Introduction
Breast cancer is the most commonly diagnosed
malignancy in women, with 21.6 new cases and 5.7 mortalities per
100,000 women reported in China in 2008 (1). In recent years, the incidence and
mortality rates of breast cancer has increased, posing a threat to
the physical and mental health of women worldwide. Globally, ~1.7
million new cases of breast cancer are diagnosed, with 521,900
deaths every year (2). Over the past
two decades, great progress has been achieved in the early
diagnosis and treatment of breast cancer through combining surgery,
adjuvant therapy and hormone therapy; however, the overall
prognosis of breast cancer remains poor due to late diagnoses,
frequent recurrence and high chemoresistance (3–6).
Therefore, understanding the underlying mechanisms of breast cancer
progression is urgently required. Doing so may aid in identifying
new biomarkers for early diagnosis and prognosis prediction.
Long non-coding RNAs (lncRNAs) are >200
nucleotides in length, but without the protein-coding ability
(7), are aberrantly expressed and
play critical regulatory roles in multiple types of cancer by
regulating tumor growth, metastasis and metabolism (8,9). In
breast cancer, a number of individual lncRNAs are dysregulated and
contribute to tumor progression. For example, LINC01121 mediates
the expression of HMGA2 to promote breast cancer cell
proliferation, migration and invasion by sponging microRNA
(miR/miRNA)-150-5p (10). In
addition, the nuclear LINC00993, which is downregulated in breast
cancer tissue, inhibits triple-negative breast cancer growth both
in vitro and in vivo by orchestrating the expression
of cell cycle regulators (11). The
lncRNA GAEA interacts with MEX3C to enhance the K27-linked
polyubiquitination of PTEN, consequently activating the
epithelial-to-mesenchymal transition (EMT) process (12). MALAT1, which is upregulated in breast
cancer tissues, promotes tumor development and progression both
in vitro and in vivo (13). However, functions of the vast
majority of lncRNAs have not yet been characterized.
MIR4435-2HG (also named LINC00978 or AK001796), a
lncRNA that is located in the 2q13 region, has been shown to
function as a tumor promoter in various types of cancer (14–17).
MIR4435-2HG interacts with EZH2 to inhibit p21 and E-cadherin
expression, promoting the progression of hepatocellular carcinoma
(HCC) (14). Through activating
β-catenin signaling and inhibiting miR-6754-5p, MIR4435-2HG
promotes lung cancer progression (15,16).
Additionally, a previous study revealed that MIR4435-2HG was
upregulated in gastric cancer and promotes tumor growth and
metastasis via activating the Wnt/β-catenin signaling pathway
(17). Several recent reports
(18,19) have revealed that MIR4435-2HG was
involved in the regulation of cell apoptosis. Knockdown of
MIR4435-2HG in cisplatin-resistant cell line HCT116R significantly
promoted cell apoptosis and restored the sensitivity to cisplatin
(18). MIR4435-2HG knockdown induced
ovarian cancer cell apoptosis via the miR-128-3p/CDK14 axis
(19). Therefore, targeting
MIR4435-2HG using antisense oligonucleotides to induce apoptosis
may be a potential advancement in cancer drug development. Notably,
Deng et al (20) reported
that MIR4435-2HG is highly expressed in breast cancer cell lines
and tissues, and could be utilized to predict prognosis in patients
with breast cancer. However, the biological functions and
underlying mechanisms of breast cancer remain to be elucidated.
The aim of the present study was to investigate the
function of MIR4435-2HG and its associated signaling pathways in
breast cancer. In addition, the effects of MIR4435-2HG knockdown on
cell proliferation, apoptosis and migration were elucidated. The
mechanisms by which MIR4435-2HG promotes tumor progression have
been explored in the present study. The findings of the present
study suggested that MIR4435-2HG may be a promising therapeutic
target for breast cancer.
Materials and methods
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), total RNA was extracted from MCF-7 and
MDA-MB-231 cells according to the manufacturer's protocol. The
first-strand cDNA was generated from 1 µg total RNA using the
PrimeScript RT reagent kit (Takara Bio, Inc.) according to the
manufacturer's protocol. For the RT-qPCR, SYBR Green Premix Ex Taq
(Takara Bio, Inc.) was used according to the manufacturer's
protocol. The relative expression levels of target genes were
calculated using the 2−∆∆Cq method (21) with GAPDH as an endogenous reference
gene. The primer sequences used in the present study were as
follows: MIR4435-2HG: Forward, 5′-AGGCCCCAGGGAATCTTTCA-3′ and
reverse, 5′-GCCTCTCCCTGAATAACTGGG-3′; GAPDH: Forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′. The experiments were performed three
times, and the RT-qPCR assays were performed in triplicate.
Lentivirus-mediated RNA
interference
The sequence for the short hairpin (sh) RNA
targeting MIR4435-2HG was:
5′-CACCGCCCAGATTTAAGGGCTATTTCAAGAGAATAGCCCTTAAATCTGGGCCTTTTTTG-3′
as previously reported (14), and
the negative control sequence (shNC) was
5′-CACCGTTCTCCGAACGTGTCACGTCAAGAGATTACGTGACACGTTCGGAGAATTTTTTG-3′.
The shMIR4435-2HG (1 µg) and shNC (1 µg) were synthesized and
inserted into the lentivirus core vector that contained an enhanced
green fluorescent protein (EGFP) reporter gene, hU6-MCS-CMV-EGFP.
Recombinant lentiviruses expressing shMIR4435-2HG or the empty
lentiviral vector shNC were produced by Shanghai Genechem Co., Ltd.
Then, 6×105 cells were infected with concentrated
lentivirus (MOI=40) in the presence of 8 µg/ml of polybrene (Sigma
Aldrich; Merck KGaA). After 72 h incubation, cells were selected
using puromycin (1 µg/ml; Sigma Aldrich; Merck KGaA) for 5 days. To
evaluate the expression level of MIR4435-2HG, RT-qPCR was used.
