Introduction
Human colorectal cancer (CRC) is the third most
common malignant tumor and the fourth most common cause of
cancer-associated mortality in the world (1,2).
Notably, the incidence rate of CRC in younger people has increased
in past years in China (3). Although
notable progress has been made in the therapy and diagnosis of CRC,
the therapeutic response and prognosis are still unsatisfactory
with a 5-year survival rate of ~50% (4). Therefore, exploration of the mechanism
underlying CRC progression and identifying effective molecular
targets are of important to improve the treatment of CRC.
It is well documented that the Wnt/β-catenin
signaling pathway is frequently hyper-activated in cancer and is
strongly implicated in carcinogenesis, including CRC (5,6).
Inhibition of the Wnt/β-catenin signaling pathway is a potential
antitumor target for CRC (7). In the
absence of Wnt, β-catenin protein is retained in cytoplasm through
forming a protein complex with axis inhibitor (Axin), adenomatous
polyposis coil (APC), casein kinase 1α and glycogen synthase kinase
3β (8). However, the free β-catenin
level is elevated in cytoplasm and then translocates into the
nucleus when Wnt signaling is activated, followed by the increased
transcription of its target genes, such as c-myc and cyclin D1,
through interacting with the T-cell factor/lymphoid enhancer factor
family of transcription factors (9,10).
Although the inactivating mutation of APC is considered an
important factor for Wnt/β-catenin activation in CRC (11–13),
other factors or genes that induce Wnt/β-catenin signaling
activation in CRC still need to be clarified.
Secreted phosphoprotein-1 (SPP-1), also known as
osteopontin, is located at 4q22.1 and a multifunctional member of
the small integrin-binding ligand N-linked glycoprotein family
(14). Evidence has demonstrated
that SPP-1 is upregulated in various malignant tumors (15,16),
including CRC (17). For example, Xu
et al (17) used
bioinformatics methods and found that SPP-1 was significantly
overexpressed in CRC tissues, and its high expression is closely
associated with the advanced clinical process and poor prognosis in
patients with CRC. Further experiments showed that downregulation
of SPP-1 significantly repressed CRC cell viability, migration and
tumor growth and induced apoptosis. Moreover, Huang et al
(18) demonstrated that
SPP-1-upregulation resulted in significant enhancements in cell
proliferation, migration and invasion, and inhibited apoptosis and
autophagy through regulating MAPK signaling. These findings
demonstrate that SPP-1 plays an oncogenic role in CRC progression,
but the underlying mechanisms remain unclear.
The present study aimed to explore the role of the
Wnt/β-catenin signaling pathway in SPP-1-induced CRC progression
with in vivo and in vitro experiments. Our results
demonstrated that β-catenin was an indispensable factor for
SPP-1-mediated CRC progression.
Materials and methods
Colorectal tissue specimens
Primary CRC tissues and adjacent normal colorectal
tissues (>2 cm from the tumor tissues) were obtained from 200
patients with CRC who underwent colectomy from May 2009 to May
2017. All patients signed the informed consent and received
colectomy as the first treatment method. Patients were excluded if
they suffered from other malignant tumors or if they received
radiotherapy and/or chemotherapy before surgery.
Tumor-Node-Metastasis (TNM) stage was evaluated according to
previously reported (19). The fresh
tissues were then immediately immersed in liquid nitrogen (−196°C)
until analysis. Experiments involving human samples were performed
according to the Helsinki Declaration and approved by The Ethical
Committee of Ganzhou People's Hospital (approval no.
Ky2019015).
Immunohistochemistry (IHC)
For IHC, the routine three-step procedure was
performed as previously described (20) with the primary antibody against SPP-1
(cat. no. ab8448; Abcam). The staining of SPP-1 was determined by
three pathologists independent from the present study in a blinded
manner. The extent of positively stained cells was scored as: 0 For
0–5%, 1 for 6–25%, 2 for 26–50%, 3 for 51–75%, and 4 for 76–100%.
The staining intensity was scored as: 0 for negative staining, 1
for weak staining, 2 for moderate staining, and 3 for strong
staining. The extent score was multiplied with intensity score to
obtain the total score of SPP-1 staining in colon tissues. Total
score of SPP-1 staining ≤ medium score (9 points) of the total
samples was defined as low expression, and > medium score was
defined as high expression.
Cell culture conditions
Human normal colon cell line CCD-18Co was purchased
from The Cell Bank of the Chinese Academy of Sciences and cultured
in RPMI-1640 medium with 10% fetal serum bovine (FBS) (both Thermo
Fisher Scientific, Inc.). Three human CRC cell lines, including
SW620, COLO 205 and SW480 were obtained from BeNa Culture
Collection. SW620 and COLO 205 cells were cultured in RPMI-1640
medium and SW480 cells were grown in DMEM-H medium (Thermo Fisher
Scientific, Inc.) with 10% FBS. All cells were maintained at 37°C
with 5% CO2.
Alteration of gene expression in
cells
To upregulate SPP-1 expression, SW480 cells were
infected with the overexpression lentiviral vector (OE-SPP-1;
Shanghai GenePharma Co., Ltd.) at a multiplicity of infection of 5
and incubated with G418 (100 µg/ml) for 14 days at 37°C to select
the stably overexpressed cells. To silence β-catenin expression,
three lentiviral vectors (sh-β-catenin-1/-2/-3; OriGene
Technologies, Inc.) and pumomycin (7 µg/ml, incubation for 14 days)
were used to select the stably transfected cells. The 2nd
generationally stable cell lines were used for further studies. Two
small interfering (si)RNAs of SPP-1 (si-SPP-1-1/-2; 100 pmol/6-well
plates) and the scrambled control (si-NC) purchased from OriGene
Technologies, Inc. were used to downregulate SPP-1 expression using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) at
37°C. After 48 h of transfection, the cells were harvested for
further analysis. The si sequences were as follows: si-SPP-1-1,
5′-UCAUAUUCUGAAUCUCAUCCU-3′; si-SPP-1-2:
5′-AGUUUCAACCGUCUUAAUCAG-3′.
RNA extraction and reverse
transcription-quantitative (RT-q)PCR
Total RNA was extracted from snap-frozen tissues or
cultured cells using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). The first-strand cDNA was synthesized
with random primers (Beijing Solarbio Science & Technology Co.,
Ltd.) using TaqMan Reverse Transcription kit (Takara Biotechnology
Co., Ltd.) at 42°C for 60 min. After that, the RT-qPCR was carried
out with SYBR Green of The SuperScript® III One-Step
RT-PCR system (Thermo Fisher Scientific, Inc.). Primers for SPP-1
were 5′-GAATCTCCTAGCCCCACAGACC-3′ (sense) and
5′-ACTCCTCGCTTTCCATGTGTG-3′ (antisense); primers for β-catenin were
5′-GCGCCATTTTAAGCCTCTCG-3′ and 5′-GGCCATGTCCAACTCCATCA-3′; primers
for GAPDH were 5′-CCACTAGGCGCTCACTGTTCTC-3′ (sense) and
ACTCCGACCTTCACCTTCCC-3′ (antisense). Reaction conditions were as
follows: 94°C For 5 min, followed by amplification for 40 cycles
(94°C for 30 sec, 57°C for 30 sec and 72°C for 30 sec), and a final
step at 72°C for 5 min. The levels of mRNAs were calculated using
the 2−ΔΔCq method (21).
