Introduction
Gastric cancer is the fifth most commonly diagnosed
cancer and the third leading cause of cancer-related mortality in
men and women, with >1 million novel cases and ~783,000 deaths
in 2018 globally (1). At present,
the pathogenesis of gastric cancer remains incompletely understood.
Dietary intake of N-nitroso compounds is associated with the
development of gastric cancer (2).
Other risk factors, including the presence of Helicobacter
pylori, genetics, alcohol consumption, obesity and
gastroesophageal reflux disease have all been demonstrated to
increase the incidence of gastric cancer (3). The therapeutic regimens used for
management of gastric cancer at present are surgery, radiotherapy,
chemotherapy and immunotherapy. However, the efficacy of these is
limited and patients may exhibit side effects. Therefore, it is
essential to identify novel anticancer agents and investigate their
anticancer mechanisms to highlight novel treatment approaches for
gastric cancer.
Macroautophagy (hereafter referred to as autophagy),
or ‘self-eating’, is a conserved cellular pathway that controls
protein and organelle degradation. It is a process that not only
facilitates nutrient recycling from damaged organelles, but is also
an adaptive mechanism in response to different stresses (4). Autophagy serves an important role in
human health and disease, and modulates numerous pathologies,
including neurodegeneration, infectious diseases, inflammation,
immunity and cancer (5). Autophagy
has important effects on the development of tumors; however, the
role of autophagy in cancer is complex and somewhat controversial
and may be cell-type dependent (6).
A previous study suggested that autophagy functions as a
double-edged sword in cancer, in that it suppresses tumor
occurrence in the earlier stages, but promotes cancer progression
following tumor formation (7).
Several previous studies have demonstrated that natural products
may inhibit carcinogenesis through regulating autophagy (8,9). For
example, a recent study reported that Apigetrin (a flavonoid)
induced autophagic cell death via the PI3K/AKT/mTOR pathway in AGS
human gastric cancer cells (10). At
present, it is well established that autophagy may serve as a novel
and promising target for cancer treatment.
Ganoderma lucidum (G. lucidum), also
known as Linzhi or Reishi, has been widely used in Asia for
>2,000 years (11). G.
lucidum may inhibit tumor growth in several types of cancer,
including colorectal cancer (12),
non-small cell lung cancer (13) and
breast cancer (14), amongst other
types. Furthermore, a previous clinical study confirmed that G.
lucidum may be an alternative or adjuvant agent to conventional
treatments as it stimulates host immunity without notable toxicity
(15). G. lucidum contains
several bioactive compounds, including polysaccharides, alkaloids,
triterpenoids, lactones, steroids and other compounds (16). Amongst all of these bioactive
components, polysaccharides and triterpenoids have been extensively
studied and are considered to be the primary contributors to the
medicinal properties of G. lucidum. Early studies
investigated the anti-cancer effects of G. lucidum
polysaccharides (GLPs) that were extracted primarily from the
fruiting body of G. lucidum. Compared with the fruiting body
of G. lucidum, the spores of G. lucidum also possess
a higher percentage of bioactive substances and exhibit higher
bioactivity (17). Several studies
have demonstrated that the spores of G. lucidum exerted
significant anticancer activity (18,19).
However, the sporoderm-broken spores of G. lucidum (BSGL)
still contain a high quantity of indigestible sporoderm, which is
primarily composed of chitin (20).
More recently, Li et al (21)
reported for the first time that the immunomodulatory effects of
triterpenoids extracted from sporoderm-removed spores of G.
lucidum (RSGL), which removed the sporoderm from BSGL, was
higher than that of BSGL. However, to the best of our knowledge,
the anti-cancer effects of the bioactive compounds, including
polysaccharides or triterpenoids, extracted from RSGL have not been
assessed.
The present study first compared the anticancer
effects of polysaccharides extracted from BSGL (BSGLP) and RSGL
(RSGLP) in three gastric cancer cell lines. In addition, the
present study investigated the role of RSGLP in regulating
autophagy and apoptosis in gastric cancer cells. To the best of our
knowledge, the present study was the first to investigate the
anticancer effects and molecular mechanisms of RSGLP in gastric
cancer cells. The results of the present study supported the
requirement for further study of RSGLP as a potential anti-gastric
cancer drug, and it was shown that its effects were mediated by
regulation autophagy.
Materials and methods
Reagents and antibodies
MTT was obtained from HXBIO. Hoechst 33342 was
purchased from Invitrogen; Thermo Fisher Scientific, Inc. Annexin
V-FITC/PI apoptosis kit was purchased from BD Pharmingen™. The
mRFP-GFP-LC3 adenoviruses were purchased from Hanbio Biotechnology
Co. Ltd. Chloroquine (CQ) was purchased from MedChemExpress.
Rapamycin (Rap) was purchased from Sigma-Aldrich; Merck KGaA.
Antibodies against PARP (cat. no. 9542; polyclonal), cleaved-PARP
(cat. no. 5625; polyclonal), total PARP (cat. no. 9532;
polyclonal), pro-caspase-3 (cat. no. 9665; monoclonal), p62 (cat.
no. 8025; polyclonal) and a secondary anti-rabbit antibody (cat.
no. 7074) were purchased from Cell Signaling Technology, Inc. Bcl-2
(cat. no. db176; polyclonal), LC3 (cat. no. db760; polyclonal) and
β-actin (cat. no. db10001; polyclonal) antibodies were purchased
from Beijing Jiachenhong Bio-Technology Co., Ltd. A bicinchoninic
acid (BCA) protein assay kit was purchased from Thermo Fisher
Scientific, Inc. Clarity Western™ ECL Substrate was purchased from
Bio-Rad Laboratories, Inc.
Cell culture
Human gastric cancer cell lines MKN28 (cat. no.
CL0368), AGS (cat. no. CL0031) and NCI-N87 (cat. no. CL0241), and
noncancerous gastric GES-1 (cat. no. CL0352) cell line were
purchased from Hunan Fenghui Biotechnology Co., Ltd., which were
originally obtained from American Type Culture Collection. The
cells were authenticated using the STR profiling method (22). All cells were tested for mycoplasma
and were confirmed to be free of contamination. Cells were
maintained in Gibco™ RPMI-1640 medium (Thermo Fisher Scientific,
Inc.), supplemented with 10% Gemini's fetal bovine serum (Thermo
Fisher Scientific, Inc.) and 1% Gibco™ penicillin-streptomycin. The
cultures were incubated in a humidified atmosphere of 5%
CO2 at 37°C, prior to the cells being harvested and
passaged at 80–90% confluence.
Preparation of G. lucidum
polysaccharide
The powder of BSGL and RSGL was obtained from the
Shouxiangu Institute of Rare Medicine Plant. The hot water
extraction and alcohol precipitation methods were used to prepare
BSGLP and RSGLP. In brief, ~5 g BSGL or RSGL was dissolved with 100
ml double-distilled water at 70°C for 12 h. Following transfer to
50 ml centrifuge tubes, the solution was centrifuged at 2,700 × g
for 15 min at room temperature to remove the insoluble substance.
Following hot water extraction, six aliquots (v/v) of cold ethanol
was added to precipitate the polysaccharide for 24 h. Next, the
crude polysaccharides were purified to remove protein using the
Sevag method (23). The samples were
then freeze-dried using the H051 freeze dryer (LaboGene) to obtain
BSGLP and RSGLP for use in subsequent experiments.
Cell viability assay
The effects of BSGLP or RSGLP on inhibition of
gastric cancer cell growth were investigated using an MTT assay. A
preliminary study suggested that RSGLP killed cells at lower
concentrations, while BSGLP killed cells at higher concentrations.
In detail, GES-1, MKN28 and AGS cells (1×104 cells/well)
were plated onto 96-well plates in RPMI-1640 medium and cultured
until they reached 40–50% confluence. Therefore, cells were treated
with 0, 7.5, 10, 12.5 or 15 mg/ml BSGLP or 0, 2.0, 3.0, 4.0 or 5.0
mg/ml RSGLP for 24, 48 or 72 h. NCI-N87 cells were seeded onto
96-well plates at a density of 3ⅹ104 cells/well, and
treated with 0, 7.5, 10, 12.5 or 15 mg/ml BSGLP or 0, 2.0, 3.0, 4.0
or 5.0 mg/ml RSGLP for 24, 48 or 72 h. The aforementioned dose
ranges of RSGLP (0–5 mg/ml) and BSGLP (0–15 mg/ml) were selected
based on different sensitivity and 50% inhibitory concentration
(IC50) values of the three gastric cancer cell lines
towards the two GLPs. Following incubation, MTT solution (5 mg/ml)
was added to each well and incubated for a further 4 h. Next,
supernatants were carefully removed and the purple formazan was
dissolved using 150 µl dimethyl sulfoxide. Following low-speed
oscillation for 10 min on a rocker, absorbance was measured at 490
nm using a Bio-Tek microplate reader (BioTek Instruments, Inc.).
Cell viability as a percentage and the IC50 of each GLP
was calculated. When investigating the effects of CQ or Rap on
RSGLP-induced cell viability, AGS cells were treated with CQ (5 µM)
or Rap (2 µM) with or without RSGLP (3.0 mg/ml) for 36 h.
Hoechst 33342 staining assay
Hoechst 33342 was used for staining the nuclei of
AGS cells. In brief, AGS cells were treated with RSGLP at 0, 2.0,
2.5, 3.0 or 3.5 mg/ml for 24 h, and cells were stained with Hoechst
33342 (10 µg/ml) for 10 min at room temperature in the dark.
