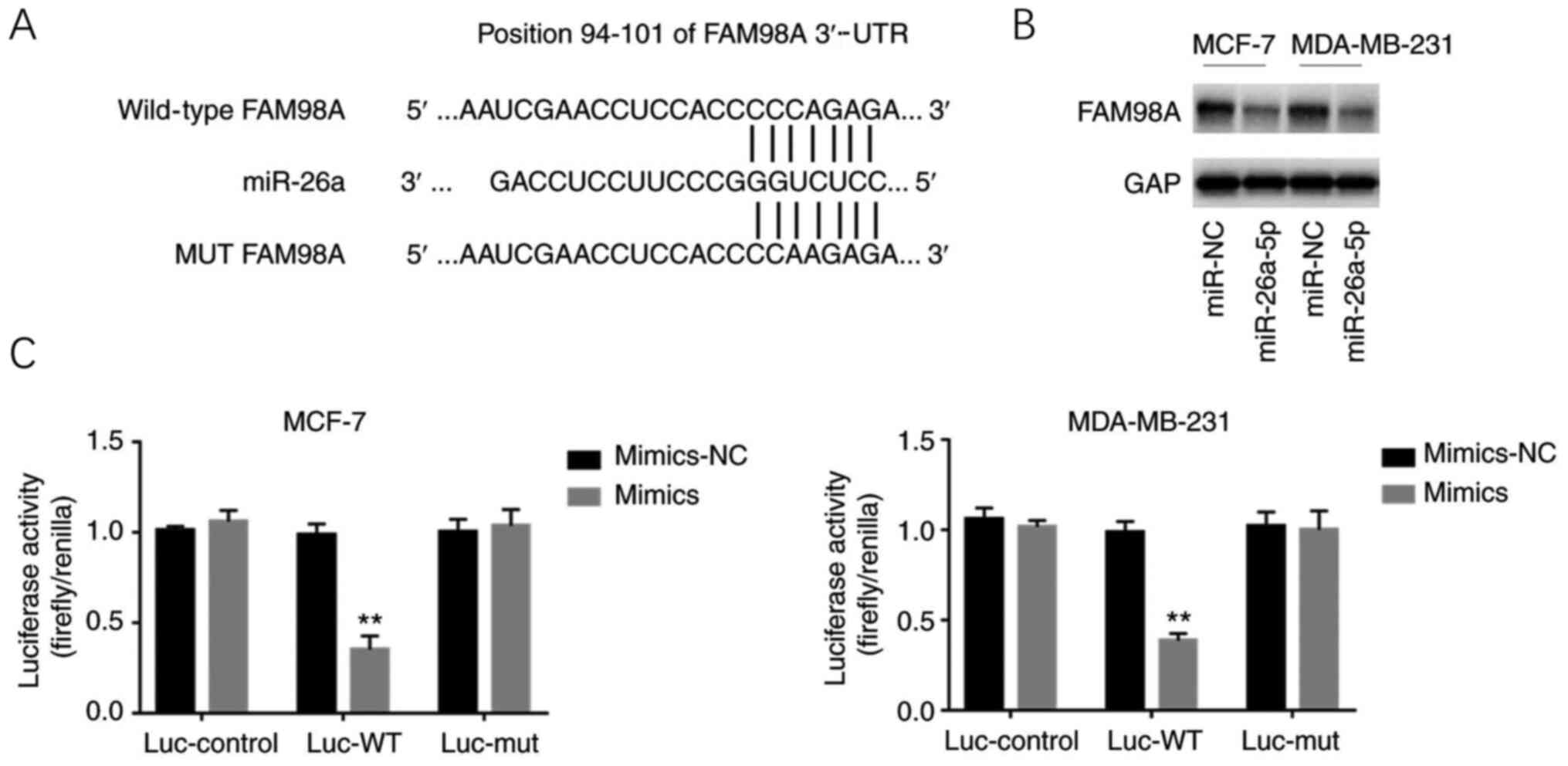
miR-26a directly targets FAM98A in
breast cancer
In order to understand how miR-26a regulates
tumorigenesis and cancer progression after identifying the
functional role of miR-26a, bioinformatics analysis (TargetScan
algorithm) was utilized to identify the putative target of miR-26a.
