Introduction
Cervical carcinoma (CC) is one of the most common
tumors among women in the world, and the incidence of which was ~7%
in globally 2020 (1). In developed
economies, the 5-year survival rate of patients with CC is >65%,
whereas this proportion is <20% in developing countries
(2). Chemotherapy is currently the
main treatment for the patients suffering from advanced or
recurrent CC (3). Cisplatin (DDP) is
widely used in chemotherapy as it blocks DNA replication, inhibits
cell cycle progression, induces apoptosis and hinders tumor growth
(4). DDP has a prominent effect on
CC treatments, DDP-based concurrent chemoradiotherapy is a standard
treatment for locally advanced cervical cancer (3); irinotecan administered alone or in
combination with DDP is useful in the treatment of recurrent
cervical cancer (4). However,
long-term use of DDP can lead to drug resistance in tumor cells,
depriving patients of favorable therapeutic efficacy (5). Hence, methods that can lower the drug
resistance of tumor cells are of great significance for improving
the treatment of patients with CC.
MicroRNA (miRNA), a class of conservative non-coding
RNA, widely exists in eukaryotic cells and participates in
biological processes, such as cell proliferation, differentiation
and apoptosis, which are closely related to tumor progression and
drug resistance of tumor cells (6–8). For
instance, miR-296-5p enhances the drug resistance of pancreatic
cancer cells leading to unfavorable prognosis in patients with
pancreatic cancer (9). miR-21 is
upregulated in DDP-resistant CC tissues and targets PTEN to promote
drug resistance of CC (10). A study
has demonstrated that miR-125a-5p reduces the resistance to
imatinib in gastrointestinal stromal tumor (11). However, in CC cells, the function and
mechanism of miR-125a-5p in regulating chemosensitivity remain
unclear.
LIM kinase 1 (LIMK1) is a serine/threonine kinase
belonging to the LIM kinase family that modulates actin
polymerization through phosphorylation of the actin-binding factor
cofilin 1, which subsequently modulates cell motility and cell
cycle (12). Studies have reported
that LIMK1 participates in the multidrug resistance of cancer
(13,14). For instance, LIMK1 promotes the
migration and the invasion of non-small cell lung cancer (NSCLC)
cells and facilitates the resistance of NSCLC cells to DDP
(14). However, in CC, the influence
of LIMK1 on the resistance of cancer cells to DDP and its mechanism
warrants further research.
The present study aimed to provide further insight
on the effect of miR-125a-5p and LIMK1 on CC cell viability and
apoptosis and validate the interaction between miR-125a-5p and
LIMK1. The findings of the present study may have important
implications for treatment of cisplatin resistance of CC.
Materials and methods
Ethics and sample collection
From April 2017 to April 2018, a total of 45
patients who had been diagnosed with CC were enrolled and the
surgically resected tumor tissues and corresponding adjacent
tissues (at least 5 cm from the tumor) were collected form the
Department of Pathology of Shengli Oilfield Central Hospital
(Dongying, China). All the resected tissues were immediately placed
in liquid nitrogen and stored in a cryogenic chamber at −80°C. All
of the patients were diagnosed via biopsy and received radical
hysterectomy for cervical cancer and did not receive any
neoadjuvant radiation, neoadjuvant chemotherapy, or immunotherapy.
The patients age range was 28–65 years (mean age, 46.5 years,
median values, 44 years). The present study was given approval by
the Medical Ethics Committee of Shengli Oilfield Central Hospital
(Dongying, China) (approval no. 2016-05). All patients provided
written informed consent prior to surgery.
Cell culture
Human CC cell lines (C-33A, CaSKi) and human
cervical epithelial cells (HUCEC) were purchased from the China
Infrastructure of Cell Line Resources, Institute of Basic Medical
Sciences, Chinese Academy of Medical Sciences. The DDP resistant
C-33A/DDP and CaSKi/DDP cell lines were established by treating
C-33A and CaSKi cells with increasing DDP concentration. The
concentration of cisplatin was increased gradually in the medium of
C-33A and CaSKi cells from 0.10, 0.50, 1.00, 1.25 to 2.50 µg/ml.
After 2 weeks of culture, the cell morphology was observed.
Subsequently, a high concentration of cisplatin, 2.50 5.00 and
10.00 µg/ml was applied to C-33A and CaSKi cells. Following this
method, C-33A and CaSKi cells were continuously cultured for 6
months. Finally, C-33A/DDP and CaSKi/DDP cells were cultured in
medium supplemented with 10.00 µg/ml cisplatin to maintain the
phenotype of cisplatin resistance. All of the cell lines mentioned
above were maintained in Dulbecco's modified Eagle's medium (DMEM;
HyClone; Cytiva) containing 10% fetal bovine serum (FBS; Hyclone,
Cytiva) 100 U/l penicillin and 100 mg/l streptomycin (both from
Thermo Fisher Scientific Inc.) at 37°C in 5% CO2. Medium
was changed every 2 days and subculture was carried out when the
cells reached ~85% confluence.
Cell transfection
Over-expressing-LIMK1 plasmid (LIMK1-OE), empty
plasmid (NC), siRNA targeting LIMK1 (si-LIMK1), siRNA negative
control (si-NC), miRNA mimics normal control (miR-NC), miR-125a-5p
mimics (miR-125a-5p), miRNA inhibitors normal control (inh-NC) and
miR-125a-5p inhibitors (miR-125a-5p-in) were provided by Guangzhou
RiboBio Co., Ltd. The sequences were as follows: si-LIMK1,
5′-CTCCAGAGGGCTAAGTGTT-3′; si-NC, 5′-CTCGAGCGGAATTGCAGTT-3′;
miR-125a-5p mimics, 5′-TCCCTGAGACCCTTTAACCTGTGA-3′; miR-NC,
5′-UUCUCCGAACGUGUCACGUTT-3′; miR-125a-5p-in,
5′-TCACAGGTTAAAGGGTCTCAGGGA-3′; and inh-NC,
5′-ACGAUACACGUUCGGAGAATT-3′. When cells confluence was ~50%,
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific Inc.) according to the manufacturer's instructions and
all the aforementioned plasmids, siRNAs, inhibitors and mimics were
transfected into CC cells. The transfection temperature was 37°C
and lasted for 24 h. The final concentration of oligonucleotides
the was 50 nM and labeled as miR-NC group, miR-125a-5p group,
inh-NC group, miR-125a-5p-in group, NC group, LIMK1 group, si-NC
group, si-LIMK1 group, LIMK1+miR-125a-5p group and
si-LIMK1+miR-125a-5p-in group, respectively. After 24 h, cells were
analyzed to determine transfection efficiency using reverse
transcription-quantitative (RT-q) PCR or used for subsequent
experimentation.
RT-qPCR
After homogenization of the CC tissues or CC cells
(C-33A, CaSKi, C-33A/DDP and CaSKi/DDP), TRIzol®
(Invitrogen; Thermo Fisher Scientific Inc.) reagent was added to
extract total RNA. Then, reverse transcription was performed using
Takara reverse transcription kit (Takara Biotechnology Co., Ltd.).