GAPDH was used as endogenous control to normalize the mRNA levels
of SPP-1 and β-catenin.
Western blotting
Protein was extracted using RIPA lysis buffer
(Shanghai Yeasen Biotechnology Co., Ltd.) and protease and
phosphatase inhibitors (Beijing Solarbio Science & Technology
Co., Ltd.). After centrifugation at 4°C for 25 min at 12,000 × g,
the protein samples were quantified by using the Bicinchoninic acid
Protein Assay kit (Thermo Fisher Scientific, Inc.) and then 25 µg
protein from each group was loaded per lane and separated by 10%
SDS-PAGE. After that, the proteins were transferred into the
polyvinylidene difluoride membranes (Thermo Fisher Scientific),
following by being blocked with 5% non-fat milk for 1 h at room
temperature. Then, the membranes were probed with the indicated
primary antibodies, SPP-1 (1:3,000 dilution; cat. no. ab844;
Abcam), Axin (1:3,000 dilution; cat. no. ab32197; Abcam), β-catenin
(1:2,000 dilution; cat. no. ab16051; Abcam), Frz (1:1,000 dilution;
cat. no. AF1617; R&D Systems, Inc.), DVL1 (1:2,000 dilution;
cat. no. TA329899; OriGene Technologies, Inc.), TCF (1:2,500
dilution; cat. no. #9383; Cell Signaling Technology, Inc.), c-myc
(1:2,000 dilution; cat. no. M4439; Sigma-Aldrich; Merck KGaA), APC
(1:2,000 dilution; cat. no. ab15270; Abcam) and GAPDH (1:6,000
dilution; cat. no. 2118; Cell Signaling Technology, Inc.) at 4°C
overnight and the HRP-conjugated secondary antibodies (1:10,000
dilution; cat. no. SA00001-1 and SA00001-2; ProteinTech Group,
Inc.) for 1 h at room temperature in succession. The protein
signaling was visualized using electrochemiluminescence reagent
(EMD Millipore) and quantified by ImageJ software (version 1.48;
National Institutes of Health).
Co-immunoprecipitation (Co-IP)
The interaction between different proteins was
assessed using a Co-IP assay. SW480 cells were rinsed with cold PBS
and lysed in IP lysis buffer (Beijing Solarbio Science &
Technology Co., Ltd.), followed by centrifugation at 4°C for 25 min
at 12,000 × g. Then, the proteins (200 µg) were incubated with 10
µl of Dynabeads® protein G (Thermo Fisher Scientific,
Inc.) for 1 h. Next, the proteins were incubated with 2 µg of SPP-1
antibody or IgG antibody (negative control) overnight at 4°C, and
Dynabeads® protein G for 1 h. The immunocomplex was
washed five times with IP lysis buffer and boiled at 100°C for 8
min, and then subjected to western blotting analysis.
Immunofluorescence microscopy
SW480 cells with and without stable SPP-1 stable
overexpression were cultured in culture glass slides in 24-well
plates and incubated at 37°C for 48 h. Then, the cells were fixed
with 4% paraformaldehyde for 15 min at room temperature, followed
by incubation with 1% Triton-100 for 10 min for cell membrane
permeabilizing. Next, the cells were rinsed with phosphate buffer,
blocked with 5% goat serum (Beijing Solarbio Science &
Technology Co., Ltd.) for 1 h at room temperature and incubated
with the primary antibody against β-catenin (cat. no. ab16051;
Abcam) overnight at 4°C and the Alexa Fluor® 488
Conjugated secondary antibody (1:1,000 dilution; cat. no. 4412;
Cell Signaling Technology, Inc.) for 1 h at room temperature. DAPI
(Invitrogen; Thermo Fisher Scientific, Inc.) was used for nuclear
staining at a concentration of 1:5,000 for 5 min at room
temperature. The subcellular location of β-catenin was analyzed on
a laser scanning microscope (magnification, ×60; TCSSP2-AOBS-MP;
Leica Microsystems, Inc.).
Cell proliferation detection
After 6 h of cell transfection with OE-SPP-1, OE-NC,
si-SPP-1, si-NC or OE-SPP-1, SW480 cells were seeded into 96-well
plates at 3,000 cells/well density and cultured at 37°C for 1, 2,
3, 4 or 5 days. Cell proliferation was tested by using a
Cell-Counting Kit 8 (CCK-8; Dojindo Molecular Technologies, Inc.)
based on the manufacturer's description.
Apoptosis detection
The effect of SPP-1/β-catenin axis on SW480
apoptosis was assessed by using the Annexin V/propidium iodide (PI)
kit (Roche Diagnostics). After incubation with the Annexin V-FITC
and PI solution in the dark for 15 min, SW480 cells were collected
for flow cytometry using CytoFLEX (Beckman Coulter, Inc.) and cell
apoptotic rates were analyzed by FlowJo 7.6 software (FlowJo LLC).
FITC−/PI− quadrant were viable cells,
FITC−/PI+ were necrosis cells,
FITC+/PI− were early apoptotic cells and
FITC+/PI+ were late apoptotic cells.
In vivo tumor formation assay
SW480 cells were transfected with OE-NC+sh-NC,
OE-SPP-1+sh-NC, OE-NC+sh-NC and OE-NC+sh-β-catenin, and then
incubated with 100 µg/ml G418 and 7 µg/ml puromycin for 14 days to
establish the stable transfection cell lines. Then,
5×106 of the aforementioned cells were resuspended in
PBS buffer and injected subcutaneously into the flanks of
4–6-week-old male nude mice (n=20; 20±2 g; Experimental Animal
Center Of The Fourth Military Medical University). Mice were fed
with common feed and sterile water ad libitum, and housed in
22±1°C with 55±1% humidity with a 12 h light/dark cycle. After 28
days of injection, mice were euthanized via cervical dislocation
and the tumors were removed to weigh and photograph. Mice are
considered as dead if the chests did not rise or fall and the
hearts do not beat. Animal health and behavior were monitored every
4 days. Mice were sacrificed if the tumor diameter was >1.8 cm.
No mouse died prior to the end of the study. These animal
experiments were carried out in accordance to the Institutional
principles for the concern and use of animals and the protocol was
approved by The Ethical Committee of Ganzhou People's Hospital
(approval no. Ky2019015).