It was found that miR-26a could directly target FAM98A (Fig. 5A). A luciferase reporter assay was
then conducted to test whether miR-26a binds to the 3′-UTR of
FAM98A. The results suggested that miR-26a significantly repressed
the relative luciferase activity of the WT 3′-UTR of FAM98A,
whereas no significant difference in the luciferase activity of the
MT 3′-UTR of FAM98A was detected (Fig.
5C). Furthermore, western blotting was performed to verify how
miR-26a regulated FAM98A, and the results indicated that the
expression level of the FAM98A protein was decreased in
miR-26a-overexpressing cells (Fig.
5B).
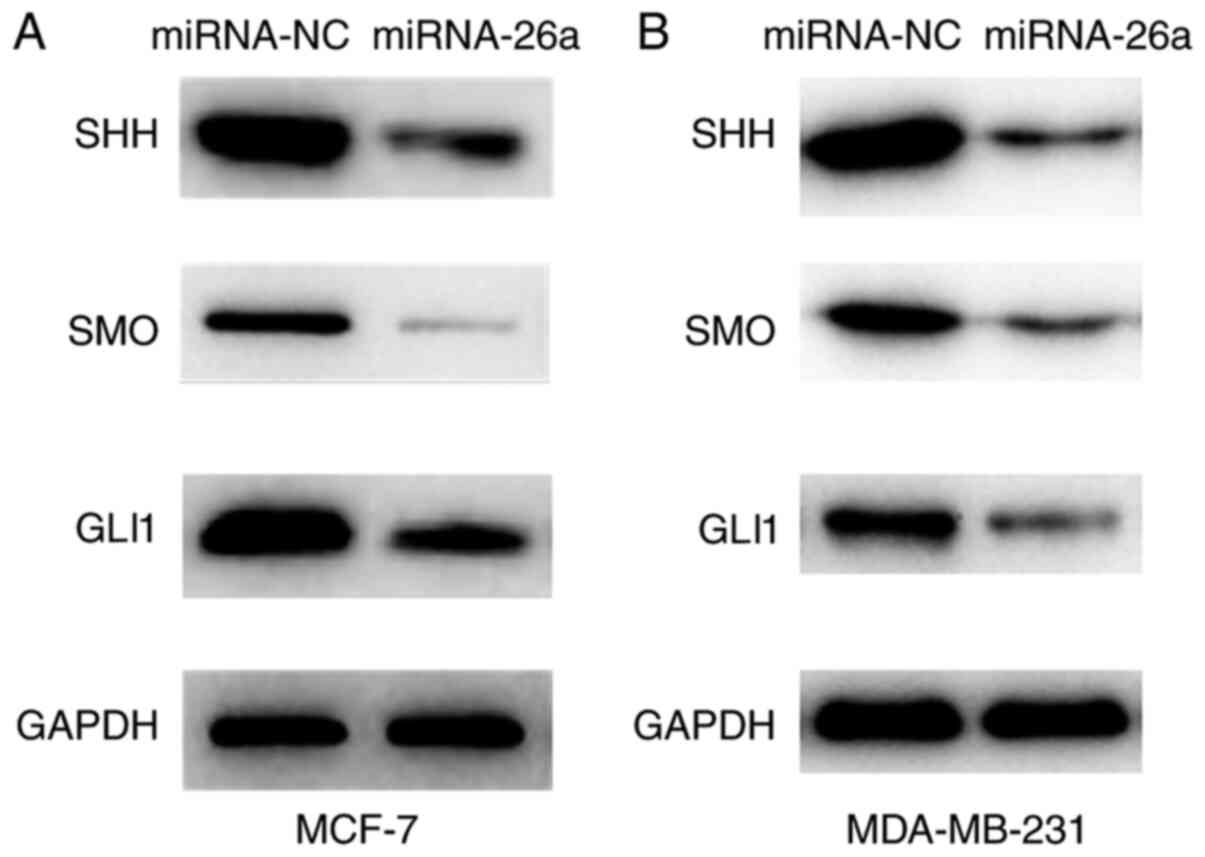
miR-26a suppresses the Hh signaling
pathway
As previously stated, miR-26a attenuated the
malignant characteristics and enhanced the sensitivity of cancer
cells to docetaxel. It was found that miR-26a targeted FAM98A and
downregulated FAM98A expression. Previous studies have reported
that the dysregulation of the Hh pathway may promote cancer cell
survival and invasion, and is associated with drug resistance
(19,20). Thus, it was investigated whether
miR-26a may exert its effects via aberrant activation of the Hh
pathway. The western blot analysis demonstrated that the expression
levels of SHH, SMO and GlI1 were significantly decreased in
miR-26a-overexpressing cells (Fig. 6A
and B).