Following washing samples, apoptotic cells were detected using an
EVOS FL Cell Imaging System (Thermo Fisher Scientific, Inc.).
mRFP-GFP-LC3 adenovirus transfection
and colocalization analysis
The effect of RSGLP, CQ or Rap on the
autophagosome-lysosome fusion was determined by mRFP-GFP-LC3
adenovirus transfection and colocalization analysis. A total of
1×104 AGS cells/well were seeded into a 4-chamber
glass-bottom dish with RPMI-1640 medium until cells reached 40%
confluence. It has been well recognized that among all currently
available viral vectors, adenovirus is the most efficient gene
delivery system that can deliver genes directly into target cells
(24). Hence, AGS cells for all
treatments, including the control, were then transfected directly
with the mRFP-GFP-LC3 adenoviruses (without any transfection
reagent) in cell culture medium at 37°C for 6 h. Following washing
with PBS twice, the cells were cultured for an additional 24 h in
RPMI-1640 medium. Next, cells were treated with RSGLP (3 mg/ml),
Rap (2 µM) or CQ (5 µM) for an additional 24 h. Following
incubation, a Zeiss LSM880 confocal microscope (magnification,
×630) (Zeiss GmbH) was used to acquire images. When mRFP and GFP
was overlayed, yellow fluorescence spots and red fluorescence spots
indicated early autophagosomes and late autolysosomes, respectively
(25).
Flow cytometry
The effect of RSGLP on the induction of apoptosis in
AGS cells was determined using flow cytometry. In brief, AGS cells
(1×104 cells/well) were seeded into 6-well plates and
treated with 0, 2.0, 2.5, 3 or 3.5 mg/ml of RSGLP for 24 or 36 h.
The cells were dissociated by trypsinization and washed with PBS.
According to the manufacturer's protocols, the cells were
resuspended in 1X binding buffer to a density of 1×106
cells/ml and 5 µl Annexin V-FITC and 10 µl PI was added to each
tube, and further incubated for 5 min at room temperature in the
dark. Samples were analyzed using a Beckman Coulter flow cytometer
(Beckman Coulter, Inc.). The percentage of early, late and total
apoptotic cells between different groups were calculated and
compared. When investigating the effects of CQ or Rap on
RSGLP-induced apoptosis, AGS cells were treated with CQ (5 µM) or
Rap (2 µM) with or without RSGLP (3.0 mg/ml) at room temperature
for 36 h. The data analysis was performed using CytExpert v.2.3
software (Beckman Coulter, Inc.).
Western blot analysis
Following treatment of AGS cells as described
earlier, cells were lysed on ice using RIPA lysis buffer (PBS, 1%
Igepal, 10% SDS, 0.5% Sodium Deoxycholate). A BCA assay was used
for measuring the total protein concentration. A total of 25 µg of
protein/lane was resolved by electrophoresis on a 10 or 12%
SDS-gel. Following separation, the proteins were transferred to a
PVDF membrane at 100 V for 2 h on ice. Membranes were blocked in 5%
skimmed dry milk in 1X TBST for 1 h at room temperature.
Subsequently, the membranes were incubated overnight at 4°C with
the following primary antibodies: PARP (dilution, 1:1,000),
cleaved-PARP (dilution, 1:1,000), total PARP (dilution, 1:1,000),
pro-caspase-3 (dilution, 1:1,000), p62 (dilution, 1:1,000). Bcl-2
(dilution 1:1,000), LC3 (dilution, 1:1,000) and β-actin (dilution,
1:2,000). Following washing with 1X TBST 3 times, 7 min per wash,
the membranes were incubated with a secondary anti-rabbit antibody
(dilution, 1:2,000) at room temperature for 1 h. Signals were
visualized using Clarity™ Western ECL substrate and examined using
a Minichemi™ 610 chemical imaging system. Densitometric analysis
was performed using ImageJ version 1.41 software (National
Institutes of Health).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism version 6.0 (GraphPad Software, Inc.). Data are presented as
the mean ± standard error of the mean of at least three
experiments. Differences in data sets were compared using a one-way
analysis of variance (ANOVA) or two-way ANOVA for multiple
comparisons, followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
RSGLP is more effective than BSGLP in
inhibiting cell viability in three gastric cancer cell lines
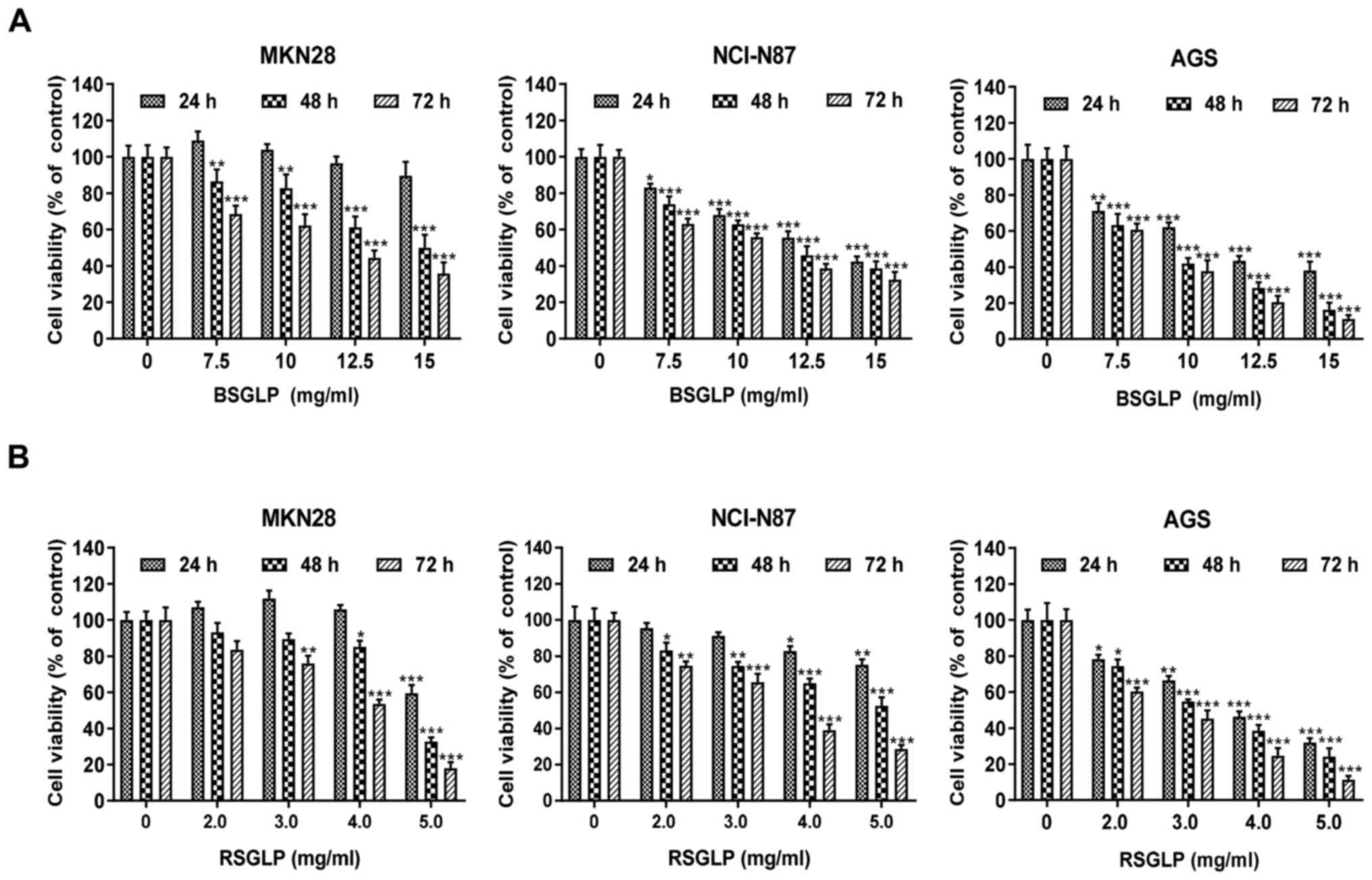
To begin with, the inhibitory effects of BSGLP and
RSGLP on cell viability in MKN28, NCI-N87 and AGS gastric cancer
cell lines were compared. MTT assays demonstrated that BSGLP and
RSGLP significantly decreased cell viability, and this inhibitory
effect was time- and dose-dependent (Fig. 1). In addition, the inhibitory effects
on cell viability were notably greater after 48 and 72 h of
treatment with the two GLPs (Fig.
1). Compared with BSGLP, RSGLP was more effective in inhibiting
cell viability, which was evidenced by the much lower
IC50 values of RSGLP (Table
I). For RSGLP, the IC50 values for MKN28, NCI-N87
and AGS cells were 5.03±1.62 mg/ml, 8.08±1.39 mg/ml and 3.76±2.85
mg/ml after 24 h; 4.68±2.58 mg/ml, 5.36±2.58 mg/ml and 3.26±1.24
mg/ml after 48 h; and 3.88±1.09 mg/ml, 3.50±0.95 mg/ml and
2.60±1.47 mg/ml after 72 h, respectively (Table I).
 | Table I.IC50 of BSGLP and RSGLP
against MKN28, NCI-N87 and AGS gastric cell lines. |
Table I.
IC50 of BSGLP and RSGLP
against MKN28, NCI-N87 and AGS gastric cell lines.
|
|
| IC50,
mg/ml |
|---|
|
|
|
|
|---|
| Group | Hour | MKN28 | NCI-N87 | AGS |
|---|
| BSGLP | 24 h | 18.88±1.58 | 13.44±0.73 | 11.76±1.16 |
|
| 48 h | 14.83±1.49 | 12.09±1.76 | 9.03±2.08 |
|
| 72 h | 10.58±2.89 | 10.36±2.54 | 8.56±1.36 |
| RSGLP | 24 h | 5.03±1.62 | 8.08±1.39 | 3.76±2.85 |
|
| 48 h | 4.68±2.58 | 5.36±2.58 | 3.26±1.24 |
|
| 72 h | 3.88±1.09 | 3.50±0.95 | 2.60±1.47 |
Subsequently, whether the cytotoxic effects of the
two GLPs are specific to gastric cancer cells was investigated. The
results of the present study demonstrated that BSGLP (≤10 mg/ml)
and RSGLP (≤3 mg/ml) had no toxicity effect in GES-1 cells
(Fig. S1). However, 4 mg/ml RSGLP
and 12.5 mg/ml BSGLP demonstrated cytotoxic effects in GES-1 cells
at 48 h and at 72 h. Subsequently, concentrations of the two GLPs
were extended to a wider range. For RSGLP, 0, 2, 3, 4, 8, 16 or 32
mg/ml were used and for BSGLP, 0, 7.5, 10, 12.5, 15, 30 or 60 mg/ml
were used. The MTT assay was conducted to compare the
IC50 of RSGLP and BSGLP in GES-1 cells with that of the
three cancer cell lines. As demonstrated in Table SI, the IC50 of the two
GLPs in GES-1 cells are much higher than that in the three cancer
cell lines. These results suggested that RSGLP was more potent in
inhibiting gastric cancer cell viability than BSGLP, and the two
GLPs demonstrated fewer cytotoxic effects in normal cells. In
particular, the AGS cell line was more sensitive to GLPs than the
other two gastric cancer cell lines. For these reasons, RSGLP and
AGS cells were used in subsequent experiments on the underlying
mechanisms.
