Introduction
Gastric cancer (GC) is one of the most common
malignant neoplasms of the upper gastrointestinal tract in Asia,
with an incidence rate of 64.6 per 100,000 individuals in 2012 and
exhibiting substantial mortality (1). Gastric oncogenesis is a multistep
process, involving non-atrophic gastritis, atrophic gastritis,
intestinal metaplasia, dysplasia and eventually GC (2). The exact factors involved in the
occurrence and development of GC are not yet fully understood, with
the exception of Helicobacter pylori infection. H.
pylori is a Gram-negative bacteria that is strongly associated
with GC occurrence, and it has been recognized as a group I
carcinogen by the International Agency for Research on Cancer in
1994 (3). Furthermore, the chronic
inflammation resulting from H. pylori infection is believed
to be a major step in the initiation and development of GC
(4).
Despite the high incidence of H. pylori
infection worldwide, only 1–3% of the H. pylori-infected
individuals progress to GC (5),
which suggests that other factors must be involved in GC etiology.
Human cytomegalovirus (HCMV), a member of the herpesvirus family
(6), is prevalent among the general
population, with an infectious rate ranging between 50 and 100%
(7). In the last two decades, HCMV
has been reported to serve an important role in the neoplastic
process of human malignant tumors, such as glioblastoma, salivary
gland cancer, lung carcinoma, breast cancer, prostatic carcinoma,
colorectal cancer and hepatocellular carcinoma (8–14). Our
previous study revealed that HCMV was present in 50–69.61% of GC
tissues (15). However, only a few
studies have focused on the effect of HCMV on GC. Previous studies
have revealed a difference in the expression levels of the UL133,
UL135, UL136 and UL138 genes between normal gastric and tumor
tissues (16,17). Additionally, an association between
HCMV infection and lymphatic metastasis was observed in GC
(15). Considering the high
prevalence of H. pylori and its role in GC, the synergetic
or antagonistic effects between HCMV and H. pylori could not
be ignored when investigating the role of HCMV in GC. Therefore,
the present study aimed to detect the infection with HCMV and H.
pylori in patients with GC using PCR and to explore the
potential association between them in GC.
Materials and methods
Patients, specimens and data
collection
A total of 134 patients (98 males and 36 females;
age range, 31–89 years; median age, 68 years) who underwent
elective gastrectomy for GC at The Second Affiliated Hospital of
Wenzhou Medical University (Wenzhou, China) between January 2017
and January 2018 were included in the present study. Paired gastric
tumor and peri-tumoral tissues (≥10 cm from the negative reception
margin) were collected. The histopathological diagnosis of gastric
tumor and paired non-tumor specimens was confirmed following
surgery by the pathological department of The Second Affiliated
Hospital of Wenzhou Medical University. Once collected, specimens
were deposited into tubes and preserved in liquid nitrogen
immediately until further use. Patient clinicopathological data,
including sex, age, tumor size (the longest tumor diameter), tumor
location, general type (ulcer or non-ulcer), tumor invasion,
lymphatic metastasis, TNM stage, vessel invasion (defined by the
pathological department using hematoxylin and eosin staining
according to routine hospital protocols) and differentiation, were
collected prospectively. Tumor invasion, lymphatic metastasis and
TNM stage were defined according to the National Comprehensive
Cancer Network Gastric Cancer Guidelines version 3.2015 (18). The present study was approved by the
Human Research Ethics Committee of The Second Affiliated Hospital
of Wenzhou Medical University, and written informed consent was
provided by all participants.
Genomic DNA extraction
Total genomic DNA was extracted from 134 pairs of
tumor and peri-tumoral tissue samples using a QIAamp DNA mini kit
(Tiangen Biotech Co., Ltd.) according to the manufacturer's
protocol. DNA purity and concentration were quantified using a
NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Inc.).
DNA samples were stored at −20°C until further use.
Detection of H. pylori and HCMV
infection
H. pylori and HCMV infections were determined
by PCR. The primers for the H. pylori genes 16S rRNA
(19) and UreA (20), and for the HCMV genes UL47, UL56 and
UL77 (15) are listed in Table I. GAPDH was used as an internal
control. PCR was performed in a T100™ Thermal Cycler (Bio-Rad
Laboratories, Inc.). The PCR mixture contained 1X Taq MasterMix
(Tiangen Biotech Co., Ltd.), 1 µg DNA sample and 0.2 µM each
specific forward and reverse primers in a final volume of 25 µl.
The positive controls for H. pylori and HCMV detection were
DNA from a GC tissue resected from a patient clinically diagnosed
with H. pylori infection at The Second Affiliated Hospital
of Wenzhou Medical University and a clinically isolated HCMV strain
or AD169 (American Type Culture Collection), respectively. The
negative control was sterile double-distilled water. After an
initial denaturation step at 95°C for 5 min, the reaction mixtures
were processed through 35 PCR cycles of denaturation at 95°C for 30
sec, annealing for 30 sec at 58°C for UL47, UL56, UL77 and 16S rRNA
or 50°C for UreA, and extension at 72°C for 1 min, followed by an
additional extension step at 72°C for 10 min. The final PCR
products were loaded on a 1.2% agarose gel for electrophoresis,
using GelRed for visualization. If a band was detected at the right
size (according to the relative position of the band and marker)
and the sequencing results (performed by The Beijing Genomics
Institute) indicated the correct sequence, the gene was considered
to be present in the tissue. If one of the two H. pylori
genes or one of the three HCMV genes was effectively amplified, the
tissue was considered positive for H. pylori or HCMV,
respectively. Additionally, patients were considered to have H.
pylori or HCMV infection if H. pylori or HCMV was
detected in their gastric tissues, regardless of its presence in
the tumor or adjacent peri-tumoral tissue.
 | Table I.Primer information. |
Table I.