Statistical analysis
Data are obtained from at least three times of
independent experiments and expressed as means ± standard deviation
or median + interquartile range only for the IHC score. Comparison
of the IHC scores between normal group and tumor group in Fig. 1A was carried out using the paired
Wilcoxon signed rank test. Other comparisons between two groups
were executed using two-tailed Student's t-tests and one-way ANOVA
test followed by Tukey's post hoc test was used for multiple
groups. A paired t-test was used for the analysis of tumor and
adjacent non-tumor samples of the same individuals, and unpaired
t-test was used for other comparisons between two groups.
Kaplan-Meier analysis with log rank tests was used to evaluate the
clinical significance of SPP-1 levels in predicting the prognosis
of CRC patients. χ2 tests were used for the data
comparisons listed in Table I.
P<0.05 was considered to indicate a statistically significant
difference.
 | Table I.Association between SPP-1 expression
and the clinicopathological characteristics of 200 patients with
colorectal cancer. |
Table I.
Association between SPP-1 expression
and the clinicopathological characteristics of 200 patients with
colorectal cancer.
| Parameters | Total | Low expression | High
expression | P-value |
|---|
| Sex |
|
|
| 0.689 |
|
Male | 102 | 45 | 57 |
|
|
Female | 98 | 46 | 52 |
|
| Age, years |
|
|
| 0.293 |
|
<60 | 48 | 25 | 23 |
|
|
≥60 | 152 | 66 | 86 |
|
|
Differentiation |
|
|
| 0.017 |
|
High | 52 | 31 | 21 |
|
|
Poor/moderation | 148 | 60 | 88 |
|
| TNM stage |
|
|
| 0.001 |
|
I/II | 46 | 40 | 6 |
|
|
III/IV | 154 | 51 | 103 |
|
| Tumor
infiltration |
|
|
| 0.001 |
|
T1/T2 | 32 | 26 | 6 |
|
|
T3/T4 | 168 | 65 | 103 |
|
| Lymph node
metastasis |
|
|
| 0.001 |
| N0 | 53 | 38 | 15 |
|
|
N1-N3 | 147 | 53 | 94 |
|
| Distant
metastasis |
|
|
| 0.001 |
| M0 | 121 | 72 | 49 |
|
| M1 | 79 | 19 | 60 |
|
| Vascular
invasion |
|
|
| 0.001 |
|
Absent | 102 | 61 | 41 |
|
|
Present | 98 | 30 | 68 |
|
Results
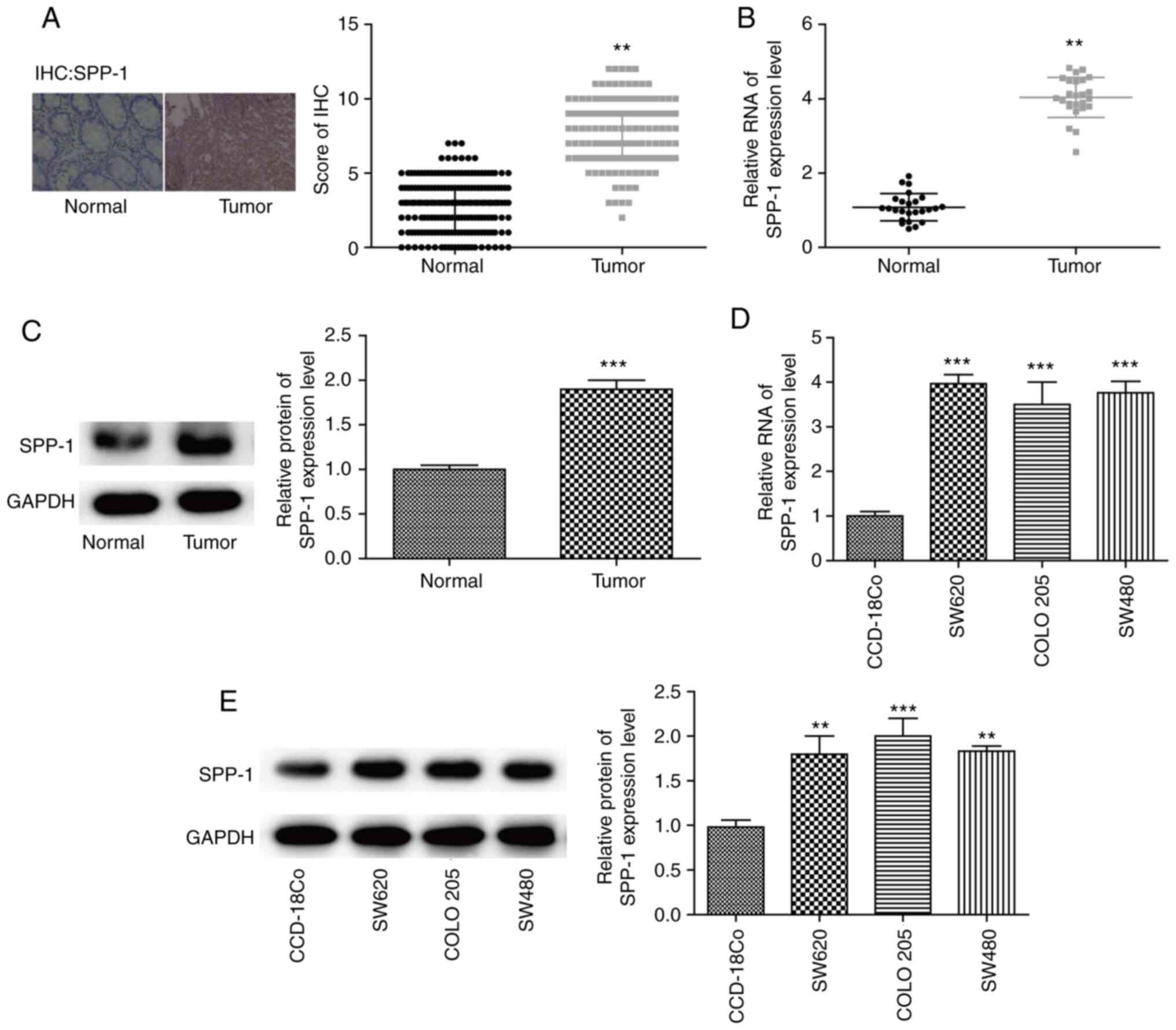
Expression of SPP-1 is increased in
CRC tissues and cells
To uncover the mechanism underlying SPP-1 in the
progression of CRC, SPP-1 expression profiles were compared between
CRC tissues and the adjacent normal colorectal tissues using IHC,
RT-qPCR and western blotting assays. It was observed that the
expression of SPP-1 was significantly increased at protein and mRNA
levels in CRC tissues, compared with that in the normal tissues
(P<0.01 and P<0.001, respectively; Fig. 1A-C). Additionally, SPP-1 expression
patterns in CRC cells and normal colon cells were evaluated using
western blotting and RT-qPCR. The results demonstrated that SPP-1
expression in CRC cell lines SW620, COLO 205 and SW480 was
significantly increased when compared with that in CCD-18Co cells
at the mRNA (P<0.01, Fig. 1D) and
protein levels (P<0.01, Fig. 1E).