RSGLP induces apoptosis in AGS
cells
Cell apoptosis serves an important role in cancer
cells following treatment with anticancer agents. Therefore, the
present study investigated the mechanism of cell death induced by
RSGLP in AGS cells by examining cell apoptosis. To begin with, AGS
cells were stained with Hoechst 33342. Compared with the control
cells, the presence of typical apoptotic bodies, nuclei coagulation
and fragmentation were observed following treatment with RSGLP for
24 h (Fig. 2A). Next, flow
cytometric analysis was used to further determine whether RSGLP
induced apoptosis in AGS cells. The results demonstrated that 3.0
and 3.5 mg/ml RSGLP significantly increased the proportion of cells
in early apoptosis from 4.93 to 14.25 and 14.99% after 24 h,
respectively (Fig. 2B). The
percentage of late apoptotic cells was increased notably following
treatment with RSGLP for 36 h, particularly by 3.0 and 3.5 mg/ml
RSGLP. Early, late and total apoptotic cell proportions were
quantified and are presented in Fig.
2C (n=3). Since Bcl-2, pro-Caspase-3 and cleaved PARP proteins
are the key players in the apoptotic pathway, the levels of these
proteins were determined. RSGLP dose-dependently downregulated the
levels of Bcl-2 and pro-Caspase-3 as determined by western blotting
(Fig. 2D; left panel). Treatment
with RSGLP for 24 h increased the expression of cleaved-PARP in a
dose-dependent manner (Fig. 2D).
Fig. 2D (right panel) presents the
densitometric analysis of Bcl-2, pro-Caspase-3 and cleaved- PARP,
using β-actin as the loading control. Taken together, these results
demonstrate that RSGLP induced apoptosis in AGS cells.
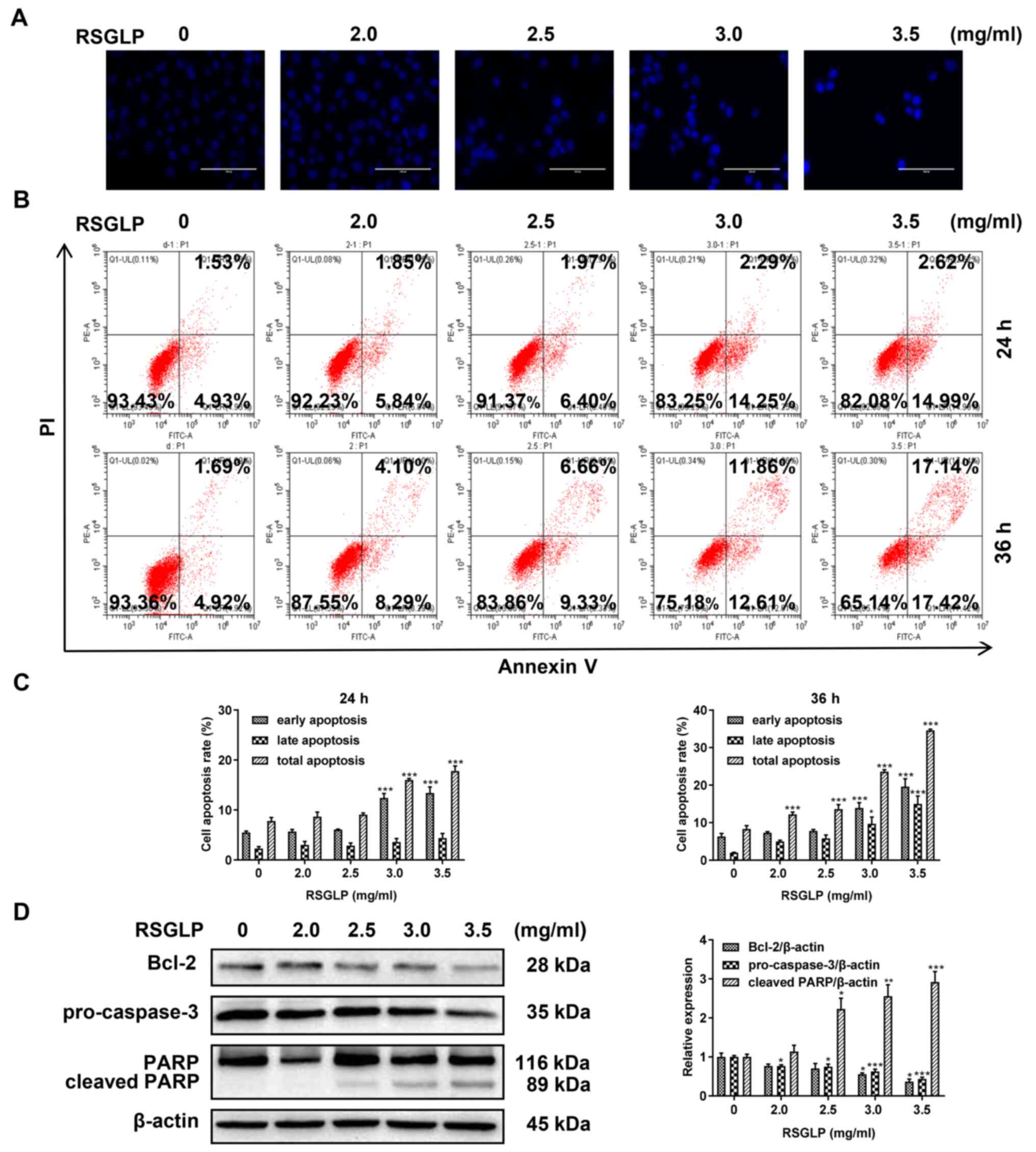 | Figure 2.RSGLP induces apoptosis in AGS cells.
(A) Hoechst 33342 staining. AGS cells were treated with 0, 2.0,
2.5, 3 or 3.5 mg/ml RSGLP for 24 h. Scale bar, 100 µm. (B) Flow
cytometric analysis of apoptosis. AGS cells were treated with 0,
2.0, 2.5, 3 or 3.5 mg/ml RSGLP for 24 or 36 h. (C) Percentage of
early apoptotic, late apoptotic and total apoptotic cells in AGS
cells following RSGLP treatment. (D) Expression of the
apoptosis-associated proteins Bcl-2, pro-Caspase-3 and cleaved-PARP
was determined by western blotting (left panel). Densitometric
analysis of the relative intensities of all proteins following
normalization against β-actin (right panel) of three individual
blots. Data are presented as the mean ± standard error of the mean
(n=3). A one-way analysis of variance, followed by a Tukey's
post-hoc test was used to compare the data. *P<0.05,
**P<0.01, ***P<0.001 vs. control. RSGLP, sporoderm-removed
spores of G. lucidum polysaccharide. |
RSGLP induces autophagy and inhibits
autophagic flux in AGS cells
To investigate whether RSGLP may regulate autophagy
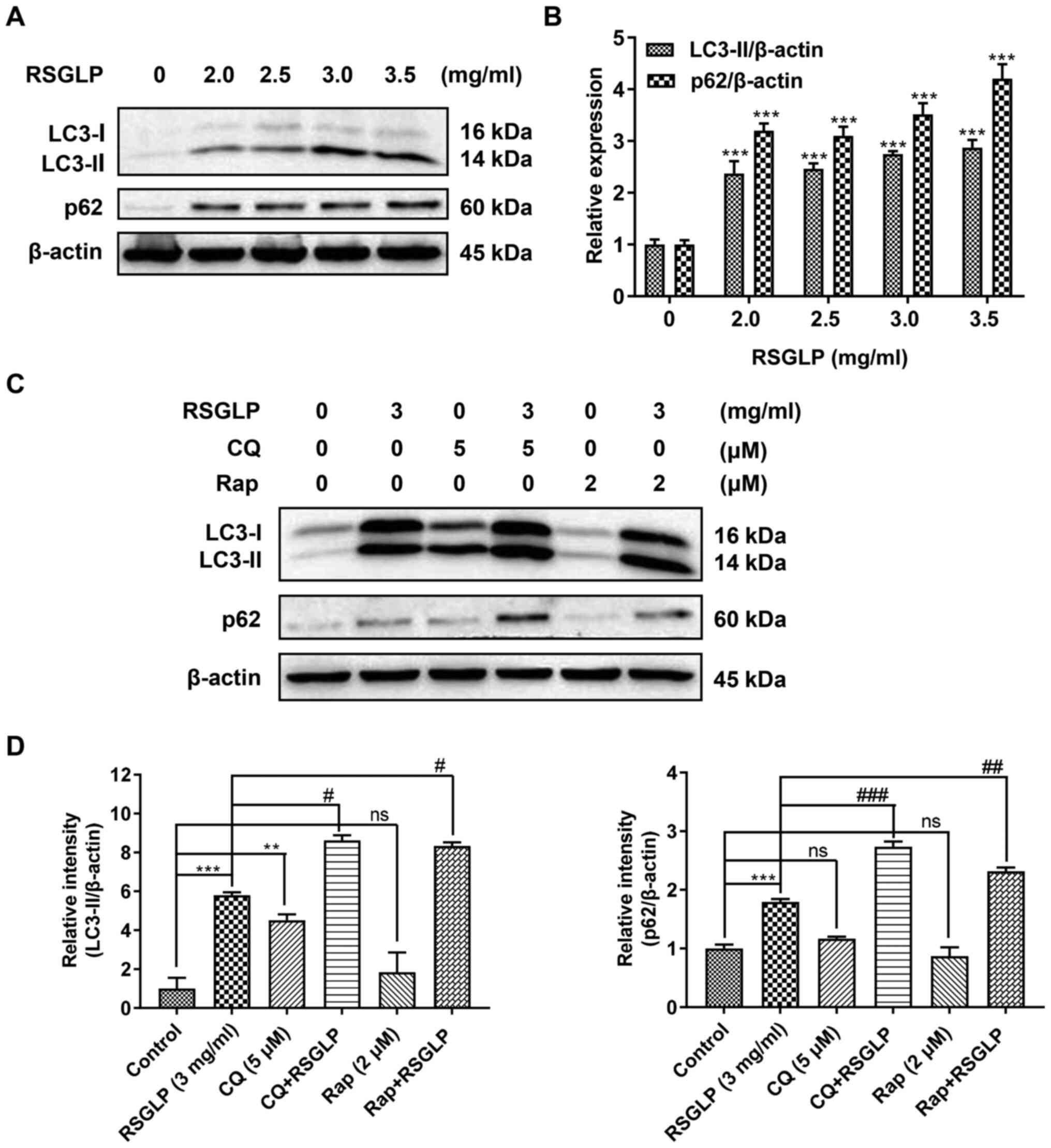
in AGS cells, the expression of autophagy-related proteins (LC3-II
and p62) was determined by western blot analysis. The levels of
LC3-II were notably increased in a dose-dependent manner in cells
treated with RSGLP for 24 h (Fig.