Temperature and duration for reverse transcription were 37°C for 15
min and 85°C for 5 min. A PCR reaction system with a final volume
of 20 µl was established according to the manufacturer's
instructions. A total of 2 µl of cDNA, 10 µl of SYBR-Green qPCR
Master Mix (Applied Biosystems), 0.5 µl of upstream and downstream
primers (10 µmol/l), and ddH2O was added to supplement
the system volume to 20 µl. The thermocycling conditions were as
follows: initial denaturation at 95°C for 5 min; 45 cycles of
denaturation at 95°C for 15 sec, annealing at 60°C for 30 sec and
extension at 72°C for 20 sec. miR-125a-5p expression was normalized
to U6, LIMK1 mRNA expression were normalized to GAPDH. The relative
expression of mRNA was measured by 2-ΔΔCq method
(15). The primers sequences used
were presented in Table I.
 | Table I.Primer sequences for RT-qPCR. |
Table I.
Primer sequences for RT-qPCR.
| Name | Sequence (5–3) |
|---|
| miR-125a-5p | F:
CTGTCCCTGAGACCCTTTAAC |
|
| R:
CGAGGAAGAAGACGGAAGAAT |
| LIMK1 | F:
CAAGGGACTGGTTATGGTGGC |
|
| R:
CCCCGTCACCGATAAAGGTC |
| U6 | F:
CTCGCTTCGGCAGCACA |
|
| R:
AACGCTTCACGAATTTGCGT |
| GAPDH | F:
AGAAGGCTGGGGCTCATTTG |
|
| R:
AGGGGCCATCCACAGTCTTC |
MTT assay
C-33A/DDP cells and CaSKi/DDP cells were harvested
and trypsinized. The cells were resuspended and inoculated into
96-well plates at a density of 2×103 cells/well. On the
1st, 2nd and 3rd day of culture, 20 µl of MTT solution (5 mg/ml)
was added per well and incubated for 4 h. Following that, the
medium was removed and 150 µl dimethyl sulfoxide (DMSO) was dripped
into each well and oscillated by hand at room temperature for 10
min. For the determination of the IC50 value of DDP,
C-33A/DDP cells and CaSKi/DDP cells were seeded into 96-well plates
and treated with a DDP concentration gradient of 0, 2.5, 5, 10, 20,
40 and 80 µg/ml for 24 h. The absorbance (A) values at were
recorded at 490 nm by a microplate reader. The cell proliferation
rate and the inhibition rate of DDP were calculated using the
following formulas: cell viability (%) = (A value of control
group-A value of specifically treated group)/A value of
specifically treated group ×100%; the inhibition rate of the drug
to the cells (%)=(1-A value of specifically treated group/A value
of control group) ×100%. IC50 was calculated by Probit
analysis using the SPSS 22.0 software (IBM Corp.).
BrdU assay
Transfected C-33A/DDP and CaSKi/DDP cells were
seeded at a density of 2,000 cells/well in a 96 well plate. Next,
48 h after transfection, cell proliferation was analyzed using the
BrdU Cell Proliferation Assay kit (AmyJet Scientific Inc.)
according to the manufacturer's instructions. All images were taken
by a fluorescence microscope (magnification, ×200). The counting of
BrdU-positive cells was performed using ImageJ software v.1.52a
(National Institutes of Health).
Flow cytometry analysis
Annexin V-FITC/propidium iodide (PI) Apoptosis
Detection kit (cat. no. 40302ES20, Yeasen Biotech Co., Ltd.) was
used to detect apoptosis. After being maintained for 72 h, the
transfected C-33A/DDP and CaSKi/DDP cells were washed three times
with PBS, then trypsinized and harvested from the medium.
Subsequently, cells were resuspended with 500 µl binding buffer,
stained with Annexin V-FITC and PI and incubated for 20 min in the
dark. Subsequently, apoptosis was detected by flow cytometry (Epics
XL; Beckman Coulter Inc.) and FlowJo software v.10.4.2 (Treestar,
Inc.) was used to analyze the data. Early and late apoptosis was
assessed.
Western blotting
P-glycoprotein (P-gp) and Glutathione
S-transferase-π (GST-π) are recognized as typical DDP
chemoresistance protein markers (16,17),
hence their protein expression was assessed. Transfected C-33A/DDP
and CaSKi/DDP cells were harvested and lysed with RIPA Lysis Buffer
containing protease inhibitors (Thermo Fisher Scientific Inc.) for
30 min. The supernatant was centrifuged at 4° at the speed of
12,000 r/min (14,440 × g) for 10 min, and the protein concentration
was determined using bicinchoninic acid (BCA) kit (Beyotime
Institute of Biotechnology). Following that, 10 µg protein of the
supernatant was subjected to 12.5% SDS-PAGE and then transferred to
PVDF membrane. Subsequently, the membrane was blocked with 5%
skimmed milk for 1 h at room temperature, incubated with primary
antibody for 24 h at 4°C, then rinsed with TBST (0.1% Tween) and
incubated with secondary antibody (1:2,000; cat. no. ab205718) for
another 1 h at room temperature. The primary antibodies used in
this study including anti-β-actin antibody (1:500; cat. no.
ab8227), anti-LIMK1 antibody (1:500; cat. no. ab81046,), anti-P-gp
(1:500; cat. no. ab103477), and anti-GST-π (1:500; cat. no.
ab242014) were purchased from Abcam. Subsequently, the membrane was
washed with TBST 3 times, and visualized using ECL (Guangzhou Xiang
Bo Biological Technology Co., Ltd.). The intensity of each band was
quantified using ImageJ software v.1.52a National Institutes of
Health).
Bioinformatics analysis
The online software TargetScan (TargetScan Human
7.0; http://www.targetscan.org/vert_70/) was used for
predicting downstream mRNAs of the miRNA.
Luciferase reporter gene assay
All luciferase reporter vectors [LIMK1-wild-type
(WT) and LIMK1-mutant (MUT)] were constructed based on the pGL3
Luciferase Reporter Vector (Promega Corporation). C-33A/DDP and
CaSKi/DDP cells were collected, and the reporter vectors were
co-transfected with miR-NC (5′-UUCUCCGAACGUGUCACGUTT-3′) or
miR-125a-5p mimics (5′-TCCCTGAGACCCTTTAACCTGTGA-3′) with
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific Inc.) according to the manufacturer's instructions.
After 24 h, the treated cells were lysed with lysate and
centrifuged at a speed of 14,440 × g for 10 min at 4°C and the
supernatant was collected. Subsequently, the Dual-Luciferase
Reporter Gene Assay kit (Promega Corporation) was used to measure
luciferase activity according to the manufacturer's instructions.
Relative luciferase activity was assayed 24 h after the
transfection and normalized with Renilla luciferase activity.
Statistical analysis
All assays were repeated at least three times and
the data were statistically analyzed using SPSS 22.0 (IBM Corp.).
All data were expressed as mean ± standard deviation (mean ± SD).
Whether the data were normally distributed was examined by
One-Sample Kolmogorov-Smirnov test. For normally distributed data,
unpaired Student's t-test was used to make the comparison between 2
groups. one-way or two-way (as appropriate) ANOVA followed by the
post hoc Tukey's test was used for multiple comparisons.
IC50 of the cells to cisplatin was calculated by Probit
analysis. Pearson's correlation analysis was used to analyze the
correlation between miR-125a-5p expression and LIMK1 expression in
CC samples. P<0.05 was considered to indicate a statistically
significant difference.