These findings confirmed that the expression of SPP-1 was increased
in CRC.
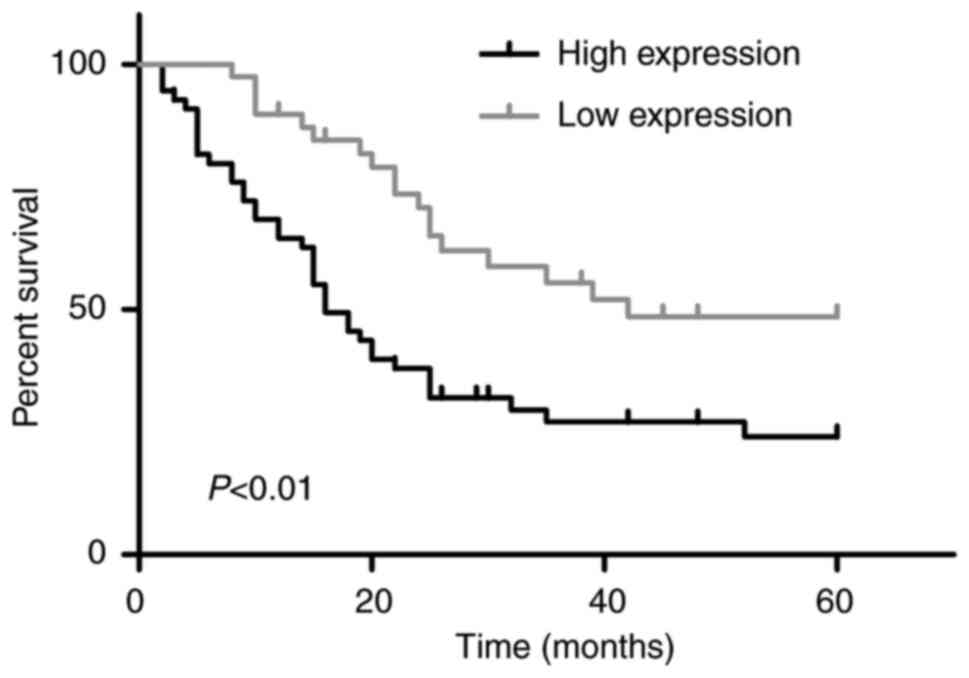
Assessment of the clinical value of
SPP-1 in CRC
To determine the clinical value of SPP-1 in CRC,
Kaplan-Meier with log-rank analysis and χ2 tests test
were used to analyze the effects of SPP-1 expression on the overall
survival rate and the clinicopathological parameters of patients
with CRC. As shown in Fig. 2, the
overall survival rates for patients with SPP-1 low expression were
significantly higher compared with that of patients with SPP-1 high
expression. Moreover, SPP-1 expression level was associated with
the differentiation of CRC tissues (P=0.017), and associated with
TNM stage (P=0.001), incidence rates of tumor infiltration
(P=0.001), lymph node metastasis (P=0.001), distant metastasis
(P=0.001) and vascular invasion (P=0.001) (Table I). These results indicated that SPP-1
played an important role in predicting the clinical stage and
prognosis in patients with CRC.
SPP-1 promotes proliferation and
inhibits the apoptosis of CRC cells
SPP-1 expression was increased in SW620, COLO 205
and SW480 cells, and SW480 cell line was selected for further study
as SPP-1-overexpression was most notable in these cells compared
with SW620 and COLO 205. Compared with the control group, SPP-1
expression level was significantly decreased following transfection
with si-SPP-1-1 and si-SPP-1-2, and OE-SPP-1 significantly enhanced
SPP-1 expression at both the mRNA and protein levels (P<0.001,
Fig. 3A-D). Compared with the
control group, downregulation of SPP-1 significantly inhibited cell
proliferation, while overexpression of SPP-1 enhanced cell
proliferation (P<0.05, Fig. 3E).
Besides, knockdown of SPP-1 significantly induced apoptosis, and
overexpression of SPP-1 resulted in the opposite result (P<0.01,
Fig. 3F-G) as compared with the
control group. These results verified that SPP-1 served as an
oncogene in CRC.
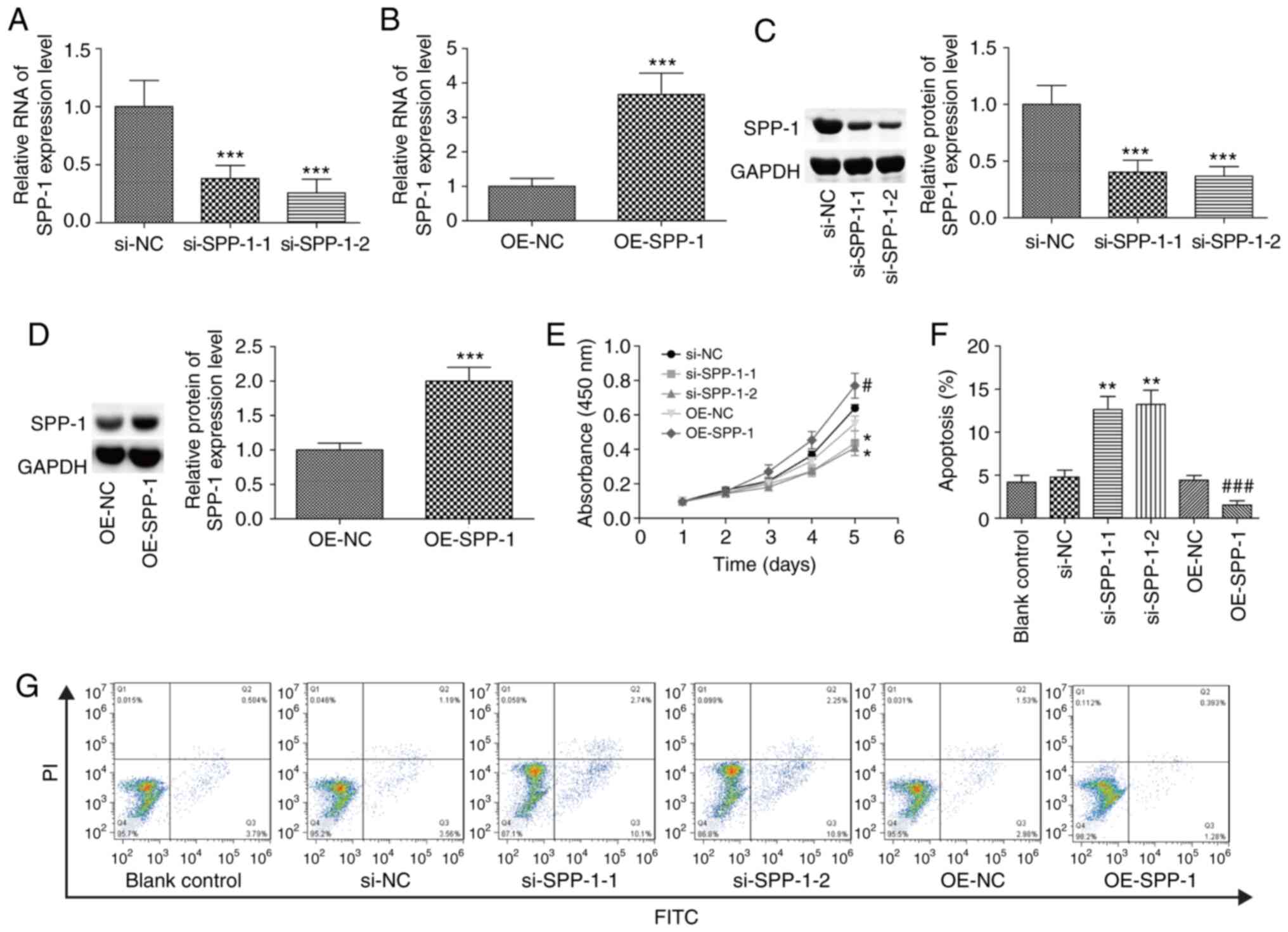 | Figure 3.Increased expression of SPP-1
enhances cell proliferation and reduces apoptosis. SW480 cells were
transiently transfected with OE-SPP-1, OE-NC, si-SPP-1 and si-NC,
then (A-B) mRNA levels were determined using RT-PCR and (C-D)
protein levels were measured by western blotting at 48 h