3A). This suggests that RSGLP induced autophagy or
autophagosome accumulation in AGS cells. Notably, RSGLP also
dose-dependently increased p62 expression (Fig. 3A), suggesting that autophagic flux
was disrupted by RSGLP. Densitometric analysis of LC3-II and p62 is
shown in Fig. 3B. Similar results
were observed in MKN28 and NCI-N87 cells (Fig. S2).
 | Figure 3.RSGLP induces autophagy and inhibits
autophagic flux. (A) AGS cells were treated with 0, 2.0, 2.5, 3 or
3.5 mg/ml RSGLP for 24 h. Western blotting of LC3-II and p62
expression. (B) Densitometric analysis of the relative intensities
of all proteins following normalization against β-actin. Data are
presented as the mean±standard error of the mean (n=3). (C) Effects
of the early and late autophagy inhibitors, Rap and CQ, on
RSGLP-mediated LC3-II and p62. AGS cells were treated with RSGLP (3
mg/ml), with or without CQ (5 µM) or Rap (2 µM) for 24 h. (D)
Densitometric analysis of the relative intensities of LC3-II and
p62 following normalization against β-actin. Data are presented as
the mean±standard error of the mean (n=3). A one-way or two-way
analysis of variance, followed by a Tukey's post-hoc test, was used
to compare the data. **P<0.01, ***P<0.001 vs. control;
#P<0.05, ##P<0.01,
###P<0.001 vs. RSGLP alone. ns, not significant vs.
control. RSGLP, sporoderm-removed spores of G. lucidum
polysaccharide; Rap, rapamycin. |
To further clarify these results, the late-stage
autophagy inhibitor, CQ (5 µM), and the autophagy inducer, Rap (2
µM), were used to investigate the effects of RSGLP on autophagy
initiation and flux. As shown in Fig.
3C, RSGLP alone increased LC3-II and p62 levels significantly.
Similarly, but to a much lower extent than RSGLP, CQ alone also
increased LC3-II and p62 levels, suggesting autophagosome
accumulation and autophagic flux impairment. In addition, CQ
treatment further increased RSGLP-induced levels of LC3-II and p62,
which confirmed that RSGLP induced autophagy initiation, and also
inhibited autophagic flux. As expected, Rap slightly upregulated
the expression of LC3-II but did not increase the expression of
p62, suggesting induction of autophagy but normal autophagic flux.
However, Rap did not decrease the RSGLP-induced accumulation of p62
(Fig. 3C), suggesting the effects of
RSGLP on autophagy were very potent, which could not be reversed by
Rap treatment. Densitometric analysis of LC3-II and p62 is shown in
Fig. 3D. Based on the densitometrric
analysis, Rap co-treatment with RSGLP further increased p62
expression (Fig. 3D). Taken
together, the results of the present study demonstrated that RSGLP
induced autophagy initiation and autophagosome accumulation, and
inhibited autophagic flux in AGS cells.
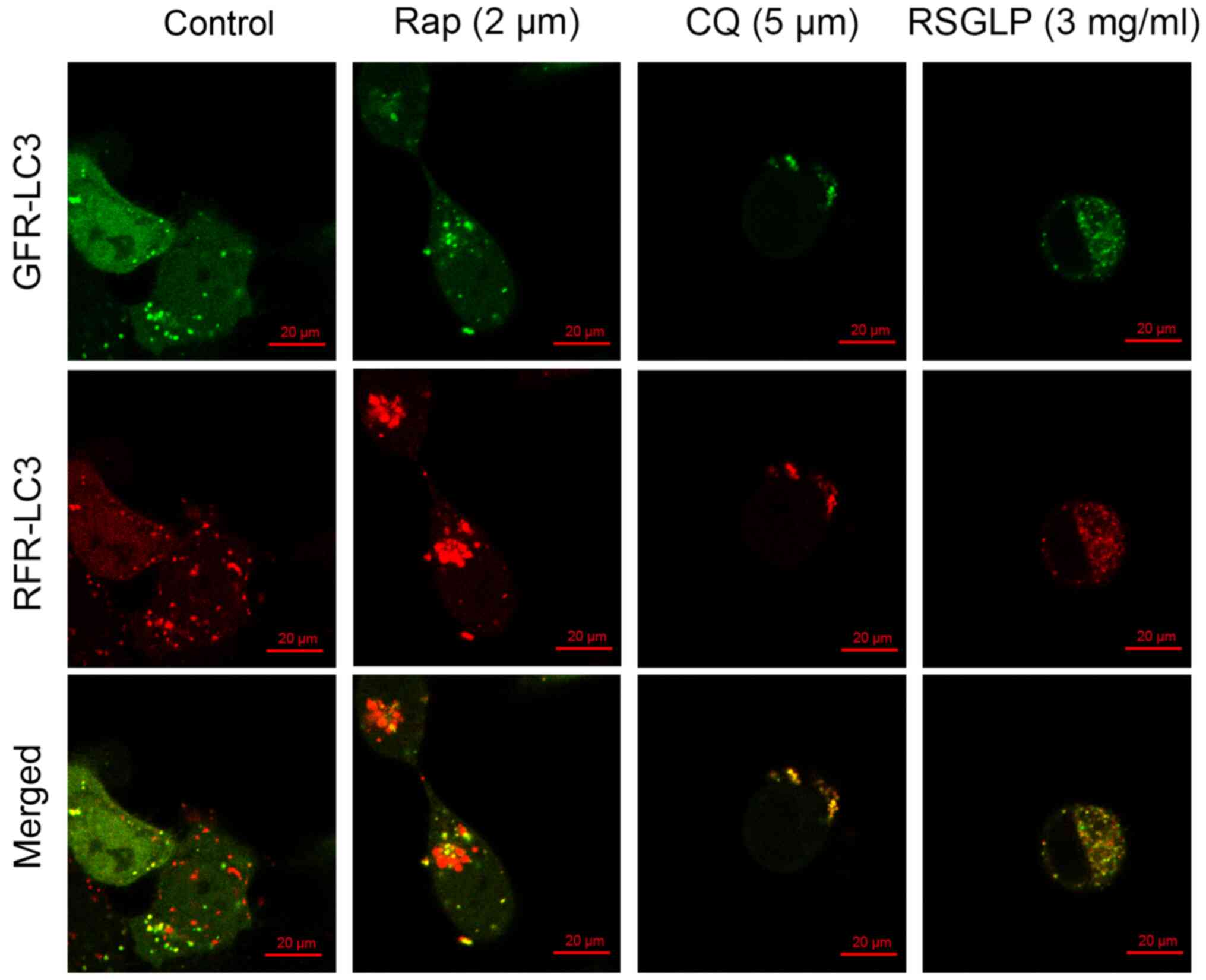
RSGLP blocks autophagosome-lysosome
fusion in AGS cells
Autophagosome-lysosome fusion is an important
process during autophagy. To further confirm the results of the
western blot analysis showing that RSGLP impaired autophagic flux
in AGS cells, AGS cells were transfected with mRFP-GFP-LC3
adenoviruses to monitor autophagosome-lysosome fusion. As shown in
Fig. 4, the autophagy inducer Rap
enhanced the presence of green and red puncta, with an increase in
the number of red puncta being visible, following merging of the
images. This suggested that Rap increased autolysosome fusion
normally in AGS cells without impairing autophagic flux. By
contrast, the late-stage autophagy inhibitor CQ showed yellow
rather than red puncta following merging of images, suggesting
disrupted autophagic flux (Fig. 4).
Similar to CQ, treatment with 3 mg/ml RSGLP also induced formation
of yellow rather than red puncta following merging, suggesting that
RSGLP blocked autophagosome-lysosome fusion and thus impaired
autophagic flux (Fig. 4). Notably,
more yellow puncta were observed in AGS cells treated with RSGLP
when compared with CQ treatment, suggesting that RSGLP may be a
more potent autophagy inhibitor than CQ.