Results
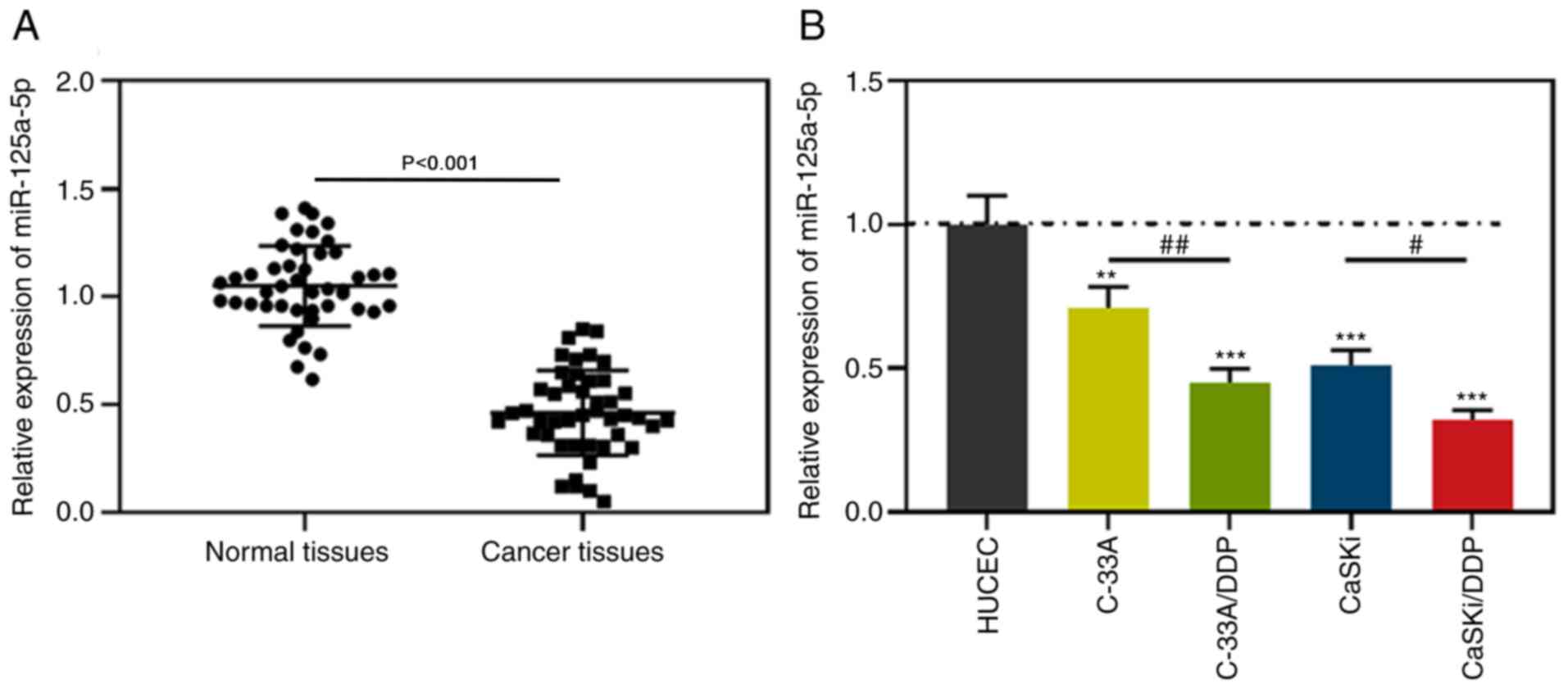
miR-125a-5p is downregulated in CC
tissues and cells compared with normal tissues and HUCECs
Firstly, RT-qPCR was used to detect the expression
of miR-125a-5p in 45 pairs of CC cancer tissues and corresponding
normal tissues, the findings of which indicated that miR-125a-5p
expression in CC tissues was downregulated compared with normal
tissues (Fig. 1A). Subsequently,
C-33A/DDP and CaSKi/DDP cell lines were established and flow
cytometry performed, the findings indicated that there were no
tumor-suppressive effects of 10 µg/ml DDP on the apoptotic rate in
C-33A/DDP and CaSKi/DDP cells (Fig.
S1A). Western blotting demonstrated that compared with C-33A
and CaSKi cells, protein expressions of P-gp and GST-π in
C-33A/DDP, and CaSKi/DDP cells were significantly upregulated
(Fig. S1B). In addition, RT-qPCR
was used to detect the expression of miR-125a-5p in normal cervical
epithelial cells (HUCEC) and CC cell lines. The results
demonstrated that compared with HUCEC cells, miR-125a-5p expression
in CC cells was significantly downregulated, while the expression
of miR-125a-5p in C-33A/DDP and CaSKi/DDP cells was significantly
lower compared with C33A and CaSKi cells (Fig. 1B). These findings implied that the
downregulation of miR-125a-5p was related to the occurrence of DDP
resistance in CC cells.
miR-125a-5p reduces drug resistance of
DDP-resistant CC cells
RT-qPCR analysis demonstrated that, compared with
miR-NC, the expression level of miR-125a-5p was significantly
increased in CaSKi/DDP cells transfected with miR-125a-5p mimics;
compared with inh-NC, while the expression level of miR-125a-5p was
significantly decreased in C-33A/DDP cells transfected with
miR-125a-5p inhibitors (Fig. 2A).
Subsequent experiments revealed that compared with the miR-NC
group, the cell viability and proliferation of the miR-125a-5p
group were reduced, the apoptosis rate of the cells was increased,
the IC50 value of DDP was reduced (Fig. 2B-G). Western blotting results
demonstrated that P-gp and GST-π protein expression was
downregulated in the miR-125a-5p mimic group. Conversely, compared
with the inh-NC group, the cell viability and proliferation of the
miR-125a-5p-in group were increased, the apoptosis rate of the
cells was reduced, the IC50 value of DDP was increased,
P-gp and GST-π protein expression was upregulated (Fig. 2B-G). These results suggested that
miR-125a-5p sensitized CC cells to DDP.