post-treatments. (E) A Cell Counting Kit-8 assay was performed to
assess the effects of altered expression of SPP-1 on the cell
proliferation. (F and G) Flow cytometry was carried out to detect
apoptosis (n=3). *P<0.05, **P<0.01 and ***P<0.001 vs.
si-NC group; #P<0.05, ###P<0.001 vs.
OE-NC group. SPP-1, secreted phosphoprotein 1; OE, overexpression,
NC, negative control; si, small interfering. |
SPP-1 promotes the expression of
β-catenin and its nuclear transportation in CRC cells
To investigate whether Wnt/β-catenin signaling was
involved in SPP-1-mediated CRC progression, the effects of SPP-1 on
the activation of Wnt/β-catenin signaling were assessed. Western
blotting showed that SPP-1-overexpression significantly increased
the expression of β-catenin and c-myc, whereas no obvious influence
on the expression levels of Frz, DVL1, APC, Axin and TCF in SW480
cells were observed (P<0.001, Fig.
4A). The Co-IP assay showed that SPP-1 could combine with
β-catenin protein (Fig. 4B).
Additionally, SPP-1-overexpression promoted the nuclear
accumulation of β-catenin (Fig. 4C).
These results suggested that SPP-1 triggered the activation of
β-catenin signaling.
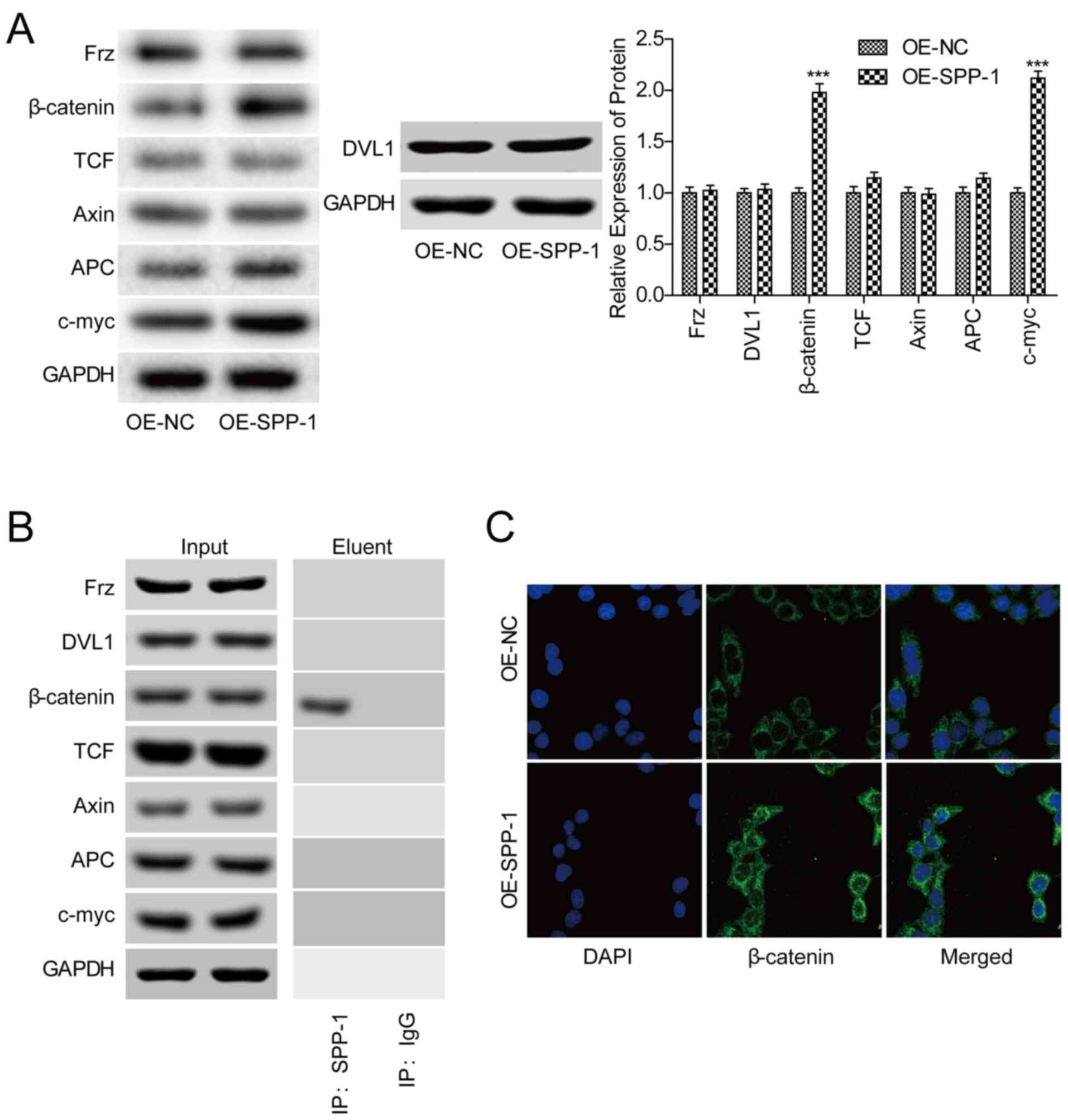 | Figure 4.Increased expression of SPP-1
increases β-catenin expression and nuclear transportation. (A)
Expression of Frz, DVL1, β-catenin, TCF, Axin, APC and c-myc were
detected using western blotting after SW480 cells were infected
OE-SPP-1 and OE-NC (n=3). (B) A co-immunoprecipitation assay was
performed to assess the interactions between SPP-1 and Frz, DVL1,
β-catenin, TCF, Axin, APC and c-myc. C) Immunofluorescence
microscopy was used to detect the effects of SPP-1 on the
subcellular location of β-catenin. ***P<0.001 vs. OE-NC. SPP-1,
secreted phosphoprotein-1; OE, overexpression; NC, negative
control; APC, adenomatous polyposis coil; Axis, axin inhibitor. |
SPP-1 facilitates CRC progression in a
β-catenin-dependent manner
Finally, it was assessed whether β-catenin was
involved in SPP-1-mediated CRC progression. Three shRNAs of
β-catenin were applied to downregulate β-catenin expression in CRC
cells, among which shRNA-1 significantly deceased β-catenin mRNA
and protein expression levels compared with the sh-NC group
(P<0.001, Fig. 5A and B).