Primer information.
| A, Helicobacter
pylori |
|---|
|
|---|
| Gene | Forward primer
(5′→3′) | Reverse primer
(5′→3′) | Target size,
bp |
|---|
| 16S rRNA |
CTTAACCATAGAACTGCATTTGAAACTAC |
GGTCGCCTTCGCAATGAGTA | 119 |
| UreA |
GCCAATGGTAAATTAGTT |
CTCCTTAATTGTTTTTAC | 411 |
|
| B, Human
cytomegalovirus |
|
| Gene | Forward primer
(5′→3′) | Reverse primer
(5′→3′) | Target size,
bp |
|
| UL47 |
GTACAGCCCCACGTTCCTG |
CCCGATACAGGTACTCGCGCT | 212 |
| UL56 |
TCCTCCACGTCCTCCCCGTA |
AGGCGCTGAGGGAGTACAAC | 202 |
| UL77 |
GCACTTTTGATCGTCACGTGCT |
ACGCAGATATTGCTGTTCGTGC | 215 |
| GAPDH |
CAGGGCTGCTTTTAACTCTGGTAA |
GGGTGGAATCATATTGGAACATGT | 101 |
Statistical analysis
Statistical analysis was performed using SPSS
version 22.0 (IBM Corp.). Univariate analyses were performed using
the χ2 test with Yates' correction or Fisher's exact
test depending on the data type. Multivariate logistic regression
analysis was performed to determine the independent risk factors.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Detection of H. pylori and HCMV
infection
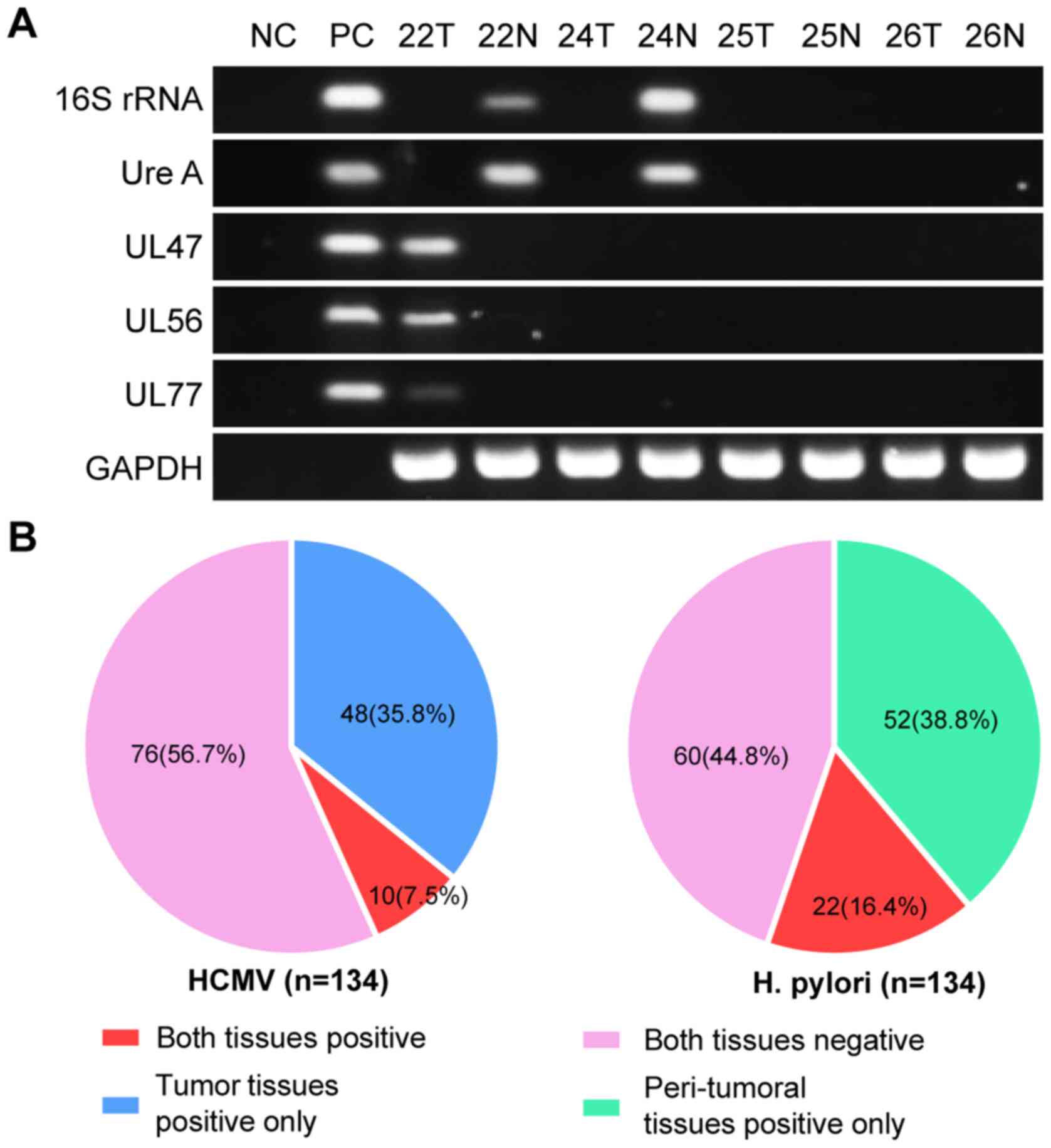
The results obtained using different primers
exhibited good consistency (Fig.
1A). As aforementioned, patients were considered to have H.
pylori or HCMV infection if H. pylori or HCMV were
detected in their gastric tissues, regardless of its presence in
the tumor or paired peri-tumoral tissue. Among the 134 patients,
H. pylori was detected in 74 cases (55.2%), including 52
cases positive only in peri-tumoral tissues and 22 cases positive
in both tumor and peri-tumoral tissues. HCMV infection was positive
in 58 cases (43.3%), comprising 48 cases positive only in tumor
tissues and 10 cases positive in both tumor and peri-tumoral
tissues (Fig. 1B). Both HCMV and
H. pylori were detected in 34 cases (25.4%); however, there
was no significant association between HCMV and H. pylori
infection (P=0.490; Table II).
 | Table II.Association between HCMV and H.
pylori in gastric tumors. |
Table II.