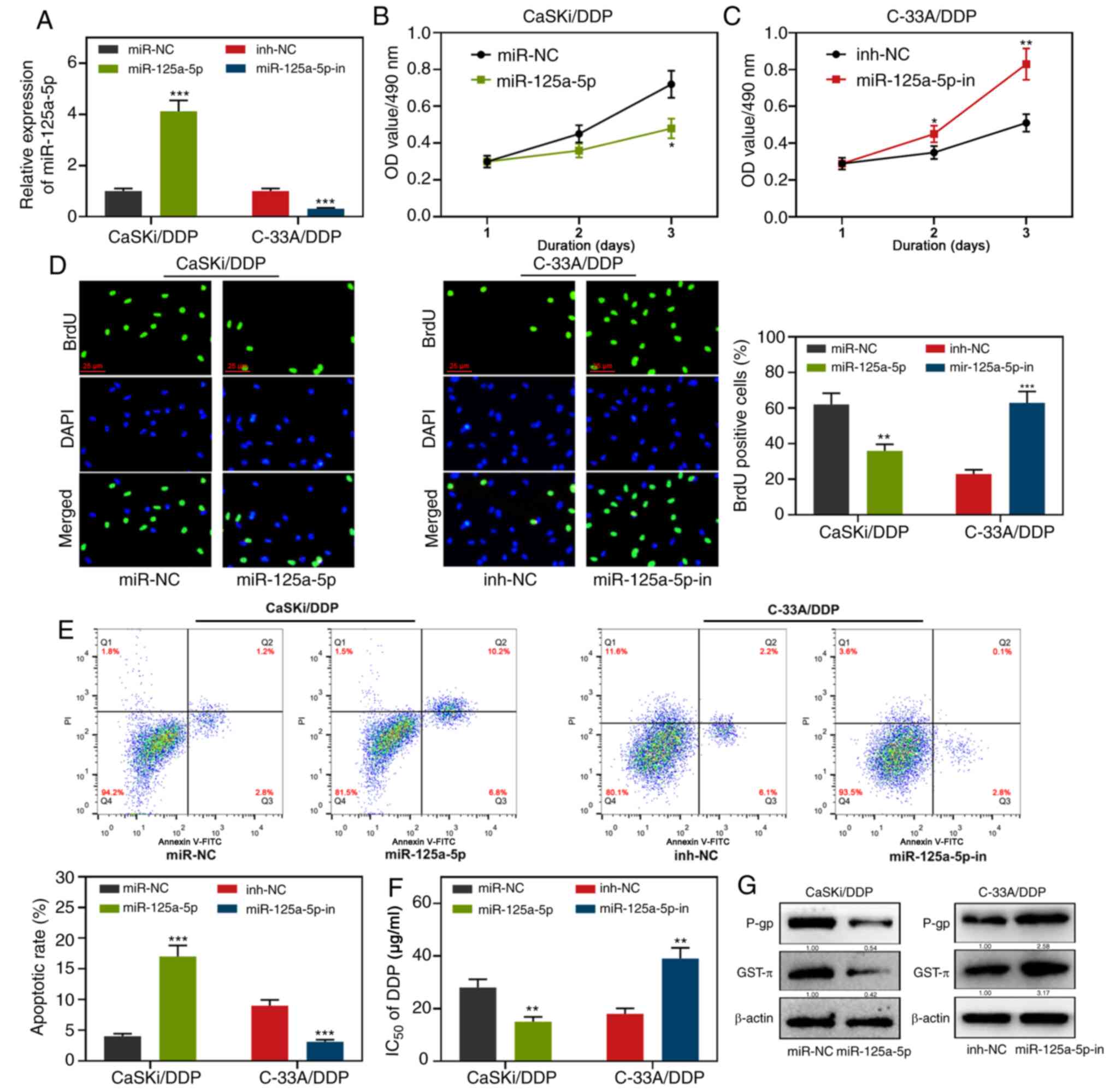 | Figure 2.Effects of miR-125a-5p on CC cell
proliferation, apoptosis, IC50 of DDP and
chemoresistance-related proteins. (A) RT-qPCR was used to detect
the transfection efficiency of miR-125a-5p mimics or miR-125a-5p
inhibitors. (B-D) MTT assay and BrdU assay were used to detect the
proliferation activity of CaSKi/DDP and C-33A/DDP cells after
transfection with miR-125a-5p mimics or miR-125a-5p inhibitors. (E)
Effects of miR-125a-5p mimics or miR-125a-5p inhibitors on
apoptosis were detected by flow cytometry analysis. (F) MTT assay
was used to detect the effect of miR-125a-5p mimics or miR-125a-5p
inhibitors on IC50 of DDP. (G) Effects of miR-125a-5p
mimics or miR-125a-5p inhibitors on the expression of apoptotic
protein were detected by western blot. *P<0.05, **P<0.01 and
***P<0.001. miR, microRNA; RT-q, reverse
transcription-quantitative; CC, cervical carcinoma; DDP, cisplatin;
NC, negative control; inh, inhibitor; OD, optical density; P-gp,
anti-P-glycoprotein; GST-π, anti-glutathione S-transferase-π. |
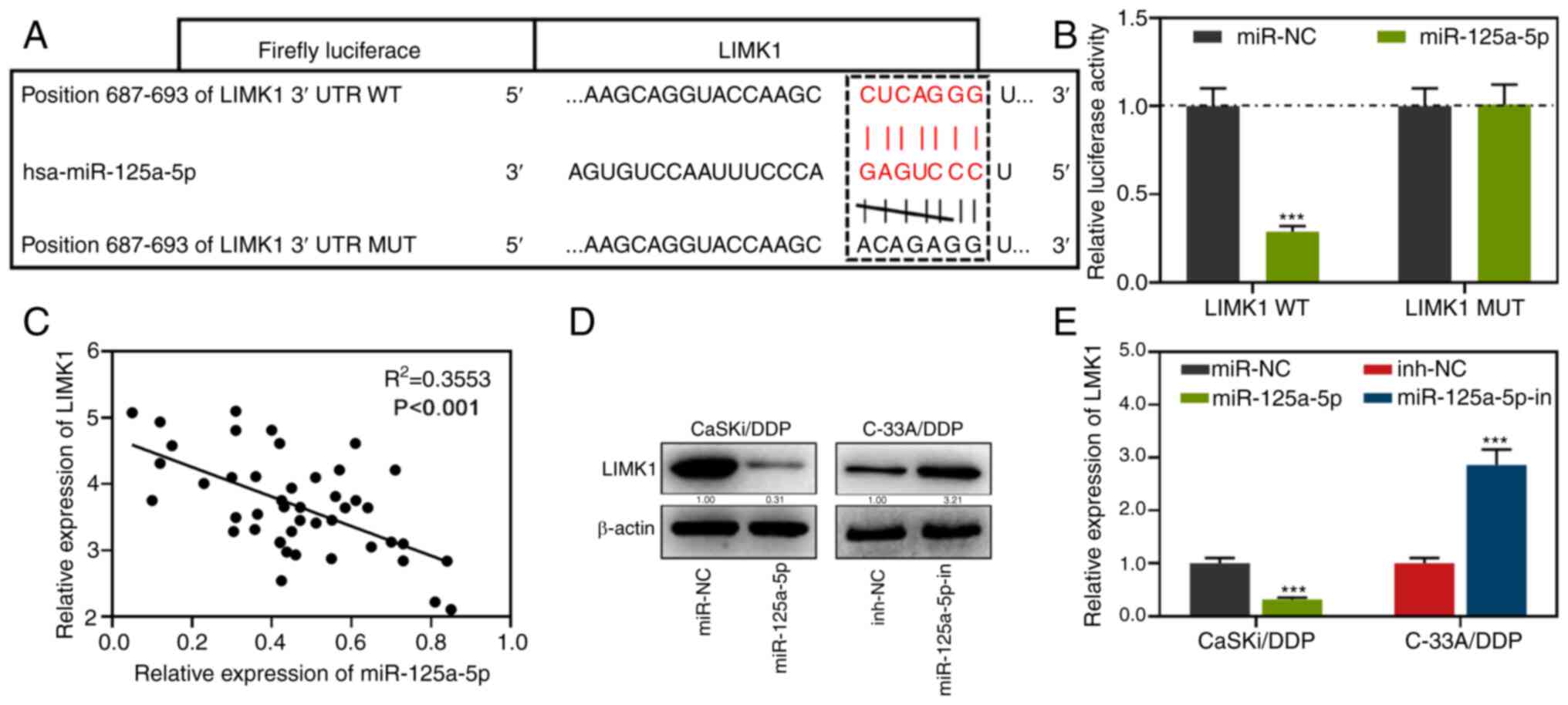
LIMK1 is a target gene of
miR-125a-5p
By an online prediction with TargetScan, it was
predicted that there was a potential binding site between LIMK1 and
miR-125a-5p (Fig. 3A). In addition,
the targeting relationship between miR-125a-5p and LIMK1 was
validated by the luciferase reporter gene assay. It was found that
the luciferase activity of LIMK1 WT cells transfected with
miR-125a-5p mimics was lower compared with the miR-NC group. There
were no significant changes observed in the luciferase activity of
LIMK1 MUT cells (Fig. 3B). Pearson's
correlation test was used to investigate the correlation between
LIMK1 and miR-125a-5p and the results demonstrated that miR-125a-5p
mRNA expression was negatively correlated with LIMK1 mRNA
expression (Fig. 3C). Additionally,
LIMK1 expression in the cells transfected with miR-125a-5p mimics
was downregulated compared with the miR-NC group; meanwhile, the
expression of LIMK1 in the cells transfected with miR-125a-5p
inhibitors was upregulated compared with the inh-NC group (Fig. 3D-E). In summary, miR-125a-5p
negatively regulated LIMK1 expression in CC cells.