Downregulation of β-catenin in SPP-1 overexpressed cells
significantly rescued the enhancement in cell proliferation induced
by SPP-1 in SW480 cells (P<0.01, Fig.
5C), and increased apoptosis rate (P<0.01, Fig. 5D) compared with the OE-SPP-1+sh-NC
group. Furthermore, SPP-1 stable overexpression in SW480 cells
significantly promoted tumor formation in vivo as compared
with the OE-NC+sh-NC group, which was abolished when β-catenin was
stably downregulated (P<0.01, Fig. 5E
and F). Additionally, the largest tumor size was ~0.18
cm3 and the largest tumor diameter was ~1.0 cm (Fig. 5F). These results suggested that SPP-1
facilitated CRC progression in a β-catenin-dependent manner.
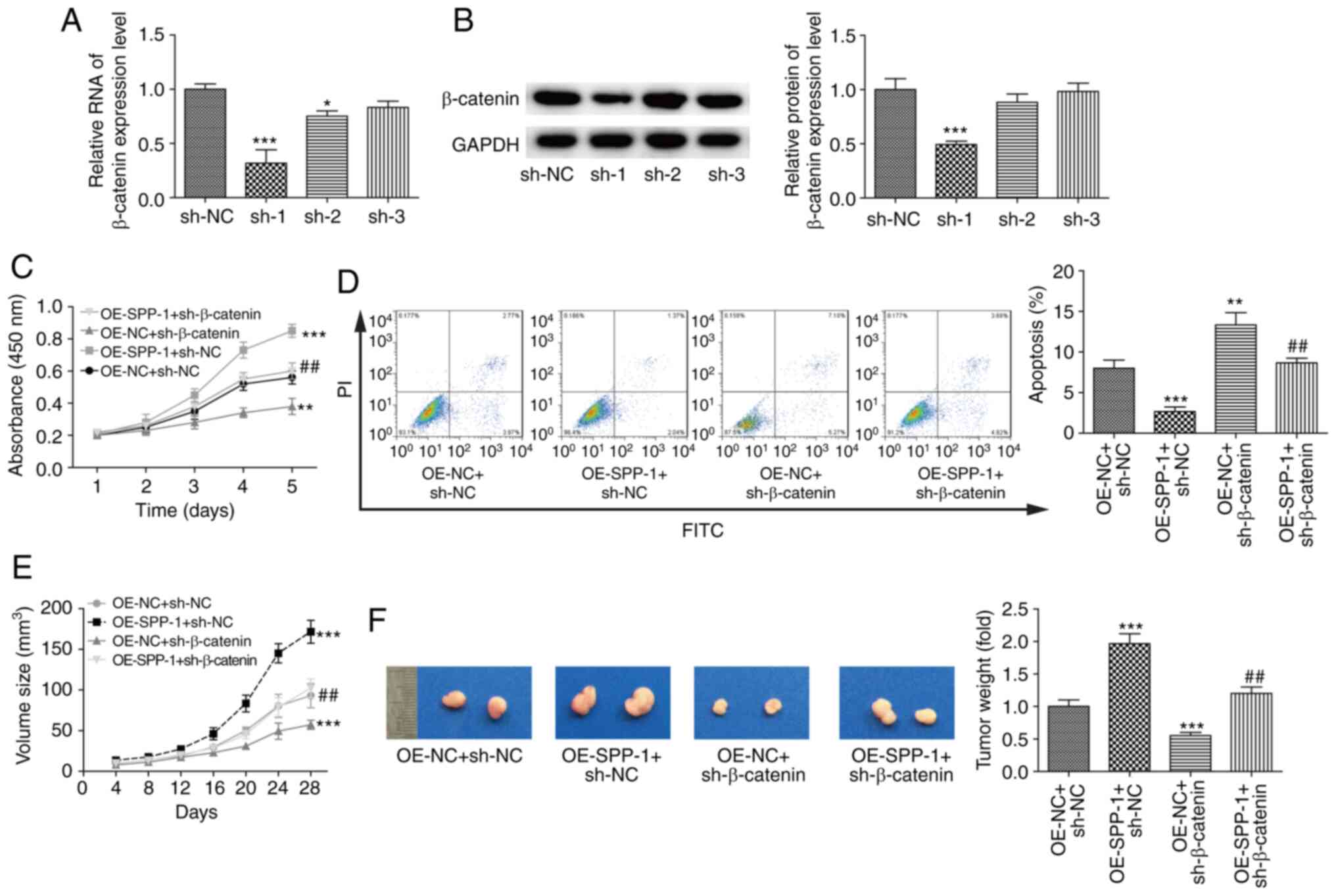 | Figure 5.Effects of SPP-1/β-catenin signaling
on cell proliferation, apoptosis and tumorigenesis. Knockdown
efficiency of sh-β-catenin in SW480 cells was determined using (A)
reverse transcription-quantitative PCR and (B) western blotting
(n=3, *P<0.05, ***P<0.001, vs. sh-NC group). SW480 cells were
treated with OE-NC+sh-NC, OE-NC+sh-β-catenin, OE-SPP-1+sh-NC and
OE-SPP-1+sh-β-catenin, then cell proliferation was determined using
a (C) Cell Counting Kit-8 assay and (D) apoptosis was assessed
using flow cytometry (n=3). (E and F) In vivo tumor
formation ability was determined by nude mice xenotransplantation
assays (n=5). (C-F) **P<0.01, ***P<0.001 vs. OE-NC+sh-NC
group; ##P<0.01 vs. OE-SPP-1+sh-NC group. SPP-1
secreted phosphoprotein 1; OE, overexpression; NC, negative
control; sh, short hairpin. |
Discussion
SPP-1 has been previously demonstrated to be highly
expressed in CRC cells and tissues (22), and overexpression of SPP-1
significantly enhances cell proliferation and motility in
vitro, and tumorigenesis and angiogenesis in vivo
(23). In addition, high SPP-1
expression predicts a poor prognosis in patients with CRC (24). These findings suggest that SPP-1
serves an important role in human CRC. The present study
demonstrated, for the first time, that SPP-1 facilitated the
progression of CRC through interacting with β-catenin and
increasing its expression and nuclear accumulation.