Cell apoptosis assay
Apoptotic cells were assessed using an annexin
V-FITC/propidium iodide apoptosis detection kit (Abcam) via flow
cytometry in MDA-MB-231 and MCF-7 cells (Cell Bank of Type Culture
Collection of the Chinese Academy of Sciences). Since shRNA
lentivirus vector expressing GFP will influence the FACS signal of
Annexin-FITC, siRNA was used to knock down MIR4435-2HG in the flow
cytometric analysis of apoptotic assays. Briefly, breast cancer
cells (5×104 cells/ml) transfected with 50 nM
siMIR4435-2HG (5′-CCCAGAUUUAAGGGCUAUUTT-3′) or siNC
(5′-UUCUCCGAACGUGUCACGUTT-3′) were seeded in 6-well culture plates.
After 48 h, cells were digested by 0.25% EDTA-free trypsin, washed
2 times with cold PBS, resuspended in 100 µl binding buffer, and
stained with Annexin V-FITC as well as propidium iodide (100
µg/ml). After 15 min incubation at 37°C in the dark, apoptotic
cells were analyzed using a FACS Calibur flow cytometer (Becton,
Dickinson and Company), and analyzed using Cell Quest software
v.3.3 (Becton, Dickinson and Company). The experiment was performed
in triplicate.
Cell culture and growth
conditions
Human transformed mammary epithelial cell line
(MCF-10A) and human breast cancer cell lines (MDA-MB-231 and MCF-7)
were obtained from the Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences. MDA-MB-231 and MCF-7 cells were
cultured in high-glucose Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) in the presence of 1% penicillin/streptomycin.
(Sigma-Aldrich; Merck KGaA). MCF-10A cells were cultured in
DMEM-F12 (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% FBS in the presence of 100 U/ml penicillin, 100 µg/ml of
streptomycin, 20 ng/ml of epidermal growth factor and 2 mM of
L-glutamine. All cells were cultured at 37°C with 5% CO2
in a standard cell culture incubator (Thermo Fisher Scientific,
Inc.).
Cell Counting Kit-8 (CCK-8)
assays
Cell proliferation was detected using CCK-8 assays
(Dojindo Molecular Technologies Inc.). Briefly, cells were seeded
in 96-well plates at a density of 1,000 cells/well. At each time
point (0, 1, 2, 3, 4 and 5 days), culture medium was replaced with
100 µl DMEM supplemented with 10 µl CCK-8 reagent (Dojindo, Japan).
Cells were then incubated at 37°C for 2 h, and then the absorbance
was measured at 450 nm. Cell proliferation rate was calculated
relative to day 0 (6 h after seeding), which were the controls.
Colony formation assays
A total of 500 cells were seeded per well onto
6-well plates. The medium was replenished with fresh medium every 3
days. After 14 days culture, the medium was removed and the cells
were fixed with 4% paraformaldehyde for 20 min at 4°C after washing
twice with PBS, stained with 0.1% crystal violet (Beyotime
Institute of Biotechnology) for 20 min at room temperature. After
another wash with water, cells were air-dried, and colonies
containing >50 cells were counted manually. Images of the
representative single colony was captured using a light microscope
(magnification, ×100).
Western blot analysis
Total protein was extracted from MDA-MB-231 and
MCF-7 cells infected with shMIR4435-2HG- or shNC-viruses using RIPA
lysis buffer (Beyotime Institute of Biotechnology). To extract
cytoplasmic and nuclear proteins, cytoplasmic and nuclear extracts
were prepared using a Nuclear and Cytoplasmic Extraction kit
(Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. The protein concentration of the
soluble materials was measured using a BCA protein assay kit
(Beyotime Institute of Biotechnology), with bovine serum albumin
(Beyotime Institute of Biotechnology) serving as a standard. Then,
the proteins (40 µg per lane) were separated via 10% SDS-PAGE and
then transferred onto a polyvinylidene difluoride (PVDF) membrane
(EMD Millipore). The membranes were blocked in 5% fat-free milk at
room temperature for 1 h followed by incubating with specific
primary antibodies at 4°C overnight, including the anti-β-actin
(1:5,000; cat. no. ab8227; Abcam), anti-PCNA (1:1,000; cat. no.
ab92552; Abcam), anti-GAPDH (1:1,000; cat. no. ab9485; Abcam),
anti-ZEB1 (1:1,000; cat. no. 3396; Cell Signaling Technology,
Inc.), anti-E-cadherin (1:1,000; cat. no. 3195; Cell Signaling
Technology, Inc.), anti-Lamin B1 (1:1,000; cat. no. ab133741;
Abcam), anti-N-cadherin (1:1,000; cat. no. 14215; Cell Signaling
Technology, Inc.), anti-Bax antibody (1:1,000; cat. no. 5023; Cell
Signaling Technology), anti-Bcl-2 (1:1,000; cat. no. 4223; Cell
Signaling Technology, Inc.), anti-Cleaved-caspase-3 (1:1,000; cat.
no. 9661; Cell Signaling Technology, Inc.), anti-β-catenin
(1:1,000; cat. no. ab68183; Abcam), anti-cleaved-PARP (1:1,000;
cat. no. 5625; Cell Signaling Technology, Inc.) and anti-vimentin
(1:1,000; cat. no. 5741; Cell Signaling Technology, Inc.).
Horseradish peroxidase-conjugated anti-rabbit (1:1,000; cat. no.
BA1054, Wuhan Boster Biological Technology, Inc.) or mouse
secondary antibodies (1:1,000; cat. no. BA1050, Wuhan Boster
Biological Technology, Inc.) were added and incubated at room
temperature for 1 h. Detection was performed using the ECL reagent
(Pierce; Thermo Fisher Scientific, Inc.). Protein levels were
quantified through densitometry using Photoshop CS4 version
v.11.0.1 (Adobe, Systems, Inc.).
Transwell cell migration and basement
membrane matrix invasion assay
Migration and invasion assays were performed to
evaluate the effect of MIR4435-2HG on breast cancer cell
metastasis. Briefly, the Transwell inserts (Costar; Corning, Inc.)
with 8 µm pore polycarbonate membranes (Corning, Inc.) coated with
Matrigel at 37°C for 4 h or without Matrigel (BD Biosciences) were
used in the invasion and migration assays, respectively. MDA-MB-231
and MCF-7 cells infected with shNC or shMIR4435-2HG viruses were
resuspended in a serum-free medium and further 6×104
cells for MCF-7 or 2×104 cells for MDA-MB-231 were
seeded into the upper chamber of the Transwell insert. The lower
chamber was filled with 500 µl DMEM (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.). After 24 h incubation at 37°C with 5%
CO2, cells in the upper chamber were gently removed
using a cotton swab, whereas cells in the lower chamber were washed
with PBS, fixed with 4% paraformaldehyde at 4°C for 20 min, and
stained with 0.1% crystal violet at room temperature for 20 min.