LIMK1 is upregulated and enhances the
drug resistance in DDP-resistant CC cells compared with normal
tissues and HUCECs
LIMK1 mRNA expression was examined by RT-qPCR, the
results indicated that compared with normal tissues, LIMK1
expression was enhanced in CC tissues (Fig. 4A). In addition, the expression of
LIMK1 in CC cells was significantly upregulated compared with HUCEC
cells, and LIMK1 expression was higher in C-33A/DDP and CaSKi/DDP
cells compared with C-33A and CaSKi cells (Fig. 4B-C). Subsequently, C-33a/DDP cells
with the lowest expression of LIMK1 were selected for
overexpression experiments and CaSki/DDP cells with the highest
expression of LIMK1 were chosen for knockdown experiments. RT-qPCR
analysis also demonstrated that the expression level of LIMK1
significantly increased in C-33A/DDP cells transfected with
LIMK1-overexpressing vector compared with NC group; while compared
with si-NC group, the expression level of LIMK1 significantly
decreased in CaSKi/DDP cells transfected with si-LIMK1 (Fig. 4D). Further experiments demonstrated
that compared with the NC group, the proliferation activity of the
CC cells in the LIMK1 overexpression group was increased, the
apoptosis rate was reduced, the IC50 value of DDP was
increased, and P-gp and GST-π protein expressions were upregulated
(Fig. 4E-I). In contrast, compared
with the control group, the cell viability of the LIMK1 knockdown
group was decreased, the apoptosis rate was enhanced, the
IC50 value of DDP was decreased, and the protein
expression level of P-gp and GST-π protein were decreased (Fig. 4E-I). These results indicated that
LIMK1 enhanced the drug resistance of DDP-resistant CC cells and
targeting LIMK1 reversed the chemoresistance.
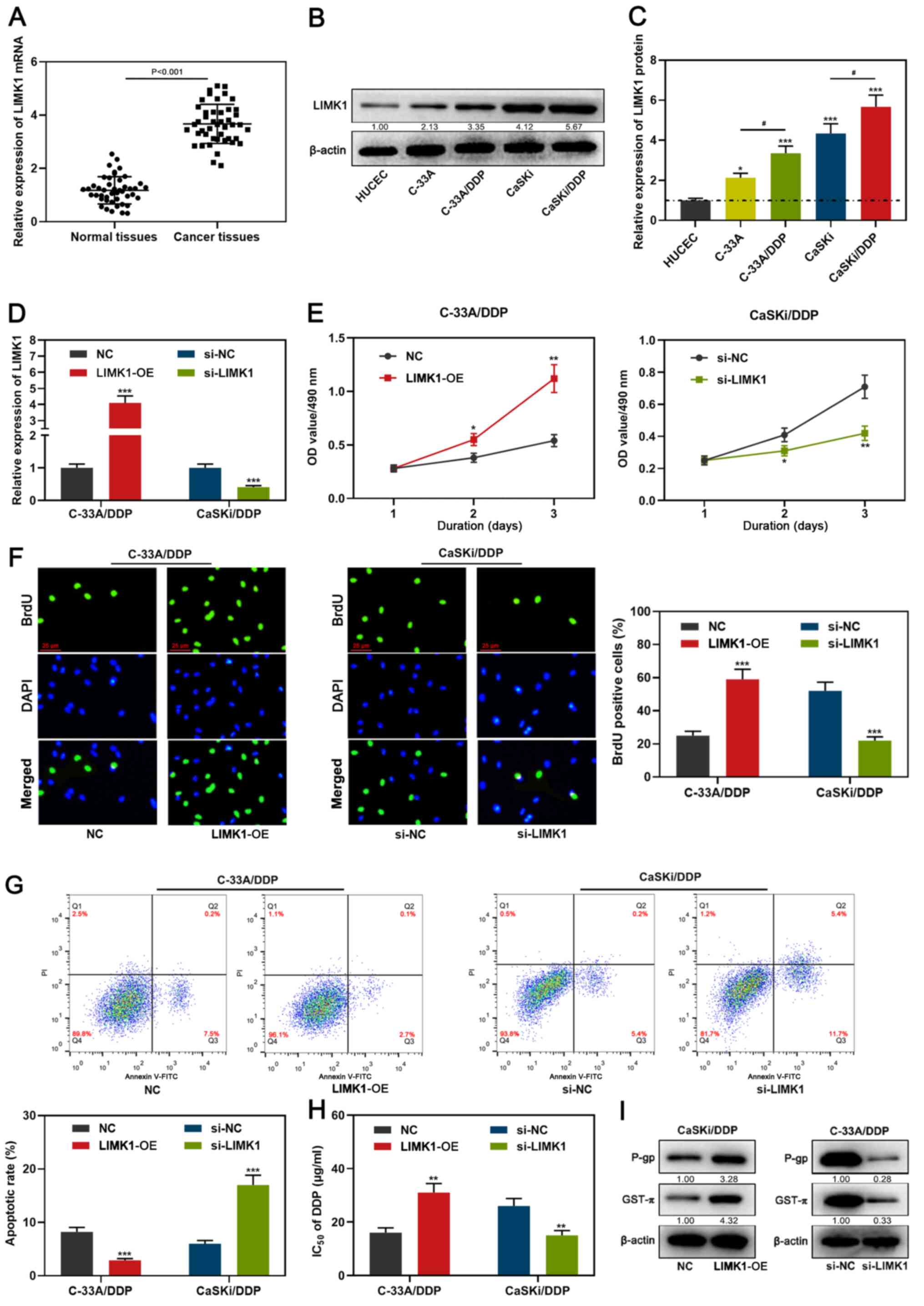 | Figure 4.LIMK1 expression pattern in CC and