Autophagosome accumulation and
autophagic flux disruption confers induction of apoptosis in AGS
cells
The association between autophagy and apoptosis is
complex. The present study investigated whether accumulation of
autophagosome and disruption of autophagic flux were involved in
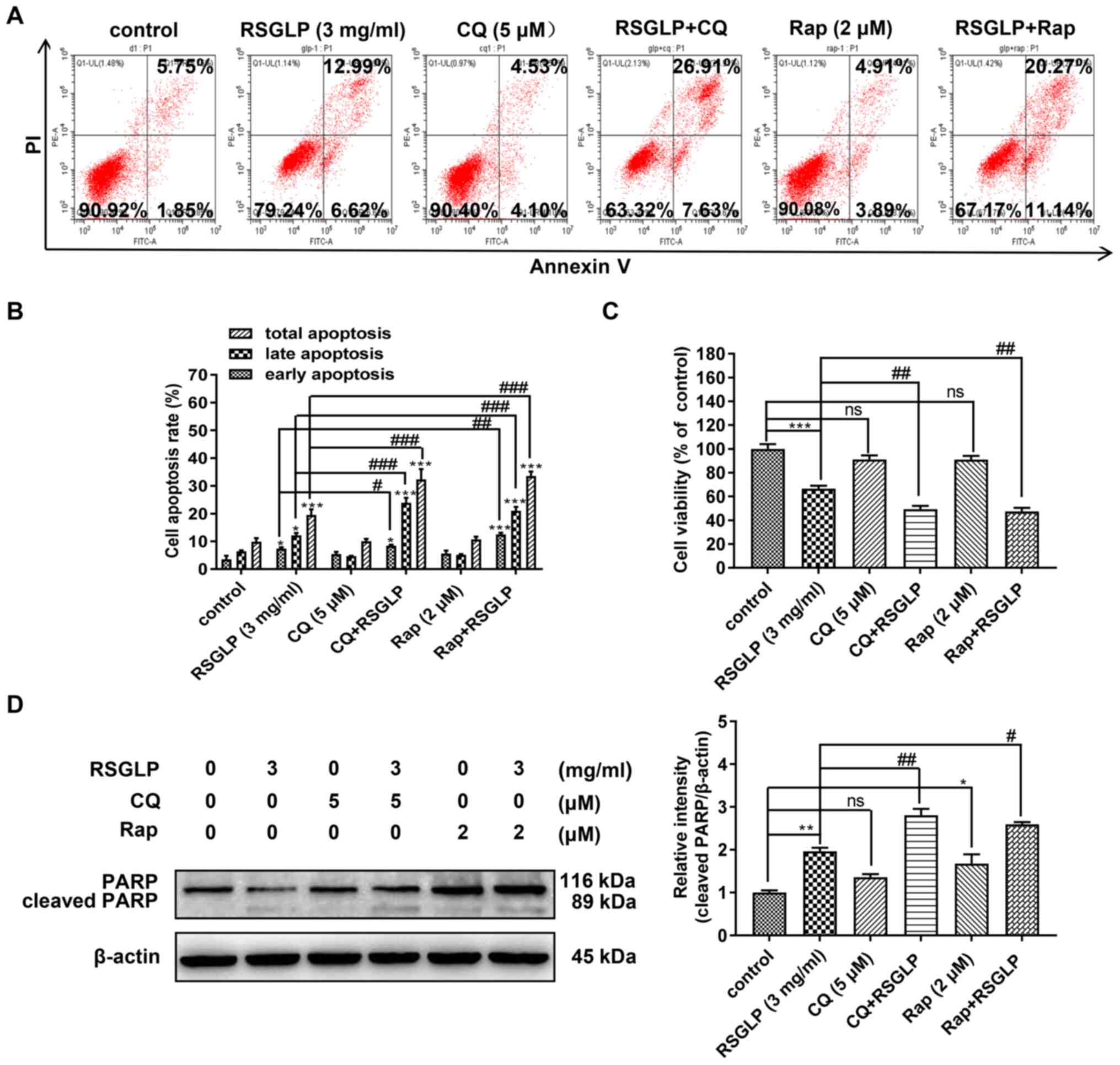
RSGLP-induced apoptosis in the AGS cells. Following AGS cells being
treated with RSGLP (3 mg/ml) with or without Rap (2 µM) or CQ (5
µM) for 36 h, flow cytometric analysis was used to assess the role
of autophagy in RSGLP-induced apoptosis in AGS cells. Compared with
RSGLP alone, RSGLP co-treatment with CQ (5 µM) or Rap (2 µM) all
further significantly increased the proportions of late apoptotic
cells from 12.99 to 26.91 or 20.27%, and increased the percentages
of total apoptotic cells from 19.61 to 34.53 or 31.41%,
respectively (Fig. 5A and B). CQ or
Rap alone had no effects on apoptosis. Total, early and late
apoptotic cells were quantified and are presented in Fig. 5B. Subsequently, the effects of CQ or
Rap co-treatment with RSGLP on cell viability were investigated. As
shown in Fig. 5C, CQ or Rap alone
has little effect on cell viability. However, compared with RSGLP
alone, RSGLP co-treatment with CQ or Rap further decreased cell
viability in AGS cells (Fig. 5C).
Furthermore, as shown in Fig. 5D,
RSGLP-induced PARP cleavage was upregulated at the protein level
following combined treatment with CQ (5 µM) or Rap (2 µM) with
RSGLP for 24 h. Using specific antibodies for total PARP and
cleaved-PARP, similar results were obtained (Fig. S3). Densitometric analysis of
cleaved-PARP is presented in Fig.
5D. Taken together, these results suggested that RSGLP-induced
cell death and apoptosis was mediated at least in part through
autophagosome accumulation and autophagic flux disruption in AGS
cells.
 | Figure 5.Autophagosome accumulation and
autophagy flux disruption further increases RSGLP-induced cell
apoptosis in AGS cells. (A) Flow cytometric analysis of apoptosis.
AGS cells were treated with RSGLP (3 mg/ml) with or without CQ (5
µM) or Rap (2 µM) for 36 h. (B) Percentage of early, late and total
apoptotic cells from three individual experiments. (C) MTT analysis
of cell viability. AGS cells were treated with RSGLP (3 mg/ml) with
or without CQ (5 µM) or Rap (2 µM) for 36 h. (D) PARP and
cleaved-PARP expression as determined by western blotting. AGS
cells were treated with RSGLP (3 mg/ml) with or without CQ (5 µM)
or Rap (2 µM) for 36 h. Densitometric analysis of the relative
intensities of cleaved-PARP following normalization against
β-actin. Data are presented as the mean ± standard error of the
mean (n=3). A two-way analysis of variance, followed by a Tukey's
post-hoc, test was used to compare the data. *P<0.05,
**P<0.01, ***P<0.001 vs. control; #P<0.05,
##P<0.01, ###P<0.001 vs. RSGLP alone.
ns, not significant vs. control. RSGLP, sporoderm-removed spores of
G. lucidum polysaccharide; CQ, chloroquine; Rap,
rapamycin. |
Discussion
G. lucidum has numerous pharmacological
properties, including immunomodulatory, antioxidative,
antidiabetic, antiallergenic, cardiovascular protective and
anticancer effects (26). G.
lucidum contains several bioactive compounds, including
polysaccharide, alkaloids, triterpenoids, lactones and steroids
(16). It is known that the
antitumor activity of G lucidum is primarily attributed to
polysaccharides and triterpenoids (27). Several studies have investigated the
effects of GLPs extracted from the fruiting body or mycelia of
G. lucidum. Recently, more attention has been focused on
BSGL, which contains a greater quantity of bioactive compounds and
possesses more potent biological effects (28,29).
However, there are no studies investigating the anticancer effects
of GLP extracted from RSGLP, or comparing the anticancer effects of
BSGLP and RSGLP, in particular in gastric cancer.
RSGLP is a more recently discovered G.
lucidum constituent, in which the sporoderm is completely
removed from the spores. The present study reported that RSGLP was
more potent than BSGLP in inhibiting gastric cancer cell viability.
The IC50 values were considerably lower for RSGLP than
the IC50 of BSGLP when assessing their cytotoxic effects
on gastric cancer cell lines. A recent study by Li et al
(21) reported that the
immunomodulatory effects of triterpenoids extracted from RSGLP were
higher than that of BSGLP. The present study further investigated
the mechanisms of the anticancer effects elicited by RSGLP. RSGLP
induced apoptosis in gastric cancer AGS cells, which was mediated
by RSGLP-induced autophagosome accumulation and disruption of
autophagic flux. To the best of our knowledge, the present study
was the first to examine the anti-cancer effects and mechanisms of
GLPs extracted from the sporoderm-removed spores of G.
lucidum in gastric cancer. The results suggested that
inhibiting autophagy may be an important anticancer mechanism of
GLPs against gastric cancer.
Using multiple well-characterized gastric cancer
cell lines to reflect the diversity of tumor phenotypes may provide
a more accurate means of investigating the anticancer mechanism of
potential anticancer drugs (30). To
investigate the cytotoxic effects of BSGLP and RSGLP in
vitro, three human gastric cell lines, MKN28, NCI-N87 and AGS,
were used. The MKN28 cell line is a moderately-differentiated
tubular adenocarcinoma, which first originated from a Japanese
70-year-old female patient in 1975 (31). NCI-N87 was derived from
well-differentiated adenocarcinomas, which was established from a
liver metastasis of a gastric carcinoma from a Caucasian patient
(32). The moderately-differentiated
AGS cell line was derived from fragments of a tumor resected from a
female Caucasian patient with gastric cancer (32). Among these three cell lines, AGS has
been the most extensively studied, and is suitable for in
vitro and in vivo xenograft tumor models (33,34). The
present study assessed the effects of RSGLP and BSGLP in these
three cell lines, and these compounds were more effective in
inhibiting cell viability in AGS cells, which had the lowest
IC50 value. Therefore, subsequent experiments were
performed on AGS cells.
Autophagy is a complex process, which consists of
several steps, including the formation of autophagosomes, fusion
with lysosomes and degradation of autophagosomes, also termed
autophagic flux (35). LC3-II, a key
player of autophagy, and is localized to autophagosomes and
autolysosomes, and is finally degraded by lysosomal hydrolases
(36). P62 is a ubiquitin-binding
scaffold protein, which binds with LC3-II in autophagosomes and is
degraded in autolysosomes (37).