Association between HCMV and H.
pylori in gastric tumors.
| H. pylori
status | Total |
HCMV− |
HCMV+ | χ2 | P-value |
|---|
| H.
pylori− | 60 | 36 | 24 | 0.477 | 0.490 |
| H.
pylori+ | 74 | 40 | 34 |
|
|
| Total | 134 | 76 | 58 |
|
|
Associations between H. pylori
infection and clinicopathological characteristics of patients with
GC
Analysis of the clinicopathological characteristics
revealed that patients in the H. pylori+ group
exhibited a higher level of distant lymph node metastasis (N2 + N3)
and TNM stage (T3 + T4), indicating that H. pylori infection
status was significantly associated with lymphatic metastasis and
TNM stage (P=0.013 and P=0.023, respectively). However, no
significant associations were detected between H. pylori
infection status and sex, age, tumor size, location, general type,
invasion, vascular invasion or pathological differentiation
(P>0.05; Table III).
 | Table III.Clinicopathological features, H.
pylori and HCMV infection in gastric tumors. |
Table III.
Clinicopathological features, H.
pylori and HCMV infection in gastric tumors.
|
| H.
pylori | HCMV |
|---|
|
|
|
|
|---|
| Clinicopathological
feature | Negative, n
(%) | Positive, n
(%) | P-value | Negative, n
(%) | Positive, n
(%) | P-value |
|---|
| Sex |
|
| 0.661 |
|
| 0.869 |
|
Female | 15 (25.0) | 21 (28.4) |
| 20 (26.3) | 16 (27.6) |
|
|
Male | 45 (75.0) | 53 (71.6) |
| 56 (73.7) | 42 (72.4) |
|
| Agea, years |
|
| 0.464 |
|
| 0.692 |
|
<68 | 27 (45.0) | 38 (51.4) |
| 38 (50.0) | 27 (46.6) |
|
|
≥68 | 33 (55.0) | 36 (48.6) |
| 38 (50.0) | 31 (53.4) |
|
| Tumor size, cm |
|
| 0.177 |
|
| 0.992 |
|
<5 | 37 (61.7) | 37 (50.0) |
| 42 (55.3) | 32 (55.2) |
|
| ≥5 | 23 (38.3) | 37 (50.0) |
| 34 (44.7) | 26 (44.8) |
|
| Tumor location |
|
| 0.609b |
|
| 0.579b |
|
Antrum | 39 (65.0) | 44 (59.5) |
| 46 (60.5) | 37 (63.8) |
|
|
Corpus | 12 (20.0) | 12 (16.2) |
| 15 (19.7) | 9 (15.5) |
|
|
Cardia | 8 (13.3) | 15 (20.3) |
| 14 (18.4) | 9 (15.5) |
|
|
Diffuse | 1 (1.7) | 3 (4.1) |
| 1 (1.3) | 3 (5.2) |
|
| General type |
|
| 0.690 |
|
| 0.806 |
|
Non-ulcer | 9 (15.0) | 13 (17.6) |
| 13 (17.1) | 9 (15.5) |
|
|
Ulcer | 51 (85.0) | 61 (82.4) |
| 63 (82.9) | 49 (84.5) |
|
| Tumor invasion |
|
| 0.897 |
|
| 0.258 |
| T1 +
T2 | 16 (26.7) | 19 (25.7) |
| 17 (22.4) | 18 (31.0) |
|
| T3 +
T4 | 44 (73.3) | 55 (74.3) |
| 59 (77.6) | 40 (69.0) |
|
| Lymphatic
metastasis |
|
| 0.013c |
|
| 0.002c |
| N0 +
N1 | 34 (56.7) | 26 (35.1) |
| 25 (32.9) | 35 (60.3) |
|
| N2 +
N3 | 26 (43.3) | 48 (64.9) |
| 51 (67.1) | 23 (39.7) |
|
| TNM stage |
|
| 0.023c |
|
| 0.026c |
|
I+II | 32 (53.3) | 25 (33.8) |
| 26 (34.2) | 31 (53.4) |
|
|
III+IV | 28 (46.7) | 49 (66.2) |
| 50 (65.8) | 27 (46.6) |
|
| Vascular
invasion |
|
| 0.332 |
|
| 0.706 |
| No | 39 (65.0) | 42 (56.8) |
| 47 (61.8) | 34 (58.6) |
|
|
Yes | 21 (35.0) | 32 (43.2) |
| 29 (38.2) | 24 (41.4) |
|
|
Differentiation |
|
| 0.814b |
|
| 0.293b |
|
Well | 3 (5.0) | 3 (4.1) |
| 3 (3.9) | 3 (5.2) |
|
|
Moderate | 8 (13.3) | 14 (18.9) |
| 9 (11.8) | 13 (22.4) |
|
|
Poor | 35 (58.3) | 43 (58.1) |
| 49 (64.5) | 29 (50.0) |
|
|
Other | 14 (23.3) | 14 (18.9) |
| 15 (19.7) | 13 (22.4) |
|
Association between HCMV infection and
clinicopathological features of patients with GC
The analysis of HCMV infection and patient
clinicopathological characteristics identified a significant
negative association between HCMV infection status and lymphatic
metastasis (P=0.002), as well as TNM stage (P=0.026). No
significant associations were observed between HCMV infection
status and sex, age, tumor size, location, general type, invasion,
vascular invasion or tumor differentiation (P>0.05; Table III).
Clinical features of HCMV and H.
pylori co-infection in patients with GC
The 134 patients were divided into HCMV+
and HCMV− groups. In these groups, the patients were
subdivided according to the presence or absence of H.
pylori. Subgroup analysis revealed that in the HCMV+
group, H. pylori infection was not significantly associated
with lymphatic metastasis (P=0.408), whereas in the
HCMV− group, H. pylori infection was
significantly associated with advanced lymphatic metastasis
(P=0.003) and TNM stage (P=0.023). Tumor invasion exhibited no
statistical significances between the two groups (Table IV).
 | Table IV.Association of H. pylori and
HCMV co-infection status with clinicopathological features. |
Table IV.