Eventually, cells that had migrated/invaded into the filter were
counted and analyzed. Images of the representative migrative or
invasive pictures were captured using a light microscope
(magnification, ×100). Each assay was performed in triplicate.
Bioinformatics/database analysis
Data from the Gene Expression Profiling Interactive
Analysis (GEPIA) database (http://gepia.cancer-pku.cn/index.html) were used to
analyze the differential expression of MIR4435-2HG and its
prognostic values in breast cancer. A gene symbol (MIR4435-2HG) was
entered into the ‘Enter gene name’ field. Then the ‘GoPIA!’ button
was clicked to obtain the expression profile of the input gene
across all tumor and normal tissues. For the profile of MIR4435-2HG
expression, input ‘MIR4435-2HG’ under ‘Gene’, the ‘ANOVA’ option
was selected under ‘Differential Methods’, choose ‘No’ under ‘Log
Scale’, choose ‘Match TCGA normal data’ under ‘Matched Normal
data’, the ‘BRCA’ option was selected under ‘Cancer name’; click
‘Plot’ button and the results were then displayed. For the boxplot
of MIR4435-2HG expression, the ‘Boxplot’ tab under the ‘Expression
DIY’ section was clicked, the ‘BRCA’ option was selected under
‘Cancer name’, choose ‘Yes’ under ‘Log Scale’, set ‘Jitter size’ as
‘0.4’, and choose ‘Match TCGA normal data’ under ‘Matched Normal
data’; the results were then displayed in box plots. For the
survival analysis, click the ‘Survival Plots’ tab under the
‘Survival’ section, the ‘Overall Survival’ or ‘Disease Free
Survival (DFS)’ was selected under ‘Methods’, choose ‘Quartile’ as
the cut-off value to separate patients into high (>75% quartile)
and low (<25% quartile) expression groups, choose ‘No’ under
‘Hazards Ratio (HR)’, choose ‘No’ under ‘95% Confidence Interval’,
choose ‘months’ under ‘Axis Units’, and the ‘BRCA’ option was
selected under ‘Cancer name’; click ‘Plot’ button and the results
were then displayed.
Statistical analysis
Data are expressed as the mean ± standard deviation
from three independent experiments unless otherwise stated. To
determine statistical significance, an unpaired Student's t-test or
one-way ANOVA (with Tukey's post hoc test) was used and analyzed
using GraphPad Prism software (version 5.0; GraphPad Software) and
SPSS software (version 22.0; IBM Corp.). Kaplan-Meier analysis was
used to evaluate disease-free survival rate and overall survival
rate. P<0.05 was considered to indicate a statistically
significant difference.
Results
MIR4435-2HG is upregulated in breast
cancer tissue and its high expression level is associated with poor
prognosis
A previous report showed that MIR4435-2HG is highly
expressed in breast cancer tissues (n=36) compared with adjacent
normal tissues (20). To confirm
this observation, the present study retrieved and analyzed the
expression level of MIR4435-2HG in a large cohort of samples from
patients with breast cancer based on The Cancer Genome Atlas
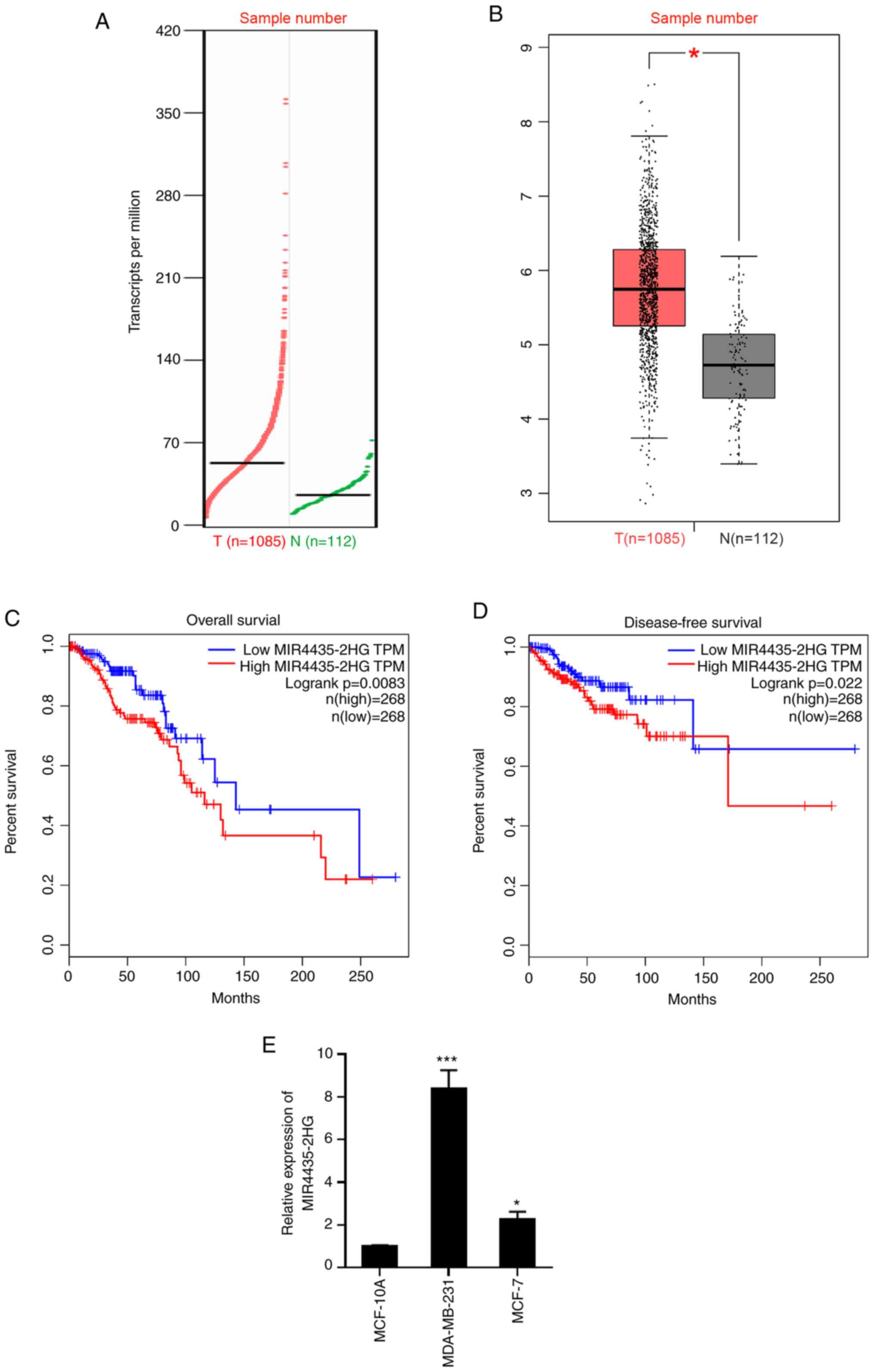
database using the online web portal GEPIA (http://gepia.cancer-pku.cn/index.html) (22). As presented in Fig. 1A and B, MIR4435-2HG expression was
significantly upregulated in breast cancer tissues compared with
normal tissues (P<0.05). Kaplan-Meier analysis demonstrated that
patients with higher MIR4435-2HG expression levels exhibited
decreased overall survival times (P=0.0083) and shorter
disease-free survival times (P=0.022) (Fig. 1C and D). In addition, the RT-qPCR
analysis revealed that MIR4435-2HG expression was higher in breast
cancer cell lines when compared with that in the normal breast cell
line MCF-10A (Fig. 1E). Taken
together, these data suggest that MIR4435-2HG may function as an
oncogene in breast cancer and its high expression is associated
with poor prognosis of patients with breast cancer.