Discussion
The dysregulated expression of miRNAs is generally
considered as a factor closely associated with oncogenesis, tumor
development and the resistance to radiotherapy and chemotherapy in
various types of cancer (21,22).
miRNA expression levels in malignant tumors may be increased or
decreased compared with those in normal tissues (6). Moreover, the differential expression of
miRNAs may play a key role in cancer by regulating the expression
levels of the encoded oncogenes or tumor suppressor genes (23). One miRNA may regulate multiple target
genes that are involved in the regulation of the biological
processes of different carcinomas. Thus, miRNAs may be valuable as
novel biological treatment targets (24,25).
The present study demonstrated that miR-26a
expression was downregulated in breast cancer tissues compared with
that in corresponding non-cancerous breast tissues. These results
were in accordance with previous research, which indicated that
miR-26a is a tumor suppressor in breast cancer (13). Previous studies have revealed that
miR-26a exerted antitumor effects in other malignancies. For
example, Li et al (26)
revealed that the increase in miR-26a expression induced cell
apoptosis and inhibited cell proliferation and invasion by
downregulating the expression level of Wnt5a. It was also found
that F-box protein 11 (FBXO11) was a downstream target regulator of
miR-26a and was upregulated in hepatocellular carcinoma (HCC)
cells. miR-26a overexpression exerts a suppressive effect on HCC
cell proliferation, migration and invasion via the negative
regulation of FBXO11 (27). Guo
et al (28) reported that
miR-26a was downregulated and SERBP1 was upregulated in prostate
cancer, they suppressed prostate cancer cell proliferation and
motility, and may represent novel prognostic biomarkers.
In the present research, in order to further assess
whether miR-26a contributes to breast cancer cell proliferation and
progression, miR-26a was overexpressed via transfection with
miR-26a mimics. The overexpression of miR-26a markedly decreased
the proliferation, clone formation ability, migration and invasion
of cancer cells. Moreover, the results of the present study
suggested that miR-26a overexpression may be a potential strategy
for reversing docetaxel resistance in patients with breast
cancer.
The present study also examined the mechanism
through which miR-26a regulates its downstream targets. TargetScan
analysis was used to identify the targeting association between
miR-26a and FAM98A. As expected, the overexpression of miR-26a
inhibited the luciferase activity of WT 3′-UTR of FAM98A, while no
changes were found in the luciferase activity of MT 3′-UTR of
FAM98A. The results indicated that FAM98A was a direct downstream
target of miR-26a. The western blot analysis revealed that FAM98A
protein expression was significantly downregulated in response to
aberrant overexpression of miR-26a. Therefore, FAM98A was selected
to be further studied as a novel target of miR-26a. The results
suggested that miR-26a may act as a tumor suppressor by regulating
the expression of FAM98A. However, little is currently known
regarding FAM98A. FAM98A is a new substrate and is methylated by
PRMT1, the expression level of which was shown to be associated
with the malignancy of cancer cells (14). PRMTs, which are abnormally expressed
in numerous malignancies, have been associated with the progression
of various types of cancer (15).
The Hh signaling pathway is important for mammalian
embryonic development, and its main components include SHH, desert
hedgehog signaling molecule, Indian hedgehog signaling molecule,
patched 1, Smo, GLI1 and GLI2 (29).
Accumulating evidence has indicated that abnormal activation of the
Hh pathway results in tumor cell survival, proliferation, stem cell
maintenance and chemotherapy resistance (30). It has also been reported that
multiple PRMTs (PRMT1, PRMT5 and PRMT7) regulate the activity of
GLI, which is the downstream effector of the Hh pathway in cancer
cells (31). Wang et al
(32) observed that GLI1 methylation
was mediated by PRMT1, and that the removal of GLI1 methylation
significantly reduces GLI1-related carcinogenic functions and
attenuates gemcitabine resistance in pancreatic cancer cells. The
present study identified that miR-26a downregulated the expression
level of FAM98A, and it also suppressed the expression of SHH, SMO
and GLI1. Therefore, these results suggested that miR-26a may
inhibit the malignancy of cancer cells and reverse the resistance
to chemotherapy drugs via the depletion of FAM98A expression and
the inactivation of the Hh signaling pathway. However, several
questions remain unanswered. In the present study, it was confirmed
that miR-26a targeted FAM98A mRNA and inhibited FAM98A protein
expression; however, it remains unclear how FAM98A affects the
proliferation, colony formation, migration and invasion of breast
cancer cells. Then, it was further demonstrated that miR-26a
suppressed the expression of SHH, SMO and GLI1, but it remains
unknown whether miR-26a expression is associated with
chemosensitivity. Therefore, further functional experiments should
be conducted in the future to validate the present findings.