Association of H. pylori and
HCMV co-infection status with clinicopathological features.
|
|
HCMV+ |
HCMV− | H.
pylori+ | H.
pylori− |
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
feature | H.
pylori+/− | P-value | H.
pylori+/− | P-value |
HCMV+/− | P-value |
HCMV+/− | P-value |
|---|
| Tumor invasion |
| 0.751 |
| 0.977 |
| 0.498 |
| 0.340 |
| T1 +
T2 | 10/8 |
| 9/8 |
| 10/9 |
| 8/8 |
|
| T3 +
T4 | 24/16 |
| 31/28 |
| 24/31 |
| 16/28 |
|
| Lymphatic
metastasis |
| 0.408 |
| 0.003a |
|
<0.001a |
| 0.202 |
| N0 +
N1 | 19/16 |
| 7/18 |
| 19/7 |
| 16/18 |
|
| N2 +
N3 | 15/8 |
| 33/18 |
| 33/15 |
| 8/18 |
|
| TNM stage |
| 0.246 |
| 0.023a |
| 0.026a |
| 0.245 |
|
I+II | 16/15 |
| 9/17 |
| 16/9 |
| 15/17 |
|
|
III+IV | 18/9 |
| 31/19 |
| 18/31 |
| 9/19 |
|
Additionally, the 134 patients were divided into
H. pylori+ and H. pylori−
groups, which were further divided according to HCMV infection
status. Subgroup analysis demonstrated that HCMV infection status
was negatively associated with lymphatic metastasis (P<0.001)
and TNM stage (P=0.026) in the H. pylori+ group.
In the H. pylori− group, there were no
significant associations between HCMV infection status and tumor
invasion, lymphatic metastasis or TNM stage (Table IV).
Univariate and multivariate analyses
based on lymphatic metastasis
The association between infection status and
lymphatic metastasis was analyzed using univariate and multivariate
analyses. Univariate analysis revealed that H. pylori
(P=0.013), HCMV (P=0.002), tumor size (P<0.001), tumor invasion
(P<0.001) and vascular invasion (P<0.001) were significantly
associated with lymphatic metastasis (Table V). Other features exhibited no
statistical significance (P>0.05). Additionally, H.
pylori [P=0.007; odds ratio (OR)=3.51], tumor invasion
(P=0.012; OR=4.31) and vessel invasion (P=0.004; OR=3.93) were
independent risk factors for lymphatic metastasis, whereas HCMV
(P=0.001; OR=0.21) was an independent protective factor for
lymphatic metastasis, according to the multivariate logistic
regression analysis (Table V).
 | Table V.Univariate and multivariate logistic
regression analyses of lymphatic metastasis and infection
status. |
Table V.
Univariate and multivariate logistic
regression analyses of lymphatic metastasis and infection
status.
|
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
feature | N0 + N1 | N2 + N3 | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| H.
pylori |
|
|
| 0.013a |
| 0.007a |
| No | 34 | 26 | 1 |
| 1 |
|
|
Yes | 26 | 48 | 2.41 (1.20,
4.86) |
| 3.51 (1.41,
8.71) |
|
| HCMV |
|
|
| 0.002a |
| 0.001a |
| No | 35 | 23 | 1 |
| 1 |
|
|
Yes | 25 | 51 | 0.32 (0.16,
0.66) |
| 0.21 (0.08,
0.53) |
|
| Sex |
|
|
| 0.730 |
| 0.759 |
|
Female | 17 | 19 | 1 |
| 1 |
|
|
Male | 43 | 55 | 1.14 (0.53,
2.46) |
| 1.17 (0.42,
3.25) |
|
| Ageb, years |
|
|
| 0.314 |
| 0.825 |
|
<68 | 32 | 33 | 1 |
| 1 |
|
|
≥68 | 28 | 41 | 1.42 (0.72,
2.81) |
| 1.11 (0.45,
2.69) |
|
| Tumor size, cm |
|
|
|
<0.001a |
| 0.060 |
|
<5 | 44 | 30 | 1 |
| 1 |
|
| ≥5 | 16 | 44 | 4.03 (1.93,
8.43) |
| 2.44 (0.96,
6.16) |
|
| Tumor location |
|
| 1.37 (0.91,
2.06) | 0.543c | 1.12 (0.67,
1.85) | 0.669 |
|
Antrum | 41 | 42 |
|
|
|
|
|
Corpus | 10 | 14 |
|
|
|
|
|
Cardia | 8 | 15 |
|
|
|
|
|
Diffuse | 1 | 3 |
|
|
|
|
| General type |
|
|
| 0.385 |
| 0.304 |
|
Non-ulcer | 8 | 14 | 1 |
| 1 |
|
|
Ulcer | 52 | 60 | 0.66 (0.26,
1.70) |
| 0.49 (0.12,
1.93) |
|
| Tumor invasion |
|
|
|
<0.001a |
| 0.012a |
| T1 +
T2 | 25 | 10 | 1 |
| 1 |
|
| T3 +
T4 | 35 | 64 | 4.57 (1.97,
10.60) |
| 4.31 (1.38,
13.40) |
|
| Vessel
invasion |
|
|
|
<0.001a |
| 0.004a |
| No | 47 | 34 | 1 |
| 1 |
|
|
Yes | 13 | 40 | 4.25 (1.98,
9.15) |
| 3.93 (1.55,
9.96) |
|
|
Differentiation |
|
|
| 0.160 |
| 0.221 |
|
Well/moderate | 17 | 11 | 1 |
| 1 |
|
|
Poor | 32 | 46 | 2.22 (0.92,
5.37) |
| 2.25 (0.73,
6.92) |
|
|
Other | 11 | 17 | 2.39 (0.82,
6.98) |
| 3.24 (0.79,
13.22) |
|
To further clarify the association between lymphatic
metastasis and co-infection, the patients were divided into
HCMV−/H. pylori−,
HCMV+/H. pylori−,
HCMV−/H. pylori+ and
HCMV+/H. pylori+ groups. Univariate
analysis revealed a significant association between lymphatic
metastasis and different co-infection statuses (P=0.001). Compared
with the HCMV−/H. pylori− group,
neither HCMV+/H. pylori− (P=0.205) nor
HCMV+/H. pylori+ (P=0.622) were
associated with lymphatic metastasis, whereas the
HCMV−/H. pylori+ group (P=0.004) was a
significant risk factor for lymphatic metastasis. Multivariate
analysis revealed that HCMV−/H.