Inhibition of MIR4435-2HG suppresses
the proliferation of breast cancer cells
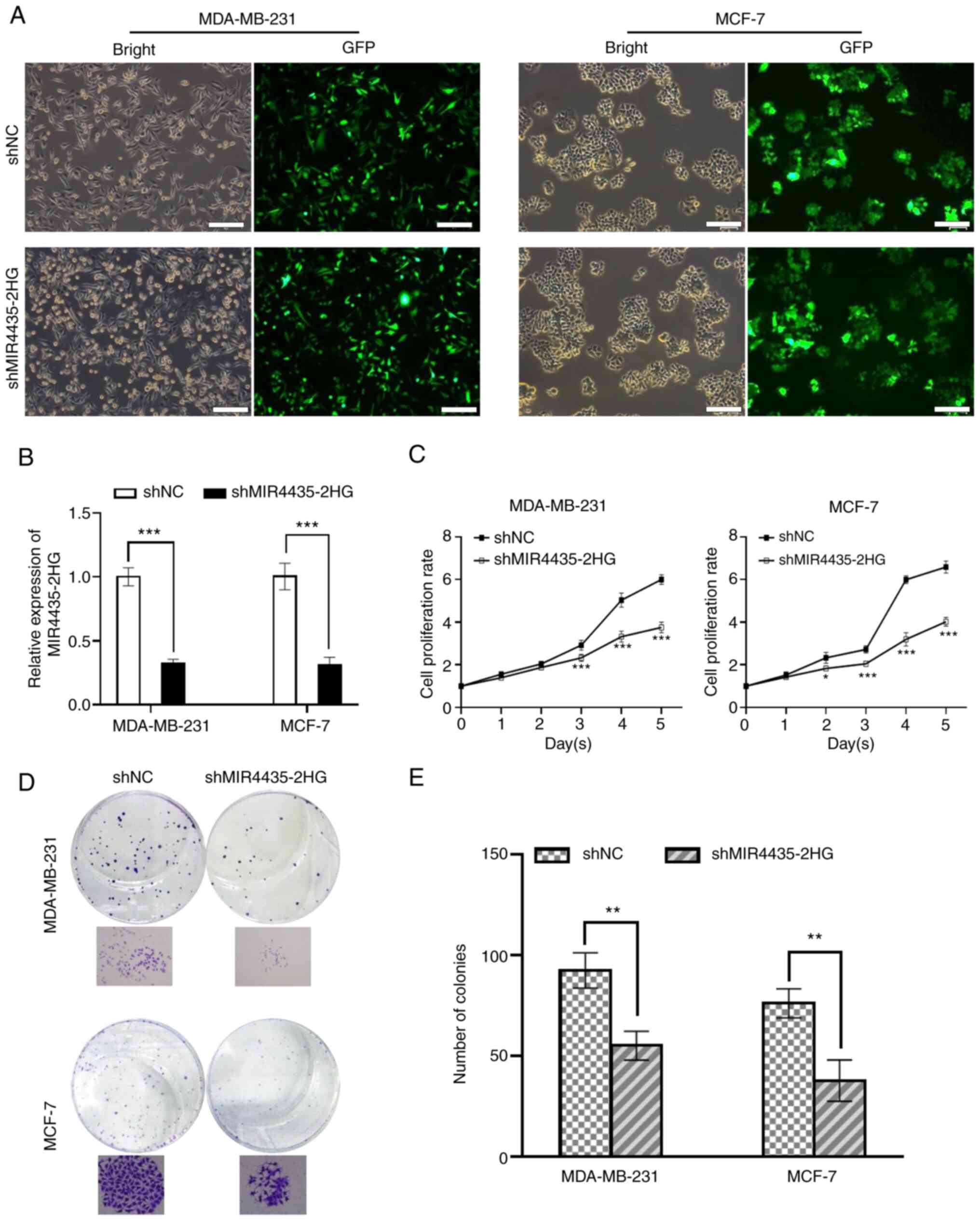
To investigate the potential function of MIR4435-2HG
in breast cancer, the present study performed MIR4435-2HG-knockdown
in MDA-MB-231 and MCF-7 cells by lentiviral infection
(shMIR4435-2HG) with an empty lentiviral vector as the shNC.