In conclusion, the expression miR-26a was found to
be decreased in breast cancer cells and tissues in the present
study, which in turn decreased cancer cell proliferation, clone
formation ability, invasion and migration, and improved the
sensitivity of cancer cells to docetaxel. It was also observed that
miR-26 targeted FAM98A and downregulated its expression.
Furthermore, it was demonstrated that miR-26 inhibited the Hh
signaling pathway. Thus, it may be inferred that miR-26a
downregulates the expression of FAM98A and inactivates the Hh
signaling pathway to function as a tumor suppressor in breast
cancer by inhibiting cell proliferation and cancer progression.
However, further in-depth research is required in order to further
elucidate the underlying mechanisms.
Acknowledgements
Not applicable.
Funding
The present study was supported by the New Medicine
Foundation of University of Science and Technology of China (grant
no. WK9110000058).
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
TL and ZMW performed the experiments and analyzed
the data. MHD helped with the collection of tissue specimens. JJW
performed part of the RTqPCR and western blotting experiments. YYP
contributed to the conception of the study and gave final approval
of the version to be published. YYP and TL have verified the
authenticity of the raw data. All the authors read and approved the
final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the First Affiliated Hospital of University of Science and
Technology of China (approval no. 2019-ky086). All the patients
provided written informed consent to participate in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
miRNAs/miR
|
microRNAs
|
|
PRMT
|
protein arginine
methyltransferases
|
|
NC
|
negative control
|
|
CCK-8
|
Cell Counting Kit-8
|
|
3′-UTR
|
3′-untranslated region
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
FAM98A
|
family with sequence similarity 98
member A
|
|
SHH
|
sonic hedgehog signaling molecule
|
|
SMO
|
smoothened, frizzled class
receptor
|
|
GLI1
|
GLI family zinc finger 1
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baltzer PAT, Kapetas P, Marino MA and
Clauser P: New diagnostic tools for breast cancer. Memo.
10:175–180. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gomez-Miragaya J, Moran S,
Calleja-Cervantes ME, Collado-Sole A, Pare L, Gomez A, Serra V,
Dobrolecki LE, Lewis MT, Diaz-Lagares A, et al: The altered
transcriptome and DNA methylation profiles of docetaxel resistance
in breast cancer PDX models. Mol Cancer Res. 17:2063–2076. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang P, Li F, Li L, You Y, Luo S, Dong Z,
Gao Q, Wu S, Brunner N and Stenvang J: lncRNA profile study reveals
the mRNAs and lncRNAs associated with docetaxel resistance in
breast cancer cells. Sci Rep. 8:179702018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fan L, Strasser-Weippl K, Li JJ, St LJ,
Finkelstein DM, Yu KD, Chen WQ, Shao ZM and Goss PE: Breast cancer
in China. Lancet Oncol. 15:e279–e289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo H, Ingolia NT, Weissman JS and Bartel
DP: Mammalian microRNAs predominantly act to decrease target mRNA
levels. Nature. 466:835–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jeffries J, Zhou W, Hsu AY and Deng Q:
miRNA-223 at the crossroads of inflammation and cancer. Cancer
Lett. 451:136–141. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Si W, Shen J, Zheng H and Fan W: The role
and mechanisms of action of microRNAs in cancer drug resistance.
Clin Epigenetics. 11:252019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He XH, Zhu W, Yuan P, Jiang S, Li D, Zhang
HW and Liu MF: miR-155 downregulates ErbB2 and suppresses
ErbB2-induced malignant transformation of breast epithelial cells.