pylori+ status was independently associated with
lymphatic metastasis (P=0.005; OR=6.00; Table VI).
 | Table VI.Univariate and multivariate logistic
regression analyses of lymphatic metastasis and co-infection
status. |
Table VI.
Univariate and multivariate logistic
regression analyses of lymphatic metastasis and co-infection
status.
|
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
feature | N0 + N1 | N2 + N3 | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Infection
status |
|
|
| 0.001a |
|
|
|
HCMV−/H.
pylori− | 10 | 12 | 1 |
| 1 |
|
|
HCMV+/H.
pylori− | 9 | 6 | 0.50 (0.17,
1.46) | 0.205b | 0.41 (0.11,
1.56) | 0.192b |
|
HCMV+/H.
pylori+ | 9 | 11 | 0.79 (0.31,
2.02) | 0.622b | 0.75 (0.23,
2.41) | 0.624b |
|
HCMV−/H.
pylori+ | 4 | 20 | 4.71 (1.66,
13.40) | 0.004a,b | 6.00 (1.71,
21.04) | 0.005a,b |
Discussion
PCR is the most widely used test in the laboratory
to detect H. pylori infection in gastric tissues, and its
positive rate is consistent with that of histopathological methods
in fresh GC tissues (21). In the
present study, the H. pylori infection rate detected using
PCR was 55.2%, which was similar to the results of a previous study
(22).
H. pylori is a pathogen recognized as a group
I carcinogen by the International Agency for Research on Cancer
(3). H. pylori infection is
considered to progress to gastric malignancy via two different
mechanisms (22,23). First, the virulence factors produced
by H. pylori, such as cytotoxin-associated gene A, directly
damage the mucosa, resulting in neoplastic transformation (23). Second, H. pylori causes
chronic inflammation; infiltration of neutrophils and lymphocytes
upregulate numerous pro-inflammatory cytokines, including
interleukin (IL)-1, IL-6, IL-8, tumor necrosis factor α and
inflammation-associated transcription factors such as NF-κB
(22). These inflammation-associated
factors, particularly NF-κB and IL-8, are important mediators in
gastric carcinogenesis (22). H.
pylori infection and consequent chronic inflammation are
considered to be a major step in the occurrence and development of
GC (22). In the present study,
analysis of the association between H. pylori infection and
clinicopathological features revealed that the H.
pylori+ group exhibited a higher rate of regional
lymph node metastasis and a higher TNM stage. Additionally,
multivariate analysis revealed that H. pylori infection was
independently associated with lymphatic metastasis.
The study of H. pylori has long been the
focus of GC research, although most studies (3,20,22,23)
have focused on H. pylori itself, ignoring the complicated
pathogenic microbiological environment of GC and neglecting the
synergistic or antagonistic effects of H. pylori with other
pathogenic microorganisms. Increasing evidence has suggested that
HCMV is detected at a very low level in various types of tumor
(12,24,25). For
example, Cobbs et al (24)
have detected HCMV genes and their products in glioblastoma
multiforme (GBM). In the present study, HCMV was detected in 43.3%
of patients with GC, and compared with our previous GC study
(15), the results obtained using
different primers exhibited improved consistency. This discrepancy
may be due to improved specimen quality control, optimization of
PCR conditions and quality of primers. The HCMV+
specimens were mostly tumor tissues, which was consistent with
studies performed in prostatic carcinoma, colorectal cancer and
malignant glioma (12,24,25).
Similarly to previously reported PCR results (26), the detection rate of H. pylori
in peri-tumoral tissues was higher than that in tumor tissues in
the present study, suggesting that H. pylori and HCMV
infections do not completely overlap spatially.
Rahbar et al (27) have demonstrated the prognostic value
of the infection status of HCMV in patients with GBM. In breast
cancer, HCMV proteins have been detected in sentinel lymph nodes,
suggesting that HCMV may contribute to metastasis (11). A number of genes encoded by HCMV,
such as UL123, UL36-38, UL97 and US28, participate in cellular
transformation (28). In addition,
several HCMV genes serve roles in apoptosis, cell cycle, invasion
and migration of cancer cells, and angiogenesis in glioblastoma
(17,28–37)
Furthermore, HCMV participates in tumor immunomodulation (38,39). In
the present study, statistical analysis revealed that lymphatic
metastasis and TNM stage were associated with a negative HCMV
status.
HCMV causes chronic inflammation similarly to other
tumor-associated pathogens, such as H. pylori (40). A previous study has reported the
potential interaction between HCMV and other pathogens (41). Compared with HCMV−/human
papilloma virus (HPV)− cases,
HCMV+/HPV+ but not
HCMV−/HPV+ patients with cervical carcinoma
had a significantly higher rate of metastasis to lymph nodes,
indicating a synergetic effect between HCMV and HPV on lymphatic
metastasis in cervical carcinoma (41). In the present study, analysis of the
clinical significance of HCMV and H. pylori co-infection
revealed that in the HCMV+ group, H. pylori
infection status was not associated with lymphatic metastasis.