Fluorescence detection showed that the transfection efficiency was
>90% according to the ratio of GFP positive cells in both cell
lines (Fig. 2A). Furthermore,
RT-qPCR analysis showed that MIR4435-HG transcript levels were
decreased by ~70% in MDA-MB-231 and MCF-7 cells expressing
shMIR4435-2HG compared with the shNC group (P<0.01; Fig. 2B). In order to investigate the
effects of MIR4435-2HG on cell proliferation, CCK-8 and colony
formation assays were performed in MDA-MB-231 and MCF-7 cells. The
results revealed that MIR4435-2HG-depleted MDA-MB-231 or MCF7 cells
proliferated significantly slower than control cells (P<0.01 and
P<0.05). Furthermore, shMIR4435-2HG-MDA-MB-231 or MCF7 cells had
significantly fewer and smaller sized colonies than control cells
(P<0.01; Fig. 2D and E). Based on
these results, it was concluded that MIR4435-2HG exerts a crucial
role in breast cancer cell proliferation, and that targeting
MIR4435-2HG may effectively inhibit breast cancer progression.
Effect of MIR4435-2HG on breast cancer
cell apoptosis
The effects of MIR4435-2HG on cell apoptosis were
assessed via flow cytometry analyses (Fig. 3A). As presented in Fig. 3B and C, MIR4435-2HG knockdown
significantly increased the proportions of apoptotic MDA-MB-231 and
MCF-7 cells compared with siNC-transfected cells (P<0.05). To
gain further insight into the molecular mechanism by which
shMIR4435-2HG induces apoptosis of breast cancer cells, the present
study measured the expression levels of several
apoptosis-associated proteins post-silencing MIR4435-2HG. The
western blot results revealed that the expression levels of Bcl-2,
an anti-apoptotic marker, and the proliferating marker PCNA, were
decreased significantly in MDA-MB-231 and MCF-7 cells after
inhibiting MIR4435-2HG. While the expression of cleaved-PARP,
cleaved-caspase-3 and Bax, which were makers of activation of
apoptosis pathways, were upregulated upon MIR4435-2HG knockdown
(Fig. 3D and F). These findings
suggested that knockdown of MIR4435-2HG induces apoptosis through
downregulation of anti-apoptotic proteins and upregulation of
proapoptotic proteins in breast cancer cells.
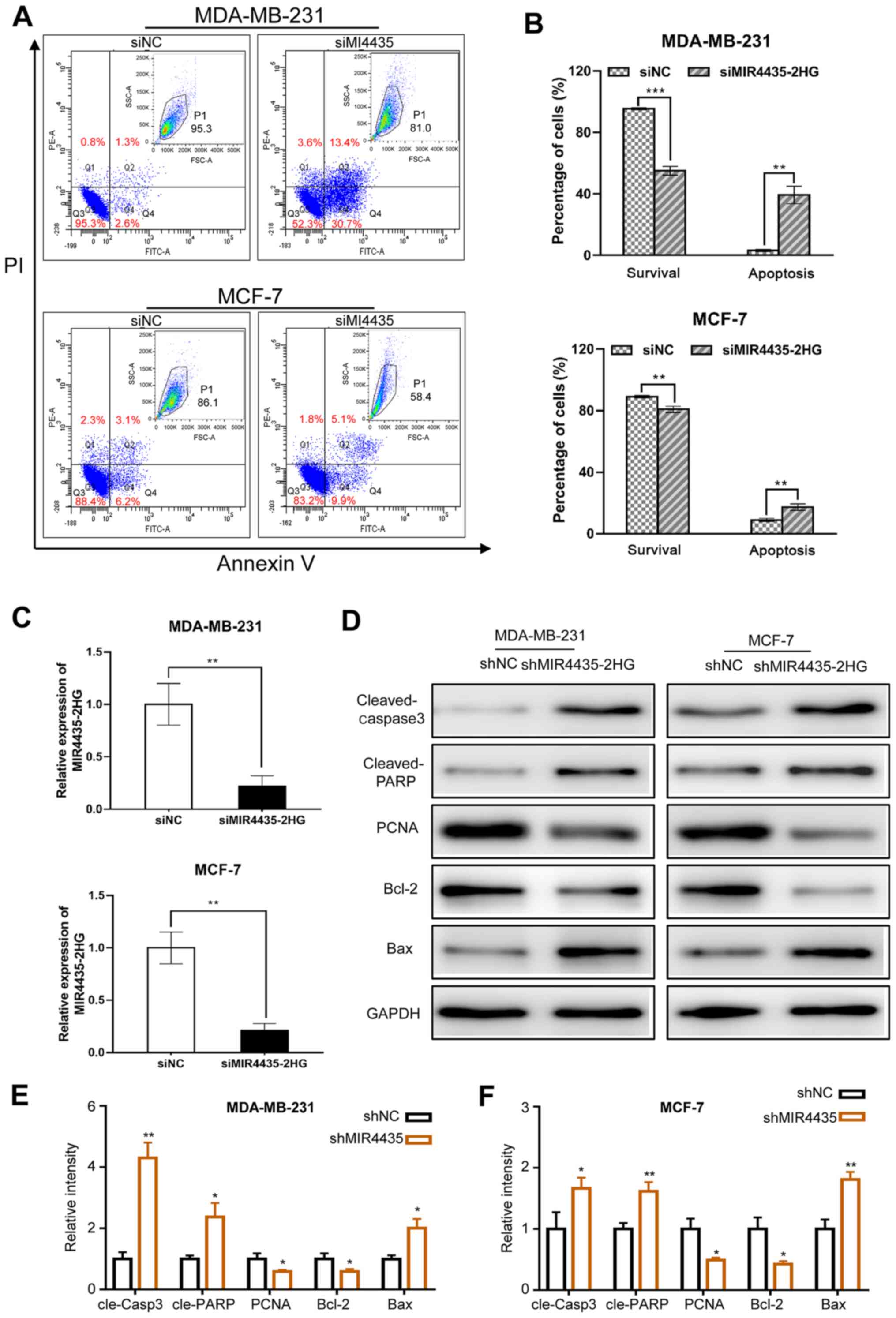 | Figure 3.Knockdown of MIR4435-2HG enhances
cell apoptosis in breast cancer cells. (A) After 48 h transfection,
cells were labelled with Annexin V-FITC and PI and then cell
apoptosis was analyzed by flow cytometry. The original flow
cytometry plots are shown in the upper-right panel of each dot
plots. (B) The percentage of apoptotic cells was calculated. The
data are presented as the mean ± standard deviation. (C) Reverse
transcription-quantitative PCR analysis of MIR4435-2HG expression
following transfection of si-NC or si-MIR4435-2HG with GAPDH
serving as the internal control. (D) Protein expression levels of
cleaved-PARP, PCNA, Bcl-2, Bax and cleaved-caspase 3 was measured
by western blotting in MDA-MB-231 cells (left panel) and MCF-7
cells (right panel). (E and F) Relative density of indicated
proteins in (D) *P<0.05, **P<0.01, ***P<0.001 vs. shNC.