SPP-1 is a secreted glycophosphoprotein, which is
expressed in multiple cell types, and is strongly implicated in
numerous biological functions, such as cell adhesion, migration,
bone calcification, immune responses and carcinogenesis (25,26). Up
to now, several studies have recognized that SPP-1 is overexpressed
in multiple types of cancer and its high expression is associated
with poor prognosis and advanced clinical process. For example, the
plasma SPP-1 level is significantly elevated in patients with renal
cell carcinoma with distant metastasis, and SPP-1 high expression
levels predicts a lower survival rate (27). SPP-1 is upregulated in oral squamous
cell carcinoma (OSCC) and stromal OSCC cells, which predicts a
higher nodal stage, higher World Health Organization clinical stage
and poor clinical prognosis in patients with OSCC (28). In addition, Loosen et al
(29) reported that the increased
expression of SPP-1 is associated with the poor survival in
patients with postoperative cholangiocarcinoma. Similarly, in CRC,
the high expression profile of SPP-1 is significantly correlated
with the lymph node metastasis, lymphatic/venous invasion and TNM
stage, as well as poor prognosis (22,30).
Consistently, the current study demonstrated that the high
expression level of SPP-1 was closely associated with a lower
overall survival rate and advanced clinical process of patients
with CRC, including TNM stage, tumor infiltration, lymph node
metastasis, distant metastasis and vascular invasion. This
suggested that SPP-1 may have value as a biomarker for CRC
diagnosis and prognosis prediction.
SPP-1 has been identified to serve as an inducer of
the aggressive behaviors in several types of tumor cell, including
CRC. For example, Xu et al (17) reported that knockdown of SPP-1
significantly represses the proliferation, colony formation,
migration and in vivo tumor growth and increased apoptosis
in CRC cells. Likui et al (31) demonstrated that SPP-1-downregulation
significantly suppresses invasion and enhances the radiosensitivity
of CRC cells. Huang et al (32) demonstrated that SPP-1-overexpression
significantly promotes the hepatic metastasis of CRC. These
findings indicate that SPP-1 plays an important role in promoting
CRC progression. Except for the oncogenic role of SPP-1 in CRC, the
present study also clarified that SPP-1 promoted the expression and
nuclear accumulation of the β-catenin protein through
protein-protein interactions. SPP-1 has been reported to induce the
phosphorylation of GSK-3β at serine 9, which is the most well
recognized means for GSK-3β inhibition (33), suggesting that SPP-1 may induce
β-catenin expression through repressing GSK-3β. Moreover, Robertson
et al (34) demonstrated that
SPP-1-upregulation could significantly promote the expression and
nuclear accumulation of β-catenin, potentially through repressing
GSK-3β expression. The present study observed that SPP-1 could
combine with β-catenin protein and promote its expression with no
obvious influence in the expression of DVL1, Frz, TCF, Axin and
APC. Evidence has shown that the increased expression of
TCF4/lymphoid enhancer-binding factor induced by β-catenin
activation can promote SPP-1 transcription (24,35).
Taken together, it was speculated that there might be a positive
feedback between SPP-1 and β-catenin, which needs to be verified in
future studies using western blotting assays.
To further explore the role of β-catenin in SPP-1
induced CRC progression, flow cytometry, CCK-8 and in vivo
tumor formation assays were performed. The results showed that the
enhancements in cell proliferation, tumorigenesis and apoptosis
inhibition induced by SPP-1-overexpression were abrogated when
β-catenin was downregulated. These results demonstrated that
β-catenin activation plays an important role in SPP-1-mediated CRC
progression.
Drug resistance is a main cause for the
dissatisfaction of CRC treatment (36), thus it is important to reveal the
mechanisms of drug resistance in CRC. One main limitation of the
present study is that the role of SPP-1 was not explored in
response to anticancer drugs, such as cis-platin-mediated apoptosis
of CRC cells. Further in vitro and in vivo
experiments should be carried out to disclose SPP-1 role in the
drug resistance of CRC.
In conclusion, the current study demonstrated that
SPP-1 functions as an oncogene in CRC, which is highly expressed in
CRC tissues and cells and is closely associated with the malignant
clinical progression and poor outcome of patients with CRC. In
addition, SPP-1 confers CRC cells with malignant phenotype in a
β-catenin dependent manner. Overall, the present study provides
evidence to support the value of SPP-1/β-catenin as a novel
therapeutic target for human CRC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CF conceived the project and revised the manuscript.
JY and YL performed the experiments, collected and interpreted the
data and wrote the manuscript. LZ analyzed the data. CF, JY, YK and
LZ confirm the authenticity of all raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by The Ethical Committee of
Ganzhou People's Hospital (approval no. Ky2019015). All patients
provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bever KM and Le DT: An expanding role for
immunotherapy in colorectal cancer. J Natl Compr Canc Netw.
15:401–410. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bode AM, Dong Z and Wang H: Cancer
prevention and control: Alarming challenges in China. Natl Sci Rev.
3:117–127. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jacome AA and Eng C: Role of immune
checkpoint inhibitors in the treatment of colorectal cancer: Focus
on nivolumab. Expert Opin Biol Ther. 19:1247–1263. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qi J, Yu Y, Akilli Ozturk O, Holland JD,
Besser D, Fritzmann J, Wulf-Goldenberg A, Eckert K, Fichtner I and
Birchmeier W: New Wnt/β-catenin target genes promote experimental
metastasis and migration of colorectal cancer cells through
different signals. Gut. 65:1690–1701. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee K, Lindsey AS, Li N, Gary B, Andrews
J, Keeton AB and Piazza GA: β-catenin nuclear translocation in
colorectal cancer cells is suppressed by PDE10A inhibition, cGMP
elevation, and activation of PKG. Oncotarget. 7:5353–5365. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Larriba MJ, Valle N, Palmer HG,
Ordóñez-Morán P, Alvarez-Díaz S, Becker KF, Gamallo C, de Herreros
AG, González-Sancho JM and Muñoz A: The inhibition of
Wnt/beta-catenin signalling by 1alpha,25-dihydroxyvitamin D3 is
abrogated by Snail1 in human colon cancer cells. Endocr Relat
Cancer. 14:141–151. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sebio A, Kahn M and Lenz HJ: The potential
of targeting Wnt/β-catenin in colon cancer. Expert Opin Ther
Targets. 18:611–615. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arce L, Yokoyama NN and Waterman ML:
Diversity of LEF/TCF action in development and disease. Oncogene.