However, in the HCMV− group, H. pylori infection
status was significantly associated with lymphatic metastasis. In
addition, the co-infection status was associated with lymphatic
metastasis, and compared with the HCMV−/H.
pylori− group, the HCMV−/H.
pylori+ status was a potential risk factor for
lymphatic metastasis. Multivariate analysis revealed that the
HCMV−/H. pylori+ status was
independently associated with high level of lymphatic metastasis.
Therefore, HCMV and H. pylori may interact in GC by exerting
an antagonistic effect on lymphatic metastasis progression. These
contradictory results in GC and cervical carcinoma may be
associated with the complexity of the GC microenvironment and with
HCMV infection itself. A recent study revealed no association
between HCMV infection and lymphatic metastasis, nor the
characteristics of co-infection with H. pylori (26). However, 65% of samples in the
aforementioned study were located at the proximal position of the
stomach, in contrast to the cohort in the present study in which
the majority (62%) of tumors were located at the distal position.
This suggests that the role of HCMV may be associated with the
tumor location in the stomach. In human malignant glioma, which is
the most studied HCMV-associated tumor, HCMV infection is mainly
regarded as a tumor promoter (42,43).
However, previous studies have revealed that HCMV may be a
protective factor in the outcome of gastrointestinal tumors, breast
cancer and hepatocellular carcinoma (44–47).
Furthermore, the HCMV genes US28 (48) and UL123 (49) can accelerate tumor growth and enhance
cancer cell stemless, while UL138 can promote the apoptosis of
cancer cells (17). A recent report
has revealed that in GC, low β-catenin-interacting protein 1
(CTNNBIP1) expression is associated with well-differentiated tumor
grades, and CTNNBIP1 downregulation is significantly associated
with HCMV infection (50),
indicating a possible tumor inhibition mechanism of HCMV. The
Epstein-Barr virus (EBV), which is a cancer promoter in Burkitt
lymphoma and nasopharyngeal carcinoma, may also serve a protective
role in GC (51–54). Several studies have demonstrated that
EBV-associated GC exhibits a lower rate of lymph node metastasis
(53,54). These results suggest that HCMV may
serve multiple roles in different types of tumor, which may be
associated with its latent and proliferative status, varied tumor
microenvironment, interaction with other tumor-associated
pathogens, diverse immunomodulatory effects of HCMV and HCMV gene
expression characteristics (51–54).
Based on the results of the present study, it can be
speculated that there may be a pathway through which HCMV inhibits
H. pylori+ GC lymphatic metastasis, which may be
used as a guide for clinicians when planning the patients'
treatment modalities. The research on H. pylori mainly
focuses on its carcinogenic role, since H. pylori is a
recognized cause of GC. Eradication of H. pylori is an
effective way to prevent GC. However, to the best of our knowledge,
there is no conclusive evidence that patients with GC can benefit
from eradication or non-eradication of H. pylori. The
present results may suggest that patients with GC who are positive
for both H. pylori and HCMV may be exempted from H.
pylori eradication.
There were certain limitations in the current study.
Only the positive rates of H. pylori and HCMV infection were
detected, without their copy numbers, so the role of the infection
level in the co-infection remains to be explored. Additionally,
only PCR was used as a detection method, not supplemented by
immunohistochemistry, in situ hybridization or other
methods, which may affect the true positive rate of the
infections.
In conclusion, the results of the present study
revealed that H. pylori infection may increase tumor
aggressiveness by promoting lymphatic metastasis. However, in
patients with HCMV and H. pylori co-infection, H.
pylori may lose its tumor-promoting ability, and this group of
patients exhibited a lower risk of developing a high level of
lymphatic metastasis. This suggests that HCMV may inhibit lymphatic
metastasis through an H. pylori-associated pathway, which
should be further investigated in future research.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81472308, 81672707,
31670922 and 31470891).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
CC and SC performed the experiments, analyzed and
interpreted the data, and drafted the manuscript. ZH collected the
clinical data and performed statistical analysis. WX designed the
study and performed the experiments. TZ, CM, LZ and XSu collected
the tissue specimens. XSh analyzed and interpreted the data,
revised the manuscript and finally approved the version of the
manuscript for publication. TK and XX contributed to the conception
and design of the study and interpreted the data; in addition, XX
supervised the study, analyzed the data, provided the project
funding, revised the manuscript and finally approved the version of
the manuscript for publication. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Human Research Ethics
Committee of the Second Affiliated Hospital of Wenzhou Medical
University (Wenzhou, China), and written informed consent was
provided by all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Correa P, Haenszel W, Cuello C, Tannenbaum
S and Archer M: A model for gastric cancer epidemiology. Lancet.
2:58–60. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Plummer M, Franceschi S, Vignat J, Forman
D and de Martel C: Global burden of gastric cancer attributable to
Helicobacter pylori. Int J Cancer. 136:487–490. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peng C, Ouyang Y, Lu N and Li N: The NF-κB
signaling pathway, the microbiota, and gastrointestinal
tumorigenesis: Recent advances. Front Immunol. 11:13872020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amieva M and Peek RM Jr: Pathobiology of
Helicobacter pylori-induced gastric cancer.
Gastroenterology. 150:64–78. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dunn W, Chou C, Li H, Hai R, Patterson D,
Stolc V, Zhu H and Liu F: Functional profiling of a human
cytomegalovirus genome. Proc Natl Acad Sci USA. 100:14223–14228.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tomtishen JP III: Human cytomegalovirus
tegument proteins (pp65, pp71, pp150, pp28). Virol J. 9:222012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Solomon IH, Ramkissoon SH, Milner DA Jr
and Folkerth RD: Cytomegalovirus and glioblastoma: A review of
evidence for their association and indications for testing and
treatment. J Neuropathol Exp Neurol. 73:994–998. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Melnick M, Sedghizadeh PP, Allen CM and
Jaskoll T: Human cytomegalovirus and mucoepidermoid carcinoma of
salivary glands: Cell-specific localization of active viral and
oncogenic signaling proteins is confirmatory of a causal
relationship. Exp Mol Pathol. 92:118–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brouchet L, Valmary S, Dahan M, Didier A,
Galateau-Salle F, Brousset P and Degano B: Detection of oncogenic
virus genomes and gene products in lung carcinoma. Br J Cancer.