PI, propidium iodide; si, small interfering; sh, short hairpin; NC,
negative control. |
Knockdown of MIR4435-2HG inhibits
breast cancer cell metastasis and invasion via the Wnt/β-catenin
pathway
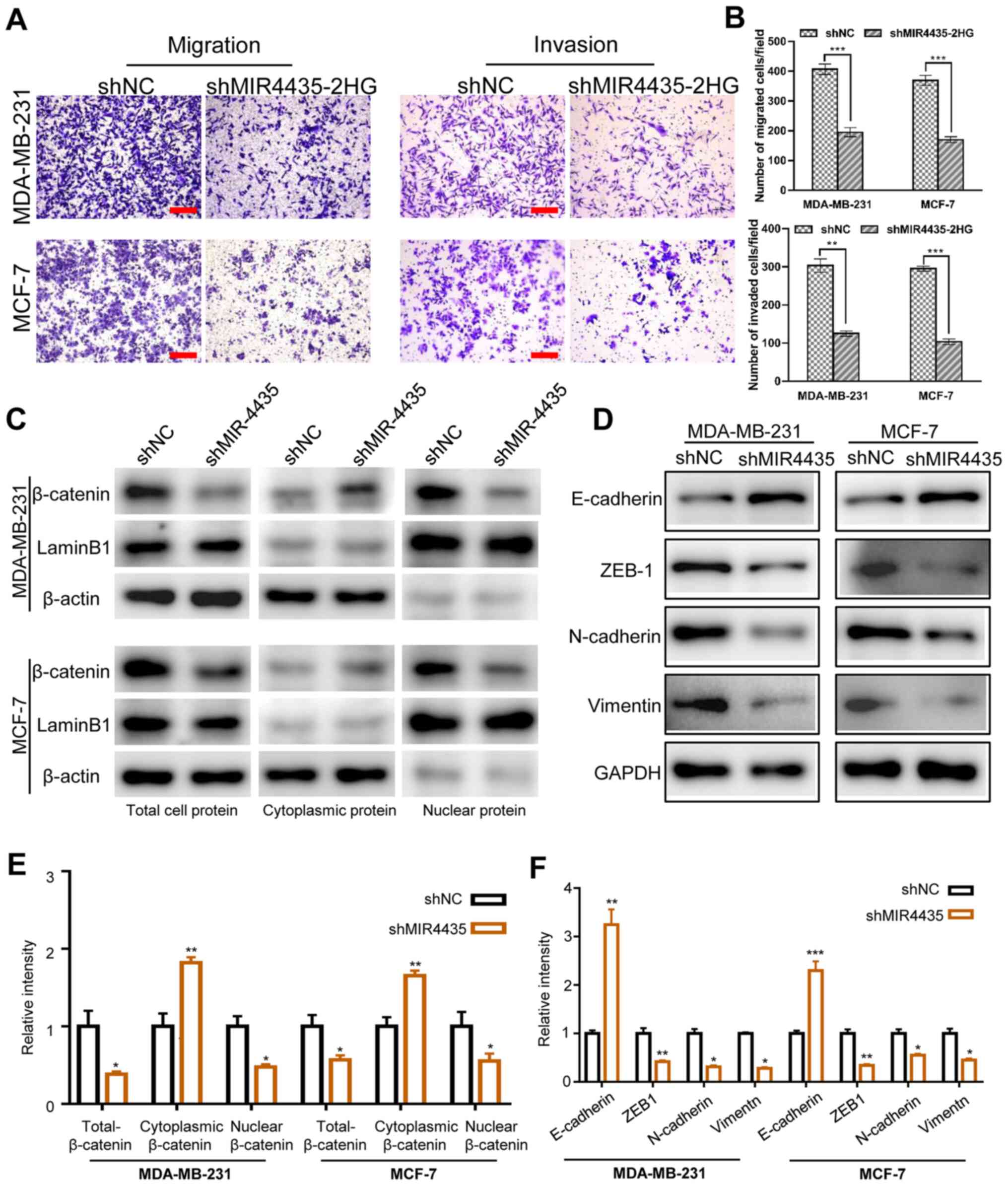
The present study performed transwell assays to
investigate the effect of MIR4435-2HG on the migration and invasion
of breast cancer cells. As presented in Fig. 4A and B, MDA-MB-231 and MCF-7 cells
displayed significantly lowered migration and invasion capabilities
compared with the control cells upon MIR4435-2HG silencing. A
previous study reported that MIR4435-2HG could inhibit gastric
cancer progression through inactivating Wnt/β-catenin pathway
(17). Given that dysregulation of
Wnt/β-catenin pathway has been shown to be involved in the
progression of various types of tumor, including breast cancer
(23), the present study examined
whether MIR4435-2HG could regulate breast cancer metastasis by
altering the Wnt/β-catenin signaling pathway. Following the western
blot analyses, it was revealed that MIR4435-2HG knockdown decreased
total and nuclear β-catenin expression, and slightly elevated the
cytoplasmic β-catenin expression (Fig.
4C and E). The present study also assessed the expression of
EMT-associated proteins via western blotting. As presented in
Fig. 4D and F, MIR4435-2HG knockdown
attenuated the expression of N-cadherin, ZEB1and vimentin, whereas
E-cadherin expression was enhanced. Collectively, the
aforementioned findings demonstrate that MIR4435-2HG contributed to
breast cancer cell metastasis through orchestrating Wnt/β-catenin
signaling.