Since p62 accumulation is considered as impairment of autophagic
flux, whereas decreased levels are indicative of autophagy
activation, p62 is regarded as a monitor of autophagic degradation
(38). GLPs induce their anti-cancer
effects through various mechanisms, including immune-regulation,
inhibition of migration, induction of apoptosis, suppression of
proliferation and suppression of tumor angiogenesis (39–41).
Furthermore, targeting autophagy is a relatively novel therapeutic
strategy for treating cancer (42).
However, whether targeting of autophagy is a potential anticancer
mechanism of GLPs in gastric cancer remains largely unknown.
The results of the present study demonstrated that
RSGLP induced autophagy and inhibited autophagic flux in AGS cells
by increasing the expression of LC3-II and p62. CQ was first
synthesized as an antimalarial drug in 1946 but was commonly used
as a late-stage autophagy inhibitor, exerting its effects through
neutralizing lysosomal pH (43). CQ
and hydroxychloroquine (HCQ) are two of the most widely tested
drugs for inhibition of autophagy, both of which have been used in
several clinical trials (44,45). A
meta-analysis demonstrated that co-treatment with CQ and HCQ
exerted better clinical responses compared with chemotherapy or
radiation therapy alone in different types of cancer (46). Based on the results of the present
study, combined treatment of AGS cells with CQ and RSGLP increased
the expression of LC3-II and p62 compared with RSGLP alone,
suggesting that RSGLP induced autophagy and impaired autophagic
flux. Notably, RSGLP inhibited the increase in expression of LC3-II
and p62 compared with CQ alone, suggesting that RSGLP may serve as
a promising autophagy inhibitor. In line with the results of the
present study, previous studies have also demonstrated that BSGLP
blocks autophagy in human colorectal cancer and osteosarcoma cancer
in vitro and in vivo (47,48).
Ginsenoside Compound K, an active metabolite of ginsenosides
isolated from Panax ginseng C.A, has been reported to block
autophagic flux in human neuroblastoma cells in vitro and
in vivo (49). However, the
role of autophagy in cancer is complex and context- dependent
(50). In contrast to our results,
the methanolic extract of G. lucidum also increased LC3-II,
but decreased p62 at the protein level, demonstrating that the
methanolic extract of G. lucidum causes induction of
autophagic flux rather than inhibition (51).
It has been proposed that the interplay between
autophagy and apoptosis may contribute toward the anti-cancer
effects of several anti-cancer agents (52,53).
Although there have been several studies showing the association
between autophagy and apoptosis, the association has not been
demonstrated clearly, and the crosstalk between autophagy and
apoptosis in cancer is controversial (54). The present study reported that
treating AGS cells with RSGLP together with either Rap or CQ
further increased RSGLP-induced PARP cleavage and the rate of
apoptotic cells, compared with RSGLP alone. In addition, it was
revealed that CQ or Rap alone has minimal effects on cell
viability. Notably, RSGLP co-treatment with CQ or Rap further
decreased cell viability in AGS cells. These results suggested that
RSGLP-induced autophagosome accumulation and autophagic flux
disruption contributed toward BSGLP-induced apoptosis and cell
death in AGS cells. However, exactly how much apoptosis or
autophagy contribute toward cell death remains unclear. More
recently, it was also reported that BSGLP induced accumulation of
autophagosomes and this disruption of autophagy was responsible for
BSGLP-induced apoptosis in colorectal cancer cells, mediated via
the MAPK/ERK signaling pathway (47). Consistent with the results of the
present study, a substantial number of studies have indicated that
excessive accumulation of autophagosomes may induce cell apoptosis
(55–58). Marsdenia tenacissima extracts
induced apoptosis and suppressed autophagy through ERK activation
in lung cancer cells (59).
Scutellaria Radix has been demonstrated to promote apoptosis
in non-small cell lung cancer cells via the induction of the
AMPK-dependent autophagy (60).
Further studies are still required to investigate
the potential signaling pathways responsible for RSGLP-induced
autophagosome accumulation and autophagic flux disruption in AGS
cells. Furthermore, the signaling pathway that from RSGLP
stimulation to Bcl-2 reduction and pro-Caspase cleavage requires
further investigation. It has been well established that Bcl-2 may
protect cells from apoptosis by acting at a point downstream from
releasing mitochondrial cytochrome C, thereby preventing a
Caspase-3-dependent proteolytic cascade (61,62). The
release of cytochrome c into the cytosol triggers Caspase-3
activation through the formation of the
cytochrome-C/Apaf-1/caspase-9-containing apoptosome complex
(62). However, Kirsch et al
(63) reported that due to a
positive feedback regulatory mechanism, cleavage of Bcl-2 is
dependent on caspase-3 activation, and thus may further promote
apoptosis. Further studies are required to investigate the
signaling cascades between Bcl-2 and caspase-3 upon RSGLP treatment
in AGS cells, which may aid in identifying the molecular targets of
RSGLP against gastric cancer.
The antitumor effects of RSGLP in gastric cancer
should also be investigated in vivo in the future. Another
limitation of the present study was that, at present, it is not
clear exactly how RSGLP stimulates p62 accumulation and LC3-II
activation. It has been reported that several anticancer agents
exert anticancer effects through inducing oxidative stress and via
activation of the nuclear factor erythroid 2-related factor 2
(Nrf2), a transcription factor that protects cells against
oxidative stress (64). Furthermore,
p62 is transcriptionally regulated by Nrf2, and forms a positive
feedback loop with Nrf2 and Kelch-like ECH-associated protein 1
(Keap1), to regulate autophagy and cell apoptosis upon oxidative
stress (65,66). Whether accumulation of p62 was the
result of RSGLP-induced oxidative stress, and whether the
Nrf2-Keap-p62 feedback loop was involved requires further
investigation.
It is worth investigating whether RSGLP enters the
gastric cancer cells via specific receptors. It has been reported
that polysaccharides may bind to receptors on immune cells,
including B-cell (67) and
macrophages (68). However, at
present how polysaccharides enter the cell membrane in cancer cells
and whether polysaccharides may bind to certain receptors in cancer
cells have not been reported. Further studies are required to
address this question. Another limitation of the present study is
that the majority of the mechanism studies are performed only in
AGS cells. As shown in the supplemental materials, RSGLP also
induced LC3 and p62 upregulation in MKN28 and NCI-N87 cell lines,
suggesting that autophagosome accumulation and autophagy flux
disruption are common in gastric cancer cells upon RSGLP
treatment.
Several studies have demonstrated that GLP exhibits
excellent anti-cancer effects; however, clinical studies
investigating the anti-cancer effects of GLP are very limited. A
meta-analysis, which included five randomized controlled trials
(RTCs) with a total of 373 patients with cancer reported that
patients who had been given G. lucidum alongside
chemo/radiotherapy were more likely to respond positively, compared
with chemo/radiotherapy alone (69).
Among these five RTCs, four studies demonstrated that patients
treated with G. lucidum had relatively improved qualities of
life, compared with the control group (70–73).
However, these studies also reported that if G. lucidum was
used alone, it did not exert any beneficial effects when used for
treatment of advanced cancer. A more recent meta-analysis, which
included 23 trials revealed that C. versicolor- and G.
lucidum-related natural products were significantly associated
with a lower risk of mortality and higher total efficacy but was
not associated with control rate compared with the control
treatment (74). Nevertheless,
clinical studies investigating the anticancer effects of GLPs are
still lacking. Therefore, the antitumor effects of GLPs,
particularly RSGLP against gastric cancer should be investigated in
clinical studies.
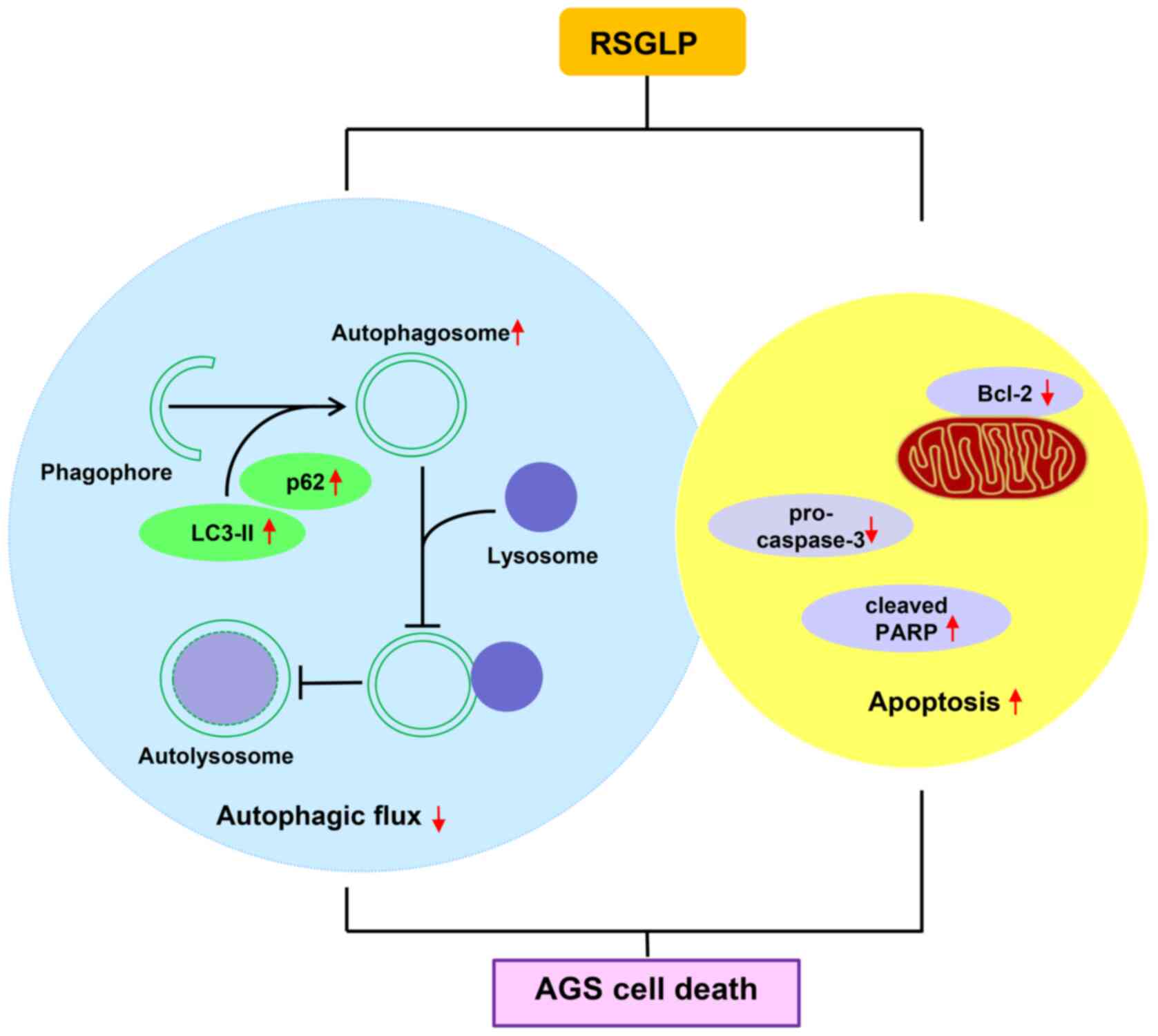
In conclusion, the results of the present study
indicated that RSGLP is more potent at inhibiting gastric cancer
cell viability than BSGLP. Additionally, RSGLP disrupted autophagic
flux and induced autophagosome accumulation, which contributed
toward RSGLP-induced apoptosis in AGS cells (Fig. 6). These observations suggested that
targeting autophagic flux and inducing autophagosome accumulation
may serve as an effective strategy to inhibit carcinogenesis. The
results provide a theoretical basis for the development of RSGLP as
an autophagy inhibitor to treat gastric and possibly other types of
cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Key
Foundation of Science and Technology Department of Zhejiang
Province (grant no. 2019C02100) and the National Natural Science
Foundation of China (grant no. 81973521).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW designed the study, provided funding support,
guided the experiments and edited the manuscript. JZ performed the
majority of the experiments, performed the data analysis, and
drafted and edited the manuscript. LF, RC, JX, DG, CJG, CLG, JC and
CC contributed toward data analysis and provided technical support
in all the experiments. XW and JZ confirmed the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kobayashi J: Effect of diet and gut
environment on the gastrointestinal formation of N-nitroso
compounds: A review. Nitric Oxide Biol Chem. 73:66–73. 2018.