92:743–746. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taher C, de Boniface J, Mohammad AA,
Religa P, Hartman J, Yaiw KC, Frisell J, Rahbar A and
Söderberg-Naucler C: High prevalence of human cytomegalovirus
proteins and nucleic acids in primary breast cancer and metastatic
sentinel lymph nodes. PLoS One. 8:e567952013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Samanta M, Harkins L, Klemm K, Britt WJ
and Cobbs CS: High prevalence of human cytomegalovirus in prostatic
intraepithelial neoplasia and prostatic carcinoma. J Urol.
170:998–1002. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cai ZZ, Xu JG, Zhou YH, Zheng JH, Lin KZ,
Zheng SZ, Ye MS, He Y, Liu CB and Xue ZX: Human
cytomegalovirus-encoded US28 may act as a tumor promoter in
colorectal cancer. World J Gastroenterol. 22:2789–2798. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lepiller Q, Tripathy MK, Di Martino V,
Kantelip B and Herbein G: Increased HCMV seroprevalence in patients
with hepatocellular carcinoma. Virol J. 8:4852011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang L, Guo G, Xu J, Sun X, Chen W, Jin
J, Hu C, Zhang P, Shen X and Xue X: Human cytomegalovirus detection
in gastric cancer and its possible association with lymphatic
metastasis. Diagn Microbiol Infect Dis. 88:62–68. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jin J, Hu C, Wang P, Chen J, Wu T, Chen W,
Ye L, Zhu G, Zhang L, Xue X and Shen X: Latent infection of human
cytomegalovirus is associated with the development of gastric
cancer. Oncol Lett. 8:898–904. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen W, Lin K, Zhang L, Guo G, Sun X, Chen
J, Ye L, Ye S, Mao C, Xu J, et al: The cytomegalovirus protein
UL138 induces apoptosis of gastric cancer cells by binding to heat
shock protein 70. Oncotarget. 7:5630–5645. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
National Comprehensive Cancer Network, .
(NCCN) Clinical Practice Guidelines in Oncology. Gastric Cancer.
Version 3.2015. https://www.nccn.orgMarch
25–2015
|
|
19
|
Tan MP, Kaparakis M, Galic M, Pedersen J,
Pearse M, Wijburg OL, Janssen PH and Strugnell RA: Chronic
Helicobacter pylori infection does not significantly alter
the microbiota of the murine stomach. Appl Environ Microbiol.
73:1010–1013. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang YL, Gan RL, Dong BH, Jiang RC and
Tang RJ: Detection and location of Helicobacter pylori in
human gastric carcinomas. World J Gastroenterol. 11:1387–1391.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fabre R, Sobhani I, Laurent-Puig P, Hedef
N, Yazigi N, Vissuzaine C, Rodde I, Potet F, Mignon M, Etienne JP,
et al: Polymerase chain reaction assay for the detection of
Helicobacter pylori in gastric biopsy specimens: Comparison
with culture, rapid urease test, and histopathological tests. Gut.
35:905–908. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang F, Meng W, Wang B and Qiao L:
Helicobacter pylori-induced gastric inflammation and gastric
cancer. Cancer Lett. 345:196–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kao CY, Sheu BS and Wu JJ: Helicobacter
pylori infection: An overview of bacterial virulence factors
and pathogenesis. Biomed J. 39:14–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cobbs CS, Harkins L, Samanta M, Gillespie
GY, Bharara S, King PH, Nabors LB, Cobbs CG and Britt WJ: Human
cytomegalovirus infection and expression in human malignant glioma.
Cancer Res. 62:3347–3350. 2002.PubMed/NCBI
|
|
25
|
Harkins L, Volk AL, Samanta M, Mikolaenko
I, Britt WJ, Bland KI and Cobbs CS: Specific localisation of human
cytomegalovirus nucleic acids and proteins in human colorectal
cancer. Lancet. 360:1557–1563. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fattahi S, Nikbakhsh N, Taheri H, Ghadami
E, Kosari-Monfared M, Amirbozorgi G, Asouri M, Pilehchian-Langroudi
M, Ranaee M, Samadani AA, et al: Prevalence of multiple infections
and the risk of gastric adenocarcinoma development at earlier age.
Diagn Microbiol Infect Dis. 92:62–68. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rahbar A, Orrego A, Peredo I, Dzabic M,
Wolmer-Solberg N, Strååt K, Stragliotto G and Söderberg-Nauclér C:
Human cytomegalovirus infection levels in glioblastoma multiforme
are of prognostic value for survival. J Clin Virol. 57:36–42. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Johnsen JI, Baryawno N and
Soderberg-Naucler C: Is human cytomegalovirus a target in cancer
therapy? Oncotarget. 2:1329–1338. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Goldmacher VS, Bartle LM, Skaletskaya A,
Dionne CA, Kedersha NL, Vater CA, Han JW, Lutz RJ, Watanabe S,
Cahir McFarland ED, et al: A cytomegalovirus-encoded
mitochondria-localized inhibitor of apoptosis structurally
unrelated to Bcl-2. Proc Natl Acad Sci USA. 96:12536–12541. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Skaletskaya A, Bartle LM, Chittenden T,
McCormick AL, Mocarski ES and Goldmacher VS: A
cytomegalovirus-encoded inhibitor of apoptosis that suppresses
caspase-8 activation. Proc Natl Acad Sci USA. 98:7829–7834. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McCormick AL, Skaletskaya A, Barry PA,
Mocarski ES and Goldmacher VS: Differential function and expression
of the viral inhibitor of caspase 8-induced apoptosis (vICA) and
the viral mitochondria-localized inhibitor of apoptosis (vMIA) cell
death suppressors conserved in primate and rodent
cytomegaloviruses. Virology. 316:221–233. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Michaelis M, Kotchetkov R, Vogel JU, Doerr
HW and Cinatl J Jr: Cytomegalovirus infection blocks apoptosis in
cancer cells. Cell Mol Life Sci. 61:1307–1316. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McCormick AL: Control of apoptosis by
human cytomegalovirus. Curr Top Microbiol Immunol. 325:281–295.