Discussion
It has previously been described that MIR4435-2HG is
upregulated and plays crucial roles in HCC (14), lung cancer (15), ovarian cancer (24), prostate cancer (25) and gastric cancer (17). Notably, MIR4435-2HG expression is
increased in breast cancer tissues and is associated with poor
prognosis (20). However, whether
MIR4435-2HG affects the biological function of breast cancer cells
remains to be elucidated. To the best of our knowledge, the present
study it the first to report on the oncogenic function of
MIR4435-2HG in breast cancer cells.
Apoptosis failure, as one of the hallmarks of cancer
(also referred to as genetically programmed cell death), has been
extensively demonstrated to be executed upon the cleavage of a
series of cysteine proteases termed pro-caspases (26). The initiators caspase-8 and −9 are
the first to be activated during apoptosis, and they subsequently
activate the executioners caspase-3 and −7, which are responsible
for the cleavage of several downstream substrates, including the
PARP protein, ultimately activating apoptosis (27,28).
Activation of caspase-3 could cleave BID to promote the
mitochondrial apoptosis pathway (29). In the present study, it was revealed
that MIR4435-2HG knockdown causes the upregulation of cleaved-PARP
and cleaved caspase-3. Furthermore, MIR4435-2HG knockdown increased
the expression of pro-apoptotic protein Bax and decreased the
expression of anti-apoptotic protein Bcl-2 in breast cancer cells.
The results of the present study suggest that the mitochondrial
pathway is associated with MIR4435-2HG knockdown-mediated apoptosis
in breast cancer cells.
Increasing evidence indicates that the EMT plays a
crucial role in the metastasis of breast cancer. EMT is a complex,
reversible and multi-step biological event characterized by the
loss of apical polarity and cell-cell contacts and acquired
mesenchymal phenotypes of mobility, plasticity and stem cell-like
properties (30,31). Notably, EMT may occur under
physiological processes, such as embryogenesis and wound healing;
however, it is active during carcinogenesis, thereby conferring the
invasive and metastatic abilities of tumor cells (32). As EMT occurs, the mesenchymal protein
markers N-cadherin and vimentin are increased and concurrently, the
epithelial protein marker E-cadherin is decreased (33). In the present study, it was revealed
that MIR4435-2HG knockdown significantly decreased the expression
of mesenchymal markers, including N-cadherin, vimentin and ZEB1,
whereas it augmented expression of epithelial marker E-cadherin.
These results indicate that MIR4435-2HG promotes metastasis by
driving EMT in breast cancer cells. Based on a previous report,
MIR4435-2HG could inhibit gastric cancer progression through
inactivating Wnt/β-catenin pathway (17). Consistently, the results of the
present study also demonstrated that knockdown of MIR4435-2HG
resulted in a decrease of total and nuclear β-catenin expression.
The Wnt/β-catenin signaling pathway has been shown to be involved
in the anti-apoptotic function of cells (34–36).
Arsenic trioxide (ATO) significantly induced HeLa cell apoptosis
via decreasing β-catenin expression at both the mRNA and protein
levels (34). Triptolide induces
breast cancer cell apoptosis by suppressing the Wnt/β-catenin
signaling pathway (35). The
generation of reactive oxygen species by thalidomide suppressed the
Wnt/β-catenin signaling pathway, with subsequent activation of the
intrinsic apoptotic pathway (36).
However, whether other signaling pathways are involved in
MIR4435-2HG-mediated tumor progression need to be further
investigated. Several previous reports have demonstrated that
MIR4435-2HG promotes cancer progression via activating the TGF-β
signaling pathway in prostate and lung cancer (25,37).
Thus, it would be of great importance to investigate whether
MIR4435-2HG modulates this pathway in breast cancer. In addition,
Kong et al (38) demonstrates
that MIR4435-2HG promotes HCC cell proliferation by upregulating
miRNA-487a. Therefore, it is rational to hypothesize that
MIR4435-2HG may modulate miRNA expression in breast cancer. Taken
together, the present study provides new insight into the oncogenic
roles of MIR4435-2HG in breast cancer progression, and thus may be
utilized as a critical therapeutic target for breast cancer
treatment. However, additional studies are needed to fully
understand the molecular mechanisms of MIR4435-2HG-mediated tumor
progression.
In conclusion, the present study demonstrated that
MIR4435-2HG was upregulated in breast cancer and predicts poor
prognosis. Notably, MIR4435-2HG knockdown promotes cell apoptosis
but inhibits breast cancer cell proliferation, migration and
invasion by impeding the Wnt/β-catenin axis. These results
suggested the potential value of MIR4435-2HG as a novel promising
diagnostic, therapeutic and prognostic target for the treatment of
breast cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Zhejiang Province Basic Public Welfare Research Project (grant no.
GF18H160036), the General Program-Education Department of Zhejiang
Province (grant no. Y201738393), the General Program-Department of
Education of Zhejiang Province (grant no. Y202045370) and the
Project of Hangzhou Science and Technology Commission (grant no.
20170533B66).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MZ and XC conceived and designed the study and wrote
the manuscript. DC, PT, FW and YW performed the experiments and
analyzed the datasets. JL and JZ performed flow cytometric analysis
and edited the manuscript. DC and XC confirmed the authenticity of
all the raw data. All authors read and approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ribeiro Pereira ACP, Koifman RJ and
Bergmann A: Incidence and risk factors of lymphedema after breast
cancer treatment: 10 years of follow-up. Breast. 36:67–73. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lemler DJ, Lynch ML, Tesfay L, Deng Z,
Paul BT, Wang X, Hegde P, Manz DH, Torti SV and Torti FM: DCYTB is
a predictor of outcome in breast cancer that functions via
iron-independent mechanisms. Breast Cancer Res. 19:252017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cuzick J, DeCensi A, Arun B, Brown PH,
Castiglione M, Dunn B, Forbes JF, Glaus A, Howell A, von Minckwitz
G, et al: Preventive therapy for breast cancer: A consensus
statement. Lancet Oncol. 12:496–503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weigelt B, Peterse JL and van't Veer LJ:
Breast cancer metastasis: Markers and models. Nat Rev Cancer.