the effects of it on proliferation, apoptosis, IC50 of
DDP and drug resistance-related proteins in CC cells. (A-C) LIMK1
expression in CC tissues and cells were detected by RT-qPCR and
western blotting. (D) RT-qPCR was used to detect the transfection
efficiency of C-33A/DDP and CaSKi/DDP cells after transfection with
LIMK1 overexpression plasmid or si-LIMK1. (E-F) MTT assay and BrdU
assay were used to detect the proliferation activity of C-33A/DDP
and CaSKi/DDP cells after transfection with LIMK1 overexpression
plasmid or si-LIMK1. (G) Effects of LIMK1 overexpression plasmid or
si-LIMK1 on apoptosis of CC cells. (H) MTT assay was used to detect
the effect of LIMK1 overexpression plasmid or si-LIMK1 on
IC50 of DDP. (I) Effects of LIMK1 overexpression plasmid
or si-LIMK1 on the expression of chemoresistance-related proteins
were detected by western blotting. *P<0.05, **P<0.01 and
***P<0.001 vs. HUCEC, NC or si-NC. #P<0.05 vs.
DDP-resistant cells. miR, microRNA; RT-q, reverse
transcription-quantitative; CC, cervical carcinoma; DDP, cisplatin;
NC, negative control empty plasmid; OD, optical density; P-gp,
anti-P-glycoprotein; GST-π, anti-glutathione S-transferase-π;
LIMK1, LIM kinase 1; si, small interfering; PI, propidium iodide;
OE, overexpression. |
miR-125a-5p reduces drug resistance of
DDP-resistant CC cells by absorbing LIMK1
To investigate the effect of miR-125a-5p targeting
LIMK1 on the chemoresistance of CC cells, miR-125a-5p mimics and
LIMK1 overexpressing plasmids, miR-125a-5p inhibitors and si-LIMK1
were co-transfected into DDP resistant cells. RT-qPCR analysis
demonstrated that the transfections were successful (Fig. 5A). Further experiments revealed that
compared with the NC group, the proliferation activity of cells in
the LIMK1 group was enhanced, while miR-125a-5p mimics inhibited
this effect (Fig. 5B-C). Also, LIMK1
inhibited the apoptosis rate of cells, while miR-125a-5p partially
reversed this effect (Fig. 5D).
Compared with the NC group, in the overexpressing LIMK1 group, the
IC50 value of DDP was increased, and P-gp and GST-π
protein expression were upregulated, but miR-125a-5p could
attenuate these effects (Fig. 5E-F).
As expected, compared with the si-NC group, LIMK1 knockdown
inhibited cell viability, promoted apoptosis, reduced the
IC50 value of DDP to cells, and downregulated P-gp and
GST-π protein expression, whereas miR-125a-5p inhibitors negatively
regulated these phenomena (Fig.
5B-F). These results implied that the function of miR-125a-5p
on modulating the chemoresistance of CC cells was dependent on
LIMK1.
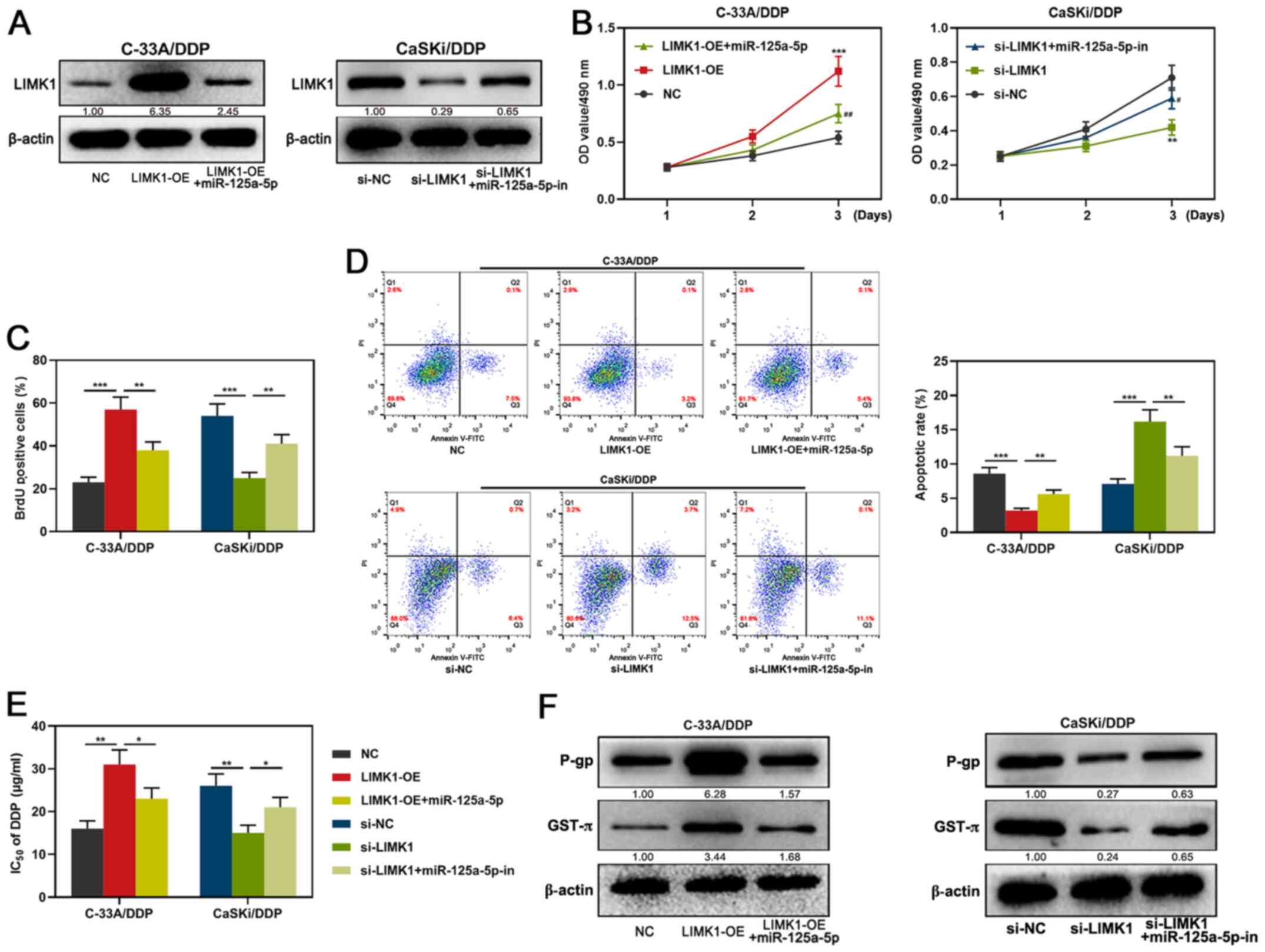 | Figure 5.Effects of miR-125a-5p/LIMK1 axis on
the proliferation, apoptosis, IC50 of DDP and drug
resistance-related proteins in CC cells. (A) Expression of LIMK1 of
C-33A/DDP and CaSKi/DDP cells after co-transfection with
miR-125a-5p mimics and LIMK1 overexpression plasmid, miR-125a-5p
inhibitors and si-LIMK1 were detected by western blotting. (B-C)
MTT assay and BrdU assay were used to detect the proliferation
activity of CC cells after transfection. (D) Flow cytometry was
used to detect the apoptosis of CC cells. (E) MTT assay was used to
detect the change of IC50 of DDP after transfection. (F)
Western blotting was used to detect the expression of
chemoresistance-related proteins after transfection. *P<0.05,
**P<0.01, and ***P<0.001 vs. NC or si-NC.
#P<0.05, and ##P<0.01 vs. LIMK1-OE or
si-LIMK1. miR, microRNA; RT-q, reverse transcription-quantitative;
CC, cervical carcinoma; DDP, cisplatin; NC, negative control empty
plasmid; OD, optical density; P-gp, anti-P-glycoprotein; GST-π,
anti-glutathione S-transferase-π; LIMK1, LIM kinase 1; si, small
interfering; inh, inhibitor; PI, propidium iodide; OE,
overexpression. |
Discussion
Chemotherapy is a crucial therapy for patients with
CC to prolong lifespan and improve quality of life, and DDP has
been widely used in clinical CC treatments (10,18).