View Article : Google Scholar
|
|
3
|
Rawla P and Barsouk A: Epidemiology of
gastric cancer: Global trends, risk factors and prevention. Prz
Gastroenterol. 14:26–38. 2019.PubMed/NCBI
|
|
4
|
Yang Z and Klionsky DJ: Eaten alive: A
history of macroautophagy. Nat Cell Biol. 12:814–822. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dikic I and Elazar Z: Mechanism and
medical implications of mammalian autophagy. Nat Rev Mol Cell Biol.
19:349–364. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
White E: Deconvoluting the
context-dependent role for autophagy in cancer. Nat Rev Cancer.
12:401–410. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
White E: The role for autophagy in cancer.
J Clin Invest. 125:42–46. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guzmán EA, Pitts TP, Cristina Diaz MC and
Wright AE: The marine natural product Scalarin inhibits the
receptor for advanced glycation end products (RAGE) and autophagy
in the PANC-1 and MIA PaCa-2 pancreatic cancer cell lines. Invest
New Drugs. 37:262–270. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim TW, Lee SY, Kim M, Cheon CH and Ko SG:
Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway
and inhibition of G9a in gastric cancer cells. Cell Death Dis.
9:8752018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim SM, Vetrivel P, Ha SE, Kim HH, Kim JA
and Kim GS: Apigetrin induces extrinsic apoptosis, autophagy and
G2/M phase cell cycle arrest through PI3K/AKT/mTOR pathway in AGS
human gastric cancer cell. J Nutr Biochem. 83:1084272020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bishop KS, Kao CH, Xu Y, Glucina MP,
Paterson RR and Ferguson LR: From 2000 years of Ganoderma
lucidum to recent developments in nutraceuticals.
Phytochemistry. 114:56–65. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Na K, Li K, Sang TT, Wu KK, Wang Y and
Wang XY: Anticarcinogenic effects of water extract of
sporoderm-broken spores of Ganoderma lucidum on colorectal
cancer in vitro and in vivo. Int J Oncol.
50:1541–1554. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang WF, Gou XH, Xue H and Liu K:
Ganoderan (GDN) regulates the growth, motility and apoptosis of
non-small cell lung cancer cells through ERK signaling pathway in
vitro and in vivo. Onco Targets Ther. 12:8821–8832. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Acevedo-Diaz A, Ortiz-Soto G,
Suarez-Arroyo IJ, Zayas-Santiago A and Martinez Montemayor MM:
Ganoderma lucidum extract reduces the motility of breast
cancer cells mediated by the RAC-Lamellipodin axis. Nutrients.
11:11162019. View Article : Google Scholar
|
|
15
|
Gao Y, Zhou S, Jiang W, Huang M and Dai X:
Effects of ganopoly (a Ganoderma lucidum polysaccharide
extract) on the immune functions in advanced-stage cancer patients.
Immunol Invest. 32:201–215. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ahmad MF: Ganoderma lucidum:
Persuasive biologically active constituents and their health
endorsement. Biomed Pharmacother. 107:507–519. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chan WK, Lam DT, Law HK, Wong WT, Koo MW,
Lau AS, Lau YL and Chan GC: Ganoderma lucidum mycelium and
spore extracts as natural adjuvants for immunotherapy. J Altern
Complement Med. 11:1047–1057. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu T, Zhou J, Li W, Rong X, Gao Y, Zhao
L, Fan Y, Zhang J, Ji C and Ma Q: Effects of sporoderm-broken
spores of Ganoderma lucidum on growth performance,
antioxidant function and immune response of broilers. Anim Nutr.
6:39–46. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu T, Ma QG, Zhao LH, Jia R, Zhang JY, Ji
C and Wang XY: Protective effects of sporoderm-broken spores of
Ganoderma lucidum on growth performance, antioxidant
capacity and immune function of broiler chickens exposed to low
level of Aflatoxin B1. Toxins. 8:2782016. View Article : Google Scholar
|
|
20
|
Zhao D, Chang MW, Li JS, Suen W and Huang
J: Investigation of ice-assisted sonication on the microstructure
and chemical quality of Ganoderma lucidum spores. J Food
Sci. 79:E2253–E2265. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Z, Shi YQ, Zhang X, Xu J, Wang H, Zhao
L and Wang Y: Screening imunoactive compounds of Ganoderma
lucidum spores by mass spectrometry molecular networking
combined with in vivo zebrafish assays. Front Pharmacol.
11:2872020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ayyoob K, Masoud K, Vahideh K and
Jahanbakhsh A: Authentication of newly established human esophageal
squamous cell carcinoma cell line (YM-1) using short tandem repeat
(STR) profiling method. Tumour Biol. 37:3197–3204. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li X, Zhao R, Zhou HL and Wu DH:
Deproteinization of polysaccharide from the stigma maydis by sevag
method. Adv Mat Res. 340:416–420. 2011.
|
|
24
|
Lee CS, Bishop ES, Zhang R, Yu X, Farina
EM, Yan S, Zhao C, Zheng Z, Shu Y, Wu X, et al: Adenovirus-mediated
gene delivery: Potential applications for gene and cell-based
therapies in the new era of personalized medicine. Genes Dis.
4:43–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rong L, Li Z, Leng X, Li H, Ma Y, Chen Y
and Song F: Salidroside induces apoptosis and protective autophagy
in human gastric cancer AGS cells through the PI3K/Akt/mTOR
pathway. Biomed Pharmacother. 122:1097262020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cör D, Knez Ž and Knez Hrnčič M:
Antitumour, antimicrobial, antioxidant and antiacetylcholinesterase
effect of Ganoderma lucidum terpenoids and polysaccharides:
A review. Molecules. 23:6492018. View Article : Google Scholar
|
|
27
|
Kladar NV, Gavarić NS and Božin BN:
Ganoderma: Insights into anticancer effects. Eur J Cancer
Prev. 25:462–471. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Min BS, Nakamura N, Miyashiro H, Bae KW
and Hattori M: Triterpenes from the spores of Ganoderma
lucidum and their inhibitory activity against HIV-1 protease.
Chem Pharm Bull. 46:1607–1612. 1998. View Article : Google Scholar
|
|
29
|
Su JY, Su L, Li D, Shuai O, Zhang YF,
Liang HJ, Jiao CW, Xu ZC, Lai Y and Xie YZ: Antitumor activity of
extract from the sporoderm-breaking spore of Ganoderma
lucidum: Restoration on exhausted cytotoxic T cell with gut
microbiota remodeling. Front Immunol. 9:17652018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park JG, Frucht H, LaRocca RV, Bliss DP,
Kurita Y, Chen TR, Henslee JG, Trepel JB, Jensen RT, Johnson BE, et
al: Characteristics of cell lines established from human gastric
carcinoma. Cancer Res. 50:2773–2780. 1990.PubMed/NCBI
|
|
31
|
Motoyama T, Hojo H and Watanabe H:
Comparison of seven cell lines derived from human gastric
carcinomas. Acta Pathol Jpn. 36:65–83. 1986.PubMed/NCBI
|
|
32
|
Mattioli E, Vogiatzi P, Sun A, Abbadessa
G, Angeloni G, D'Ugo D, Trani D, Gaughan JP, Vecchio FM, Cevenini
G, et al: Immunohistochemical analysis of pRb2/p130, VEGF, EZH2,
p53, p16(INK4A), p27(KIP1), p21(WAF1), Ki-67 expression patterns in
gastric cancer. J Cell Physiol. 210:183–191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Barati T, Haddadi M, Sadeghi F,
Muhammadnejad S, Muhammadnejad A, Heidarian R, Arjomandnejad M and
Amanpour S: AGS cell line xenograft tumor as a suitable gastric
adenocarcinoma model: Growth kinetic characterization and
immunohistochemistry analysis. Iran J Basic Med Sci. 21:678–681.