Oncogene. 35:6015–6025. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jafri MA, Al-Qahtani MH and Shay JW: Role
of miRNAs in human cancer metastasis: Implications for therapeutic
intervention. Semin Cancer Biol. 44:117–131. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen J, Zhang K, Xu Y, Gao Y, Li C, Wang R
and Chen L: The role of microRNA-26a in human cancer progression
and clinical application. Tumour Biol. 37:7095–7108. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang ZM, Ge HF, Yang CC, Cai Y, Chen Z,
Tian WZ and Tao JL: MicroRNA-26a-5p inhibits breast cancer cell
growth by suppressing RNF6 expression. Kaohsiung J Med Sci.
35:467–473. 2019.PubMed/NCBI
|
|
14
|
Akter KA, Mansour MA, Hyodo T, Ito S,
Hamaguchi M and Senga T: FAM98A is a novel substrate of PRMT1
required for tumor cell migration, invasion, and colony formation.
Tumour Biol. 37:4531–4539. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Akter KA, Mansour MA, Hyodo T and Senga T:
FAM98A associates with DDX1-C14orf166-FAM98B in a novel complex
involved in colorectal cancer progression. Int J Biochem Cell Biol.
84:1–13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Z, Li N, Sun X and Wang J: FAM98A
promotes cancer progression in endometrial carcinoma. Mol Cell
Biochem. 459:131–139. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng R, Liu Q, Wang T, Wang L and Zhang
Y: FAM98A promotes proliferation of non-small cell lung cancer
cells via the P38-ATF2 signaling pathway. Cancer Manag Res.
10:2269–2278. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Katoh M: Genomic testing, tumor
microenvironment and targeted therapy of Hedgehog-related human
cancers. Clin Sci (Lond). 133:953–970. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Riaz SK, Khan JS, Shah STA, Wang F, Ye L,
Jiang WG and Malik MFA: Involvement of hedgehog pathway in early
onset, aggressive molecular subtypes and metastatic potential of
breast cancer. Cell Commun Signal. 16:32018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma L: MicroRNA and Metastasis. Adv Cancer
Res. 132:165–207. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Braicu C, Raduly L, Morar-Bolba G,
Cojocneanu R, Jurj A, Pop LA, Pileczki V, Ciocan C, Moldovan A,
Irimie A, et al: Aberrant miRNAs expressed in HER-2 negative breast
cancers patient. J Exp Clin Cancer Res. 37:2572018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fridrichova I and Zmetakova I: MicroRNAs
contribute to breast cancer invasiveness. Cells. 8:13612019.
View Article : Google Scholar
|
|
24
|
Biswas S: MicroRNAs as therapeutic agents:
The future of the battle against cancer. Curr Top Med Chem.
18:2544–2554. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang C, Tabatabaei SN, Ruan X and Hardy P:
The dual regulatory role of MiR-181a in breast cancer. Cell Physiol
Biochem. 44:843–856. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, Wang P, Wu LL, Yan J, Pang XY and
Liu SJ: miR-26a-5p inhibit gastric cancer cell proliferation and
invasion through mediated Wnt5a. Onco Targets Ther. 13:2537–2550.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma Y, Deng F, Li P, Chen G, Tao Y and Wang
H: The tumor suppressive miR-26a regulation of FBXO11 inhibits
proliferation, migration and invasion of hepatocellular carcinoma
cells. Biomed Pharmacother. 101:648–655. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo K, Zheng S, Xu Y, Xu A, Chen B and Wen
Y: Loss of miR-26a-5p promotes proliferation, migration, and
invasion in prostate cancer through negatively regulating SERBP1.
Tumour Biol. 37:12843–12854. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu F, Zhang Y, Sun B, McMahon AP and Wang
Y: Hedgehog signaling: From basic biology to cancer therapy. Cell
Chem Biol. 24:252–280. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hanna A and Shevde LA: Hedgehog signaling:
Modulation of cancer properies and tumor mircroenvironment. Mol
Cancer. 15:242016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Abe Y and Tanaka N: Fine-Tuning of GLI
activity through arginine methylation: Its mechanisms and function.
Cells. 9:19732020. View Article : Google Scholar
|
|
32
|
Wang Y, Hsu JM, Kang Y, Wei Y, Lee PC,
Chang SJ, Hsu YH, Hsu JL, Wang HL, Chang WC, et al: Oncogenic
functions of gli1 in pancreatic adenocarcinoma are supported by its
PRMT1-Mediated methylation. Cancer Res. 76:7049–7058. 2016.
View Article : Google Scholar : PubMed/NCBI
|