However, the resistance of cancer cells to DDP during treatments
limits the efficacy of DDP (19). In
recent years, numerous studies have demonstrated that miRNAs and
mRNAs feature prominently in CC proliferation, metastasis,
apoptosis and DDP resistance (20–22). The
present study demonstrated that miR-125a-5p expression was
downregulated in CC tissues and cell lines compared with normal
tissues or HUCECs. The gain and loss of function experiments
results indicated that miR-125a-5p targeted LIMK1 to inhibit CC
cell proliferation, promote apoptosis and reduce drug resistance to
DDP.
miR-125a-5p is differentially expressed in various
cancers, such as colorectal cancer, bladder cancer, and breast
cancer and serves as a tumor suppressor and can affect tumor cell
proliferation, apoptosis and metastasis via regulating vascular
endothelial growth factor A, fucosyltransferase 4, BRCA1 associated
protein 1 and ABL proto-oncogene 2 (23–26). For
instance, miR-125a-5p suppresses colorectal cancer progression by
targeting VEGFA (23). In CC,
miR-125a-5p downregulates ABL proto-oncogene 2 expression, thereby
inhibiting cell proliferation and migration (26). With in-depth study, it has been found
that miR-125a-5p can affect not only the growth and the metastasis
of tumor cells, but also the drug resistance of tumor cells
(27). In esophageal squamous cell
carcinoma, miR-125a-5p expression is downregulated and the
sensitivity of cancer cells to DDP is enhanced via activation of
the STAT3 signaling pathway (28).
In the present study, it was found that compared with normal
tissues or HUCECs, the expression of miR-125a-5p was downregulated
in CC tissues and cell lines; miR-125a-5p mimics could inhibit the
expression of drug resistance-related proteins P-gp and GST-π,
hinder cell proliferation, promote apoptosis and decrease
IC50 value in DDP resistant cells. The results of
transfection of miR-125a-5p inhibitors were opposite to the
aforementioned results, indicating that miR-125a-5p could inhibit
DDP resistance of CC cells.
LIMK1 serves an essential regulatory role in
proliferation, metastasis and apoptosis of tumors, including
colonic, ovarian, and gastric cancer (29–31). It
has been reported that LIMK1 was highly expressed in NSCLC, and its
high expression was closely associated with advanced TNM stage and
lymph node metastasis; in addition, LIMK1 interference
significantly increased the sensitivity of NSCLC cells to DDP,
suggesting that LIMK1 has as a cancer-promoting role in NSCLC and
enhances DDP resistance (14). In
the present study, RT-qPCR and western blotting demonstrated that
compared with normal tissues or HUCECs, the expression of LIMK1 was
upregulated in CC tissues and cell lines. Overexpression of LIMK1
in the present study increased the proliferation, inhibited the
apoptosis of DDP-resistant cells C-33A/DDP and CaSKi/DDP cells,
increased the IC50, promoted the expression of
resistance-related proteins P-gp and GST-π and enhance the DDP
resistance in CC.
The miRNA-mRNA axis exerts a critical part in the
progression of tumors, including CC (32–34). In
recent years, numerous studies demonstrated that the miRNA-mRNA
axis exerts a regulatory role in DDP resistance of CC, miR-130a was
found to directly target copper transporter protein 1 resulting in
upregulation of DDP resistance in CC (35). In the present study, the potential
binding site between miR-125a-5p and LIMK1 was predicted using the
TargetScan database, and the subsequent luciferase reporter
activity assay demonstrated that miR-125a-5p significantly
decreased luciferase activity of the LIMK1 WT reporter, but didn't
reduce activity of the LIMK1 MUT reporter. Western blotting assay
results demonstrated that miR-125a-5p negatively regulated the
expression of LIMK1 protein. Additionally, by performing gain- and
loss-of-function experiments, the present study demonstrated that
miR-125a-5p impeded LIMK1 expression and reduced the resistance of
CC cells to DDP. The findings of the present study not only partly
explained the mechanism by which miR-125a-5p modulated the
chemoresistance of CC cells, but also identified LIMK1 as a novel
target gene of miR-125a-5p.
The present study had some limitations. Firstly,
other target genes of miR-125a-5p were not identified. Future
studies investigating this will help to further clarify the
mechanism of miR-125a-5p in regulating the chemosensitivity of CC
cells to DDP. Secondly, whether miR-125a-5p modulates the
chemosensitivity of another chemotherapeutic drug apart from DDP
deserves further investigation. Thirdly, the detailed mechanism by
which miR-125a-5p/LIMK1 regulates the expression of P-gp and GST-π
is still unknown and should be the focus of future work.
In summary, compared with normal tissues or HUCECs,
in CC tissue and cell lines, miR-125a-5p expression is
downregulated and LIMK1 expression is upregulated. LIMK1 is a
target gene of miR-125a-5p, which targets LIMK1 and inhibits the
resistance to DDP of CC cells. The findings of the present study
will improve the treatment of patients with CC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PN, YX and YD conceived and designed the
experiments. YX, YZ and LM performed the experiments. YX, YZ, YD
and LM analyzed the results. YX, YZ, YD and PN wrote the paper. PN
and YX confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was given approval by the Medical
Ethics Committee of Shengli Oilfield Central Hospital (Dongying,
China; approval no, 2016-05). All patients provided written
informed consent prior to surgery.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu R, Qian M, Zhou T and Cui P: TP53
mediated miR-3647-5p prevents progression of cervical carcinoma by
targeting AGR2. Cancer Med. 8:6095–6105. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Federico C, Sun J, Muz B, Alhallak K,
Cosper PF, Muhammad N, Jeske A, Hinger A, Markovina S, Grigsby P,
et al: Localized delivery of cisplatin to cervical cancer improves
its therapeutic efficacy and ninimizes its side effect profile. Int
J Radiat Oncol Biol Phys. Nov 27–2020.(Epub ahead of print). doi:
10.1016/j.ijrobp.2020.11.052. PubMed/NCBI
|
|
4
|
Matsuoka H, Murakami R, Abiko K, Yamaguchi
K, Horie A, Hamanishi J, Baba T and Mandai M: UGT1A1 polymorphism
has a prognostic effect in patients with stage IB or II uterine
cervical cancer and one or no metastatic pelvic nodes receiving
irinotecan chemotherapy: A retrospective study. BMC Cancer.
20:7292020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu H, Luo H, Zhang W, Shen Z, Hu X and
Zhu X: Molecular mechanisms of cisplatin resistance in cervical
cancer. Drug Des Devel Ther. 10:1885–1895. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng TL, Li DP, He ZF and Zhao S: miR-145
sensitizes esophageal squamous cell carcinoma to cisplatin through
directly inhibiting PI3K/AKT signaling pathway. Cancer Cell Int.
19:2502019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mesci A, Huang X, Taeb S, Jahangiri S, Kim
Y, Fokas E, Bruce J, Leong HS and Liu SK: Targeting of CCBE1 by
miR-330-3p in human breast cancer promotes metastasis. Br J Cancer.