2018.PubMed/NCBI
|
|
34
|
Barranco SC, Townsend CM, Quraishi MA,
Burger NL, Nevill HC, Howell KH and Boerwinkle WR: Heterogeneous
responses of an in vitro model of human stomach cancer to
anticancer drugs. Invest New Drugs. 1:117–127. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li X, He S and Ma B: Autophagy and
autophagy-related proteins in cancer. Mol Cancer. 19:122020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schaaf MB, Keulers TG, Vooijs MA and
Rouschop KM: LC3/GABARAP family proteins: Autophagy-(un)related
functions. FASEB J. 30:3961–3978. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lamark T, Svenning S and Johansen T:
Regulation of selective autophagy: The p62/SQSTM1 paradigm. Essays
Biochem. 61:609–624. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Klionsky DJ, Abdelmohsen K, Abe A, Abedin
MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD,
Adeli K, et al: Guidelines for the use and interpretation of assays
for monitoring autophagy (3rd edition). Autophagy. 12:1–222. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liang ZE, Yi YJ, Guo YT, Wang RC, Hu QL
and Xiong XY: Inhibition of migration and induction of apoptosis in
LoVo human colon cancer cells by polysaccharides from Ganoderma
lucidum. Mol Med Rep. 12:7629–7636. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sohretoglu D and Huang S: Ganoderma
lucidum polysaccharides as an anti-cancer agent. Anticancer
Agents Med Chem. 18:667–674. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang C, Shi S, Chen Q, Lin S, Wang R, Wang
S and Chen C: Antitumor and immunomodulatory activities of
Ganoderma lucidum polysaccharides in glioma-bearing rats.
Integr Cancer Ther. 17:674–683. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Amaravadi RK, Kimmelman AC and Debnath J:
Targeting autophagy in cancer: Recent advances and future
directions. Cancer Discov. 9:1167–1181. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kimura T, Takabatake Y, Takahashi A and
Isaka Y: Chloroquine in cancer therapy: a double-edged sword of
autophagy. Cancer Res. 73:3–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Karasic TB, O'Hara MH, Loaiza-Bonilla A,
Reiss KA, Teitelbaum UR, Borazanci E, De Jesus-Acosta A, Redlinger
C, Burrell JA, Laheru DA, et al: Effect of gemcitabine and
nab-paclitaxel with or without hydroxychloroquine on patients With
Advanced Pancreatic Cancer: A phase 2 randomized clinical trial.
JAMA Oncol. 5:993–998. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sotelo J, Briceño E and López-González MA:
Adding chloroquine to conventional treatment for glioblastoma
multiforme: A randomized, double-blind, placebo-controlled trial.
Ann Intern Med. 144:337–343. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xu R, Ji Z, Xu C and Zhu J: The clinical
value of using chloroquine or hydroxychloroquine as autophagy
inhibitors in the treatment of cancers: A systematic review and
meta-analysis. Medicine (Baltimore). 97:e129122018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pan H, Wang Y, Na K, Wang Y, Wang L, Li Z,
Guo C, Guo D and Wang X: Autophagic flux disruption contributes to
Ganoderma lucidum polysaccharide-induced apoptosis in human
colorectal cancer cells via MAPK/ERK activation. Cell Death Dis.
10:4562019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang W, Lei Z, Meng J, Li G, Zhang Y, He
J and Yan W: Water extract of sporoderm-broken spores of
Ganoderma lucidum induces osteosarcoma apoptosis and
restricts autophagic flux. Onco Targets Ther. 12:11651–11665. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Oh JM, Kim E and Chun S: Ginsenoside
compound K induces ros-mediated apoptosis and autophagic inhibition
in human neuroblastoma cells in vitro and in vivo. Int J Mol Sci.
20:42792019. View Article : Google Scholar
|
|
50
|
Kimmelman AC: The dynamic nature of
autophagy in cancer. Genes Dev. 25:1999–2010. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Reis FS, Lima RT, Morales P, Ferreira IC
and Vasconcelos MH: Methanolic extract of Ganoderma lucidum
induces autophagy of AGS human gastric tumor cells. Molecules.
20:17872–17882. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Marino G, Niso-Santano M, Baehrecke EH and
Kroemer G: Self-consumption: The interplay of autophagy and
apoptosis. Nat Rev Mol Cell Biol. 15:81–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cooper KF: Till death do us part: The
marriage of autophagy and apoptosis. Oxidative Med Cell Longev.
2018:47012752018. View Article : Google Scholar
|
|
54
|
Gump JM and Thorburn A: Autophagy and
apoptosis: What is the connection? Trends Cell Biol. 21:387–392.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Choi PR, Kang YJ, Sung B, Kim JH, Moon HR,
Chung HY, Kim SE, Park IP, Park MI, Park SJ and Kim ND:
MHY218-induced apoptotic cell death is enhanced by the inhibition
of autophagy in AGS human gastric cancer cells. Int J Oncol.
47:563–572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yi H, Wang K, Du B, He L, Ho H, Qiu M, Zou
Y, Li Q, Jin J, Zhan Y, et al: Aleuritolic acid impaired autophagic
flux and induced apoptosis in hepatocellular carcinoma HepG2 cells.
Molecules. 23:13382018. View Article : Google Scholar
|
|
57
|
Kaneko A, Kiryu-Seo S, Matsumoto S and
Kiyama H: Correction: Damage-induced neuronal endopeptidase (DINE)
enhances axonal regeneration potential of retinal ganglion cells
after optic nerve injury. Cell Death Dis. 11:5412020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yang C, Wu C, Xu DJ, Wang M and Xia Q:
Astragaloside II inhibits autophagic flux and enhances
chemosensitivity of cisplatin in human cancer cells. Biomed
Pharmacother. 81:166–175. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jiao YN, Wu LN, Xue D, Liu XJ, Tian ZH,
Jiang ST, Han SY and Li PP: Marsdenia tenacissima extract induces
apoptosis and suppresses autophagy through ERK activation in lung
cancer cells. Cancer Cell Int. 18:1492018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kim HI, Hong SH, Ku JM, Lim YS, Lee SJ,
Song J, Kim TY, Cheon C and Ko SG: Scutellaria Radix promotes
apoptosis in non-small cell lung cancer cells via induction of
AMPK-dependent autophagy. Am J Chin Med. 47:691–705. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Swanton E, Savory P, Cosulich S, Clarke P
and Woodman P: Bcl-2 regulates a caspase-3/caspase-2 apoptotic
cascade in cytosolic extracts. Oncogene. 18:1781–1787. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kirsch DG, Doseff A, Chau BN, Lim DS, de
Souza-Pinto NC, Hansford R, Kastan MB, Lazebnik YA and Hardwick JM:
Caspase-3-dependent cleavage of Bcl-2 promotes release of
cytochrome c. J Biol Chem. 274:21155–21161. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sporn MB and Liby KT: NRF2 and cancer: The
good, the bad and the importance of context. Nat Rev Cancer.
12:564–571. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tang Z, Hu B, Zang F, Wang J, Zhang X and
Chen H: Nrf2 drives oxidative stress-induced autophagy in nucleus
pulposus cells via a Keap1/Nrf2/p62 feedback loop to protect
intervertebral disc from degeneration. Cell Death Dis. 10:5102019.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wu X, Sun R, Wang H, Yang B, Wang F, Xu H,
Chen S, Zhao R, Pi J and Xu Y: Enhanced p62-NRF2 feedback loop due
to impaired autophagic flux contributes to Arsenic-induced
malignant transformation of human keratinocytes. Oxid Med Cell
Longev. 2019:10389322019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhang X, Ding R, Zhou Y, Zhu R, Liu W, Jin
L, Yao W and Gao X: Toll-like receptor 2 and Toll-like receptor
4-dependent activation of B cells by a polysaccharide from marine
fungus Phoma herbarum YS4108. PLoS One. 8:e607812013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Chen S, Yin DK, Yao WB, Wang YD, Zhang YR
and Gao XD: Macrophage receptors of polysaccharide isolated from a
marine filamentous fungus Phoma herbarum YS4108. Acta Pharmacol
Sin. 30:1008–1014. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Jin X, Ruiz Beguerie J, Sze DM and Chan
GC: Ganoderma lucidum (Reishi mushroom) for cancer
treatment. Cochrane Database Syst Rev. 4:CD0077312016.PubMed/NCBI
|
|
70
|
Gao Y, Dai X, Chen G, Ye J and Zhou S: A
randomized, placebo-controlled, multicenter study of Ganoderma
lucidum (w.curt.:fr.) Lloyd (aphyllophoromycetideae)
polysaccharides (ganopoly) in patients with advanced lung cancer.
Int J Med Mushrooms. 5:369–381. 2003. View Article : Google Scholar
|
|
71
|
He W and Yi J: Study of clinical eficacy
of Lingzhi spore capsule on tumour patients with
chemotherapy/radiotherapy. Clin J Trad Chin Med. 9:292–293.
1997.
|
|
72
|
Yan B, Wei Y and Li Y: Efect of Laojunxian
Lingzhi oral liquid combined with chemotherapy on non-parvicellular
lung cancer at stage II and III. Trad Chin Drug Res Clin Pharmacol.
9:78–80. 1998.
|
|
73
|
Zhang X, Jia Y, Li Q, Niu S, Zhu S and
Shen C: Clinical curative efect investigation of Lingzhi tablet on
lung cancer. Chin Trad Pat Med. 22:486–488. 2000.
|
|
74
|
Zhong LD, Yan PJ, Lam WC, Yao L and Bian
ZX: Coriolus Versicolor and Ganoderma Lucidum related
natural products as an adjunct therapy for cancers: A systematic
review and meta-analysis of randomized controlled trials. Front
Pharmacol. 10:7032019. View Article : Google Scholar : PubMed/NCBI
|