5:591–602. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rinn JL and Chang HY: Genome regulation by
long noncoding RNAs. Annu Rev Biochem. 81:145–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu YP, Jin YP, Wu XS, Yang Y, Li YS, Li
HF, Xiang SS, Song XL, Jiang L, Zhang YJ, et al: LncRNA-HGBC
stabilized by HuR promotes gallbladder cancer progression by
regulating miR-502-3p/SET/AKT axis. Mol Cancer. 18:1672019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin C and Yang L: Long noncoding RNA in
cancer: Wiring signaling circuitry. Trends Cell Biol. 28:287–301.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Z, Wang P, Cao L, Li F, Duan S, Yuan
G, Xiao L, Guo L, Yin H, Xie D, et al: Long intergenic non-coding
RNA 01121 promotes breast cancer cell proliferation, migration, and
invasion via the miR-150-5p/HMGA2 axis. Cancer Manag Res.
11:10859–10870. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo S, Jian L, Tao K, Chen C, Yu H and Liu
S: Novel breast-specific long non-coding RNA LINC00993 acts as a
tumor suppressor in triple-negative breast cancer. Front Oncol.
9:13252019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu Q, Li C, Wang S, Li Y, Wen B, Zhang Y,
Liang K, Yao J, Ye Y, Hsiao H, et al: LncRNAs-directed PTEN
enzymatic switch governs epithelial-mesenchymal transition. Cell
Res. 29:286–304. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jin C, Yan B, Lu Q, Lin Y and Ma L:
Reciprocal regulation of Hsa-miR-1 and long noncoding RNA MALAT1
promotes triple-negative breast cancer development. Tumour Biol.
37:7383–7394. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu X, Gu J, Ding X, Ge G, Zang X, Ji R,
Shao M, Mao Z, Zhang Y, Zhang J, et al: LINC00978 promotes the
progression of hepatocellular carcinoma by regulating EZH2-mediated
silencing of p21 and E-cadherin expression. Cell Death Dis.
10:7522019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qian H, Chen L, Huang J, Wang X, Ma S, Cui
F, Luo L, Ling L, Luo K and Zheng G: The lncRNA MIR4435-2HG
promotes lung cancer progression by activating β-catenin
signalling. J Mol Med (Berl). 96:753–764. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X, Ren Y and Zuo T: Long noncoding RNA
LINC00978 promotes cell proliferation and invasion in nonsmall cell
lung cancer by inhibiting miR-6754-5p. Mol Med Rep. 18:4725–4732.
2018.PubMed/NCBI
|
|
17
|
Wang H, Wu M, Lu Y, He K, Cai X, Yu X, Lu
J and Teng L: LncRNA MIR4435-2HG targets desmoplakin and promotes
growth and metastasis of gastric cancer by activating Wnt/β-catenin
signaling. Aging (Albany NY). 11:6657–6673. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luo P, Wu SG, Ji KB, Yuan X, Li HM, Chen
JP, Tian YF, Qiu Y and Zhong XM: LncRNA MIR4435-2HG mediates
cisplatin resistance in HCT116 cells by regulating Nrf2 and HO-1.
PLoS One. 15:e02230352020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu LJ, Wang AH, Gao M, Duan XY and Li ZH:
LncRNA MIR4435-2HG triggers ovarian cancer progression by
regulating miR-128-3p/CKD14 axis. Cancer Cell Int. 20:1452020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deng LL, Chi YY, Liu L, Huang NS, Wang L
and Wu J: LINC00978 predicts poor prognosis in breast cancer
patients. Sci Rep. 6:379362016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pohl SG, Brook N, Agostino M, Arfuso F,
Kumar AP and Dharmarajan A: Wnt signaling in triple-negative breast
cancer. Oncogenesis. 6:e3102017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu S, Qiao Z, Ma Q, Liu X and Ma X:
LncRNA CYTOR and MIR4435-2HG in ovarian cancer and its relationship
with clinicopathological features. Panminerva Med. 2019.(Epub ahead
of print).
|
|
25
|
Zhang H, Meng H, Huang X, Tong W, Liang X,
Li J, Zhang C and Chen M: lncRNA MIR4435-2HG promotes cancer cell
migration and invasion in prostate carcinoma by upregulating
TGF-β1. Oncol Lett. 18:4016–4021. 2019.PubMed/NCBI
|
|
26
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
McIlwain DR, Berger T and Mak TW: Caspase
functions in cell death and disease. Cold Spring Harb Perspect
Biol. 5:a0086562013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Degterev A, Boyce M and Yuan J: A decade
of caspases. Oncogene. 22:8543–8567. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Suliman A, Lam A, Datta R and Srivastava
RK: Intracellular mechanisms of TRAIL: Apoptosis through
mitochondrial-dependent and -independent pathways. Oncogene.
20:2122–2133. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang YP, Guo PT, Zhu Z, Zhang H, Xu Y,
Chen YZ, Liu F and Ma SP: Pleomorphic adenoma gene like-2 induces
epithelial-mesenchymal transition via Wnt/β-catenin signaling
pathway in human colorectal adenocarcinoma. Oncol Rep.
37:1961–1970. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang P, Zhao X, Zhang W, He A, Lei B,
Zhang W and Chen Y: Leukemia-associated gene MLAA-34 reduces
arsenic trioxide-induced apoptosis in HeLa cells via activation of
the Wnt/β-catenin signaling pathway. PLoS One. 12:e01868682017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shao HM, Ma JH, Guo TH and Hu RR:
Triptolide induces apoptosis of breast cancer cells via a mechanism
associated with the Wnt/β-catenin signaling pathway. Exp Ther Med.
8:505–508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Knobloch J, Schmitz I, Götz K,
Schulze-Osthoff K and Rüther U: Thalidomide induces limb anomalies
by PTEN stabilization, akt suppression, and stimulation of
caspase-dependent cell death. Mol Cell Biol. 28:529–538. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang M, He X, Huang X, Wang J, He Y and
Wei L: LncRNA MIR4435-2HG-mediated upregulation of TGF-beta1
promotes migration and proliferation of nonsmall cell lung cancer
cells. Environ Toxicol. 35:582–590. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kong Q, Liang C, Jin Y, Pan Y, Tong D,
Kong Q and Zhou J: The lncRNA MIR4435-2HG is upregulated in
hepatocellular carcinoma and promotes cancer cell proliferation by
upregulating miRNA-487a. Cell Mol Biol Lett. 24:262019. View Article : Google Scholar : PubMed/NCBI
|