116:1350–1357. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qiu Z, Li H, Wang J and Sun C: miR-146a
and miR-146b in the diagnosis and prognosis of papillary thyroid
carcinoma. Oncol Rep. 38:2735–2740. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okazaki J, Tanahashi T, Sato Y, Miyoshi J,
Nakagawa T, Kimura T, Miyamoto H, Fujino Y, Nakamura F, Takehara M,
et al: MicroRNA-296-5p promotes cell invasion and drug resistance
by targeting Bcl2-related ovarian killer, leading to a poor
prognosis in pancreatic cancer. Digestion. 101:794–806. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feng Y, Zou W, Hu C, Li G, Zhou S, He Y,
Ma F, Deng C and Sun L: Modulation of CASC2/miR-21/PTEN pathway
sensitizes cervical cancer to cisplatin. Arch Biochem Biophys.
623-624:20–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang WK, Akçakaya P, Gangaev A, Lee L,
Zeljic K, Hajeri P, Berglund E, Ghaderi M, Åhlén J, Bränström R, et
al: miR-125a-5p regulation increases phosphorylation of FAK that
contributes to imatinib resistance in gastrointestinal stromal
tumors. Exp Cell Res. 371:287–296. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi B, Ma C, Liu G and Guo Y: miR-106a
directly targets LIMK1 to inhibit proliferation and EMT of oral
carcinoma cells. Cell Mol Biol Lett. 24:12019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang H, Wang Y, Xing F, Wang J, Wang Y,
Wang H, Yang Y and Gao Z: Overexpression of LIMK1 promotes
migration ability of multidrug-resistant osteosarcoma cells. Oncol
Res. 19:501–509. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Q, Jiao D, Hu H, Song J, Yan J, Wu L
and Xu LQ: Downregulation of LIMK1 level inhibits migration of lung
cancer cells and enhances sensitivity to chemotherapy drugs. Oncol
Res. 20:491–498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xue F, Xu Y, Song Y, Zhang W, Li R and Zhu
X: The effects of sevoflurane on the progression and cisplatinum
sensitivity of cervical cancer cells. Drug Des Devel Ther.
13:3919–3928. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiao Y, Liang MR, Liu CC, Wang YN, Zeng Y,
Zhou J, Zhu HT, Wang Q, Zou Y and Zeng SY: Overexpression of P16
reversed the MDR1-mediated DDP resistance in the cervical
adenocarcinoma by activating the ERK1/2 signaling pathway. Cell
Div. 14:62019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Conte E, Bresciani E, Rizzi L, Cappellari
O, De Luca A, Torsello A and Liantonio A: Cisplatin-Induced
skeletal muscle dysfunction: Mechanisms and counteracting
therapeutic strategies. Int J Mol Sci. 21:E12422020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yi SA, Kim GW, Yoo J, Han JW and Kwon SH:
HP1γ sensitizes cervical cancer cells to cisplatin through the
suppression of UBE2L3. Int J Mol Sci. 21:59762020. View Article : Google Scholar
|
|
20
|
Xu X, Jiang X, Chen L, Zhao Y, Huang Z,
Zhou H and Shi M: miR-181a promotes apoptosis and reduces cisplatin
resistance by inhibiting osteopontin in cervical cancer cells.
Cancer Biother Radiopharm. 34:559–565. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang LL and Rao W: SiRNA interfering
STAT3 enhances DDP sensitivity in cervical cancer cells. Eur Rev
Med Pharmacol Sci. 22:4098–4106. 2018.PubMed/NCBI
|
|
22
|
Xia J, Yu X, Song X, Li G, Mao X and Zhang
Y: Inhibiting the cytoplasmic location of HMGB1 reverses cisplatin
resistance in human cervical cancer cells. Mol Med Rep. 15:488–494.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang X, Qiu J, Kang H, Wang Y and Qian J:
miR-125a-5p suppresses colorectal cancer progression by targeting
VEGFA. Cancer Manag Res. 10:5839–5853. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Y, Zhang D, Lv J, Wang S and Zhang
Q: miR-125a-5p suppresses bladder cancer progression through
targeting FUT4. Biomed Pharmacother. 108:1039–1047. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yan L, Yu MC, Gao GL, Liang HW, Zhou XY,
Zhu ZT, Zhang CY, Wang YB and Chen X: miR-125a-5p functions as a
tumour suppressor in breast cancer by downregulating BAP1. J Cell
Biochem. 119:8773–8783. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qin X, Wan Y, Wang S and Xue M:
MicroRNA-125a-5p modulates human cervical carcinoma proliferation
and migration by targeting ABL2. Drug Des Devel Ther. 10:71–79.
2015.PubMed/NCBI
|
|
27
|
Liu R, Wang M, Li E, Yang Y, Li J, Chen S,
Shen WJ, Azhar S, Guo Z and Hu Z: Dysregulation of microRNA-125a
contributes to obesity-associated insulin resistance and
dysregulates lipid metabolism in mice. Biochim Biophys Acta Mol
Cell Biol Lipids. 1865:1586402020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao Y, Ma K, Yang S, Zhang X, Wang F,
Zhang X, Liu H and Fan Q: MicroRNA-125a-5p enhances the sensitivity
of esophageal squamous cell carcinoma cells to cisplatin by
suppressing the activation of the STAT3 signaling pathway. Int J
Oncol. 53:644–658. 2018.PubMed/NCBI
|
|
29
|
Su J, Zhou Y, Pan Z, Shi L, Yang J, Liao
A, Liao Q and Su Q: Downregulation of LIMK1-ADF/cofilin by DADS
inhibits the migration and invasion of colon cancer. Sci Rep.
7:456242017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen P, Zeng M, Zhao Y and Fang X:
Upregulation of Limk1 caused by microRNA-138 loss aggravates the
metastasis of ovarian cancer by activation of Limk1/cofilin
signaling. Oncol Rep. 32:2070–2076. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
You T, Gao W, Wei J, Jin X, Zhao Z, Wang C
and Li Y: Overexpression of LIMK1 promotes tumor growth and
metastasis in gastric cancer. Biomed Pharmacother. 69:96–101. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wei WF, Zhou CF, Wu XG, He LN, Wu LF, Chen
XJ, Yan RM, Zhong M, Yu YH, Liang L, et al: MicroRNA-221-3p, a
TWIST2 target, promotes cervical cancer metastasis by directly
targeting THBS2. Cell Death Dis. 8:32202017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Peng Y, Zhang X, Ma Q, Yan R, Qin Y, Zhao
Y, Cheng Y, Yang M, Wang Q, Feng X, et al: MiRNA-194 activates the
Wnt/β-catenin signaling pathway in gastric cancer by targeting the
negative Wnt regulator, SUFU. Cancer Lett. 385:117–127. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu S, Zheng Q, Wu H, Wang C, Liu T and
Zhou W: miR-532 promoted gastric cancer migration and invasion by
targeting NKD1. Life Sci. 177:15–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Feng C, Ma F, Hu C, Ma JA, Wang J, Zhang
Y, Wu F, Hou T, Jiang S, Wang Y, et al: SOX9/miR-130a/CTR1 axis
modulates DDP-resistance of cervical cancer cell. Cell Cycle.
17:448–458. 2018. View Article : Google Scholar : PubMed/NCBI
|