25:7492–7504. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang S, Li Y, Wu Y, Shi K, Bing L and Hao
J: Wnt/β-catenin signaling pathway upregulates c-Myc expression to
promote cell proliferation of P19 teratocarcinoma cells. Anat Rec
(Hoboken). 295:2104–2113. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dow LE, O'Rourke KP, Simon J,
Tschaharganeh DF, van Es JH, Clevers H and Lowe SW: Apc restoration
promotes cellular differentiation and reestablishes crypt
homeostasis in colorectal cancer. Cell. 161:1539–1552. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brabletz T, Jung A, Reu S, Porzner M,
Hlubek F, Kunz-Schughart LA, Knuechel R and Kirchner T: Variable
beta-catenin expression in colorectal cancers indicates tumor
progression driven by the tumor environment. Proc Natl Acad Sci
USA. 98:10356–10361. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Phelps RA, Chidester S, Dehghanizadeh S,
Phelps J, Sandoval IT, Rai K, Broadbent T, Sarkar S, Burt RW and
Jones DA: A two-step model for colon adenoma initiation and
progression caused by APC loss. Cell. 137:623–634. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kang YS, Jeong EJ, Seok HJ, Kim SK, Hwang
JS, Choi ML, Jo DG, Kim Y, Choi J, Lee YJ, et al: Cks1 regulates
human hepatocellular carcinoma cell progression through osteopontin
expression. Biochem Biophys Res Commun. 508:275–281. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ouyang X, Huang Y, Jin X, Zhao W, Hu T, Wu
F and Huang J: Osteopontin promotes cancer cell drug resistance,
invasion, and lactate production and is associated with poor
outcome of patients with advanced non-small-cell lung cancer. Onco
Targets Ther. 11:5933–5941. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ferreira LB, Eloy C, Pestana A, Lyra J,
Moura M, Prazeres H, Tavares C, Sobrinho-Simões M, Gimba E and
Soares P: Osteopontin expression is correlated with differentiation
and good prognosis in medullary thyroid carcinoma. Eur J
Endocrinol. 174:551–561. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu C, Sun L, Jiang C, Zhou H, Gu L, Liu Y
and Xu Q: SPP1, analyzed by bioinformatics methods, promotes the
metastasis in colorectal cancer by activating EMT pathway. Biomed
Pharmacother. 91:1167–1177. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang RH, Quan YJ, Chen JH, Wang TF, Xu M,
Ye M, Yuan H, Zhang CJ, Liu XJ and Min ZJ: Osteopontin promotes
cell migration and invasion, and inhibits apoptosis and autophagy
in colorectal cancer by activating the p38 MAPK signaling pathway.
Cell Physiol Biochem. 41:1851–1864. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xin B, He X, Wang J, Cai J, Wei W, Zhang T
and Shen X: Nerve growth factor regulates CD133 function to promote
tumor cell migration and invasion via activating ERK1/2 signaling
in pancreatic cancer. Pancreatology. 16:1005–1014. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Likui W, Hong W and Shuwen Z: Clinical
significance of the upregulated osteopontin mRNA expression in
human colorectal cancer. J Gastrointest Surg. 14:74–81. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Irby RB, McCarthy SM and Yeatman TJ:
Osteopontin regulates multiple functions contributing to human
colon cancer development and progression. Clin Exp Metastasis.
21:515–523. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rohde F, Rimkus C, Friederichs J,
Rosenberg R, Marthen C, Doll D, Holzmann B, Siewert JR and Janssen
KP: Expression of osteopontin, a target gene of de-regulated Wnt
signaling, predicts survival in colon cancer. Int J Cancer.
121:1717–1723. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Denhardt DT and Guo X: Osteopontin: A
protein with diverse functions. FASEB J. 7:1475–1482. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Denhardt DT, Giachelli CM and Rittling SR:
Role of osteopontin in cellular signaling and toxicant injury. Annu
Rev Pharmacol Toxicol. 41:723–749. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ramankulov A, Lein M, Kristiansen G, Meyer
HA, Loening SA and Jung K: Elevated plasma osteopontin as marker
for distant metastases and poor survival in patients with renal
cell carcinoma. J Cancer Res Clin Oncol. 133:643–652. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Avirovic M, Matusan-Ilijas K, Damante G,
Fabrro D, Cerović R, Juretić M, Grahovac B, Jonjić N and Lučin K:
Osteopontin expression is an independent factor for poor survival
in oral squamous cell carcinoma: A computer-assisted analysis on
TMA sections. J Oral Pathol Med. 42:620–626. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Loosen SH, Roderburg C, Kauertz KL,
Pombeiro I, Leyh C, Benz F, Vucur M, Longerich T, Koch A,
Braunschweig T, et al: Elevated levels of circulating osteopontin
are associated with a poor survival after resection of
cholangiocarcinoma. J Hepatol. 67:749–757. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wei R, Wong JPC, Lyu P, Xi X, Tong O,
Zhang SD, Yuen HF, Shirasawa S and Kwok HF: In vitro and clinical
data analysis of Osteopontin as a prognostic indicator in
colorectal cancer. J Cell Mol Med. 22:4097–4105. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Likui W, Hong W, Shuwen Z, Yuangang Y and
Yan W: The potential of osteopontin as a therapeutic target for
human colorectal cancer. J Gastrointest Surg. 15:652–659. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang J, Pan C, Hu H, Zheng S and Ding L:
Osteopontin-enhanced hepatic metastasis of colorectal cancer cells.
PLoS One. 7:e479012012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McManus EJ, Sakamoto K, Armit LJ,
Ronaldson L, Shpiro N, Marquez R and Alessi DR: Role that
phosphorylation of GSK3 plays in insulin and Wnt signalling defined
by knockin analysis. EMBO J. 24:1571–1583. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Robertson BW and Chellaiah MA: Osteopontin
induces beta-catenin signaling through activation of Akt in
prostate cancer cells. Exp Cell Res. 316:1–11. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
El-Tanani MK, Barraclough R, Wilkinson MC
and Rudland PS: Regulatory region of metastasis-inducing DNA is the
binding site for T cell factor-4. Oncogene. 20:1793–1797. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Van der Jeught K, Xu HC, Li YJ, Lu XB and
Ji G: Drug resistance and new therapies in colorectal cancer. World
J Gastroenterol. 24:3834–3848. 2018. View Article : Google Scholar : PubMed/NCBI
|