2008.PubMed/NCBI
|
|
34
|
Moorman NJ, Cristea IM, Terhune SS, Rout
MP, Chait BT and Shenk T: Human cytomegalovirus protein UL38
inhibits host cell stress responses by antagonizing the tuberous
sclerosis protein complex. Cell Host Microbe. 3:253–262. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Norris KL and Youle RJ: Cytomegalovirus
proteins vMIA and m38.5 link mitochondrial morphogenesis to Bcl-2
family proteins. J Virol. 82:6232–6243. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Michaelis M, Doerr HW and Cinatl J: The
story of human cytomegalovirus and cancer: Increasing evidence and
open questions. Neoplasia. 11:1–9. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Soroceanu L, Matlaf L, Bezrookove V,
Harkins L, Martinez R, Greene M, Soteropoulos P and Cobbs CS: Human
cytomegalovirus US28 found in glioblastoma promotes an invasive and
angiogenic phenotype. Cancer Res. 71:6643–6653. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Foster H, Ulasov IV and Cobbs CS: Human
cytomegalovirus- mediated immunomodulation: Effects on glioblastoma
progression. Biochim Biophys Acta Rev Cancer. 1868:273–276. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guo G, Ye S, Xie S, Ye L, Lin C, Yang M,
Shi X, Wang F, Li B, Li M, et al: The cytomegalovirus protein US31
induces inflammation through mono-macrophages in systemic lupus
erythematosus by promoting NF-ΚB2 activation. Cell Death Dis.
9:1042018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Soroceanu L and Cobbs CS: Is HCMV a tumor
promoter? Virus Res. 157:193–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen TM, Chang CF, Chen YH, Chen CA, Wu CC
and Hsieh CY: Coexistence of human cytomegalovirus and human
papillomavirus type 16 correlates with lymph node metastasis in
cervical cancer. J Cancer Res Clin Oncol. 122:629–632. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hortal AM, Vermeulen JF, Van Hecke W and
Bovenschen N: Oncogenic role of cytomegalovirus in medulloblastoma?
Cancer Lett. 408:55–59. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Joseph GP, McDermott R, Baryshnikova MA,
Cobbs CS and Ulasov IV: Cytomegalovirus as an oncomodulatory agent
in the progression of glioma. Cancer Lett. 384:79–85. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen HP, Jiang JK, Chen CY, Yang CY, Chen
YC, Lin CH, Chou TY, Cho WL and Chan YJ: Identification of human
cytomegalovirus in tumour tissues of colorectal cancer and its
association with the outcome of non-elderly patients. J Gen Virol.
97:2411–2420. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Oberstein A and Shenk T: Cellular
responses to human cytomegalovirus infection: Induction of a
mesenchymal-to-epithelial transition (MET) phenotype. Proc Natl
Acad Sci USA. 114:E8244–E8253. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kumar A, Coquard L, Pasquereau S, Russo L,
Valmary-Degano S, Borg C, Pothier P and Herbein G: Tumor control by
human cytomegalovirus in a murine model of hepatocellular
carcinoma. Mol Ther Oncolytics. 3:160122016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Erkes DA, Wilski NA and Snyder CM:
Intratumoral infection by CMV may change the tumor environment by
directly interacting with tumor-associated macrophages to promote
cancer immunity. Hum Vaccin Immunother. 13:1778–1785. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Heukers R, Fan TS, de Wit RH, van Senten
JR, De Groof TWM, Bebelman MP, Lagerweij T, Vieira J, de Munnik SM,
Smits-de Vries L, et al: The constitutive activity of the virally
encoded chemokine receptor US28 accelerates glioblastoma growth.
Oncogene. 37:4110–4121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Soroceanu L, Matlaf L, Khan S, Akhavan A,
Singer E, Bezrookove V, Decker S, Ghanny S, Hadaczek P, Bengtsson
H, et al: Cytomegalovirus immediate-early proteins promote stemness
properties in glioblastoma. Cancer Res. 75:3065–3076. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kosari-Monfared M, Nikbakhsh N, Fattahi S,
Ghadami E, Ranaei M, Taheri H, Amjadi-Moheb F, Godazandeh GA,
Shafaei S, Pilehchian-Langroudi M, et al: CTNNBIP1 downregulation
is associated with tumor grade and viral infections in gastric
adenocarcinoma. J Cell Physiol. 234:2895–2904. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Camargo MC, Kim WH, Chiaravalli AM, Kim
KM, Corvalan AH, Matsuo K, Yu J, Sung JJ, Herrera-Goepfert R,
Meneses-Gonzalez F, et al: Improved survival of gastric cancer with
tumour Epstein-Barr virus positivity: An international pooled
analysis. Gut. 63:236–243. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shinozaki-Ushiku A, Kunita A and Fukayama
M: Update on Epstein-Barr virus and gastric cancer (review). Int J
Oncol. 46:1421–1434. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yanagi A, Nishikawa J, Shimokuri K, Shuto
T, Takagi T, Takagi F, Kobayashi Y, Yamamoto M, Miura O, Yanai H,
et al: Clinicopathologic characteristics of epstein-barr
virus-associated gastric cancer over the past decade in Japan.
Microorganisms. 7:3052019. View Article : Google Scholar
|
|
54
|
Osumi H, Kawachi H, Yoshio T, Ida S,
Yamamoto N, Horiuchi Y, Ishiyama A, Hirasawa T, Tsuchida T, Hiki N,
et al: Epstein-Barr virus status is a promising biomarker for
endoscopic resection in early gastric cancer: Proposal of a novel
therapeutic strategy. J Gastroenterol. 54:774–783. 2019. View Article : Google Scholar : PubMed/NCBI
|